Abstract
The ability to accurately determine cell viability is essential to performing a well-controlled biological experiment. Typical experiments range from standard cell culturing to advanced cell-based assays that may require cell viability measurement for downstream experiments. The traditional cell viability measurement method has been the trypan blue (TB) exclusion assay. However, since the introduction of fluorescence-based dyes for cell viability measurement using flow or image-based cytometry systems, there have been numerous publications comparing the two detection methods. Although previous studies have shown discrepancies between TB exclusion and fluorescence-based viability measurements, image-based morphological analysis was not performed in order to examine the viability discrepancies. In this work, we compared TB exclusion and fluorescence-based viability detection methods using image cytometry to observe morphological changes due to the effect of TB on dead cells. Imaging results showed that as the viability of a naturally-dying Jurkat cell sample decreased below 70 %, many TB-stained cells began to exhibit non-uniform morphological characteristics. Dead cells with these characteristics may be difficult to count under light microscopy, thus generating an artificially higher viability measurement compared to fluorescence-based method. These morphological observations can potentially explain the differences in viability measurement between the two methods.
Keywords: Trypan blue exclusion, Image cytometry, Cellometer, Morphology, Fluorescence, Acridine orange, Propidium iodide, Viability
Introduction
Determining cell viability is a vital component in many biological experiments that range from standard cell culturing to the use of primary cells for examining the efficacy of pharmacological agents on tumors. One of the earliest and most common methods for measuring cell viability is the trypan blue (TB) exclusion assay (Altman et al. 1993; Louis and Siegel 2011). Trypan blue is a ~960 Da molecule that is cell membrane impermeable and therefore only enters cells with compromised membranes. Upon entry into the cell, TB binds to the intracellular proteins thereby rendering the cells a blue color. The TB exclusion assay allows for a direct identification and enumeration of live (unstained) and dead (blue) cells in a given population.
Although TB exclusion assay has been the traditional method for measuring cell viability, it has several potential drawbacks. The toxic nature of TB allows only a short period of time from cell staining to counting (Tsaousis et al. 2012). Secondly, because TB binds to cellular proteins, there is a potential of binding to non-specific cellular artifacts, especially in clinical and primary cell samples. Thirdly, there is currently no standardization of TB concentration for the measurement of cell viability. Finally, the detection method for TB exclusion is manual counting using a hemacytometer under a simple light microscope, this can be time-consuming and have operator-dependent variation.
The use of fluorescent-based dyes to determine cell viability has provided an alternative method to the TB exclusion assay. A variety of fluorescent viability dyes, such as DAPI, Hoechst 33342, ethidium bromide, propidium iodide (PI), SYTOX orange/red, acridine orange (AO), SYTO9/13, DRAQ5/7, calcein AM, and CFDA have been used for viability measurement in flow or image-based cytometers (Wallen et al. 1980; Al-Rubeai et al. 1997; Foglieni et al. 2001; Chan et al. 2012). These dyes have the capability to fluorescently label live and dead cells. The AO and PI dual staining method has been routinely used to measure viability of nucleated cell (Wallen et al. 1980; Darzynkiewicz et al. 1992; Mascotti et al. 2000; Foglieni et al. 2001; Ling et al. 2003; Solomon et al. 2010; Chan et al. 2012, 2013). AO is a cell membrane permeable dye that binds to DNA and RNA in live cells by intercalation or electrostatic attraction. PI is a membrane impermeable molecule (~668 Da) that is capable of binding to DNA and RNA only upon the loss of cellular membrane integrity in dying, dead, and necrotic cells. Cell viability is then calculated by examining the ratio of the number of live and dead fluorescing cells (Wallen et al. 1980).
Over the last two decades, there have been various publications on comparison of TB exclusion and fluorescence-based cellular viability assays (Black and Berenbaum 1964; Jones and Senft 1985; Altman et al. 1993; Mascotti et al. 2000). Previous results have shown that in a time-course measurement of a standard cell culture, TB exclusion assays measured significantly higher viability in comparison to the fluorescence-based methods (Jones and Senft 1985; Mascotti et al. 2000). These previous studies have only shown comparison results, but did not provide evidence on potential reasons for the differences between the two methods. In this work, we performed a time-course study to compare cellular viability measured with TB and AO/PI using an automated image-based cytometry method (Cellometer, Nexcelom Bioscience, Lawrence, MA, USA). In concert with earlier studies, we observed that TB produced higher viability measurements when the cell viability decreased below 70 %. Utilizing image-based cytometry, morphological changes of TB stained cells were observed, which may explain the differences between the two methods. Results showed that as cells begin to die in both cell culture and primary cells, numerous dying cells displayed large blue diffused profiles when stained with TB, which may be missed during manual cell counting. In addition, multiple concentrations of TB were tested that yielded higher viability results in comparison to the fluorescence-based methods.
Materials and methods
Jurkat cell preparation
Jurkat cells (ATCC, Manassas, VA, USA) were cultured in RPMI-1640 Medium (ATCC) supplemented with 10 % FBS (LONZA, Verviers, Belgium) and 1 % penicillin/streptomycin (LONZA) in a 37 °C and 5 % CO2 incubator. For the naturally-dying viability comparison, 5 ml of cells (~4 × 106 cells/ml) in one T25 flask were taken out of the incubator and placed into a bench-top drawer. The flask was kept at room temperature in the drawer for the remainder of the experiment. A small aliquot (200 μl) of cells was removed from the flask under sterile conditions for each tested time point (0, 6, 12, 24, 48, 72, 96, and 168 h). A similar experiment was conducted to study the effect of different TB concentrations on Jurkat cell viability, which was also measured at time points (0, 3, 6, 9, 12, 27, and 33 h). The time points were shorter than the first experiment because it was targeted towards the time frame, where the viability difference was the most apparent. For the controlled heat-killed viability comparison, 5 ml of Jurkat cells were aliquoted into a 15 ml eppendorf tube and placed in boiling water for 15 min. After heat-killing, five samples were prepared at viabilities of 0, 25, 50, 75, and 100 % by mixing the heat-killed and fresh Jurkat cells at the appropriate volumes to produce a final sample volume of 2 ml.
