Abstract
Plants are still to be explored for new anti-cancer compounds because overall success in cancer treatment is still not satisfactory. As a new possible source for such compounds, the lichens are recently taking a great attention. We, therefore, explored both the genotoxic and anti-growth properties of lichen species Parmelia sulcata Taylor. The chemical composition of P. sulcata was analyzed with comprehensive gas chromatography–time of flight mass spectrometry. Anti-growth effect was tested in human breast cancer cell lines (MCF-7 and MDA-MB-231) by the MTT and ATP viability assays, while the genotoxic activity was studied by assays for micronucleus, chromosomal aberration and DNA fragmentation in human lymphocytes culture. Cell death modes (apoptosis/necrosis) were morphologically assessed. P. sulcata inhibited the growth in a dose-dependent manner up to a dose of 100 μg/ml and induced caspase-independent apoptosis. It also showed genotoxic activity at doses (>125 μg/ml) higher than that required for apoptosis. These results suggest that P. sulcata may induce caspase-independent apoptotic cell death at lower doses, while it may be genotoxic at relatively higher doses.
Keywords: Parmelia sulcata, Cell death, Apoptosis, Breast cancer, Treatment, DNA damage
Introduction
Lichens are dual organisms because they are symbiotic associations between two (or sometimes more) entirely different types of microorganisms, and they produce a great variety of secondary metabolites which are unique to these groups. Approximately 1,050 lichen substances have been identified in the literature, and lichen secondary metabolites have diverse biological activities, including antibacterial, antiviral, antifungal, antitumor, antiherbivore, antiprotozoal, antimutagenic, antioxidant, antiinflammatory, allelochemical, analgesic, antipyretic, antiproliferative and cytotoxic effects (Müller 2001; Oksanen 2006; Molnar and Farkas 2010; Mitrović et al. 2011).
The number of studies related to the cytotoxicity and genotoxicity of lichens and their secondary metabolites have increased in recent years. In our previous study, our findings demonstrated that Hypogymnia physodes extract had an anti-growth effect at relatively lower concentrations, while relatively higher concentrations were required for genotoxic activity (Ari et al. 2012). Koparal et al. (2006) investigated the cytotoxic and genotoxic activities of (+)-usnic and (−)-usnic acid isolated from the Ramalina farinacea and Cladonia foliacea on human lymphocytes, and they observed no genotoxicity against human lymphocytes and high level of cytotoxicity against cancer cells. In another study, it was found that acetone extract of Cetraria aculeata had cytotoxic activity in some cancer cell lines (Zeytinoglu et al. 2008).
Several studies were published related to antiproliferative effects of lichens or compounds from lichen extracts on cancer cell lines. Cytotoxic potential of chloroform, ethyl acetate and methanol extracts of Thamnolia vermicularis var. subuliformis was evaluated, and the researchers observed that ethyl acetate and chloroform extracts had cytotoxic effects 72 h after the treatment in HeLa cells (Manojlović et al. 2010). The extracts of 69 lichens species were evaluated in terms of their biological activities, and lichen extracts demonstrated antimicrobial, antiviral and cytotoxic activities (Perry et al. 1999). In a study, the cytotoxic activities of eight lichen extracts were investigated in vitro using two murine and four human cancer cell lines (Bezivin et al. 2003). A significant cytotoxic activity on the tested cancer cell lines was determined in at least one extract of each lichen species. In another study, the antiproliferative activity of methanol extract of Xanthoria parietina was evaluated against murine myeloma cells. The methanol extract of X. parietina inhibited the growth of cancer cells at approximately 75 % (Triggiani et al. 2009).
Parmelia sulcata belongs to the family of Parmeliaceae and contains salazinic acid, atranorin (Kosanić et al. 2011; Purvis et al. 1994) and sometimes lobaric acid (Brodo et al. 2001). According to a recent study, P. sulcata contains salazinic acid, atranorin, chloroatranorin and protocetraric acid (Manojlović et al. 2012). It is notable that only limited data are present on cytotoxic activity of P. sulcata in the literature. A study showed that acetone extracts of P. sulcata had anticancer activity in human melanoma and colon cancer cells (Kosanić et al. 2012). In another study, salazinic acid which is a major metabolite in P. sulcata was found to possess high cytotoxic activity in human melanoma and colon cancer cell lines (Manojlović et al. 2012). However, to the best of our knowledge, there is no information regarding the genotoxic activity of P. sulcata in the relevant literature. In the present study, it was aimed to elucidate whether or not P. sulcata has beneficial anticancer properties or any harmful genotoxic effect. Therefore, cytotoxic and genotoxic activities of the methanol extract of P. sulcata were investigated in breast cancer cell lines and human lymphocytes, respectively.
Materials and methods
Collection and identification of lichen samples
Parmelia sulcata was collected from the trunks of Quercus sp. (Uludag Mountain, Bursa, Turkey) in May 2010 and identified with the aid of flora books (Purvis et al. 1994; Wirth 1995). A voucher specimen has been deposited in the Herbarium of Uludag University (BULU), Bursa, Turkey.
Extraction of lichen sample
Air-dried lichen sample was carefully cleansed of extraneous materials and ground into powder. 15 g of the ground material was extracted consecutively by adding 150 ml of methanol (Merck, Darmstadt, Germany) and water in a Soxhlet extractor for 24 h. The crude extracts were concentrated using a rotary evaporator at 40 °C; thereafter, the residues were lyophilized and stored at −20 °C until the next use.
Direct thermal desorption (DTD)
To evaluate the volatile compounds in lichen sample, DTD followed by analysis with gas chromatography–time of flight mass spectrometry (GCXGC–TOF/MS) was performed. There was no sample preparation, and plant was directly loaded into the system. A GCXGC–TOF/MS system was used together with a dual stage commercial thermal desorption injector. This incorporated a thermal desorption unit (TDU) connected to a programmable-temperature vaporization (PTV) injector, CIS-4 plus (Gerstel, Mülheim an der Ruhr, Germany) using a heated transfer line. The injector was equipped with a MPS autosampler (Gerstel). Empty glass thermodesorption tubes were conditioned at 400 °C for 2 h prior to each use. Approximately 20–30 mg of sample was placed into the thermodesorption tubes using tweezers to ensure no contamination of the sample. Initial desorption of the sample was carried out by heating the TDU from 40 °C (initial time 0.2 min) to 150 °C at a rate of 120 °C/min with a final hold time of 10 min under a helium flow of 1.5 ml/min in splitless mode. Volatile analytes released from this heating were cryo-focused at −40 °C in the CIS cooled with liquid nitrogen prior to injection. The CIS was then heated at a rate of 10 °C/s to a final temperature of 150 °C. Analytes were transferred splitless to the GC column during the CIS temperature ramp.
