Summary
Declarative memories are thought to be stored within anatomically distributed neuronal networks requiring the hippocampus; however, it is unclear how neocortical areas participate in memory at the time of encoding. Here, we use a c-fos-based genetic tagging system to selectively express the channelrhodopsin variant, ChEF, and optogenetically reactivate a specific neural ensemble in retrosplenial cortex (RSC) engaged by context fear conditioning. Artificial stimulation of RSC was sufficient to produce both context-specific behavior and downstream cellular activity commensurate with natural experience. Moreover, optogenetically, but not contextually-elicited responses were insensitive to hippocampal inactivation, suggesting that although the hippocampus is needed to coordinate activation by sensory cues, a higher-order cortical framework can independently subserve learned behavior, even shortly after learning.
Keywords: associative memory, c-fos, optogenetics, retrosplenial cortex, fear conditioning
Introduction
The ability to form lasting associations is a critical evolutionary adaptation; yet, relatively little is known about how these experiences are biologically represented within distributed neuroanatomical networks. Since the early studies of amnesic patient H.M., much of memory research has focused on the hippocampus, a subcortical structure known to be essential for the formation of explicit memories, which are generally accessible to conscious recall in humans (Squire, 1986). Although cortical networks are known to undergo activation and communicate with hippocampal circuits during both sensory experience and memory-related activity (Bontempi et al., 1999; Frankland et al., 2004; Frankland et al., 2006; Goshen et al., 2011; Maviel et al., 2004; McClelland and Goddard, 1996; Smith et al., 2012; Tse et al., 2011; Wang and Morris, 2010), the consequences of their interactions for memory encoding and retrieval is unknown. To date, several theoretical views of systems-level memory consolidation have emerged based on the observation that hippocampal damage in both humans and rats causes temporally graded retrograde amnesia, such that newer memories are lost, while older memories are spared (Anagnostaras et al., 1999; Bayley et al., 2003; Bontempi et al., 1999; Reed and Squire, 1998). Although different in abstract principles, these ideas converge on a common premise that hippocampal networks provide an index, map or unified representation of memory based on multiple cortically processed sensory features (Eichenbaum, 2000; Morris, 2006; Nadel and MacDonald, 1980; Nadel et al., 2000; O’Reilly and Rudy, 2001; Tse et al., 2007). According to prevailing models, cortical representations gradually acquire independence from the hippocampus over time (Teng and Squire, 1999; Zola-Morgan and Squire, 1990), either through synaptic strengthening via internal replay of patterned activity, or by the formation of separate traces, incorporated into memory during retrieval to generate a flexible and anatomically distributed framework for long-term associations.
Several circuit-based mechanisms could account for the observed interactions between hippocampal and cortical systems: 1) learning-induced cellular activity or plasticity within the hippocampus itself sufficient to store information and engage the cortex during retrieval; 2) concurrent encoding of task-relevant information in both the hippocampus and cortex, which synergistically retrieve memory within a coordinated network; or 3) direct acquisition of information in neocortical ensembles, with hippocampal neurons providing location-specific input to higher-order circuits during retrieval. Recently published work has reported that sparse neural ensembles are recruited during contextual fear conditioning (Garner et al., 2012) and when targeted in the dentate gyrus (DG) can be directly stimulated to induce context-specific fear expression (Liu et al., 2012; Ramirez et al., 2013). Although this finding supports the view that learning-related patterns of cellular activity in the hippocampus can be used to artificially recapitulate memory retrieval, these studies do not address whether DG circuits serve as a unique cellular signature with an obligatory function in contextual memory. Here, we investigate if patterns of activity generated in higher-level cortical structures at the time of learning are sufficient to drive contextual memory recall. By optogenetically bypassing the hippocampus, we revealed a targetable circuit in retrosplenial cortex (RSC), able to drive context-specific behavior less than two days after training.
We combined a c-fos based genetic tagging system with optogenetic stimulation to functionally target and “tag” cells recruited during contextual fear conditioning. We focused on the RSC because this region has strong reciprocal connectivity with the hippocampus, entorhinal cortex, and numerous sensory and subcortical areas involved in emotional learning, making it a likely participant in hippocampal learning mechanisms. In addition, this area is essential for spatial cognition and memory, and has been shown to participate in the retrieval of both recent and remote contextual fear memory (Bucci and Macleod, 2007; Corcoran et al., 2011; Keene and Bucci, 2008a, b, c, 2009; Robinson et al., 2011; Robinson et al., 2012). We found that stimulation of the neural ensembles activated in the RSC with contextual learning were sufficient to produce fear memory retrieval. Moreover, artificially induced retrieval activated downstream cell populations in the central and basal nuclei of the amygdala that were common to those activated by natural memory recall. Finally, we show that while natural contextual fear memory retrieval was impaired by hippocampal inactivation, optogenetically reactivated memory, through direct stimulation of RSC ensembles, could bypass this requirement, leading to expression of contextual fear even in the absence of hippocampal activity. Taken together, these results are consistent with the idea that a redundant encoding mechanism enables recent contextual memories to drive behavior through dissociable cortical and hippocampal pathways.
Results
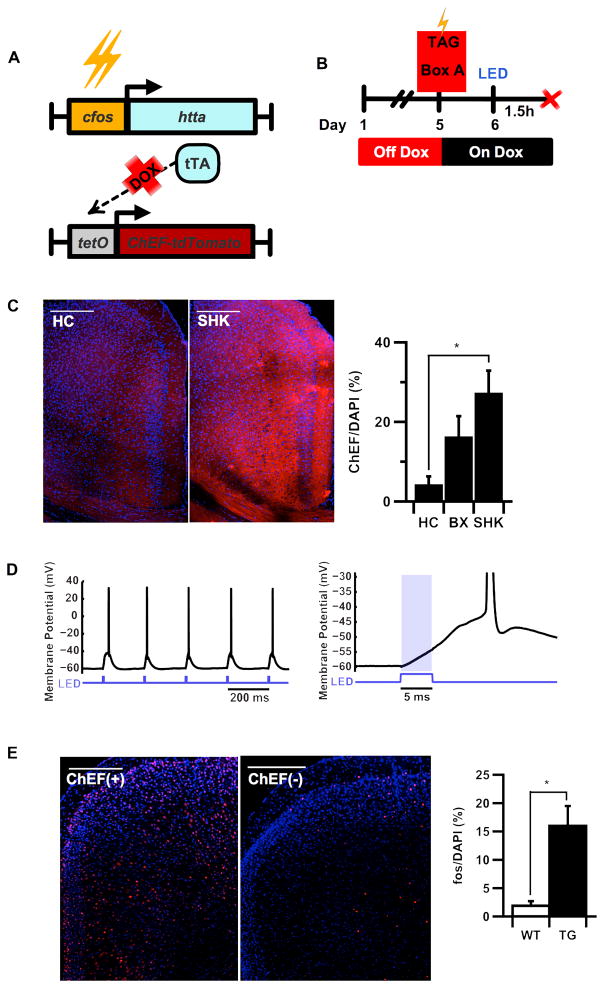
To test the idea that RSC might contribute to a cellular representation of contextual memory, we used the Tet Tag mouse line, (Reijmers et al., 2007), which expresses the tetracycline transactivator (tTA) under control of the neural activity-responsive c-fos promoter. To generate the tet-off bitransgenic line used in this study, Tet Tag mice were crossed with a tetO-ChEF-tdTomato transgenic line, enabling us to selectively express the channelrhodopsin variant, ChEF (Lin et al., 2009) in active neurons, while also allowing us to use doxycycline (Dox) to globally restrict transgene expression to a defined temporal window. As shown in Figure 1A, mice were removed from Dox for four days to open a window for c-fos-mediated ChEF transgene expression (Fig. 1B). Mice that received a series of footshocks (SHK) on the fifth day after Dox removal showed a significant increase in RSC levels of ChEF-tdTomato compared to homecage controls (HC) (Fig. 1C). We did not observe a significant difference in the total number of RSC cells tagged in box- compared to shock-tagged mice using either of two cell counting thresholds (Fig. 1C; Supp. Fig. S1A). These numbers are comparable to those previously reported for hippocampus (Matsuo et al., 2008) and are consistent with physiological data from RSC (Smith et al., 2012). Thus, context-driven activity in RSC appears to engage a cellular ensemble that either overlaps with context-shock-responsive cells, or is unique but similar in size. Immunofluorescence confirmed that multiple cell types express fos-induced ChEF in RSC after natural learning-related activity, thus the relevant ensemble includes both excitatory and inhibitory contributions (Supp. Fig S1E–F). 5Hz stimulation of ChEF(+) RSC neurons with light (454nm) was sufficient to trigger action potential firing in an acute slice preparation (Fig. 1D), and significantly increase c-fos protein expression after in vivo delivery of light pulses through a cranial window (Fig. 1E). Moreover, analysis of direct overlap between ChEF-expressing neurons tagged during training in Box A and retrieval-induced endogenous c-fos expression (Supp. Fig. S2A) showed a significantly higher percentage of reactivated (fos-expressing) ChEF(+) cells after a 24h memory test in Box A, compared to mice exposed to novel Box B (Supp. Fig. S2B). Similarly, 30–50% percentage of ChEF+ cells colocalized with c-fos 90 min. after LED stimulation (Supp. Fig. 1C).
