Abstract
Caco-2 cells spontaneously differentiate into enterocyte-like cells and secrete apolipoprotein B (apoB) lipoproteins. We evaluated the effect of different extracellular matrix proteins on lipoprotein secretion by these cells. Caco-2 cells grown on human amnion connective tissue (HACT) secreted twice as much apoB as control cells on Transwells, but secreted similar amounts of apoA1. Cells cultured on fibrillar collagen type I secreted increased amounts of apoB similar to the cells cultured on HACT, but cells cultured on non-fibrillar collagen type I, type IV collagen or laminin-1 did not. The increased secretion was nullified by a function inhibiting anti-integrin β1 monoclonal antibody. Therefore, interactions between type I collagen and β1 integrins augment apoB secretion by Caco-2 cells. Cells on HACT formed a more uniform columnar epithelium with lipid droplets polarized to the basolateral membrane. We also studied the effect of extracellular matrix proteins on transepithelial resistance (TER) of differentiated Caco-2 cells. TER in cells cultured on HACT was similar to that on Transwells, but cells on laminin-1 and collagen IV exhibited higher TER. Thus, various extracellular matrix proteins regulate apoB secretion and TER differently. This new observation that extracellular matrix proteins can enhance apoB secretion in Caco-2 cells could be useful to explore the modulation of lipid transport by these proteins.
Keywords: ApoB, Caco-2 cell, Collagen, Amnion, Integrin, Transepithelial resistance, Differentiation, Extracellular matrix protein
1. Introduction
Enterocytes are highly differentiated intestinal epithelial cells specialized for nutrient absorption. As in all organs, these epithelial cells are in contact with the extracellular matrix proteins present in the basement membrane. Extracellular matrix proteins play an important role in the development and differentiation of the gut [6]. Furthermore, they have a significant effect on the growth, differentiation, and survival of the epithelial cells of the small intestine [4]. The intestinal epithelial basement membrane contains various collagens and laminins. The epithelial cells interact with these extracellular matrix proteins via integrins. Integrins are heterodimeric proteins composed of α and β subunits. Both of these subunits have several isoforms and their combination determines specificity. For example, α2β1 binds collagen type I whereas α1β1 binds collagen type IV [60].
A major function of enterocytes is to synthesize chylomicrons to transport dietary fat and fat-soluble vitamins. Assembly and secretion of chylomicron requires a structural protein, apolipoprotein B (apoB), as well as a dedicated chaperone, microsomal triglyceride transfer protein (MTP). Caco-2 cells, human colon carcinoma, have been used extensively as a model system to study various enterocyte functions [5,23,24]. These cells proliferate when cultured under sub-confluent conditions and differentiate into enterocyte-like cells when cultured for longer periods on plastic or in Transwells. After differentiation, these cells exhibit polarized columnar epithelial morphology, form tight junctions, and show transepithelial electrical resistance (TER). In addition, they assemble and secrete apoB-containing lipoproteins and have been used to study the molecular mechanisms involved in their assembly and secretion [8,11,13,14,22,31,34,55]. These cells secrete both apoB100 and apoB48-containing lipoproteins, predominantly apoB100, mainly toward the basolateral side. A number of factors, such as acyl-CoA: cholesterol acyltransferase and MTP inhibitors [42,54], butyrate [35], calcium antagonist [20], colchicine [12], IL-1β, IL-6, TNF [39–41], okadaic acid [37], quercetin [7], and sphingomyelinase [15] have been shown to decrease apoB secretion by Caco-2 cells. On the other hand, the assembly and secretion of these particles by differentiated Caco-2 cells are increased by the addition of lipids such as oleic acid to the apical surface [8,34,53]. In addition, TGFβ has been shown to increase synthesis and secretion of apoB by Caco-2 cells [41,50]. Thus, several studies indicate that the culture environment can modulate the assembly and secretion of apoB-lipoproteins by Caco-2 cells.
Cell culture studies are generally performed on plastic surfaces. However, numerous studies show that culturing cells on matrix proteins substantially alters their phenotype and proliferation. For example, Caco-2 cells grown on extracellular matrix proteins express more brush border enzymes compared to those grown on plastic [5]. While previous studies have used Caco-2 cells to examine the effects of extracellular matrix on cell adhesion [21,33,49,51], integrin localization, cytoskeletal organization [21], spreading [5,21,49], migration [49], proliferation [5,51,61], and differentiation [5,33,45,48,51], little has been done to examine the role of extracellular matrix proteins in the assembly and secretion of lipoproteins. We hypothesized that differentiation of Caco-2 cells on different extracellular matrix proteins would promote the assembly and secretion of apoB-lipoproteins.
2. Experimental procedures
2.1. Reagents
Antibodies used for the determination of apoB and apoA1 have been described [3,25,27]. Monoclonal antibodies against human integrin β1 (AIIB2) and human integrin α5 (BIIG2) were developed by Dr. Caroline H. Damsky and were obtained from Developmental Studies Hybridoma Bank (Iowa City, IA). Anti-integrin α1 (FB12), α2 (P1E6), and α3 (P1B5) antibodies were from Chemicon (Temecula, CA). Another anti α1 antibody 5E8D9 was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Mouse laminin-1 and collagen type IV were purchased from Becton Dickinson (Bedford, MA). Rat-tail tendon collagen type I was kindly provided by Dr. George Ojakian (SUNY Downstate Medical Center, Brooklyn, NY). Oleic acid (OA) and taurocholate (TC) were from Sigma (St. Louis, MO).
2.2. Preparation of extracellular matrix protein coated filters
Polycarbonate filters (Transwell® 24 mm diameter, 3.0 μm pore size, Corning Incorporated, Corning, NY) were cut out and attached to Lexan cylinders (0.71 cm2 area, Fig. 1A) by silicone adhesive (DAP, Dow Corning Corp., Midland, MI). These were autoclaved and then sterile coated with collagen type IV or laminin-1 according to the manufacturer’s directions. Briefly, the stock collagen type IV was diluted in 0.05 N HCl to 100 μg/ml, 100 μl was added to each cylinder, and the cylinders were incubated at room temperature for 1 h. The excess was removed and the collagen type IV coated filters were rinsed three times with calcium and magnesium free phosphate buffered saline (CMF-PBS) and once with Dulbecco’s modified Eagle’s medium (DMEM) to remove any residual acid immediately before plating cells. For laminin-1 coating, the stock laminin-1 was diluted to 50 μg/ml in serum-free DMEM, 100 μl was added to each cylinder, and the cylinders were incubated at room temperature for 1 h. The excess was removed and the laminin-1 coated filters were rinsed three times with DMEM+10% FBS immediately before plating cells. Type I collagen can have different structures depending on its preparation [for recent reviews, 19,57]. We prepared cylinders coated with either non-fibrillar collagen films or fibrillar collagen gels to evaluate the effect of different collagen structures on apoB secretion by differentiated Caco-2 cells. Filters attached to Lexan cylinders were coated with non-fibrous collagen type I film (100 μl/cylinder at 50 μg/ml in 0.1% acetic acid) for 1 h at room temperature. The supernatant solution was discarded, the filters were allowed to air dry, and were stored at room temperature until used. Immediately before plating cells, the filters were rinsed three times with CMF-PBS and once with DMEM to remove any residual acetic acid. To prepare fibrillar collagen type I gels, 300 μg/ml collagen in 0.1% acetic acid was mixed with 10× DMEM and the acid was neutralized with 0.34 M NaOH to pH 7.2. This solution (250 μl) was added to each sterile Transwell® polycarbonate filter/Lexan cylinder and was incubated at 37 °C for 1 h. Caco-2 cells were plated directly onto the gel without rinsing.
Fig. 1.
Schematic diagrams of Lexan cylinders with either polycarbonate filter (A) or human amnion connective tissue (B).
