Abstract
Enteroviruses (EVs) are the most common human viral pathogens. They cause a variety of pathologies, including myocarditis and meningoencephalopathies, and have been linked to the onset of type I diabetes. These pathologies result from the death of cells in the myocardium, central nervous system, and pancreas, respectively. Understanding the role of EVs in inducing cell death is crucial to understanding the etiologies of these diverse pathologies. EVs both induce and delay host cell death, and their exquisite control of this balance is crucial for their success as human viral pathogens. Thus, EVs are tightly involved with cell death signaling pathways and interact with host cell signaling at multiple points Here, we review the literature detailing the mechanisms of EV-induced cell death. We discuss the mechanisms by which EVs induce cell death, the signaling pathways involved in these pathways, and the strategies by which EVs antagonize cell death pathways. We also discuss the role of cell death in both the resulting pathology in the host and in the facilitation of viral spread.
1. Introduction
Viruses are ubiquitous infectious agents that are astoundingly diverse in their size, shape, and tropism. Despite this diversity, all viruses must accomplish certain tasks in order to replicate and propagate their genetic material. Viruses must enter a living host cell, replicate within that cell, and, finally, exit the cell only to begin the cycle anew. This entire process can occur in a matter of hours in the case of a lytic viral infection or can take decades in the case of latent viral infection.
Enteroviruses (EVs) are the most common human viral pathogens (1,2). These small (~30nm), positive-sense RNA viruses with ~7 kB genome in the picornavirus family cause a range of syndromes from mild, upper respiratory infections to severe neurological pathology and dilated cardiomyopathy (3–7). Here, we focus on the non-rhinovirus EVs, which include poliovirus (PV) and coxsackieviruses (CVA or CVB), amongst others. EVs are fecal-oral pathogens that can infect a variety of cell types in their human hosts. As fecal-oral pathogens, they infect the polarized epithelial cells lining the intestinal tract (but can also target the respiratory tract) upon their initial ingestion. If dissemination occurs, EVs can infect pancreatic cells, cardiomyocytes, and neurons; the most serious pathologies arise as a consequence of viral replication at these secondary sites.
The enteroviral life cycle in such diverse human cell types is dependent upon eliciting cell death in order to facilitate egress. It is worth noting that there is emerging evidence that a small subpopulation of enteroviral virions may also exit the cell in a cell death-independent manner; this will be discussed below (Section 4.2). Despite the necessary role of cell death in viral egress, cell death can also be considered an antiviral response, and when executed correctly can serve to control infection (8). In this review, we provide an overview of the existing literature addressing host cell death initiated upon EV infection. We begin by detailing the literature available regarding the role of cell death in pathogenesis during enteroviral infections (Section 2). Next, we review the mechanisms of cell death induction during EV infections (Section 3) and examine the types of cell death that occur upon EV infection (namely, apoptosis (Section 3.1) and necrosis (Section 3.2)). We then focus on the mechanisms by which cell death facilitates cell to cell viral spread (Section 4). While EVs do indeed rely upon cell death in order to exit the cell, cell death can also be a potent innate immune mechanism enacted against intracellular pathogens by the host; the death of an infected cell before the intracellular pathogen completes its life cycle removes the environment required for the pathogen to replicate. With this in mind, we then review the innovative and fascinating ways in which EVs actively control host cell death pathways during their life cycles (Section 5).
2. Cell death and pathology
While cell death is indeed a necessary event for viral spread, EV-induced cell death is also responsible for pathological outcomes of EV infection. In this section, we review the literature detailing how EV-induced cell death induces these pathologies. We focus on the pathological effects of EV infections in the heart, pancreas, and central nervous system (CNS). EVs display tropism for each of these three tissues, and a recent case study of twin newborns who died from CVB4 infections found high levels of CVB4 nucleic acid at each of these three sites (9). It is important to note that replication at these sites is not necessary for successful transmission of the EV to a new host. Indeed, the majority of people infected with EVs efficiently transmit the virus without experiencing any of the clinical outcomes detailed here. Replication in these organs may be considered a dead end for the virus, and unfortunately also leads to serious pathologies and even death for infected individuals (10).
2.1 Myocarditis
One of the most serious potential outcomes of an EV infection is the dissemination of the virus to the heart and the subsequent development of myocarditis, which can then further progress to dilated cardiomyopathy. These conditions are major causes of heart failure, particularly among children and adolescents (11). Evidence of an EV infection is found in up to 50% of cardiomyopathy cases (12–14). EV infections contribute to development of cardiomyopathy in two distinct ways—[1] EVs can directly infect and induce the cell death of cardiomyocytes or [2] they can trigger an autoimmune response in which the host’s own immune system destroys cardiomyocytes and leads to the characteristic inflammation seen in cardiomyopathy. EV infection does indeed directly cause apoptosis of cardiomyocytes in vitro. Primary cardiomyocytes isolated from mice or rats are killed upon CVB infection (15,16), and a murine cardiac muscle cell line, HL-1 (17), undergoes apoptosis upon CVB infection (18). In vivo, mouse models of CVB-induced myocarditis showed apoptotic lesions in myocardial tissue as detected by TUNEL staining, a method that detects DNA fragmentation resulting from apoptosis (19,20). Human patients presenting with EV-induced myocarditis who had cardiac biopsies taken showed a strong positive correlation between cardiomyocytes staining positive for EV capsid protein and those staining positive by TUNEL staining (21). Perhaps the most convincing evidence for direct EV-mediated cell death comes from SCID (Severe Combined Immunodeficiency) mice. These mice lack all mature T- and B- cell responses, and therefore are incapable of developing autoimmunity. Even in a SCID mouse model of CVB-induced myocarditis, death of cardiomyocytes correlates with the development of cardiomyopathy (22). Despite these convincing results, it remains probable that autoimmune responses contribute to EV-induced myocarditis, acting in combination with cell death caused directly by EV replication. In mouse models of CVB-induced myocarditis, CVB infection leads to the generation of heart specific autoantibodies (23), and human patients with viral-induced myocarditis also have autoantibodies in their sera (24). Therefore, it is likely that both EV-induced cell death and EV-induced autoimmunity contribute to the destruction of cardiomyocytes in EV-associated cardiomyopathy.
2.2 Type I Diabetes
The contribution of environmental factors to the onset of type I diabetes in children has long been suspected, and there is strong evidence suggesting that viral infections, and EV infections in particular, can precipitate its development (25). A meta-analysis of the available literature showed that patients with type I diabetes were significantly more likely to have evidence of an acute EV infection than the general population (26), and patients with recent-onset type I diabetes were significantly more likely to have evidence of enteroviral protein in their pancreatic islets than non-diabetic controls (27). In perhaps the most convincing evidence for EV-induced diabetes, CVB4 was isolated from the pancreas of a young patient who died from diabetic ketoacidosis and this strain of CVB4 was able to cause death of β-cells and hyperglycemia in mice (28). In type I diabetes, the insulin producing β-cells of the pancreatic islet are destroyed, with the subsequent dearth of insulin serving to induce diabetes. As is the case for EV-induced cardiomyopathy (Section 2.1) there is evidence both for direct EV-induced death of β-cells and for activation of an autoimmune response upon EV infection that results in β-cell destruction. Direct infection and destruction of β-cells by EVs seems to play a particularly important role in development of a clinically distinct subtype of type I diabetes known as fulminant diabetes, marked by extremely rapid onset, ketoacidosis, and a lack of the autoantibodies directed against β-cells typically seen in type I diabetics (29,30). Primary β-cells isolated from rats and infected with the diabetogenic EV CVB5 died via intrinsic mitochondrial apoptosis (31). Adding to the evidence that EVs can infect and directly induce apoptosis of pancreatic islet cells in vitro, many different EVs efficiently infect β-cells in islets isolated from human pancreases, and this results in the abrogation of stimulated insulin release (32). It is more difficult to find evidence of direct infection and apoptosis induction of pancreatic β-cells in vivo. In one small study, three pancreases from individuals with fulminant type I diabetes each were found to have EV protein in their islets, with immunohistochemistry showing that those cells containing EV protein also showed evidence of cell death by morphological changes in their nuclei (33). A second study found that the pancreases of three of six diabetic patients had EV protein in their pancreatic islets with corresponding alterations in nuclear morphology (34). There is also evidence that β-cell destruction upon EV-infection may be due to molecular mimicry, in which an EV protein bears molecular similarity to an autoantigen (35,36), or by the release of a normally sequestered autoantigen by EV-infection of pancreatic islets (37), both of which lead to an autoimmune response directed against the pancreatic islets. Clearly, EV infections can both directly and indirectly contribute to the β-cell destruction that results in development of type I diabetes.