Figure 1.
(A) Schematic of the fos/tTA-tetO/ChEF-tdTomato bitransgenic system and (B) experimental protocol used to tag memory-related circuits in RSC. (C) Behavioral induction of transgene expression in RSC measured 1 day after training off dox. Footshock (SHK) induced significantly greater expression of ChEF-tdTomato protein than homecage (HC) (ANOVA, F(2,20) = 3.907, main effect P= 0.037*, post-hoc Fisher LSD, FC x HC, P=0.012*; BX x HC, P =0.188, n.s.). Images show ChEF-tdTomato expressed in RSC (red) 24h post-induction counterstained with DAPI (blue). (D) Whole cell recording of an RSC layer 2/3 pyramidal cell in an acute slice preparation (n=7 neurons in 2 mice). 5Hz light pulses evoked large short latency depolarizations (latency < 1 ms) in two regular spiking (RS) neurons (putative pyramidal cells) and reliably evoked action potentials when cells were held at −60 mV (top). The time-course of activation indicates direct optical activation of this cell by light (bottom). (E) Transgenic mice receiving LED stimulation show signficantly higher levels of c-fos protein 90min after LED stimulation of RSC (t-test, t(18.512)=3.936, P=0.001**). Confocal images depict optical induction of c-fos protein (red) with DAPI counterstain (blue) in transgenic and wildtype brains perfused 90′ after LED stimulation. Error bars indicate s.e.m.; scale bar = 250uM; *indicates statistical significance at the level of P=0.05; **indicates statistical significance at the level of P=0.001.
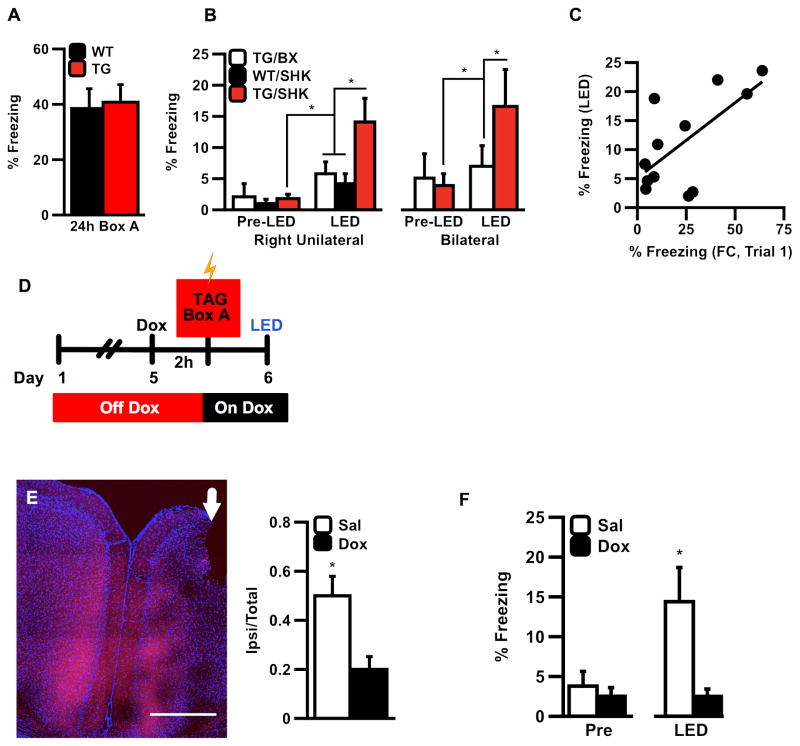
Expression of ChEF protein in transgenic (TG) mice did not affect normal contextual fear memory compared to wildtype (WT) controls (Fig. 2A). To determine if RSC ensemble stimulation was sufficient to drive fear-related behavior (freezing), we fear conditioned mice off Dox with four 1mA shocks (SHK) so as to genetically tag an active ensemble in the RSC of TG mice. Mice were then returned to high-dose Dox chow overnight to suppress further ChEF expression, and the next day received either right unilateral or bilateral 5Hz light stimulation of RSC in a neutral arena (light was delivered by an LED mounted to the skull). Results confirmed that neither un-shocked transgenic box controls (TG/BX) nor shocked wildtype (WT/SHK) littermates froze significantly above baseline (Pre) during LED stimulation. In contrast, TG mice that were fear conditioned (SHK) froze significantly more than mice in either of the control conditions during LED stimulation, regardless of whether light was applied bilaterally or right unilaterally (Fig. 2B, Supp. Table S1). In addition, mean LED-induced freezing among TG/SHK mice was significantly correlated with shock-induced freezing triggered in the first post-shock interval (a measure of initial learning, Fig. 2C).
Figure 2.
