Abstract
Palm oil is the major oil produced, with annual world production in excess of 50 million tonnes. About 85% of global palm oil produced is used in food applications. Over the past three decades, research on nutritional benefits of palm oil have demonstrated the nutritional adequacy of palm oil and its products, and have resulted in transitions in the understanding these attributes. Numerous studies have demonstrated that palm oil was similar to unsaturated oils with regards to effects on blood lipids. Palm oil provides a healthy alternative to trans-fatty acid containing hydrogenated fats that have been demonstrated to have serious deleterious effects on health. The similar effects of palm oil on blood lipids, comparable to other vegetable oils could very well be due to the structure of the major triglycerides in palm oil, which has an unsaturated fatty acid in the stereospecific numbers (sn)-2 position of the glycerol backbone. In addition, palm oil is well endowed with a bouquet of phytonutrients beneficial to health, such as tocotrienols, carotenoids, and phytosterols. This review will provide an overview of studies that have established palm oil as a balanced and nutritious oil.
Keywords: Health, Lipid, Nutrition, Palm oil, Palm olein, Phytonutrient
Introduction
Palm oil contributes significantly to the world's oils and fats market. The oil is used globally, with Malaysia being a major exporter, selling to over 150 countries worldwide. This global acceptance of palm oil is due to its competitive price vis-a-vis other oils and its suitability in various food applications, such as frying, specialty fats, margarines, shortenings, vegetable ghee, etc. In addition, there is now increasing awareness of its health attributes. This may be yet another major factor contributing to the growing demand for the oil.
Almost 85% of the world's palm oil is used as food and this has meant that the nutritional properties of palm oil and its fractions must be adequately demonstrated through research. The fatty acid composition of palm oil, of almost 50% saturated fatty acids (SFA), has been the focus of attention in determining its nutritional adequacy in relation to coronary heart disease (CHD) risk. Palmitic acid (44%) is the major SFA in palm oil, counter-balanced by almost 39% monounsaturated oleic acid and 11% polyunsaturated linoleic acid. This composition is very different from palm kernel oil (obtained as a co-product from the seed or kernel) which is almost 80% saturated. In addition, the fractionation of palm oil produces a more liquid form of the oil referred to as palm olein (POo), which contains up to 44% oleic acid and 13% linoleic acid. Besides having a balanced fatty acid composition, palm oil is also rich in a number of phytonutrients—carotenoids, tocopherols, tocotrienols, sterols, squalene, coenzyme Q10, phospholipids, and polyphenols. Although these minor components constitute less than 1% of the oil, they nevertheless play an important role in the stability and quality of the oil. In addition, all these phytonutrients have antioxidant properties and some of them exhibit nutritional and health benefits beyond their antioxidant function.
Using a carefully evolved research strategy, the Malaysian Palm Oil Board (MPOB) has focused on multi-pronged nutrition trials in animals and humans to prove the nutritional worthiness of palm oil and its products. This has resulted in over 200 publications in high impact peer reviewed journals. Collaborative projects have been undertaken at biomedical centres of excellence abroad where palm oil was compared to the indigenous oils used in the various countries. The results have shown that palm oil is as good as the indigenous oils, in some instances even better in its cholesterolemic response to the other oils and fats studied. The studies have yielded results that not only demonstrate the nutritional adequacy of palm oil and its products but also transitions in the science of edible oils and fatty acid effects on CHD.
The results from these studies have helped palm oil gain market share and positioned it as a safe and nutritious oil. The worldwide focus on trans fats as unhealthy has also opened the door further for palm oil as the healthy natural substitute. MPOB has developed many trans free formulations for margarines and shortenings.
sn-2 hypothesis
The nutritional properties of palm oil have been demonstrated since the 80's and specifically reported by Hornstra et al. (1987) [1] where it was described that although palm oil contains 50% long chain saturated fats, it has a distinct antithrombotic effect based on observations from an animal study on arterial thrombogenesis in vivo. These observations became more prominent in many more studies carried out subsequently (described elsewhere in this review). Ong and Goh (2002) [2] later hypothesized that palm oil does not increase cardiovascular risk because the oil is highly structured to contain predominantly oleic acid at the stereospecific numbers (sn)-2 position in the major triacylglycerols (TAG). More recently, the report of an Expert Consultation on Fats and Fatty Acids in Human Nutrition (2010) [3] has noted that “there is possible evidence to suggest that the TC and low density lipoprotein cholesterol (LDL-C) raising effects of palmitic acid are lower for vegetable than animal sources because it is present predominantly in the sn-1 and sn-3 position as opposed to sn-2 position as in animal fats such as lard”. As such, palm oil, like other vegetable oils, including olive oil, has the favourable oleic acid in this position. Therefore, this will lead to an acceptance that palm oil is as good as olive oil. This will also explain why even though 50% saturated, palm oil behaves more like a monounsaturated fat.
Significance of the sn-2 hypothesis
The body uses fats for long-term energy storage because they provide about six times as much energy as an equal weight of hydrated glycogen [4]. Many different fats and oils are sources of TAG in the human diet. These oils originate from fruits (e.g., palm and olive oils) or from seeds (corn, rapeseed, and soybean oils) as well as animal and fish sources. Animal fats like butter and lard are solid at room temperature while vegetable oils like corn, soybean, and peanut oils are liquid. However, their structures are chemically similar.
Fats and oils are made up of a mixture of TAG which consists of a glycerol backbone to which three fatty acids are esterified. The positions of fatty acid attachment on the glycerol backbone are referred to by (sn) -1, -2 and -3. The attachment of which fatty acid to which position has an important effect on the fat/oil properties [5]. Figure 1 shows the schematic structure of a TAG with three fatty acids bonded to the glycerol backbone.
Figure 1.

TAG structure showing the stereospecific numbering of sn-1, -2 and -3.
The fatty acids in fats and oils are classified as SFA, monounsaturated fatty acids (MUFA), or polyunsaturated fatty acids (PUFA). With this classification, palm kernel oil, which has 80% SFA (lauric acid, C12:0, myristic acid, C14:0 and palmitic acid, C16:0), and very little MUFA and PUFA, is a saturated fat. Olive oil, on the other hand, has 80% oleic acid (C18:1) and 9% PUFA but only 10% SFA, and so is predominantly monounsaturated. Sunflower oil has 70% linoleic acid (C18:2) and only 12% SFA, and hence is polyunsaturated.
In vegetable oils, oleic acid (a MUFA) is predominantly situated at the sn-2 position, while in animals fats it is predominantly palmitic acid or stearic acid (C16:0 or C18:0-saturated fat) that is situated there. Even though POo and lard have similar proportions of SFA, MUFA, and PUFA, they differ significantly in their positional distribution on the TAG molecule. POo TAG contains only 7–11% palmitic acid at the sn-2 position while about 87% is unsaturated fatty acids (oleic acid and linoleic acid). Lard has the highest amount of palmitic acid in the sn-2 position at 70%. In human milk, palmitic acid is predominantly present in sn-2 (53–57%) while cow milk fat contains less palmitic acid in the sn-2 position (38%) [6]. It is now believed that the distribution of fatty acids in the TAG is more important than the fatty acid composition (FAC) alone in conferring the oils’ “saturated” or “unsaturated properties.”
Lipid digestion and metabolism
The digestion of fat occurs when the enzyme lipase is present. The lipases involved in this process are lingual, gastric, pancreatic, and co-pancreatic, found in the mouth, stomach, and small intestine, respectively.
In the stomach, predigestion of 10–30% of the fat occurs in the presence of lingual and gastric lipases, and bile salts produced by the liver. The lingual and gastric lipases cleave the sn-3 fatty acids, resulting in the formation of 1,2-diacylglycerols (1,2-DAG) and sn-3 free fatty acids (FFA). The acidic medium in the stomach then facilitates conversion of sn-1,2-DAG to sn-1,3-DAG. The sn-1,3-DAG and sn-3 FFA (if <12 carbons) are readily absorbed in the intestine. The schematic diagram below shows the hydrolysis route of TAG at different locations in the human body (Fig. 2).
Figure 2.
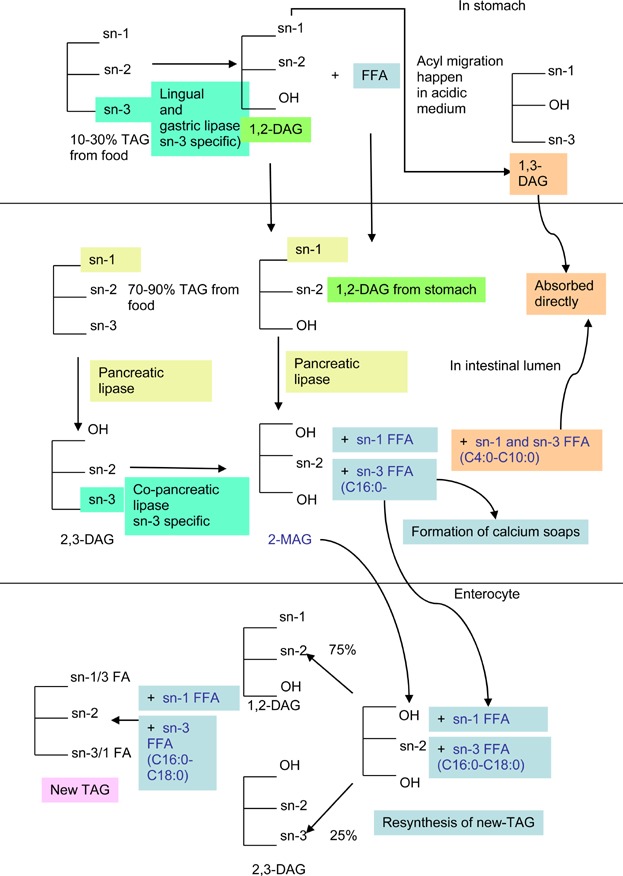
Hydrolysis route of TAG at different locations.