2.3 Meningitis and encephalitis
Neurological complications arising from EV infections are well-studied, mainly due to the severe and global nature of PV-induced poliomyelitis. Until the introduction of the PV vaccine in the 1960s, epidemic poliomyelitis was a serious public health concern, and even now in certain developing nations PV remains endemic (38). Poliomyelitis is characterized by aseptic meningitis (an inflammation of the meninges not due to a culturable bacterial agent), and paralysis of the extremities (39). These symptoms arise from PV-induced damage of motor neurons, and it has long been noted that PV is present in neuronal axons during poliomyelitis (40). Apoptosis was evident in the CNS of mice transgenically expressing the PV receptor (PVR) and infected with PV, and this apoptosis was further shown to occur specifically in PV-infected neurons (41). In vitro studies in a human neuroblastoma cell line further investigated PV-induced apoptotic cell death and found that PV infection triggered apoptosis through the mitochondrion-dependent intrinsic pathway (42), discussed in detail below (Section 3). In addition to PV, there are other EVs that can cause pathologies in the CNS. Both aseptic meningitis and encephalitis, in which the brain itself is inflamed in addition to the meninges, can result from EV infection (43). EV71 is of special note, as in recent years it has been the source of serious outbreaks of meningoencephalitis in the Asia-Pacific region (44). This pathology is thought to result in largely the same manner as PV, from viral-induced death of motor neurons. In studies of organs from fatal cases of EV71 infections, viral antigen was found specifically in the neuronal cells of the CNS (45,46). In vitro, EV71 infections of human neuronal cell lines results in apoptosis (47,48). In mice transgenically expressing the EV71 receptor SCARB2, intravenous EV71 infection resulted in viral dissemination to the brain and spinal cord, with immunohistochemistry showing that EV71 was specifically infecting neurons and the authors observing that EV71 infection induced cell death (49). Both CVA and CVB infections are also neurovirulent, in much the same manner as EV71 infections (43). Neonates are at particular risk from CV infections (50). In a neonatal mouse model of CVB3 infection, viral protein was found exclusively in neurons and neuronal stem cells and the authors observed death of infected cells (51). Additionally, neonatal mice infected with sublethal doses of CVB3 showed reduction in brain weight due to CVB3-induced apoptosis of neuronal stem cells and consequent decreased neuronal proliferation (52).
3. Induction of cell death upon enterovirus infection
It is clear that cell death caused by EVs has serious consequences for human health. We now turn to the mechanistic question of how cell death is initiated and carried out upon EV infection. Cells possess diverse routes of cell death initiation, from cell surface receptors such as the Tumor Necrosis Factor Receptor (TNFR) (53), to intracellular receptors known as pattern recognition receptors (PRRs) that sense pathogen or damage associated molecular patterns (PAMPs or DAMPs, respectively) (54), to the detection of DNA damage (55). In vitro studies of EV infections in cell culture models reveal that multiple redundant pathways are likely triggered to initiate cell death upon EV infection. Additionally, distinct forms of cell death can occur downstream of these pathways. Apoptosis, in either its caspase-dependent or caspase-independent form, is a tightly regulated form of cell death that results in chromatin condensation, DNA fragmentation, and the eventual disruption of the cell into apoptotic blebs (56). Caspases are proteases that require proteolytic processing for activation, and, once activated, cleave downstream substrates to induce apoptotic cell death (57,58). Apoptosis is further categorized as intrinsic or extrinsic. Intrinsic apoptosis is mediated by the mitochondria and is dependent on the release of cytochrome C from the mitochondria, a process which is regulated by the Bcl-2 family of proteins, whose pro-apoptotic family members act to release cytochrome C from the mitochondria while the anti-apoptotic family members act to prevent cytochrome C release. Extrinsic apoptosis, on the other hand, proceeds from cell surface receptors and is independent of the mitochondria (59). Necrosis was long thought to be an unregulated form of cell death, characterized by loss of membrane integrity and a release of intracellular contents into the extracellular space that provoked a highly inflammatory reaction. Recently, programmed forms of necrosis have been discovered, with tightly regulated signaling cascades that result in either membrane permeabilization by the mixed lineage kinase like protein (MLKL) or generation of reactive oxygen species (ROS) and lead to the necrotic death phenotype (60). Because the necrotic pathway was only recently recognized as a distinct, regulated process, some of the older literature examining EV-induced cell death lacks precision in distinguishing between the various forms of cell death. The literature is further complicated by the use of the word ‘necrosis’ in clinically oriented literature to refer to any tissue death, not a mechanistically distinct form of cellular death. Here we seek to clarify and distill the literature surrounding cell death initiation and execution upon EV infection.
3.1 Apoptosis
As detailed above (Section 2), there is much evidence to suggest that apoptosis is a relevant in vivo form of cell death induced by EVs. EV-infected cardiomyocytes from patients with myocarditis and PV-infected neurons from a mouse model of poliomyelitis were apoptotic (21,41), and primary rat pancreatic cell cultures died by apoptosis upon CVB5 infection (31). Many groups have further addressed in vitro the mechanisms by which this apoptotic cell death may be occurring. Infection of a variety of cell lines with diverse EVs leads to apoptotic cell death, as shown by cleavage of downstream caspase substrates, DNA fragmentation, and flipping of phosphatidyl serine to the outer leaflet of the cell membrane. Work with various CVB serotypes in HeLa cells has shown that infection leads to cytochrome C release from the mitochondria (61), a hallmark of intrinsic apoptosis. This release is followed by caspase-activation (62), specifically caspase 9 (61,63), an initiator caspase that cleaves other caspases. Activated caspase 9 then goes on to process caspases-2, -3, -6, -7, and -8 (61,62). PV infection in HeLa cells or a neuronal cell line also caused cytochrome C release and caspase-dependent apoptosis (42,64–66). A third EV, EV71, also induces caspase-dependent intrinsic apoptosis in HeLa cells (67,68), but also induces caspase-independent apoptosis, dependent on truncation of apoptosis inducing factor (AIF) by calpains. Inhibition of both of these pathways showed an additive effect on increasing cell viability upon EV71 infection (68). It is possible that caspase-independent apoptosis plays a role in other EV infections, but to our knowledge this has not been described.
3.1.1 Apoptosis Signaling
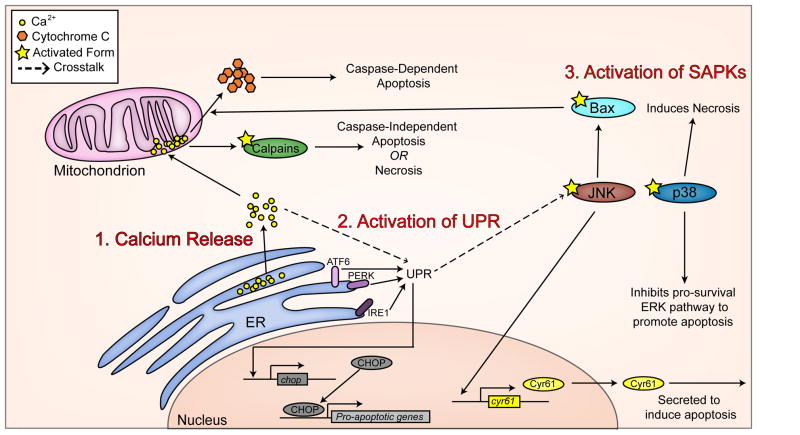
Although the studies described above clearly show that EVs induce apoptotic cell death in specific cell types, it remains to be explained how this apoptosis is initially induced during viral infection. EV infection induces apoptosis through at least three distinct mechanisms. The activation of calpains upon EV71 infection, as stated above (68), provides one clue as to how apoptosis initiation may occur. Calpains are calcium-dependent proteases whose activity is regulated by the concentration of Ca2+ ions within the cell (69) and it is known that an increase in intracellular Ca2+ can lead to apoptosis (70). Indeed, PV infection in neuronal cells causes an efflux of Ca2+ from the endoplasmic reticulum (ER) where stores of Ca2+ are maintained into the cytosol, and then into the mitochondria. Blocking the accumulation of Ca2+ in the mitochondria by treating the cells with an intracellular calcium chelator also blocks PV-induced cytochrome C release from the mitochondria and reduces downstream apoptosis (65). In the case of calpain-dependent EV71 induced apoptosis, blocking mitochondrial Ca2+ channels blocked death (68). CVB infection in HeLa cells also led to Ca2+ release from the ER (71), though in this case Ca2+ release may have anti-apoptotic functions (reviewed in (72), and discussed thoroughly in Section 5). In addition to causing the release of Ca2+ to induce apoptosis, EVs also induce apoptosis through the activation of the unfolded protein response (UPR). The UPR, under normal cellular circumstances, is a cell-wide response to the accumulation of unfolded proteins in the ER (73), and can trigger apoptotic cell death (74). CVB infection in HeLa cells induced the UPR through each of the three distinct UPR initiation pathways (the PERK, IRE1, and ATF6 dependent pathways) (75), and resulted in cleavage of caspase-12, a UPR specific caspase (76), and up-regulation of CHOP, a pro-apoptotic transcription factor classically implicated in UPR-initiated cell death (77). Finally, EV infection can trigger apoptosis through the activation of stress activated protein kinases (SAPKs). SAPKs, including JNK and p38MAPK, are responsible for sensing cell stressors and transducing that signal to trigger induce responses to those stressors, including induction of cell death pathways (78,79). JNK was rapidly phosphorylated upon infection with PV in a neuronal cell line (within 15 minutes post-infection). Pharmacological inhibition of JNK blocked translocation of Bax, a pro-apoptotic protein Bcl-2 family member, to the mitochondria and release of cytochrome C from the mitochondria (42). CVB infection of HeLa cells also caused JNK phosphorylation, though not until 6 hours post-infection, and in this case, cell death downstream of JNK was linked to the secretion of the pro-apoptotic protein Cyr61 (80), although a separate study found no effect of inhibition of JNK on cell death in HeLa cells infected with CVB (81). p38MAPK was also phosphorylated and activated upon CVB infection of HeLa cells or a murine cardiomyocyte cell line (81,82), and pharmacological inhibition of p38MAPK reduced CVB-induced cell death, though only cell viability, not apoptotic indicators, were measured here (81). These mechanisms of apoptosis induction are summarized in Figure 1.