(A) Natural long-term memory recall in wildtype (WT) and transgenic (TG) mice re-exposed to the fear conditioning chamber (Box A) 24h post-training. (B) Transgenic/Shock (TG/SHK) mice froze significantly more than controls in response to right unilateral (left) and bilateral (right) stimulation of RSC. Pre-LED freezing did not differ between groups (ANOVA, Pre-LED Freezing, F(2,17)=0.145; P=0.866, n.s), whereas LED-induced freezing was significantly increased only in TG/SHK mice during the LED test. (ANOVA, main effect of LED Test x Group F(2,17)=3.939, main effect, P=0.039*; Test x Group interaction (F(2,19)=4.94, 0.02); post-hoc Fisher LSD, TG/SHK x TG/BX, P=0.039*; TG/SHK x WT/SHK, P=0.020*). Post-hoc analysis of significant main effects obtained from comparisons of Pre vs. LED test (main effect, F(1,20)=23.62, P=0.0002**) and Group vs. LED test revealed a significant interaction (F(2,19)=4.94, P=0.020*) revealed that of the three groups, only TG/SHK mice froze significantly more during LED stimulation, as measured by a within-subject comparison (by group) of the calculated difference between Pre-LED and LED freezing (see also Supp. Table S1 for all statistics). (C) Across all uni- or bilaterally stimulated TG/SHK mice (N=12 total), LED-induced freezing was significantly correlated with trial 1 of learning (R=0.665, P=0.018*). (D) To assess the anatomical and temporal specificity of the training induced tag, mice received pre-training intra-RSC microinfusions of Dox (50ug, right side/white arrow). (E) Micro-infusion of Dox significantly reduced expression of ChEF-tdTomato ipsilateral to the site of infusion (t-test, t(13)=2.815, P=0.015*). (F) Mice infused with Dox showed significantly reduced LED-triggered freezing (t-test, t(8.34)=2.728, P=0.025) than mice infused with saline. Error bars indicate s.e.m.; scale bars = 1000uM; *indicates statistical significance at the level of P=0.05.
To confirm that the observed behavioral difference was specifically attributable to the expression of ChEF in RSC cells during training, rather than due to fibers of passage or background activity in homecage, we blocked c-fos-induced ChEF expression by microinfusion of either Dox or saline directly into RSC 2h prior to fear conditioning (Fig. 2D–E). We reasoned that if a relevant pattern of ChEF was expressed in RSC during training, then acute blockade of learning-related expression would impair the efficacy of LED-induced recall the next day. Consistent with this hypothesis, we observed that animals receiving Dox froze significantly less than saline-infused mice during LED stimulation trials (Fig. 2F). These results suggest that RSC participates in the initial phase of context fear memory formation.
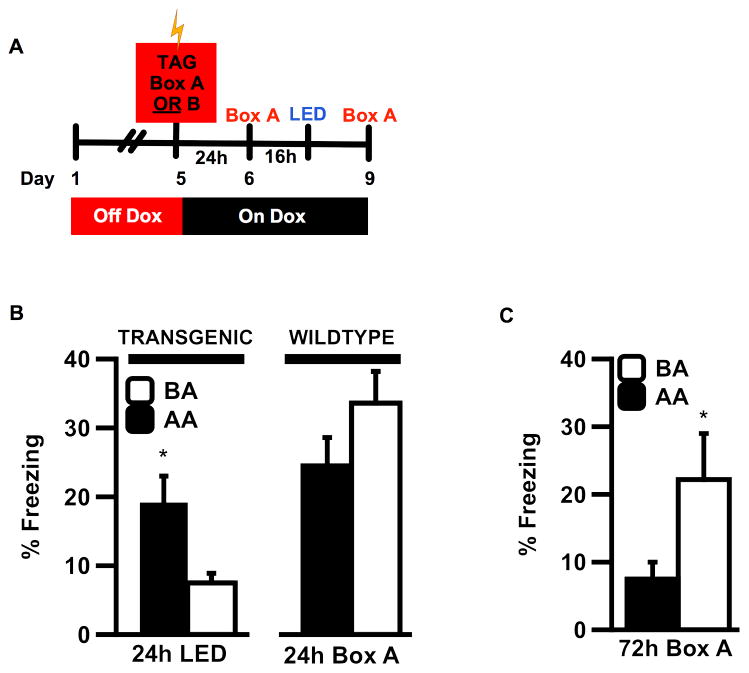
Previous studies suggest that some place-responsive neurons in the hippocampus and RSC undergo context-specific shifts in their firing patterns after the location is paired with a salient emotional cue (Moita et al., 2003; Smith et al., 2012). Since our initial experiments involved tagging mice during an aversive experience and comparing responses from shocked mice to those from un-shocked mice, our next question was whether RSC encodes a specific representation of the CS (context), the US (shock) or a novel representation of the conjunction of the two stimuli. We therefore tagged active neural ensembles with ChEF prior to conditioning in a neutral (Box A or Box B) and tested LED-induced freezing after subsequent training in Box A (Fig. 3A). Thus, instead of tagging mice for 40min in Box A during training, mice were tagged for 40min in either Box A or Box B the day prior to training. Following tagging, mice were returned to Dox to prevent further expression of ChEF, and all mice in both groups received training in Box A the next day, followed by LED stimulation of RSC. Thus, mice receiving the (A/A) sequence were shocked in the same box where contextual tagging (CS only) had occurred, while mice receiving the (B/A) sequence were shocked in a completely different context. Three possible outcomes could be anticipated from this experiment: 1) only A/A mice freeze during LED stimulation, suggesting that the tagged Box A representation is sufficiently stable to be linked to the shock during subsequent conditioning in Box A; 2) both A/A and B/A mice express LED-induced freezing, suggesting that reactivation of any tagged network in RSC is sufficient to reactivate a shock association, suggesting encoding of the US shock component; or 3) Neither A/A nor B/A mice express LED-induced freezing, suggesting that the Box A association with shock forms a unique representation that cannot be accessed by reactivating cells tagged in a neutral context.
Figure 3.
(A) Timecourse for experiments testing the contextual specificity of a tagged neuronal representation. Mice were tagged with exposure to Box A or B, returned to dox, and received fear conditioning in Box A 24h later. (B) TG mice pre-exposed to Box A showed significantly more LED-induced freezing than mice pre-exposed to Box B (left) (ANOVA, main effect of group during LED (N=8/6, F(1,12)=5.053, P=0.044) and did not significantly differ from WT mice tested for natural Box A memory at the same timepoint after training (right) (group x genotype interaction, P=0.001*; post-hoc t-test for TG vs WT in AA: t(12)=−0.955, P=0.358, n.s.). (C) 24h after stimulation (72h post-training), transgenic mice in the A/A group froze significantly less than mice in the B/A group (t-test, N=8/6, t(12)= −2.246, P=0.043*). Error bars indicate s.e.m.; *indicates statistical significance at the level of P=0.05.
Analysis of the data from this experiment showed that, consistent with the idea of a context-specific tag, mice in the A/A group froze significantly more than mice in the B/A group (Fig. 3B, left). Moreover, the level of LED-induced freezing in A/A mice was not significantly different from natural level of context fear memory observed in WT mice that were similarly pre-exposed to Box A or B, trained in Box A, and tested for natural LTM in Box A at the same time-point post-training (Fig. 3B, right). These results suggest that even when tagging occurs prior to training, RSC retains a sufficiently stable context representation to permit reactivation of a fear memory formed later in the same context.