Chromatographic analysis
The GCxGC–TOF/MS system consisted of an Agilent 6890 (Agilent Technologies, Palo Alto, CA, USA) gas chromatograph and a Pegasus III TOF–MS (LECO, St. Joseph, MI, USA). The modulator between first and second GC columns was based on a Leco (Stockport, UK) liquid nitrogen two stage cold jet system. The modulation time was 5 s. The first column was a non-polar BPX5 (30 m × 0.32 mm i.d. × 0.25 μm film thickness) and the second column a BPX50 (1.5 m × 0.10 mm i.d. × 0.10 μm film thickness) both from SGE Analytical Science (Sydney, VIC, Australia). The initial temperature of the first dimension column was 60 °C for 1 min and the subsequent temperature programme had a heating rate of 5 °C/min until 310 °C was reached and held isothermally for a further 1 min. The initial temperature of the second dimension column was 75 °C for 1 min and a 5 °C/min heating rate was used until 325 °C was reached and held isothermally for further 1 min. Helium was used as a carrier gas at a constant flow of 1.0 ml/min. The first dimensional separation was based on separation by volatility in a non-polar column. The second dimensional separation was based on separation by polarity using a more polar column. The combination of separations produced the overall two dimensional chromatogram. Peak identification was made using TOF/MS with electron ionization. The mass spectrometer used a push plate frequency of 5 kHz, with transient spectra averaging to give unit resolved mass spectra between 45 and 500 amu at a rate of 50 spectra/s. Mass spectra were compared against the NIST 2005 mass spectral library.
Determination of cytotoxicity
Cell culture and chemicals
Breast cancer cell lines (MCF-7 and MDA-MB-231) were from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI 1640 supplemented with penicillin G (100 U/ml), streptomycin (100 μg/ml), l-glutamine, and 5 % fetal calf serum (Invitrogen, Paisley, UK) at 37 °C in a humidified atmosphere containing 5 % CO2. Lyophilized P. sulcata was dissolved in DMSO at a concentration of 100 mg/ml as a stock solution. Further dilutions of P. sulcata extract (PSE) were prepared in culture medium. PSE was used at different concentrations ranging from 0.20 to 100 μg/ml.
The MTT viability assay
MCF-7 or MDA-MB-231 cells were seeded in 200 μl culture medium in triplicates at a density of 5 × 103 cells per well of a 96-well plate. Cells were incubated either alone (for control which included 0.1 % DMSO) or in the presence of the PSE for 72 h. Each experiment was conducted twice in triplicates. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) viability assay was performed as previously described (Ulukaya et al. 2008). MTT was first prepared as a stock solution of 5 mg/ml in phosphate buffer (PBS, pH 7.2) and was filtered. At the end of the treatment period (72 h), 20 μl of MTT solution (5 mg/ml PBS, pH 7.2) was added to each well. After incubation for 4 h at 37 °C, 100 μl of solubilizing buffer (10 % sodium dodecyl sulfate dissolved in 0.01 N HCl) was added to each well. After overnight incubation, the absorbance (Abs) was read by an ELISA plate reader at 570 nm to determine the cell viability. Viability of treated cells was calculated in reference to the untreated control cells by using the following formula:
The ATP viability assay
The seeding of cells and treatment conditions as well as the calculation of viability was performed similar to that of the MTT assay (see above). The ATP assay was based on the highly sensitive ‘‘firefly’’ reaction to determine the level of cellular ATP as a surrogate marker for the number of alive cells (Andreotti et al. 1995). It was performed to confirm the results of the MTT assay due to the possibility of the interference of the MTT assay itself with the PSE. At the end of the treatment period (72 h), the ATP assay was used for luminometric measurement of cell growth (viability) according to the standard protocol with a little modification of the manufacturer (ATP Bioluminescent Somatic Cell Assay Kit, Sigma, St. Louis, MO, USA). The ATP was extracted from the cells by the addition of 50 μl of tumor cell extraction reagent to each well. After mixing thoroughly, the microplates were allowed to stand on the bench for 20 min at room temperature before 50 μl medium from each well was transferred to a white plate. Next, the 50 μl luciferin–luciferase counting reagent was added. The microplates were measured using a count integration time of 1 s with a luminometer (Bio-Tek, Winooski, VT, USA).
Assessment of morphological changes in cells
The morphological changes were observed under a phase contrast microscope (Olympus CKX41, Tokyo, Japan) after the cells were exposed to 100 μg/ml of the PSE for 72 h.
Measurement of programmed cell death (apoptosis)
Apoptosis was assayed by measuring the level of caspase-cleaved keratin 18 (ccK18, M30) by a commercially available immunoassay kit (M30-Apoptosense ELISA kit, Peviva AB, Bromma, Sweden) according to the manufacturer’s instructions. This kit measures the levels of the CK18-Asp396 neo-epitope (M30), which is a well-known marker of apoptosis. 1x104 cells were seeded per well of a 96-well plate in 200 μl culture medium in triplicates. MCF-7 or MDA-MB-231 cells were treated for 72 h with 100 μg/ml of the PSE. Paclitaxel (3.12 μM) was used as a positive control for apoptosis as this agent is considered as an appropriate apoptosis-inducer (Park et al. 2004). At the end of the treatment period, the cells were lysed with 10 % NP-40 for 10 min on a shaker. The content of identical wells were pooled and centrifuged at 2,000 rpm for 10 s to remove the debris. All samples were placed into wells coated with a mouse monoclonal antibody as a catcher. After washing, a horseradish peroxidase conjugated antibody (M30) was used for detection. The absorbance was determined with an ELISA reader at 450 nm (FLASH Scan S12, Eisfeld, Germany).
Fluorescence imaging for apoptosis
The detection of cell death mode was based on both the nuclear morphology of cells and the cell membrane integrity using a fluorescent microscope. Therefore, the nuclear morphology of living (not fixed) cells was examined after staining them with a nucleus-staining fluorescent Hoechst dye 43332. The Hoechst dye stains all of the alive or dead (primary necrotic or secondary necrotic) cells. Primary necrosis was decided on the basis of swelling of cells, but lack of fragmented or pyknotic nuclei. Secondary necrosis was decided on the basis of pyknotic or fragmented nuclei with damaged cell membrane, which is also considered as the late stage of apoptosis. Briefly, MCF-7 and MDA-MB-231 cells were seeded in a 6-well plate at the density of 5 × 105 cells per well, and then exposed to a PSE at the concentration of 100 μg/ml for 72 h. After the treatment, the cells were washed with PBS, and incubated with the Hoechst dye 43332 (5 μg/ml) solution for 15 min in the dark at 37 °C.