Figure 1. Mechanisms for the induction of apoptosis.
Apoptosis can be induced through a number of mechanisms upon EV infection which include (1) release of ER-derived Ca2+ stores, (2) activation of the unfolded protein response (UPR), and (3) activation of stress activated protein kinases (SAPKs).
3.1.2 Apoptotic Triggers
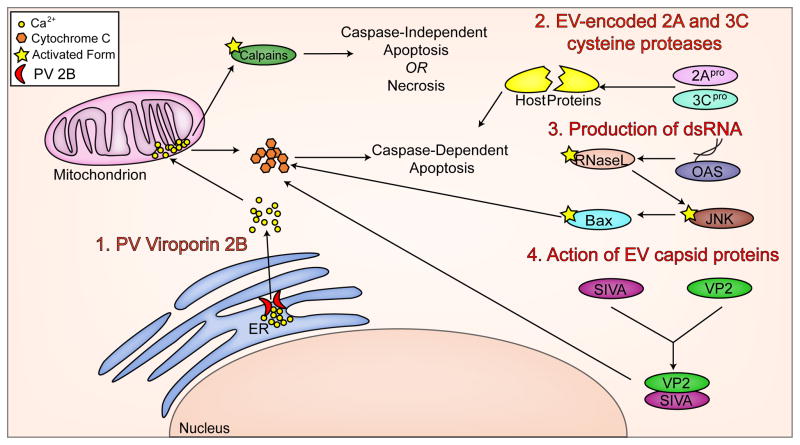
The data summarized above provide a description of the multiple signaling pathways involved in inducing apoptosis upon EV infection. We now turn to the identity of the virus-associated triggers for these apoptotic pathways. EVs encode two cysteine proteases, 2Apro and 3Cpro, that are necessary for processing the viral polypeptide, but also target numerous cellular proteins for cleavage and are well-conserved amongst EVs (83–85). Transient overexpression of 2Apro or 3Cpro cloned from CVB in HeLa cells is sufficient to reduce cell viability, activate caspases, and induce cytochrome C release from the mitochondria (86), and expression of either of those same proteases cloned from PV or EV71 also induced caspase-dependent apoptosis (67,87–89). The mechanism of death induction by these proteases is dependent on proteolytic activity, as a catalytically inactive mutant of 3Cpro cloned from EV71 was incapable of inducing the apoptotic cell death induced by the wild-type protease in a glioblastoma cell line (89). Cleavage of host cell proteins by these proteases likely feeds into the apoptotic pathways discussed above in multiple ways. A second mechanism by which apoptosis is triggered during EV infections may be through production of double-stranded (ds)RNA during the course of genome replication. dsRNA is a highly immunogenic PAMP that can also lead to the induction of cell death (54,90). Noninfectious dsRNA from bovine enterovirus (BEV) was sufficient to cause cell death (91), suggesting that sensors of viral dsRNA are sufficient to induce cell death signaling cascades. Multiple intracellular sensors of dsRNA exist and serve to activate innate immune signaling and can cross-talk with cell death pathways. MDA5, a cytosolic dsRNA sensor, and TLR3, an endosomal dsRNA sensor are both key sensors of EV infection (92), and CVB infection induces expression of another cytosolic dsRNA sensor, PKR (93). Further, PV-induced apoptosis in HeLa cells was reduced in the presence of a dominant negative inhibitor of RNase L, a key downstream enzyme of the dsRNA sensing 2′–5′ oligoadenylate synthetase pathway (OAS) (94), suggesting that viral dsRNA may be sensed by OAS and downstream OAS signaling may feed into apoptotic pathways, such as the activation of JNK signaling (95). Finally, viral capsid proteins themselves may serve to initiate apoptosis. In a mechanism that seems to be specific to CVB3, VP2, one of four viral proteins that compose EV capsids (83), physically interacts with the pro-apoptotic host protein SIVA (98,99). SIVA functions by binding to BCL-XL, an anti-apoptotic member of the Bcl-2 family and sequestering it, thus preventing it from carrying out its anti-apoptotic function (100). Infection of a mouse model with CVB3 containing a mutation in VP2 preventing SIVA binding showed lower levels of cell death in the pancreas than wild-type CVB3, despite similar viral titers (98). The mechanism(s) by which VP2 binding to SIVA enhances its apoptotic function is not fully understood. Additionally, a single point mutation in the VP1 capsid protein of a strain of CVB2 that does not normally cause cell death in rhabdomyosarcoma cells was sufficient to cause the strain to begin inducing cell death (101). Viral capsid protein may or may not be necessary to induce the JNK signaling pathway, depending on the EV. Whereas one group found that UV-inactivated PV still caused JNK phosphorylation (42), a second found that UV-inactivated CVB did not (81). These triggers are summarized in Figure 2.
Figure 2. Mechanisms for the induction of apoptosis in EV-infected cells.
The induction of apoptosis during an EV infection occurs in a number of ways including (1) the release of ER-derived Ca2+ stores by the PV virally-encoded virporin 2B, (2) the direct cleavage of host proteins by the virally-encoded cysteine protease 2Apro and 3Cpro, (3) the detection of dsRNA produced as a replication intermediate by components associated with the innate immune system, and (4) direct binding of the EV capsid protein VP2 to the pro-apoptotic host cell component SIVA.
3.2 Necrosis
In contrast to the large amount of data available on apoptotic cell death during EV infection, much less is known about the contribution of necrotic cell death during EV infections. One reason for this dearth of data is the only recent development of the programmed necrosis (‘necroptosis’) field. Another is that in many of the cell types studied in vitro, the main cell death pathway induced upon EV infection is apoptosis and necrosis seems to be uninvolved, or not involved to any significant degree (Section 3.1). However, we recently showed that a polarized intestinal epithelial cell line undergoes Ca2+-dependent necrotic cell death upon CVB3 infection (102). As described above for PV-infection in neurons (65), cell death induction was dependent on Ca2+ release from the ER upon CVB3 infection (102). However, whereas this Ca2+ release in PV-infected neurons leads to apoptotic cell death, it induces calpain-mediated necrotic cell death in intestinal epithelial cells infected with CVB3 (65,102). It is therefore likely that additional, as yet unidentified, cell type-specific differences exist and are important for determining the manner of EV-induced cell death signaling. Another study showed that a pancreatic islet cell line that undergoes apoptosis when infected with CVB5 at a low multiplicity of infection (MOI), may instead undergo necrosis at higher MOIs. However, the method used here to characterize and quantify necrosis was not robust (103). It is likely that both necrosis and apoptosis are relevant forms of cell death in vivo, as EV infections must cross a polarized epithelial cell barrier to cause initial viremia, and intestinal epithelial cells die necrotically in vitro (above, (102)) whereas apoptotic cell death seems most relevant at the distal sites of infection resulting in pathology (myocardium, pancreatic islets, and the CNS, Sections 2, 3.1). The cell types reviewed here that are known to be productively infected by EVs and the type of cell death caused by EV-infection are summarized in Table 1.
Table 1.
A summary of the manner of cell death induced by EV infection in the indicated cell types.
| Cell type | Cell death | Reference |
|---|---|---|
| Murine cardiomyocyte line (HL-1) | Apoptosis | 18, 82 |
| In vivo murine cardiomyocytes | Apoptosis | 19, 20, 99 |
| Biopsied human cardiomyocytes | Apoptosis | 21 |
| Primary rat β-cells | Intrinsic apoptosis | 31 |
| Biopsied human pancreatic islets | Likely apoptosis, by nuclear morphology | 33, 34 |
| In vivo murine neurons and neuronal stem cells | Apoptosis | 41, 49, 52 |
| Human neuronal cell lines (IMR5, SK-N-MC) | Apoptosis | 42, 47 |
| Human glioblastoma cell line (SF268) | Apoptosis | 48 |
| Human cervical adenocarcinoma cell line (HeLa) | Apoptosis | 61–68, 75, 80, 86, 102, 121, 124 |
| Human cervical adenocarcinoma cell line (HeLa) | Caspase-independent apoptosis | 68 |
| In vivo murine pancreatic tissue | Apoptosis | 98, 99 |
| Human intestinal epithelial cell line (Caco-2) | Necrosis | 102 |
| Human kidney cell line (HEK293) | Apoptosis | 126 |
4. Enteroviral infections and cell death
Classically, a cell productively infected with an EV will soon undergo cell death. These fast-replicating, lytic viruses cause cell death in the ways described above (Section 3). Here, we discuss the role of cell death in facilitating viral spread. We also touch on exceptions to this paradigm, reviewing data suggesting that EVs may in some cases exit the cell before cell death and the existence of persistent EV infections.
