Abstract
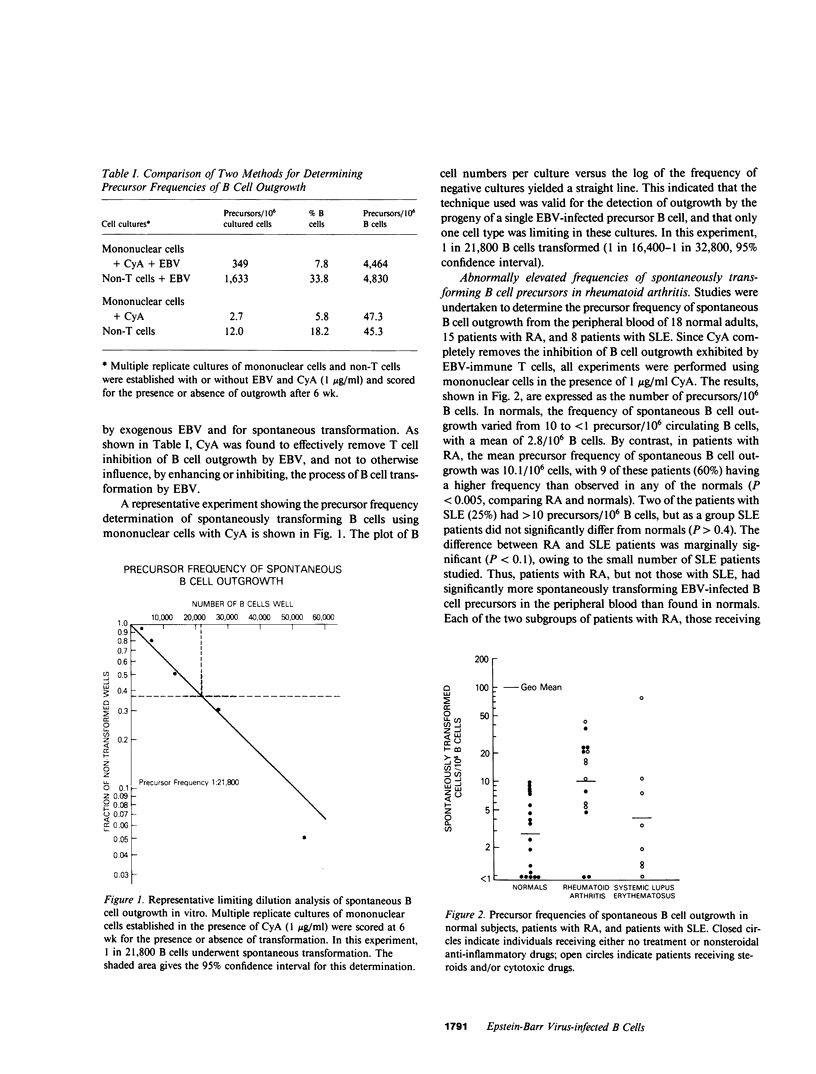
Patients with rheumatoid arthritis (RA) are known to have in vitro regulatory T cell abnormalities relating to Epstein-Barr virus (EBV). In this report, we asked whether patients with RA have more circulating EBV-infected B cells than normals. To address this question, we determined the frequency of spontaneously transforming B cells in the peripheral blood of 18 normals, 15 patients with RA, and 8 patients with systemic lupus erythematosus (SLE). The mean frequency of spontaneously transforming B cells in RA patients was 10.1/10(6) B cells, which was significantly greater than that of the normal controls, 2.8/10(6) B cells (P less than 0.005). The group of patients with SLE did not differ from the normals (P greater than 0.4). In further studies undertaken to investigate as to whether RA B cells are more easily transformed by EBV than normal B cells, we determined that the frequencies of transforming B cells in the presence of exogenous EBV were similar in RA patients and normals. Lymphocytes obtained from patients with RA demonstrate a profound T cell defect in their EBV-specific suppression, as measured in vitro; there was no direct correlation, however, between this in vitro T cell abnormality and the number of circulating EBV-infected B cells. Thus, patients with RA, as a group, have abnormally elevated numbers of circulating EBV-infected B cells, and this abnormality most likely derives from a complex dysregulation of the defense mechanisms for infection with EBV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh M. A., Henle G., Lennette E. T., Henle W. Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J Clin Invest. 1981 Apr;67(4):1134–1140. doi: 10.1172/JCI110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Jensen F. C., Rabin H., Tan E. M. Lymphocytes transformed by Epstein-Barr virus. Induction of nuclear antigen reactive with antibody in rheumatoid arthritis. J Exp Med. 1978 Apr 1;147(4):1018–1027. doi: 10.1084/jem.147.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwick P. A., Bluestein H. G., Zvaifler N. J., Depper J. M., Seegmiller J. E. Altered regulation of Epstein-Barr virus induced lymphoblast proliferation in rheumatoid arthritis lymphoid cells. Arthritis Rheum. 1980 Jun;23(6):626–632. doi: 10.1002/art.1780230603. [DOI] [PubMed] [Google Scholar]
- Bird A. G., McLachlan S. M., Britton S. Cyclosporin A promotes spontaneous outgrowth in vitro of Epstein-Barr virus-induced B-cell lines. Nature. 1981 Jan 22;289(5795):300–301. doi: 10.1038/289300a0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Bluestein H. G., Zvaifler N. J. Impaired regulation of Epstein-Barr virus-induced lymphocyte proliferation in rheumatoid arthritis is due to a T cell defect. J Immunol. 1981 Nov;127(5):1899–1902. [PubMed] [Google Scholar]
- Depper J. M., Zvaifler N. J. Epstein-Barr virus. Its relationship to the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1981 Jun;24(6):755–761. doi: 10.1002/art.1780240601. [DOI] [PubMed] [Google Scholar]
- Diehl V., Henle G., Henle W., Kohn G. Demonstration of a herpes group virus in cultures of peripheral leukocytes from patients with infectious mononucleosis. J Virol. 1968 Jul;2(7):663–669. doi: 10.1128/jvi.2.7.663-669.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P., Lucas S., Nonoyama M., Perlin E., Goldstein L. I. Oral excretion of Epstein-Barr virus by healthy subjects and patients with infectious mononucleosis. Lancet. 1972 Nov 11;2(7785):988–989. doi: 10.1016/s0140-6736(72)92402-6. [DOI] [PubMed] [Google Scholar]