In the small intestine, particularly the duodenum, digestion of 70–90% of the fat occurs in the presence of pancreatic and co-pancreatic lipases. Pancreatic lipase hydrolyses the sn-1 fatty acids, while co-pancreatic lipase hydrolyses the sn-3 fatty acids. The products from these hydrolyses are 2-monoacylglycerol (2-MAG), and sn-1 and sn-3 FFA.
The sn-2 fatty acid as 2-MAG is transported by chylomicrons which now contain 93% of new TAG (solely from the food source) in its core. These new TAG are a result of resynthesis of 2-MAG and FFA (majority long-chain SFA) in the intestine. Short and medium chain fatty acid absorption is not via chylomicrons. Chylomicrons are then secreted into the blood stream via the lymphatic system. Lipoprotein lipase which lines the blood vessel walls hydrolyzes the new TAG in the chylomicrons. Chylomicron remnants, 2-MAG, and FFA are then produced. The chylomicron remnants carrying the cholesterol ester and TAG are transported back to the liver, while 2-MAG and FFA are used for liver TAG synthesis or energy supply and storage. Eating long-chain SFA and with elaidic acid (trans isomer of oleic acid) at sn-2 of TAG may slow down hydrolysis of the chylomicron TAG, liver uptake, and clearance of the chylomicron remnants. The presence of a large amount of chylomicron remnants in the blood can lead to increased plasma cholesterol and atherogenesis which can be detrimental to health.
In the intestines, the sn-1 and sn-3 short- and medium-chain FFA are absorbed directly after hydrolysis. Long-chain SFA will either be absorbed or predominantly react with 2-MAG for resynthesis of new TAG and chylomicron formation. The sn-1 and sn-3 long-chain free SFA are not absorbed or have delayed absorption as their melting points are above body temperature. Furthermore, in the presence of calcium in the intestines, the long-chain free SFA tend to precipitate as calcium soaps which are excreted (Table1). Therefore, long chain SFA when situated at sn-1 and -3 positions of the glycerol backbone are more difficult to be absorbed.
Table 1.
Summary of absorption for some common fatty acids at the sn-1 and sn-3 positions in tag
| Common name | Fate after hydrolysis |
|---|---|
| Short-chain fatty acids (C4–C6) | Absorbed directly |
| Medium-chain fatty acids (C8–C10) | Absorbed directly |
| Long-chain saturated fatty acids | Delayed absorption by minor phosphatidic acid pathway or form calcium soaps and excreted |
| Long-chain polyunsaturated fatty acids | Delayed formation of TAG and reduced supply of 2-MAG |
Surprisingly, pancreatic lipase and its co-lipase have low activity on long-chain PUFA with 20 carbons and more, especially arachidonic acid (C20:4n-6), eicopentaenoic acid (C20:5n-3), and docosahexaenoic acid (C22:6n-3), although they are located at sn-3 [7]. These long-chain PUFA occur as 2,3-DAG instead of at sn-3. The 2,3-DAG are only hydrolyzed by hepatic lipase in the liver. They are retained in the chylomicron remnants transported to the liver to produce 2-MAG and free long-chain PUFA. The slow hydrolysis of long-chain PUFA at sn-3 in TAG reduces the supply of sn-2 MAG and delays the resynthesis of new TAG in the intestine. Hence, sn-3 positioned long-chain PUFA are not directly absorbed by the body, while sn-2 long-chain PUFA are directly absorbed as 2-MAG. This might also lead to lesser excretion of long-chain PUFA from the body during the process of digestion.
The absorption and digestion of fat in infants are slightly different from those in adults. At birth, infants have to adapt to the high fat content of breast milk after relying mainly on glucose for energy during foetal development. Pancreatic secretion of lipase is low and the immature liver cannot provide sufficient bile salts to solubilize the digested lipids. Hence, new-born babies digest fats less efficiently than adults. However, breast milk contains lipoprotein lipase and bile-salt-stimulated-lipase that may assist the baby digest milk TAG. In addition, the baby itself secretes a TAG lipase from glands in its stomach and tongue. The milk lipase (only occurs in babies) in the intestinal lumen hydrolyses 2-MAG to glycerol and FFA for direct absorption before resynthesis of new TAG. This milk lipase shortens the route of digestion and absorption of sn-2 long-chain SFA in infants. Besides, human milk with palmitic acid (long-chain SFA) predominantly at sn-2 forms mixed micelles with bile salts in the milk itself. This again allows good and rapid absorption of TAG by infants [8–10]. If an infant formulation contains long-chain SFA at sn-1 and sn-3, absorption will be delayed as the milk lipase may only act on 2-MAG formed in the intestine before new TAG is resynthesized. Hence, the tendency to form long-chain calcium soap results in hard stools or constipation, and reduced calcium absorption in infants. The TAG structure of human milk is unique as it optimizes the absorption of palmitic acid. This is why sn-2 palmitic acid is preferred in infants over sn-1 and sn-3 palmitic acid.
Human clinical trials [11],[12],[32] and animal studies [21],[22] have been carried out to determine the effects of stereospecific fats on their lipid profiles. The position of a SFA in TAG exerts two effects on plasma TC. If long-chain SFA occurs at sn-1 and sn-3, they are either neutral or tend to lower total cholesterol (TC). If at sn-2, they generally raise TC.
sn-2 hypothesis and palm oil
In palm oil, the long-chain SFA (palmitic acid) is predominantly at sn-1 and sn-3, and mainly excreted through the formation of calcium soaps (evidence from human infants and animals). The other main fatty acid in palm oil, oleic acid, is situated at sn-2 where it is absorbed into the body and induces beneficial effects similar to olive oil (∼80% oleic acid at sn-2) [11–13].
Hence, it is wrong to group palm oil with the traditional saturated fats. In addition, plasma lipid response to a palm oil-rich diet was found to be mild, and appeared more dependent on age, gender, high body mass index (BMI), daily cholesterol ingestion [11],[13],[25] and the synthetic naure of the test oils used [24]. More scientific evidence with adequate and well controlled study designs are required to clear the misconception over palm oil and its nutritional implications, especially in human adults, and is currently the focus of research at MPOB.
Effects of palm oil and its fractions on blood lipids and lipoproteins
Animal studies
In a study to investigate the effects of different dietary fats on arterial thrombosis using the aorta loop technique in rats, it was demonstrated that palm oil exhibited a distinct anti-thrombotic effect comparable to PUFA oils including rapeseed, linseed, and sunflower seed oils [1]. Rand et al. (1988) [14] measured collagen activated platelet aggregation in rats fed 50% energy as palm oil or sunflower oil. They reported greater platelet aggregation in the sunflower oil fed rats compared to the palm oil fed rats. Subsequently, several animal studies compared palm oil with the more unsaturated oils and saturated fats for their effects on blood lipids, lipoproteins and other cardiovascular risk factors.
Oluba et al. (2008) [15] examined the effects of palm oil and soybean oil (both at 5%E level) on serum lipid and some serum enzymes over 6 wk. At the end of the trial, serum TC and TAG were significantly reduced in the rats fed palm oil from those fed soybean oil. This shows that palm oil consumption better ameliorates coronary heart disease risk than soybean oil. In a very recent study, Gouk et al. (2013) [16] fed mice with POo, chemically interesterified palm olein (IPOo), and soy bean oil and found that mice receiving the soy bean oil diet gained significantly higher amounts of subcutaneous fat and total fat compared with the POo group, despite similar body mass gain being recorded. Mice fed with the IPOo diet gained 14.3% more fat per food consumed when compared with the POo group, despite their identical total fatty acid compositions. The authors attributed this observation to the higher content of long chain SFA at the sn-1, 3 positions of TAG in POo. In a subsequent paper, Gouk et al. (2014) [17] also reported that SFA of different chain length at sn-1 and -3 positions exert profound effects on fat accretion.
In recent years, the hamster, especially the golden Syrian hamster and gerbil have been used extensively as animal models to elucidate sterol synthesis and LDL-C metabolism. Using them, the effects of dietary fatty acids and dietary cholesterol on plasma cholesterol synthesis and LDL-C metabolism in vivo were investigated. In the hamster model, dietary cholesterol feeding induced significant changes in plasma TC and LDL-C, whereas these parameters were relatively unaffected in rats. Several studies with palm oil included as dietary fat have been carried out and are examined below.
Khosla et al. (1997) [18] examined the effects of dietary oleic and palmitic acid on plasma lipids and lipoprotein metabolism in hamsters fed purified diets with low cholesterol but different quantities of fat (low fat 20%E and high fat 40%E, with constant levels of myristic and linoleic acids). Increasing dietary fat from 20%E to 30%E, and 40%E, by increasing oleic or palmitic acid had no effects on plasma lipid or lipoprotein cholesterol. The similarity in plasma and lipoprotein cholesterol levels was further confirmed by kinetics studies using radio-labeled native/methylated LDL-C or HDL-C. Consistent with circulating LDL-C and high density lipoprotein cholesterol (HDL-C) concentrations, there were also no differences in the clearance of LDL-C and HDL-C.
Wilson et al. (2005) [19] compared the effects of different palm oil preparations (RPO, RBDPO, and RBD + RPO-PO) with coconut oil on plasma cholesterol concentrations and aortic cholesterol accumulation in hypercholesterolemic hamsters. The hamsters fed the three palm oil preparations had lower plasma TC and non HDL-C, but higher HDL-C concentrations while accumulating less aortic cholesterol compared with the coconut oil-fed hamsters.
van Jaarsveld et al. (2002) [20] assessed the effect of POo in a moderate fat diet on the plasma lipoprotein profile and aortic atherosclerosis in a non-human primate model after 25.5 months of dietary exposure. The vervet monkeys fed a moderate fat/moderate cholesterol diet (MFD) with 11%E from lard, POo, and sunflower oil (SFO) had a polyunsaturated/saturated (P/S) ratio of 0.4 for 24 months. Plasma lipids were measured at 6-monthly intervals and atherosclerosis was assessed in the aorta and arteries after 25.5 months of dietary exposure. POo, relative to SFO and lard, significantly reduced the risk of developing early lesions in the peripheral arteries. Therefore, in this primate model of atherogenesis, the isocaloric substitution of lard with POo seems beneficial.