If LED stimulation of a ChEF-expressing neural ensemble is sufficient to directly reactivate memory of Box A, then LED-induced retrieval might modify properties of the original memory circuit, just as extinction training (box re-exposure without shock) alters activity in cells previously sensitive to fear-inducing stimuli. To determine if any such change in the original memory occurs, we completed the experiment by testing transgenic animals for natural memory of Box A 48h after the LED stimulation. Interestingly, A/A mice froze significantly less than B/A mice (Fig. 3C), suggesting that by targeting artificial LED stimulation to cells tagged in Box A, we were able to induce a behavioral modification to the natural Box A association, possibly similar to extinction. In contrast, stimulation of cells tagged in Box B, which remained neutral and unrelated to training Box A, had significantly less effect on the expression of natural LTM in Box A.
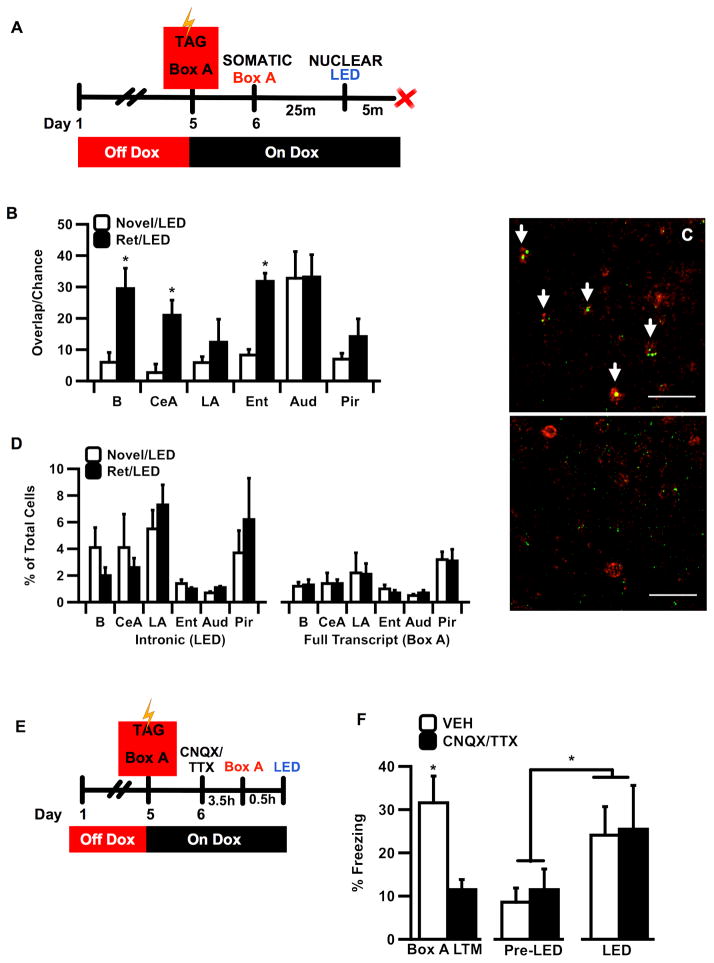
Optogenetic activation of neural ensembles differs markedly from natural sensory-based recall in its lack of temporal patterning or synchronization to endogenous rhythms. Although it is difficult to know the nature of the mouse’s experience, our behavioral results imply that local stimulation of a relevant ensemble of RSC neurons is sufficient to produce a complex perceptual experience similar to natural recall. If natural and optogenetic stimulation are indeed similar in terms of their ability to drive behavior via common patterns of cellular activity, then downstream ensembles engaged by both optogenetic and natural recall should overlap, even in areas distant from RSC. To test this hypothesis we used cellular compartment analysis of temporal activity by fluorescence in situ hybridization (catFISH) (Guzowski et al., 1999; Guzowski et al., 2006) to determine if bouts of activity triggered by natural recall and artificial RSC stimulation would recruit overlapping neurons in the amygdala, a brain region critical for the storage and regulation of aversive memories (Fig. 4A). In addition, we measured mRNA expression in entorhinal, auditory and piriform cortices.
Figure 4.
(A) Timecourse for behaviorally induced synthesis of full length (somatic) and intronic (nuclear) c-fos mRNA transcripts after sequential bouts of activity triggered by natural and optogenetic recall events, respectively. (B) Compared to animals that were exposed to a novel box prior to LED stimulation (Novel/LED, N=3), animals that received a retrieval test in Box A (Ret/LED, N=5) showed significantly higher co-expression (overlap observed/chance) of somatic and nuclear mRNA in basal amygdala (B) (t-test, t(6)= −2.637; P=0.039*), Central Amygdala (CeA) (t(6)= −2.716; P=0.035*) and Entorhinal Cortex (Ent) (t(4)= −6.272; P=0.003*), whereas no significant group differences were observed in lateral amygdala (LA) (t(6)= −0.666, P=0.53), auditory cortex (Aud) (t(6)= −0.035, P=0.973), or piriform cortex (Layer 2) (Pir) (t(6)=0.949, P=0.379). Extended statistical analysis in Supp. Table S2 shows ANOVA and post hoc Fisher LSD test results comparing observed % overlap with chance for each brain region and experimental group. Chance overlap was calculated as P(intronic) x P(full length)). (C) Confocal image from basal amygdala tissue in a RET-LED individual. White arrows indicate examples of cellular overlap of somatic (red) with intronic (green) c-fos mRNA transcripts in RET mice (top) compared to NR mice (bot), which showed significantly less transcript overlap; scale bar = 50uM; (D) Total expression of mRNA did not significantly differ between the RET and NR groups in any brain region analyzed. (E) Schematic of behavioral paradigm used for hippocampal inactivation (F) Mice infused with CNQX/TTX expressed significantly less freezing than Veh when tested for natural memory in Box A (ANOVA, F(1,9)=10.414, P=0.01*); however; mice receiving dHPC microinfusions of CNQX/TTX and Vehicle froze significantly above baseline (Pre-LED) levels during LED stimulation of RSC (repeated measures ANOVA N=5/6, F(1,9)=14.925, P=0.004*) with no significant difference between groups. Post-hoc comparison of Pre-LED versus LED-induced freezing for each group revealed significantly increased freezing during LED stimulation in both the Veh and Drug treatment conditions (Fisher LSD, P=0.022, Veh; P=0.024, CNQX/TTX). B, basal amygdala; CeA, central amygdala; LA, lateral amygdala; Ent, entorhinal cortex; Aud, primary auditory cortex; Pir, layer 2 piriform cortex. dHPC, dorsal hippocampus. Error bars indicate s.e.m; *indicates statistical significance at the level of P=0.05.
Mice were context fear conditioned in Box A and the following day received either a 5-minute retrieval test in Box A (Ret), or an equivalent novel box exposure (Novel). Twenty-five minutes later, all mice were given LED stimulation and were perfused for catFISH 5 minutes later. To differentiate cellular activation from the first (natural retrieval) versus the second (optogenetic) bouts of activity, tissue was co-labeled with RNA probes directed against either the full-length (somatic) or intronic (nuclear foci) c-fos transcript, respectively. Cell counts obtained from confocal scans of hybridized sections revealed that the c-fos intronic and full-length mRNA transcripts induced by the sequential bouts of activity overlapped at significantly higher levels in basal amygdala (B), central amygdala (CeA), and entorhinal cortex (Ent) from Ret/LED mice compared to Novel/LED mice (Fig. 4B, C). In contrast, overall levels of mRNA expression did not differ between groups in any region analyzed at either time-point (Fig. 4D). Statistical analysis of cells with somatic and/or nuclear mRNA localization revealed significant region-specific differences in the percent overlap of labeled cells compared to overlap expected by chance (Supp. Table S2) (Reijmers et al., 2007; Tayler et al., 2013). Labeled cells within amygdala subregions and Ent overlapped significantly above chance in reactivated (Ret/LED) mice, while cellular overlap did not differ from chance in non-reactivated controls (Novel/LED). Interestingly, labeled cells in piriform and auditory cortices overlapped significantly above chance in both experimental groups, perhaps reflecting patterned activity triggered by ambient background stimuli (olfactory or auditory) or other coordinated network activity within these primary sensory areas.