- Heller M., Henderson A., Kieff E. Repeat array in Epstein-Barr virus DNA is related to cell DNA sequences interspersed on human chromosomes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5916–5920. doi: 10.1073/pnas.79.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Miller G., Robinson J., Heston L. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology. 1977 Jan;76(1):152–163. doi: 10.1016/0042-6822(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci M. G., Bejarano M. T., Masucci G., Klein E. Large granular lymphocytes inhibit the in vitro growth of autologous Epstein-Barr virus-infected B cells. Cell Immunol. 1983 Mar;76(2):311–321. doi: 10.1016/0008-8749(83)90374-x. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Wallace L. E., Epstein M. A. Sequential appearance of Epstein-Barr virus nuclear and lymphocyte-detected membrane antigens in B cell transformation. Nature. 1981 Jun 25;291(5817):664–666. doi: 10.1038/291664a0. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Wallace L. E., Epstein M. A. HLA-restricted T-cell recognition of Epstein-Barr virus-infected B cells. Nature. 1980 Feb 28;283(5750):865–867. doi: 10.1038/283865a0. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Smith D., Niederman J. Plasmacytic differentiation of circulating Epstein-Barr virus-infected B lymphocytes during acute infectious mononucleosis. J Exp Med. 1981 Feb 1;153(2):235–244. doi: 10.1084/jem.153.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi G., Felici A., Ragona G., Heinz A. Quantitative evaluation of Epstein-Barr-virus-infected mononuclear peripheral blood leukocytes in infectious mononucleosis. N Engl J Med. 1977 Jan 20;296(3):132–134. doi: 10.1056/NEJM197701202960302. [DOI] [PubMed] [Google Scholar]
- Rosén A., Gergely P., Jondal M., Klein G., Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977 May 5;267(5606):52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Chess L., Strominger J. L. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J Exp Med. 1977 Aug 1;146(2):495–508. doi: 10.1084/jem.146.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The suppression of Epstein-Barr virus infection in vitro occurs after infection but before transformation of the cell. J Immunol. 1980 Feb;124(2):745–751. [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Blaese R. M. T cell-mediated immunoregulation of Epstein Barr virus- (EBV) induced B lymphocyte activation in EBV-seropositive and EBV-seronegative individuals. J Immunol. 1982 Feb;128(2):575–579. [PubMed] [Google Scholar]
- Tosato G., Pike S. E., Koski I. R., Blaese R. M. Selective inhibition of immunoregulatory cell functions by cyclosporin A. J Immunol. 1982 May;128(5):1986–1991. [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Carson D. A., Fong S., Slovin S. F., Fox R. I., Vaughan J. H. Lysis of autologous Epstein-Barr virus-infected B cells by cytotoxic T lymphocytes of rheumatoid arthritis patients. Clin Immunol Immunopathol. 1982 Jul;24(1):8–14. doi: 10.1016/0090-1229(82)90083-6. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Tosato G., Blaese R. M., Simon R. M., Nelson D. L. Limiting dilution analysis of Epstein-Barr virus-induced immunoglobulin production by human B cells. J Exp Med. 1983 Jan 1;157(1):1–14. doi: 10.1084/jem.157.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]