Abstract
The epithelial-mesenchymal transition (EMT) is an essential mechanism in embryonic development and tissue repair. EMT also contributes to the progression of disease, including organ fibrosis and cancer. EMT, as well as a similar transition occurring in vascular endothelial cells called endothelial-mesenchymal transition (EndMT), results from the induction of transcription factors that alter gene expression to promote loss of cell-cell adhesion, leading to a shift in cytoskeletal dynamics and a change from epithelial morphology and physiology to the mesenchymal phenotype. Transcription program switching in EMT is induced by signaling pathways mediated by transforming growth factor β (TGF-b) and bone morphogenetic protein (BMP), Wnt–β-catenin, Notch, Hedgehog, and receptor tyrosine kinases. These pathways are activated by various dynamic stimuli from the local microenvironment, including growth factors and cytokines, hypoxia, and contact with the surrounding extracellular matrix (ECM). We discuss how these pathways crosstalk and respond to signals from the microenvironment to regulate the expression and function of EMT-inducing transcription factors in development, physiology, and disease. Understanding these mechanisms will enable the therapeutic control of EMT to promote tissue regeneration, treat fibrosis, and prevent cancer metastasis.
Introduction
The epithelial-mesenchymal transition (EMT) is an important cellular mechanism in embryonic development, tissue repair, and disease. First described in the 1980s as a cellular phenomenon in the primitive streak of chick embryos, EMT governs many developmental processes such as gastrulation, neural crest development, somite dissociation, and palate and lip fusion (1, 2). A similar process termed the endothelial-mesenchymal transition (EndMT) involves the transformation of vascular endothelial cells to mesenchymal cells, which regulates the formation of the valves and septa of the developing heart (3). In the adult, these developmental programming mechanisms can be awoken and often result in the development of various diseases. Both organ fibrosis and cancer metastasis are driven by EMT, the latter driven perhaps by the generation of cancer stem cells capable of colonizing other tissues to form secondary tumors (4, 5). EndMT also has a role in fibrosis (6) and cancer progression (7) and is involved in the formation of stem-like cells that differentiate into osteoprogenitor cells during heterotopic ossification (8) and vascular calcification (9).
Epithelial cells form polarized sheets that are held together through various cell adhesion molecules, such as claudins and E-cadherin (10). Beneath this cell layer, the basement membrane anchors epithelial cells to the matrix surface and maintains apical-basal polarity through connections between intermediate filaments and hemidesmosomes. Adhesion to both the basement membrane and adjacent cells is critical for maintaining the epithelial phenotype (10). During EMT, cells lose these epithelial characteristics, gaining instead an invasive and migratory mesenchymal phenotype, which permits these cells to leave the tissue parenchyma and enter the systemic circulations during cancer metastasis (11). Hallmarks of EMT include the loss of expression or function of E-cadherin and reduced abundance of tight junction proteins [such as zona occludens 1 (ZO-1) and occludin] and cytokeratins, as well as concomitant increase in abundance of mesenchymal markers, such as vimentin, fibronectin, fibroblast specific protein 1 (FSP-1), α-smooth muscle actin (α-SMA), and N-cadherin (12). Loss of adherens junctions between cells triggers a change in cytoskeletal composition and an arrangement that alters cell polarity to form spindleshaped cells (Fig. 1). These newly formed mesenchymal cells invade their basal extracellular matrix (ECM) and migrate into underlying tissues along a secreted matrix of fibronectin (12).
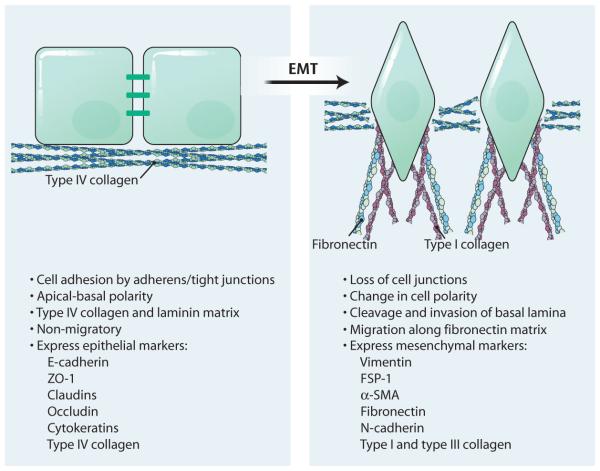
Fig. 1. Cellular changes associated with EMT.
Epithelial cells demonstrate apical-basal polarity, show strong cell-cell adhesion through adherens junctions and tight junctions, and have a basal matrix consisting primarily of type IV collagen and laminin (left). Upon induction of EMT, the cells lose their adhesion and change morphology and acquire front end-to-back end polarity (right). These cells cleave and invade the basal lamina and migrate along a newly formed matrix of fibronectin and type I collagen. The abundance of epithelial biomarkers is reduced, whereas that of mesenchymal markers is increased.
Whereas EMT is a well-characterized process during development, its involvement in disease pathology is more readily debated (13). Many studies implicate EMT in the generation of cancer stem cells within primary tumors that may be capable of metastasizing (5). Additionally, the role of EMT in stemness has become a topic of particular interest, given the studies demonstrating that the production of induced pluripotent stem cells requires an initial mesenchymal-epithelial transformation (MET) (14, 15). These topics are beyond the scope of this review, but have been recently reviewed elsewhere (13, 16).
It is tempting to think of EMT and the reverse process of MET as existing as binary states. However, developing evidence suggests that EMT may be better described as a spectrum of partial EMT states. In the embryo, multiple rounds of EMT and MET are necessary to complete gastrulation and primitive streak formation, highlighting the reversibility of this process (16). Initial dedifferentiation to a mesenchymal phenotype enables cells to migrate and then undergo MET to give rise to multiple different cell types in the notochord, somites, primordia of the urogenital system, and the splanchnopleura and somatopleura. Development of the cardiac valves also requires EMT and MET (16).
However, in some cases, a partial EMT or MET is necessary, as is the case with differentiation and tubule formation during kidney development (2, 17). In Drosophila, collective migration of groups of neural crest cells occurs without the loss of cell-cell contacts, suggesting that these cells have characteristics of both epithelial and mesenchymal cells (18). The flexible nature of the epithelial/mesenchymal state renders this process very elusive to observe.
Expression and activation of EMT-inducing transcription factors occurs in response to various signaling pathways, including those mediated by transforming growth factor β (TGF-β), bone morphogenetic protein (BMP), epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), Wnt, Sonic Hedgehog (Shh), Notch, and integrin signaling (19–24). These pathways signal through intracellular kinase cascades to induce transcription factors that activate the expression of EMT-associated genes. Some of these signals may be predominant in driving EMT at particular stages during reprogramming. Many EMT-inducing signals tend to be cell- and tissue type–specific, hinting that cells may integrate particular signals differently or react to extracellular molecules with differing sensitivities, depending on their microenvironment and cell state (17). Other signals from the ECM and hypoxic conditions have demonstrated their ability to induce EMT in various systems, and may also vary with tissue type and location within the parenchyma (25). Here, we describe many of the common signaling mechanisms that promote EMT, and we discuss how these intracellular cascades participate in crosstalk to integrate cues from the microenvironment and drive epithelial cell reprogramming.
EMT-Inducing Transcription Factors
Several transcription factors induce EMT. These include the zinc-finger binding transcription factors Snail1 and Snail2 (also known as Slug) and several other basic helix-loop-helix (bHLH) factors such as zinc finger E-box–binding homeobox 1 (ZEB1), ZEB2, and Twist (26, 27). A T cell factor (TCF) transcription factor family member called lymphoid enhancer binding factor-1 (LEF-1) can directly induce EMT (28). These proteins bind to the promoter region of genes associated with cell-cell adhesion and repress their transcription, which is the key initiating step of EMT.
The Snail family of transcriptional repressors plays a critical role in regulating EMT. Snail1 and Snail2 bind the promoter of CDH1, encoding E-cadherin, to repress its transcription (29, 30). Accumulation of Snail1 in the nucleus is associated with decreased E-cadherin abundance and the induction of metastatic phenotypes in breast cancer (31). Circulating tumor cells isolated from patients with metastatic hepatocellular carcinoma (HCC) were found to have 20 times more Snail1 than cells isolated from patients with nonmetastatic HCC (32). Snail2 also has a well-established role in the induction of EMT during cancer metastasis and is involved in gastrulation and the development and migration of the neural crest (17, 33). During gastrulation in Drosophila, Snail cooperates with co-repressors CtBP (C-terminal binding protein) and Ebi to form a complex with the histone deacetylase HDAC3 (34–36). Together, these proteins trigger switching from E-cadherin to N-cadherin, the form found in mesenchymal cells (35). Genetic deletion of Snai2, which encodes Snail2, does not seem to perturb normal EMT during gastrulation in mice (37). Interestingly, in the chick embryo, Snail2 enhances the development and migration of the neural crest but not the trunk crest, in which overexpression of Snail2 has no effect (38). Overexpression of either Snail1 or Snail2 induces EMT and correlates with increased tumor metastasis in vivo, consistent with the theme of developmental reprogramming becoming reactivated in metastatic carcinomas in disparate ways, depending on location within the organism (29, 30).
Twist1 and Twist2 belong to a bHLH transcription family and play an essential role in cancer metastasis (27). During determination of the ventral furrow in Drosophila, the expression of Twi and Sna (encoding Twist and Snail) are increased, and their respective protein products have central roles in invagination of ventral mesoderm and delamination of mesodermal cells (39). In human mammary cells, Twist1 binds the SNAI2 promoter and stimulates its expression to induce EMT, consistent with reports of a higher abundance of Twist1 in metastatic mammary tumors compared with their less metastatic counterparts (27, 40). Studies in a mouse model of atypical ductal hyperplasia, an early stage of primary breast tumor development, show increased expression of Twist1 (41). Regulation of transcription is not mediated by Twist1 alone; however, BMI1, a polycomb-group repressor complex protein, acts in a concerted fashion with Twist to repress E-cadherin and the cell cycle inhibitor p16INK4α (42). Additionally, Twist1 is responsible for the expression of several microRNAs (miRNAs), which mediate inhibition of HOXD1 expression and its downstream target genes (43). One of these targets is RhoC (Ras homolog gene family, member C), which has a well-established role in cytoskeletal reorganization and cancer metastasis (44).
The ZEB family of transcriptional repressors have an essential role in neural crest development and have attracted substantial interest as regulators of cancer progression (45). ZEB1 and ZEB2 interact with the bipartite E-box regions of DNA that flank the CDH1 gene to repress its promoter activity (Fig. 2) (46, 47). In the nucleus, ZEB1 cooperates with the deacetylase sirtuin 1 to modify histone H3 and reduce binding of RNA polymerase II at the CDH1 promoter (48). Ectopic expression of ZEB proteins in mammary epithelial cells is sufficient to induce the dissociation of adherens junctions, presumably through suppressing the expression of genes encoding plakophilin-2 and ZO-3, both critical for maintaining the epithelial phenotype (47, 49). They also increase the expression of genes encoding matrix metalloproteinases (MMPs), implicating ZEB1 and ZEB2 in various matrix remodeling mechanisms associated with EMT (50). These remodeling events may trigger the transmission of additional extracellular signals, which are discussed in later sections. The miR-200 family, a group of five miRNAs that share similar targeting sequences, inhibit the abundance of ZEB proteins on the CDH1 promoter (51, 52). In turn, ZEB1 and ZEB2 bind to the E-box promoters of miR-200, creating a reciprocal feedback loop controlling EMT (51, 53). Interestingly, expression of miR-200 family members enhances the recolonization of metastatic cells, highlighting the role of this regulatory loop in maintaining the mesenchymal phenotype and demonstrating the reversibility of EMT (54).