4.1 Cell death and viral spread
Many viruses are capable of exiting a living host cell by budding out of the host cell membrane or a variety of other mechanisms. In contrast, for the preponderance of their spread, EVs rely on cell death to escape the cell (exceptions discussed in Section 4.2). Indeed, a wide range of studies with various EVs in various cell lines have shown that blocking cell death reduces and/or delays the release of extracellular virus. For example, treating a neuronal cell line with an intracellular Ca2+ chelator to reduce apoptosis upon PV infection also significantly delayed the release of virus into the cell culture medium (65). Delayed PV release was also seen when apoptotic cell death was blocked with JNK inhibitors (42). Blocking apoptotic cell death in HeLa cells upon CVB3 infection with caspase inhibitors or by overexpressing anti-apoptotic members of the Bcl-2 family of mitochondrial proteins that block intrinsic apoptosis lead to large reductions in release of extracellular virus (61). Conversely, increasing apoptotic cell death in CVB3 infected HeLa cells through ectopic expression of the proapoptotic protein Cyr61 led to an increase in extracellular virus release, while siRNA-mediated knockdown of Cyr61 decreased extracellular virus release (80). Finally, blocking CVB3-induced necrosis in intestinal epithelial cell lines through a variety of mechanisms all led to a decrease in extracellular virus titers (102). The fact that diverse mechanisms of blocking cell death in different cell lines upon EV-infection all resulted in a reduction or delay of viral release strongly implicates cell death in the process of viral egress and minimizes the possibility that inhibitors of cell death were having an off target effect on a separate aspect of the viral life cycle.
4.2 Viral spread without cell death
Despite the strong evidence cited above for the link between viral egress and cell death, there is also data that suggests that there may be secondary routes of viral egress that occur temporally before and independently of cell death. One such route may involve autophagy, which under normal conditions serves as a cellular recycling mechanism and is generally considered to be a pro-survival response. Neural progenitor cells infected with CVB3 shed extracellular microvesicles derived from autophagic pathways containing infectious CVB3 virions before any signs of cell death (104). Additionally, pharmacologically blocking the autophagy pathway in CVB3-infected HeLa cells resulted in a reduction of extracellular virus, though this data is difficult to interpret in the context of autophagy as an egress pathway as treatment also reduced intracellular virus replication (105). Finally, autophagosomes in HeLa cells infected with PV showed reduced motility, and when their motility was restored through nocodazole treatment, there was an earlier release of virus without any corresponding change in the timing of cell death initiation (106).
4.3 Persistent Infections
Up until this point, we have operated under the assumption that cell death is a necessary part of the EV life cycle. And while this is generally thought to be true, the data discussed above (Section 4.2) that demonstrate viral spread without cell death leave open the possibility of persistent viral infections, with virus continuously being shed from an infected cell without killing that cell. Indeed, there is some evidence for this in vitro. It has long been observed that individuals who developed poliomyelitis could shed PV in their feces for up to 6 weeks after the development of symptoms (107). In addition, immunodeficient individuals given the live attenuated PV vaccine can shed vaccine-derived PV for much longer, in one case up to 2 years (108). In a mouse model of CVB3-induced myocarditis, viral RNA was found in cardiomyocytes up to 30 days post-infection in mice experiencing chronic myocarditis (109). Given the extensive and overlapping triggers for cell death invoked upon EV infection discussed above (Section 3.1.2), how might such a persistent infection occur? Some mechanistic work has been done in vitro to examine this question and suggests that a complex interplay between the host immune system and genetic variation in both the virus and the host exists. Persistent EV infections have been established in human intestinal cell culture lines (110), neuroblastoma lines (111), and erythroblastoid lines (112), with continuous viral shedding and low levels of cell death. In primary human pancreatic islets, CVB3 could establish persistent infections through a mechanism involving IFNα signaling (113). In the case of persistent PV-infections, alterations of the interaction between PVR and PV through genetic variation of either the host or the virus seems to be key in establishing a persistent infection (114–116), with these altered interactions leading to a reduction in cell death (117). It is not entirely understood how a given EV manages to suppress the cell death pathways triggered by infection in order to establish a persistent infection, and discussion of the literature on persistent viral infections is beyond the scope of this review. However, EVs do possess mechanisms that serve to antagonize cell death pathways during lytic infections, discussed below (Section 5), and many of these mechanisms may also come into play during establishment of a persistent infection.
5. Antagonism of cell death pathways by enteroviruses
As discussed in the introduction, cell death can serve as a mechanism to eliminate intracellular pathogens before they complete their replication cycle. EVs possess several mechanisms of delaying cell death, in order to counter the fact that EV infection potently induces cell death pathways (Section 3). One such mechanism is through the manipulation of host cell signaling pathways. The phosphatidylinositol 3-kinase (PI3K) signaling pathway is a pro-survival pathway activated by many viruses to delay or inhibit cell death (118). PV infection of a neuroblastoma cell line led to early activation of PI3K and subsequent phosphorylation of downstream effector kinase, Akt. Inhibition of this pathway led to more apoptosis and earlier PV release. These data may be complicated by the additional role PI3K plays in regulating autophagy (119,120), as effects on EV release upon PI3K inhibition may additionally be due to inhibition of autophagy. The PI3K/Akt pathway seems to antagonize the pro-apoptotic JNK pathway, as its inhibition also led to an increase in activated JNK (66). A similar pro-survival mechanism involving activation of the PI3K/Akt pathway was shown in HeLa cells infected with CVB3 (121). One group showed that the PI3K/Akt pathways could be activated in CVB3-infected HeLa cells through the action of interferon-γ-inducible GTPase (IGTP)(122), a protein upregulated in CVB3-infected murine myocardial tissue (123). Activating transcription factor 3 (ATF3) has been shown to sensitize HeLa cells to CVB3-induced apoptosis, but CVB3 infection leads to an abrupt and significant reduction in ATF3 protein levels that serves to reduce cell death (124). ATF3 downregulation also leads to the degradation of p53 (124), and the degradation of p53 reduces PV-induced apoptosis (125).
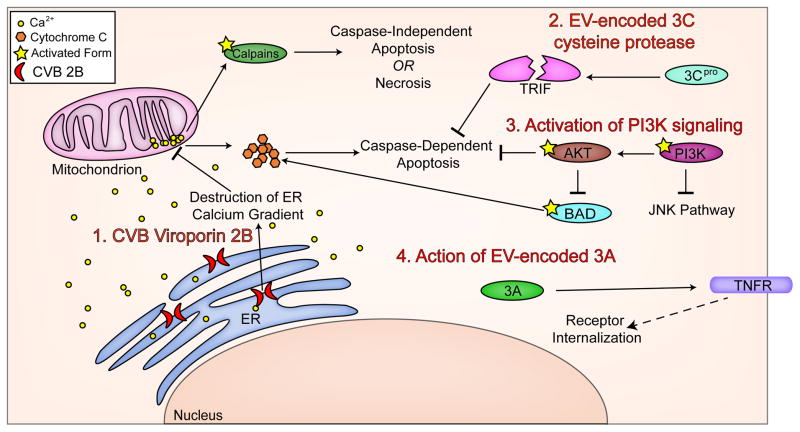
EV infections can also directly target host proteins involved in cell death signaling for proteolytic cleavage and inactivation via the virally encoded cysteine proteases discussed above (Section 3). For example, 3Cpro of CVB3 cleaves Toll/IL-1 receptor domain containing adaptor inducing interferon-beta (TRIF), a protein involved in apoptotic signaling, and inhibits its apoptotic functions (126). Another example of a viral protein directly inhibiting cell death is the viral protein 2B. Expression of the CVB nonstructural protein 2B causes Ca2+ release through its ability to from pores in the ER membrane (71). 2B is highly conserved among enteroviruses and the PV 2B also is responsible for Ca2+ release from the ER (127). Because Ca2+ efflux from the ER and its subsequent influx into the mitochondria is known to cause apoptosis (65), it may seem that 2B would then be a pro-apoptotic protein. And indeed, in some cases viral proteins causing release of Ca2+ stores are pro-apoptotic (128). However, 2B of CVB3 seems to prevent apoptosis through destruction of the Ca2+ gradients necessary for the cell to initiate intrinsic apoptosis (72,129). The virally-encoded 3A can also inhibit apoptosis through yet another mechanism. 3A from PV interferes with protein trafficking to greatly reduce the levels of TNFR on the cell surface, thereby downregulating extrinsic apoptosis (130), and during PV infection levels of other apoptosis inducing cell surface receptors are reduced as well (131). These mechanisms are summarized in Figure 3.