Time-course naturally-dying viability for Jurkat cells
The viability and concentration of the naturally-dying Jurkat cells were determined and compared using 4 detection methods: (1, 2) measuring viability staining with PI or AO/PI and counting with Cellometer Vision, (3) staining with 0.4 % TB and counting with Cellometer AutoT4, and (4) staining with 0.4 % TB and manual counting with hemacytometer. The AO/PI staining procedure was performed using concentrations at 10 and 190 μM as previously described (Bank 1987). Fifty microliters of each naturally-dying cell sample at 0, 6, 12, 24, 48, 72, 96, and 168 h was mixed with 50 μl of PI, AO/PI, or 0.4 % TB (Sigma-Aldrich, St. Louis, MO, USA), and analyzed immediately with Cellometer Vision, AutoT4, and hemacytometer. The viability and concentration were measured in quadruplicate (n = 4) for all time points.
Time-course viability of naturally dying murine bulk splenocytes
Fresh murine bulk splenocyte sample was a kind donation from Christina A. Kuksin at the University of Massachusetts Amherst. Lysing protocol was first performed to lyse the red blood cells in the splenocyte sample. Cells were spun down and re-suspended in one ml of ACK lysing buffer (Life Technologies, Carlsbad, CA, USA). After 5 min of incubation the cells were again spun down and re-suspended in medium (RPMI with 10 % FBS). The viability of splenocytes was measured at 0, 33, and 50 h by staining with TB at 0.4 and 0.1 %, PI, and AO/PI to compare the viability differences between staining methods for primary cells. TB was measured by manual hemacytometer counting, while AO/PI and PI were measured using Cellometer Vision. The viability was measured in quadruplicate (n = 4) for all time points.
Trypan blue concentration dependence viability comparison
The effect of TB concentration on viability of naturally-dying Jurkat cells were determined and compared using 2 detection methods: (1) measuring viability by staining with AO/PI and counting with Cellometer Vision, and (2) staining with various concentrations of TB and manual counting with hemacytometer. Fifty microliters of each naturally-dying cell sample at 0, 3, 6, 9, 12, 27, and 33 h was mixed with 50 μl of AO/PI or 0.4, 0.2, 0.1, 0.05, 0.025, 0.0125 % of TB (Sigma-Aldrich, St. Louis, MO, USA, and analyzed immediately with Cellometer Vision and hemacytometer. The viability and concentration were measured in quadruplicate (n = 4) for all time points.
Controlled heat-killed viability comparison
Freshly collected Jurkat cells were separated into two equal aliquots. One was heat-killed by boiling the samples for 15 min and the other was not. The cells in the heat-killed sample were then 100 % non-viable and the other sample was considered 100 % viable. The cells from these two aliquots were then mixed at different ratios to produce cell suspensions. The viability and concentration of each heat-killed/live viability mixture (0, 25, 50, 75 and 100 %) was determined and compared using 3 detection methods: (1) Measuring viability staining with PI (Nexcelom Bioscience, Lawrence, MA, USA) and counting with Cellometer Vision, (2) staining with 0.4 % TB (Sigma-Aldrich) and counting with Cellometer AutoT4, and (3) staining with 0.4 % TB and manual counting with hemacytometer. Fifty microliters of each heat-killed/live mixture sample was mixed with 50 μl of PI or 0.4 % TB, and analyzed immediately with image cytometer and hemacytometer. The viability and concentration for each of the three independent experiments were measured in quadruplicate (n = 4) for all five mixtures.
Image cytometry automated cell counting protocol
Jurkat cells stained with PI, AO/PI, or TB (20 μl) were pipetted immediately after mixing into a Nexcelom disposable counting chamber. The Cellometer Vision utilizes fluorescence (FL) optics modules of VB-535-402 (pseudo-color green) and VB-660-502 (pseudo-color red) to detect AO and PI, respectively. The Cellometer AutoT4 uses a color camera to detect TB stained Jurkat cells. Both systems capture multiple images at different locations on the counting chamber for cell count and viability measurements. For TB image analysis, live cells with bright center and dark cells stained with TB in the bright-field (BR) were enumerated. For PI image analysis, total cells in the BR and dead cells in the FL channel were enumerated. For AO/PI image analysis, AO-stained live cells and PI-stained dead cells in green and red FL channels were enumerated. The measured cell count was then used to automatically calculate both concentration and viability of each sample.
Hemacytometer manual cell counting protocol
Jurkat cells stained with TB (10 μl) were pipetted immediately into a Neubauer hemacytometer. Live and dead cells were enumerated manually under light microscopy for samples from both controlled heat-killed and naturally-dying Jurkat cells stained. For Figs. 7 and 8 Jurkat cell samples with an approximate 50 % viability were used for manual counting.
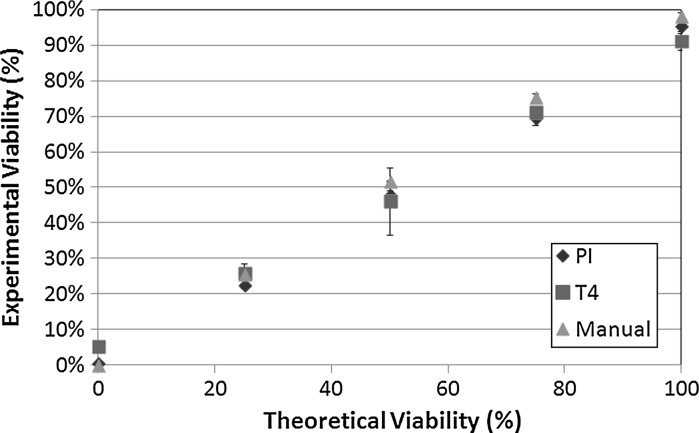
Fig. 7.