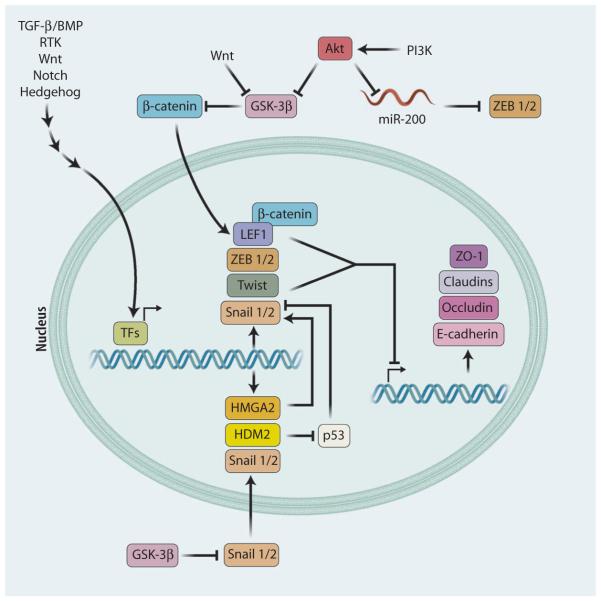
Fig. 2. EMT-inducing transcription factors.
EMT is triggered by transcription factors that bind and inhibit the expression of genes encoding adherens junction and tight junction molecules, such as E-cadherin, ZO-1, claudins, and occludin. These transcription factors include Snail1/2, ZEB1/2, Twist, and LEF-1, the expression of which is induced by various signaling pathways. Several regulatory molecules can inhibit the function of these transcription factors. GSK-3β can inhibit β-catenin–induced activation of LEF-1 and can also inhibit the stability and nuclear translocation of Snail1/2. The miR-200 family of miRNAs can inhibit the expression of ZEB1/2. GSK-3β and miR-200 can be blocked by the kinase Akt, which is activated by most EMT signaling pathways.
LEF-1 is another key transcription factor that can directly induce EMT through its repression of E-cadherin (55). Overexpression of LEF-1 in colon carcinoma cell lines promotes EMT through its activation by β-catenin (56). Furthermore, inhibiting LEF-1 activity, either with a dominant-negative form of the protein or with targeted small interfering RNA, inhibits EMT in many systems (28, 57, 58).
Despite a host of other factors known to be involved in epithelial transformation, the repression of E-cadherin by the various transcription factors outlined above is often considered to be the critical event during EMT. This predominating idea is supported by the observation that genetic deletion of E-cadherin in mice results in the formation of migratory lobular carcinomas (59), as well as long-standing evidence that loss of E-cadherin can be used as an indicator of carcinoma progression and poor prognosis in multiple tumor types (60). Furthermore, breast ductal carcinomas with low abundance of claudin (a critical component of tight junctions) are shown to have decreased expression of E-cadherin in addition to increased expression of EMTinducing transcription factors, such as Snail1, Twist, and ZEB2, thus linking the expression of E-cadherin with the classical EMT factors in a clinically relevant fashion (61). Although these studies seem to provide compelling evidence for a definitive link between E-cadherin and EMT, the loss of E-cadherin alone is not necessarily indicative of a migratory phenotype. Differential expression of CDH1 within particular tissues has been reported, with particular sets of carcinomas lacking expression of CDH1 during the early stage of the disease, resulting in cells with a static EMT phenotype (62–65).
Because numerous transcription factors are involved in the activation of EMT programs, it is perhaps not surprising that an array of phenotypes have been observed during epithelial cell reprogramming. Regulation of EMT-inducing transcription factors involves multiple layers of control that have been recently reviewed (66, 67). Evolving evidence suggests that many of the EMT-inducing transcription factors act synergistically with one another and use common pathways, yet some studies show that inhibition of a single transcription factor is sufficient to block EMT (68).
TGF-β and BMP Signaling in EMT
TGF-β signaling is the most well-characterized pathway that is known to induce EMT, and acts through various intracellular messengers. Signaling is typically activated by the TGF-β superfamily of ligands, which include—among others—three isoforms of TGF-β (TGF-β1, 2, and 3) and six isoforms of BMP (BMP2 through BMP7). Most systems in which EMT is observed, including cancer and fibrosis, are regulated by TGF-β1 (69), whereas TGF-β2 primarily controls EndMT in heart development (70), and TGF-β3 mediates EMT in the developing palate (28). BMP2 and BMP4 induce EMT in cancer (71–73) and EndMT in the developing heart (74) as well as during heterotopic ossification (8). During development, an increasing gradient of BMP4 directs the patterning of the mesoderm along the mediolateral axis (75). BMP4 is highly increased in invasive epithelium compared with normal colonic mucosa, further highlighting the widespread reliance on this protein in several different tissues and its involvement in reactivating development processes (76, 77). Conversely, BMP7 has been shown to counteract EMT in breast cancer (78) and fibrosis (4), and is the most common factor promoting an epithelial cell phenotype. In addition, BMP5 attenuates TGF-β–induced EMT, underscoring the specificity of particular isoforms of BMP in development and disease (79).
TGF-β signaling occurs through the formation of a heterotetrameric receptor complex composed of type I and type II TGF-β receptors (TGF-βRI and TGF-βRII). There are currently seven type I receptors and five type II receptors identified in mammals (80–82). Upon ligand binding, TGF-βRII trans-phosphorylates TGF-βRI, facilitating its kinase activity. Combinations of different receptors enable differential signaling to occur when activated by the same ligand (82). Varying combinations of type I and type II receptors enable differential ligand binding to occur, thus enabling them to respond to various isoforms of the TGF-β superfamily (82, 83). The TGF-β type III receptors β-glycan and endoglin, as well as accessory proteins (such as Cripto), can modulate ligand binding affinity at the membrane in epithelial and endothelial cells (84, 85). BMP signaling occurs in a similar manner to TGF-β signaling, except that a specific type II BMP receptor is involved instead of TGF-βRII (86, 87). Different BMP ligands can induce activation of various type I BMP receptors termed activin-like kinases (ALKs), which initiate the signaling cascade at the cell surface (88).
SMAD-dependent signaling
Phosphorylation of TGF-βRI by the Ser/Thr kinase activity of TGF-βRII creates a docking site in the Gly/Ser (GS)–rich domain of TGF-βRI, which recruits the transcription factors SMAD2 and SMAD3 [mothers against decapentaplegic homologs 2 and 3 (SMAD2/3)] (Fig. 3). SMADs are consequently phosphorylated at Ser residues in their C-terminal domain, facilitating formation of a complex with coactivator SMAD4. R-SMADs and SMAD4 contain conserved MH1 and C-terminal MH2 domains, which influence their ability to form complexes and bind to the minor groove of DNA (80, 81, 89). Phosphorylation of MH2 domains by TGF-βR1 is required for oligomerization of SMAD2/3 with SMAD4, and also enables the nuclear import of the R-SMAD/SMAD4 complex by exposing the nuclear localization signal. This signal permits binding of importins b1, 7, and 8 to the complex and their subsequent translocation into the nucleus (90–92). Additionally, Lys-rich localization sequences in the MAD homology 1 (MH1) domain of R-SMADs enable nuclear import of SMAD1 and SMAD3 (93). In addition to activating SMADs, TGF-β signaling is also capable of activating inhibitory SMAD proteins (SMAD6 and SMAD7), which bind to TGF-βRI and prevent the recruitment of effector SMADs (80, 81, 89). This system introduces a layer of control designed to prevent aberrant activation of this pathway. In BMP signaling, the basic pathway remains the same; however, BMP receptors make use of phosphorylated SMAD1/5/8 in place of SMAD2/3 complexes (94, 95).
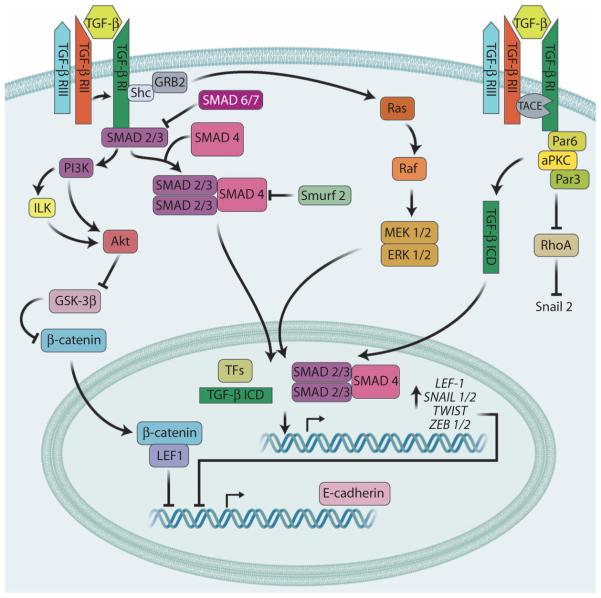
Fig. 3. TGF-β signaling in EMT.
TGF-β ligands bind to their type II and type III receptors (TGF-βRII and TGF-βRIII), which causes recruitment and phosphorylation of the type I receptor (TGF-βRI). This activates various signaling pathways, including those mediated by SMAD2/3, Ras, and PI3K (simplified here), which activate transcription factors that induce the expression of genes encoding EMT-inducing transcription factors. At the surface of the cell, TACE cleaves the intracellular domain of TGF-β RI, which can then act as a transcriptional regulator to mediate EMT. Additionally, the Par3–Par6–aPKC (atypical PKC) complex also associates with TGF-βRs at the cell membrane and is involved in cytoskeletal remodeling to promote the mesenchymal phenotype. SMAD-independent pathways (such as through PI3K and ILK) can activate Akt, which in turn can inhibit the function of GSK-3β, a kinase that inhibits nuclear translocation of Snail and β-catenin. Inhibition of SMAD signaling is mediated by SMAD6/7, which prevent the binding and phosphorylation of SMAD2/3 at TGF-βRs, and by Smurf2, which is known to degrade the activated complex of SMAD2/3/4.
Inhibition of signaling may also be mediated by posttranslational degradation of SMADs. The HECT ubiquitin protesome E3 ligase family members Smurf1 and Smurf2 (SMAD ubiquitin regulatory factors 1 and 2) are capable of interacting with SMADs, targeting them for proteasome degradation and suppressing TGF-β signaling (96). Another E3 ligase, PIAS1 [protein inhibitor of activated STAT (signal transducer and activator of transcription), 1], is also inhibited by TGF-β signaling. Without the activity of PIAS1, the sumoylation of Ski-related novel protein N (SnoN) is decreased, eventually leading to the degradation of SnoN and enabling SMAD complexes to access and regulate target genes (97).
Once inside the nucleus, SMAD complexes bind regulatory elements and induce the transcription of key genes associated with EMT. Complexes of R-SMADs are capable of binding directly to the promoter of SNAI1 to induce its transcription, and can form complexes with Snail1 to suppress the expression of genes encoding E-cadherin and occludin (98, 99). Other factors directly influenced by the binding of R-SMADs include the ZEB transcription factors and the high mobility group factor HGMA2, which also regulates the expression of SNAI1, SNAI2, and TWIST (Fig. 2) (99, 100). R-SMAD/SMAD4 complexes can induce the transcription of the gene encoding the E3 ubiquitin ligase HDM2, which promotes the degradation of p53 (Fig. 2). Studies show that the loss of p53 function induces Snail1-mediated EMT by inhibiting the action of regulatory miRNAs, such as miR-34 and members of the miR-200 family that suppress the action of EMT-inducing transcription factors (101–103). TGF-β and the tumor suppressor p12 may also influence the expression of the gene encoding Twist2, which promotes EMT through suppression of E-cadherin (104).