2.3. Preparation of cylinders with human amnion connective tissue (HACT)
HACT was selected as a substrate because it provides a source of human connective tissue and it has enabled other types of epithelial cells to express many of the morphological and physiological characteristics that distinguish them in vivo [17]. HACT was prepared by a modification of the method of Liotta et al. [32]. Placentas were obtained from normal vaginal deliveries at SUNY Downstate Medical Center, usually within 6 h after delivery. Under aseptic conditions, the amnion reflecta was separated from the chorion by blunt dissection. The amnion was fastened to Lexan cylinders (Fig. 1B) with Viton orings (C. E. Conover and Co., Inc., Philadelphia, PA). The amniotic epithelium faced the basal chamber while the connective tissue formed the substrate for cell attachment. The cylinders were cut from the remainder of the amniotic tissue, washed extensively in CMF-PBS containing penicillin (500 IU/ml) and streptomycin (50 μg/ml), and incubated with sterile 0.25 M NH4OH for 2 h at room temperature. The amniotic epithelial layer was removed from the basal surface of the cylinder by gentle scraping with sterile cotton swabs. The tissue was again washed extensively with CMF-PBS and was stored in CMF-PBS containing penicillin (500 IU/ml) and streptomycin (50 μg/ml) at −20 °C until used. Immediately before plating cells onto the HACT, the cylinders were rinsed four times with CMF-PBS and once with DMEM to remove the excess antibiotics. In some experiments, the completed cylinders were further subjected to boiling for 15 min, trypsinization for 30 min at 37 °C, soaking in isopropyl alcohol for 1 h at room temperature, fixation in 2.5% glutaraldehyde for 1 h at room temperature followed by extensive rinsing in 50 mM NH4Cl to neutralize free aldehyde groups, or 2 times rapid freeze and thaw cycles.
2.4. Cell culture
Caco-2 cells were routinely cultured in DMEM (4.5 g/l Glucose and L-Glutamine, Mediatech, Inc., Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 IU/ml) and streptomycin (10 μg/ml) (GibcoBRL), maintained in a 5% CO2/95% air atmosphere at 37 °C. For experiments, cells from sub-confluent flasks were seeded at 2.8×105 cells/ml density, 0.5 ml/cylinder into prepared cylinders and incubated suspended in 24-well tissue culture plates (Becton Dickinson Co., Franklin Lakes, NJ). Cultures were fed every 2–3 days with fresh medium in both the apical (0.5 ml/cylinder) and basal (1.5 ml/well) compartments. Eighteen hours prior to collecting media for apoB and apoA1 determinations, the basal medium was replaced with fresh medium containing 1% FBS and the apical medium was replaced with fresh medium containing 10% FBS. When experiments required the addition of monoclonal antibodies or inhibitors, these were incorporated into both apical and basal medium at the indicated concentrations for the final 18 h of incubation. ApoB and apoA1 were quantified using a sandwich ELISA assay [3,25].
2.5. Transepithelial electrical resistance (TER) measurements
Measurements of TER were performed as previously described using a chamber specially designed (patent no. 4686190) to hold the Lexan cylinders, 3 M KCl/agarose salt bridges, and Hg/HgCl2 electrodes [9,36,38]. Briefly, 10 μA of direct current was passed across the cell monolayers through Hg/HgCl2 electrodes connected to each compartment by 3 M KCl-agarose bridges. The voltage change was measured on a digital multimeter, and this measurement was used to calculate TER of the epithelial monolayer.
2.6. Chylomicron separation by density gradient ultracentrifugation
For chylomicron secretion, cultures were supplemented with oleic acid:taurocholate (OA:TC) at 1.6:0.5 mM in the apical compartment for the final 18 h of incubation [34]. Conditioned basal medium pooled from 3 cultures (3.8 ml/condition) was brought to 4 ml volume in SW41 ultracentrifuge tubes and adjusted to a density of 1.12 g/ml by the addition of 0.565 g of KBr [34]. The media were sequentially overlaid with 3 ml of 1.063 g/ml, 3 ml of 1.019 g/ml, and 2 ml of 1.006 g/ml density solutions. The tubes were centrifuged (SW41 rotor, 40,000 rpm, 33 min, 15 °C) and the top 1 ml was collected. This fraction contained large chylomicrons (Sf > 400) [34]. The gradients were then overlaid with 1 ml of 1.006 g/ml solution, centrifuged (40,000 rpm, 3 h and 30 min, 15 °C), and the top 1 ml was aspirated (small chylomicrons fraction). The gradients were overlaid with a final 1 ml of 1.006 g/ml solution, centrifuged (40,000 rpm, 17 h, 15 °C), and the top 1 ml was collected. This fraction contained the VLDL size particles. The remaining solutions were aspirated into 1.5 ml fractions labeled 1–7.
2.7. Light microscopy
For examination of 1-micron sections of monolayers by light microscope, filters or amnion were fixed for 1 h in 2.5% v/v glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) and then washed in buffer. The monolayers were postfixed for 1 h in 1% v/v OsO4 in 0.1 M phosphate buffer (pH 7.3), washed in 0.85% saline, dehydrated in ethanol, transferred to propylene oxide, and embedded in Epon 812. Thick sections, stained with trypan blue were examined by light microscopy under oil at 1000×.
2.8. Statistics
Values are presented as the mean±s.d. or s.e.m. Standard deviation was used to describe the variability of individual cultures in a single experiment. Standard error of the mean was used to describe the variability of the mean when the values represent the average of the means of multiple experiments. Linear regression analysis and determination of statistical differences between paired or unpaired groups were done using GraphPad Software Prism 3.0.
3. Results
3.1. Caco-2 cells cultured on HACT secrete more apoB
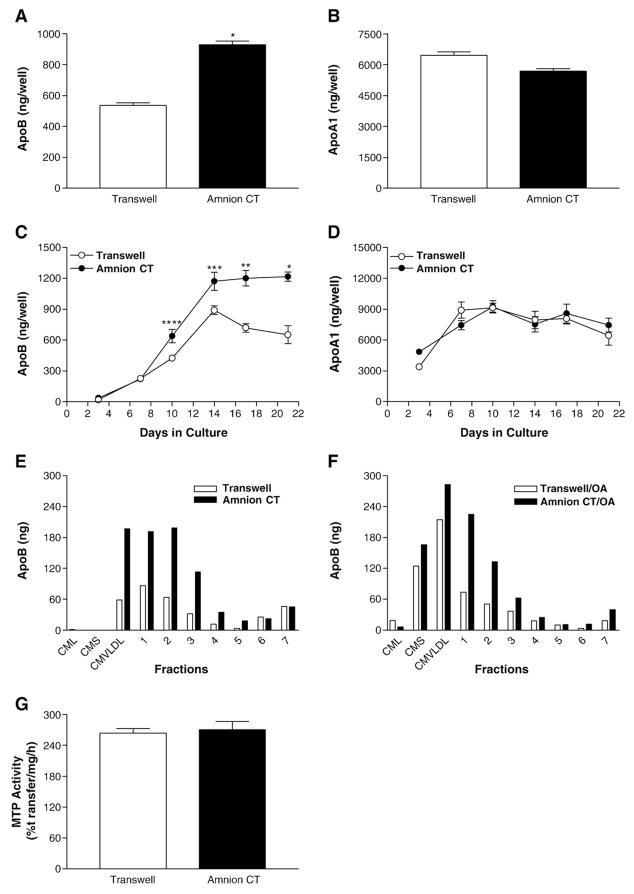
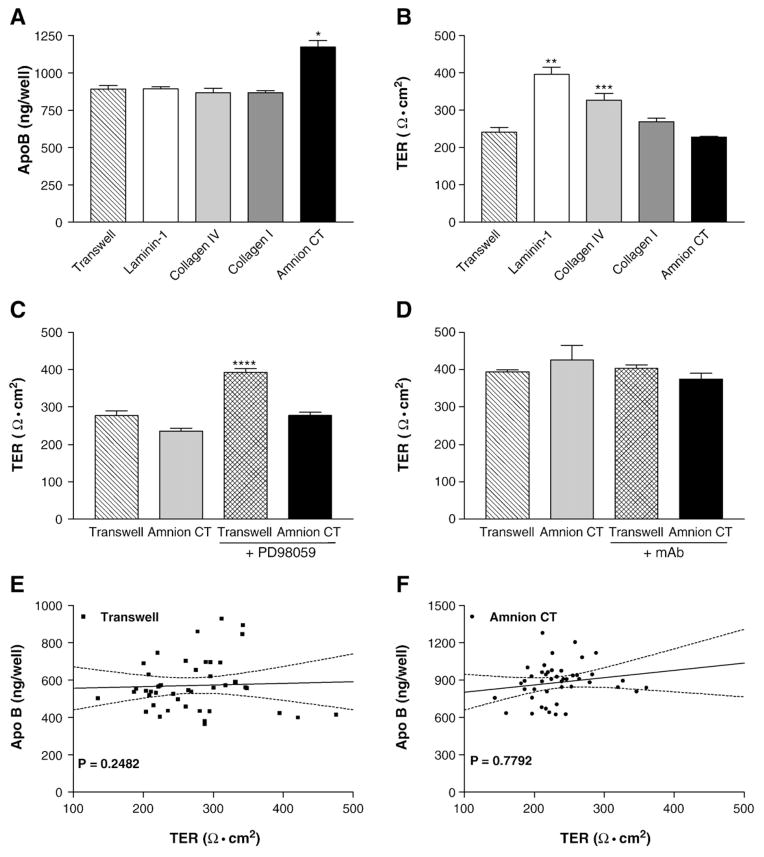
To study the effect of extracellular matrix proteins on the secretion of apolipoproteins B (apoB), Caco-2 cells were cultured on either HACT or Transwell® filters for 14 days. Cells grown on HACT secreted significantly more apoB than cells grown on Transwells (Fig. 2A, p<0.0001). However, secretion of apoA1 by these cells was not affected by their culture in these two different conditions (Fig. 2B). Therefore, cells on HACT specifically secrete more apoB.