Measurement of active caspase-3 and cleaved PARP levels
MCF-7 and MDA-MB-231 cells (1 × 106) were seeded in 25 cm2 flasks and treated with 100 μg/ml of the PSE for 72 h in order to detect active caspase-3 and cleaved PARP [poly(ADP-ribose) polymerase] levels, which are the markers for apoptosis. After treatment, cells were washed in ice-cold PBS, and lysed in lysis buffer (Cell Signaling, Danvers, MA, USA), containing protease inhibitors (P2714, Sigma St. Louis, MO, USA) and 1 mM of PMSF (Phenylmethylsulfonyl fluoride) (78830, Sigma, St. Louis, MO, USA). Cells were extracted at 4 °C for 5 min, and centrifuged at 4 °C for 10 min at 14,000g. The level of cleaved PARP was estimated using the PARP Cleavage [214/215] Human ELISA Kit (Invitrogen Corporation, Camarillo, CA, USA) and Caspase-3 (Active) Human ELISA Kit (Invitrogen Corporation) according to the protocols described in the manufacturer’s instructions. For determination of active caspase-3, 100 μl of cell lysates were incubated in the microplate wells provided in the kit at room temperature for 2 h. The samples were aspirated and washed four times with washing buffer and incubated with 100 μl of detection antibody (Anti-active caspase-3) for 1 h at room temperature. After removal of the antibody solution, the wells were washed again and incubated with 100 μl of HRP anti-rabbit antibody for 30 min at room temperature. After the aspiration of the anti-rabbit antibody, blue color was developed by adding 100 μl of stabilized chromogen solution for 20 min at room temperature. The reaction was stopped after the addition of 100 μl of stopping solution. For determination of cleaved PARP levels, 50 μl from each lysate was incubated with Anti-cleaved PARP (detection antibody) for 3 h. After washing, each sample in a well was incubated with a secondary antibody, IgG–HRP solution for 30 min. Stabilized chromogen was added to each well for another 30 min followed by addition of stop solution. The absorbance of each well was read at 450 nm using a microplate reader.
Genotoxicity assays
Heparinized peripheral blood obtained from four healthy and non-smoking donors (two males and two females, ages 18–23) was used in all experiments. The cultures were set up by adding 0.3 ml of whole blood to RPMI 1640 medium (1X, Sigma, St. Louis, MO, USA) supplemented with 20 % foetal calf serum (Biochrom AG, Berlin, Germany), 100 IU/ml penicillin-100 μg/ml streptomycin (Biological Industries, Beit Haemek, Israel), 0.5 mg/ml l-glutamine and 6 μg/ml phytohemagglutinin (PHA-L, Biochrom AG, Berlin, Germany). Lymphocytes were incubated at 37 °C for 72 h. As positive control, ethyl methanesulphonate (EMS, 1250 μg/ml) was dissolved in sterile distilled water and drug solution was prepared immediately before use to avoid degradation of the drug. PSE was dissolved in 50 % DMSO. The cells were treated separately with a mutagenic agent, 50 % DMSO as solvent control and diluted sterile PSE (125, 250 and 500 μg/ml), which were chosen after a preliminary study (50, 100, 125, 250, 400, 500 and 750 μg/ml), for 24 h prior to harvest. Metaphases were obtained by adding colcemid (0.2 μg/ml final concentration, Sigma, St. Louis, MO, USA) 2 h prior to harvest. For micronuclei assay, Cytochalasin B (6 μg/ml) was added at 44 h to block the cytokinesis process and lymphocyte cultures were harvested after 72 h (Fenech 2000). Chromosomes were prepared using standard procedures (Benn and Perle 1992). Staining of the micronuclei was performed by immersing the air-dried slides in a 2 % Giemsa solution.
The alkaline version of comet assay was used with some modifications (Singh et al. 1988). Collected venous blood samples from healthy donors (0.5 ml) were mixed with 5 ml of RPMI 1640 medium supplemented with 20 % fetal calf serum, 1 % penicillin/streptomycin (100 μg/ml) and 2.5 % phytohemagglutinin. The lymphocyte culture was incubated for 72 h at 37 °C. PSE lichen extract (100 μg/ml), and ethyl methanesulfonate (EMS, 1,250 μg/ml, as positive control) were added 24 h prior the end of incubation. The lymphocytes were isolated with Histopaque from the culture medium. 100 μl of the cell suspension was mixed with 250 μl 0.65 % low melting point agarose and rapidly laid on slides coated with 0.65 % normal melting point agarose.
The cells were lysed (2.5 M NaCl, 100 mM EDTA disodium salt, 10 mM Tris, 1 % Triton ×-100 and 10 % DMSO) overnight at 4 °C. The slides were submerged into a running buffer (0.3 M NaOH, 1 mM EDTA disodium salt, pH > 13) kept at 4 °C for electrophoresis and left to stand for 15 min allowing the DNA to unwind. The electrophoresis was performed at 25 V for 30 min. The slides were neutralized in 0.4 M Tris (pH 7.5).
Microscopic evaluation
Chromosomal aberrations (CA)
The analysis of chromosomal aberrations (CAs) was performed in 50 metaphases for each culture. Total chromosome aberrations were given in two forms, one includes gaps and pulverizations (TGAP) and the other excluding gaps and pulverizations (TGEP). The CAs were classified according to the Environmental Health Criteria (EHC) 51 for Short-term Tests for Mutagenic and Carcinogenic Chemicals (IPCS 1985).
Micronuclei (MN)
Two thousand binucleated cells for each experimental point were examined following the scoring criteria adopted by the Human Micronucleus Project (Bonassi et al. 2001). We evaluated the binucleated micronucleated lymphocytes (BNMN) frequency as the number of binucleated lymphocytes containing one or more MN per 1,000 binucleated cells.
Mitotic index (MI) and nuclear division index (NDI)
The mitotic index was calculated from the number of metaphases in 2,000 cells analyzed per culture for each dose group and donor in CA assay. In micronuclei assay, 500 lymphocytes were scored to evaluate the percentage of binucleated cells, and the nuclear division index (NDI) was calculated according to the following formula:
where MONO, BN, TRI and TETRA were mononuclear, binucleated, trinucleated and tetranucleated lymphocytes respectively.
COMET assay
The slides stained with ethidium bromide were visualized under a microscope with a fluorescence attachment. The images were analyzed by using specialized software for COMET analysis (Kameram 21, Argenit, Istanbul, Turkey). Comet square, comet length, comet density, tail length, tail DNA percentage, tail moment length, olive moment length, head DNA %, genetic damage index (GDI) and percentage of damaged cell parameters were evaluated for each cell.
Genetic damage index was calculated according to the following formula used by COMET analysis programme (Singh et al. 1988),
(ΣType 0: Total of undamaged cells; ΣType 1: Total of very lowly damaged cells; ΣType 2: Total of lowly damaged cells; ΣType 3: Total of highly damaged cells; ΣType 4: Total of very highly damaged cells).