Viability comparison between manual, automated (Cellometer AutoT4) TB, and automated PI measurements of heat-killed Jurkat cells. The y-axis indicates the experimental viability percentages measured using the three described detection methods. The x-axis indicates the mixtures of fresh and heat-killed Jurkat cells to yield 0, 25, 50, 75, and 100 % theoretically viability percentages
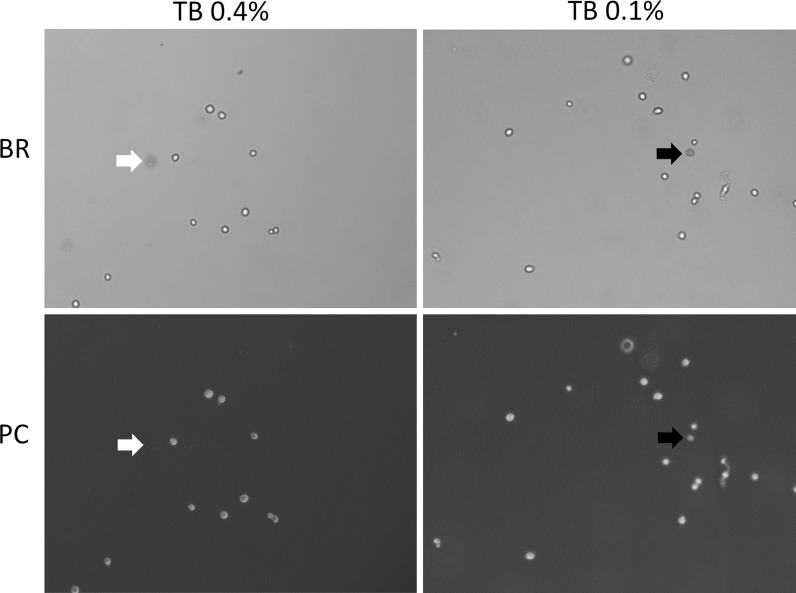
Fig. 8.
Phase contrast and BR microscopy images. Both phase contrast and bright-field images were captured using digital camera on a light microscope. The red arrow indicated the dim diffused cell in 0.4 % TB-stained Jurkat cells, where it was not observed in the phase contrast image. The green arrow indicated the dark dead cell in 0.1 % TB-stained Jurkat cells, where it was also observed in the phase contrast image. (Color figure online)
Phase contrast microscopy
Jurkat cells stained with 0.4 and 0.1 % TB were imaged using light microscopy with digital camera. Both bright-field (BR) and phase contrast (PC) images were captured to identify the dim diffused Jurkat cells.
Results
Time-course viability of naturally dying Jurkat cells
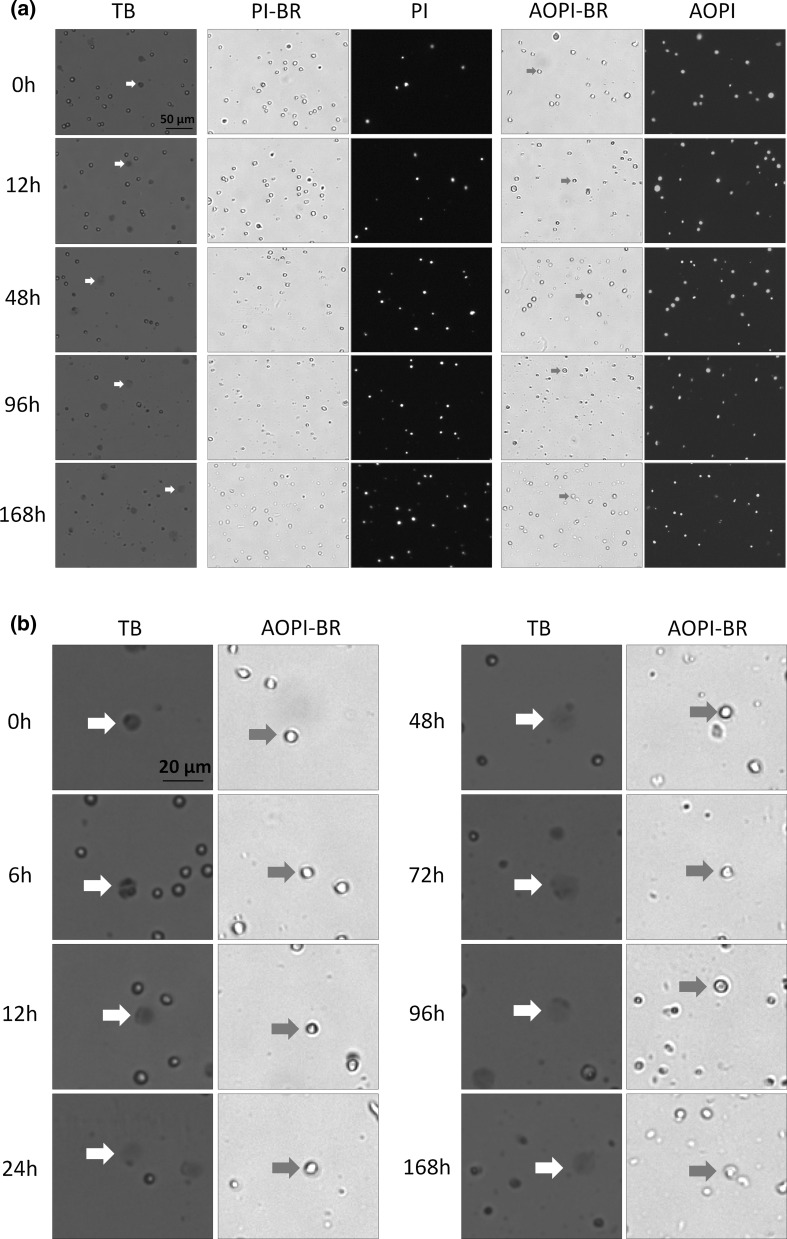
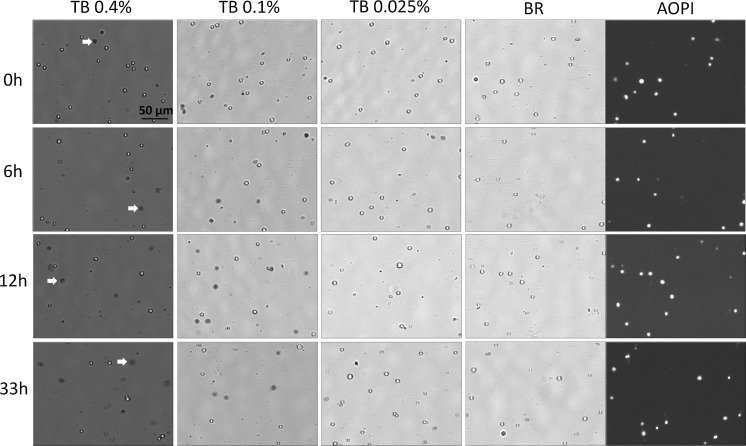
TB exclusion and fluorescence-based viability methods were used to compare the viability of (non-heat-killed) naturally-dying Jurkat cells in a time-course study. Cell death was stimulated by continuously incubating the flask in a desk drawer at room temperature from 0 to 168 h. As gradual cell death occurred, samples from the flask were removed, stained with TB, PI, and AO/PI and imaged (Fig. 1a). By examining and comparing BR images, from 0 to 168 h, we observed a gradual morphological change in the cell membranes. Cells from 0 to 12 h resembled those that were heat-killed; the outer cell membranes in these samples appeared to be dark, and well defined. By 168 h, however, the cells that gradually underwent a morphological change now appeared grayish, and undefined (green arrows in Fig. 1b). Similar morphological characteristics, where cell membranes changed from well-defined to amorphous, were observed in TB-stained Jurkat cells. The morphology of those cells changed from tight dark blue shapes to large dim diffused shapes, which can be visually observed in the TB stained images (red arrows in Fig. 1b). While the number of TB stained large dim cells increased with time, the morphological characteristics of Jurkat cells stained with AO or PI did not change over the same period of time. As the cells were incubated at room temperature, we can visually confirm that the number of stained (PI, AO/PI, and TB) dead Jurkat cells increased over time. For PI image analysis, the total number of cells in the BR and total number of dead cells in the FL channel were examined. We observed that the composition of the cellular membrane in Jurkat cells, at later time points (96 and 168 h), also became less defined in the BR images, which may increase the difficulty of obtaining accurate cell viability using PI image analysis (Fig. 1a). The most optimal method for measuring cell viability is using the AO/PI method. This method allows for dual-fluorescence detection of AO (live) and PI (dead), thus eliminating the potential inaccurate counting of membrane-poor cells populations: late apoptotic and necrotic cells, as well as eliminating the counting of cellular debris in the BR images.