Figure 3. Mechanisms by which EVs antagonize host cell death pathways.
EVs utilize a variety of diverse mechanisms to alter host cell death signaling which include (1) destruction of Ca2+ gradients by the production of virally-encoded viroporins such as 2B, (2) direct cleavage of pro-apoptotic host cell components by the virally-encoded 2Apro and 3Cpro cysteine proteases, (3) activation of PI3K/Akt signaling pathways, and (4) internalization of TNFR by the virally-encoded 3A protein.
6. Conclusion
Cell death is a tightly regulated and controlled process. Unwarranted death of many cell types, especially neuronal cells, is extremely deleterious to the health of an individual and must be avoided. However, under some circumstances, including the presence of an intracellular pathogen, it is to the overall benefit of the host for infected cells to be eliminated. This elimination can be triggered through a variety of pathways that can lead to necrotic or apoptotic cell death, according to the identity of the trigger and the identity of the cell.
Enteroviral infections can lead to serious pathologies, and these pathologies often result from cell death. Myocarditis, type I diabetes, and meningoencephalopathies can all result from cell death due to EV infections in the myocardium, pancreas, or CNS. In addition to causing pathology, cell death is an essential mechanism of enteroviral egress, and thereby is responsible for cell-to-cell spread. An EV that infects a host cell has a variety of mechanisms by which it triggers cell death. Depending on the species or serotype of the EV and the identity of the infected host cell, any number of these mechanisms may be used simultaneously to induce cell death. Broadly, EVs induce cell death through modulation of Ca2+ gradients, induction of the UPR, and/or activation of MAPK signaling pathways, all of which can be precipitated by various aspects of viral replication, including expression of EV-encoded cysteine proteases, generation of immunogenic dsRNA, and interaction of EV capsid protein with host cell receptors. Cells then undergo necrotic or apoptotic cell death, depending on the identity of the infected cell. The balance between cell death induction and cell death prevention/delay is finely tuned during an EV infection, as evidenced by the evolutionary success of these viruses. EVs are efficient at replicating within a cell and are successful at spreading both to neighboring cells and to a new host. This success is partially due to their exquisite interaction with the cell death signaling pathways of their host. Understanding the ways in which EVs induce and control cell death is critical in understanding the pathogenesis and spread of these important human pathogens.
Highlights.
Enteroviruses commonly induce cell death as a means to promote egress and spread.
The strategies by which enteroviruses induce and alter cell death pathways are complex.
Here, we review the mechanisms of cell death associated with enterovirus infections.
Acknowledgments
We apologize to any colleagues whose work we may have neglected to cite due to space limitations. This project was supported by NIH R01-AI081759 (C.B.C) and NIH T32-AI049820 and T32-AI060525 (K.G.H.). In addition, C.B.C is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award and K.G.H is a recipient of the ARCS Foundation Scholar Award.
Biographies
 Carolyn Coyne received a B.S. degree in Biochemistry from Florida State University in Tallahassee, Florida and went on to obtain her Ph.D. in Pharmacology from the University of North Carolina at Chapel Hill in Chapel Hill, NC. Following her graduate work, Dr. Coyne performed a post-doctoral fellowship with Dr. Jeffrey Bergelson at the Children’s Hospital of Philadelphia/University of Pennsylvania in Philadelphia, PA where she characterized the cell entry mechanisms of the enteroviruses coxsackievirus B (CVB) and poliovirus (PV) into polarized epithelial and endothelial cell types. Dr. Coyne joined the faculty at the University of Pittsburgh in 2007 and is now an Associate Professor of Microbiology and Molecular Genetics. Dr. Coyne’s research interests remain centered on the mechanisms by which enteroviruses enter and infect polarized cell types, including the mechanisms by which these viruses induce and abrogate cell death signaling in these cell types.
Carolyn Coyne received a B.S. degree in Biochemistry from Florida State University in Tallahassee, Florida and went on to obtain her Ph.D. in Pharmacology from the University of North Carolina at Chapel Hill in Chapel Hill, NC. Following her graduate work, Dr. Coyne performed a post-doctoral fellowship with Dr. Jeffrey Bergelson at the Children’s Hospital of Philadelphia/University of Pennsylvania in Philadelphia, PA where she characterized the cell entry mechanisms of the enteroviruses coxsackievirus B (CVB) and poliovirus (PV) into polarized epithelial and endothelial cell types. Dr. Coyne joined the faculty at the University of Pittsburgh in 2007 and is now an Associate Professor of Microbiology and Molecular Genetics. Dr. Coyne’s research interests remain centered on the mechanisms by which enteroviruses enter and infect polarized cell types, including the mechanisms by which these viruses induce and abrogate cell death signaling in these cell types.
 Katharine Harris received her B.S. in Microbiology from Purdue University in West Lafayette, IN where she performed undergraduate research in the laboratory of Dr. Michael Rossmann. Ms. Harris entered the Interdisciplinary Biomedical Graduate Program (IBGP) at the University of Pittsburgh in 2010 and joined the laboratory of Dr. Carolyn Coyne and entered the Molecular Virology and Microbiology (MVM) graduate program in 2011. Ms. Harris’ research focuses on the mechanisms by which coxsackievirus B (CVB) attenuates innate immune and cell death signaling pathways.
Katharine Harris received her B.S. in Microbiology from Purdue University in West Lafayette, IN where she performed undergraduate research in the laboratory of Dr. Michael Rossmann. Ms. Harris entered the Interdisciplinary Biomedical Graduate Program (IBGP) at the University of Pittsburgh in 2010 and joined the laboratory of Dr. Carolyn Coyne and entered the Molecular Virology and Microbiology (MVM) graduate program in 2011. Ms. Harris’ research focuses on the mechanisms by which coxsackievirus B (CVB) attenuates innate immune and cell death signaling pathways.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Centers for Disease Control. Non-Polio Enterovirus Infections. 2011. [Google Scholar]
- 2.International Committee on Taxonomy of Viruses. Virus Taxonomy: 2012 Release (current) 2012. [Google Scholar]
- 3.Huang C, Morse D, Slater B, Anand M, Tobin E, Smith P, Dupuis M, Hull R, Ferrera R, Rosen B, Grady L. Multiple-year experience in the diagnosis of viral central nervous system infections with a panel of polymerase chain reaction assays for detection of 11 viruses. Clin Infect Dis. 2004;39:630–635. doi: 10.1086/422650. [DOI] [PubMed] [Google Scholar]
- 4.Koskiniemi M, Rantalaiho T, Piiparinen H, von Bonsdorff CH, Farkkila M, Jarvinen A, Kinnunen E, Koskiniemi S, Mannonen L, Muttilainen M, Linnavuori K, Porras J, Puolakkainen M, Raiha K, Salonen EM, Ukkonen P, Vaheri A, Valtonen V. Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J Neurovirol. 2001;7:400–408. doi: 10.1080/135502801753170255. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Zhaori G, Vene S, Shen K, Zhou Y, Magnius LO, Wahren B, Linde A. Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr Infect Dis J. 1996;15:1018–1024. doi: 10.1097/00006454-199611000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Fowlkes AL, Honarmand S, Glaser C, Yagi S, Schnurr D, Oberste MS, Anderson L, Pallansch MA, Khetsuriani N. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J Infect Dis. 2008;198:1685–1691. doi: 10.1086/592988. [DOI] [PubMed] [Google Scholar]
- 7.Khetsuriani N, Fowlkes AL, Oberste MS, Pallansch MA Centers for Disease Control. Enterovirus Surveillance --- United States, 1970–2005. Morbidity and Mortality Week Report (MMWR) 2006;55:1–20. [PubMed] [Google Scholar]
- 8.Upton JW, Chan FK. Staying alive: cell death in antiviral immunity. Molecular cell. 2014;54:273–280. doi: 10.1016/j.molcel.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissel SJ, Winkler CC, Deltondo J, Wang G, Williams K, Wiley CA. Coxsackievirus B4 myocarditis and meningoencephalitis in newborn twins. Neuropathology: official journal of the Japanese Society of Neuropathology. 2014 doi: 10.1111/neup.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344:9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Rose NR, Kaya Z. The Autoimmune Diseases. 5. 2014. Myocarditis and Dilated Cardiomyopathy; pp. 1033–1048. [Google Scholar]
- 12.Andreoletti L, Leveque N, Boulagnon C, Brasselet C, Fornes P. Viral causes of human myocarditis. Arch Cardiovasc Dis. 2009;102:559–568. doi: 10.1016/j.acvd.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Tam PE. Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol. 2006;19:133–146. doi: 10.1089/vim.2006.19.133. [DOI] [PubMed] [Google Scholar]