Kritchevsky et al. (2000a) [21] compared the atherogenic effects of refined, bleached, and deodorized (RBD) palm oil with those of randomized RBD palm oil. The RBD palm oil contained 41.2% palmitic acid, of which 2.6% was at the sn-2 position. In the randomized palm oil, 13.6% palmitic acid was at the sn-2 position. The randomized palm oil was significantly more atherogenic for rabbits than was the RBD palm oil.
In a different study, Kritchevsky et al. (2000b) [22] showed that increasing the amount of palmitic acid at the sn-2 position of a fat led to an increased atherogenic effect. Rabbits were fed with four synthetic fats with triglyceride structures of SOS, SSO, POP, and PPO (S = stearic acid, P = palmitic acid, O = oleic acid) and were evaluated for their atherogenic potential. The fats were incorporated into semisynthetic diets containing 15% fat, of which 58% was the synthetic fat, 24% was sunflower oil, and 18% was high-oleic safflower oil. All of the diets contained 0.05% cholesterol and the rabbits were fed for 20 wk. The blood lipid levels (TC, % HDL, and triglycerides) were similar in all four groups. Average atherosclerosis was similar in rabbits fed SOS compared to SSO (2.4 or 28% of stearic acid in the sn-2 position, respectively); however, it was much greater in rabbits fed PPO compared to POP. The authors noted that these findings confirmed their earlier observations that randomization of fats affects their atherogenicity but not their lipidemic effects. Specifically, fats bearing palmitic acid (but not stearic acid) in the sn-2 position have increased atherogenicity compared to fats with palmitic acid in the sn-1 or sn-3 positions.
Human studies
Palm oil versus saturated fats
While there is general perception that SFA (C12:0–16:0) increase TC and LDL-C in comparison with MUFA and PUFA, several studies on normocholesterolemic and hypercholesterolemic subjects have established that C16:0 from palm oil elicits significantly lower plasma cholesterol responses compared to C12:0- and C14:0-rich diets and have similar effects as C18:0 (Table2). A study by Ng et al. (1991) [23] found that feeding a moderate fat diet (31%E from fat) rich in C12:0 + C14:0 for 4 wk to 31 healthy normocholesterolemic subjects resulted in a significant increase in serum TC, LDL-C, and HDL-C over baseline levels. However, when the subjects were switched to a C16:0-rich diet, these levels were reduced significantly. In addition, Zock et al. (1994) [24] reported that C14:0 is about 1.5 times more cholesterol raising than C16:0. Sundram et al. (1994) [25] demonstrated that by exchanging 5%E of C12:0 + C14:0 to C16:0 resulted in a significant reduction (9%) in serum TC, primarily a 11% reduction in LDL-C and a more moderate decrease in HDL-C compared to the C12:0 + C14:0 rich diet.
Table 2.
Summary of human dietary intervention studies: palm oil versus saturated fats
| Reference | Subjects (n) | Age (y) | BMI (kg/m2) | Design | Dietary fatty acids (%E) |
Cholesterol (mg) | Lipids (mmol/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total fat | 12:0 | 14:0 | 16:0 | 18:0 | 18:1 | TC | TAG | LDL-C | HDL-C | ||||||
| Nestel et al. (1998) | 12 men, | 51 ± 7 | 26.2 ± 3.9 | RCT, 2 wk run in, 5 wk | 41 | - | – | 13.1 | 2.5 | 21.3 | 0 | 5.5 | 1.6 | 3.7 | 1.1 |
| 8 women | 42 | 3.8 | 14.3 | 20.2 | 0 | 5.4 | 1.5 | 3.7 | 1.1 | ||||||
| Sundram et al. (1994) | 17 men | 19–21 | 20.1 ± 1.8 | RCT, 3 wk run in, 4 wk | 30.7 | 0.8 | 1.2 | 11.1 | 1.5 | 10.8 | <200 | 4.0 | 1.1 | 2.4 | 1.1 |
| 30.5 | 4.6 | 2.7 | 6.9 | 1.1 | 10.3 | 4.4* | 1.0 | 2.7* | 1.2 | ||||||
| Zock et al. (1994) | 36 women, | 18–62 | 17.9–32.4 | RTC, 3 wk | 39.2 | 0.3 | 1.1 | 14.9 | 4.1 | 11.6 | <400 | 5.0 | 1.0 | 3.0 | 1.5 |
| 23 men | 39.6 | 0.4 | 11.3 | 4.7 | 4.3 | 10.9 | 5.2* | 1.0 | 3.1* | 1.7* | |||||
| Ng et al. (1991) | 27 per group | <30 | <26 | Parallel, 5 wk | 30.0 | 0.3 | 0.5 | 11.6 | 1.5 | 12.9 | <200 | 4.8 | 0.9 | 3.2 | 1.3 |
| 30.8 | 11.0 | 4.8 | 4.4 | 1.1 | 3.3 | 4.0** | 0.9 | 2.5** | 1.1** | ||||||
Significantly different from palmitic acid diet (P < 0.05).
Significantly different from entry levels (P < 0.05).
Furthermore, studies comparing C16:0 with C18:0 demonstrated similar effects on the lipid profile. Nestel et al. (1998) [26] compared the effects on plasma lipids of a C18:0-rich diet versus C16:0 in hypercholesterolemic subjects and demonstrated that plasma TC concentrations with the low-fat, C18:0-rich, and the C16:0-rich diets were not significantly different but lower than those measured during the habitual diet period. Neither HDL-C nor plasma TAG differed significantly among the three study diets. Kelly et al. (2002) [27] concluded that there is no significant differences between the two diets enriched in C18:0 and C16:0 in plasma lipoprotein concentrations. Based on the food items, the diet enriched in C16:0 was a mixture of fats with triglyceride structures of POP and POo where palmitic acids were esterified at sn-1 or sn-3 positions which have relatively neutral effects on cholesterol levels.
Saturated fatty acids with chain lengths C12:0–C14:0 produce a detrimental effect on blood lipids compared to C16:0 in healthy normocholesterolemic young men. C16:0 may be similar to C18:0 in its effects on plasma lipids subject to the fatty acids occupied in the sn-2 position.
Palm oil versus unsaturated oils
Several studies have shown that in healthy, normocholesterolemic humans, dietary C16:0 can be exchanged for C18:1 without adverse effects on serum lipid or lipoprotein levels. Ng et al. (1992) [11] and Choudhury et al. (1995) [12] evaluated POo against olive oil on serum lipids and lipoproteins (Table3). A study by Ng et al. (1992) [11] exchanged 7%E between C16:0 and C18:1 (with energy from C18:2 maintained at 3%) and resulted in identical serum TC, LDL-C, HDL-C, and TAG levels. Truswell et al. (1992) [28] reported that HDL-C were 8% lower on canola oil as compared to POo. Similarly, a study by Choudhury et al. (1995) [12] reported that substituting 5%E from C18:1 with C16:0 elicited similar plasma TC, LDL-C, and HDL-C levels. Voon et al. (2011) [29] reported that POo and olive oil have similar effects on blood lipid profile. Hence, in a one-to-one exchange basis, POo demonstrated similar TC, LDL-C, HDL-C, and TAG levels with olive oil in healthy normolipidemic subjects. Sundram et al. (1995) [30] reported that exchange of 4%E between C16:0 and C18:1 while maintaining 6%E from C18:2 resulted in similar plasma lipid levels. Oleic acid has been proven neutral in its cholesterolemic effect. However, the optimum requirement for oleic acid to confer beneficial lipoprotein profiles has not yet been ascertained. In this context, POo containing 44–48% oleic acid was equal in its plasma cholesterol and modulating lipoprotein effects to the higher oleic acid-containing oils, such as olive [2]. Table4 presents the percentage of total and sn-2 FAC of palm oil and olive oil.
Table 3.
Summary of human dietary intervention studies: palm olein versus olive oil
| Reference | Subjects (n) | Age (y) | BMI (kg/m2) | Design | Dietary fatty acids (%E) |
Cholesterol (mg) | Lipids (mmol/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total fat | 16:0 | 18:1 | 18:2 | TC | TAG | LDL-C | HDL-C | ||||||
| Choudhury et al. (1995) | 12 males, 12 females | 19–44 | <25 | RCT, | 30 | 12.1 | 13.0 | 3.2 | 175 | 4.7* | 1.0* | 3.3 | 0.9* |
| 30 days | 31 | 3.3 | 24.1 | 2.0 | 194 | 4.6* | 1.0* | 3.4 | 0.8* | ||||
| Ng et al. (1992) | 20 males, 13 females | 22–41 | <28 | RCT, | 34 | 6.3 | 21.4 | 2.6 | <200 | 4.9 | 1.2 | 3.3 | 1.1 |
| 6 wk | 34 | 13.4 | 13.8 | 3.5 | 4.9 | 1.2 | 3.4 | 1.1 | |||||
| Voon et al. (2011) | 9 males, 36 females | 30.1 ± 8.3 | 23.1 ± 3.7 | RCT, | 30.6 ± 2.3 | 9.7 | 12.3 | 4.0 | <300 | 4.8 | 0.85 | 3.2 | 1.3 |
| 5 wk | 31 ± 2.8 | 4.8 | 19.1 | 3.5 | 4.7 | 0.84 | 3.1 | 1.3 | |||||
Significantly different from entry levels (P < 0.05).
Table 4.