Fig. 1.
Bright-field and FL images of PI, AO/PI, and TB stained naturally-dying Jurkat cells. a Samples were measured at time points 0, 6, 12, 24, 48, 72, 96, and 168 h post incubation at room temperature. b Higher magnification of a showing TB time-course study. Large, dim, and diffused shaped cells in the TB and bright-field images are shown with red and green arrows, respectively. (Color figure online)
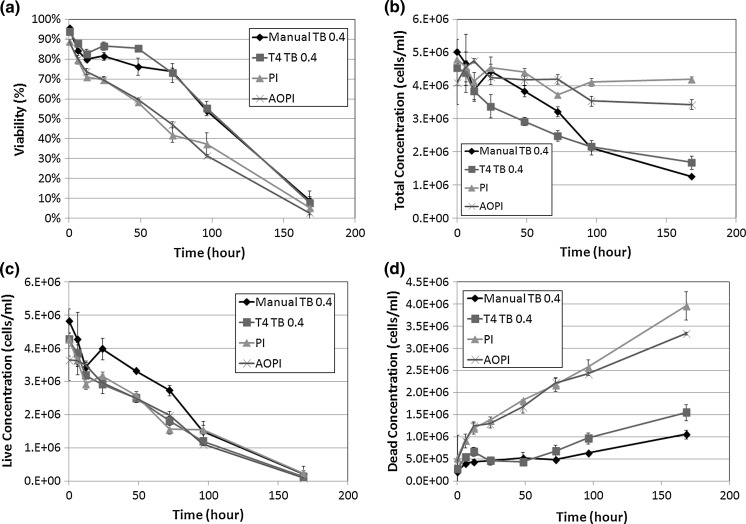
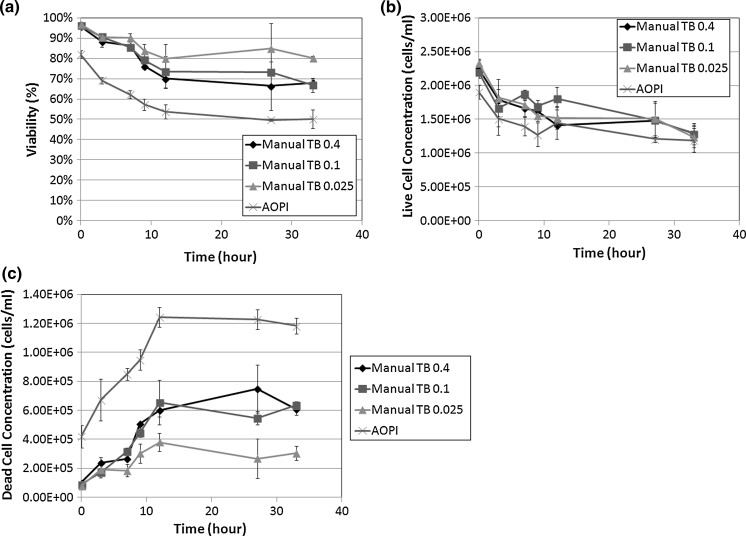
Having assessed the morphological changes, we also examined the viability and concentration measurements from both image cytometry and manual hemacytometer counts, which are shown in Fig. 2. The comparison of viability measurements between the 4 detection methods showed clear differences between fluorescence-based and TB exclusion assays (Fig. 2a). At 12 h post incubation at room temperature we observed a sharp decrease in Jurkat cell viability measured by fluorescence-based detection methods (AO/PI and PI). A 2-sample T test was calculated at 12 h time point for comparing AO/PI to PI and AO/PI to TB staining. The results showed that AO/PI was comparable to PI staining method (p > 0.05), while AO/PI was significantly different from TB counting (p ≤ 0.05). Although the cell viability, measured by AO/PI, showed that the viability of Jurkat cells was reduced to 70 %, the measured viability by TB method showed an inflated viability measurement of 82.8 %. These data are consistent with earlier published reports that show cell samples at lower viability (80 % and below) stained with TB, display an overestimation in the measured cell viability (Mascotti et al. 2000). In correlation with the viability measurements we also recorded the measured cell concentrations for every time point and for each testing parameter. While the fluorescence-based method measured consistent total cell concentrations over the length of the experiment, TB exclusion showed a gradual decrease in cell concentration (Fig. 2b). To further analyze this result, the measured live cell concentration was plotted separately from the measured dead cell population (Fig. 2c, d). For live cell concentrations, all detection methods showed similar concentration measurements over time (Fig. 2c). However, the dead cell concentrations were significantly different from each other. By the end of the time trial, both the manual and TB measured concentrations were more than two times lower than the fluorescence-based detection methods. Furthermore, the initial deviation in the number of measured dead cells by TB began at 12 h post incubation at room temperature which is the same time at which the measured cell viability decreased to ~70 % by AO/PI. These data suggest that as the cell viability decreases, the TB exclusion method undercounts the number of dead cells thereby producing an inflated viability measurement.