Fig. 2.
Effect of human amnion connective tissue on the secretion of apoB and apoA1 and cellular MTP activity. Panels A and B: Secretion of apoB and apoA1 by Caco-2 cells cultured on HACT. Caco-2 cells were cultured for 14 days on uncoated Transwell® filters, or on HACT (Amnion CT). Eighteen hours prior to collection, the basal medium was exchanged with fresh DMEM+1% FBS, and the apical medium was replaced with DMEM+10% FBS. Basolateral media were used to measure apoB (A, *p<0.0001) or apoAI (B) using ELISA. Panels C and D: Time course of apoB and apoA1 secretion by Caco-2 cells cultured on HACT. The basal medium was exchanged with fresh DMEM containing 1% FBS 18 h prior to each collection time. At the times indicated, basal medium was collected and immediately assayed by ELISA for apoB (C, *p<0.0001, **p<0.0002, ***p<0.004, ****p<0.006) or apoA1 (D). Average±s.d. values are plotted against time (n=4). Panels E and F: Secretion of different apoB-lipoproteins by Caco-2 cells cultured on HACT. Eighteen hours prior to collection, the basal medium was exchanged with fresh DMEM+1% FBS, and the apical medium was replaced with DMEM+10% FBS+0.5 mM taurocholate (panel E) or DMEM+10% FBS supplemented with 1.6 mM oleic acid:0.5 mM taurocholate (panel F). Basal medium was collected and immediately fractionated by density gradient centrifugation and the fractions were assayed by ELISA for apoB. The data are from one experiment and are representative of three independent experiments. Panel G: Activity of microsomal triglyceride transfer protein (MTP) in Caco-2 cells cultured on HACT. Cells were washed and used to measure triglyceride transfer activity of MTP. Data are representative of three independent experiments performed in triplicate.
We further studied the effect of HACT on apoB secretion during differentiation. Caco-2 cells were grown on Transwell® filters alone or cylinders with HACT. In a time course study of 21 days, Caco-2 cells grown on HACT secreted significantly greater amounts of apoB than cells grown on Transwell® filters (Fig. 2C). This increase became apparent on day 10 and continued to day 21. Therefore, not only did HACT significantly increase apoB secretion, it also sustained the maximum secretion throughout the study period. Cells cultured on Transwell® filters secreted maximum amounts of apoB on day 14. Subsequently, the amounts of apoB secretion by these cells appear to decline. In contrast to apoB, secretion of apoA1 from Caco-2 cells was similar during the same time span, regardless of the extracellular matrix protein used (Fig. 2D). These studies indicate that cells grown on HACT secrete more apoB for a longer period.
Next, we asked whether growth on HACT would affect the types of lipoproteins secreted by these cells. When no additional lipid was added to the cultures, Caco-2 cells grown on Transwell® filters secreted apoB in the VLDL and other smaller lipoproteins (Fig. 2E) as described earlier [34]. Growth of these cells on HACT significantly increased the amounts of apoB in the VLDL and other smaller lipoproteins. No additional less buoyant particles were present in the conditioned media.
We then studied the effect of fatty acids on the secretion of different types of lipoproteins (Fig. 2F). This treatment has been shown to induce assembly and secretion of more buoyant chylomicron-size particles without affecting the secretion of apoAI-lipoproteins [34]. The addition of oleic acid to the cultures for the final 18 h of incubation caused greater secretion of apoB and stimulated chylomicron secretion in cells grown on both human amnion connective tissue and Transwell® filters (Fig. 2F). Again, amounts of apoB secreted in VLDL and other smaller lipoproteins by cells cultured on HACT were higher than those secreted by cells cultured on Transwell® filters. However, there were no significant differences in the amounts of chylomicrons secreted under two different culture conditions. These data indicate that cells grown on HACT increase apoB secretion without significantly altering the types of lipoproteins produced.
ApoB lipoprotein secretion is critically dependent on microsomal triglyceride transfer protein (MTP). To test, if MTP activity was altered during different culture conditions, we allowed Caco-2 cells to differentiate on Transwell® and cylinders with HACT. Cells were used to measure triglyceride transfer activity of MTP [2,47]. Analysis of MTP activity in cells revealed that the MTP activity was similar under both culture conditions (Fig. 2G). Thus, differentiation of Caco-2 cells on HACT has no effect on MTP activity.
3.2. Growth of Caco-2 cells on fibrillar collagen type I increases apoB secretion
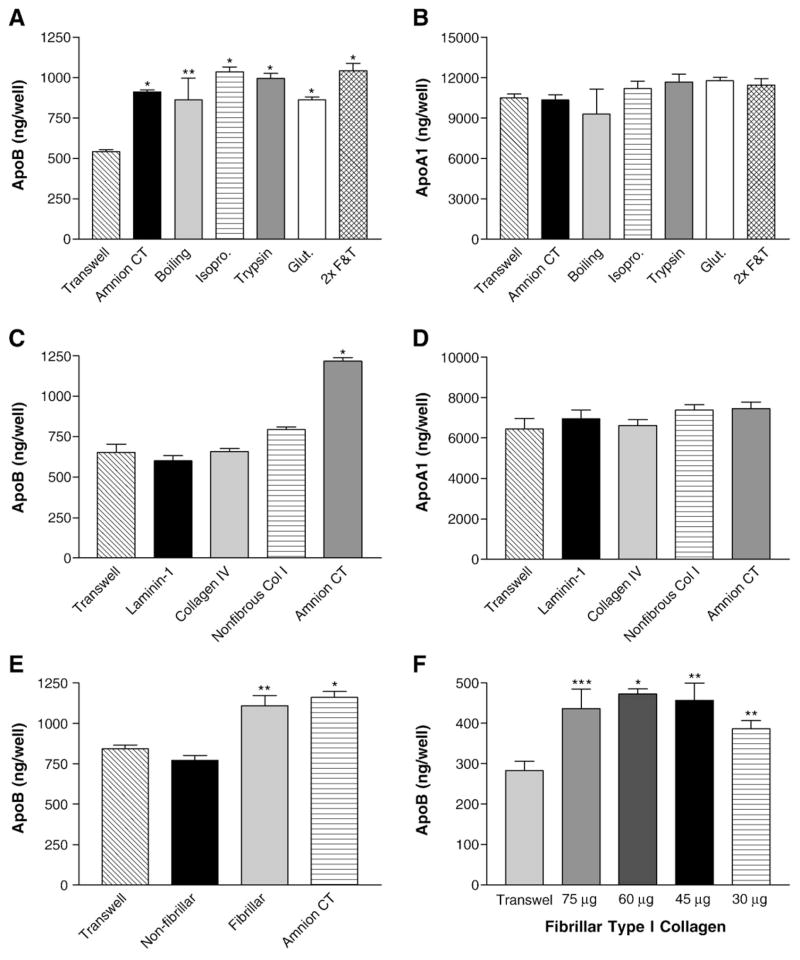
Attempts were made to identify the active component in HACT that increases apoB secretion. To determine if labile components of the amnion were responsible for the increased apoB secretion, a variety of treatments were employed to denature or remove them. Cylinders with HACT were subjected to boiling, soaking in isopropanol, trypsinization, glutaraldehyde fixation, or freeze and thaw cycles. Equal numbers of cells were then plated in these cylinders and in cylinders with untreated HACT and Transwell® filters. Cells plated on HACT that had been denatured in various ways still secreted elevated levels of apoB similar to cells grown on untreated HACT (Fig. 3A) indicating that the stimulating factor was not affected by these treatments. Amounts of apoA1 secreted by cells after various treatments were similar to those secreted by cells grown on either untreated HACT or on Transwell® filter (Fig. 3B). These results indicate that a labile molecule in the HACT was not responsible for the enhanced apoB secretion.