Total and sn-2 fatty acid composition (fac) of palm oil and olive oil (%)
| Type of oil | Fatty acid | Total FAC (%) | FA in sn-2 position (%) |
|---|---|---|---|
| Palm oil | C16:0 | 44.3 | 11 |
| C18:0 | 4.6 | 2 | |
| C18:1 | 39 | 65 | |
| C18:2 | 10.5 | 22 | |
| Olive Oil | C16:0 | 13.1 | 1.4 |
| C18:0 | 2.6 | − | |
| C18:1 | 71.8 | 82.9 | |
| C18:2 | 9.8 | 14 |
Source: A. S. H Ong and S. H. Goh, 2002 [2].
Scoltz et al. (2004) [31] evaluated the effect of consuming 33–38%E POo with sunflower oil in healthy subjects (Table5). POo significantly increased serum TC and LDL-C compared to sunflower oil. The relatively high linoleic acid content (24%E) in SFO may have contributed to its beneficial effect. In contrast to this finding, palm oil was comparable to peanut oil in serum lipids profile in a group of mildly hypercholesterolemic Chinese subjects [32] and in a healthy Indian population [33]. In addition, Marzuki et al. (1991) [34] reported that consumption of POo elicited similar effects on TC, LDL-C, and HDL-C in comparison to a soybean oil diet. The soybean oil diet, however, increased TAG compared to a POo diet. Ng et al. (1991) [23] reported a decrease in TC, LDL-C, and an increase in HDL-C in subjects fed a diet enriched with POo and corn oil at 30%E fat in comparison with baseline values.
Table 5.
Summary of human dietary intervention studies: palm oil versus polyunsaturated oils
| Reference | Subjects (n) | Age (y) | BMI (kg/m2) | Design | Dietary fatty acids (%E) |
Cholesterol (mg) | Lipids (mmol/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total fat | 16:0 | 18:1 | 18:2 | TC | TAG | LDL-C | HDL-C | ||||||
| ‘Scholtz et al. (2004) | 18–20 males + females | 21–59 | <30 | RCT, 4 wk | 33–38 | 13.5 | 13.8 | 3.7 | 207–224 | 5.6 | 1.6 | 3.6 | 1.1 |
| 2.2 | 6.9 | 24.2 | 5.0** | 1.3 | 3.3** | 1.0 | |||||||
| Zhang et al. (1997) | Groundnut oil: | 32–68 | − | Parallel, 6 wk | 30 | 9.8 | 11.6 | 5.9 | 163 | 5.7* | 1.4 | 4.1* | 1.3 |
| 15 males, 11 females | 30.2 | 4.8 | 11.9 | 9.5 | 166 | 5.9 | 1.3 | 4.4 | 1.2 | ||||
| Red palm oil: | |||||||||||||
| 16 males, | |||||||||||||
| 9 females, | |||||||||||||
| mildly hyperchole-sterolemic | |||||||||||||
| Ghafoorunissa et al. (1995) | 12 per group | 29–52 | 16–30 | RCT, 8 wk, 6 wk washout | 32 | 12.0 | 11.7 | 3.4 | <100 | 4.4 | 0.8 | 2.5 | 0.8 |
| 6.0 | 11.4 | 7.1 | 4.3 | 0.7 | 2.5 | 0.7 | |||||||
| Ng et al. (1991) | Palm oil: | 20–34 | Palm: 19.5 ± 2.0 | Parellel, double-blind, 5 wk | 30 | 11.3 | 12.6 | 3.3 | ∼200 | 4.0* | 0.9 | 2.5* | 1.1* |
| 20 males, | Corn: 19.4 ± 2.3 | 6.7 | 9.8 | 10.6 | 3.2* | 0.9* | 1.8* | 1.0* | |||||
| 7 females | |||||||||||||
| Corn oil | |||||||||||||
| 19 males, | |||||||||||||
| 7 females | |||||||||||||
| Marzuki et al. (1991) | 110 healthy males | 16–17 | – | RCT, 5 wk, 6 wk washout | 36 | 13.8 | 15.6 | 3.9 | 343 | 3.9 | 0.7 | 2.4 | 1.3 |
| 34 | 3.6 | 7.3 | 18.9 | 342 | 4.0 | 1.1** | 2.4 | 1.3 | |||||
Significantly lower from entry diet (P < 0.05).
Significantly different from palm oil diet (P < 0.05).
In comparison with diets enriched with peanut, corn, or soybean oils, POo appeared comparable in its ability to modulate lipids and lipoproteins. Studies that have inferred a hypercholesterolemic effect of C16:0 may be associated with the dietary fat content, cholesterol load, and the metabolic status of the subjects. It has been hypothesized that C16:0 is only hypercholesterolemic in situations in which the LDL receptors are down regulated [35]. In addition, Hayes and Khosla (1992) [35] also concluded that C16:0 has a variable cholesterolemic effect, where in normocholesterolemic subjects (TC < 225 mg/dL) consuming dietary cholesterol <300 mg/day, it has no impact on plasma cholesterol with the recommended intake of 18:2n-6 [36],[37]. Apart from the fatty acid composition, the minor components in POo, especially tocotrienols and other phytonutrients, have been reported to exert their antioxidant properties by modulating cholesterol levels [38]. These findings merit the re-evaluation of palm oil as a cholesterol-raising fat.
Palm oil versus hydrogenated oils
Compelling data have linked dietary trans fatty acids to increased risk of cardiovascular heart disease [39–41]. Evidence from large epidemiological studies, involving 667 to 80,082 men and women in different age groups, followed over 6 to 20 years, consistently found a positive association between trans fatty acid intake and risk of cardiovascular heart disease [42–46]. Controlled feeding dietary intervention studies have demonstrated that trans fatty acids intake compared to cis-unsaturated fats and saturated fats increases LDL-C concentrations, reduces HDL-C concentrations, which consequently increases the TC:HDL-C ratio, a better predictor of cardiovascular heart risk [47]. The recent interim report from FAO/WHO Expert Consultation also stated that there is convincing evidence that intake of trans fatty acid decreases HDL-C and increases the TC:HDL-C ratio in comparison to SFA (C12:0-C16:0), cis MUFA and PUFA. Mozaffarian et al. (2009) [41] also reported that a 2% increase in energy intake from partially hydrogenated fats or trans fatty acids was associated with a 23% increase in the incidence of cardiovascular heart disease. Current evidence also suggests that trans fatty acids may be implicated in the risk of sudden cardiac death and metabolic syndrome components.
The increasing use of artificially produced trans fats in foods therefore drew grave concern from the public, and led to stricter regulatory measures globally. Consequently, the US Food and Drug Administration required mandatory trans fatty acids labelling on packaged foods from January 1, 2006, prompting food manufacturers to find alternatives to commercially-hydrogenated vegetable oils for bakery products, margarines, and fried foods. In this context, palm oil provides the best natural replacement for commercially-produced hydrogenated vegetable oils, as it is naturally solid.
Sundram et al. (1997) [48] demonstrated that a 5.5%E intake of trans fatty acid (elaidic acid) was more deleterious than a greater intake of palmitic acid (11%E) contributed by POo (Table6). The finding showed that trans fatty acids increased TC, LDL-C, Lp(a) but decreased HDL-C. In contrast with this finding, other researchers have shown that palmitic acid and trans fatty acids elicited identical effects on TC and LDL-C, but not on HDL-C [49],[50]. Our laboratory recently conducted a randomized crossover intervention to investigate the effects of a high oleic POo versus partially hydrogenated soybean oil and palm stearin diets [51] in a group of healthy individuals. The trans fatty acids-rich diet significantly increased the TC:HDL-C ratio (a robust risk marker for CVD risk) compared to high oleic POo. In addition, trans fatty acids were also found to increase serum high-sensitivity C-reactive protein (hsCRP) in comparison with high oleic POo and palm stearin. hsCRP is recognized as a novel inflammatory marker for CVD risk. From our findings, we support the use of vegetable oils, such as palm oil, in their natural state over one that has undergone hydrogenation for modulating blood lipids and inflammation.
Table 6.
Summary of human dietary intervention studies: palm oil versus hydrogenated oils
| Reference | Subjects (n) | Age (y) | BMI (kg/m2) | Design | Dietary fatty acids (%E) |
Cholesterol (mg) | Lipids (mmol/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total fat | 16:0 | 18:1t | 18:1 | 18:2 | TC | TAG | LDL-C | HDL-C | ||||||
| Teng et al. (2010) | 33 female, 8 male completed | 28.8 ± 9.1 | 21.9 ± 3.9 | RCT, 5 wk, 1 wk washout | 33.5 | 8.7 | nd | 15.3 | 5.8 | <300 | 4.48 | 0.83 | 2.69 | 1.63 |
| 32.3 | 8.1 | 9.9 | 7.2 | 3.9 | 4.72* | 0.89* | 3.11* | 1.42* | ||||||
| 31.7 | 13.9 | nd | 10.7 | 3.6 | 4.66* | 0.88* | 2.95* | 1.55* | ||||||
| Pedersen et al. (2005) | 30 female, 27 completed | 19–42 | 26.5 ± 4.1 (20–36) | RCT, 17 days, 1 wk washout | 31.0 | 10.5 | Nd | 11.7 | 5.0 | 86 | 4.74 | 0.90 | 2.90 | 1.47 |
| 30.1 | 3.3 | 6.8 | 10.6 | 4.1 | 56 | 4.61 | 0.92 | 2.88 | 1.32 | |||||
| Sundram et al. (1997) | 20 men, 9 women, 27 completed | 19–39 | 22.7 ± 2.6 | RCT, double-blind, 4 wk | 31.7 | 11.4 | nd | 13.7 | 3.3 | 207 | 4.9 | 0.9 | 3.2 | 1.3 |
| (19–30) | 31.6 | 4.6 | 6.9 | 10.8 | 5.3 | 210 | 5.2* | 0.8 | 3.8* | 1.1 | ||||
| Nestel et al. (1992) | 27 mildly hypercholes-terolemic men | 46.8 ± 9.6 | 80.2 ± 8.9 | RCT, 3 wk | 37.0 | 9.8 | <1 | 12.9 | 5.7 | 186 | 226 | 128 | 161 | 42 |
| (30–63) | 37.0 | 4.9 | 5.7 | 11.3 | 6.6 | 168 | 229 | 142 | 165 | 38 | ||||
Nd, not detectable.
