Significance
Although understanding the precise nature of oxytocin’s influence on complex human social behavior has proven difficult, increasing evidence points to an anxiolytic effect. We use an imaging epigenetic approach to further parse oxytocin’s effects by examining a biological marker within the oxytocin system, DNA methylation of the oxytocin receptor gene (OXTR). Importantly, this epigenetic modification directly impacts the expression of oxytocin’s receptor, which is critical for the actions of oxytocin to have an effect. We find that higher levels of OXTR methylation are associated with increased neural response and decreased functional coupling within regions supporting social perception and emotion processing. This pattern of activity may be indicative of diminished emotion regulation to negative stimuli and increased risk of pathology.
Keywords: oxytocin receptor, OXTR, epigenetics, fMRI, social perception
Abstract
In humans, the neuropeptide oxytocin plays a critical role in social and emotional behavior. The actions of this molecule are dependent on a protein that acts as its receptor, which is encoded by the oxytocin receptor gene (OXTR). DNA methylation of OXTR, an epigenetic modification, directly influences gene transcription and is variable in humans. However, the impact of this variability on specific social behaviors is unknown. We hypothesized that variability in OXTR methylation impacts social perceptual processes often linked with oxytocin, such as perception of facial emotions. Using an imaging epigenetic approach, we established a relationship between OXTR methylation and neural activity in response to emotional face processing. Specifically, high levels of OXTR methylation were associated with greater amounts of activity in regions associated with face and emotion processing including amygdala, fusiform, and insula. Importantly, we found that these higher levels of OXTR methylation were also associated with decreased functional coupling of amygdala with regions involved in affect appraisal and emotion regulation. These data indicate that the human endogenous oxytocin system is involved in attenuation of the fear response, corroborating research implicating intranasal oxytocin in the same processes. Our findings highlight the importance of including epigenetic mechanisms in the description of the endogenous oxytocin system and further support a central role for oxytocin in social cognition. This approach linking epigenetic variability with neural endophenotypes may broadly explain individual differences in phenotype including susceptibility or resilience to disease.
Understanding the biological influences that shape normal and pathological social behavior is of significant interest to the social and medical sciences. Although numerous biological pathways have been implicated in animal systems, translating this work to complex social behavior in humans has proven difficult. Limitations exist both with phenotypic descriptions, where behavior is often broadly assessed through self-reports or disease diagnosis, as well as at the biological level, where research is restricted to biomarkers accessible with noninvasive methods. To make further progress in these efforts, we must consider how we measure individual variability and explore novel biological mechanisms, including epigenetic modifications, that can be assessed from peripheral tissue.
One particularly relevant biomarker for social behavior is oxytocin. A peripheral hormone and central neuromodulator, oxytocin influences a variety of social and affective processes including affiliative behaviors (1), care-giving (2), and stress reactivity (3). Intranasally administered, oxytocin has also been implicated in specialized components of social cognition, such as trust (4), envy (5), and mentalizing (6).
However, in addition to its role as a neuromodulator influencing social behavior, oxytocin acts on many peripheral organs with gastrointestinal, reproductive, and cardiovascular effects, among others (7). Phylogenetically, it is improbable that a biochemical with such wide-ranging targets evolved to have a highly focused effect on specialized processes, like trust or envy. Rather, oxytocin likely has a more general effect on basic biological systems that ultimately support these complex social–cognitive constructs. One way oxytocin may influence behavior is through anxiety reduction; intranasal oxytocin has been shown to have anxiolytic effects on brain systems supporting affective responses to negatively arousing stimuli (8–11). These findings support oxytocin’s role in anxiety reduction and make it an attractive candidate in neurobiological models of psychiatric disorders.
Within the endogenous oxytocin system, plasma oxytocin levels are highly variable across individuals and are often hypothesized to be a biomarker of disease or disorder (12–17). However, establishing direct links between oxytocin levels and specific social abilities has proven difficult, and the majority of studies have mixed results (18, 19). These inconsistences may occur because the action of oxytocin is dependent upon the expression of its receptor, which is encoded by the oxytocin receptor gene (OXTR, hg38_chr3, 8750409–8769614) (20). Therefore, variability in OXTR expression likely plays a major role in the function of the endogenous oxytocin system. One active area of research attempts to associate OXTR genetic variation with social phenotypes including social and affective disorders, such as autism spectrum disorders (ASDs), anxiety, and depression (21–24), and individual differences in complex social behaviors, including dispositional differences in empathy, stress reactivity, and positive affect (25–28). These studies have primarily focused on identifying common genetic variants such as single nucleotide polymorphisms (SNPs) associated with these phenotypic differences. However, the functional impact of these SNPs on OXTR is unknown, making it difficult to build a meaningful mechanistic model of how oxytocin impacts biological systems and how these systems lead to various social behaviors.