Fig. 3.
Effect of different extracellular matrix proteins on the secretion of apoB and apoA1 by Caco-2 cells. Panels A and B: Effect of various treatments of HACT on apoB secretion. Prior to plating the Caco-2 cells, the HACT was boiled, soaked in isopropanol (isopro.), treated with trypsin, fixed in glutaraldehyde (Glut.) or freeze/thawed twice (2×F & T) to determine the effect on the secretion of apoB (A, *p<0.0001, **p<0.04) or apoA1 (B). Caco-2 cells were cultured for 14 days on the treated and untreated substrates. Basal media were collected and immediately assayed by ELISA. Average±s.d. values are depicted (n=4). Panels C and D: Effect of different extracellular matrix proteins on apoB secretion. Caco-2 cells were cultured for 21 days on uncoated Transwell® filters, filters which had been coated with laminin-1, collagen type IV or non-fibrillar collagen type I (non-fibrous Col 1), and on HACT (Amnion CT). The basal medium was exchanged with fresh DMEM containing 1% FBS. After 18 h, basal medium was collected and immediately assayed by ELISA for apoB (C, *p<0.0001) or apoA1 (D). Average±s.d. values are plotted (n=4). Panel E: Effect of fibrillar and non-fibrillar collagen type I on the secretion of apoB by Caco-2 cells. Caco-2 cells were cultured for 14 days on uncoated Transwell® filters, filters coated with non-fibrillar or fibrillar collagen type I, or on HACT (Amnion CT). Basal medium was collected and immediately assayed by ELISA for apoB (*p<0.0005, **p<0.008). Panel F: Effect of different amounts of fibrillar collagen type I on the secretion of apoB by Caco-2 cells. Caco-2 cells were cultured for 14 days on uncoated Transwell® filters, or filters with different amounts of fibrillar collagen type I added to the cylinder. Basal medium was collected and immediately assayed by ELISA for apoB as described before (*p<0.001, **p<0.02, ***p<0.03). Average±s.d. values are depicted (n=4).
We then focused on the major extracellular matrix proteins. Immunogold labeling and transmission electron microscopy studies of HACT show that it contains collagen Type I, collagen Type IV and laminins [10], however their percent composition is unknown. Therefore, Caco-2 cells were grown on Transwell® filters alone or these filters coated with laminin 1, collagen IV or non-fibrillar collagen type I (Fig. 3C). The amounts of apoB secreted by these cells grown on different matrix proteins were then compared with those secreted by cells grown on HACT. Caco-2 cells grown on HACT secreted significantly greater amounts of apoB than cells grown on any other matrix protein (Fig. 3C). In contrast to apoB, secretion of apoA1 from Caco-2 cells was similar regardless of the extracellular matrix proteins used (Fig. 3D).
Since these extracellular matrix proteins did not increase apoB secretion, we determined if the morphological structure of the collagen was the stimulating factor [16,28] and tested the ability of Transwell® filters coated with fibrillar collagen type I to stimulate apoB secretion. As seen in Fig. 3E, fibrillar collagen caused a similar increase in apoB secretion as HACT. This increase in secretion occurred independent of the amounts of collagen used (Fig. 3F). In fact, the lowest amounts, which were barely sufficient to form a layer on the Transwell® filters, were still able to enhance apoB secretion (Fig. 3F, 30 μg). These studies identify fibrillar collagen type I as the active component responsible for increased apoB secretion by Caco-2 cells grown on HACT.
3.3. Fibrillar collagen type I and β1 integrin are involved in augmenting apoB secretion
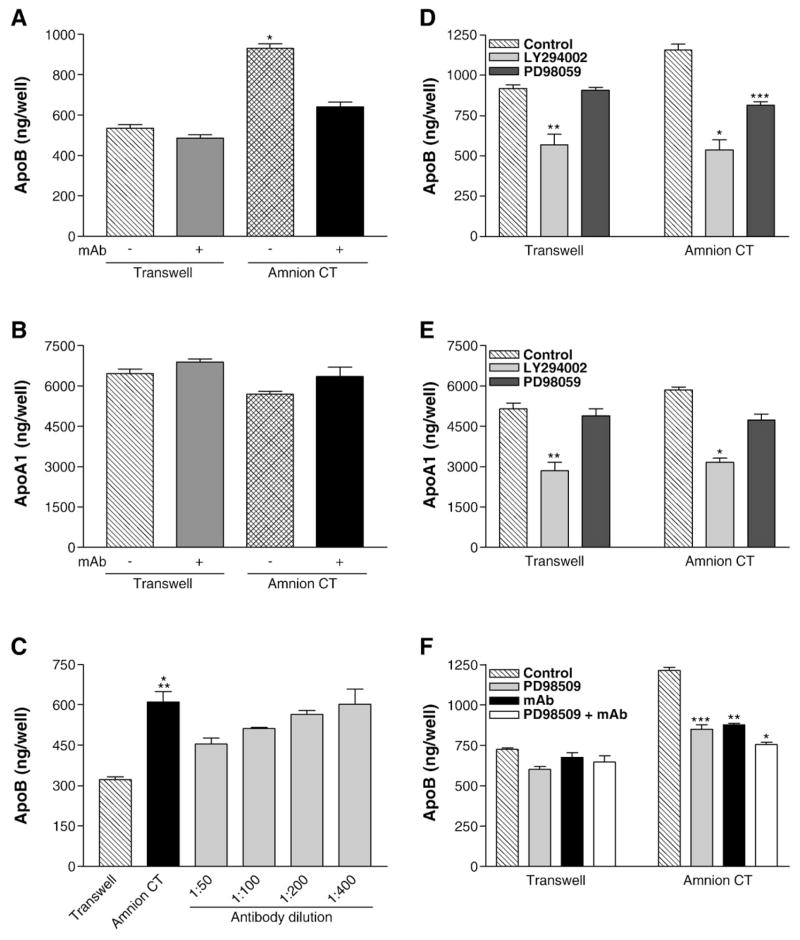
There are four integrin heterodimers which can function as collagen type I receptors: α1β1, α2β1, α10β1, and α11β1 [60]. Since all these integrins contain the β1 subunit, we used anti-functional antibodies against the β1 subunit to determine if the binding of collagen type I to integrins is necessary for enhanced apoB secretion. To evaluate the role of the β1 integrin subunit, 14-day old cultures were incubated with monoclonal antibody against integrin β1 (mAb AIIB2) for 18 h. mAb AIIB2 had no effect on the secretion of apoB (Fig. 4A) and apoA1 (Fig. 4B) by cells grown on Transwell® filters. However, treatment of cells grown on HACT with anti-integrin β1 antibody significantly reduced the secretion of apoB (Fig. 4A, p<0.0001) without altering apoA1 secretion (Fig. 4B). When anti-integrin β1 monoclonal antibody was added in decreasing concentrations to the cultures grown on HACT, the decrease in apoB secretion was shown to be dose responsive (Fig. 4C). Addition of these antibodies after 14 days of culture decreased apoB secretion indicating that interactions between these cells and extracellular matrix proteins are dynamic and susceptible to changes in extracellular milieu. Similar experiments using various anti α1 (FB12 and 5E8D9), α2 (P1E6), α3 (P1B5), and α5 (BIIG2) integrin antibodies did not reduce apoB secretion (data not shown) most likely because more than one α subunits might be involved. We interpret these data to suggest that the binding of collagen type I to β1 integrins is important for the enhanced apoB secretion.