TGF-β signaling can directly increase LEF1 expression through SMADs and inhibit glycogen synthase kinase 3β (GSK-3β) through the phosphoinositide 3-kinase (PI3K)–Akt pathway, which enables β-catenin–dependent activation of LEF-1 to induce EMT (58, 105). Additionally, induction of LEF-1 may occur in a β-catenin–independent manner through TGF-β3 by forming SMAD2/SMAD4/LEF-1 transcription complexes that suppress E-cadherin abundance during EMT in palate medial edge epithelial cells (28). Snail1 and Snail2 can indirectly increase the abundance of TGF-β3, which in turn stimulates LEF1 expression (57).
SMAD-independent signaling
In addition to acting through SMAD proteins, signaling through TGF-β receptor complexes involves a number of SMAD-independent pathways most commonly observed in receptor tyrosine kinase (RTK) signaling. Multiple roles exist for PI3K-Akt signaling in controlling EMT. Mechanistically, it is likely that TGF-β activates PI3K machinery directly through its own receptors (106) or through trans-activation of EGF and PDGF receptors (107, 108). Activation of PI3K and Akt signaling by TGF-β has been identified in multiple cell types (106, 109–111). Activated PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to make phosphatidylinositol 3,4,5- trisphosphate (PIP3), a phospholipid membrane protein that binds Akt (Fig. 4). Upon binding, Akt is phosphorylated by phosphoinositide-dependent kinase 1 (PDK1) (112). Mutations in p110α, the catalytic subunit of PI3K, are often present in tumors and cause increased PIP3 production and aberrant activation of Akt. Phosphorylation of Akt may also be induced through stimulation of integrins, which activate integrin-linked kinase (ILK) (113). Dephosphorylation of PIP3 is mediated by phosphatase and tensin homolog (PTEN), which is often absent in metastatic cancers (114).
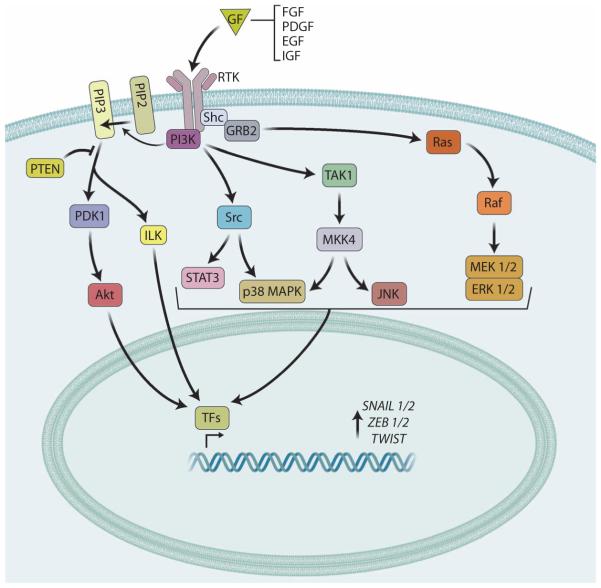
Fig. 4. RTK signaling in EMT.
Growth factors (GFs), such as FGF, EGF, PDGF, or IGF, stimulate RTKs, which activate various signaling pathways (simplified here), including those mediated by Ras, PI3K, Src, and ILK. These signaling cascades activate transcription factors (TFs) that bind to the promoters of genes that encode EMT-inducing transcription factors, such as Snail1/2, ZEB1/2 and Twist, which induce EMT by inhibiting the expression genes encoding cell adhesion molecules. When present and functional, the tumor suppressor PTEN can suppress PI3K-mediated induction of EMT.
Mammalian cells have three Akt isoforms, each with distinct and often opposing functions. Silencing Akt2 using RNA interference is correlated with loss of EMT phenotype and increased expression of E-cadherin (115). When activated, Akt2 phosphorylates heterogeneous nuclear ribonucleoprotein E1 (hnRNPE1), which, when not phosphorylated, binds to the 3′ UTR (untranslated region) of mRNAs and inhibits their translation. Phosphorylation of hnRNPE1 by Akt2 causes its dissociation from DAB2 and ILE1 mRNA (encoding disabled homolog 2 and interleukin-like EMT inducer, respectively), enabling translation of their respective protein products, which are then capable of promoting expression of EMT-inducing transcription factors (116, 117). Akt also inhibits GSK-3β, which phosphorylates Snail1, marking it for degradation by β-transducin repeat– containing protein (β-TRCP) (118). In addition, Akt induces the transcription of SNAI1 through the activation of nuclear factor kB (NF-κB), inducing EMT in squamous cell carcinoma cells (119). Still other examples of PI3K signaling are found in the Src family kinases, which activate β-catenin and EMT in pancreatic carcinoma cells (120). These kinases also signal through STAT3 to affect transcriptional changes in epithelial cells (121). Src kinases are associated with integrin signaling, consistent with a role for ILK in activating Akt (122). Additionally, activation of Akt by TGF-β activates both mammalian target of rapamycin complex 1 (mTORC1) and mTORC2, both of which contribute to EMT in different ways: Motility and invasion, protein synthesis, and particularly cell size are controlled by mTORC1, whereas mTORC2 is essential for the phenotype transformation itself (123, 124).
TGF-β1 is a potent activator of various other kinase pathways including extracellular signal–regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK). These pathways have been elucidated both independently of SMAD signaling and through regulation of SMAD complexes. MAPK kinase (MEK)–ERK signaling is implicated in the activation of EMT and in crosstalk with canonical SMAD signaling pathways. Early evidence from studies using dominant-negative SMADs suggests that MAPK pathways might be involved in TGF-β signaling independently of transcription (125), and that the activation of SMADs by TGF-βRI was not necessary for p38 MAPK activation (126). The oncogenic guanosine triphosphatase (GTPase) Ras activates ERK signaling (127), consistent with the observation that induction of EMT transcription factors, such as Twist1, requires concomitant Ras signaling to observe changes in phenotype (128). Activation of TGF-β receptors leads to Tyr phosphorylation of the adaptor protein Shc (Src homology 2 domain–containing transforming protein), permitting the GRB2 (growth factor receptor–bound 2)–SOS1 (son of sevenless homolog 1) complex to dock and activate Ras and the downstream ERK pathway (129). Abrogating the activation of ERK1 and ERK2 (ERK1/2) by inhibiting MEK1/2 inhibits the delocalization of E-cadherin and ZO-1, highlighting a necessary role for ERK signaling in EMT (68).
The JNK and p38 MAPK cascades are initiated by the E3 ligase member TRAF6. Association of TRAF6 with TGF-βRI causes activation of TGF-β–activated kinase 1 (TAK1), which is involved in EMT of mammary epithelial cells in a SMAD-independent manner (130). Depletion of TRAF6 in the mammary epithelium leaves TGF-β unable to activate p38 MAPK or JNK and induce EMT, but does not affect SMAD signaling (130, 131). Furthermore, TGF-βRIs lacking the docking sites for SMADs are still capable of activating JNK signaling, underscoring the existence of multiple signaling pathways induced by TGF-β receptors (132). Activation of TAK1 is postulated to promote NF-κB signaling, which may lead to EMT (133). In addition to its role in mediating p38/JNK pathways, TRAF6 is also involved in stimulation of EMT by disrupting junction assembly at the plasma membrane (130). Close to the cell surface, the metalloproteinase TACE and protein kinase C (PKC) facilitate TRAF6-mediated cleavage of the intracellular portion of TGF-βRI, which may co-operate with nuclear SMAD complexes to induce transcription of SNAI1 (130, 134).
In addition to signaling through SMADs and other kinases that transmit signals to the nucleus, TGF-β induces changes proximally at the plasma membrane. Stimulation of TGF-β receptors leads to phosphorylation of Par6 by TGF-βRII and subsequent recruitment of Smurf1. In addition to marking SMAD proteins for degradation, Smurf1 marks RhoA GTPase for degradation, and the loss of RhoA promotes EMT through changes in the organization of actin filaments and the loss of tight junctions and adherens junctions (135). It is suspected that a lack of RhoA activity induces SNAI1 expression (136).
Interestingly, other studies have demonstrated that RhoA and Cdc42 (another Rho GTPase) are involved in the assembly of stress fibers and activation of p38 MAPK during the formation of lamellipodia, contrasting the role of RhoA in the regulation of epithelial polarity and motility as discussed above (137).
RTK Signaling in EMT
Various growth factors activate RTKs and through downstream signaling can induce EMT. The TGF-β receptors have kinase activity, and thus, many of the non-SMAD signaling cascades induced by TGF-β [such as Ras, PI3K, focal adhesion kinase (FAK), Src, and TAK] are also induced by RTK activation in response to growth factors, such as EGF, FGF, insulin growth factor (IGF), and PDGF (Fig. 4) (23, 107, 108, 138). Growth factor binding to RTKs induces receptor dimerization and trans-phosphorylation of the intracellular domains, thus transducing the signal to intracellular cascades (139). In addition to participating in the signaling pathways discussed above, many of these ligands also increase cell proliferation and contribute to the formation of partial EMT (140).
FGF is involved in EMT in various ways. FGF-4 and FGF-8 play a role in controlling mesenchyme formation to mediate the patterning of the mesodermal layer during gastrulation (141). FGF-2 can induce EMT in tubularepithelial cells by increasing the expression of genes encoding vimentin and FSP-1 and inducing the activity of MMP-2 to increase cell motility (142). These results are consistent with observations that the abundance of FGF-2 is increased in fibrotic renal tissue (143). FGF-2 is also capable of inducing changes in the actin cytoskeleton through crosstalk between Rho GTPases and PI3K, which promotes the elongated mesenchymal phenotype associated with cell motility (144). Cell motility is also stimulated by EGF in an a2 integrin–dependent manner in mammary epithelial cells (145). The additional observation that EGF dephosphorylates and inactivates FAK suggests that EGF-mediated inhibition of FAK facilitates cell detachment from the ECM to enable cell motility (146). How cell migration then proceeds through ECM-induced reactivation of FAK is discussed in a later section.
Similarly, overexpression of insulin-like growth factor 1 (IGF-1) in mammary epithelial cells leads to EMT, increased migration, and decreased abundance of E-cadherin through the activation of β-catenin (138). IGF-1 activates NF-κB to increase SNAI1 expression in the mammary epithelium and increases ZEB1 expression through the activation of ERK (138, 147). Interestingly, hepatocyte growth factor (HGF) is also involved in EMT through the induction of Snail1 or Snail2 in a cell type–dependent manner (148, 149). Destabilization of desmosomes is also carried out by HGF, demonstrating the ability of RTKs to induce changes in multiple ways in response to ligand binding (148).
PDGF controls the expression of CDH2 (encoding N-cadherin) in mesoderm by activating the PI3K pathway, providing directional information for primitive streak formation (150). Inhibition of N-cadherin occurs primarily through the loss of phosphorylated Akt. Additionally, PDGF induces the phosphorylation of the nuclear RNA helicase p68, which promotes EMT through the nuclear translocation of β-catenin in a Wnt-independent manner in various epithelial cancer cell lines (114). Decreased MMP-2 activity and subsequent inhibition of mesenchymal cell migration is observed after ablation of the PDGF receptor, resulting in craniofacial and heart valve malformations (151).