Significantly different from palm oil.
Minor components in palm oil and their health benefits
Palm oil is a rich source of beneficial phytonutrients, which are present to 1% of its weight. The most prevalent are tocols (600–1000 parts per million (ppm), carotenes (500–700 ppm), phytosterols (300–620 ppm), squalene (250–540 ppm), coenzyme Q10 (10–80 ppm), polyphenols (40–70 ppm), and phospholipids (20–100 ppm) [52],[53].
Seventy percent of the vitamin E in palm oil occurs as tocotrienols and the remainder as tocopherols [54]. The structure of tocotrienol and tocopherol are shown in Fig. 3. Both tocotrienol and tocopherol have four isomers each and the former has three double bonds in its isoprenoid side chain. Tocotrienols are unique as they are able to penetrate tissues with saturated fatty layers freely thus performing more efficient metabolic function than tocopherols. Thus, tocotrienols are more powerful antioxidant than tocopherols. MPOB has successfully isolated individual tocotrienol isomers from palm oil by using supercritical fluid chromatography [55] which provides high purity products through green and environmentally friendly processes. Each individual tocotrienol isomer (α-, β-, γ-, δ-) has unique beneficial properties. Accumulation of tocotrienols in tissue imparts tremendous health benefits [56]. Tocotrienols could help reduce blood cholesterol [57–59] and arteriosclerotic functions [60–62], encompass possible anti-angiogenic functions [63–66], exhibit efficient antioxidant activity [67–69] as well as anticancer [58],[70–77] anti-inflammatory effects [78], prevention of arthritis [79], prevention of osteoporosis [80],[81], prevention of skin diseases [82–84], anti-diabetic [85],[86], and neuroprotective properties [87–89].
Figure 3.

The structure of tocopherol and tocotrienol molecules (adapted from The AOCS Lipid Library).
Carotenoids are natural pigments responsible for the brilliant orange-red colour of palm oil. About 600 types of naturally occurring carotenoids are known but only 13 found in palm oil. Among them, the major ones are in the form of β-carotene, α-carotene, lycopene, phytoene, and phytofluene. Crude palm oil is considered one of the world's richest sources of carotenoids and contains 500–700 ppm. Carotenoids from commercial crude palm oil are concentrated during extraction and fractionation [90] as shown in Table7.
Table 7.
Carotenoid content of various palm oil fractions [89]
| Concentration (ppm) | |
|---|---|
| Crude palm oil | 630–700 |
| Crude palm olein | 680–760 |
| Crude palm stearin | 380–540 |
| Residual oil from fiber | 4000–6000 |
| Second-pressed oil | 1800–2400 |
Carotenoids act as precursors of vitamin A which is required to prevent night blindness [91], improve the vitamin A status of lactating women and their infants [92],[93] improve serum retinol concentrations [94] and combat vitamin A deficiency [95],[96]. Carotenoids can also protect against cardiovascular diseases [97] and suppress the growth of various cancer cells such as breast [98–101], lung, and liver as well as colon tumors. Carotenoids have been shown to enhance cell to cell communication in exerting their anti-cancer properties [100].
Coenzyme Q10, also known as ubiquinone, is a natural coenzyme in palm oil. It is claimed to possess ten times greater antioxidant property than vitamin E, although the carotenes and vitamin E in their far greater concentrations mask its functionality [102]. Besides being a powerful antioxidant and free radical scavenger [103], Coenzyme Q10 also plays a vital role in the mitochondrial electron transport chain and has been shown to exhibit membrane stabilizing properties. It has been used in the treatment of many cardiovascular ailments [104],[105] and studies have also demonstrated its anticancer effects [106].
Squalene is a valuable triterpene enormously found in shark liver oil. It is present in trace amounts in palm oil. Squalene is an oxygen transmitter and can aid cardiovascular health [104],[105]. It also has reported antitumor activity in rodents [107], suppresses hyperproliferation of cancer cells [107–110] in addition to exhibiting radioprotective effects [107].
Phytosterols are naturally-occurring substances in all plants and plant-based raw materials in foods. The major phytosterols in crude palm oil are β-sitosterol, campesterol, and stigmasterol. The main interest in palm phytosterols is their cholesterol lowering properties [111–113]. Besides research has also proven that they possess anticancer properties [114] and enhance immune functions [115]. Based on studies carried out at MPOB, palm fruits can serve as an inexpensive source of phenolic antioxidants, the market for which is currently monopolized by grape seed and tea extracts. Although the fat-soluble components have been receiving considerable attention, relatively little importance is given to the water-soluble components of palm oil. Polyphenols are a large family of natural compounds that can be classified as phenolic acids and flavonoids. The major palm phenolics (OPP) include p-hydroxybenzoic, cinnamic, ferulic and coumaric acids, and the flavonoid rutin hydrate [116],[117].
Flavonoids are touted as the most potent free radical scavenger and ion chelator. Phenolics, on the other hand, act as free radical terminators [118]. MPOB has developed and patented a breakthrough process [119] to recover the concentrations of OPP from palm oil mill effluent (POMEI). These compounds are also known to possess anti-carcinogenic [120–122] and cardioprotective properties [114].
Phospholipids form the main building block in all living forms. The main phospholipids in palm oil are phosphotidylcholine, phosphotidylethanolamine, phosphotidylinositol, and phosphotidylglycerol [123]. Phospholipids are essential components of lipoproteins and biological membranes [124] and they are essential for enhancing brain function [125], energy endurance [124],[126], structural integrity of cells as well as easing digestion and nutrient absorption [126].
Conclusions
To date, our extensive nutritional studies have shown that palm oil with high monounsaturation at sn-2 position is comparable to monounsaturated oils (e.g., olive, groundnut, and canola oils) in its effect on lipid profile. New concepts related to the cholesterol saturated fat hypothesis have slowly emerged from these studies. The interactive roles of the fatty acids and minor components in palm oil have also contributed significantly to nutritional science. Coupled to this factor is the well-known cost effective attributes of palm oil and its compatibility in various food formulations. All these suggest that palm oil will continue to play a leading role in the world oils and fats market with much greater acceptance among consumers.
CYM is the Director-General of the Malaysian Palm Oil Board and this review article is by invitation for the EuroFedLipid Highlights 2014, October 2014. KN is the Regional Manager for Malaysian Palm Oil Board, Europe Regional Office in Brussels, Belgium.
Glossary
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DAG
diacylglycerols
- E
energy
- FAO
Food Agricultural Organisation
- FFA
free fatty acids
- HDL-C
high density lipoprotein cholesterol
- hsCRP
high-sensitivity C-reactive protein
- IPOo
interesterified palm olein
- LDL
Clow density lipoprotein cholesterol
- MAG
monoacylglycerol
- MPOB
Malaysian Palm Oil Board
- MUFA
monounsaturated fatty acids
- POo
palm olein
- PUFA
polyunsaturated fatty acids
- P/S
polyunsaturated/saturated
- RBDPO
refined bleached deodorized palm oil
- RPO
red palm olein
- SFA
saturated fatty acid
- SFO
sunflower oil
- sn
stereospecific numbers
- TAG
triacylglycerols
- TC
total cholesterol
- VLDL-C
very low density lipoprotein cholesterol
- WHO
World Health Organisation
References
- [1].Honstra G. In: Polyunsaturated Fatty Acids and Eicosanoids. Lands WEM, editor. Champaign, Illinois: Americal oil Chemists Society; 1987. pp. 408–412. [Google Scholar]
- [2].Augustine Ong SH, Goh SH. Palm oil: A healthful and cost-effective dietary component. Food Nutr. Bull. 2002;23:11–22. doi: 10.1177/156482650202300102. [DOI] [PubMed] [Google Scholar]
- [3].Interim Summary of Conclusions and Dietary Recommendations On Total Fat & Fatty Acids. Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition, November 10–14, 2008, WHO HQ, Geneva. http://www.fao.org/ag/agn/nutrition/docs/Fats%20and%20Fatty%20Acids%20Summary.pdf.