Previous studies have shown that contextual representations are formed in the dentate gyrus during learning; however, our data suggest that this subcortical memory is mirrored by a higher-level representation that encodes meaningful context-specific information at the time of initial learning. Based on these findings, we asked if contextual representations in RSC are sufficient, in and of themselves, to drive reactivation of fear memory independent of a hippocampal contribution. Two groups of TG mice were first trained and tagged in Box A, as previously described. The next day, we pharmacologically inactivated the dorsal hippocampus (dHPC) by local microinfusion of the glutamate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and the sodium channel blocker, tetrodotoxin (TTX), which have previously been shown to impair context memory retrieval (Kitamura et al., 2009; Wiltgen et al., 2010). Mice were then tested for LTM in Box A, followed, 30 minutes later by LED stimulation of RSC in a novel context. Consistent with the known involvement of the dHPC in memory retrieval (Amaral et al., 2007; Conejo et al., 2013; Holt and Maren, 1999; Lorenzini et al., 1996; Moser and Moser, 1998; Riedel et al., 1999), CNQX/TTX-infused mice expressed significantly attenuated memory during the natural memory test in Box A (Fig. 4F) and expressed a significant reduction in c-fos expression in CA1 (Supp. Fig. S3). However, both drug and vehicle groups froze at equivalent levels during direct RSC stimulation induced recall (Fig. 4F). This result indicates that the pattern of neural activity in the RSC at the time of learning encodes a coherent and independent representation of the context that can drive behavior when stimulated directly but requires hippocampal activity during natural recall.
Discussion
The role of the hippocampus in declarative memory has been studied extensively; however, its cellular contribution to memory, and its involvement in higher-level cortical processing remain matters of controversy in systems neuroscience. Nevertheless, the presence of a cooperative hippocampal-cortical dynamic is widely acknowledged as essential for the formation of conjunctive representations, where individual sensory elements are bound to a global contextual identity that can be uniquely associated with aversive or rewarding events, like those occurring in Pavlovian fear conditioning (Rudy and O’Reilly, 2001). Despite the common view of cortical circuits as primarily important for late-stage consolidation, some intriguing evidence suggests that these ensembles are recruited into the memory circuit within hours of acquisition (Corcoran et al., 2011; Lesburgueres et al., 2011). Our findings go further to suggest that unitary cellular representations of context, which have often been assumed to exist within hippocampal circuits, are concurrently established in RSC at the time of learning or shortly thereafter. Moreover, the finding that direct RSC stimulation is sufficient to drive context-specific responding in the absence of hippocampal engagement, suggests that RSC instantiates a coherent cellular framework for memory that can drive behaviorally relevant activity as an independent memory network. Interestingly, the well-documented finding that dorsal hippocampal silencing impairs natural context-elicited behavior remains consistent with the view that, short of direct top-down stimulation, hippocampal processing of ongoing spatial cues is needed to recruit the appropriate cortical circuits.
Recent reports of contextual recall by optogenetic stimulation of hippocampal ensembles have focused attention on hippocampal networks as the primary cellular site for memory storage. Our results, however, suggest that complex contextual representations may not be exclusive to hippocampal ensembles. Instead, a representation of contextually relevant information memory also appears to occur within higher-level RSC circuits at the time of initial learning. Our data are therefore consistent with the theory of a hippocampal map or index for associations that may be stored redundantly in cortical systems (Moscovitch et al., 2005; Nadel, 1992; O’Keefe, 1990; O’Keefe and Black, 1977). Our findings diverge from these ideas, however, in demonstrating that memory-related information can be directly accessed via anatomically localized cortical stimulation, suggesting that although the hippocampus may participate in retrieval under natural circumstances, it can also be uncoupled from the memory recall process if cortical networks are directly engaged.
Compared to the hippocampus, much less research has focused on the involvement of RSC in memory; yet a surprisingly consistent and compelling body of literature in both humans and rodents has emerged, demonstrating critical functions of RSC in the ability to retrieve episodic memories and emotional associations (Aggleton, 2010; Katche et al., 2013b; Keene and Bucci, 2008a, c); to integrate relationships among distinct sensory cues (Bucci and Macleod, 2007; Keene and Bucci, 2008b; Smith et al., 2012); to navigate (Auger and Maguire, 2013; Clark et al., 2010; Cooper and Mizumori, 2001); and to identify familiar or meaningful places in space (Auger et al., 2012; Cho and Sharp, 2001; Smith et al., 2012). Moreover, the large surface area of this structure, its densely interconnected organization as a site of anatomical convergence among sensory, limbic and spatial systems (Shibata et al., 2009), and its known physiological interactions with the medial temporal lobe (Albasser et al., 2007; Cooper and Mizumori, 2001; Dumont et al., 2010; Robinson et al., 2012; Sherrill et al., 2013; Smith et al., 2012), make RSC uniquely suited to host a cellular record of contextual experience. Given the striking level of intra-cortical connectivity observed in RSC across multiple sensory modalities, this area could even be theorized to serve as an index for contextual memory itself.
Despite major technological advances in optogenetic tools, studies using animal models to study brain circuit function are limited by their reliance on primitive behavioral readouts that do not provide true measures of subjective perceptual experience. As a result, experiments using artificial stimulation to drive behavior often rely on the untested assumption that if natural and artificial patterns of circuit activity produce the same behavioral output, they must do so by a common neural mechanism. How can we confirm that artificially induced freezing triggers patterns of brain activity that accurately recapitulate the stimulus-driven process of memory recall? The current study begins to address this question by examining patterns of overlapping cellular activity in brain areas downstream of the RSC after individual mice were subjected to both optogenetic and natural recall. These data revealed a high degree of overlap, in both amygdala and entorhinal cortex, among cells activated by the optogenetic tag and those activated by the fear-inducing context itself. This observation suggests that, at least at the level of neural circuits, optogenetic stimulation of specific RSC circuits can be validly related to the natural physiological process of memory retrieval. Future studies taking advantage of the CatFISH approach in conjunction with artificial stimulation protocols may provide deeper insights into how best to interpret optogenetically generated behaviors.
Taken together, these data provide novel evidence that recently formed cortical representations of memory can coherently drive learned behaviors that mimic stimulus-driven retrieval at the level of single cells. In addition, artificial activation of the RSC trace was sufficient to independently initiate an otherwise hippocampus-dependent response, thereby unmasking the presence of a redundant pathway for new memory encoding and maintenance. Moreover, these findings supports the idea that information accessed through hippocampal networks is neither exclusive nor unique to that locus, and that cortical circuits are concurrently recruited to establish a stable record of experience (Goshen et al., 2011; Katche et al., 2013a; Smith et al., 2012). Future investigations will be necessary to determine how other cortical connections involving prefrontal, anterior cingulate, and entorhinal cortex also participate in storing and conveying relevant contextual information to subcortical structures like the amygdala. Finally, these results lend credence to the long-standing, yet previously untested assumption that targeted artificial stimulation of relevant pathways can generate behavior through patterns of brain activity that directly converge with those triggered by natural stimuli.