In addition to promoting a migratory phenotype through FAK inhibition, as described above, EGF is capable of inducing EMT through repression of E-cadherin in a number of ways. Upon binding of EGF to its receptor, E-cadherin is internalized from the cell membrane, reducing cell-cell contacts and weakening the epithelial layer (152). In addition, EGF also induces the expression of SNAI1 and TWIST, leading to repression of the CDH1 promoter (153). During development, expression of the gene encoding the glycoprotein Cripto1 is associated with formation of the primitive streak and specification toward mesodermal and endodermal lineages (154). Consistent with this observation are data demonstrating an increase in the mesenchymal markers N-cadherin and vimentin when Cripto1 is expressed in mammary epithelium (155). Cripto1 is thought to act less like a ligand, relying instead on its ability to interact with Nodal and contribute to Wnt signaling (156, 157). This is covered in more detail in subsequent sections of this review.
RTK signaling is not only involved in the induction of classical EMT transcription factors, but it also regulates the deposition of ECM, which provides important ligands for EMT and promotes an invasive phenotype. Pharmacological inhibition of JNK or PI3K impairs the production of type I collagen by mammary epithelial cells (158), and activation of p38 MAPK induces the production of b1 or b3 integrin subunits, which bind the ECM (122, 159–161). ECM proteins induce various signaling mechanisms, and certain ECM proteins provide a suitable microenvironment for EMT, as outlined in subsequent sections of this review. The close relationship between the production of ECM and the induction of signaling pathways related to ECM binding further supports the viewpoint that EMT is a dynamic process that is continuously regulated to maintain the cell state. EGF signaling also mediates the production and secretion of proteolytic enzymes, such as MMP-2 and MMP-9, and activates ERK and ILK signaling. Withdrawal of EGF reverses cellular morphology back to the epithelial state and decreases the activity of the ILK and MEK-ERK pathways; however, inhibition of MEK and ILK abrogates cell migration and MMP production, but does not cause any change in cell morphology. This study demonstrates the ability of EGF, which may be produced by various sources within the tissue, to enact changes in epithelial cell reprogramming and basement membrane remodeling through multiple different mechanisms (162).
Even in a brief overview of the signaling pathways involving growth factors and RTKs, it is quickly apparent how many different extracellular signals can be involved in the regulation of EMT. Many of these growth factors act through similar or redundant pathways, yet the effects of activation by a particular ligand appear to be highly tissue- and cell type–dependent in some instances. This observation hints at the existence of an additional layer of control that may be present to integrate many cues from the extracellular space. For example, differential autocrine and paracrine signals in the surrounding tissue and the biophysical functionality of the basement membrane may be integrated together to induce EMT in a particular way. A major source of TGF-β within tumors is the stromal fibroblasts, which provide extracellular signals that induce EMT (163). Once they have left the tissue and entered the bloodstream, many circulating cancer cells associate with platelet cells, which also provide a rich source of TGF-β (164). Whereas both environments provide a source of the necessary growth factors for maintenance of the mesenchymal phenotype, they surely also provide other soluble and nonsoluble cues that may become integrated with TGF-β and other factors to cause differential activation of cellular programs.
Wnt Signaling in EMT
Wnt signals are transduced across the plasma membrane by Frizzled and low-density lipoprotein receptor–related protein (LRP) receptors. In the absence of signaling, β-catenin is phosphorylated by a complex of GSK-3β, Axin, and the tumor suppressor adenomatous polyposis coli (APC), which sequesters β-catenin in the cytoplasm and marks it for proteasomal degradation (165). Activation of Frizzled by Wnt ligands results in phosphorylation of LRP6 by GSK-3β and the recruitment of Dishevelled (Dvl) and Axin to the plasma membrane. Unable to form a complex with Axin, GSK-3β cannot then phosphorylate β-catenin, enabling it to translocate to the nucleus. Restriction of GSK-3β to the cytoplasm after Wnt–β-catenin activation also prevents the destabilizing phosphorylation of Snail1 (31).
Nuclear β-catenin binds to members of the TCF/LEF family of transcription factors to promote EMT. During gastrulation, β-catenin forms a complex with LEF-1 to bind and inhibit the transcription of CDH1 (Fig. 5) and induce EMT (166). Wnt signaling is critical for the formation of the primary axis during vertebrate embryogenesis: mouse embryos deficient in Wnt3 do not form and develop anterior-posterior neural patterning (167). Premature formation of the endoderm occurs as a result of stabilization of β-catenin during early development, emphasizing the importance of this pathway in development (168). In numerous cancers, Wnt signaling is inappropriately active (24, 169) and directly induces SNAI1 and SNAI2 expression (118, 170, 171). The Wnt–GSK-3β–β-TRCP1 (β-transducin repeat–containing protein 1) axis induces the activity of Snail2, which promotes EMT and, by binding its promoter and recruiting a histone demethylase, inhibits the expression of BRCA1 (encoding breast cancer 1, early onset). The loss of BRCA1 is associated with aggressive basal-like breast cancer (172). Wnt-mediated induction of EMT through Snail2 is consistent with other reports of decreased E-cadherin and increased fibronectin after the accumulation of β-catenin in the nucleus (173, 174). Wnt has also been linked to increased expression of TWIST in mammary epithelial cells (175). Mutations in APC and other components of the β-catenin signaling pathway have been identified in metastatic colorectal cancer, highlighting the importance of the Wnt pathway in EMT during cancer progression (176, 177).
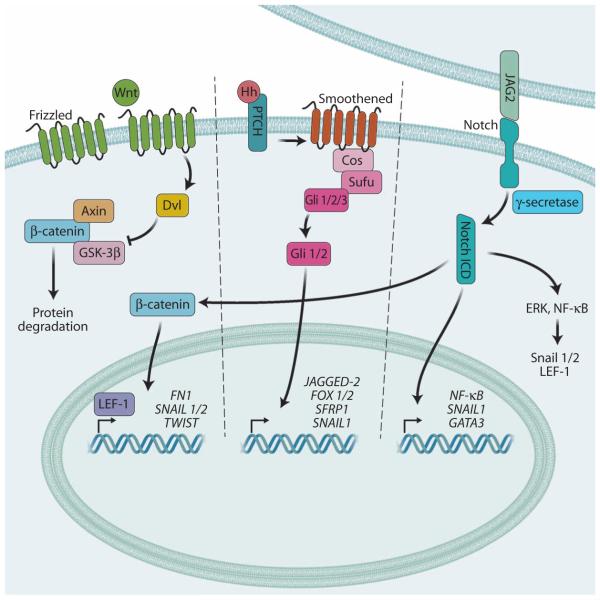
Fig. 5. Wnt, Notch, and Hedgehog signaling in EMT.
Wnt ligands bind and activate Frizzled receptors, which promote Dvl-dependent inhibition of GSK-3β, a kinase that causes degradation of cytoplasmic β-catenin. This enables the accumulation and nuclear localization of β-catenin to activate the LEF-1 transcription factor, which promotes the expression of various EMT-associated genes. The intercellular interaction between JAG2 and its receptor Notch induces the g-secretase–mediated cleavage and release of the Notch ICD, which can directly activate target genes associated with EMT signaling. The Notch ICD can also stabilize cytoplasmic β-catenin and activate other pathways, like ERK and NF-κB, that induce the Snail1/2 and LEF-1 transcription factors. Hh signaling induces EMT-associated gene expression through activating Gli transcription factors.
Several layers of crosstalk between the TGF-β and Wnt signaling pathways have been identified; for example, it is apparent that LEF-1 may be activated by binding to β-catenin or SMAD proteins (178, 179). Additionally, activation of TGF-β in pancreatic carcinoma cells induces the expression of the genes encoding homeobox transcription factor CUTL1 and downstream target Wnt-5A, a canonical activating Wnt ligand that induces EMT and mediates the invasive capacity of pancreatic tumor cells (180).
Notch Signaling in EMT
The Notch receptor is composed of an extracellular domain and an intracellular domain (NICD) that contains motifs for nuclear localization. Upon interaction with a neighboring Notch receptor, g-secretase and TACE cleave NICD, facilitating its translocation to the nucleus (181, 182). The NICD binds to DNα-bound CSL [CBF1, Su(H), LAG1] transcription repressor complexes to activate the expression of genes encoding proteins involved in tumor development, such as NF-κB, Akt, and p21 (182–184). Notch signaling can regulate expression of SNAI1 both directly (185, 186) and indirectly through the induction of hypoxiα-inducible factor 1α (HIF-1α). Binding of HIF-1α to the promoter of LOX (encoding lysyl oxidase) leads to its transcription and subsequent LOX-mediated stabilization of Snail1 (187). Snail2 interacts with Notch and is essential for Notch-mediated repression of E-cadherin and β-catenin activation (126, 127). Overexpression of Notch in endothelial cells results in loss of vascular endothelial (VE)– cadherin and subsequent EndMT (185). Additionally, inhibition of Notch1 in lung adenocarcinoma cells decreases their invasive phenotype and partially reverses EMT (188).
In addition to the direct effects mediated by its intracellular domain, Notch also indirectly regulates EMT through various signaling pathways, including NF-κB and β-catenin, and through the action of various regulatory miRNAs (189). Inhibition of Notch in pancreatic cancer cell lines decreases the DNA binding potential of NF-κB and lowers the expression of MMP9, which encodes a critical MMP involved in remodeling the ECM to facilitate extravasation of pancreatic cancer cells (190). Binding of the Notch ligand Jagged2 (JAG2) promotes EMT through the induction of GATα-binding protein 3, which inhibits the miR-200 family (189). Recent reports have highlighted a role for miR-200 in targeting JAG1 to regulate Notch activation in a feedback loop (173).
Hedgehog Signaling in EMT
Members of the Hedgehog (Hh) family include Shh, Desert Hedgehog (Dhh), and Indian Hedgehog (Ihh) (191, 192). Hh ligands bind to patched homolog 1 (PTCH1) and PTCH2, which function to inhibit the activity of Smoothened (Smo) in the absence of ligand binding (193, 194). Activation of PTCH1/2 releases Smo and initiates an intracellular cascade (195) that activates Gli family transcription factors, which promote transcription of target genes such as PTCH, WNT, and SNAI1 (24, 196). Expression of GLI1 in kidney epithelium results in the loss of E-cadherin and an increase in Shh signaling at the invasive front of neuroendocrine tumors (196, 197). Additionally, Hh signaling induces TGF-β1 secretion to increase motility (198) and induces JAG2 expression, resulting in cleavage of the Notch intracellular domain (NICD) (187). Other known targets include secreted Frizzled-related protein 1 (SFRP1) (199), which modulates Wnt signaling in a concentration-dependent context, as well as FOX1 and FOX2, two mesenchymal forkhead transcription factors that control the intracellular accumulation of β-catenin and have been shown to limit Wnt signaling in the gut (200).