- [4].McMurray J. Biomolecules: Lipids. Organic Chemistry. Thomson Learning; 2000. 5th Edn. [Google Scholar]
- [5].Goh SH. Oils and fats in nutrition and health: 2. Chemistry, digestion and metabolism. Malays. Oil Sci. Technol. 2006;15:43–63. [Google Scholar]
- [6].Straarup EM, Lauritzen L, Faerk J, Hoy CE, Michaelsen KF. The stereospecific triacylglycerol structures and fatty acid profiles of human milk and infant formulas. J Pediatr. Gastroenterol. Nutr. 2006;42:293–299. doi: 10.1097/01.mpg.0000214155.51036.4f. [DOI] [PubMed] [Google Scholar]
- [7].Bottino NR, Vandenburg GA, Reiser R. Resistance of certain long-chain polyunsaturated fatty acids of marine oils to pancreatic lipase hydrolysis. Lipids. 1967;2:489–493. doi: 10.1007/BF02533177. [DOI] [PubMed] [Google Scholar]
- [8].Filer LJ, Mattson F, Fomon SJ. Triglyceride configuration and fat absorption by the human infant. J. Nutr. 1969;99:293–298. doi: 10.1093/jn/99.3.293. [DOI] [PubMed] [Google Scholar]
- [9].Bracco U. Effect of triglyceride structure on fat absorption. Am. J. Clin. Nutr. 1994;60 (suppl):1002S–1009S. doi: 10.1093/ajcn/60.6.1002S. [DOI] [PubMed] [Google Scholar]
- [10].Innis SM, Dyer R, Lien EL. Formula containing randomized fats with palmitic acid (16:0) in the 2-position increases 16:0 in the 2-position of plasma and chylomycron tryacylglycerols in formula fed piglets to levels approaching those of piglets fed sow's milk. J. Nutr. 1997;127:1362–1370. doi: 10.1093/jn/127.7.1362. [DOI] [PubMed] [Google Scholar]
- [11].Ng TKW, Hayes KC, De Witt GF, Jegathesan M. Plamitic and oleic acids exert similar effects on lipid profiles in normocholesterolemic humans. J. Am. Coll. Nutr. 1992;11:383–390. doi: 10.1080/07315724.1992.10718241. et al. [DOI] [PubMed] [Google Scholar]
- [12].Choudhury N, Tan L, Truswell AS. Comparison of POo and olive oil: Effects on plasma lipids and vitamin E in young adults. Am. J. Clin. Nutr. 1995;61:1043–1051. doi: 10.1093/ajcn/61.4.1043. [DOI] [PubMed] [Google Scholar]
- [13].Voon PT, Ng TKW, Lee VKM, Nesaretnam K. Diets high in palmitic acid (16: 0), lauric and myristic acids (12: 0+ 14: 0), or oleic acid (18: 1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am. J. Clin. Nutr. 2011;94:1451–1457. doi: 10.3945/ajcn.111.020107. [DOI] [PubMed] [Google Scholar]
- [14].Rand ML, Hennissen AA, Hornstra G. Effects of dietary palm oil on arterial thrombosis, platelet responses and platelet membrane fluidity in rats. Lipids. 1988;23:1019–1023. doi: 10.1007/BF02535646. [DOI] [PubMed] [Google Scholar]
- [15].Oluba OM, Adeyemi O, Ojieh GC, Aboluwoye CO, Eidangbe GO. Comparative effect of soybean oil and palm oil on serum lipids and some serum enzymes in cholesterol-fed rats. Eur. J. Sci. Res. 2008;23:559–566. [Google Scholar]
- [16].Gouk SW, Cheng SF, Mok JSL, Ong ASH, Chuah CH. Long-chain SFA at the sn-1, 3 positions of TAG reduce body fat deposition in C57BL/6 mice. Br J Nutr. 2013;110:1987–1995. doi: 10.1017/S0007114513001475. [DOI] [PubMed] [Google Scholar]
- [17].Gouk SW, Cheng SF, Ong ASH, Chuah CH. Stearic acids at sn-1, 3 positions of TAG are more efficient at limiting fat deposition than palmitic and oleic acids in C57BL/6 mice. Br J Nutr. 2014;111:1174–1180. doi: 10.1017/S0007114513003668. [DOI] [PubMed] [Google Scholar]
- [18].Khosla P, Pronzuk A, Hajri T, Hayes KC. Dietary oleic and palmitic acid exert similar effects on plasma lipids and lipoprotein metabolism in hamster fed purified diets with low cholesterol but different quantities of fat. Asia Pac. J. Clin. Nutr. 1997;6:26–30. [PubMed] [Google Scholar]
- [19].Wilson TA, Nicolosi RJ, Kotyla T, Sundram K, Kritchevsky D. Different palm oil preparations reduce plasma cholesterol concentrations and aortic cholesterol accumulation compared to coconut oil in hypercholesterolemic hamsters. J. Nutr. Biochem. 2005;16:633–640. doi: 10.1016/j.jnutbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [20].Van Jaarsveld PJ, Benade AJ. Effects of POo oil in a moderate-fat diet on low-density lipoprotein composition in non-human primates. Asia Pac. J. Clin. Nutr. 2002;11:S416–423. doi: 10.1046/j.1440-6047.11.s.7.2.x. [DOI] [PubMed] [Google Scholar]
- [21].Kritchevsky D, Tepper SA, Kuksis A, Wright S, Czarnecki SK. Cholesterol vehicle in experimental atherosclerosis. 22. Refined, bleached, deodorized (RBD) palm oil, randomized palm oil and red palm oil. Nutr. Res. 2000a;20:887–892. [Google Scholar]
- [22].Kritchevsky D, Tepper SA, Chen SC, Meijer GW, Krauss RM. Cholesterol vehicle in experimental atherosclerosis. 23. Effects of specific synthetic triglycerides. Lipids. 2000b;35:621–625. doi: 10.1007/s11745-000-0565-3. [DOI] [PubMed] [Google Scholar]
- [23].Ng TKW, Hassan K, Lim JB, Lye MS, Ishak R. Non hypercholesterolemic effects of a palm-oil diet in Malaysian volunteers. Am. J. Clin. Nutr. 1991;53:1015S–1020S. doi: 10.1093/ajcn/53.4.1015S. [DOI] [PubMed] [Google Scholar]
- [24].Zock PL, De Vries JH, Katan MB. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler. Thromb. 1994;14:567–575. doi: 10.1161/01.atv.14.4.567. [DOI] [PubMed] [Google Scholar]
- [25].Sundram K, Hayes KC, Siru OH. Dietary palmitic acid results in lower serum cholesterol than does a lauric-myristic acid combination in normolipemic humans. Am. J. Clin. Nutr. 1994;59:841–846. doi: 10.1093/ajcn/59.4.841. [DOI] [PubMed] [Google Scholar]
- [26].Nestel PJ, Pomeroy S, Kay S, Sasahara T, Yamashita T. Effect of a stearic acid-rich, structured triacylglycerol on plasma lipid concentrations. Am. J. Clin. Nutr. 1998;68:1196–1201. doi: 10.1093/ajcn/68.6.1196. [DOI] [PubMed] [Google Scholar]
- [27].Kelly FD, Sinclair AJ, Mann NF, Turner AH. Short-term diets enriched in stearic or palmitic acids do not alter plasma lipids, platelet aggregation or platelet activation status. Eur. J. Clin. Nutr. 2002;56:490–499. doi: 10.1038/sj.ejcn.1601332. et al. [DOI] [PubMed] [Google Scholar]
- [28].Truswell AS, Choudhury N, Roberts DC. Double blind comparison of plasma lipids in healthy subjects eating potato crisps fried in palm olein or canola oil. Nutr. Res. 1992;12:S43–S52. [Google Scholar]
- [29].Voon PT, Ng TKW, Kar ML, Nesaretnam K. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprantidal or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am. J. Clin. Nutr. 2011;94:1451–1457. doi: 10.3945/ajcn.111.020107. [DOI] [PubMed] [Google Scholar]
- [30].Sundram K, Hayes KC, Siru OH. Both dietary 18:2 and 16:0 may be required to improve the serum LDL/HDL cholesterol ratio in normocholesterolemic men. J. Nutr. Biochem. 1995;4:179–187. [Google Scholar]
- [31].Scholtz SC, Pieters M, Oosthuizen W, Jerling JC. The effect of red palm olein and refined palm olein on lipids and haemostatic factors in hyperfibrinogenaemic subjects. Thromb. Res. 2004;113:13–25. doi: 10.1016/j.thromres.2004.02.004. et al. [DOI] [PubMed] [Google Scholar]
- [32].Zhang J, Wang C, Dai J, Chen X, Ge K. Palm oil may benefit midly hypercholesterolemic Chinese adults. Asia Pac. J. Clin. Nutr. 1997;6:22–25. [PubMed] [Google Scholar]
- [33].Ghafoorunissa, Reddy V, Sesikaran B. Palm olein and groundnut oil have comparable effects on blood lipids and platelet aggregation in healthy Indian subjects. Lipids. 1995;30:1163–1169. doi: 10.1007/BF02536619. [DOI] [PubMed] [Google Scholar]
- [34].Marzuki A, Arshad F, Razak TA, Jaarin K. Influence of dietary fat on plasma lipid profiles of Malaysian adolescents. Am. J. Clin. Nutr. 1991;53:1010S–1014S. doi: 10.1093/ajcn/53.4.1010S. [DOI] [PubMed] [Google Scholar]
- [35].Hayes KC, Khosla P. Dietary fatty acid thresholds and cholesterolemia. FASEB J. 1992;6:2600–2607. doi: 10.1096/fasebj.6.8.1592210. [DOI] [PubMed] [Google Scholar]
- [36].Clandinin MT, Cook SL, Konrad SD, Goh YK, French MA. The effect of palmitic acid on lipoprotein cholesterol levels and endogenous cholesterol synthesis in hyperlipidemic subjects. Lipids. 1999;34:S121–124. doi: 10.1007/BF02562257. [DOI] [PubMed] [Google Scholar]
- [37].Clandinin MT, Cook SL, Konrad SD, French MA. The effect of palmitic acid on lipoprotein cholesterol levels. Int. J. Sci. Nutr. 2000;51 (suppl):S61–71. [PubMed] [Google Scholar]
- [38].Mukherjee S, Mitra A. Health effects of palm oil. J. Hum. Ecol. 2009;26:197–203. [Google Scholar]
- [39].Mozaffarian D, Katan MB, Ascherio A, Stampfer M, Willett W. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- [40].Mozaffarian D, Abdollahi M, Campos H, Houshiarrad A, Willett WC. Consumption of trans fats and estimated effects on coronary heart disease in Iran. Eur. J. Clin. Nutr. 2007;61:1004–1010. doi: 10.1038/sj.ejcn.1602608. [DOI] [PubMed] [Google Scholar]
- [41].Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur. J. Clin. Nutr. 2009;63 (Suppl 2):S22–33. doi: 10.1038/sj.ejcn.1602976. [DOI] [PubMed] [Google Scholar]
- [42].Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D. Dietary fat and risk of coronary heart disease in men: Cohort follow-up study in the United States. BMJ. 1996;313:84–90. doi: 10.1136/bmj.313.7049.84. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hu FB, Stampfer MJ, Manson JE, Rimm E. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. et al. [DOI] [PubMed] [Google Scholar]
- [44].Pietinen P, Ascherio A, Korhonen P, Hartman AM. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The alpha-tocopherol, beta-carotene cancer prevention study. Am. J. Epidemiol. 1997;145:876–887. doi: 10.1093/oxfordjournals.aje.a009047. et al. [DOI] [PubMed] [Google Scholar]
- [45].Oomen CM, Ocke MC, Feskens EJ, Van Erp-Baart MA. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: A prospective population-based study. Lancet. 2001;357:746–751. doi: 10.1016/s0140-6736(00)04166-0. et al. [DOI] [PubMed] [Google Scholar]
- [46].Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurse's Health Study. Am. J. Epidemiol. 2005;161:672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- [47].Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- [48].Sundram K, Ismail A, Hayes KC, Jeyamalar R, Pathmanathan R. Trans (elaidic) fatty acids adversely affect the lipoprotein profile relative to specific saturated fatty acids in humans. J. Nutr. 1997;127:514S–520S. doi: 10.1093/jn/127.3.514S. [DOI] [PubMed] [Google Scholar]
- [49].Nestel P, Noakes M, Belling B, Mcarthur R. Plasma lipoprotein lipid and Lp (a) changes with substitution of elaidic acid for oleic acid in the diet. J. Lipid Res. 1992;33:1029–1036. et al. [PubMed] [Google Scholar]
- [50].Pedersen JI, Muller H, Seljeflot I, Kirkhus B. Palm oil versus hydrogenated soybean oil: Effects on serum lipids and plasma haemostatic variables. Asia Pac. J. Clin. Nutr. 2005;14:348–357. [PubMed] [Google Scholar]
- [51].Teng K-T, Voon P-T, Cheng H-M, Nesaretnam K. Effects of partially hydrogenated, semi-saturated, and high oleate vegetable oils on inflammatory markers and lipids. Lipids. 2010;45:385–392. doi: 10.1007/s11745-010-3416-1. [DOI] [PubMed] [Google Scholar]
- [52].Goh SH, Choo YM, Ong SH. Minor constituents of palm oil. J. Am. Oil Chem. Soc. 1985;62:237–240. [Google Scholar]
- [53].Choo YM, Lau HL, Puah CW, Bong SC. 2002. et al., Production of phytonutrients (carotenes, vitamin e, sterols, squalene, coenzyme Q and phospholipids) from palm methyl esters. MPOB Information Series.MPOB TT No. 348.