Statistical analyses
All statistical analyses were performed using the SPSS 20.0 statistical software for Windows. The significance was calculated using one-way analysis of variance (ANOVA) and Tukey Honest Significant Difference (HSD) tests with 95 % confidence intervals. A value of p < 0.05 was considered statistically significant. Results were expressed as mean ± standard deviation (SD).
Results
Chemical compositions of Parmelia sulcata extract
The volatile compounds of PSE were analyzed by using direct thermal desorption method coupled with comprehensive gas chromatography–time of flight mass spectrometry. Direct thermal desorption (DTD) has significant advantages over other methods such as the ability to be directly coupled to GC–MS, the requirement of only a small amount of sample, swiftness of method for qualitative and quantitative analysis of volatile compounds, and the capability to quantify volatiles (Ozel et al. 2006). The volatile compounds in PSE were analyzed by GCXGC–TOF/MS system and the qualitative and quantitative compositions are presented in Table 1. Twenty-six compounds were identified in PSE and the major components were of it was eicosane (24.43 %), methyl 2,4-dihydroxy-3,6-dimethylbenzoate (20.65 %), 2-methylnonadecane (16.18 %), (E,E)-3,7,11-trimethyl-2,6-dodecadien-1-ol (5.60 %), 2-methyloctadecane (5.28 %), heneicosane (4.66 %), 9-octadecenal (4.65 %), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (2.54 %), n-hexadecanoic acid (2.43 %). All other components were present at <2 %.
Table 1.
Chemical compositions of Parmelia sulcata extract
| Compounda | 1tbR | 2tbR | Areac (%) |
|---|---|---|---|
| Acetic acid | 430 | 1.30 | 0.94 |
| Methyl 2,4-dihydroxy-3,6-dimethylbenzoate | 1,875 | 2.37 | 20.65 |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 1,965 | 1.4 | 2.54 |
| n-Hexadecanoic acid | 2,240 | 1.455 | 2.43 |
| Decalactone | 2,385 | 1.92 | 0.13 |
| 9-Octadecynoic acid | 2,405 | 1.885 | 0.23 |
| Dodecanamide | 2,440 | 1.82 | 0.42 |
| Pentadecane | 2,480 | 1.75 | 0.22 |
| 9-Octadecenal | 2,580 | 1.78 | 4.65 |
| Methyl isoheptadecanoate | 2,760 | 1.485 | 0.26 |
| 2-Methylnonadecane | 2,765 | 1.485 | 16.18 |
| Farnesane | 2,810 | 1.37 | 1.33 |
| 2-Nonadecanone | 2,815 | 1.61 | 0.24 |
| 1-Acetoxynonadecane | 2,835 | 1.475 | 0.47 |
| Eicosane | 2,900 | 1.365 | 24.43 |
| Hexyl 2-phenylethyl ester-succinic acid | 2,915 | 1.73 | 0.52 |
| 1-Heneicosanol | 2,925 | 1.515 | 1.00 |
| (E,E)-3,7,11-trimethyl-2,6-dodecadien-1-ol | 2,945 | 1.49 | 5.60 |
| Ethyl icosanoate | 2,995 | 1.5 | 0.86 |
| Allyl octadecyl oxalate | 3,000 | 1.435 | 0.25 |
| 1-Docosanol, acetate | 3,010 | 1.505 | 1.59 |
| Squalene | 3,015 | 1.58 | 0.26 |
| 2-Hexyl-1-octanol | 3,070 | 1.4 | 0.25 |
| 2-Methyloctadecane | 3,075 | 1.46 | 5.28 |
| Heneicosane | 3,080 | 1.57 | 4.66 |
| Unknown | 4.63 |
aAs identified by GCxGC–TOF/MS software; names according to NIST 2005 mass spectral library
btR and 2tR, retention times in the first and second dimension, respectively
cPercentage of each component is calculated as peak area of analyte divided by peak area of total ion chromatogram times 100
Anti-growth activity of Parmelia sulcata extract by the MTT and ATP viability assays
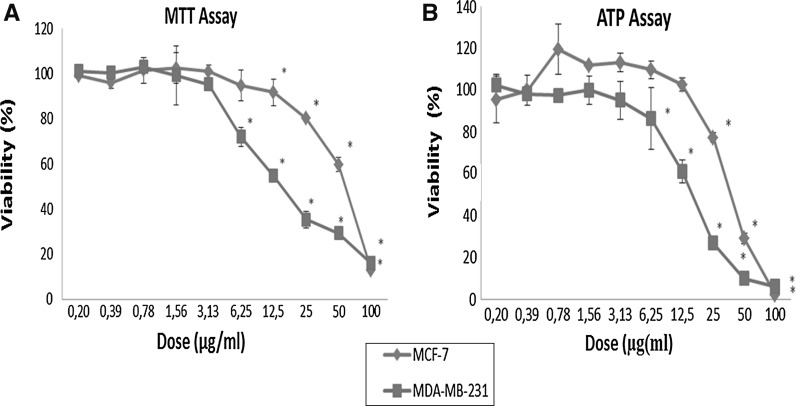
Anti-growth effect of the PSE was assessed by the MTT assay, and then the results were confirmed by a more sensitive assay that is the ATP assay. The anti-growth effect of PSE (0.20–100 μg/ml, for 72 h) was assessed against the MCF-7 and MDA-MB-231 breast cancer cells (Fig. 1a). PSE reduced cell viability in a dose-dependent manner (p < 0.05). Given the results of both the MTT and ATP assays, relatively higher doses showed significant anti-growth activity in both cell lines. The intracellular ATP level significantly decreased after PSE treatments at 25, 50 and 100 μg/ml doses in both types of cells (p < 0.05) (Fig. 1b).
Fig. 1.
Assessment of viability of MCF-7 and MDA-MB-231 cell lines after 72 h treatment with varying doses of Parmelia sulcata extract. Asterisks denote statically significant differences in comparison with control (p < 0.05)
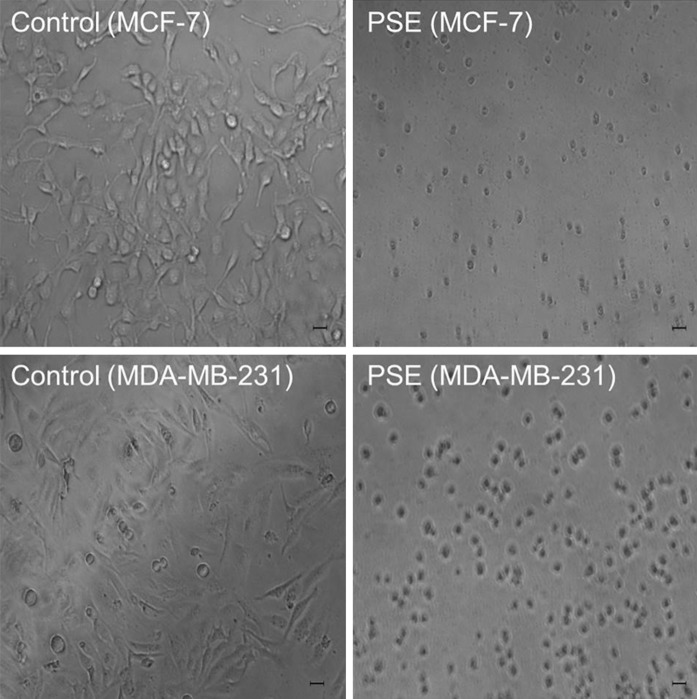
IC50 and IC90 values were calculated on the basis of the results of the ATP assay (Table 2). MDA-MB-231 cells were found to be more sensitive than MCF-7 cells according to the IC50 values (16.5 μg/ml for MDA-MB-231 and 39.1 μg/ml for MCF-7). The morphological evaluation by phase contrast microscopy showed that anti-growth effect was resulted from cell death (cytotoxicity). Cells were treated with PSE (100 μg/ml) as shown in Fig. 2, PSE was cytotoxic when compared with the control in both MCF-7 and MDA-MB-231 breast cancer cells.