Oncogenic forms of Shh have been identified in basal cell carcinoma and are misregulated in prostate adenocarcinoma, esophageal and stomach cancer, and pancreatic adenocarcinoma (201). Disruption of Shh with cyclopamine, a steroidal alkaloid that binds and deactivates Smo (202), inhibits EMT in pancreatic cancer cell lines (203). Other studies have observed Hh-mediated inhibition of Wnt signaling through paracrine mechanisms involving local mesenchymal stromal cells (204). It is possible that the paracrine signaling from stromal cells in the microenvironment maintains an epithelial cell phenotype in the tumors. Those cells existing at the invasive front may be relatively free from such paracrine signaling, enabling EMT to occur. This once again underscores the dynamic nature of EMT regulation, relying on progressive gradients of different extracellular signals rather than an initial stimulus sufficient to induce and retain the mesenchymal phenotype indefinitely.
Matrix Signaling in EMT
Signaling mediated by the ECM is a critical regulator of epithelial cell fate, working both independently and in conjunction with the pathways described above. Not only does binding of epithelial cells to particular matrix elements initiate intracellular signaling cascades, but it also results in the remodeling of the matrix to activate additional signaling pathways that contribute to a migratory phenotype. EMT was initially discovered when isolated embryonic epithelium was incubated in three-dimensional gels of type I collagen in culture. Over time, the cells acquired a mesenchymal morphology and migrated along the collagen fibrils away from the implanted epithelial tissue (205). The abundance of type I and type III collagen is increased during EMT, and directly seeding epithelial cells on these matrices can induce EMT through various signaling pathways (12), summarized below.
During EMT, mesenchymal cells cut through the basal lamina of type IV collagen and laminin, laying down their own matrix of type I collagen and fibronectin. The formation of lamellipodia and extruding filopodia at the cell surface provides directional motility to the cell, and the release of MMPs degrades the matrix to facilitate cell invasion (206, 207). The dissolution of epithelial junctions causes the formation of actin stress fibers and clustering of integrins at the migratory front of the cell (208) where MMPs are released. Unsurprisingly, EMT signaling pathways have been shown to induce expression of genes encoding MMP-2 and MMP-9, which cleave type IV collagen in the basal lamina to promote post-EMT invasion of underlying tissues (7, 12). MMP-3 has also been shown to directly induce EMT through activation of Rac1 GTPase–ROS (reactive oxygen species) signaling, which promotes expression of SNAI1 (209). Additionally, MMPs are capable of degrading E-cadherin in the cell membrane, disrupting adherens junctions, and weakening the epithelial sheet (210). This process enables cells to acquire a mesenchymal phenotype and migrate into the matrix.
At the cell membrane, integrins bind to matrix proteins and activate intracellular cascades that mediate EMT (Fig. 6) (12, 211, 212). The diversity in binding affinities of different integrin proteins enables cells to respond to a vast array of extracellular elements and mediate different signaling cascades in response to a changing matrix environment. As the composition of the ECM changes, so does the abundance of integrins at the cell surface, promoting the progression of EMT under the control of the pericellular environment. aVb3 integrins facilitate Src-mediated phosphorylation of TGF-βRII, creating a docking site for ShcA and GRB2, which signal through the p38 MAPK pathway to induce EMT (122, 159). Interactions between b3 integrin subunit and TGF-βRII complexes may also be bridged by FAK as an essential mediator of EMT and metastasis in breast cancer (213, 214). b1 integrin subunits also function as critical mediators of the p38 MAPK pathway in addition to their role in JNK signaling and regulation of DAB2 expression (161, 215). Other studies highlighting the crosstalk of integrins with TGF-β signaling show that phosphorylated β-catenin and SMAD2 form complexes through the cooperation of TGF-β and a3b1 integrin signaling, which promote EMT in vitro and in vivo in a model of lung fibrosis (216). The latent form of TGF-β contains an integrin-binding motif. The binding of latent TGF-β to integrins aVb6 and aVb8 induces the proteolytic release of the latency-associated peptide (LAP), hence activating TGF-β at the cell membrane; aVb8 mediates this activation through the protease activity of membrane type 1 MMP (also known as MMP-14) (217, 218). Release of LAP from TGF-β in the matrix further amplifies the EMT mechanism through the various signaling cascades activated by soluble TGF-β. This in turn promotes the synthesis of type I collagen and fibronectin, which contribute further to EMT (140). In this way, matrix remodeling not only changes the types of matrix proteins that interact with the cell membrane but also influences the soluble cytokine environment that affects EMT.
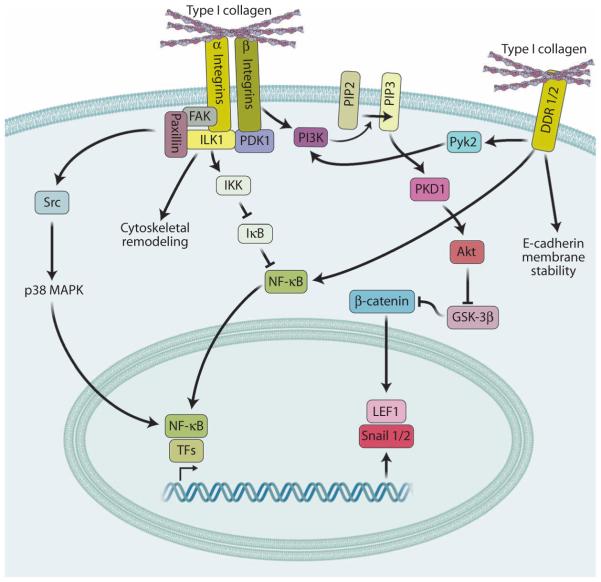
Fig. 6. Matrix signaling in EMT.
Type I collagen induces EMT through integrin or DDR1/2 signaling. Both types of receptors activate NF-κB and other transcription factors (TFs) that promote expression of SNAI1/2 and LEF1. Other pathways simplified here, such as those mediated by PI3K, ILK, proline-rich tyrosine kinase 2 (PYK2)–PDK1, and FAK-paxillin, are activated by type I collagen through integrins and DDRs, ultimately promoting the stabilization and activity of the EMT-associated transcription factors Snail1/2 and LEF-1. DDR1 is also known to form complexes with E-cadherin at the cell surface, which are disrupted upon binding to type I collagen.
As they invade the basement membrane, mesenchymal cells synthesize a fibronectin matrix, which provides a track for migration of mesenchymal cells during EMT and maintains the mesenchymal phenotype (219). Its deposition in the ECM increases substrate rigidity, which is involved in progression of mammary tumors (220–222) and is controlled by SMADs and JNK signals activated by TGF-β (132, 223). An increased amount of fibronectin in conjunction with activated Ras in mammary epithelial cells causes the replacement of a6b3 integrins with a5b1 integrins, which improves the migratory capacity of cells by increasing cell adhesion to fibronectin (224). Increased amounts of type I collagen and fibronectin in the matrix are also correlated with integrins switching to a high-affinity ligand-binding state, increasing signaling activity through FAK and ILK and promoting EMT (225, 226).
The importance of type I collagen in EMT is evident in various cellular systems. Lack of type I collagen in mouse embryos results in abnormal craniofacial development and mandibular growth, highlighting the importance of proper matrix remodeling for the migration of mesenchymal cells during development (227). In the adult, type I collagen is associated with EMT in lung, breast, and pancreatic carcinomas, highlighting the importance of the matrix environment in metastasis as well (158, 228, 229). Induction of EMT is dependent on the interaction between type I collagen fibers and a2b1 integrin, which triggers the intracellular cascade (230). Type I collagen causes ILK-dependent phosphorylation of IkB (inhibitor of kB) to increase the abundance of nuclear-localized NF-κB, which promotes the expression of SNAI1 and LEF1 to induce EMT (231). Increased abundance of type I collagen also activates JNK pathways, consistent with studies demonstrating that pharmacological inhibition of JNK signaling abrogates type I collagen–mediated migration and metastasis of breast cancer cells (232, 233). More recent studies highlight a ligand-independent role for collagen in promoting both canonical and noncanonical TGF-β signaling (234). The interaction of b1 integrin subunits with type I collagen found in the pericellular matrix is correlated with direct suppression of E-cadherin and indirect induction of N-cadherin (233, 235).
Lastly, type I collagen is also shown to induce EMT through binding and activating the discoidin domain receptors DDR1 and DDR2. In cooperation with integrins, DDR1 promotes EMT through the JNK1–c-Jun pathway (233). The expression of both DDR2 and COL1A1 (which encodes type I collagen) is increased in response to TGF-β signaling, and DDR2 mediates type I collagen–induced EMT through activation of the NF-κB and LEF-1 transcription factors (236).
Hypoxia Signaling in EMT
Various systems in which EMT occurs, such as fibrosis and cancer, experience hypoxia as a result of ischemic conditions. Therefore, it should come as no surprise that low-oxygen tension is capable of enacting changes in cellular phenotype and cooperating with other pathways to induce EMT. Lack of oxygen inhibits prolyl hydroxylases, such as PHD2 and PHD3, which act to catalyze and degrade HIF-1α under normoxic conditions (Fig. 7). HIF-1α is a transcription factor that induces expression of a number of EMT-associated genes, such as TGF-β, TWIST, and LOX (237).
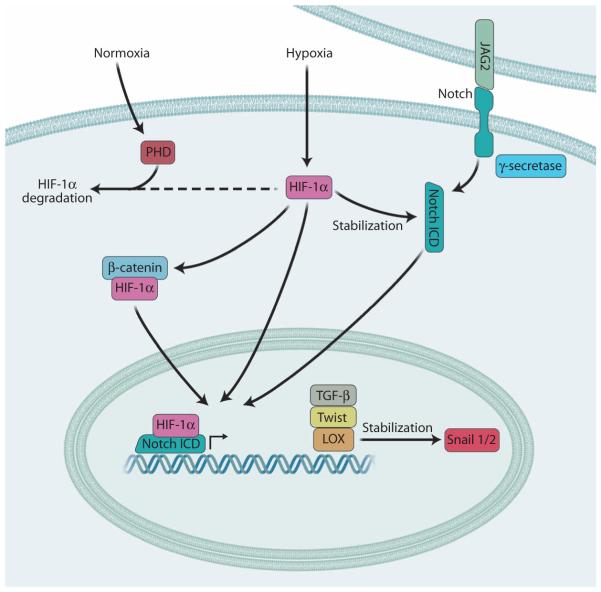
Fig. 7. Hypoxia signaling in EMT.
Under normoxic conditions, prolyl hydroxylases (PHD) promote the degradation of the transcription factor HIF-1α. Under hypoxic conditions, PHDs are inactivated, thus enabling the accumulation and functional activation of HIF-1α, which induces the expression of genes associated with EMT, such as TGF-β, TWIST, and LOX, and the stabilization of the Snail1/2 transcriptional repressors. HIF-1α has also been shown to stabilize β-catenin as well as the Notch ICD after its JAG2-induced cleavage by g-secretase. Both β-catenin and the Notch ICD also promote the expression of EMT-associated genes.
HIF-1α is capable of binding directly to the hypoxiα-responsive element of the TWIST1 promoter (238). These data are supported by studies in mouse models demonstrating similar phenotypes between HIF-1α mutants and Twist knockout mice (239, 240). During hypoxiα-induced EMT, HDAC3 abundance is increased by HIF-1α. HDAC3 binds to the promoters of CDH1 and JUP (encoding plakoglobin, also known as g-catenin) and cooperates with Snail1 to inhibit their transcription. Additionally, HDAC3 mediates the formation of histone methyltransferase complexes that are necessary to induce the expression of mesenchymal markers, such as vimentin and N-cadherin (241). In hepatocellular carcinoma cells, hypoxia facilitates EMT and enhances metastatic potential through the activation of β-catenin (242). Interestingly, hypoxia is able to reverse the inhibitory function of SMAD7 and convert it into a promoter of cell invasion, demonstrating the modular nature of these effector proteins and their flexibility to react to environmental cues (243).