Imaging genetics studies allow for more precise, albeit limited, associations between genetic variants and neural endophenotypes. OXTR SNPs have been associated with structural differences in oxytocinergic brain regions including amygdala and hypothalamus (29–31), as well as functional differences during social perceptual tasks including emotional face processing (30, 32). Similar to the results from intranasal oxytocin studies, OXTR genetic variants have been found to influence the perception of negative emotion, further suggesting an anxiolytic effect for oxytocin (30, 32, 33). However, this approach associating allelic variation with individual phenotypic variability has led to somewhat inconsistent findings (23, 32, 34, 35). For example, whereas one imaging study found an association between OXTR SNP rs53576 and amygdala activity evoked during emotion perception (30), a second study failed to replicate this finding in a much larger sample (32). It is possible that phenotypic variability related to the endogenous oxytocin system is concealed by the variability that exists within groups when dichotomizing by allele. This phenotypic variability may reflect the influence of other genetic variants or perhaps be due to moderating environmental variables. For example, Loth et al. (32) found that carriers of the same allele show different behavioral outcomes under different environmental contexts. A potential mechanism driving this differential susceptibility may be epigenetic factors that impact the expression of the gene without changing the underlying genetic structure.
Methylation of 5′-Cytosine-phosphate-Guanine-3′ (CpG) dinucleotide pairs in DNA is a highly investigated epigenetic modification that may influence behavioral phenotypes. DNA methylation within the promoter region of OXTR is variable within the population (36, 37), and methylation of specific OXTR CpG sites reduces transcription of the gene (38). High levels of OXTR methylation at these same sites have been associated with autism (36), callous unemotional traits (39), and anorexia nervosa (40), suggesting the utility of OXTR methylation as a biomarker of phenotypic variability. Furthermore, the use of peripheral tissue derived from blood in these studies lends validity to this noninvasive biological marker.
Here, we investigate the impact of OXTR methylation on individual variability in neural response during a core component of social perception, emotional face processing. Specifically, we hypothesized that OXTR methylation would be associated with neural activity within systems responsible for responding to negatively arousing stimuli and previously linked to pathophysiology of social dysfunction. To test this hypothesis, we recruited 98 participants to provide a blood sample for epigenotyping and complete an emotional face-matching task while undergoing functional magnetic resonance imaging (fMRI). Methylation level was assessed for our candidate CpG site (–934) within the promoter of OXTR. First, we assessed the relationship between OXTR methylation and blood oxygenation level-dependent (BOLD) response to emotional faces within amygdala. Second, we performed an exploratory analysis to pinpoint an association between OXTR methylation and activity within additional brain regions that support social perception and emotion processing. Third, we performed task-specific amygdala functional connectivity analyses to identify how OXTR methylation mediates coordination within a network of brain regions in response to emotional faces.
Results
Ninety-eight healthy Caucasian adults (42 males) aged 18–30 y were recruited for blood collection and fMRI. OXTR methylation was assessed at CpG site –934 (36), a functional CpG site previously shown to impact gene expression (36, 38). In the present sample, OXTR methylation level at this site ranged from 33% to 72% methylated (M = 48.97, SD = 7.00). There was a significant effect of sex such that females (M = 50.62, SD = 7.01) have a higher level of methylation at site –934 than males (M = 46.77, SD = 6.43), t(96) = 2.79, P = 0.0063. Age was not a significant predictor of methylation level (Beta = –0.008, P = 0.97, R2 = 0.000013).
Participants completed an emotional face-matching fMRI task previously shown to elicit a neural response within areas critical for social perception (41). Our primary analysis strategy focused on both a priori regions of interest (ROIs) based on prior literature as well as exploratory analyses that considered differences in both regional activation and functional connectivity. All analyses used OXTR methylation as a regressor to predict individual variability in the processing of emotional face expressions and were corrected for multiple comparisons using the false discovery rate (FDR) q < 0.05 voxel significance level and spatial extent threshold (k) ≥ 10 contiguous voxels.
OXTR Methylation Impacts Amygdala Response to Angry and Fearful Faces.
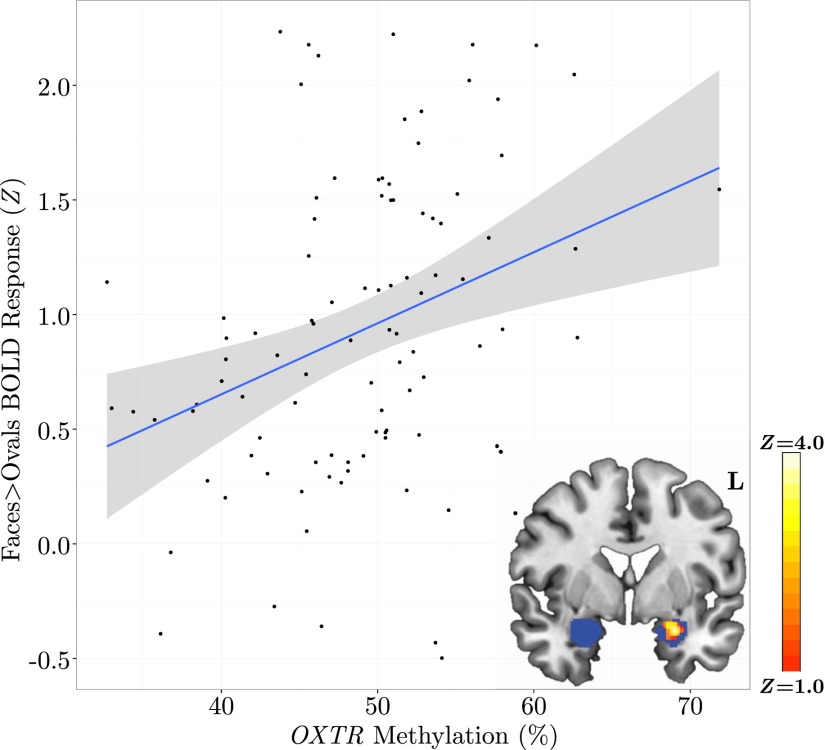
We conducted a primary ROI analysis to assess the effect of OXTR methylation on activity within amygdala—a region activated by this task (41) and previously associated with genetic variability in OXTR (30). This ROI analysis revealed a significant main effect of OXTR methylation on task-specific activity such that increased methylation levels predicted increased BOLD activity in left amygdala (Fig. 1). Peak activity (Z = 3.64) for this cluster of voxels (k = 103) occurred at x = –30, y = 2, z = –20. No voxels within right amygdala showed a significant main effect of OXTR methylation. Analyses testing for a sex difference in amygdala activation and a Methylation × Sex interaction were nonsignificant.