Table 2.
Anti-growth parameters for Parmelia sulcata extract determined by the ATP assay after the treatment of MCF-7 and MDA-MB-231 breast cancer cells for 72 h
| Cells | IC50 (μg/ml)* | IC90 (μg/ml) |
|---|---|---|
| MCF-7 | 39.1 | 85.6 |
| MDA-MB-231 | 16.5 | 50.1 |
* IC50 is defined as the 50 % inhibitory concentration
* IC90 is defined as the 90 % inhibitory concentration
Fig. 2.
Images of phase contrast microscopy. Cells were treated with 100 μg/ml Parmelia sulcata extract (PSE) for 72 h. Controls included untreated cells. Scale bars represent 10 μm. Upper panels indicate MCF-7 cells, while bottom panels indicate MDA-MB-231 cells
Apoptosis-inducing effect of Parmelia sulcata extracts
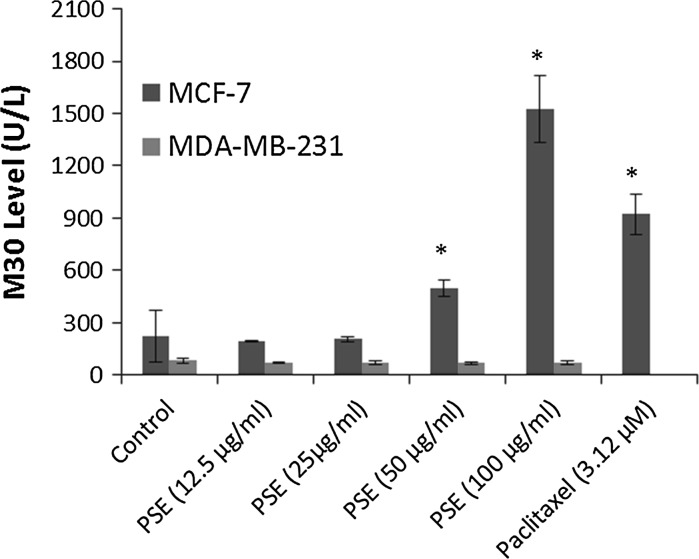
Taking the morphological evaluation following the treatment into account, the growth inhibiting effect of the PSE seemed to be the result of the cytotoxic activity against the cells as explained above. Therefore, the apoptotic activity of PSE was further investigated by examining caspase-cleaved cytokeratin 18 (M30) which is a well-known marker of apoptosis. Paclitaxel at the dose of 3.12 μM was used as a positive control for the induction of apoptosis. Figure 3, shows that M30 level was significantly increased in MCF-7 cells after the treatment with 50 and 100 μg/ml PSE (p < 0.05), but remained unchanged in MDA-MB-231 cells.
Fig. 3.
M30 (U/L) levels 72 h after treatment with Parmelia sulcata extract (PSE) in MCF-7 and MDA-MB-231 breast cancer cells. Asterisks denote statistically significant change from control (p < 0.05)
Fluorescence staining for confirmation of cell death mode
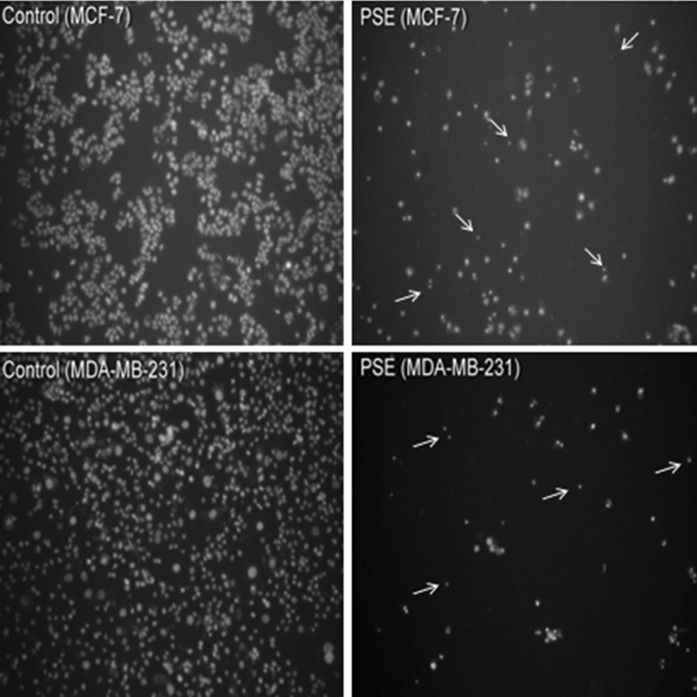
According to the MTT and ATP assay results, PSE displayed a potent cytotoxic activity at 100 μg/ml dose against both cell lines. Therefore, we further evaluated/confirmed the mode of cell death induced by PSE by fluorescence imaging based on nuclear morphology. We observed that PSE resulted in cell/nuclear shrinkage (pyknosis) in some cells, which is also a hallmark of apoptosis (Fig. 4).
Fig. 4.