Fig. 2.
Concentration and viability time-course measurement of TB, PI, and AO/PI-stained Jurkat cells. a Viabilities of each method were measured from 0 to 168 h experimental duration. At the 24 h time point, the viability measured by TB showed approximately 80 %, while the AO/PI or PI showed 70 %. b The total concentrations were also measured by AO/PI and PI methods, which showed consistent results, whereas the TB method showed a decrease over time. c The live cell concentrations were measured and showed no differences between the two methods, however d the dead cells showed higher concentrations for AO/PI and PI methods than the TB method over time
Time-course naturally-dying viability for murine bulk splenocytes
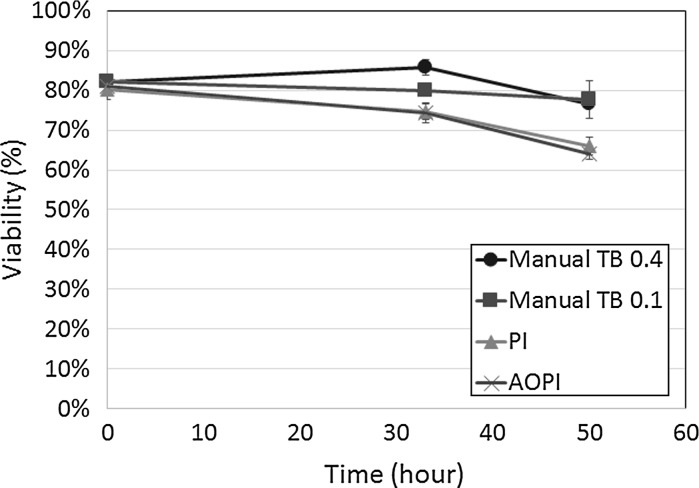
A similar time-course study was also performed for primary murine bulk splenocytes. As with cultured Jurkat cells, similar viability results were observed. Shown in Fig. 3, the AO/PI and PI viability measurements showed a gradual decrease from ~80 % at time 0 h to ~65 % viability at 50 h post incubation at room temperature. In contrast, the TB stained cells at 0.4 and 0.1 % concentration, showed a viability decrease of only ~5 %. A 2-sample T test was calculated that showed significant difference in viability between AO/PI and TB (p ≤ 0.05). In addition, AO/PI viability was comparable to PI (p > 0.05). These results showed the viability differences between TB and FL measurements, which mirrored the results obtained for cultured Jurkat experiments.
Fig. 3.
Viability time-course measurement of TB, PI, and AO/PI-stained primary murine splenocytes. The viabilities were measured at time 0, 33, and 50 h, which showed differences amongst the various staining methods
Trypan blue concentration dependence viability comparison
Further experiments were conducted to observe the effect of TB concentrations on the viability measurement and morphology of naturally-dying Jurkat cells in comparison to AO/PI stained cells. Images of cells stained with TB at concentrations of 0.4, 0.2 (not shown), 0.1, 0.05 (not shown), 0.025 %, and as well as cells stained with AO/PI are shown in Fig. 4. As expected, TB at the high concentration (0.4 %) stained not only more cells, but also displayed more large dim diffused shapes (red arrows in Fig. 4). At lower TB concentrations (0.1 and 0.05 %) although fewer cells were stained, those that were stained appeared as tighter dark shapes and not large dim diffused shapes. This is especially evident at the 12 h time point and beyond. AO/PI staining remained consistent with the previous experiment, showing increase in PI positive cells over the course of the experiment. The viability results followed the same trend that was observed in the time-course assay experiment (Fig. 5a). As before, the viabilities measured from each TB concentration were higher than the counts from AO/PI-stained cells. Because the cells in this experiment had a lower starting viability (~80 %), the difference between TB and AO/PI counts were seen immediately. The TB viability counts were consistently higher than the AO/PI counts. Similarly, the live cell concentrations were comparable to each other when 2-sample T test was calculated for AO/PI against 0.4, 0.1, and 0.025 % (p > 0.05, for all TB concentrations). The measured concentration of dead cells for each experimental parameter was significantly different (p ≤ 0.05) for AO/PI against all TB concentrations (Fig. 5b, c). The largest difference could be seen between the AO/PI measurement and the 0.025 % TB measurement. This difference is most likely due to the low concentration of TB. At this TB concentration, there is low level of observable trypan staining, which is confirmed in the acquired images. However, for 0.05, 0.1, and 0.4 % TB, it should be expected that the amount of dead cells detected is proportional to the number of PI positive cells. And as in the previous time course experiment, even at high TB concentrations, we observe a considerably lower number of reported dead cells. These data support not only our previous findings that TB viability measurement overestimates measured sample viability percentage, but it also shows that this phenomenon occurs at every TB tested concentration.
Fig. 4.
Bright-field and fluorescent images of TB concentration series and AO/PI stained Jurkat cells. a Samples were measured at time points 0, 6, 12, and 33 h post incubation at room temperature. In addition to the increase of large dim diffused shaped cells over time (shown with red arrows), high concentration of TB exhibited more of these cells in comparison to low concentration in the images. (Color figure online)
Fig. 5.