Fig. 1.
Individuals with increased methylation of OXTR display elevated amygdala response to angry and fearful faces. Mean Z statistic values are plotted against percent OXTR methylation for each participant (n = 98). Gray shading indicates 95% confidence interval around the best-fit line. (Inset) Z statistic map of voxels shows significant main effect of OXTR methylation depicted in MNI space (y = 0), FDR corrected at q < 0.05. ROI is depicted in blue.
Exploratory ROI Analysis Indicates OXTR Methylation Influences Areas Important for Face Perception and Emotion Processing.
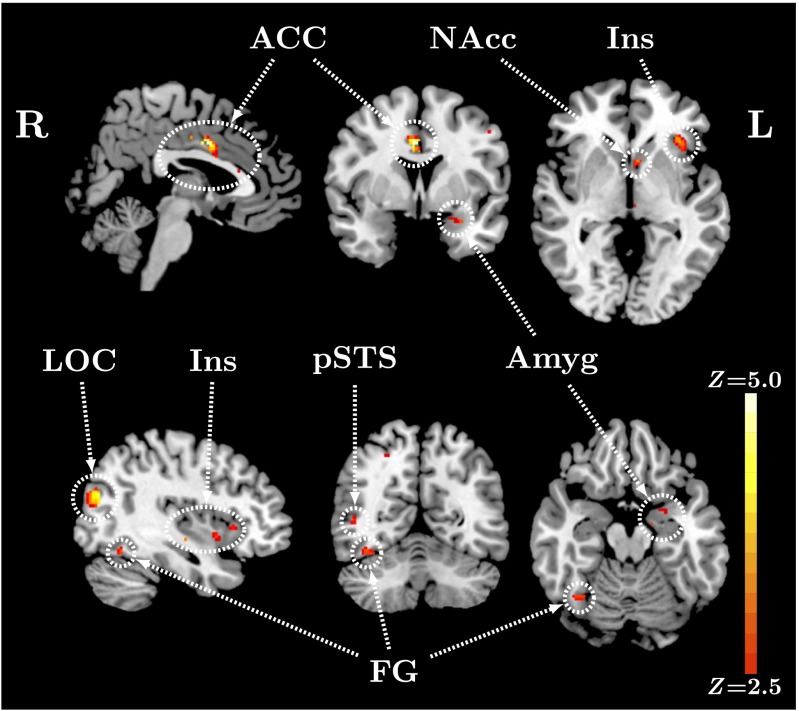
We then performed an exploratory functional ROI analysis targeting regions involved in face perception and emotion processing as defined by a meta-analytic approach using Neurosynth.org feature set keywords “face” and “emotion” (depicted in Fig. S1). This functional ROI analysis revealed a significant positive main effect of OXTR methylation on task-specific activity in multiple brain regions (Fig. 2), including areas associated with emotion regulation, such as amygdala, insular cortex, and dorsal anterior cingulate cortex (dACC), as well as regions associated with social perception, such as fusiform gyrus and posterior superior temporal sulcus. See Table 1 for local maxima statistics for this analysis. The pattern of activation in all areas identified was such that increased levels of methylation predicted increased BOLD activity (Fig. S2). We also conducted an exploratory whole-brain analysis to identify potential relationships between OXTR methylation and brain activity in regions not included in our a priori hypotheses. Results for this analysis (Table S1 and Figs. S3 and S4) revealed similar regions with the same relationship between OXTR methylation and brain activity. Analyses testing for a sex difference in activation and a Methylation × Sex interaction were nonsignificant.
Fig. 2.
Methylation of OXTR predicts activity in brain regions previously associated with emotion and face perception. Z statistic map of voxels shows a significant positive main effect of OXTR methylation on BOLD activity for faces > ovals contrast within ROI, FDR corrected at q < 0.05. Images are depicted in MNI space (Top, x = 2, y = 3, z = –2; Bottom, x = 38, y = –59, z = –18). ACC, anterior cingulate cortex; Amyg, amygdala; FG, fusiform gyrus; Ins, insular cortex; LOC, lateral occipital cortex; NAcc, nucleus accumbens; pSTS, posterior superior temporal sulcus.
Table 1.