- [54].Nesaretnam K, Guthrie N, Chambers AF, Carroll KK. Effect of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids. 1995;30:1139–1142. doi: 10.1007/BF02536615. [DOI] [PubMed] [Google Scholar]
- [55].Ng MH, May CY. Chromatographic analyses of tocopherols and tocotrienols in palm oil. J. Chromatogr. Sci. 2012;50:283–286. doi: 10.1093/chromsci/bms002. [DOI] [PubMed] [Google Scholar]
- [56].Das S, Lekli I, Das M, Szabo G. Cardioprotection with palm oil tocotrienols: Comparison of different isomers. Am. J. Physiol. Heart. Clin. Physiol. 2008;294:70–78. doi: 10.1152/ajpheart.01200.2007. [DOI] [PubMed] [Google Scholar]
- [57].Qureshi AA, Qureshi N, Wright JJK, Shen Z. Lowering of serum cholesterol in hypercholesterolemic humans by tocotrienols (palmvitee) Am. J. Clin. Nutr. 1991;53:1021S–1026S. doi: 10.1093/ajcn/53.4.1021S. et al. [DOI] [PubMed] [Google Scholar]
- [58].Parker RA, Pearce BC, Clark RW, Gordon DA. Tocotrienols regulate cholesterol production in mammalian cells by post transcriptional suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Biol. Chem. 1993;268:11230–11238. [PubMed] [Google Scholar]
- [59].Song BL, Boyd RAD. Insign-dependent ubiquination and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase stimulated by δ- and γ tocotrienols. J. Biol. Chem. 2006;281:25054–25061. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- [60].Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids. 1995;30:1179–1183. doi: 10.1007/BF02536621. [DOI] [PubMed] [Google Scholar]
- [61].Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran and in hypercholesterolemic humans. Atherosclerosis. 2002;161:199–207. doi: 10.1016/s0021-9150(01)00619-0. [DOI] [PubMed] [Google Scholar]
- [62].Rasool AH, Rahman AR, Yuen KH, Wong AR. Arterial compliance and vitamin E blood levels with a self-emulsifying preparation of tocotrienol rich vitamin E. Arch. Pharm. Res. 2008;31:1212–1217. doi: 10.1007/s12272-001-1291-5. [DOI] [PubMed] [Google Scholar]
- [63].Miyazawa T, Shibata A, Nakagawa K, Tsuzuki T. Anti-angiogenic function of tocotrienol. Asia Pac. J. Clin. Nutr. 2008;7:253–256. [PubMed] [Google Scholar]
- [64].Shibata A, Nakagawa K, Sookwong P, Tsuzuki T. Tumor anti-angiogenic effect and mechanism of action of δ tocotrienol. Biochem. Pharmacol. 2008;76:330–339. doi: 10.1016/j.bcp.2008.05.017. et al. [DOI] [PubMed] [Google Scholar]
- [65].Wong WY, Selvaduray KR, Ming CH, Nesaretnam K. Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis. Nutr. Cancer. 2009;61:367–373. doi: 10.1080/01635580802582736. [DOI] [PubMed] [Google Scholar]
- [66].Selvaduray KR, Radhakrishnan AK, Kutty MK, Nesaretman K. Palm tocotrienols decrease levels of pro-angiogenic markers in human umbilical vein endothelial cells (HUVEC) and murine mammary cancer cells. Genes Nutr. 2012;7:53–61. doi: 10.1007/s12263-011-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Suarna C, Hood RL, Dean RT, Stocker R. Comparative antioxidant activity of tocotrienols and other natural lipid-soluble antioxidants in a homogeneous system, and in rat and human lipoproteins. Biochim. Biophys. Acta. 1993;1166:163–170. doi: 10.1016/0005-2760(93)90092-n. [DOI] [PubMed] [Google Scholar]
- [68].Azlina MFN, Nafeera MI, Khalid BAK. Effect of tocotrienol on lipid peroxidation in experimental gastritis induced by restraint stress. Pak. J. Nutr. 2005;4:69–72. [Google Scholar]
- [69].Suzana M, Suhana M, Zalinah A, Gapor MT, Wan Ngah WZ. Comparative effects of alpha-tocopherol and gamma-tocotrienol on lipid peroxidation status in Hep G2 cell line transfected with CYP2E1 gene. Eur. J. Sci. Res. 2005;7:41–56. [Google Scholar]
- [70].Nesaretnam K, Stephen R, Dils R, Darbre P. Tocotrienols inhibits the growth of human breast cancer cells irrespective of estrogen receptor status. Lipids. 1998;33:461–469. doi: 10.1007/s11745-998-0229-3. [DOI] [PubMed] [Google Scholar]
- [71].Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A. Tocotrienol-rich fraction from palm oil affects gene expression in tumors resulting from MCF-7 cell inoculation in athymic mice. Lipids. 2004;39:459–467. doi: 10.1007/s11745-004-1251-1. et al. [DOI] [PubMed] [Google Scholar]
- [72].Nesaretnam K, Koon TH, Selvaduray KR, Bruno RS, Ho E. Modulation of cell growth and apoptosis response in human prostate cancer cells supplemented with tocotrienols. Eur. J. Lipid Sci. Technol. 2008;110:23–31. [Google Scholar]
- [73].Yu FL, Gapor A, Bender W. Evidence for the preventive effect of the polyunsaturated phytol side chain in tocotrienols on 17β-estradiol epoxidation. Cancer Detect. Prev. 2005;29:383–388. doi: 10.1016/j.cdp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [74].Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2006;346:447–453. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- [75].Selvaduray KR, Radhakrishnan AK, Kutty MK, Nesaretnam K. Palm tocotrienols inhibit proliferation of murine mammary cancer cells and induce expression of interleukin-24 mRNA. J. Interferon. Cytokine. Res. 2010;30:909–916. doi: 10.1089/jir.2010.0021. [DOI] [PubMed] [Google Scholar]
- [76].Hafid SRA, Radhakrishnan AK, Nesaretnam K. Tocotrienols are good adjuvants for developing cancer vaccines. BMC Cancer. 2010;10:5. doi: 10.1186/1471-2407-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hafid SRA, Chakravarthi S, Nesaretnam K, Radhakrishnan AK. Tocotrienol-adjuvanted dendritic cells inhibit tumor growth and metastasis: A murine model of breast cancer. PloS One. 2013;8:e74753. doi: 10.1371/journal.pone.0074753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids. 2009;44:787–797. doi: 10.1007/s11745-009-3326-2. [DOI] [PubMed] [Google Scholar]
- [79].Zainal Z, Shahrim Z. Gamma-tocotrienol from Palm Oil for Athritis (12MY39) MALAYSIAN Patent Application No. PI 2011005682.