- 14.Martino T, Liu P, Petric M, Sole M. Enteroviral myocarditis and dilated cardiomyopathy: A review of clinical and experimental studies. In: Rotbart H, editor. Human Enterovirus Infections. American Society for Microbiology; Washington, DC: 1995. pp. 291–351. [Google Scholar]
- 15.Herzum M, Ruppert V, Kuytz B, Jomaa H, Nakamura I, Maisch B. Coxsackievirus B3 infection leads to cell death of cardiac myocytes. Journal of molecular and cellular cardiology. 1994;26:907–913. doi: 10.1006/jmcc.1994.1108. [DOI] [PubMed] [Google Scholar]
- 16.Ahn J, Joo CH, Seo I, Kim D, Kim YK, Lee H. All CVB serotypes and clinical isolates induce irreversible cytopathic effects in primary cardiomyocytes. Journal of medical virology. 2005;75:290–294. doi: 10.1002/jmv.20269. [DOI] [PubMed] [Google Scholar]
- 17.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miteva K, Haag M, Peng J, Savvatis K, Becher PM, Seifert M, Warstat K, Westermann D, Ringe J, Sittinger M, Schultheiss HP, Tschope C, Van Linthout S. Human cardiac-derived adherent proliferating cells reduce murine acute Coxsackievirus B3-induced myocarditis. PloS one. 2011;6:e28513. doi: 10.1371/journal.pone.0028513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joo CH, Hong HN, Kim EO, Im JO, Yoon SY, Ye JS, Moon MS, Kim D, Lee H, Kim YK. Coxsackievirus B3 induces apoptosis in the early phase of murine myocarditis: a comparative analysis of cardiovirulent and noncardiovirulent strains. Intervirology. 2003;46:135–140. doi: 10.1159/000071453. [DOI] [PubMed] [Google Scholar]
- 20.Kyto V, Lapatto R, Lakkisto P, Saraste A, Voipio-Pulkki LM, Vuorinen T, Pulkki K. Glutathione depletion and cardiomyocyte apoptosis in viral myocarditis. European journal of clinical investigation. 2004;34:167–175. doi: 10.1111/j.1365-2362.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 21.Venteo L, Bourlet T, Renois F, Douche-Aourik F, Mosnier JF, Maison GL, Pluot M, Pozzetto B, Andreoletti L. Enterovirus-related activation of the cardiomyocyte mitochondrial apoptotic pathway in patients with acute myocarditis. European heart journal. 2010;31:728–736. doi: 10.1093/eurheartj/ehp489. [DOI] [PubMed] [Google Scholar]
- 22.Chow LH, Beisel KW, McManus BM. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Laboratory investigation; a journal of technical methods and pathology. 1992;66:24–31. [PubMed] [Google Scholar]
- 23.Wolfgram LJ, Beisel KW, Rose NR. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. The Journal of experimental medicine. 1985;161:1112–1121. doi: 10.1084/jem.161.5.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maisch B, Berg PA, Kochsiek K. Autoantibodies and serum inhibition factors (sif) in patients with myocarditis. Klinische Wochenschrift. 1980;58:219–225. doi: 10.1007/BF01476967. [DOI] [PubMed] [Google Scholar]
- 25.Craig ME, Nair S, Stein H, Rawlinson WD. Viruses and type I diabetes: a new look at an old story. Pediatric Diabetes. 2013;14:149–158. doi: 10.1111/pedi.12033. [DOI] [PubMed] [Google Scholar]
- 26.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. Bmj. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. The New England journal of medicine. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 29.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nature clinical practice. Endocrinology & metabolism. 2007;3:36–45. doi: 10.1038/ncpendmet0351. quiz 32p following 69. [DOI] [PubMed] [Google Scholar]
- 30.Imagawa A, Hanafusa T, Makino H, Miyagawa JI, Juto P. High titres of IgA antibodies to enterovirus in fulminant type-1 diabetes. Diabetologia. 2005;48:290–293. doi: 10.1007/s00125-004-1624-z. [DOI] [PubMed] [Google Scholar]
- 31.Colli ML, Nogueira TC, Allagnat F, Cunha DA, Gurzov EN, Cardozo AK, Roivainen M, Op de beeck A, Eizirik DL. Exposure to the Viral By-Product dsRNA or Coxsackivirus B5 Triggers Pancreatic Beta Cel Apoptosis via a Bim/Mcl-1 Imbalance. PLoS Pathogens. 2011:7. doi: 10.1371/journal.ppat.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T. Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia. 2002;45:693–702. doi: 10.1007/s00125-002-0805-x. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, Suzuki M, Shimura H, Takizawa S, Takahashi M, Akiyama D, Arai-Yamashita S, Furuya F, Kawaguchi A, Kaneshige M, Katoh R, Endo T, Kobayashi T. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes. 2009;58:2285–2291. doi: 10.2337/db09-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Del Prato S, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vreugdenhil GR, Geluk A, Ottenhoff TH, Melchers WJ, Roep BO, Galama JM. Molecular mimicry in diabetes mellitus: the homologous domain in coxsackie B virus protein 2C and islet autoantigen GAD65 is highly conserved in the coxsackie B-like enteroviruses and binds to the diabetes associated HLA-DR3 molecule. Diabetologia. 1998;41:40–46. doi: 10.1007/s001250050864. [DOI] [PubMed] [Google Scholar]
- 36.Bason C, Lorini R, Lunardi C, Dolcino M, Giannattasio A, d’Annunzio G, Rigo A, Pedemonte N, Corrocher R, Puccetti A. In type 1 diabetes a subset of anti-coxsackievirus B4 antibodies recognize autoantigens and induce apoptosis of pancreatic beta cells. PloS one. 2013;8:e57729. doi: 10.1371/journal.pone.0057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nature medicine. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Poliomyelitis Fact Sheet Number 114. 2014. [Google Scholar]
- 39.Modlin JF. Poliomyelitis and Poliovirus Immunization. In: Rotbart H, editor. Human Enterovirus Infections. American Society for Microbiology; 1995. pp. 195–220. [Google Scholar]
- 40.Mueller S, Wimmer E, Cello J. Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus research. 2005;111:175–193. doi: 10.1016/j.virusres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Girard S, Couderc T, Destombes J, Thiesson D, Delpeyroux F, Blondel B. Poliovirus induces apoptosis in the mouse central nervous system. Journal of virology. 1999;73:6066–6072. doi: 10.1128/jvi.73.7.6066-6072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Autret A, Martin-Latil S, Mousson L, Wirotius A, Petit F, Arnoult D, Colbere-Garapin F, Estaquier J, Blondel B. Poliovirus induces Bax-dependent cell death mediated by c-Jun NH2-terminal kinase. Journal of virology. 2007;81:7504–7516. doi: 10.1128/JVI.02690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotbart H. Meningitis and Encephalitis. In: Rotbart H, editor. Human Enterovirus Infections. American Society for Microbiology; 1995. pp. 271–289. [Google Scholar]
- 44.Yip CC, Lau SK, Woo PC, Yuen KY. Human enterovirus 71 epidemics: what’s next? Emerging health threats journal. 2013;6:19780. doi: 10.3402/ehtj.v6i0.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong KT, Munisamy B, Ong KC, Kojima H, Noriyo N, Chua KB, Ong BB, Nagashima K. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. Journal of neuropathology and experimental neurology. 2008;67:162–169. doi: 10.1097/nen.0b013e318163a990. [DOI] [PubMed] [Google Scholar]
- 46.Wong KT, Ng KY, Ong KC, Ng WF, Shankar SK, Mahadevan A, Radotra B, Su IJ, Lau G, Ling AE, Chan KP, Macorelles P, Vallet S, Cardosa MJ, Desai A, Ravi V, Nagata N, Shimizu H, Takasaki T. Enterovirus 71 encephalomyelitis and Japanese encephalitis can be distinguished by topographic distribution of inflammation and specific intraneuronal detection of viral antigen and RNA. Neuropathology and applied neurobiology. 2012;38:443–453. doi: 10.1111/j.1365-2990.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen TC, Lai YK, Yu CK, Juang JL. Enterovirus 71 triggering of neuronal apoptosis through activation of Abl-Cdk5 signalling. Cellular microbiology. 2007;9:2676–2688. doi: 10.1111/j.1462-5822.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 48.Chang SC, Lin JY, Lo LY, Li ML, Shih SR. Diverse apoptotic pathways in enterovirus 71-infected cells. J Neurovirol. 2004;10:338–349. doi: 10.1080/13550280490521032. [DOI] [PubMed] [Google Scholar]
- 49.Fujii K, Nagata N, Sato Y, Ong KC, Wong KT, Yamayoshi S, Shimanuki M, Shitara H, Taya C, Koike S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14753–14758. doi: 10.1073/pnas.1217563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control. Increased Detection and Severe Neonatal Disease Associated with Coxsackievirus B1 Infection --- United states, 2007. 2008. [PubMed] [Google Scholar]
- 51.Feuer R, Mena I, Pagarigan RR, Harkins S, Hassett DE, Whitton JL. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. The American journal of pathology. 2003;163:1379–1393. doi: 10.1016/S0002-9440(10)63496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruller CM, Tabor-Godwin JM, Van Deren DA, Jr, Robinson SM, Maciejewski S, Gluhm S, Gilbert PE, An N, Gude NA, Sussman MA, Whitton JL, Feuer R. Neural stem cell depletion and CNS developmental defects after enteroviral infection. The American journal of pathology. 2012;180:1107–1120. doi: 10.1016/j.ajpath.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Seminars in cancer biology. 2003;13:135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X, Ai M, Guo Y, Zhou X, Wang L, Li X, Yao C. Poly I:C-induced tumor cell apoptosis mediated by pattern-recognition receptors. Cancer biotherapy & radiopharmaceuticals. 2012;27:530–534. doi: 10.1089/cbr.2012.1226. [DOI] [PubMed] [Google Scholar]