Concentration and viability time-course measurement of TB concentration series (0.4–0.025 %) versus AO/PI-stained Jurkat cells. a The viabilities were measured compared from 0 to 33 h experimental duration. b Similarly, both b live, and c dead cell concentrations were measured and compared between various TB concentrations and AO/PI method
Controlled heat-killed viability comparison
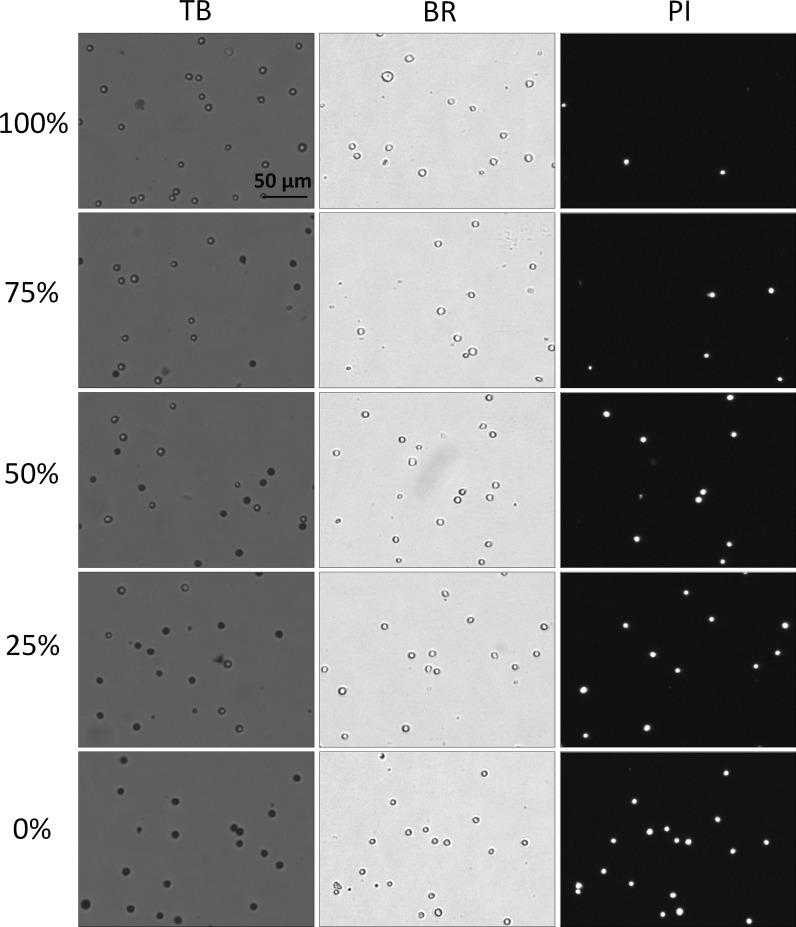
In this experiment, Jurkat cells were used to show that the morphological characteristic differences between the heat-killed and “naturally dying” cells plays a role in the manner in which cells are counted. The bright-field, fluorescent, and TB images of 0, 25, 50, 75, and 100 % viability mixtures are shown in Fig. 6. It is clear that there were morphological differences between the heat-killed and non-heat-killed dead cells. Because the heat-killed cells did not undergo natural apoptotic cell death and necrosis, the stained Jurkat cells appear uniform in shape, color, and circularity. In contrast, cells that were incubated at room temperature experienced naturally induced cell death and necrosis and therefore exhibit non-uniform cellular morphology and non-uniform TB staining pattern. The heat-killed TB-stained Jurkat cells clearly showed very tight and dark blue staining pattern, which is similar to dead cells from cultures that have overall high viability (>80 %) (Fig. 1a). Due to the morphological uniformity of heat-killed cells, as expected, the viability counts between TB and PI showed a high degree of correlation (Fig. 7). In this experiment, PI was used instead of AO/PI due to the fact that heat-killed cells did not generate cellular debris, thus viability was determined by measuring the number of total and dead cells in BR and FL images, respectively. In contrast, naturally-dying cells generated large amounts of cellular debris, thus when measured using BR image analysis, this method could generate inaccurate counting results. As a result, AO/PI was required to accurately determine the viability of nucleated cells in the sample without interference from the cellular debris (Chan et al. 2013).
Fig. 6.
Bright-field and fluorescent images of TB and PI-stained heat-killed Jurkat cells at various viability percentages. The heat-killed and fresh Jurkat cells were mixed to produce five different viability percentages (0–100 %). In these heat-killed Jurkat samples the TB images showed a clear distinction between live and dead cells; where the dead cells exhibited a tight dark and well-defined morphology. Similarly, the dead cells in the bright-field images maintained an easily detectable and sharp cellular membrane
Phase contrast microscopy
The bright-field (BR) and phase contrast (PC) images of TB-stained Jurkat cells are shown in Fig. 8. At 0.4 % TB-stained Jurkat cell was observed and identified as dim diffuse dead cell in the BR image, but was too dim to be observed in the PC image (red arrow). However, at 0.1 % TB-stained cell was observed to have dark cellular morphology in the BR image and the same cell was observed in the PC image (green arrow). This result shows that appropriate trypan blue dye concentration and the method of detection are vital for obtaining an accurate viability reading.
Discussion
The purpose of this study is to investigate the differences in viability measurement between TB exclusion assay and fluorescence-based viability stains. These experiments were repeated three times and resulted in similar viability characteristics over the experimental time frame (data not shown). Utilizing image-based cytometric analysis, we were able to observe morphological changes of cells stained with TB at different viability ranges. The membranes of naturally-dying Jurkat cells undergo extreme changes during cell death. In samples with high viability, the TB stained dead cells exhibited tight dark uniform characteristics. This observation suggests that even though the cells were permeable to TB, cellular membranes remained intact enough to retain the TB dye in the cytosol. In samples with low viability, the TB stained dead cells exhibited large, dim, non-uniform diffused characteristics, which suggests functional loss of cell membrane integrity. It appears that in those cells, TB was not contained within the cytosol, which led to the formation of the observed large dim cells. We also observed that the dim diffused shapes can range in size and brightness. Because the dimmer cells may be difficult to count in hemacytometer under light microscopy, the TB exclusion assay may artificially produce higher viability counts due to the unintended exclusion of dead cells. As a result, the measured viability between the two methods showed significant discrepancy, as high as 30 %, where similar observations have been made in previous publications (Mascotti et al. 2000). Note that in the TB column in Fig. 1b, the dimly stained cells are on the same focal plane as the bright live cells. We have adjusted the focus and observed no change in the dim diffused shape (data not shown). This leads us to conclude that we are not observing staining artifacts or possible stained contamination.