Crosstalk of EMT Signaling Pathways
Given the vast number of processes in development that are controlled by EMT, it is not surprising that many overlapping pathways may be involved. Similarly, a wide array of extracellular signals (both soluble and matrix-bound) must be able to affect this process to carry out multiple rounds of EMT and MET in a spatially defined manner. Given the emerging evidence indicating the existence of partial EMT states, it is worthwhile revisiting the myriad of ways in which these different pathways interact with one another and postulating whether these interactions may be dependent on the existence of a particular cellular microenvironment or cell state.
Crosstalk between hypoxia and Notch signaling is a well-established phenomenon that contributes to EMT signaling. The expression of HIF-1α increases the abundance of LOX, an enzyme required for FAK activity and intercellular and cell-matrix adhesion (244). LOX stabilizes the activity of Snail1 by deaminating trimethylated histone H3 Lys4 in the CDH1 promoter (187, 245). Aberrant activation of Notch signaling by hypoxia can induce EMT during tumor progression. Not only does HIF-1α stimulate Notch signaling, but it also stabilizes nuclear NICD, which is involved in the induction of many EMT-associated genes (246, 247). Additionally, hypoxia represses miR-34α, which inhibits the Notch signaling pathway (248).
TGF-β signaling is capable of interacting with various pathways through its ability to activate many different effector proteins. Notch and TGF-β signaling function together in endocardial cushion formation (185). Other development processes mediated by joint signals from TGF-β and other signaling pathways include gastrulation and neural crest delamination, which involve a triad of TGF-β, Wnt, and FGF signaling (249, 250). As cellular junctions begin to disintegrate under the control of TGF-β, β-catenin accumulates in the nucleus, where it enhances signaling through complex formation with LEF-1 (251). LEF-1 is also capable of forming a complex with SMAD proteins to repress transcription of CDH1 (252). TGF-β is capable of activating PI3K directly through its own receptors (106) or through trans-activation of EGF and PDGF receptors (107, 108). Additionally, the ERK pathway is involved in crosstalk with TGF-β and other growth factors to induce EMT (253).
SMAD proteins often connect TGF-β and other signaling cascades. Some of the existing evidence regarding interactions with SMADs (and crosstalk with TGF-β signaling in general) seems to be conflicting, such as the observation that HGF inhibits TGF-β–induced EMT and prevents myofibroblast differentiation in renal fibrosis through the involvement of SMADs (254). This is surprising, given that TGF-β interacts positively with other growth factors, such as PDGF (255). The role of SMAD proteins is, in some cases, not clearly defined and can change in response to changes in cell environment (243).
RTK pathways are capable of signaling independently of SMADs. However, consistent lines of evidence highlight the crosstalk between SMAD and non-SMAD kinase pathways for EMT induction. Although PI3K signaling and SMAD signaling can act independently of each another, chemical inhibition of PI3K activity has been shown to abrogate EMT and reduce phosphorylation of SMAD2 (106). Both ATF-2 (activating transcription factor-2, a substrate of p38 MAPK pathways) and c-Jun (a substrate of JNK) are capable of interacting with SMADs (80, 81, 89). This convergence of pathways is consistent with observations that p38 MAPK and ERK1/2 are required for canonical TGF-β–induced EMT, despite the mechanistic ability of SMAD complexes to promote the induction of EMT-associated transcription factors (126, 253, 256).
One of the clearest examples of signal crosstalk occurs in positive feedback loops created through autocrine signaling and matrix remodeling. Snail1 cooperates with the transcription factor ETS1 downstream of MAPK to activate MMP expression (257). Additionally, binding of MMP3 induces the expression of RAC1 and increases the production of ROS and Snail1 (258, 259), which activate EMT and lead to production of other MMPs, such as MMP-2 and MMP-9. These MMPs degrade the basement membrane, releasing latent TGF-β and enabling preferential binding to type I collagen and fibronectin (17). These ECM proteins activate intracellular EMT signaling cascades of their own, as previously discussed (225, 226). The synthesis of fibronectin is also associated with Wnt activation and accumulation of nuclear β-catenin, providing further evidence of multiple signaling pathways converging to produce ECM capable of supporting migration (173). It thus becomes clear that the process of epithelial reprogramming and acquiring an invasive phenotype is one that is continually fed back upon through signal crosstalk at various points within the cascade, particularly in interactions between the cell and its dynamic environment.
With so many pathways being activated at once, it is necessary to have effective mechanisms that integrate signals and dampen aberrant activation of EMT. GSK-3β exists at the intersection of many of these pathways and is responsible for mediating much of the crosstalk between pathways. For example, GSK-3β phosphorylates Snail1 and SMAD proteins and marks them for degradation, preventing aberrant activation of EMT. However, both Wnt and AKT inhibit the action of GSK-3β and thus stabilize Snail1 (118, 260, 261).
Concluding Remarks
EMT is a remarkable mechanism that is essential for proper embryogenesis, without which developmental defects or embryonic lethality can occur. Although essential for development, EMT can have detrimental consequences in the adult organism, promoting the progression of diseases, such as fibrosis and cancer metastasis. Identifying and understanding the signaling mechanisms that promote EMT may lead to novel therapeutic strategies to inhibit this cellular transformation in systems of human disease. Of particular importance is the recognition of the physiological context and dynamic nature of this process in the development of drugs that target EMT to combat disease because the effects of such drugs may need to be finely tuned for a particular tissue microenvironment. Moreover, many of the mechanisms that are involved in inducing EMT are also capable of maintaining the dormant state of circulating cancer cells (140). Great care must be taken not to inadvertently promote reestablishment of the epithelial phenotype in mesenchymal cells where they may induce the formation of secondary tumors. EMT is tightly regulated by particular cellular microenvironments; thus, careful investigation of the effects that these different extracellular contexts may have is necessary before designing novel therapeutics.
Acknowledgments
Funding: This work was supported by grants to D.M. from the NIH (R01HL112860 and P20GM104937) and the John Butler Mulliken Foundation. Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Lim J, Thiery JP. Epithelial-mesenchymal transitions: Insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 3.Garside VC, Chang AC, Karsan A, Hoodless PA. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell. Mol. Life Sci. 2013;70:2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Medici D, Kalluri R. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin. Cancer Biol. 2012;22:379–384. doi: 10.1016/j.semcancer.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br. J. Cancer. 2008;99:1375–1379. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Boström KI. A role for the endothelium in vascular calcification. Circ. Res. 2013;113:495–504. doi: 10.1161/CIRCRESAHA.113.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nawshad A, Lagamba D, Polad A, Hay ED. Transforming growth factor-β signaling during epithelial-mesenchymal transformation: Implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179:11–23. doi: 10.1159/000084505. [DOI] [PubMed] [Google Scholar]
- 12.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieto MA, Cano A. The epithelial–mesenchymal transition under control: Global programs to regulate epithelial plasticity. Semin. Cancer Biol. 2012;22:361–368. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA, Pei D. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 17.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev. Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack N, O’Dea S. Regulation of epithelial to mesenchymal transition by bone morphogenetic proteins. Cell. Signal. 2013;25:2856–2862. doi: 10.1016/j.cellsig.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341:41–45. doi: 10.1016/j.canlet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelialmesenchymal transition in human carcinomas. Front. Biosci. 2012;4:671–684. doi: 10.2741/s292. [DOI] [PubMed] [Google Scholar]
- 23.Katoh Y, Katoh M. FGFR2-related pathogenesis and FGFR2-targeted therapeutics (Review) Int. J. Mol. Med. 2009;23:307–311. doi: 10.3892/ijmm_00000132. [DOI] [PubMed] [Google Scholar]
- 24.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 25.Philip B, Ito K, Moreno-Sánchez R, Ralph SJ. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis. 2013;34:1699–1707. doi: 10.1093/carcin/bgt209. [DOI] [PubMed] [Google Scholar]
- 26.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 2004;48:365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Nawshad A, Hay ED. TGFβ3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J. Cell Biol. 2003;163:1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 30.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 31.Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ. A Wnt–Axin2–GSK3b cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 32.Min AL, Choi JY, Woo HY, Kim JD, Kwon JH, Bae SH, Yoon SK, Shin SH, Chung YJ, Jung CK. High expression of Snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin. Exp. Metastasis. 2009;26:759–767. doi: 10.1007/s10585-009-9275-6. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Qi D, Bergman M, Aihara H, Nibu Y, Mannervik M. Drosophila Ebi mediates Snaildependent transcriptional repression through HDAC3-induced histone deacetylation. EMBO J. 2008;27:898–909. doi: 10.1038/emboj.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-βased cell–cell adhesion system during Drosophila gastrulation. Dev. Biol. 1998;203:435–450. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- 36.Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. dCtBP mediates transcriptional repression by Knirps, Krüppel and Snail in the Drosophila embryo. EMBO J. 1998;17:7009–7020. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev. Biol. 1998;198:277–285. [PubMed] [Google Scholar]
- 38.del Barrio MG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development. 2002;129:1583–1593. doi: 10.1242/dev.129.7.1583. [DOI] [PubMed] [Google Scholar]
- 39.Stathopoulos A, Levine M. Linear signaling in the Toll-Dorsal pathway of Drosophila: Activated Pelle kinase specifies all threshold outputs of gene expression while the bHLH protein Twist specifies a subset. Development. 2002;129:3411–3419. doi: 10.1242/dev.129.14.3411. [DOI] [PubMed] [Google Scholar]
- 40.Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Yang MH, Hsu DSS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OKS, Wu KJ. Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nat. Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 43.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 44.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 45.Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y. Mice lacking Zfhx1b, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease–mental retardation syndrome. Am. J. Hum. Genet. 2003;72:465–470. doi: 10.1086/346092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Remacle JE, Kraft H, Lerchner W, Wuytens G, Collart C, Verschueren K, Smith JC, Huylebroeck D. New mode of DNA binding of multi-zinc finger transcription factors: dEF1 family members bind with two hands to two target sites. EMBO J. 1999;18:5073–5084. doi: 10.1093/emboj/18.18.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 48.Romano LA, Runyan RB. Slug is an essential target of TGFβ2 signaling in the developing chicken heart. Dev. Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- 49.Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell–cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, Miyazaki K. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br. J. Cancer. 2004;90:1265–1273. doi: 10.1038/sj.bjc.6601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 53.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 54.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim K, Lu Z, Hay ED. Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell. Biol. Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 57.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through β-catenin–T-cell factor-4-dependent expression of transforming growth factor-β3. Mol. Biol. Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medici D, Hay ED, Goodenough DA. Cooperation between Snail and LEF-1 transcription factors is essential for TGF-β1-induced epithelial-mesenchymal transition. Mol. Biol. Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derksen PWB, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am. J. Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic sub-type of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- 63.Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berx G, Becker KF, Höfler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Hum. Mutat. 1998;12:226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 65.Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S. E-Cadherin gene mutations in human gastric carcinoma cell lines. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1858–1862. doi: 10.1073/pnas.91.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–1763. doi: 10.1038/onc.2013.128. [DOI] [PubMed] [Google Scholar]