- 55.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends in molecular medicine. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British journal of cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 58.Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 59.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy JM, Silke J. Ars Moriendi; the art of dying well - new insights into the molecular pathways of necroptotic cell death. EMBO reports. 2014;15:155–164. doi: 10.1002/embr.201337970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carthy CM, Yanagawa B, Luo H, Granville DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson CB, Hunt DW, McManus BM. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology. 2003;313:147–157. doi: 10.1016/s0042-6822(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 62.Carthy CM, Granville DJ, Watson KA, Anderson DR, Wilson JE, Yang D, Hunt DW, McManus BM. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. Journal of virology. 1998;72:7669–7675. doi: 10.1128/jvi.72.9.7669-7675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomes R, Guerra-Sa R, Arruda E. Coxsackievirus B5 induced apoptosis of HeLa cells: effects on p53 and SUMO. Virology. 2010;396:256–263. doi: 10.1016/j.virol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Agol VI, Belov GA, Bienz K, Egger D, Kolesnikova MS, Raikhlin NT, Romanova LI, Smirnova EA, Tolskaya EA. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology. 1998;252:343–353. doi: 10.1006/viro.1998.9438. [DOI] [PubMed] [Google Scholar]
- 65.Brisac C, Teoule F, Autret A, Pelletier I, Colbere-Garapin F, Brenner C, Lemaire C, Blondel B. Calcium flux between the endoplasmic reticulum and mitochondrion contributes to poliovirus-induced apoptosis. Journal of virology. 2010;84:12226–12235. doi: 10.1128/JVI.00994-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Autret A, Martin-Latil S, Brisac C, Mousson L, Colbere-Garapin F, Blondel B. Early phosphatidylinositol 3-kinase/Akt pathway activation limits poliovirus-induced JNK-mediated cell death. Journal of virology. 2008;82:3796–3802. doi: 10.1128/JVI.02020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuo RL, Kung SH, Hsu YY, Liu WT. Infection with enterovirus 71 or expression of its 2A protease induces apoptotic cell death. The Journal of general virology. 2002;83:1367–1376. doi: 10.1099/0022-1317-83-6-1367. [DOI] [PubMed] [Google Scholar]
- 68.Lu JR, Lu WW, Lai JZ, Tsai FL, Wu SH, Lin CW, Kung SH. Calcium flux and calpain-mediated activation of the apoptosis-inducing factor contribute to enterovirus 71-induced apoptosis. The Journal of general virology. 2013;94:1477–1485. doi: 10.1099/vir.0.047753-0. [DOI] [PubMed] [Google Scholar]
- 69.Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome biology. 2007;8:218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. The EMBO journal. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Kuppeveld FJ, de Jong AS, Melchers WJ, Willems PH. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends in microbiology. 2005;13:41–44. doi: 10.1016/j.tim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature reviews. Molecular cell biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 74.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis: an international journal on programmed cell death. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 75.Zhang HM, Ye X, Su Y, Yuan J, Liu Z, Stein DA, Yang D. Coxsackievirus B3 infection activates the unfolded protein response and induces apoptosis through downregulation of p58IPK and activation of CHOP and SREBP1. Journal of virology. 2010;84:8446–8459. doi: 10.1128/JVI.01416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 77.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell death and differentiation. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 78.Herr I, Wilhelm D, Meyer E, Jeremias I, Angel P, Debatin KM. JNK/SAPK activity contributes to TRAIL-induced apoptosis. Cell death and differentiation. 1999;6:130–135. doi: 10.1038/sj.cdd.4400467. [DOI] [PubMed] [Google Scholar]
- 79.Paul A, Wilson S, Belham CM, Robinson CJ, Scott PH, Gould GW, Plevin R. Stress-activated protein kinases: activation, regulation and function. Cellular signalling. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 80.Kim SM, Park JH, Chung SK, Kim JY, Hwang HY, Chung KC, Jo I, Park SI, Nam JH. Coxsackievirus B3 infection induces cyr61 activation via JNK to mediate cell death. Journal of virology. 2004;78:13479–13488. doi: 10.1128/JVI.78.24.13479-13488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Si X, Luo H, Morgan A, Zhang J, Wong J, Yuan J, Esfandiarei M, Gao G, Cheung C, McManus BM. Stress-activated protein kinases are involved in coxsackievirus B3 viral progeny release. Journal of virology. 2005;79:13875–13881. doi: 10.1128/JVI.79.22.13875-13881.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jensen KJ, Garmaroudi FS, Zhang J, Lin J, Boroomand S, Zhang M, Luo Z, Yang D, Luo H, McManus BM, Janes KA. An ERK-p38 subnetwork coordinates host cell apoptosis and necrosis during coxsackievirus B3 infection. Cell host & microbe. 2013;13:67–76. doi: 10.1016/j.chom.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hellen C, Wimmer E. Enterovirus Structure and Assembly. In: Rotbart H, editor. Human Enterovirus Infections. American Society for Microbiology; Washington, D.C: 1995. pp. 155–174. [Google Scholar]
- 84.Haller A, Semler B. Translation and Host Cell Shutoff. In: Rotbart H, editor. Human Enterovirus Infections. American Society for Microbiology; Washington, D.C: 1995. pp. 113–133. [Google Scholar]
- 85.Schlegel A, Kirkegaard K. Cell Biology of Enterovirus Infection. In: Rotbart H, editor. Human Enterovirus Infections. American Society for Microbiology; Washington, D.C: 1995. pp. 135–154. [Google Scholar]
- 86.Chau DH, Yuan J, Zhang H, Cheung P, Lim T, Liu Z, Sall A, Yang D. Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis: an international journal on programmed cell death. 2007;12:513–524. doi: 10.1007/s10495-006-0013-0. [DOI] [PubMed] [Google Scholar]
- 87.Calandria C, Irurzun A, Barco A, Carrasco L. Individual expression of poliovirus 2Apro and 3Cpro induces activation of caspase-3 and PARP cleavage in HeLa cells. Virus research. 2004;104:39–49. doi: 10.1016/j.virusres.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 88.Barco A, Feduchi E, Carrasco L. Poliovirus protease 3C(pro) kills cells by apoptosis. Virology. 2000;266:352–360. doi: 10.1006/viro.1999.0043. [DOI] [PubMed] [Google Scholar]
- 89.Li ML, Hsu TA, Chen TC, Chang SC, Lee JC, Chen CC, Stollar V, Shih SR. The 3C protease activity of enterovirus 71 induces human neural cell apoptosis. Virology. 2002;293:386–395. doi: 10.1006/viro.2001.1310. [DOI] [PubMed] [Google Scholar]
- 90.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 91.Cordell-Stewart B, Taylor MW. Effect of viral double-stranded RNA on mammalian cells in culture: cytotoxicity under conditions preventing viral replication and protein synthesis. Journal of virology. 1973;12:360–366. doi: 10.1128/jvi.12.2.360-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harris KG, Coyne CB. Enter at your own risk: how enteroviruses navigate the dangerous world of pattern recognition receptor signaling. Cytokine. 2013;63:230–236. doi: 10.1016/j.cyto.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ylipaasto P, Kutlu B, Rasilainen S, Rasschaert J, Salmela K, Teerijoki H, Korsgren O, Lahesmaa R, Hovi T, Eizirik DL, Otonkoski T, Roivainen M. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia. 2005;48:1510–1522. doi: 10.1007/s00125-005-1839-7. [DOI] [PubMed] [Google Scholar]
- 94.Castelli JC, Hassel BA, Wood KA, Li XL, Amemiya K, Dalakas MC, Torrence PF, Youle RJ. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. The Journal of experimental medicine. 1997;186:967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li G, Xiang Y, Sabapathy K, Silverman RH. An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and c-Jun NH2-terminal kinase. The Journal of biological chemistry. 2004;279:1123–1131. doi: 10.1074/jbc.M305893200. [DOI] [PubMed] [Google Scholar]
- 96.Malathi K, Paranjape JM, Bulanova E, Shim M, Guenther-Johnson JM, Faber PW, Eling TE, Williams BR, Silverman RH. A transcriptional signaling pathway in the IFN system mediated by 2′–5′-oligoadenylate activation of RNase L. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14533–14538. doi: 10.1073/pnas.0507551102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gil J, Esteban M. The interferon-induced protein kinase (PKR), triggers apoptosis through FADD-mediated activation of caspase 8 in a manner independent of Fas and TNF-alpha receptors. Oncogene. 2000;19:3665–3674. doi: 10.1038/sj.onc.1203710. [DOI] [PubMed] [Google Scholar]