Fig. 4.
Effect of anti-integrin β1 monoclonal antibody and/or protein kinase inhibitors on the secretion of apoB and apoA1 by Caco-2 cells. Caco-2 cells were cultured for 14 days on uncoated Transwell® filters or filters coated with on HACT (Amnion CT). The apical and basal media were supplemented with fresh DMEM with or without mAb AIIB2, an anti-functional integrin β1 antibody. After 18 h, basal medium was collected and immediately assayed by ELISA for apoB (A, *p<0.0001 Amnion CT vs Transwell with or without mAb AIIB2 or Amnion CT+mAb AIIB2) or apoA1 (B). Average±s.d. values are depicted. The data are from one experiment performed in quadruplicate and are representative of four independent experiments. Panel C shows a separate experiment in which cells were incubated with different indicated dilutions of the monoclonal antibody. Average±s.d. values are plotted (*p<0.0004 Amnion CT vs Transwell, **p<0.03 Amnion CT vs Amnion CT+1:50 mAb, n=4). Panels D–F: The apical and basal media were exchanged with fresh DMEM with or without protein kinase inhibitors LY294002 (25 μM) or PD98059 (50 μM) and/or mAb AIIB2. Basal medium was collected after 18 h and immediately used to quantify apoB (D, *p<0.006, **p<0.009 Control vs LY294002, ***p<0.005 Control vs PD98059, F, *p<0.0003, **p<0.004, ***p<0.008) and apoA1 (E, *p<0.001, **p<0.008 Control vs LY294002). Average±s.d. values are depicted (n=3).
Binding of collagen type I to integrins is known to induce outside-in signaling involving different kinases. Two major kinases involved in the signaling are PI3-kinase and MAP kinase kinase. To evaluate their role in apoB secretion, Caco-2 cells grown on Transwell® filters or amnion connective tissue were incubated with either PI3-kinase inhibitor, LY294002 [56], or PD98059 [43], which inhibits MAP kinase kinase. LY294002 significantly reduced secretion of apoB (Fig. 4D, p<0.009) and apoA1 (Fig. 4E) by Caco-2 cells grown on both Transwell® filters and amnion connective tissue to the same level. In contrast, PD98059 significantly reduced apoB secretion by cells grown on amnion connective tissue only (Fig. 4D). These data indicate that PI3-kinase plays an important role in apoB and apoA1 secretion by differentiated Caco-2 cells. However, the amnion connective tissue induced apoB secretion involves the MAP kinase pathway.
MAP kinase kinase is activated by several stimuli. To determine if PD98059 effect was due to the inhibition of the signaling elaborated by the binding of type I collagen to β1 integrin subunit, we used both anti-integrin β1 antibodies and PD98059 (Fig. 4F). The apoB secretion by cells grown on Transwell® filters was not affected by the addition of PD98059, mAb AIIB2, or PD98059 and mAb AIIB2. In contrast, cells grown on amnion connective tissue secreted significantly lower amounts of apoB in the presence of the inhibitor and the monoclonal antibody (Fig. 4F). Both these reagents individually decreased apoB secretion by ~30%. When PD98059 and mAb AIIB2 were added together no additive inhibitory effect was observed indicating that both the antibody and the inhibitor affect the same pathway. These studies indicate that MAP kinase kinase is involved in the enhanced secretion of apoB by the outside-in signaling induced by fibrillar collagen type I.
3.4. Effect of HACT on cell morphology and on intracellular lipid droplets
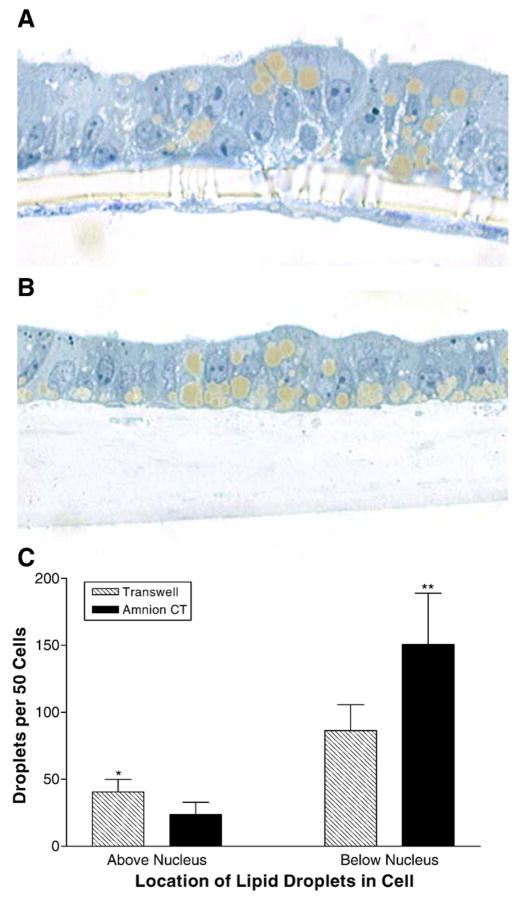
Since the extracellular matrix is known to play a significant role in the differentiation of cells, we compared the morphology of the Caco-2 cells grown on Transwell® (Fig. 5A) and HACT (Fig. 5B). Cells grown on Transwell® filters for 14 days showed some multilayering with nuclei at different levels and distended intercellular space (Fig. 5A). These cells extended their pseudopods through the pores of the filter and formed a layer along the basal surface of the filter. In contrast, Caco-2 cells grown on HACT showed a simpler columnar epithelial monolayer with most of the nuclei at the same level (Fig. 5B). Another feature was the presence of lipid droplets. Cells grown on both surfaces and supplemented with fatty acids contained large lipid droplets. In contrast to cells grown on Transwell® filters (Fig. 5A), the organization of the lipid droplets in cells grown on HACT (Fig. 5B) were more polarized with numerous lipid droplets lining the basal surface of the cells. In Fig. 5C, the number and location of lipid droplets with respect to the location of the nuclei were compared. Cells grown on HACT had significantly more lipid droplets below the nucleus. No attempt was made to determine if these droplets contain apoB or the amounts of apoB within these cells. These studies show that cells grown on HACT form a more uniform columnar epithelial cell layer and contain lipid droplets predominately below the nucleus, i.e., the basolateral side where apoB-lipoproteins are secreted.
Fig. 5.
Effect of HACT on cell morphology and intracellular lipid droplets. Caco-2 cells were cultured for 14 days on uncoated Transwell® filters, or on HACT (Amnion CT). The cultures were treated for 18 h with oleic acid:taurocholate (1.6:0.5 mM), and then fixed in 2.5% glutaraldehyde and embedded for sectioning. Cross-sections show typical growth on uncoated Transwell® filters (A), or on HACT (B). Lipid droplets were counted for 50 consecutive cells on each substrate according to their location within each cell (above or below the nucleus, C). Average±s.d. are depicted (*p<0.05, **p<0.03, n=4).