Fluorescence imaging for determination of cell death mode. The cells were treated with 100 μg/ml Parmelia sulcata extract (PSE) for 72 h. Arrows indicate apoptosis-like cells with nuclear shrinkage (pyknosis). Compared to control/untreated cells, much lower cell density is also evident as a sign of anti-growth activity against both types of cells
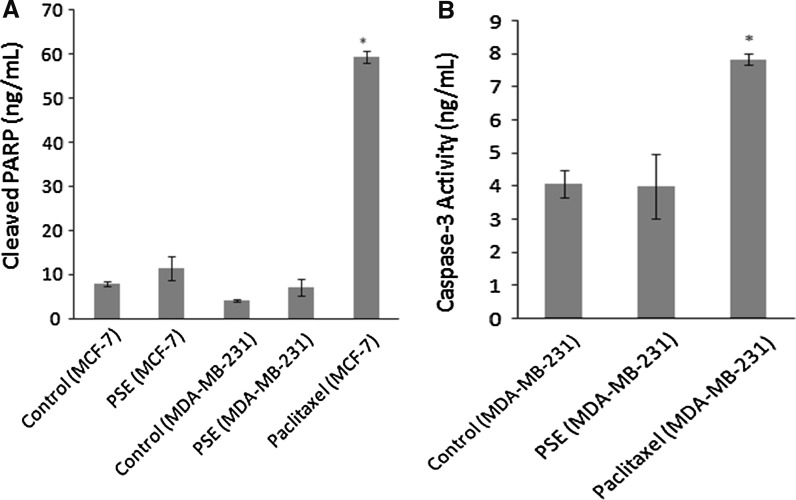
Effect of Parmelia sulcata on caspase-3 and PARP
Caspase-3 also known as CPP32 (32 kDa cysteine protease) or apopain is a cysteine protease with aspartate specificity and a well-characterized effector of apoptosis. Caspase-3 is synthesized as an inactive proenzyme, which upon cleavage at Asp175/Ser176, is converted to the active enzyme. As caspase-3 activation followed by PARP cleavage is a key event in the process of apoptosis, and as these two events are used as markers for apoptosis induction we sought to determine the role of PSE on caspase-3 activation and PARP cleavage. The effect of PSE on caspase-3 activation and PARP cleavage is shown in Fig. 5. As can be seen in Fig. 5a and b, exposure of MCF-7 and MDA-MB-231 cells to PSE resulted in no change in the caspase-3 activation and PARP cleavage compared with untreated control cells.
Fig. 5.
Effect of Parmelia sulcata extract (PSE) on PARP cleavage (a) and caspase-3 activity (b). The cells were treated with 100 μg/ml PSE and 3.12 μM Paclitaxel (as a positive control) for 72 h. Active caspase-3 not shown in part b as MCF-7 cells are not able to produce this enzyme. Asterisks denote statistically significant change from control (p < 0.05)
Genotoxic activities of Parmelia sulcata extracts
The results of genotoxicity tests are summarized in Tables 3 and 4. The doses lower than 125 μg/ml had no genotoxic effect (data not shown). Table 3 shows the effect of PSE (125, 250 and 500 μg/ml), positive (EMS 1,250 μg/ml), solvent (DMSO, 50 %) and negative controls (distilled water) on the mitotic and nuclear division index, the number of chromosomal aberrations, the frequency of abnormal metaphases and micronuclei frequency in human lymphocyte cell cultures. As presented in Table 3, 250 and 500 μg/ml PSE treatments significantly increased CAs when gap and pulverized metaphase were both included and excluded compared with the solvent control group (p < 0.05 and p < 0.005, respectively).
Table 3.
Frequencies of chromosome aberrations (CA), micronuclei (MN), nuclear division index (NDI) and mitotic index (MI %) in cultured human lymphocytes treated with Parmelia sulcata extract (PSE), positive and negative controls (mean ± SD)
| Treatments | MI ± SDa | Number of chromosome aberration | TGAP ± SDa | TGEP ± SDa | MN ± SDa | NDI ± SDa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CG | ICG | CB | ICB | EXC | SE | PLV | ||||||
| Negative control | 8.62 ± 2.24 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.02 ± 0.02 | 0.00 ± 0.00 | 13.10 ± 0.86 | 1.20 ± 0.05 |
| Solvent control (DMSO) | 8.04 ± 0.61 | 58 | 4 | 12 | 16 | 0 | 0 | 0 | 1.06 ± 0.22 | 0.48 ± 0.09 | 18.60 ± 0.66 | 1.41 ± 0.06 |
| 125 μg/ml PSE | 7.72 ± 0.42 | 105 | 36 | 22 | 40 | 0 | 5 | 0 | 1.43 ± 0.32 | 0.72 ± 0.15 | 19.80 ± 0.49 | 1.37 ± 0.07 |
| 250 μg/ml PSE | 7.14 ± 0.35 | 68 | 17 | 28 | 58 | 0 | 15 | 0 | 1.76 ± 0.33* | 0.97 ± 0.24** | 19.88 ± 2.46 | 1.30 ± 0.08 |
| 500 μg/ml PSE | 6.59 ± 0.29 | 82 | 29 | 46 | 40 | 2 | 19 | 0 | 1.91 ± 0.36** | 0.88 ± 0.19* | 25.18 ± 5.71* | 1.12 ± 0.03*** |
| Positive control (EMS) | 3.34 ± 1.92 | 38 | 24 | 26 | 12 | 21 | 2 | 7 | 1.61 ± 0.37b | 0.66 ± 0.19b | 34.05 ± 2.12c | 1.09 ± 0.34d |
CG chromatid gap, ICG iso-chromatid gap, CB chromatid break, ICB iso-chromatid break, EXC exchange figure, SE spiralization error, PLV pulverization, MI mitotic index, TGAP total chromosome aberration including gaps and pulverizations, TGEP total chromosome aberration excluding gaps and pulverizations, MN micronuclei frequency (%), NDI nuclear division index, PSE Parmelia sulcata methanolic extract, SD standard deviation, DMSO 50 % dimethyl sulphoxide as solvent control, EMS methyl methane sulphonate as positive control (1,250 μg/ml). Distilled water as negative control
aSignificancy of PSE doses compared with DMSO solvent control at * p < 0.05; ** p < 0.005; *** p < 0.001
bSignificancy of EMS compared with negative control at p < 0.05
cSignificancy of EMS compared with negative control at p < 0.005
dSignificancy of EMS compared with negative control at p < 0.001
Table 4.