- 67.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 68.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-β1–induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akhurst RJ, Derynck R. TGF-β signaling in cancer—A double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 70.Camenisch TD, Molin DGM, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFβ ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 71.Kang MH, Kang HN, Kim JL, Kim JS, Oh SC, Yoo YA. Inhibition of PI3 kinase/Akt pathway is required for BMP2-induced EMT and invasion. Oncol. Rep. 2009;22:525–534. doi: 10.3892/or_00000467. [DOI] [PubMed] [Google Scholar]
- 72.Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–456. [PubMed] [Google Scholar]
- 73.Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003;24:1445–1454. doi: 10.1093/carcin/bgg100. [DOI] [PubMed] [Google Scholar]
- 74.McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev. Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 76.Deng H, Ravikumar TS, Yang WL. Bone morphogenetic protein-4 inhibits heat-induced apoptosis by modulating MAPK pathways in human colon cancer HCT116 cells. Cancer Lett. 2007;256:207–217. doi: 10.1016/j.canlet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp. Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 78.Buijs JT, Henriquez NV, van Overveld PGM, van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clézardin P, Papapoulos SE, Cecchini MG, Löwik CWGM, van der Pluijm G. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 79.Bramlage CP, Müller GA, Tampe B, Bevanda J, Maatouk I, Koziolek M, Lange K, Ahrens K, Schmid H, Cohen CD, Bramlage P, Kretzler M, Strutz F. The role of bone morphogenetic protein-5 (BMP-5) in human nephrosclerosis. J. Nephrol. 2011;24:647–655. doi: 10.5301/JN.2011.6330. [DOI] [PubMed] [Google Scholar]
- 80.Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-β family members through Smad proteins. Eur. J. Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 81.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-β signal transduction. J. Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 82.Derynck R, Feng XH. TGF-β receptor signaling. Biochim. Biophys. Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 83.Gilboa L, Nohe A, Geissendörfer T, Sebald W, Henis YI, Knaus P. Bone morphogenetic protein receptor complexes on the surface of live cells: A new oligomerization mode for serine/threonine kinase receptors. Mol. Biol. Cell. 2000;11:1023–1035. doi: 10.1091/mbc.11.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yan YT, Liu JJ, Luo Y, Haltiwanger CE,RS, Abate-Shen C, Shen MM. Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol. Cell. Biol. 2002;22:4439–4449. doi: 10.1128/MCB.22.13.4439-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blobe GC, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor β (TGF-β) signaling. Functional modulation of type III TGF-β receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 86.Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 89.Massagué J. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 90.Xu L, Chen YG, Massagué J. The nuclear import function of Smad2 is masked by SARA and unmasked by TGFβ-dependent phosphorylation. Nat. Cell Biol. 2000;2:559–562. doi: 10.1038/35019649. [DOI] [PubMed] [Google Scholar]
- 91.Kurisaki A, Kose S, Yoneda Y, Heldin CH, Moustakas A. Transforming growth factor-β induces nuclear import of Smad3 in an importin-β1 and Ran-dependent manner. Mol. Biol. Cell. 2001;12:1079–1091. doi: 10.1091/mbc.12.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yao X, Chen X, Cottonham C, Xu L. Preferential utilization of Imp7/8 in nuclear import of Smads. J. Biol. Chem. 2008;283:22867–22874. doi: 10.1074/jbc.M801320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao Z, Watson N, Rodriguez C, Lodish HF. Nucleocytoplasmic shuttling of Smad1 conferred by its nuclear localization and nuclear export signals. J. Biol. Chem. 2001;276:39404–39410. doi: 10.1074/jbc.M103117200. [DOI] [PubMed] [Google Scholar]
- 94.Hoodless PA, Haerry T, Abdollah S, Stapleton M, O’Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling path-ways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 95.Derynck R, Zhang Y, Feng XH. Transcriptional activators of TGF-β responses: Smads. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 96.Arora K, Warrior R. A new Smurf in the village. Dev. Cell. 2001;1:441–442. doi: 10.1016/s1534-5807(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 97.Netherton SJ, Bonni S. Suppression of TGFβ-induced epithelial-mesenchymal transition like phenotype by a PIAS1 regulated sumoylation pathway in NMuMG epithelial cells. PLOS One. 2010;5:e13971. doi: 10.1371/journal.pone.0013971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vincent T, Neve EPA, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1–SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat. Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peinado H, Quintanilla M, Cano A. Transforming growth factor β-1 induces Snail transcription factor in epithelial cell lines: Mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 100.Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J. Biol. Chem. 2008;283:33437–33446. doi: 10.1074/jbc.M802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, Liu M, Chen CT, Yu D, Hung MC. p53 regulates epithelial– mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, Hermeking H. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10:4256–4271. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 103.Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, Ozturk A, Hicks GG, Hannon GJ, He L. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol. 2011;13:1353–1360. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu G. Epithelialmesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 106.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- 107.Murillo MM, del Castillo G, Sánchez A, Fernández M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-β1 in hepatocytes. Oncogene. 2005;24:4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- 108.Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H, Grünert S. Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 110.Lin CC, Chiang LL, Lin CH, Shih CH, Liao YT, Hsu MJ, Chen BC. Transforming growth factor-β1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-κB pathways in human lung epithelial cells. Eur. J. Pharmacol. 2007;560:101–109. doi: 10.1016/j.ejphar.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 111.Lien SC, Usami S, Chien S, Chiu JJ. Phosphatidylinositol 3-kinase/Akt pathway is involved in transforming growth factor-β1-induced phenotypic modulation of 10T1/2 cells to smooth muscle cells. Cell. Signal. 2006;18:1270–1278. doi: 10.1016/j.cellsig.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 112.Lamouille S, Derynck R. Emergence of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin axis in transforming growth factor-β-induced epithelial-mesenchymal transition. Cells Tissues Organs. 2011;193:8–22. doi: 10.1159/000320172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—Essential roles in physiology and cancer biology. J. Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 114.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelialmesenchymal transition. J. Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-β-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat. Cell Biol. 2010;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MCJ, Merrick WC, Howe PH. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol. Cell. 2011;41:419–431. doi: 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat. Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 119.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-κB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 120.Vogelmann R, Nguyen-Tat MD, Giehl K, Adler G, Wedlich D, Menke A. TGFβ-induced downregulation of E-cadherin-βased cell-cell adhesion depends on PI3-kinase and PTEN. J. Cell Sci. 2005;118:4901–4912. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- 121.Cao D, Tal TL, Graves LM, Gilmour I, Linak W, Reed W, Bromberg PA, Samet JM. Diesel exhaust particulate-induced activation of Stat3 requires activities of EGFR and Src in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L422–L429. doi: 10.1152/ajplung.00204.2006. [DOI] [PubMed] [Google Scholar]
- 122.Galliher AJ, Schiemann WP. b3 Integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamouille S, Derynck R. Cell size and invasion in TGF-β–induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-β-mediated transcription. J. Biol. Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 126.Yu L, Hébert MC, Zhang YE. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yue J, Mulder KM. Activation of the mitogen-activated protein kinase pathway by transforming growth factor-β. Methods Mol. Biol. 2000;142:125–131. doi: 10.1385/1-59259-053-5:125. [DOI] [PubMed] [Google Scholar]
- 128.Morel AP, Hinkal GW, Thomas C, Fauvet F, Courtois-Cox S, Wierinckx A, Devouassoux-Shisheboran M, Treilleux I, Tissier A, Gras B, Pourchet J, Puisieux I, Browne GJ, Spicer DB, Lachuer J, Ansieau S, Puisieux A. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLOS Genet. 2012;8:e1002723. doi: 10.1371/journal.pgen.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol. 2009;19:385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 130.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol. Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landström M. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 132.Itoh S, Thorikay M, Kowanetz M, Moustakas A, Itoh F, Heldin CH, ten Dijke P. Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J. Biol. Chem. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- 133.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, Hermansson A, Dimitriou H, Bengoechea-Alonso MT, Ericsson J, Heldin CH, Landström M. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat. Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 136.Hutchison N, Hendry BM, Sharpe CC. Rho isoforms have distinct and specific functions in the process of epithelial to mesenchymal transition in renal proximal tubular cells. Cell. Signal. 2009;21:1522–1531. doi: 10.1016/j.cellsig.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 137.Edlund S, Landström M, Heldin CH, Aspenström P. Transforming growth factor-β– induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol. Biol. Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-κB and Snail. Mol. Cell. Biol. 2007;27:3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsai JH, Yang J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: A role for FGFR1 in morphogenetic movement through the primitive streak. Development. 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- 142.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 143.Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Müller GA. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 144.Lee JG, Kay EP. Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF-2. Invest. Ophthalmol. Vis. Sci. 2006;47:2358–2368. doi: 10.1167/iovs.05-1490. [DOI] [PubMed] [Google Scholar]
- 145.Matthay MA, Thiery JP, Lafont F, Stampfer F, Boyer B. Transient effect of epidermal growth factor on the motility of an immortalized mammary epithelial cell line. J. Cell Sci. 1993;106:869–878. doi: 10.1242/jcs.106.3.869. Pt. 3. [DOI] [PubMed] [Google Scholar]
- 146.Lu Z, Jiang G, Blume-Jensen P, Hunter T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell. Biol. 2001;21:4016–4031. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Graham TR, Zhau HE, Odero-Marah VA, Osunkoya AO, Kimbro KS, Tighiouart M, Liu T, Simons JW, O’Regan RM. Insulin-like growth factor-I–dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2008;68:2479–2488. doi: 10.1158/0008-5472.CAN-07-2559. [DOI] [PubMed] [Google Scholar]
- 148.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein Slug causes desmo-some dissociation, an initial and necessary step for growth factor–induced epithelial– mesenchymal transition. J. Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grotegut S, von Schweinitz D, Christofori G, Lehembre F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 2006;25:3534–3545. doi: 10.1038/sj.emboj.7601213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–3530. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- 151.Robbins JR, McGuire PG, Wehrle-Haller B, Rogers SL. Diminished matrix metalloproteinase 2 (MMP-2) in ectomesenchyme-derived tissues of the Patch mutant mouse: Regulation of MMP-2 by PDGF and effects on mesenchymal cell migration. Dev. Biol. 1999;212:255–263. doi: 10.1006/dbio.1999.9373. [DOI] [PubMed] [Google Scholar]
- 152.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 153.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am. J. Pathol. 2012;180:2188–2200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J. Cell. Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 156.Morkel M, Huelsken J, Wakamiya M, Ding J, van de Wetering M, Clevers H, Taketo MM, Behringer RR, Shen MM, Birchmeier W. β-Catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development. 2003;130:6283–6294. doi: 10.1242/dev.00859. [DOI] [PubMed] [Google Scholar]
- 157.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal Wnt11 activates the canonical Wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 158.Shintani Y, Wheelock MJ, Johnson KR. Phosphoinositide-3 kinase–Rac1–c-Jun NH2-terminal kinase signaling mediates collagen I–induced cell scattering and upregulation of N-cadherin expression in mouse mammary epithelial cells. Mol. Biol. Cell. 2006;17:2963–2975. doi: 10.1091/mbc.E05-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 160.Galliher-βeckley AJ, Schiemann WP. Grb2 binding to Tyr284 in TbR-II is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin b1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 162.Ahmed N, Maines-βandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am. J. Physiol. Cell. Physiol. 2006;290:C1532–C1542. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- 163.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 164.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fodde R, Brabletz T. Wnt/β-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 166.Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of β-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 167.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 168.Mohamed OA, Clarke HJ, Dufort D. β-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev. Dyn. 2004;231:416–424. doi: 10.1002/dvdy.20135. [DOI] [PubMed] [Google Scholar]