The overestimation of viability can also be observed in primary murine bulk splenocytes. By comparing viability measurement of murine bulk splenocytes using AO/PI, PI, and TB, the results clearly showed significant difference between TB and FL staining methods (p ≤ 0.05), which further supported the overestimation from TB manual counting. It is also important to note that overestimation of viability when using TB on samples at or below 70 % occurs in both cultured and primary cell lines.
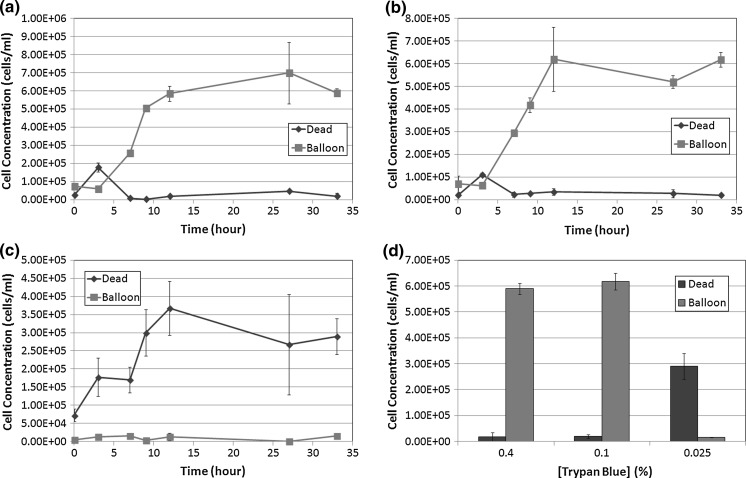
In addition, we examined the potential morphological changes that may be caused by different concentrations of TB. Upon staining the cells with a high concentration of TB (0.4 %), we observed a rapid formation of large dim diffused circular shapes (Fig. 9). Here, we refer to these cells as “balloons”. The characteristics of the “balloons” can be observed under light microscopy and Cellometer, which are expanded and diffused after staining with TB. Furthermore, the balloons appear to have different size characteristics and brightness, some are clearly more diffuse than others. We observed the formation of initial balloons within the first 30 s of staining the cells with 0.4 % TB. Additional balloons, although fewer in number formed within the next 60 s of staining. At low TB concentrations (0.025 %) we did not observe many balloon-like cells (Fig. 9). It can be visually confirmed that there are more large dim diffused shapes at higher TB concentration. At 0.025 % TB, the concentration of the dye may be too low to adequately stain all dead cells. Even though the nature of the balloons made them difficult to count, the balloons were carefully identified and manually counted under a light microscope for the 0.4, 0.1, and 0.025 % of TB. In a separate count, the typical dark dead cells were also counted for each trypan concentration. The concentration of each population was analyzed and plotted in Fig. 10. We observed that at the 33 h time point, for both 0.4 and 0.1 % concentrations there are significantly higher number of diffused balloon cells than the dark dead cells (p ≤ 0.05, for both TB concentrations). At 0.025 % concentration there was not only a reversal in the number of counted balloons versus the number of counted dead cells (p ≤ 0.05), but there was also a lower number of total counted cells as well; which can be attributed to low dye concentration. Together these data suggest that TB, at high concentration, has a morphological effect on membrane compromised dead cells—thus producing balloon like cells. At lower concentrations of TB these dye-induced effects were not observed. Finally, because these dead cells appear as faint diffused colored balloon structures they are very difficult to see and count, which leads to an overestimation of viability measurement in the sample. Although the BR background changed due to the TB concentration, the samples were manually counted, thus it did not affect the measured results.
Fig. 9.
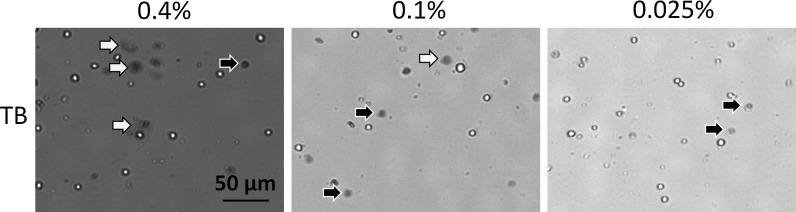
Morphological characteristics observation of trypan concentration series using image cytometry. At 0.4 %, the white arrows points to the dead cells that have expanded into the large dim diffused shapes, while the black arrows point to the dead cells that maintained a dark tight morphology
Fig. 10.
Time-course concentration measurement of large dim cells (“balloon”) and dark tight (dead) shaped Jurkat cells at TB concentrations. a 0.4 %, b 0.1 %, and c 0.025 %. d Concentration results (at 33 h post incubation at room temperature) showed that at higher TB concentration, the large dim diffused cells or “balloons” were very high in number compared to the lowest TB concentration
Other potential reasons for the differences between TB and fluorescence-based method can also be hypothesized. For example, PI has been shown to enter cells earlier than TB, thus it has the potential to measure more dead cells, hence lower viability. This could be due to the molecular weight of PI at ~668 Da versus TB at ~960 Da, which could have allowed PI to enter cells more readily. Also, since TB is a cytoplasmic dye that stains intracellular proteins, dying cells with variation in membrane degradation may not retain TB because of the loss of membrane integrity. As a result, the number of dead cells counted may be lowered which in turn can generate higher viability measurement. Meanwhile PI, an intercalating dye, binds to the nucleic acid of the cells and is retained within the nucleus regardless of membrane integrity. It is because of these properties that PI is routinely used to stain and identify late apoptotic and necrotic cell populations (Denecker et al. 2000; Yedjou et al. 2012). The staining consistency of fluorescence-based viability dyes makes them the preferred method for viability analysis over TB exclusion method.