3.5. Effect of extracellular matrix proteins on TER and on apoB secretion
The ability of the HACT to stimulate Caco-2 cells to form a columnar epithelial monolayer with polarized lipid droplets and to increase apoB secretion suggests that this substrate might be uniformly increasing the differentiation of these cells. If this is true then different characteristics of the differentiated cells are expected to behave similarly. Therefore, we compared the ability of apoB secretion of differentiated cells with their ability to form tight junctions. To examine this, Caco-2 cells were grown on different extracellular matrix proteins and then used to study apoB secretion and TER, a measure of tight junction formation. Cells grown on Transwells, and Transwells coated with laminin-1, collagen IV and non-fibrillar collagen I secreted similar amounts of apoB (Fig. 6A). In contrast, cells grown on HACT secreted enhanced amounts of apoB consistent with previous data. Surprisingly, the TER of cells grown on HACT was similar to that observed in cells grown on Transwells (Fig. 6B). However, the TER was significantly increased in cells grown on laminin-1 and collagen type IV (p<0.005 and p<0.0076, respectively). We next examined the effects of PD98059 and mAb AIIB2 on the TER of Caco-2 cells grown on Transwell® filters or amnion connective tissue. PD98059 which had no effect on apoB secretion by cells grown on Transwell® filters (Fig. 4D) significantly increased the TER in these cells (Fig. 6C, p<0.0001). However, although PD98059 reduced apoB secretion in Caco-2 cells grown on HACT, it had no effect on the TER. The anti-integrin β1 monoclonal antibody had no effect on the TER of Caco-2 cells grown on Transwell® filters or amnion connective tissue (Fig. 6D). This antibody, like PD98059, significantly reduced apoB secretion by cells grown on HACT (Fig. 4A). When we compared the amount of apoB secretion to the TER in Caco-2 cells grown on Transwell® filters (Fig. 6E) or HACT (Fig. 6F), we found no correlation between these parameters. These data indicate that apoB secretion and TER are two independently regulated phenotypes of the differentiated Caco-2 cells.
Fig. 6.
Effect of extracellular matrix proteins on the TER and apoB secretion by Caco-2 cells. Panels A and B: Caco-2 cells were cultured for 14 days on Transwell® filters coated with laminin-1, collagen type IV, non-fibrillar collagen type I, or HACT (Amnion CT). Uncoated filters (Transwell) served as controls. The basal medium was exchanged with fresh DMEM containing 1% FBS and, 18 h later, was collected and immediately assayed by ELISA for apoB (*p<0.005, Transwell vs Amnion CT, n=4, A). The TER was assessed on day 14 in DMEM+10% FBS (**p<0.005 Transwell vs Laminin-1, ***p<0.01 Transwell vs Collagen IV, n=4, B). Panels C and D: Caco-2 cells cultured for 14 days on uncoated Transwell® filters or on amnion connective tissue were incubated for 18 h with fresh DMEM containing 1% FBS±50 μM PD98059 (****p<0.0001 Transwell® vs Transwell®+PD98059, n=6, C) or mAb AIIB2 (D) and used to assess TER in DMEM+10% FBS. Panels E and F: Correlation between apoB secretion and TER. Caco-2 cells were cultured for 14 days on Transwell® filters or on HACT. The basal medium was changed to fresh DMEM containing 1% FBS, collected after 18 h, and immediately assayed for apoB. The cultures were then assessed for TER in DMEM+10% FBS. Paired values of apoB and TER from 45 cultures from 12 independent experiments are depicted for Transwell® filters (E) and HACT (F). Linear regression slope is not significantly different from zero (Transwell® filters p=0.7792, Amnion CT p=0.2482).
4. Discussion
Caco-2 cells grown on HACT secrete approximately twice as much apolipoprotein B over an 18-hour period compared to cultures grown on tissue culture treated Transwell® filters. The enhancement in apoB secretion was not due to a generalized increase in secreted proteins, as apoA1 secretion was not augmented under the same conditions. Morphological studies revealed that cells grown on HACT have a more regular, columnar epithelial organization with lipid droplets accumulating along the basolateral membrane. Biochemical studies revealed that the increase in apoB secretion was dependent on type I collagen in a fibrillar, cross-linked format, since non-fibrillar type I collagen as well as laminin and collagen IV did not cause this increase. The increased apoB secretion was dependent on integrin β1 as incubation with a function inhibiting anti-integrin β1 monoclonal antibody reduced apoB secretion. Furthermore, the MAP kinase kinase inhibitor PD98059 reduced apoB secretion to about the same level as those seen in Transwell® filter cultures. A combination of PD98059 and anti-integrin β1 monoclonal antibody did not show additive reduction in apoB secretion. Thus, it is likely that interactions between extracellular type I collagen and cell surface β1 integrins activate MAPK pathway to increase apoB secretion in Caco-2 cells.
Differentiation of Caco-2 cells results in the acquisition of phenotype characteristic of intestinal epithelial cells. Two major characteristic features of differentiated Caco-2 cells are apoB-lipoprotein assembly and formation of tight junctions [1,18]. TER measures the tightness of the occluding junctions. We reasoned that increased apoB secretion by cells cultured on fibrillar type I collagen might be related to more efficient differentiation of these cells. However, comparison of two different differentiation phenotypes gave unanticipated results. While HACT and fibrous type I collagen increased apoB secretion, they had no effect on the TER of the Caco-2 cells. In contrast, the tightness of the occluding junctions as measured by TER was increased by laminin-1 and type IV collagen. These extracellular matrix proteins, however, had no effect on apoB secretion. Thus, various extracellular matrix proteins regulate these two Caco-2 cell differentiation phenotypes differently.
Caco-2 cells grown on Transwell® filters express brush border enzymes such as alkaline phosphatase, dipeptidyl peptidase, sucrase-isomaltase and lactase [44,52] after differentiation. ApoB secretion is also dependent upon differentiation of Caco-2 cells, as it increases with time in culture reaching a maximum at 12 days post-confluence [48]. This is in good agreement with our findings, since our cultures reached confluence at about day 2 to 3 after plating, and reached their maximum secretion at day 14. Our studies show that the ability of Caco-2 cells to secrete apoB can be significantly improved when they are grown on HACT or fibrillar type I collagen.
Growth of Caco-2 cells on different extracellular matrix proteins has been shown to affect expression of different enzymes in Caco-2 cells. For example, laminin-1 promotes an earlier expression of sucrase-isomaltase in Caco-2 cells than laminins 2, 5 or 10 [51]. Cells cultured on both laminin-1 and type IV collagen substrates exhibit greater specific activities of sucrase-isomaltase, alkaline phosphatase and dipeptidyl peptidase than cells grown on non-fibrous type I collagen or plastic substrates [5]. Thus, we had anticipated that laminin-1 and type IV collagen in the HACT might enhance Caco-2 cell differentiation and apoB secretion. Surprisingly, both of these basement membrane constituents did not affect apoB secretion. Nonetheless, cells grown on these substrates exhibited increase in the TER, another measure of cellular differentiation. Thus, various extracellular matrix proteins have differential effects on the expression of individual proteins.
Type I collagen, both in fibrillar and non-fibrillar forms, has been used extensively as a substrate for the growth of a variety of cell types. Wang et al. [58] showed that fibrillar type I collagen stimulated the production and activation of pro-matrix metalloproteinases in cultured hepatic stellate cells while non-fibrillar type I collagen did not. In contrast, Fujisaki and Hattori [16] showed that human foreskin keratinocytes grown on fibrillar type I collagen undergo apoptosis while those grown on non-fibrillar collagen do not. Ichii et al. [26] reported that smooth muscle cells on fibrillar type I collagen show suppressed proliferation and migration compared to cells on a monomeric non-fibrillar collagen. Hansen et al. [19] showed that fibrillar collagen type I gels induce a highly differentiated phenotype of growth arrested primary hepatocytes. In contrast, a film of non-fibrillar monomeric collagen promotes dedifferentiation and cell division. Therefore a variety of cell types show different responses when grown on various forms of type I collagen. In our experiments, non-fibrillar type I collagen had no effect on apoB secretion. However, growth on fibrillar type I collagen significantly increased apoB secretion without altering the TER of the Caco-2 cell monolayers.
Cells grown on HACT show significant lipid droplets below the nuclei, therefore the increased secretion of apoB to the basal chamber may be due to better targeting of the apoB to the basolateral membrane. However, the overall increased secretion could also result from either increased synthesis due to enhanced transcription or due to decreased posttranslational degradation. For example, the increase in apoB secretion following addition of oleic acid plus taurocholate [34] to Caco-2 cultures is not due to increased synthesis, but due to reduced degradation [11,13,46], while the increased apoB secretion following stimulation with transforming growth factor β has been shown to result from increased transcription of the apoB gene [41,50]. Further experiments are required to determine if reduced degradation and/or increased transcription is responsible for the increased apoB secretion due to growth of Caco-2 cells on HACT.