Our data and previous studies in the DG (Liu et al., 2012; Ramirez et al., 2013) demonstrate that the c-fos-based tagging system can be used to artificially induce contextual memory recall. This is surprising given the artificial nature of the activity produced with ChEF, as well as the fact that the genetic tagging system is likely to label a variety of neurons not specific to the context (eg. persistently active cells, cells associated with homecage, handling, room noise etc.). Future studies will be needed to determine exactly how many cells are required for retrieval and how much noise is tolerated by given network. The fact that RSC stimulation is sufficient to drive a meaningful and resolvable network signal, despite potential interference from ongoing sensory input, internally generated ensembles, and spontaneous firing, is consistent with the observation that emotional associations are readily excitable and disruption-resistant. These data also suggest that memory reactivation is less contingent on precise temporal and spatial firing patterns than previously thought.
Experimental Procedures
Subjects
Double transgenic c-fos-tTA/tetO-ChEF mice were generated by crossing single transgenic c-fos-tTA (Reijmers et al 2007) and tetO-ChEF-tdTomato mice bred on a C57BL/6J background. Mice used in behavioral experiments were 8–16 weeks old and were group housed on a 12h/12h light dark cycle with food and water ad libitum. Control experiments involving wildtype mice were conducted using single transgenic littermates of experimental subjects. All procedures were conducted in compliance with The Scripps Research Institute and National Institutes of Health guidelines for humane care and use of animals.
LED attachment
For bilateral stimulation, mice were anesthetized with an oxygen/isoflurane mix and the skull was thinned to create a 1mm2 translucent window above retrosplenial cortex (anterioposterior −1.58mm; mediolateral: ±0.5mm at window center), extending 0.5mm into each hemisphere from the mid-sagittal suture). Area of light penetration was limited by surrounding entire skull surface except for thinned skull window with a layer of opaque black lacquer. Water/heat-proof silicon-encased SMD 5050 tri-chip ultra-bright LEDs (oznium.com) were purchased pre-wired with resistors and affixed with clear superglue over skull window. Light intensity at brain surface ranged from 3.5 to 4.1 mWmm−2 (mean=3.8mWmm−2), obtained by pulsing light at 5Hz through a 1mm2 thinned skull window (removed post-mortem from an experimental animal) and placed over a light sensor (ThorLabs, S302C) connected to a power meter (ThorLabs, PM100USB). Estimated light spread is ≤250uM from surface, based on previously published estimates (Huber et al., 2008).
Drug Infusions
For local infusions of doxycycline (50μg dissolved in 0.5uL 0.9% saline) into RSC, drug or vehicle was unilaterally infused below the center of the LED (anterioposterior −1.5mm; mediolateral: ±0.5mm, dorsoventral: 0.5mm) (Paxinos & Watson 2001). Infusions took place over 10 minutes at a rate of 0.2μL/minute. For intra-hippocampal infusions, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20mM, Wako) and tetrodotoxin (TTX, 3mM, Tocris) were dissolved 0.9% saline and bilaterally micro-infused into the dorsal hippocampus at a rate of 0.2μL/min (0.5μL per side, anterioposterior −2.0mm; mediolateral: ±1.7mm, dorsoventral: 1.5mm; Paxinos & Watson 2001).
Behavior/Optical Stimulation
Four days after removal from low-dose doxycycline chow (40mg/kg), mice were context fear conditioned (Med Associates). Training occurred over 40 minutes and began with a 4-minute period of chamber acclimation, followed by four 1-second footshocks (1mA, mean ITI=100s). 15–20h after training, mice received LED stimulation (5ms pulses, 5Hz) in a novel open field arena. Stimulation was preceded by six minutes of context acclimation, followed by three 1-minute trials of light pulses, each separated by a 1-minute period of light off. After stimulation, mice were returned to their homecages and perfused for immunofluorescence 90 minutes later. For A/A-B/A experiments, mice were tagged during a single 40-minute exposure to ‘Box A’ (fear conditioning chamber) or ‘Box B’ (novel arena B). Mice were trained (on dox) in Box A 16h later (4x 1s 1mA footshocks, 525s total session) and received LED stimulation in Box C (novel arena C) 24h post-training.
Immunofluroescence
Following completion of all behavioral procedures, mice were deeply anesthetized with isoflurane and transcardially perfused with 4% paraformadelhyde. Brains were post-fixed in PFA overnight and were sectioned by vibratome at 50uM. Sections were blocked in 10% Normal goat serum/0.2% Triton X and were probed with an anti-cFos IgG (Chemicon, 1:750) followed by an anti-rabbit Alexa-488 or Alexa-647 fluorescent dye-conjugated secondary antibody (Invitrogen, 1:700). Sections were mounted, counterstained with DAPI and cover-slipped before imaging (Invitrogen, Slowfade with DAPI).
In situ hybridization
Brains were perfused with 4% paraformaldehyde and 40 μm free-floating sections were incubated in formamide hybridization buffer (KPL) with Digoxigenin (DIG)- and fluorscein isothiocyanate (FITC)-labeled RNA probes targeting the c-fos first intron and full-length mRNA transcripts. Primer sequences for the intronic probe were 5′-CTTTGTGTAGCCGCCAGGTC-3′ (Forward) and 5′-AAAAAGAGGAAAGCGGAGGTGAGC-3′ (Reverse); and primers for the full-length probe were 5′-GGCTCTCCTGTCAACACACA-3′ (Forward) and 5′-TAAGTAGTGCAGCCCGGAGT-3′ (Reverse). Signals were amplified using Cy5- and FITC-tyramide (PerkinElmer) and visualized using Cy5- and FITC-conjugated antibodies (Roche). Sections were mounted using SlowFade Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (DAPI) as a general nuclear counterstain (Invitrogen).
Microscopy and cell counting
50μM sections were imaged at 20x magnification using an A1 Nikon Confocal microscope. Whole coronal stitched images were acquired as single plane optical sections at (6.5 μM) at a scale of 0.62 pixels/μM. All imaging was done using standardized laser settings held constant for samples from the same experimental dataset. Quantifications derive from cell counts averaged across mice, where the value for each subject represents the mean of 1–2 single-plane ROIs (left and right hemisphere). The z-plane for each section was adjusted to the level where DAPI emission was highest. ROIs were determined blind to experimental group using the DAPI channel and published anatomical guidelines (Paxinos and Franklin, 2001). Two different methods were used to confirm estimates ChEF-tdTomato cell quantifications in RSC (see Supp. Methods). For c-fos nuclear counts, an ImageJ macro was used to apply a standard MIT to images in a data set. An ImageJ macro was applied to count nuclei above this threshold that overlapped with in-plane DAPI+ nuclei. For counts of overlap between ChEF and c-fos (see representative example of co-localization in Supp. Fig. S1) (Tayler et al., 2013), only those cells identified as positive for both ChEF and c-fos were counted as “overlapping” % overlap by chance was calculated by: [ChEF/DAPI x fos/DAPI × 100] and % overlap was calculated by: [overlap/DAPI × 100], and % overlap was normalized to % chance overlap by: [overlap/chance × 100]. Calculation of % ChEF+ cells reactivated (i.e. % of ChEF+ cells that co-express c-fos) was obtained by: [overlap/total ChEF × 100].