As reported, the morphological characteristics of TB-stained heat-killed Jurkat cells were tight, dark, and very confined within the cell membrane. It could be possible that the healthy cells with highly intact membrane were permeated (heat-killed) at the same time, thus all of the permeated cells can exhibit excellent TB staining characteristics. By using the heat-killed method, viabilities measured with TB exclusion and fluorescence-based methods were highly correlated.
The morphological differences between naturally-dying and heat-killed cells can potentially explain the variations in viability reporting of comparison between TB and fluorescence-based methods. Even by PC microscopy, there was a clear difference between a dim diffused cell versus a dark dead cell, where the “balloon” cell was not observed, thus could contribute to the counting error in manual TB counting method (Fig. 8). Over the years, there have been numerous comparisons between the two viability detection methods, but the reports have not stated reasons linking to the morphological changes caused by TB. By using image-based cytometry, we were able to capture and examine images of TB-stained Jurkat cells, which allowed for visual confirmation of morphological differences between high and low viability samples. There are also other fluorescence-based dyes that can be used to measure viability, such as Hoechst/PI or 7AAD. We selected AO/PI due to their stability and popularity for image-based viability analysis. Furthermore, similar work could be performed on standard fluorescent microscopy, however, the lack of automation would increase the difficulty of capturing multiple samples for cell population analysis. Future studies can be conducted to examine the effect of TB incubation time on cell morphology, as well as time-course video recording of cells stained with TB using image cytometry.
Acknowledgments
The authors would like to thank Christina A. Kuksin at the University of Massachusetts Amherst for her generous donation of primary murine bulk splenocytes.
Conflict of interest
The authors, L.L.C., D.K., S.S., and J.Q. declare competing financial interests, and the work performed in this manuscript utilized the instruments of Nexcelom Bioscience, LLC.
References
- Al-Rubeai M, Welzenbach K, Lloyd DR, Emery AN. A rapid method for evaluation of cell number and viability by flow cytometry. Cytotechnology. 1997;24:161–168. doi: 10.1023/A:1007910920355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman SA, Randers L, Rao G. Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnol Prog. 1993;9:671–674. doi: 10.1021/bp00024a017. [DOI] [PubMed] [Google Scholar]
- Bank HL. Assessment of islet cell viability using fluorescent dyes. Diabetologia. 1987;30:812–816. doi: 10.1007/BF00275748. [DOI] [PubMed] [Google Scholar]
- Black L, Berenbaum MC. Factors affecting the dye exclusion test for cell viability. Exp Cell Res. 1964;35:9–13. doi: 10.1016/0014-4827(64)90066-7. [DOI] [PubMed] [Google Scholar]
- Chan LL, Wilkinson AR, Paradis BD, Lai N. Rapid image-based cytometry for comparison of fluorescent viability staining methods. J Fluoresc. 2012;22:1301–1311. doi: 10.1007/s10895-012-1072-y. [DOI] [PubMed] [Google Scholar]
- Chan LLY, Laverty DJ, Smith T, Nejad P, Hei H, Gandhi R, Kuksin D, Qiu J. Accurate measurement of peripheral blood mononuclear cell concentration using image cytometry to eliminate RBC-induced counting error. J Immunol Methods. 2013;388:25–32. doi: 10.1016/j.jim.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Bruno S, Bino GD, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Denecker G, Dooms H, Loo GV, Vercammen D, Grooten J, Fiers W, Declercq W, Vandenabeele P. Phosphatidyl serine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett. 2000;465:47–52. doi: 10.1016/S0014-5793(99)01702-0. [DOI] [PubMed] [Google Scholar]
- Foglieni C, Meoni C, Davalli AM. Fluorescent dyes for cell viability: an application on prefixed conditions. Histochem Cell Biol. 2001;115:223–229. doi: 10.1007/s004180100249. [DOI] [PubMed] [Google Scholar]
- Jones KH, Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Ling E, Shirai K, Kanekatsu R, Kiguchi K. Classification of larval circulating hemocytes of the silkworm Bombyx mori, by acridine orange and propidium iodide staining. Histochem Cell Biol. 2003;120:505–511. doi: 10.1007/s00418-003-0592-6. [DOI] [PubMed] [Google Scholar]
- Louis KS, Siegel AC. Cell viability analysis using trypan blue: manual and automated methods. In: Stoddart MJ, editor. Mammalian cell viability: methods and protocols. Berlin: Springer; 2011. pp. 7–12. [DOI] [PubMed] [Google Scholar]
- Mascotti K, McCullough J, Burger SR. HPC viability measurement: trypan blue versus acridine orange and propidium iodide. Transfusion. 2000;40:693–696. doi: 10.1046/j.1537-2995.2000.40060693.x. [DOI] [PubMed] [Google Scholar]
- Solomon M, Wofford J, Johnson C, Regan D, Creer MH. Factors influencing cord blood viability assessment before cryopreservation. Transfusion. 2010;50:820–830. doi: 10.1111/j.1537-2995.2009.02491.x. [DOI] [PubMed] [Google Scholar]
- Tsaousis KT, Kopsachilis N, Tsinopoulos IT, Dimitrakos SA, Kruse FE, Welge-Luessen U. Time-dependent morphological alterations and viability of cultured human trabecular cells after exposure to trypan blue. Clin Exp Ophthalmol. 2012;41:484–490. doi: 10.1111/ceo.12018. [DOI] [PubMed] [Google Scholar]
- Wallen CA, Higashikubo R, Dethlefsen LA. Comparison of two flow cytometric assays for cellular RNA—acridine orange and propidium iodide. Cytometry. 1980;3:155–160. doi: 10.1002/cyto.990030303. [DOI] [PubMed] [Google Scholar]
- Yedjou CG, Saeed MA, Hossain MA, Dorsey W, Yu H, Tchounwou PB (2012) Basic apoptotic and necrotic cell death in human liver carcinoma (HepG(2)) cells induced by synthetic azamacrocycle. Environ Toxicol 1–7. doi:10.1002/tox.21786 [DOI] [PubMed]