It is known that the MAP kinase pathway is important in regulating tight junctions in epithelia. PD98059 has been shown to cause a decrease in the TER of MDCK cells [29] while it has caused an increase in the TER in human corneal epithelial cells [59] and in a Raf-1 transfected mouse hepatic cell line [30]. In our studies, PD98059 caused a significant increase in TER in Caco-2 cells grown on Transwell® filters but not when grown on human amnion connective tissue. In contrast, PD98059 reduced the secretion of apoB when Caco-2 cells were grown on human amnion connective tissue. This indicates that the MAP kinase pathway regulates multiple functions within these cells, and its effects appear to be influenced by the substrate these cells are grown on.
The HACT substrate model for Caco-2 cells described here promotes a more normal architecture of intestinal epithelia, shows a targeting of lipid droplets toward the basolateral membrane, and specifically promotes greater secretion of apoB than comparable cultures grown on Transwell® filters. We show that β1 integrin/collagen type I interactions augment apoB secretion. This model may be an effective tool for further understanding the modulation of apoB production pathways.
Abbreviations
- HACT
human amnion connective tissue
- MTP
microsomal triglyceride transfer protein
- apoAI
apolipoprotein AI
- apoB
apolipoprotein B
- CMF-PBS
calcium and magnesium free phosphate buffered saline
- FBS
fetal bovine serum
- ELISA
enzyme linked immunoassay
- TER
transepithelial electrical resistance
- DMEM
Dulbecco’s modified Eagle’s medium
References
- 1.Anderson JM, Van Itallie CM, Peterson MD, Stevenson BR, Carew EA, Mooseker MS. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J Cell Biol. 1989;109:1047. doi: 10.1083/jcb.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004;45:764. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Bakillah A, Zhou Z, Luchoomun J, Hussain MM. Measurement of apolipoprotein B in various cell lines: correlation between intracellular levels and rates of secretion. Lipids. 1997;32:1113. doi: 10.1007/s11745-997-0143-8. [DOI] [PubMed] [Google Scholar]
- 4.Basson MD. Invited research review: cell–matrix interactions in the gut epithelium. Surgery. 2003;133:263. doi: 10.1067/msy.2003.24. [DOI] [PubMed] [Google Scholar]
- 5.Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225:301. doi: 10.1006/excr.1996.0180. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JF. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- 7.Casaschi A, Wang Q, Dang K, Richards A, Theriault A. Intestinal apolipoprotein B secretion is inhibited by the flavonoid quercetin: potential role of microsomal triglyceride transfer protein and diacylglycerol acyltransferase. Lipids. 2002;37:647. doi: 10.1007/s11745-002-0945-8. [DOI] [PubMed] [Google Scholar]
- 8.Chateau D, Pauquai T, Delers F, Rousset M, Chambaz J, Demignot S. Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J Cell Physiol. 2005;202:767. doi: 10.1002/jcp.20173. [DOI] [PubMed] [Google Scholar]
- 9.Conyers G, Milks L, Conklyn M, Showell H, Cramer E. A factor in serum lowers resistance and opens tight junctions of MDCK cells. Am J Physiol. 1990;259:C577–C585. doi: 10.1152/ajpcell.1990.259.4.C577. [DOI] [PubMed] [Google Scholar]
- 10.Cooper LJ, Kinoshita S, German M, Koizumi N, Nakamura T, Fullwood NJ. An investigation into the composition of amniotic membrane used for ocular surface reconstruction. Cornea. 2005;24:722. doi: 10.1097/01.ico.0000154237.49112.29. [DOI] [PubMed] [Google Scholar]
- 11.Dashti N, Smith EA, Alaupovic P. Increased production of apolipoprotein B and its lipoproteins by oleic acid in Caco-2 cells. J Lipid Res. 1990;31:113. [PubMed] [Google Scholar]
- 12.Field FJ, Albright E, Mathur SN. Regulation of triglyceride-rich lipoprotein secretion by fatty acids in Caco-2 cells. J Lipid Res. 1988;29:1427. [PubMed] [Google Scholar]
- 13.Field FJ, Born E, Chen HS, Murthy S, Mathur SN. Lysophosphatidylcholine increases the secretion of cholesteryl ester-poor triacylglycerol-rich lipoproteins by CaCo-2 cells. Biochem J. 1994;304:35. doi: 10.1042/bj3040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field FJ, Born E, Chen HS, Murthy S, Mathur SN. Regulation of apolipoprotein B secretion by biliary lipids in CaCo-2 cells. J Lipid Res. 1994;35:749. [PubMed] [Google Scholar]
- 15.Field FJ, Chen HS, Born E, Dixon B, Mathur S. Release of ceramide after membrane sphingomyelin hydrolysis decreases the basolateral secretion of triacylglycerol and apolipoprotein-B in cultured human intestinal cells. J Clin Invest. 1993;92:2609. doi: 10.1172/JCI116876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisaki H, Hattori S. Keratinocyte apoptosis on type I collagen gel caused by lack of laminin 5/10/11 deposition and Akt signaling. Exp Cell Res. 2002;280:255. doi: 10.1006/excr.2002.5639. [DOI] [PubMed] [Google Scholar]
- 17.Furie MB, Cramer EB, Naprstek BL, Silverstein SC. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984;98:1033. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 19.Hansen LK, Wilhelm J, Fassett JT. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr Top Dev Biol. 2006;72:205. doi: 10.1016/S0070-2153(05)72004-4. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Gohda M, Matzno S, Kubo Y, Kido H, Yamauchi T, Nakamura N. Possible mechanism of action of AE0047, a calcium antagonist, on triglyceride metabolism. J Pharmacol Exp Ther. 1997;282:882. [PubMed] [Google Scholar]
- 21.Honore S, Pichard V, Penel C, Rigot V, Prev tC, Marvaldi J, Briand C, Rognoni JB. Outside-in regulation of integrin clustering processes by ECM components per se and their involvement in actin cytoskeleton organization in a colon adenocarcinoma cell line. Histochem Cell Biol. 2000;114:323. doi: 10.1007/s004180000189. [DOI] [PubMed] [Google Scholar]
- 22.Hughes TE, Sasak WV, Ordovas JM, Forte TM, Lamon-Fava S, Schaefer EJ. A novel cell line (Caco-2) for the study of intestinal lipoprotein synthesis. J Biol Chem. 1987;262:3762. [PubMed] [Google Scholar]
- 23.Hussain MM, Glick JM, Rothblat GH. In vitro model systems: cell cultures used in lipid and lipoprotein research. Curr Opin Lipidol. 1992;3:173. [Google Scholar]
- 24.Hussain MM, Kancha RK, Zhou Z, Luchoomun J, Zu H, Bakillah A. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim Biophys Acta. 1996;1300:151. doi: 10.1016/0005-2760(96)00041-0. [DOI] [PubMed] [Google Scholar]
- 25.Hussain MM, Zhao Y, Kancha RK, Blackhart BD, Yao Z. Characterization of recombinant human apoB-48-containing lipoproteins in rat hepatoma McA-RH7777 cells transfected with apoB48 cDNA: overexpression of apoB-48 decreases synthesis of endogenous apoB-100. Arterioscler Thromb Vasc Biol. 1995;15:485. doi: 10.1161/01.atv.15.4.485. [DOI] [PubMed] [Google Scholar]
- 26.Ichii T, Koyama H, Tanaka S, Kim S, Shioi A, Okuno Y, Raines EW, Iwao H, Otani S, Nishizawa Y. Fibrillar collagen specifically regulates human vascular smooth muscle cell genes involved in cellular responses and the pericellular matrix environment. Circ Res. 2001;88:460. doi: 10.1161/01.res.88.5.460. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal J, Anwar K, Hussain MM. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J Biol Chem. 2003;278:31610. doi: 10.1074/jbc.M301177200. [DOI] [PubMed] [Google Scholar]