- 98.Henke A, Nestler M, Strunze S, Saluz HP, Hortschansky P, Menzel B, Martin U, Zell R, Stelzner A, Munder T. The apoptotic capability of coxsackievirus B3 is influenced by the efficient interaction between the capsid protein VP2 and the proapoptotic host protein Siva. Virology. 2001;289:15–22. doi: 10.1006/viro.2001.1082. [DOI] [PubMed] [Google Scholar]
- 99.Henke A, Launhardt H, Klement K, Stelzner A, Zell R, Munder T. Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein siva. Journal of virology. 2000;74:4284–4290. doi: 10.1128/jvi.74.9.4284-4290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu F, Borthakur A, Sun X, Barkinge J, Gudi R, Hawkins S, Prasad KV. The Siva-1 putative amphipathic helical region (SAH) is sufficient to bind to BCL-XL and sensitize cells to UV radiation induced apoptosis. Apoptosis: an international journal on programmed cell death. 2004;9:83–95. doi: 10.1023/B:APPT.0000012125.01799.4c. [DOI] [PubMed] [Google Scholar]
- 101.Gullberg M, Tolf C, Jonsson N, Polacek C, Precechtelova J, Badurova M, Sojka M, Mohlin C, Israelsson S, Johansson K, Bopegamage S, Hafenstein S, Lindberg AM. A single coxsackievirus B2 capsid residue controls cytolysis and apoptosis in rhabdomyosarcoma cells. Journal of virology. 2010;84:5868–5879. doi: 10.1128/JVI.02383-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bozym RA, Patel K, White C, Cheung KH, Bergelson JM, Morosky SA, Coyne CB. Calcium signals and calpain-dependent necrosis are essential for release of coxsackievirus B from polarized intestinal epithelial cells. Molecular biology of the cell. 2011;22:3010–3021. doi: 10.1091/mbc.E11-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rasilainen S, Ylipaasto P, Roivainen M, Lapatto R, Hovi T, Otonkoski T. Mechanisms of coxsackievirus B5 mediated beta-cell death depend on the multiplicity of infection. Journal of medical virology. 2004;72:586–596. doi: 10.1002/jmv.20043. [DOI] [PubMed] [Google Scholar]
- 104.Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, Nguyen DP, Sayen MR, Hilton BJ, Doran KS, Segall AM, Wolkowicz R, Cornell CT, Whitton JL, Gottlieb RA, Feuer R. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog. 2014;10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Le X, Zonghui X, Xiaolin M, Feng H, Hailan Y, Zhewei L. Coxsackievirus B3 induces crosstalk between autophagy and apoptosis to benefit its release after replicating in autophagosomes through a mechanism involving caspase cleavage of autophagy-related proteins. Infection, Genetics and Evolution. 2014;26:95–102. doi: 10.1016/j.meegid.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Taylor MP, Burgon TB, Kirkegaard K, Jackson WT. Role of microtubules in extracellular release of poliovirus. Journal of virology. 2009;83:6599–6609. doi: 10.1128/JVI.01819-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alexander JP, Jr, Gary HE, Jr, Pallansch MA. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J Infect Dis. 1997;175(Suppl 1):S176–182. doi: 10.1093/infdis/175.supplement_1.s176. [DOI] [PubMed] [Google Scholar]
- 108.Martin J, Dunn G, Hull R, Patel V, Minor PD. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. Journal of virology. 2000;74:3001–3010. doi: 10.1128/jvi.74.7.3001-3010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Labadie K, Pelletier I, Saulnier A, Martin J, Colbere-Garapin F. Poliovirus mutants excreted by a chronically infected hypogammaglobulinemic patient establish persistent infections in human intestinal cells. Virology. 2004;318:66–78. doi: 10.1016/j.virol.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 111.Colbere-Garapin F, Christodoulou C, Crainic R, Pelletier I. Persistent poliovirus infection of human neuroblastoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7590–7594. doi: 10.1073/pnas.86.19.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lloyd RE, Bovee M. Persistent infection of human erythroblastoid cells by poliovirus. Virology. 1993;194:200–209. doi: 10.1006/viro.1993.1250. [DOI] [PubMed] [Google Scholar]
- 113.Chehadeh W, Kerr-Conte J, Pattou F, Alm G, Lefebvre J, Wattre P, Hober D. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. Journal of virology. 2000;74:10153–10164. doi: 10.1128/jvi.74.21.10153-10164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pelletier I, Duncan G, Colbere-Garapin F. One amino acid change on the capsid surface of poliovirus sabin 1 allows the establishment of persistent infections in HEp-2c cell cultures. Virology. 1998;241:1–13. doi: 10.1006/viro.1997.8954. [DOI] [PubMed] [Google Scholar]
- 115.Pavio N, Couderc T, Girard S, Sgro JY, Blondel B, Colbere-Garapin F. Expression of mutated poliovirus receptors in human neuroblastoma cells persistently infected with poliovirus. Virology. 2000;274:331–342. doi: 10.1006/viro.2000.0462. [DOI] [PubMed] [Google Scholar]
- 116.Duncan G, Pelletier I, Colbere-Garapin F. Two amino acid substitutions in the type 3 poliovirus capsid contribute to the establishment of persistent infection in HEp-2c cells by modifying virus-receptor interactions. Virology. 1998;241:14–29. doi: 10.1006/viro.1997.8955. [DOI] [PubMed] [Google Scholar]
- 117.Gosselin AS, Simonin Y, Guivel-Benhassine F, Rincheval V, Vayssiere JL, Mignotte B, Colbere-Garapin F, Couderc T, Blondel B. Poliovirus-induced apoptosis is reduced in cells expressing a mutant CD155 selected during persistent poliovirus infection in neuroblastoma cells. Journal of virology. 2003;77:790–798. doi: 10.1128/JVI.77.1.790-798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. The Journal of general virology. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 119.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nature cell biology. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang J, McManus BM. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. Journal of virology. 2004;78:4289–4298. doi: 10.1128/JVI.78.8.4289-4298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang HM, Yuan J, Cheung P, Luo H, Yanagawa B, Chau D, Stephan-Tozy N, Wong BW, Zhang J, Wilson JE, McManus BM, Yang D. Overexpression of interferon-gamma-inducible GTPase inhibits coxsackievirus B3-induced apoptosis through the activation of the phosphatidylinositol 3-kinase/Akt pathway and inhibition of viral replication. The Journal of biological chemistry. 2003;278:33011–33019. doi: 10.1074/jbc.M305352200. [DOI] [PubMed] [Google Scholar]
- 123.Yang D, Yu J, Luo Z, Carthy CM, Wilson JE, Liu Z, McManus BM. Viral myocarditis: identification of five differentially expressed genes in coxsackievirus B3-infected mouse heart. Circulation research. 1999;84:704–712. doi: 10.1161/01.res.84.6.704. [DOI] [PubMed] [Google Scholar]
- 124.Hwang HY, Kim JY, Lim JY, Chung SK, Nam JH, Park SI. Coxsackievirus B3 modulates cell death by downregulating activating transcription factor 3 in HeLa cells. Virus research. 2007;130:10–17. doi: 10.1016/j.virusres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 125.Pampin M, Simonin Y, Blondel B, Percherancier Y, Chelbi-Alix MK. Cross talk between PML and p53 during poliovirus infection: implications for antiviral defense. Journal of virology. 2006;80:8582–8592. doi: 10.1128/JVI.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mukherjee A, Morosky SA, Delorme-Axford E, Dybdahl-Sissoko N, Oberste MS, Wang T, Coyne CB. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011;7:e1001311. doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aldabe R, Irurzun A, Carrasco L. Poliovirus protein 2BC increases cytosolic free calcium concentrations. Journal of virology. 1997;71:6214–6217. doi: 10.1128/jvi.71.8.6214-6217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Madan V, Castello A, Carrasco L. Viroporins from RNA viruses induce caspase-dependent apoptosis. Cellular microbiology. 2008;10:437–451. doi: 10.1111/j.1462-5822.2007.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Campanella M, de Jong AS, Lanke KW, Melchers WJ, Willems PH, Pinton P, Rizzuto R, van Kuppeveld FJ. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. The Journal of biological chemistry. 2004;279:18440–18450. doi: 10.1074/jbc.M309494200. [DOI] [PubMed] [Google Scholar]
- 130.Neznanov N, Kondratova A, Chumakov KM, Angres B, Zhumabayeva B, Agol VI, Gudkov AV. Poliovirus protein 3A inhibits tumor necrosis factor (TNF)-induced apoptosis by eliminating the TNF receptor from the cell surface. Journal of virology. 2001;75:10409–10420. doi: 10.1128/JVI.75.21.10409-10420.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neznanov N, Chumakov KP, Ullrich A, Agol VI, Gudkov AV. Unstable receptors disappear from cell surface during poliovirus infection. Medical science monitor: international medical journal of experimental and clinical research. 2002;8:BR391–396. [PubMed] [Google Scholar]