ROI analysis local maxima statistics
| Anatomical region | Hem | x | y | z | Z | k |
| Amygdala | L | −30 | 4 | −20 | 4.03 | 12 |
| Anterior cingulate gyrus | L | −4 | −10 | 40 | 4.34 | 32 |
| Anterior cingulate gyrus | R | 4 | 2 | 36 | 4.75 | 78 |
| Fusiform gyrus | R | 38 | −60 | −16 | 3.63 | 16 |
| Insular cortex | L | −38 | 22 | −4 | 4.09 | 48 |
| Insular cortex | R | 32 | 16 | 6 | 4.15 | 38 |
| Insular cortex | R | 36 | 8 | −4 | 3.99 | 15 |
| Lateral occipital cortex | L | −28 | −84 | 24 | 4.09 | 10 |
| Lateral occipital cortex | R | 34 | −78 | 26 | 4.86 | 196 |
| Middle temporal gyrus | R | 46 | −58 | 4 | 3.6 | 12 |
| Superior parietal gyrus | R | 26 | −54 | 54 | 3.7 | 18 |
| Supramarginal gyrus | L | −62 | −34 | 36 | 4.2 | 24 |
Significant cluster threshold: FDR (q) < 0.05, k ≥ 10 voxels. Hem, hemisphere; L, left; R, right; x, y, z, coordinates of local maxima in MNI space; Z, maximum Z statistic.
OXTR Methylation Moderates Connectivity in Regions Associated with Affect Appraisal and Emotion Regulation.
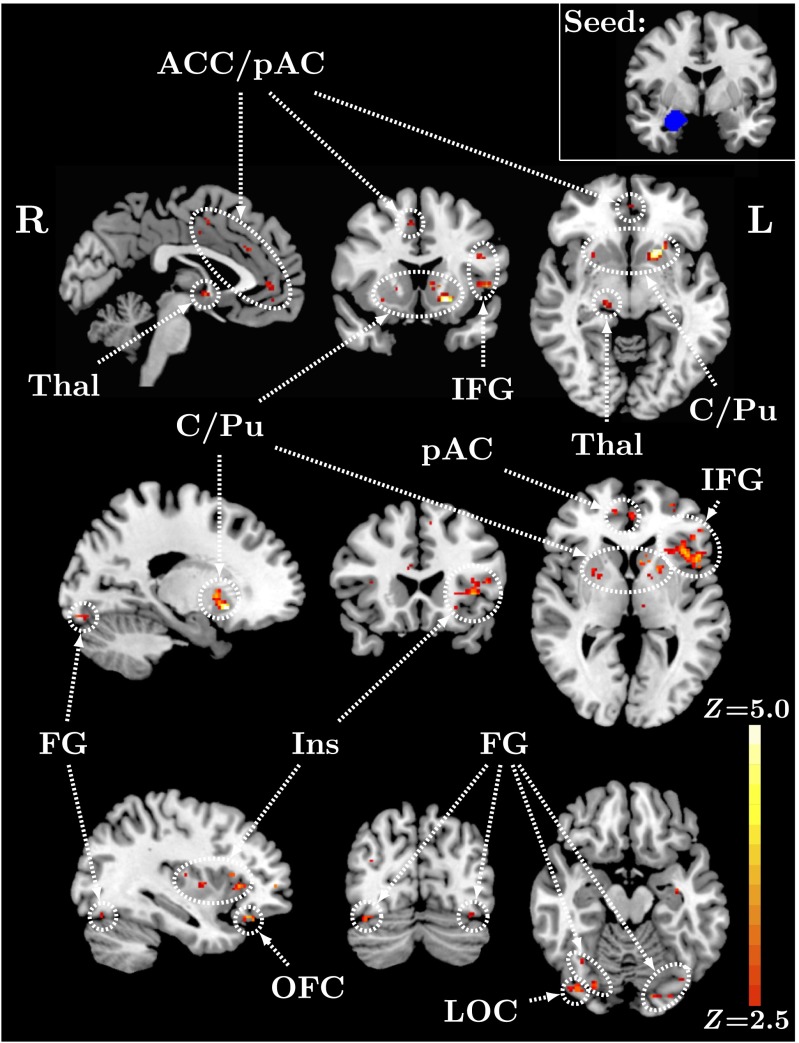
We conducted a psychophysiological interaction (PPI) analysis (42) to examine the effect of OXTR methylation on task-specific amygdala functional connectivity. This analysis revealed a significant negative main effect of OXTR methylation on right amygdala functional connectivity with multiple brain regions within the functional ROI (Fig. 3), including areas associated with emotion regulation, such as insular cortex, cingulate cortex, and orbitofrontal cortex, as well as regions associated with face perception, such as fusiform gyrus. The pattern of activation in all areas identified was such that higher levels of methylation predicted decreased levels of functional connectivity (Fig. S5). See Table 2 for local maxima statistics for the PPI analysis. Results for an unmasked, whole-brain PPI analysis (Table S2 and Figs. S6 and S7) identified similar regions with the same pattern of activation. Analyses using a left amygdala seed region revealed no significant voxels.
Fig. 3.
OXTR methylation predicts amygdala connectivity within brain regions important for emotion regulation and face perception. Z statistic map of voxels shows a significant negative main effect of OXTR methylation on right amygdala functional connectivity for faces > ovals contrast within ROI, FDR corrected at q < 0.05. Images are depicted in MNI space (Top, x = –3, y = 14, z = –7; Middle, x = –20, y = 22, z = 2; Bottom, x = –36, y = –74, z = –16). (Inset) Right amygdala seed region (y = –4). ACC, anterior cingulate cortex; C/Pu, caudate/putamen; FG, fusiform gyrus; IFG, inferior frontal gyrus; Ins, insular cortex; LOC, lateral occipital cortex; OFC, orbitofrontal cortex; pAC, paracingulate gyrus; Thal, thalamus.