Acute brain slice preparation
Mice were anesthetized with isoflurane, and the brain quickly removed and placed into a sucrose based cutting solution (in mM): sucrose 222, D-Glucose 11, NaHCO3 26, NaH2PO4 1, KCl 3, MgCl2 7, CaCl2 0.5, aerated with 95% O2, 5 % CO2. 300 μm coronal slices containing retrosplenial cortex were cut with a Leica VT1000S Vibratome. Slices were allowed to recover at 37°C for 30 minutes, and then at room temperature in ACSF (in mM): NaCl 124, KCl 2.5, NaHCO3 26 NaH2PO4 1.25, D-Glucose 10, Sucrose 4, CaCl2 2.5, MgCl2 2, aerated with 95% O2, 5 % CO2.
Electrophysiology/Optical Stimulation
Cells were visualized using an upright Olympus BX51 microscope with a 40X water-immersion lens using epifluorescence and DIC illumination. Whole-cell recordings of fluorescent cells were made in current clamp mode or voltage clamp mode using a Multiclamp (Molecular Devices) patch clamp amplifier under visual guidance using DIC optics and epifluorescence with an Olympus BX51 microscope equipped with a custom made analog-controlled LED illumination system. Recordings were performed at 31–33°C. The internal solution contained (in mM): KGluc 115, KCl 20, HEPES 10, Phosphocreatine 10, ATP-Mg2+ 4, GTP-Na+ 0.3. Micropipettes were 3–5 MOhms in resistance. Series resistance was below 30 MOhms and fully compensated in current clamp mode. Electrophysiological signals were low pass-filtered at 4 kHZ and digitized at 10 kHz using NIDAQ boards controlled by Strathclyde WinWCP software. After a whole-cell recording was established, pulsed blue light stimuli (470nm) were delivered through the epifluorescence pathway of the microscope using a custom-made LED light array driven by analog pulses delivered through the NIDAQ card. The illumination intensity was measured through the objective as 1–7 mWmm−2.
Supplementary Material
Figure S1. Characterization of ChEF transgene expression (Related to Fig. 1). A) Confirmation of ChEF quantification by Method 2 for thresholding (see Supp. Methods). Method 1 counts in Fig 1C) are obtained using a single stringent minimum intensity threshold applied globally to all images in a data set. In Method 2, images were counted at a less stringent thresholds determined on an image-by-image basis by the mean pixel intensity of the whole ROI (see Methods). This approach allowed the inclusion of low-intensity ChEF(+) cells that were excluded in Method 1. Regardless of the thresholding method, there was a significant main effect of condition on the number of ChEF(+) cells (ANOVA, F(2,20)=6.909, P=0.005), with post-hoc comparisons showing that significantly more ChEF(+) cells in FC and BX mice compared to HC mice (Tukey-Kramer, P=0.013 (HC vs BX); P=0.006 (HC vs FC); P=) but not in BX compared to FC mice (P>0.05). On average, cell counts for each group were similar by Method 1 and Method 2 but only Method 2 produced a significant post-hoc difference between BX and HC. There were no significant differences in mean cell pixel intensity between groups using either method, and all three groups included both low- and high- intensity ChEF-expressing individuals. B) Representative image illustrating individual ChEF(+) cells (Red) identified in quantifications to overlap with c-fos (green) C) Quantification of the percentage of ChEF+ cells directly reactivated (c-fos-expressing) 90-minutes after LED stimulation (Stim+, N=5) compared to ChEF+ cells that were not exposed to light (Stim−, N=3), t(6) =3.22, P=0.018. D) Anatomical expression gradient distinguishing RSC from neighboring cortical areas. White dotted line indicates approximate cortical boundary of RSC (Paxinos & Watson, 2001). Representative confocal images from fixed RSC tissue obtained from ChEF transgenic mice and immunohistochemically stained for E) the astrocytic marker, glial fibrillary acidic protein (GFAP, green) and F) parvalbumin-expressing interneurons (PV, green) overlayed with ChEF (red). Samples were counterstained with DAPI (blue). * indicates P<0.05; error bars represent s.e.m. Scale bars = 50μM.
Figure S2. RSC ChEF(+) cells tagged during Box A training were reactivated by re-exposure to Box A (Related to Figs. 3). A) Mice were initially trained off dox in Box A to induce ChEF expression and were subsequently re-exposed to either training Box A or novel Box B to induce c-fos protein. Overall levels of either ChEF and c-fos protein did not differ between groups; however, B) a greater percentage of reactivated cells, determined on the basis of c-fos immunoreactivity, were colocalized to ChEF-expressing neurons in mice tested in Box B (N=5/6 per group; t(9) = 3.60, P=0.006). Quantification specifically reflects non-astrocytic labeling in the granular A subregion of RSC, the area most directly targeted during optical stimulation. ** indicates P<0.01; error bars represent s.e.m.
Figure S3. Infusion of CNQX/TTX significantly reduced endogenous c-fos expression in hippocampal area CA1 90min after LED stimulation (Related to Fig. 4). A) Cell counts of c-fos (+) cells confirm that CNQX/TTX had significantly reduced hippocampal activation at the time of LED stimulation. CA1 N=5/6 per group; 2-tailed t-test, t(9) = 2.63, P=0.027; scale bar = 50μM; B) Merged 20x confocal images from CA1 show endogenous c-fos expression (green) overlapping with ChEF-tdTomato (red) and DAPI counterstain (blue) in mice infused with CNQX/TTX (bot) or vehicle (top); C) Correct bilateral placement of micro-injectors was histologically confirmed for all experimental animals. Representative image counterstained with DAPI shows the entry site of an injector tip indicated by the arrow. Scale bar = 250μM. * indicates P<0.05; error bars represent s.e.m.
Table S1 (Related to Figure 2). Statistical output of ANOVA tests and post-hoc analysis performed on data shown in in Figure 2B. Red values indicate P < 0.05, indicating a statistically significant difference.
Table S2 (Related to Experimental Procedures and Fig. 4). Summary of additional statistical information based on fos-CatFISH regional mRNA overlap data presented in Figure 4B. For each region of interest, post-hoc tests provide a comparison between “observed overlap” and “overlap expected by chance,” (intronic/DAPI * cyto/DAPI). These comparisons do not account for any underlying differences in network behavior that may intrinsically differentiate sensory from associative areas. Therefore, the comparisons in Table S2 should be interpreted separately from the main group differences shown in Figure 4B. Red values indicate P < 0.05, indicating a statistically significant difference.