- 28.Jiang F, Horber H, Howard J, Muller DJ. Assembly of collagen into microribbons: effects of pH and electrolytes. J Struct Biol. 2004;148:268. doi: 10.1016/j.jsb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Kiely B, Feldman G, Ryan MP. Modulation of renal epithelial barrier function by mitogen-activated protein kinases (MAPKs): mechanism of cyclosporine A-induced increase in transepithelial resistance. Kidney Int. 2003;63:908. doi: 10.1046/j.1523-1755.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 30.Lan M, Kojima T, Osanai M, Chiba H, Sawada N. Oncogenic Raf-1 regulates epithelial to mesenchymal transition via distinct signal transduction pathways in an immortalized mouse hepatic cell line. Carcinogenesis. 2004;25:2385. doi: 10.1093/carcin/bgh248. [DOI] [PubMed] [Google Scholar]
- 31.Levy E, Mehran M, Seidman E. Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB J. 1995;9:626. doi: 10.1096/fasebj.9.8.7768354. [DOI] [PubMed] [Google Scholar]
- 32.Liotta LA, Lee CW, Morakis DJ. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980;11:141. doi: 10.1016/0304-3835(80)90105-6. [DOI] [PubMed] [Google Scholar]
- 33.Lorentz O, Duluc I, Arcangelis AD, Simon-Assmann P, Kedinger M, Freund JN. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol. 1997;139:1553. doi: 10.1083/jcb.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells: nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem. 1999;274:19565. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- 35.Marcil V, Delvin E, Garofalo C, Levy E. Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. J Nutr. 2003;133:2180. doi: 10.1093/jn/133.7.2180. [DOI] [PubMed] [Google Scholar]
- 36.Marmorstein AD, Mortell KH, Ratcliffe DR, Cramer EB. Epithelial permeability factor: a serum protein that condenses actin and opens tight junctions. Am J Physiol. 1992;262:C1403–C1410. doi: 10.1152/ajpcell.1992.262.6.C1403. [DOI] [PubMed] [Google Scholar]
- 37.Mathur SN, Born E, Bishop WP, Field FJ. Effect of okadaic acid on apo B and apo A-I secretion by CaCo-2 cells. Biochim Biophys Acta. 1993;1168:130. doi: 10.1016/0005-2760(93)90117-r. [DOI] [PubMed] [Google Scholar]
- 38.Mortell KH, Marmorstein AD, Cramer EB. Fetal bovine serum and other sera used in tissue culture increase epithelial permeability. In Vitro Cell Dev Biol. 1993;29A:235. doi: 10.1007/BF02634190. [DOI] [PubMed] [Google Scholar]
- 39.Murthy S, Mathur SN, Bishop WP, Field FJ. Inhibition of apolipoprotein B secretion by IL-6 is mediated by EGF or an EGF-like molecule in CaCo-2 cells. J Lipid Res. 1997;38:206. [PubMed] [Google Scholar]
- 40.Murthy S, Mathur SN, Field FJ. Tumor necrosis factor-alpha and interleukin-1beta inhibit apolipoprotein B secretion in CaCo-2 cells via the epidermal growth factor receptor signaling pathway. J Biol Chem. 2000;275:9222. doi: 10.1074/jbc.275.13.9222. [DOI] [PubMed] [Google Scholar]
- 41.Murthy S, Mathur SN, Varilek G, Bishop W, Field FJ. Cytokines regulate apolipoprotein B secretion by Caco-2 cells: differential effects of IL-6 and TGF-β1. Am J Physiol Gastrointest Liver Physiol. 1996;270:G94–G102. doi: 10.1152/ajpgi.1996.270.1.G94. [DOI] [PubMed] [Google Scholar]
- 42.Pal S, Allister E, Thomson A, Mamo JC. Cholesterol esters regulate apoB(48) secretion in CaCo(2) cells. Atherosclerosis. 2002;161:55. doi: 10.1016/s0021-9150(01)00630-x. [DOI] [PubMed] [Google Scholar]
- 43.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 44.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J, Cell Sci. 1992;102(Pt 3):581. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 45.Pons V, Peres C, Teulie JM, Nauze M, Mus M, Rolland C, Collet X, Perret B, Gassama-Diagne A, Hullin-Matsuda F. Enterophilin-1 interacts with focal adhesion kinase and decreases beta1 integrins in intestinal Caco-2 cells. J Biol Chem. 2004;279:9270. doi: 10.1074/jbc.M309764200. [DOI] [PubMed] [Google Scholar]
- 46.Ranheim T, Gedde-Dahl A, Rustan AC, Drevon CA. Effect of chronic incubation of CaCo-2 cells with eicosapentaenoic acid (20:5, n−3) and oleic acid (18:1, n−9) on triacylglycerol production. Biochem J. 1994;303:155. doi: 10.1042/bj3030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005;46:1779. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Reisher SR, Hughes TE, Ordovas JM, Schaefer EJ, Feinstein SI. Increased expression of apolipoprotein genes accompanies differentiation in the intestinal cell line Caco-2. Proc Natl Acad Sci U S A. 1993;90:5757. doi: 10.1073/pnas.90.12.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via alpha1beta1- and alpha2beta1-integrin-dependent Src kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G547–G557. doi: 10.1152/ajpgi.00262.2003. [DOI] [PubMed] [Google Scholar]
- 50.Singh K, Batuman OA, Akman HO, Kedees MH, Vakil V, Hussain MM. Differential, tissue-specific, transcriptional regulation of apolipoprotein B secretion by transforming growth factor beta. J Biol Chem. 2002;277:39515. doi: 10.1074/jbc.M205513200. [DOI] [PubMed] [Google Scholar]
- 51.Turck N, Gross I, Gendry P, Stutzmann J, Freund JN, Kedinger M, Simon-Assmann P, Launay JF. Laminin isoforms: biological roles and effects on the intracellular distribution of nuclear proteins in intestinal epithelial cells. Exp Cell Res. 2005;303:494. doi: 10.1016/j.yexcr.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Vachon PH, Perreault N, Magny P, Beaulieu JF. Uncoordinated, transient mosaic patterns of intestinal hydrolase expression in differentiating human enterocytes. J Cell Physiol. 1996;166:198. doi: 10.1002/(SICI)1097-4652(199601)166:1<198::AID-JCP21>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 53.Van Greevenbroek MMJ, Erkelens DW, De Bruin TWA. Caco-2 cells secrete two independent classes of lipoproteins with distinct density: effect of the ratio of unsaturated to saturated fatty acid. Atherosclerosis. 2000;149:25. doi: 10.1016/s0021-9150(99)00289-0. [DOI] [PubMed] [Google Scholar]
- 54.Van Greevenbroek MMJ, Robertus-Teunissen MG, Erkelens DW, De Bruin TWA. Participation of the microsomal triglyceride transfer protein in lipoprotein assembly in Caco-2 cells: interaction with saturated and unsaturated dietary fatty acids. J Lipid Res. 1998;39:173. [PubMed] [Google Scholar]
- 55.Van Greevenbroek MMJ, vanMeer G, Erkelens DW, De Bruin TWA. Effects of saturated, mono-, and polyunsaturated fatty acids on the secretion of apo B containing lipoproteins by Caco-2 cells. Atherosclerosis. 1996;121:139. doi: 10.1016/0021-9150(95)05712-9. [DOI] [PubMed] [Google Scholar]
- 56.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241. [PubMed] [Google Scholar]
- 57.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev. 2003;55:1631. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Wang DR, Sato M, Li LN, Miura M, Kojima N, Senoo H. Stimulation of pro-MMP-2 production and activation by native form of extracellular type I collagen in cultured hepatic stellate cells. Cell Struct Funct. 2003;28:505. doi: 10.1247/csf.28.505. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Zhang J, Yi XJ, Yu FS. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption inhuman corneal epithelial cells. Exp Eye Res. 2004;78:125. doi: 10.1016/j.exer.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 60.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36:1405. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Cromwell JW, Kunjummen BD, Yee D, Garcia-Aguilar J. The alpha2 and alpha3 integrins are required for morphologic differentiation of an intestinal epithelial cell line. Surgery. 2003;133:429. doi: 10.1067/msy.2003.107. [DOI] [PubMed] [Google Scholar]