Table 2.
PPI analysis local maxima statistics
| Anatomical region | Hem | x | y | z | Z | k |
| Anterior cingulate gyrus | L | −6 | 42 | 6 | 3.75 | 26 |
| Anterior cingulate gyrus | R | 6 | 30 | 20 | 3.41 | 18 |
| Caudate | L | −8 | 14 | 2 | 3.97 | 16 |
| Frontal pole | L | −34 | 48 | 4 | 4.35 | 13 |
| Fusiform gyrus | L | −18 | −90 | −14 | 4.05 | 23 |
| Inferior frontal gyrus | L | −50 | 10 | 4 | 4.49 | 142 |
| Inferior frontal gyrus | L | −44 | 16 | 22 | 4.42 | 22 |
| Inferior frontal gyrus | L | −46 | 32 | 0 | 3.98 | 19 |
| Insular cortex | L | −36 | −2 | 6 | 3.61 | 12 |
| Lateral occipital cortex | R | 38 | −76 | −18 | 4.02 | 27 |
| Orbitofrontal cortex | L | −36 | 30 | −18 | 4.13 | 19 |
| Paracingulate gyrus | R | 6 | 12 | 46 | 3.68 | 17 |
| Precentral gyrus | L | −48 | 0 | 28 | 3.83 | 13 |
| Putamen | L | −18 | 14 | −8 | 5.25 | 93 |
| Thalamus | L | −12 | −16 | 4 | 3.56 | 10 |
| Thalamus | R | 16 | −22 | −6 | 3.94 | 13 |
Significant cluster threshold: FDR (q) < 0.05, k ≥ 10 voxels. Hem, hemisphere; L, left; R, right; x, y, z, coordinates of local maxima in MNI space; Z, maximum Z statistic.
Discussion
Using an imaging epigenetic approach, we provide evidence that a functional epigenetic modification within OXTR predicts individual variability in neural response to negative social stimuli. Specifically, we found that higher levels of OXTR methylation were associated with increased activity in amygdala and regions of the extended face perception system when viewing negative facial expressions. Furthermore, connectivity between neural systems supporting social perception was attenuated by OXTR methylation. These results provide compelling evidence to suggest that social perceptual and emotional processes thought to involve the endogenous oxytocin system may be governed by epigenetic processes and that this relationship may be predicted from tissue acquired through noninvasive means.
Prior research has proposed two roles for oxytocin in human social perception: Oxytocin may promote social and affiliative behaviors through (i) reduced threat reactivity and (ii) increased sensitivity to social salience. Our current results are congruent with previous intranasal studies that support anxiolytic properties of oxytocin. These studies indicate that increased availability of oxytocin through intranasal administration is associated with a decrease in amygdala activation to negatively valenced stimuli (6, 8, 9, 43–45; but see ref. 46 for conflicting results in women). Methylation of OXTR directly impacts gene expression such that increased levels of methylation are associated with decreased gene transcription (36, 38). Therefore, lower levels of methylation may indicate increased ability to use available oxytocin due to increased receptor expression. As expected by this model, individuals in the current study with lower levels of methylation showed decreased amygdala activation when viewing angry and fearful face stimuli. Amygdala activity was specific to left hemisphere, as commonly found for emotional stimuli (see ref. 47 for review).
Results from intranasal literature also indicate a valence-specific tempering of amygdala activation. For example, Gamer et al. (43) found that after oxytocin administration, amygdala response decreased in response to angry faces but increased when viewing happy faces. We therefore hypothesize that attenuated amygdala response may indicate enhanced ability to regulate affective response to negative stimuli among individuals with lower OXTR methylation. The results from our functional ROI and PPI analyses support this prediction. Across multiple brain regions crucial for processing social and emotional stimuli including fusiform, lateral occipital cortex, insular cortex, and dACC, individuals with lower methylation levels show an attenuated BOLD response and simultaneously increased amygdala coupling.
Further supporting the hypothesized relationship between OXTR methylation and emotion regulation ability is our reported increased coupling between amygdala and left insula for those low in OXTR methylation. Previous research indicates amygdala–insula connectivity increases with repeated viewing of negative stimuli, leading to successful desensitization and habituation (48). Additionally, our reported increased connectivity between amygdala and dACC could reflect more efficient affective appraisal for those low in OXTR methylation. Several other studies have also proposed a strong link between the endogenous oxytocin system and dACC structure and function (44, 45, 49). In fact, dysfunction in this area is often implicated in developmental and psychiatric disorders (50–52). Finally, additional regions not functionally activated by this task arise in the connectivity analysis including thalamus, caudate, putamen, and precentral gyrus. Lesion and imaging studies suggest that right amygdala specifically may be involved in early processing of and potential to react to threatening stimuli (53–55). Although functional lateralization effects in amygdala are well documented (47), potential hemispheric specificity in amygdala connectivity is less understood and should be further explored in the future.
Taken together, our results indicate that the actions of OXTR methylation may be twofold. First, individuals lower in methylation and who theoretically have greater access to endogenous oxytocin show attenuated response to negative stimuli. Second, these individuals also display greater connectivity between amygdala and structures supporting affective appraisal of and ability to respond to the stimulus, potentially leading to better habituation processes and less likelihood of disordered social perception.