- [80].Hermizi H, Faizah O, Ima-Nirwana S. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in Sprague–Dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009;84:65–74. doi: 10.1007/s00223-008-9190-x. [DOI] [PubMed] [Google Scholar]
- [81].Ima Nirwana S, Wang M, Roshayati AB, Nursyahrina AH. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012;2012:532862. doi: 10.1155/2012/532862. et al.,, Article ID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yamada Y, Obayashi M, Ishikawa T, Kiso Y. Dietry tocotrienl reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J. Nutr. Sci. Vitaminol. 2008;54:117–123. doi: 10.3177/jnsv.54.117. et al. [DOI] [PubMed] [Google Scholar]
- [83].Shibata A, Nakagawa K, Kawakami Y, Tsuzuki T, Miyazawa T. Suppression of gamma-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J. Agric. Food Chem. 2010;58:7013–7020. doi: 10.1021/jf100691g. [DOI] [PubMed] [Google Scholar]
- [84].Pedrelli VF, Lauriola MN, Pigatto PD. Clinical evaluation of photoprotective effect by a topical antioxidants combination (tocopherols and tocotrienols) JEADV. 2011;26:1449–1453. doi: 10.1111/j.1468-3083.2011.04219.x. [DOI] [PubMed] [Google Scholar]
- [85].Budin SB, Othman F, Louis SR, Bakar MA. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics. 2009;64:235–244. doi: 10.1590/S1807-59322009000300015. et al.,. doi: 10.1590/S1807-59322009000300015.PMC2666447. PMID 19330251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Muharis SP, Md Top AG, Murugan D, Mustafa MR. Palm oil tocotrienol fractions restore endothelium dependent relaxation in aortic rings of streptozotocin-induced diabetic and spontaneously hypertensive rats. Nutr. Res. 2010;30:209–216. doi: 10.1016/j.nutres.2010.03.005. [DOI] [PubMed] [Google Scholar]
- [87].Sen CK, Khanna S, Roy S, Parker L. Molecular basis of vitamin E action: Tocotrienol potently inhibits glutamate-induced pp60c-Src kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 2000;275:13049–13055. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]
- [88].Sen CK, Rink C, Khanna S. Palm oil-derived natural vitamin E alpha-tocotrienol in brain health and diseases. J. Am. Coll. Nutr. 2010;29:314S–323S. doi: 10.1080/07315724.2010.10719846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Khanna S, Roy S, Slivka A, Craft TK. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36:2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Choo YM. Palm oil carotenoids. Food Nutr. Bull. 1994;15 [Google Scholar]
- [91].Wattanapenpaiboon N, Wahlqvist ML. Phytonutrient deficiency: The place of palm fruit. Asia Pac. J. Clin. Nutr. 2003;12:363–368. [PubMed] [Google Scholar]
- [92].Canfield LM, Kaminsky RG. Red palm oil in the maternal diet improves the vitamin status of lactating mothers and their infants. Food Nutr. Bull. 2000;21:144–148. [Google Scholar]
- [93].Lietz G, Henry CJK, Mulokozi G, Mugyabuso J. Use of red palm oil for the promotion of maternal vitamin A status. Food Nutr. Bull. 2000;21:215–218. et al. [Google Scholar]
- [94].Stuijvenberg MEV, Benade AJS. South Africa experience with the use of red palm oil to improve the vitamin A status of primary schoolchildren. Food Nutr. Bull. 2000;21:212–214. [Google Scholar]
- [95].Rao NBS. Potential use of red palm oil in combating vitamin deficiency in India. Food Nutr. Bull. 2000;21:202–211. [Google Scholar]
- [96].Scrimshaw NS. Nutritional potential of red palm oil for combating vitamin A deficiency. Food Nutr. Bull. 2000;21:195–201. [Google Scholar]
- [97].Rooyen JV, Esterhuyse AJ, Engelbrecht AM, Toit EF. Health benefits of a natural carotenoid rich oil: A proposed mechanism of protection against ischemia/reperfusion injury. Asia Pac. J. Clin. Nutr. 2008;17:316–319. [PubMed] [Google Scholar]
- [98].Nesaretnam K, Radhakrishnan A, Selvaduray KR, Reimann K. Effect of palm oil carotene on breast cancer tumorigenicity in nude mice. Lipids. 2002;37:557–560. doi: 10.1007/s11745-002-0932-0. et al. [DOI] [PubMed] [Google Scholar]
- [99].Toniola P, Kappel ALV, Akhmedkhanov A, Ferrari P. Serum carotenoids and breast cancer. Am. J. Epidemiol. 2001;153:1142–1147. doi: 10.1093/aje/153.12.1142. et al. [DOI] [PubMed] [Google Scholar]
- [100].Zhang S, Hunter DJ, Forman MR, Rosner BA. Dietarycarotenoids and vitamins A, C, and E and risk of breast cancer. J. Natl. Cancer Inst. 1999;91:547–556. doi: 10.1093/jnci/91.6.547. et al. [DOI] [PubMed] [Google Scholar]
- [101].Tamimi RM, Hankinson SE, Campos H, Spiegelman D. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am. J. Epidemiol. 2005;161:153–160. doi: 10.1093/aje/kwi030. et al. [DOI] [PubMed] [Google Scholar]
- [102].Ng MH, Choo YM, Ma AN, Chuah CH, Hashim MA. Separation of coenzyme Q10 in palm oil by supercritical fluid chromatography. Am. J. Appl. Sci. 2006;3:1929–1932. [Google Scholar]
- [103].Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: Defense against oxidative damage. Int. J. Biol. Sci. 2007;3:257–262. doi: 10.7150/ijbs.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Verma DD, Hartner WC, Thakkar V, Levchenko TS, Torchilin VP. Protective effect of coenzyme Q10-loaded liposomes on the myocardium in rabbits with an acute experimental myocardial infarction. Pharm. Res. 2007;24:2131–2137. doi: 10.1007/s11095-007-9334-0. [DOI] [PubMed] [Google Scholar]
- [105].Burke B, Neuenschwander E, Olson RRD. Randomized, double-blind, placebo-controlled trial of coenzyme q10 in isolated systolic hypertension. South. Med. J. 1994;94:1112–1117. doi: 10.1097/00007611-200111000-00015. [DOI] [PubMed] [Google Scholar]
- [106].Portakal O, Ozkaya O, Inai ME, Bozan B. Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients. Clin. Biochem. 2000;33:279–284. doi: 10.1016/s0009-9120(00)00067-9. et al. [DOI] [PubMed] [Google Scholar]
- [107].Smith TJ, Yang GY, Seril DN; Liao J, Kim S. Inhibition of 4 (methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis by dietary olive oil and squalene. Carcinogenesis. 1998;19:703–706. doi: 10.1093/carcin/19.4.703. [DOI] [PubMed] [Google Scholar]
- [108].Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of squalene on colon cancer. Carcinogenesis. 1998;19:287–290. doi: 10.1093/carcin/19.2.287. [DOI] [PubMed] [Google Scholar]
- [109].Murakoshi M, Nishino H, Satomi Y, Takayasu J. Potent preventive action of α-carotene against carcinogenesis: Spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by α-carotene than by β-carotene. Cancer Res. 1992;52:6583–6587. et al. [PubMed] [Google Scholar]
- [110].Miettinen TA, Vuoristo M, Nissinen M, Järvinen HJ, Gylling H. Serum, biliary, and fecal cholesterol and plant sterols in colectomized patients before and during consumption of stanol ester margarine 1-3. Am. J. Clin. Nutr. 2000;71:1095–1102. doi: 10.1093/ajcn/71.5.1095. [DOI] [PubMed] [Google Scholar]
- [111].Jones PJH, Raeini-Sarjaz M, St-Onge M. Phytosterols in low- and non-fat beverages as part of a controlled diet fail to lower plasma lipid levels. J. Lipid Res. 2003;44:1713–1719. doi: 10.1194/jlr.M300089-JLR200. [DOI] [PubMed] [Google Scholar]
- [112].Zadak Z, Hyspler R, Ticha A, Solichova D. Poly-unsaturated fatty acids, phytosterols and cholesterol metabolism in the Mediterranean diet. Acta. Medica. 2006;49:23–26. et al. [PubMed] [Google Scholar]
- [113].Awad A, Fink BCS. Phytosterol as anticancer dietary components: Evidence and mechanism of action. Am. Soc. Nutr. Sci. 2000;130:2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- [114].Bouic PJD, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K. Beta-sitosterol and Beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- [115].Tan YA, Sambanthamurthi R, Sundram K, Wahid MB. Valorisation of palm byproducts as functional components. Eur. J. Lipid Sci. Technol. 2007;109:380–393. [Google Scholar]
- [116].Sambanthanurthi R, Tan YA, Sundram K. 2011. Treatment of vegetation liquors derived from oil-bearing fruits. United States patent 7387802 )
- [117].Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 2008;7:3188–3192. [Google Scholar]
- [118].Sambanthamurthi R, Tan YA, Sundram K. 2008. Treatment of vegetation liquors derived from oil-bearing fruit. Malaysian Palm Oil Board: United States Patent US 7387802, B2 )
- [119].Fink BN, Steck SE, Wolff MS, Britton JA. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am. J. Epidemiol. 2007;165:514–523. doi: 10.1093/aje/kwk033. et al. [DOI] [PubMed] [Google Scholar]
- [120].Nair HK, Rao KVK, Aalinkeel RM, Supriya M. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin. Diag. Lab. Immunol. 2004;11:63–69. doi: 10.1128/CDLI.11.1.63-69.2004. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Guthrie N, Gapor A, Chambers AF, Carroll KK. Palm oil tocotrienols and plant flavonoids act synergistically with each other and with Tamoxifen in inhibiting proliferation and growth of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells in culture. Asia Pac. J. Clin. Nutr. 1997;6:41–45. [PubMed] [Google Scholar]
- [122].Aviram M, Fuhrman B. Polyphenolic flavonoids inhibit macrophage- mediated oxidation of LDL and attenuate atherogenesis. Atherosclerosis. 1998;137:S45–S50. doi: 10.1016/s0021-9150(97)00306-7. [DOI] [PubMed] [Google Scholar]
- [123].Jager R, Purpura M, Kingsley M. Phospholipids and sports performance. J. Int. Soc. Sports Nutr. 2007;4:1–8. doi: 10.1186/1550-2783-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Suzuki S, Yamatoya H, Sakai M, Kataoka A. Oral administration of soybean lecithin transphosphatidylated phosphatidylserine improves memory impairment in aged rats. J. Nutr. 2001;131:2951–2956. doi: 10.1093/jn/131.11.2951. et al. [DOI] [PubMed] [Google Scholar]
- [125].Starks MA, Starks SL, Kingsley M, Purpura M, Jager R. The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J. Int. Soc. Sports Nutr. 2008;5:1–6. doi: 10.1186/1550-2783-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lochmann R, Brown R. Soybean–lecithin supplementation of practical diets for juvenile goldfish (Carassius auratus. J. Am. Oil Chem. Soc. 1997;74:149–152. [Google Scholar]