Results of comet analysis in cultured human lymphocytes treated with Parmelia sulcata extract (PSE), positive and negative controls (mean ± SD)
| Treatments | Tail lengtha | Tail DNAa (%) | Olive tail momenta | Head diametera | Mean density of heada | Head DNAa (%) | Genetic damage indexa | Damage cella (%) |
|---|---|---|---|---|---|---|---|---|
| Negative Control | 5.98 ± 7.97 | 3.71 ± 6.76 | 0.79 ± 2.32 | 25.87 ± 2.89 | 0.21 ± 0.02 | 96.29 ± 6.76 | 0.14 ± 0.06 | 0.03 ± 0.03 |
| Solvent Control (DMSO) | 7.17 ± 13.6 | 5.81 ± 15.89 | 1.78 ± 7.3 | 27.78 ± 4.94 | 0.20 ± 0.02 | 94.19 ± 15.89 | 0.21 ± 0.06 | 0.05 ± 0.03 |
| 125 μg/ml PSE | 11.05 ± 16.41 | 7.86 ± 14.12 | 2.39 ± 6.26 | 27.18 ± 5.81 | 0.18 ± 0.03* | 92.14 ± 14.12 | 0.46 ± 0.09*** | 0.14 ± 0.01*** |
| 250 μg/ml PSE | 15.66 ± 26.12** | 12.96 ± 24.32* | 5.14 ± 12.29* | 24.22 ± 4.54*** | 0.20 ± 0.04 | 87.04 ± 24.32* | 0.62 ± 0.02*** | 0.18 ± 0.01*** |
| 500 μg/ml PSE | 13.33 ± 20.64 | 11.22 ± 18.33 | 3.6 ± 9.44 | 23.74 ± 5.19*** | 0.18 ± 0.04*** | 88.79 ± 18.38 | 0.65 ± 0.01*** | 0.23 ± 0.03*** |
| EMS | 110.50 ± 19.22 | 96.31 ± 10.25 | 56.82 ± 10.54 | 5.96 ± 3.37 | 0.13 ± 0.05 | 3.69 ± 10.25 | 3.88 ± 0.05 | 0.98 ± 0.02 |
PSE Parmelia sulcata methanolic extract, SD standard deviation, DMSO 50 % dimethyl sulphoxide as solvent control, EMS methyl methane sulphonate as positive control (1,250 μg/ml), distilled water as negative control
aSignificancy of PSE doses compared with DMSO solvent control at * p < 0.05; ** p < 0.005; *** p < 0.001
Chromatid gaps and breaks, iso-chromatid gaps and breaks and other subchromatid aberrations were the most common chromosomal abnormalities. An insignificant decrease in the frequency of mitotic index was detected for the treatment with PSE (p > 0.05). A significant increase in the frequency of the CAs when gap and pulverized metaphase were both included (TGAP, p < 0.05–0.005) and excluded (TGEP, p < 0.05–0.005) compared with the control was observed at concentrations up to 125 μg/ml.
The positive control, EMS at 1,250 μg/ml concentration significantly increased the number of abnormal metaphases and the total structural chromosome aberrations, respectively, compared with the negative control (p < 0.05, 0.005 and 0.001, respectively) (Table 3).
The results of the MN assay are also shown in Table 3. Increase in MN frequency at all doses of PSE was statistically significant (p < 0.05). When we compared the nuclear division index (NDI), we also found significant differences at the highest dose of PSE (p < 0.001, Table 3). Table 4 shows the effect of PSE, positive, solvent and negative controls on the comet frequency and genetic damage indices in human lymphocyte cell cultures. The percentages of DNA in the ‘comet tails’, which is indicative of the degree of DNA damage, were significantly different from solvent control cells (p < 0.005, Table 4). PSE also induced a significant increase in genetic damage index and damage frequency compared with the control at all tested concentrations (p < 0.001, Table 4).
Discussion
In this study, the cytotoxic effects of the methanol extract of P. sulcata were investigated in breast cancer cell lines and genotoxic effects in human lymphocytes. Our study showed that the PSE exhibited significant anti-growth activity against breast cancer cell lines. In the literature, there is no study dealing with the cytotoxic activity of PSE against breast cancer cell lines. In this aspect, this study is the first report on cytotoxic activity.
In recent years, some natural sources were reported in terms of their protective activity against cancer cell formation. Therefore, lichen species as alternative materials to be extracted for natural therapeutic compounds have attracted considerable attention of biomedical scientists. There is evidence that lichens contain compounds with a relatively high antioxidant and genotoxic/antigenotoxic activity (Kosanić et al. 2012; Mitrović et al. 2011; Molnar and Farkas 2010; Manojlović et al. 2010). Several authors have reported increased antimutagenic/antigenotoxic activities of lichen compounds (Aslan et al. 2012; Zeytinoglu et al. 2008). On the contrary, our results demonstrated that PSE at the tested concentrations had genotoxic effects in human lymphocyte cultures because PSE induced DNA damage that was evidenced by all of the three tests employed in our study (Tables 3, 4). Importantly, the genotoxic activity on lymphocytes was found to be evident only at the relatively higher concentrations than at the doses which showed cytotoxicity. To the best of our knowledge, this is the first report on the genotoxic activity of P. sulcata as there are no relevant published data.
In the present study, different concentrations (ranging from 0.20 to 100 μg/ml) of PSE were tested in MCF-7 and MDA-MB-231 breast cancer cells. PSE showed significant antigrowth activity against these cancer cell lines in a dose-dependent manner. MDA-MB-231 cells were found to be more sensitive than MCF-7 cells according to the IC50 values (16.5 μg/ml for MDA-MB-231 and 39.1 μg/ml for MCF-7). This is in agreement with a study, in which different IC50 values for acetone extracts of P. sulcata in human melanoma (18 μg/ml) and colon cancer cells (22 μg/ml) were reported (Kosanić et al. 2012). Nevertheless, in a different colon cancer cell line, the extract of P. sulcata did not induce a significant inhibition of cell growth in a dose- and time- depended manner (Mitrović et al. 2011). These differential effects seem to be dependent on the type of cancer. In addition, we examined the mode of cell death. We found that PSE resulted in increments in M30 levels (sign for apoptosis) in MCF-7 cells. However, MDA-MB-231 cells did not exhibit any increase in M30 levels. This lack of increase might be result from two reasons: (a) the mode of cell death in this cell line may not be apoptosis, (b) the proteomic status of this cell line might play a role. Regarding the latter, it was reported that in the breast cancer cell lines expressing high amount of vimentin, the cytokeratin levels may be low (Sommers et al. 1989). This may explain why we detected very low levels of M30 in this cell line although these cells die by apoptosis (as evidenced by pyknotic nuclei and caspase-3 activity). Hence, the M30 assay may not be an ideal test to detect apoptosis in such cell lines. Thus, we further evaluated the cell death mode by fluorescence imaging on the basis of nuclear morphology. PSE resulted in cell shrinkage (pyknosis) in some cells, which is one of the hallmarks of apoptosis.
Caspase activation (cleavage of procaspase to active caspase) is a hallmark of apoptosis. Caspase-3 is a central effector caspase in many types of cells and mediates the cleavage of other caspase-3 molecules around, other downstream caspases, and other caspase substrates such as cytokeratin 18, and PARP (Gown and Willingham 2002). Therefore, we further investigated the effect of PSE on caspase-3 activity and PARP-cleavage in MCF-7 and MDA-MB-231 cells. We observed no change neither in the caspase-3 activation nor PARP cleavage compared with untreated control cells. In fact, in the literature, to the best of our knowledge, there is no information on the mechanism of action of P. sulcata which causes cell death. It is tentatively suggested that P. sulcata may induce apoptotic cell death probably through the caspase-independent pathway in these cells or other caspases (caspase 6 or 7) instead of caspase-3 may be involved in the process.