There are two important limitations to the current study. Methylation is highly tissue specific for some CpG sites (56–59), and when studying healthy humans, we are limited to assessments from peripheral tissue rather than the causal tissue for the social phenotype, the brain. However, recent studies have demonstrated that, for some CpG sites, methylation may be correlated between tissues and stable across the lifespan (60–63). For the CpG site tested here, there is evidence for the maintenance of methylation levels between brain and blood (36), suggesting our peripheral marker is likely to correlate with methylation levels in the brain. Furthermore, other studies have also found this CpG site to have high intersubject variability indicative of differential social outcomes, suggesting the functional significance of this site for social behavior (36–39, 64). A second limitation to the current study is a lack of behavioral evidence to reveal how these epigenetic and neural markers impact the overt social phenotype. Future work aimed at establishing relationships between genetic, epigenetic, and phenotypic variability should consider the full spectrum of behavior, from disordered to healthy. Despite these limitations, the current study reveals how epigenetic variability in the endogenous oxytocin system impacts brain systems supporting social cognition and is an important step to better characterize relationships between genes, brain, and behavior.
A major advantage of our approach is that the reported epigenetic marker is a continuous variable directly relevant to gene function and capable of predicting individual variability without having to dichotomize by allele groups. However, an important future direction for the field will be to explore allele- and sex-linked methylation differences. In particular, consideration of potential genetic–epigenetic relationships may reveal the mechanism by which previously reported OXTR polymorphisms impact social and emotional processing. Molecular and animal studies will help to establish these links, and once established, a large human sample may clarify how this interaction explains neural and behavioral variability. These studies will require greater sample sizes with adequate power to detect potentially small effect sizes between groups (65) and careful control of additional biological factors. Finally, because methylation levels have been shown to differ as a function of race (66), we restricted our sample to Caucasians of European descent. Future work should therefore explore the impact of epigenetic differences within and between racial groups.
Our findings are of broad significance for several reasons. First, at a basic level, we provide evidence that the epigenetically governed oxytocin system influences neural systems involved in the perception of emotion and that the actions of this system support an anxiolytic role for oxytocin. These findings are in line with previous intranasal oxytocin studies and hold significance for neurobiological models of affect appraisal and regulation. Second, our findings have potential relevance for clinical studies. Recently, several groups have considered the use of intranasal oxytocin to treat social deficits associated with ASD. Given that the actions of oxytocin are dependent upon its receptor and expression of the receptor is under epigenetic control, any gains one sees in social cognitive abilities with oxytocin administration will likely be mediated by OXTR methylation. Moreover, our results linking variability in social perceptual processes to OXTR methylation indicate that the effectiveness of behavioral interventions targeting such social perceptual processes may also be modulated by OXTR methylation. Finally, our results add to an important and growing literature implicating epigenetic variability as a driver of individual variability in complex behavior. Epigenetics will likely play an increasing role in our understanding of gene–behavior relationships and has the potential to expand models of differential susceptibility for psychiatric and developmental disorders.
Materials and Methods
Participants.
Ninety-nine healthy adults with normal or corrected-to-normal vision were recruited for the current study. Only self-identified Caucasians of European descent were included to avoid population stratification artifacts. All individuals gave written informed consent for a protocol approved by the University of Virginia (UVA) Institutional Review Board (Protocol 15051; principal investigator, Jessica J. Connelly) and were paid $50 for participation. One individual was excluded due to an fMRI data collection error. Data from 98 participants (42 males) aged 18–30 y (M = 22.82 y, SD = 3.37) were included in the final analysis.
Blood Collection and DNA Extraction.
Venipuncture was performed at the UVA Fontaine Research Park. Eight milliliters of blood were collected from each subject in mononuclear cell separation tubes (BD Vacutainer CPT with sodium citrate, BD Biosciences). Upon collection, blood samples were immediately spun at 1,800 relative centrifugal force for 30 min at 23 °C to separate the mononuclear cell fraction per product protocol. The mononuclear cells were then lysed, and DNA was extracted using the reagents supplied in the Gentra Puregene Blood Kit (Qiagen). DNA was stored at −20 °C before further analysis.
Epigenotyping Procedures.
Analysis of the level of DNA methylation at CpG site –934 in OXTR was performed as previously reported in Jack et al. (37). Briefly, DNA derived from peripheral blood mononuclear cells was bisulfite converted, and pyrosequencing was performed using Qiagen PyroMark Q24 Classic and Pyromark reagents. Samples were analyzed in singlicate as previous data (37) indicated low replicate variability (on average, samples deviated from the mean ±1.7%).
Imaging Procedures.
Scanning was performed at the UVA Fontaine Research Park on a Siemens 3 Tesla MAGNETOM Trio high-speed imaging device equipped with 12-channel head-coil, integrated mirror, and head stabilizers. As part of a series of functional tasks, participants completed an emotional face-matching block-design task (41) in which they identify via left/right button press which of two probes at the bottom of the screen matches a target stimulus at the top of the screen. Each 20-s block consists of four stimuli presented sequentially for 5 s. During the emotion identification condition (six blocks), participants match facial expressions (all face stimuli depict angry or fearful expressions acquired from the NimStim Stimulus Set) (67); during the sensorimotor control condition (6 blocks), participants match oval orientation.
fMRI Analysis.