- 169.Verras M, Sun Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 170.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: Implications for the epithelial-mesenchymal transition. J. Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou BP, Hung MC. Wnt, Hedgehog and Snail: Sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle. 2005;4:772–776. doi: 10.4161/cc.4.6.1744. [DOI] [PubMed] [Google Scholar]
- 172.Wu ZQ, Li XY, Hu CY, Ford M, Kleer CG, Weiss SJ. Canonical Wnt signaling regulates Slug activity and links epithelial–mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16654–16659. doi: 10.1073/pnas.1205822109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kirchner T, Brabletz T. Patterning and nuclear β-catenin expression in the colonic adenoma-carcinoma sequence. Analogies with embryonic gastrulation. Am. J. Pathol. 2000;157:1113–1121. doi: 10.1016/s0002-9440(10)64626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Howe LR, Watanabe O, Leonard J, Brown AMC. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63:1906–1913. [PubMed] [Google Scholar]
- 176.Clevers H. Wnt breakers in colon cancer. Cancer Cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 177.Vermeulen L, De Sousa E Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 178.Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-β signalling pathways during formation of Spemann’s organizer. Nature. 2000;403:781–785. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- 179.Labbé E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and Wnt pathways. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ripka S, König A, Buchholz M, Wagner M, Sipos B, Klöppel G, Downward J, Gress T, Michl P. WNT5A—Target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 181.Kopan R. Notch: A membrane-βound transcription factor. J. Cell Sci. 2002;115:1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- 182.Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr. Cancer Drug Targets. 2006;6:313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 183.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J. Cell. Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 184.Miele L. Notch signaling. Clin. Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 185.Timmerman LA, Grego-βessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick F, Izpisúa-βelmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Sluginduced repression of E-cadherin. J. Exp. Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xie M, Zhang L, He C, Xu F, Liu J, Hu Z, Zhao L, Tian Y. Activation of notch-1 enhances epithelial–mesenchymal transition in gefitiniβ-acquired resistant lung cancer cells. J. Cell. Biochem. 2012;113:1501–1513. doi: 10.1002/jcb.24019. [DOI] [PubMed] [Google Scholar]
- 189.Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA, Goodall GJ, Kurie JM. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200–dependent pathway in mice. J. Clin. Invest. 2011;121:1373–1385. doi: 10.1172/JCI42579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Z, Banerjee S, Li Y, Rahman KMW, Zhang Y, Sarkar FH. Down-regulation of Notch-1 inhibits invasion by inactivation of nuclear factor-κB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 191.Marigo V, Roberts DJ, Lee SM, Tsukurov O, Levi T, Gastier JM, Epstein DJ, Gilbert DJ, Copeland NG, Seidman CE. Cloning, expression, and chromosomal location of SHH and IHH: Two human homologues of the Drosophila segment polarity gene hedgehog. Genomics. 1995;28:44–51. doi: 10.1006/geno.1995.1104. [DOI] [PubMed] [Google Scholar]
- 192.Katoh Y, Katoh M. Comparative genomics on Sonic hedgehog orthologs. Oncol. Rep. 2005;14:1087–1090. [PubMed] [Google Scholar]
- 193.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr., Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 194.van den Heuvel M, Ingham PW. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 195.Gallet A, Therond PP. Temporal modulation of the Hedgehog morphogen gradient by a patched-dependent targeting to lysosomal compartment. Dev. Biol. 2005;277:51–62. doi: 10.1016/j.ydbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 196.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene. 2006;25:609–621. doi: 10.1038/sj.onc.1209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fendrich V, Waldmann J, Esni F, Ramaswamy A, Mullendore M, Buchholz M, Maitra A, Feldmann G. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr. Relat. Cancer. 2007;14:865–874. doi: 10.1677/ERC-07-0108. [DOI] [PubMed] [Google Scholar]
- 198.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-β-mediated activation of the ALK5–Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 199.Katoh Y, Katoh M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int. J. Mol. Med. 2006;17:171–175. [PubMed] [Google Scholar]
- 200.Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 201.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 202.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 203.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 204.He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, Luxon BA, Xie J. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J. Biol. Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 205.Greenburg G, Hay ED. Cytoskeleton and thyroglobulin expression change during transformation of thyroid epithelium to mesenchyme-like cells. Development. 1988;102:605–622. doi: 10.1242/dev.102.3.605. [DOI] [PubMed] [Google Scholar]
- 206.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 207.McNiven MA. Breaking away: Matrix remodeling from the leading edge. Trends Cell Biol. 2013;23:16–21. doi: 10.1016/j.tcb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nelson WJ. Remodeling epithelial cell organization: Transitions between front-rear and apical-βasal polarity. Cold Spring Harb. Perspect. Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stallings-Mann ML, Waldmann J, Zhang Y, Miller E, Gauthier ML, Visscher DW, Downey GP, Radisky ES, Fields AP, Radisky DC. Matrix metalloproteinase induction of Rac1b, a key effector of lung cancer progression. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004062. 142ra95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nisticò P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: General principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb. Perspect. Biol. 2012;4:a011908. doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 212.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 213.Wendt MK, Smith JA, Schiemann WP. p130Cas is required for mammary tumor growth and transforming growth factor-β-mediated metastasis through regulation of Smad2/3 activity. J. Biol. Chem. 2009;284:34145–34156. doi: 10.1074/jbc.M109.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-β signaling and metastasis. Breast Cancer Res. 2009;11:R68. doi: 10.1186/bcr2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor β (TGFβ)-stimulated fibronectin synthesis through TGFβ-activated kinase 1 and activation of the JNK pathway. J. Biol. Chem. 2005;280:25920–25927. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- 216.Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. Integrin a3b1–dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J. Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin avb6 binds and activates latent TGF b1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 218.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin avb8 mediates epithelial homeostasis through MT1-MMP–dependent activation of TGF-β1. J. Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals— Distinct functions of a5b1 and avb3 integrins in regulating cell behaviour. IUBMB Life. 2009;61:731–738. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chandler EM, Saunders MP, Yoon CJ, Gourdon D, Fischbach C. Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Phys. Biol. 2011;8:015008. doi: 10.1088/1478-3975/8/1/015008. [DOI] [PubMed] [Google Scholar]
- 221.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 222.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 225.Imamichi Y, Menke A. Signaling pathways involved in collagen-induced disruption of the E-cadherin complex during epithelial-mesenchymal transition. Cells Tissues Organs. 2007;185:180–190. doi: 10.1159/000101319. [DOI] [PubMed] [Google Scholar]
- 226.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: The shape of things to come. Biochem. J. 1999;339:481–488. Pt. 3. [PMC free article] [PubMed] [Google Scholar]
- 227.Lavrin IO, McLean W, Seegmiller RE, Olsen BR, Hay ED. The mechanism of palatal clefting in the Col11a1 mutant mouse. Arch. Oral. Biol. 2001;46:865–869. doi: 10.1016/s0003-9969(01)00044-9. [DOI] [PubMed] [Google Scholar]
- 228.Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab. Invest. 1997;76:651–660. [PubMed] [Google Scholar]
- 229.Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin–mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662–4671. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- 230.Vallés AM, Boyer B, Tarone G, Thiery JP. a2b1 integrin is required for the collagen and FGF-1 induced cell dispersion in a rat bladder carcinoma cell line. Cell Adhes. Commun. 1996;4:187–199. doi: 10.3109/15419069609014222. [DOI] [PubMed] [Google Scholar]
- 231.Medici D, Nawshad A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-κB and LEF-1. Matrix Biol. 2010;29:161–165. doi: 10.1016/j.matbio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH2-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–11753. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- 233.Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I–mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Garamszegi N, Garamszegi SP, Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL, Scully SP. Extracellular matrix-induced transforming growth factor-β receptor signaling dynamics. Oncogene. 2010;29:2368–2380. doi: 10.1038/onc.2009.514. [DOI] [PubMed] [Google Scholar]
- 235.Gonzalo P, Moreno V, Gálvez BG, Arroyo AG. MT1-MMP and integrins: Handto-hand in cell communication. Biofactors. 2010;36:248–254. doi: 10.1002/biof.99. [DOI] [PubMed] [Google Scholar]
- 236.Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial–mesenchymal transition. Matrix Biol. 2011;30:243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: Master regulators of metastasis. Clin. Cancer Res. 2010;16:5928–5935. doi: 10.1158/1078-0432.CCR-10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1a promotes metastasis. Nat. Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 239.Ryan HE, Lo J, Johnson RS. HIF-1 a is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 241.Wu MZ, Tsai YP, Yang MH, Huang CH, Chang SY, Chang CC, Teng SC, Wu KJ. Interplay between HDAC3 and WDR5 is essential for hypoxia-induced epithelialmesenchymal transition. Mol. Cell. 2011;43:811–822. doi: 10.1016/j.molcel.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 242.Aryee DNT, Niedan S, Kauer M, Schwentner R, Bennani-βaiti IM, Ban J, Muehlbacher K, Kreppel M, Walker RL, Meltzer P, Poremba C, Kofler R, Kovar H. Hypoxia modulates EWS-FLI1 transcriptional signature and enhances the malignant properties of Ewing’s sarcoma cells in vitro. Cancer Res. 2010;70:4015–4023. doi: 10.1158/0008-5472.CAN-09-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Heikkinen PT, Nummela M, Jokilehto T, Grenman R, Kähäri VM, Jaakkola PM. Hypoxic conversion of SMAD7 function from an inhibitor into a promoter of cell invasion. Cancer Res. 2010;70:5984–5993. doi: 10.1158/0008-5472.CAN-09-3777. [DOI] [PubMed] [Google Scholar]
- 244.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KSK, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Herranz N, Dave N, Millanes-Romero A, Morey L, Díaz VM, Lórenz-Fonfría V, Gutierrez-Gallego R, Jerónimo C, Di Croce L, García de Herreros A, Peiró S. Lysyl oxidase-like 2 deaminates lysine 4 in histone H3. Mol. Cell. 2012;46:369–376. doi: 10.1016/j.molcel.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 246.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Dev. Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 247.Zheng X, Linke S, Dias JM, Zheng X, Gradin K, Wallis TP, Hamilton BR, Gustafsson M, Ruas JL, Wilkins S, Bilton RL, Brismar K, Whitelaw ML, Pereira T, Gorman JJ, Ericson J, Peet DJ, Lendahl U, Poellinger L. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 2008;105:3368–3373. doi: 10.1073/pnas.0711591105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, Wang H, Huang C, Sun S. Hypoxiainduced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLOS One. 2012;7:e30771. doi: 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Heisenberg CP, Solnica-Krezel L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr. Opin. Genet. Dev. 2008;18:311–316. doi: 10.1016/j.gde.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 251.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 252.Nawshad A, Medici D, Liu CC, Hay ED. TGFβ3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J. Cell Sci. 2007;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Iekushi K, Taniyama Y, Azuma J, Sanada F, Kusunoki H, Yokoi T, Koibuchi N, Okayama K, Rakugi H, Morishita R. Hepatocyte growth factor attenuates renal fibrosis through TGF-β1 suppression by apoptosis of myofibroblasts. J. Hypertens. 2010;28:2454–2461. doi: 10.1097/HJH.0b013e32833e4149. [DOI] [PubMed] [Google Scholar]
- 255.Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J. Am. Soc. Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 256.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFβ-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 257.Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, Evers BM, Zhou BP. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J. Clin. Invest. 2012;122:1469–1486. doi: 10.1172/JCI57349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin. Exp. Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 260.Aragón E, Goerner N, Zaromytidou AI, Xi Q, Escobedo A, Massagué J, Macias MJ. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev. 2011;25:1275–1288. doi: 10.1101/gad.2060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]