Conclusion
In conclusion, the findings suggest that methanol extract of P. sulcata has a strong anti-cancer activity against breast cancer cell lines by inducing apoptosis, depending on the type of tumor cell. These results suggest that P. sulcata may deserve more attention regarding its use as anticancer plant and warrants further in vivo experiments.
Acknowledgments
We appreciate the Research Fund of Uludag University for the Project that is numbered UAP(F)-2011/42 as for providing us with the kits/chemicals. The authors would like to thank Neslihan Onder Ozdemir (Lecturer of Medical English, Uludag University) for her comments on linguistic improvement. We thank Prof. Dr. Ayhan Bilir (Istanbul University, Turkey) for the cell lines and thank Nazlihan Aztopal, Mehmet Sarimahmut and Sezin Bozdemir for technical assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, Gleiberman I, Caruso PA, Ricks SH, Untch M. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995;55:5276–5282. [PubMed] [Google Scholar]
- Ari F, Celikler S, Oran S, Balikci N, Ozturk S, Ozel MZ, Ozyurt D, Ulukaya E (2012) Genotoxic, cytotoxic and apoptotic effects of Hypogymnia physodes (L.) Nyl. on breast cancer cells. Environ Toxicol. doi:10.1002/tox.21809 [DOI] [PubMed]
- Aslan A, Gulluce M, Agar G, Karadayi M, Bozari S, Orhan F. Mutagenic and antimutagenic properties of some lichen species grown in the Eastern Anatolia Region of Turkey. Tsitol Genet. 2012;46:36–42. [PubMed] [Google Scholar]
- Benn PA, Perle MA. Chromosome staining and banding techniques. In: Rooney DE, Czepulkowsky BH, editors. Human cytogenetics: a practical approach I–II. Oxford: IRL Press; 1992. pp. 7–83. [Google Scholar]
- Bezivin C, Tomasi S, Lohezic-Le Devehat F, Boustie C. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine. 2003;10:499–503. doi: 10.1078/094471103322331458. [DOI] [PubMed] [Google Scholar]
- Bonassi S, Fenech M, Lando C. Human micronucleus project: international database comparison for results with the cytokinesis block micronucleus assay in human lymphocytes: effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ Mol Mutagen. 2001;37:31–45. doi: 10.1002/1098-2280(2001)37:1<31::AID-EM1004>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. New Haven: Yale University Press; 2001. [Google Scholar]
- Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- Gown AM, Willingham MC (2002) Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem 50:449–454 [DOI] [PubMed]
- IPCS (1985) Guide to short-term tests for detecting mutagenic and carcinogenic chemicals, vol. 208. Environmental Health Criteria, World Health Organization, Geneva, pp 102–103
- Koparal AT, Tüylü BA, Türk H. In vitro cytotoxic activities of (+)-usnic acid and (−)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat Prod Res. 2006;20:1300–1307. doi: 10.1080/14786410601101910. [DOI] [PubMed] [Google Scholar]
- Kosanić M, Ranković B, Vukojević J. Antioxidant properties of some lichen species. J Food Sci Technol. 2011;48:584–590. doi: 10.1007/s13197-010-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosanić M, Ranković RB, Stanojković TP. Antioxidant, antimicrobial and anticancer activities of three Parmelia species. J Sci Food Agric. 2012;92:1909–1916. doi: 10.1002/jsfa.5559. [DOI] [PubMed] [Google Scholar]
- Manojlović NT, Vasiljevic P, Juskovic M, Najman S, Jankovic S, Milenkovic-Andjelkovic A. HPLC analysis and cytotoxic potential of extracts from the lichen, Thamnoliavermicularis var. subuliformis. J Med Plant Res. 2010;49:817–823. [Google Scholar]
- Manojlović N, Ranković B, Kosanić M, Vasiljević P, Stanojković T. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine. 2012;19:1166–1172. doi: 10.1016/j.phymed.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Mitrović T, Stamenković S, Cvetković V, Tośić S, Stanković M, Radojević I, Stefanović O, Ljiljana Č, Dragana Ð, Ćurčić M, Marković S. Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci. 2011;12:5428–5448. doi: 10.3390/ijms12085428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar K, Farkas E. Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch. 2010;65:157–173. doi: 10.1515/znc-2010-3-401. [DOI] [PubMed] [Google Scholar]
- Müller K. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol. 2001;56:9–16. doi: 10.1007/s002530100684. [DOI] [PubMed] [Google Scholar]
- Oksanen I. Ecological and biotechnological aspects of lichens. Appl Microbiol Biotechnol. 2006;73:723–734. doi: 10.1007/s00253-006-0611-3. [DOI] [PubMed] [Google Scholar]
- Ozel MZ, Gogus F, Lewis AC. Determination of Teucrium chamaedrys volatiles by using direct thermal desorption–comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J Chromatogr A. 2006;1114:164–169. doi: 10.1016/j.chroma.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR. Taxol induces caspase-10-dependent apoptosis. J Biol Chem. 2004;279:51057–51067. doi: 10.1074/jbc.M406543200. [DOI] [PubMed] [Google Scholar]
- Perry NB, Benn MH, Brennan NJ, Burgess EJ, Elliss G, Galloway DJ, Lorimer SD, Tangney RS. Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist. 1999;31:627–636. doi: 10.1017/S002428299900081X. [DOI] [Google Scholar]
- Purvis OW, Coppins BJ, Hawskworth DL, James PW, Moore DM (1994) The lichen flora of Great Britain and Ireland. Natural History Museum Publications. The British Lichen Society, London, pp 421–437
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sommers CL, Walker-Jones D, Heckford SE, Worland P, Valverius E, Clark R, McCormick F, Stampfer M, Abularach S, Gelmann EP. Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines and in oncogene-transformed mammary epithelial cells. Cancer Res. 1989;49:4258–4263. [PubMed] [Google Scholar]
- Triggiani D, Ceccarelli D, Tiezzi A, Pisani T, Munzi S, Gaggi C, Loppi S (2009) Antiproliferative activity of lichen extracts on murine myeloma cells. Biologia 64:59–62
- Ulukaya E, Ozdikicioglu F, Oral AY, Demirci M. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol In Vitro. 2008;22:232–239. doi: 10.1016/j.tiv.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Wirth W (1995) Die Flechten Baden-Württembergs. Teil 1-2. Verlag Eugen Ulmer, Stuttgart, pp 637–669
- Zeytinoglu H, Incesu Z, Tuylu BA, Turk AO, Barutca B. Determination of genotoxic, antigenotoxic and cytotoxic potential of the extract from lichen Cetraria aculeata (Schreb.) Fr. in vitro. Phytother Res. 2008;22:118–123. doi: 10.1002/ptr.2279. [DOI] [PubMed] [Google Scholar]