Image acquisition and preprocessing details are provided in SI Materials and Methods. Data analysis was conducted using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB Software Library) (68). Subject-level time-series statistical analysis was carried out using FSL’s improved linear model with local autocorrelation correction (69). Regressors for each condition (faces and ovals) were modeled by convolving the time course with a gamma hemodynamic response function (HRF), adding a temporal derivative, and applying temporal filtering. A faces > ovals contrast was conducted, and the contrast of parameter estimates from this analysis was carried forward to higher level analysis.
Group-level analyses were conducted using FSL’s local analysis of mixed effects stage 1 (70–72). To determine the effect of OXTR methylation on task-specific activity, mean faces > ovals activation and de-meaned percent methylation were entered into the model, and contrasts testing for positive and negative main effects of methylation were computed. To test for sex differences in faces > ovals activation, a two-sample unpaired t test was computed. Finally, to assess if the linear relationship between methylation and BOLD response differs across sexes, a Methylation × Sex interaction analysis was conducted by entering mean faces > ovals activation and grand-mean–centered percent methylation for each sex separately into the model.
A primary ROI analysis was first conducted within amygdala, a region activated by this task (30, 41). Left and right amygdala ROIs were created using FSL’s Harvard–Oxford Subcortical Atlas (73–76) with a probabilistic threshold = 60%. We then conducted an exploratory functional ROI analysis targeting regions involved in face perception and emotion processing as defined by a meta-analytic approach using Neurosynth.org feature set keywords “face” and “emotion.” See Fig. S1 for ROI images. Finally, we conducted an exploratory unmasked analysis to probe the effect of OXTR methylation on the whole brain. All analyses were FDR corrected for multiple comparisons at q < 0.05 and k ≥ 10. Clusters of voxels that survived correction were registered to subject space, and mean Z statistic values were extracted for each participant from these clusters. Anatomical labels were identified using FSL’s Harvard–Oxford Structural Atlases.
Functional Connectivity Analysis.
We then conducted a PPI analysis (42) to examine the effect of OXTR methylation on task-specific amygdala functional connectivity. Separate analyses were conducted using left and right amygdala seed regions created with FSL’s Harvard–Oxford Subcortical Atlas with a probabilistic threshold = 60%.
First-level analysis was carried out in FEAT, with time-series statistical analysis carried out using FSL’s improved linear model with local autocorrelation correction. Regressors of interest included the psychological variable, faces > ovals (convolved with a double-gamma HRF, temporal derivative added, temporal filtering applied); the physiological variable, mean seed-region time series; and the PPI variable, interaction term between the psychological regressor (zero-centered about min and max values) and the physiological regressor (de-meaned). The faces + ovals time course (convolved with a double-gamma HRF, temporal derivative added, temporal filtering applied) was also included as a nuisance regressor to account for shared variance between conditions. Higher level statistical inference testing for a main effect of methylation within the ROI and whole brain proceeded exactly as described for the fMRI analysis except each participant’s PPI contrast of parameter estimate replaced the faces > ovals COPE.
Supplementary Material
Acknowledgments
We thank Themistoclis Karaoli, Zoë Raymond, Meghan Cronk, Allison Jack, Holly Earls, Katie Lancaster, and Tyler Santander for their assistance in this project. This research was supported by National Science Foundation Grant 1228522, “Examining an Epigenetic Biomarker of Social Perception” (to J.J.C. and J.P.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1422096112/-/DCSupplemental.
References
- 1.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18(11):965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 3.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 5.Shamay-Tsoory SG, et al. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66(9):864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Kiss A, Mikkelsen JD. Oxytocin—Anatomy and functional assignments: A minireview. Endocr Regul. 2005;39(3):97–105. [PubMed] [Google Scholar]
- 8.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labuschagne I, et al. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labuschagne I, et al. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol. 2011;15(07):1–14. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- 11.Riem MME, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol Psychiatry. 2011;70(3):291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Jobst A, et al. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2014;26(6):347–355. doi: 10.1017/neu.2014.20. [DOI] [PubMed] [Google Scholar]
- 13.Brown EC, et al. Social approach and avoidance behaviour for negative emotions is modulated by endogenous oxytocin and paranoia in schizophrenia. Psychiatry Res. 2014;219(3):436–442. doi: 10.1016/j.psychres.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Taurines R, et al. Oxytocin plasma concentrations in children and adolescents with autism spectrum disorder: Correlation with autistic symptomatology. Atten Defic Hyperact Disord. 2014;6(3):231–239. doi: 10.1007/s12402-014-0145-y. [DOI] [PubMed] [Google Scholar]
- 15.Yuen KW, et al. Plasma oxytocin concentrations are lower in depressed vs. healthy control women and are independent of cortisol. J Psychiatr Res. 2014;51:30–36. doi: 10.1016/j.jpsychires.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan T, Uysal C, Asdemir A, Kılıç E. May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology. 2013;38(12):2890–2896. doi: 10.1016/j.psyneuen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Cyranowski JM, et al. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70(9):967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker KJ, et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci USA. 2014;111(33):12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37(8):1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58(1):74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Costa B, et al. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34(10):1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Jacob S, et al. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36(1):144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creswell KG, et al. OXTR polymorphism predicts social relationships through its effects on social temperament. Soc Cogn Affect Neurosci. 2014 doi: 10.1093/scan/nsu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucht MJ, et al. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):860–866. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogan A, et al. Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proc Natl Acad Sci USA. 2011;108(48):19189–19192. doi: 10.1073/pnas.1112658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue H, et al. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol Psychiatry. 2010;68(11):1066–1072. doi: 10.1016/j.biopsych.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Tost H, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA. 2010;107(31):13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36(6):891–897. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loth E, et al. IMAGEN Consortium Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol Psychiatry. 2014;76(5):367–376. doi: 10.1016/j.biopsych.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 33.Riem MME, Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Soc Cogn Affect Neurosci. 2011;6(3):294–300. doi: 10.1093/scan/nsq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tansey KE, et al. Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: Genetic and molecular studies. Neurosci Lett. 2010;474(3):163–167. doi: 10.1016/j.neulet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 35.Apicella CL, et al. No association between oxytocin receptor (OXTR) gene polymorphisms and experimentally elicited social preferences. PLoS ONE. 2010;5(6):e11153. doi: 10.1371/journal.pone.0011153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory SG, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7(1):62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jack A, Connelly JJ, Morris JP. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front Hum Neurosci. 2012;6:280. doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusui C, et al. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys Res Commun. 2001;289(3):681–686. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- 39.Dadds MR, et al. Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev Psychopathol. 2014;26(1):33–40. doi: 10.1017/S0954579413000497. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y-R, Kim J-H, Kim MJ, Treasure J. Differential methylation of the oxytocin receptor gene in patients with anorexia nervosa: A pilot study. PLoS ONE. 2014;9(2):e88673. doi: 10.1371/journal.pone.0088673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 42.O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107(20):9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28(26):6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riem MME, et al. No laughing matter: Intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology. 2012;37(5):1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Domes G, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Denny BT, et al. Insula-amygdala functional connectivity is correlated with habituation to repeated negative images. Soc Cogn Affect Neurosci. 2014;9(11):1660–1667. doi: 10.1093/scan/nst160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koch SBJ, et al. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: Salience processing and fear inhibition processes. Psychoneuroendocrinology. 2014;40:242–256. doi: 10.1016/j.psyneuen.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 50.Bijanki KR, Hodis B, Brumm MC, Harlynn EL, McCormick LM. Hippocampal and left subcallosal anterior cingulate atrophy in psychotic depression. PLoS ONE. 2014;9(10):e110770. doi: 10.1371/journal.pone.0110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krause-Utz A, et al. Amygdala and anterior cingulate resting-state functional connectivity in borderline personality disorder patients with a history of interpersonal trauma. Psychol Med. 2014;44(13):2889–2901. doi: 10.1017/S0033291714000324. [DOI] [PubMed] [Google Scholar]
- 52.Boehme S, Mohr A, Becker MP, Miltner WH, Straube T. Area-dependent time courses of brain activation during video-induced symptom provocation in social anxiety disorder. Biol Mood Anxiety Disord. 2014;4:6. doi: 10.1186/2045-5380-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung Y, et al. Unattended emotional faces elicit early lateralized amygdala-frontal and fusiform activations. Neuroimage. 2010;50(2):727–733. doi: 10.1016/j.neuroimage.2009.12.093. [DOI] [PubMed] [Google Scholar]
- 54.Gläscher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright CI, et al. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 56.Byun H-M, et al. Epigenetic profiling of somatic tissues from human autopsy specimens identifies tissue- and individual-specific DNA methylation patterns. Hum Mol Genet. 2009;18(24):4808–4817. doi: 10.1093/hmg/ddp445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ladd-Acosta C, et al. DNA methylation signatures within the human brain. Am J Hum Genet. 2007;81(6):1304–1315. doi: 10.1086/524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Illingworth R, et al. A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biol. 2008;6(1):e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rakyan VK, et al. DNA methylation profiling of the human major histocompatibility complex: A pilot study for the human epigenome project. PLoS Biol. 2004;2(12):e405. doi: 10.1371/journal.pbio.0020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finer S, Holland ML, Nanty L, Rakyan VK. The hunt for the epiallele. Environ Mol Mutagen. 2011;52(1):1–11. doi: 10.1002/em.20590. [DOI] [PubMed] [Google Scholar]
- 61.Waterland RA, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6(12):e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris RA, Nagy-Szakal D, Kellermayer R. Human metastable epiallele candidates link to common disorders. Epigenetics. 2013;8(2):157–163. doi: 10.4161/epi.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaminsky Z, et al. A multi-tissue analysis identifies HLA complex group 9 gene methylation differences in bipolar disorder. Mol Psychiatry. 2012;17(7):728–740. doi: 10.1038/mp.2011.64. [DOI] [PubMed] [Google Scholar]
- 64.Kumsta R, Hummel E, Chen FS, Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: Implications for behavioral neuroscience. Front Neurosci. 2013;7:83. doi: 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biol Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang FF, et al. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6(5):623–629. doi: 10.4161/epi.6.5.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tottenham N, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 69.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 70.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 71.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 72.Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 73.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 74.Frazier JA, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 75.Goldstein JM, et al. Hypothalamic abnormalities in schizophrenia: Sex effects and genetic vulnerability. Biol Psychiatry. 2007;61(8):935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 76.Makris N, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83(2-3):155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.