Acknowledgments
This work was supported by NIH funding awards R01MH057368-15 and R01DA028300-03 (to MM). We are grateful to Roger Tsien for providing the ChEF construct used to generate this mouse and to Peter Cameron for contributing the mRNA probes used in fos-CatFISH experiments. We also thank Anton Maximov and Denise Cai for providing helpful discussion and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP. Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia. 2010;48:2328–2338. doi: 10.1016/j.neuropsychologia.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Albasser MM, Poirier GL, Warburton EC, Aggleton JP. Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex: distal dysfunctions in a spatial memory system. The European journal of neuroscience. 2007;26:1254–1266. doi: 10.1111/j.1460-9568.2007.05753.x. [DOI] [PubMed] [Google Scholar]
- Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behavioural brain research. 2007;180:113–118. doi: 10.1016/j.bbr.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Maguire EA. Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49:2904–2913. doi: 10.1016/j.cortex.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, Maguire EA. Retrosplenial cortex codes for permanent landmarks. PloS one. 2012;7:e43620. doi: 10.1371/journal.pone.0043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature. 1999;400:671–675. doi: 10.1038/23270. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Macleod JE. Changes in neural activity associated with a surprising change in the predictive validity of a conditioned stimulus. The European journal of neuroscience. 2007;26:2669–2676. doi: 10.1111/j.1460-9568.2007.05902.x. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behavioral neuroscience. 2001;115:3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, Taube JS. Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:5289–5302. doi: 10.1523/JNEUROSCI.3380-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo NM, Cimadevilla JM, Gonzalez-Pardo H, Mendez-Couz M, Arias JL. Hippocampal inactivation with TTX impairs long-term spatial memory retrieval and modifies brain metabolic activity. PloS one. 2013;8:e64749. doi: 10.1371/journal.pone.0064749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:3986–4001. doi: 10.1523/JNEUROSCI.21-11-03986.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Guedea AL, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JR, Petrides M, Sziklas V. Fornix and retrosplenial contribution to a hippocampo-thalamic circuit underlying conditional learning. Behavioural brain research. 2010;209:13–20. doi: 10.1016/j.bbr.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature reviews Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learning & memory. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner AR, Rowland DC, Hwang SY, Baumgaertel K, Roth BL, Kentros C, Mayford M. Generation of a synthetic memory trace. Science. 2012;335:1513–1516. doi: 10.1126/science.1214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Dorman G, Gonzalez C, Kramar CP, Slipczuk L, Rossato JI, Cammarota M, Medina JH. On the role of retrosplenial cortex in long-lasting memory storage. Hippocampus. 2013a;23:295–302. doi: 10.1002/hipo.22092. [DOI] [PubMed] [Google Scholar]
- Katche C, Dorman G, Slipczuk L, Cammarota M, Medina JH. Functional integrity of the retrosplenial cortex is essential for rapid consolidation and recall of fear memory. Learning & memory. 2013b;20:170–173. doi: 10.1101/lm.030080.112. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral neuroscience. 2008a;122:89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behavioral neuroscience. 2008b;122:651–658. doi: 10.1037/0735-7044.122.3.651. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral neuroscience. 2008c;122:1070–1077. doi: 10.1037/a0012895. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiology of learning and memory. 2009;91:408–414. doi: 10.1016/j.nlm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Lesburgueres E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B. Early tagging of cortical networks is required for the formation of enduring associative memory. Science. 2011;331:924–928. doi: 10.1126/science.1196164. [DOI] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophysical journal. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484:381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: a tetrodotoxin functional inactivation study. Brain research. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. The European journal of neuroscience. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of anatomy. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Distributed encoding and retrieval of spatial memory in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L. Multiple memory systems: what and why. Journal of cognitive neuroscience. 1992;4:179–188. doi: 10.1162/jocn.1992.4.3.179. [DOI] [PubMed] [Google Scholar]
- Nadel L, MacDonald L. Hippocampus: cognitive map or working memory? Behavioral and neural biology. 1980;29:405–409. doi: 10.1016/s0163-1047(80)90430-6. [DOI] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. A computational theory of the hippocampal cognitive map. Progress in brain research. 1990;83:301–312. doi: 10.1016/s0079-6123(08)61258-3. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Black AH. Single unit and lesion experiments on the sensory inputs to the hippocampal cognitive map. Ciba Foundation symposium. 1977:179–198. doi: 10.1002/9780470720394.ch9. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological review. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. p. 2. [Google Scholar]
- Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S. Creating a false memory in the hippocampus. Science. 2013;341:387–391. doi: 10.1126/science.1239073. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: findings from four new cases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317:1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature neuroscience. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behavioral neuroscience. 2011;125:578–587. doi: 10.1037/a0024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, Bucci DJ. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12076–12086. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, affective & behavioral neuroscience. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19304–19313. doi: 10.1523/JNEUROSCI.1825-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Honda Y, Sasaki H, Naito J. Organization of intrinsic connections of the retrosplenial cortex in the rat. Anatomical science international. 2009;84:280–292. doi: 10.1007/s12565-009-0035-0. [DOI] [PubMed] [Google Scholar]
- Smith DM, Barredo J, Mizumori SJ. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus. 2012;22:1121–1133. doi: 10.1002/hipo.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232:1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Current biology : CB. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RG. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annual review of psychology. 2010;61:49–79. C41–44. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, Li W, Silva AJ. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Current biology : CB. 2010;20:1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of ChEF transgene expression (Related to Fig. 1). A) Confirmation of ChEF quantification by Method 2 for thresholding (see Supp. Methods). Method 1 counts in Fig 1C) are obtained using a single stringent minimum intensity threshold applied globally to all images in a data set. In Method 2, images were counted at a less stringent thresholds determined on an image-by-image basis by the mean pixel intensity of the whole ROI (see Methods). This approach allowed the inclusion of low-intensity ChEF(+) cells that were excluded in Method 1. Regardless of the thresholding method, there was a significant main effect of condition on the number of ChEF(+) cells (ANOVA, F(2,20)=6.909, P=0.005), with post-hoc comparisons showing that significantly more ChEF(+) cells in FC and BX mice compared to HC mice (Tukey-Kramer, P=0.013 (HC vs BX); P=0.006 (HC vs FC); P=) but not in BX compared to FC mice (P>0.05). On average, cell counts for each group were similar by Method 1 and Method 2 but only Method 2 produced a significant post-hoc difference between BX and HC. There were no significant differences in mean cell pixel intensity between groups using either method, and all three groups included both low- and high- intensity ChEF-expressing individuals. B) Representative image illustrating individual ChEF(+) cells (Red) identified in quantifications to overlap with c-fos (green) C) Quantification of the percentage of ChEF+ cells directly reactivated (c-fos-expressing) 90-minutes after LED stimulation (Stim+, N=5) compared to ChEF+ cells that were not exposed to light (Stim−, N=3), t(6) =3.22, P=0.018. D) Anatomical expression gradient distinguishing RSC from neighboring cortical areas. White dotted line indicates approximate cortical boundary of RSC (Paxinos & Watson, 2001). Representative confocal images from fixed RSC tissue obtained from ChEF transgenic mice and immunohistochemically stained for E) the astrocytic marker, glial fibrillary acidic protein (GFAP, green) and F) parvalbumin-expressing interneurons (PV, green) overlayed with ChEF (red). Samples were counterstained with DAPI (blue). * indicates P<0.05; error bars represent s.e.m. Scale bars = 50μM.
Figure S2. RSC ChEF(+) cells tagged during Box A training were reactivated by re-exposure to Box A (Related to Figs. 3). A) Mice were initially trained off dox in Box A to induce ChEF expression and were subsequently re-exposed to either training Box A or novel Box B to induce c-fos protein. Overall levels of either ChEF and c-fos protein did not differ between groups; however, B) a greater percentage of reactivated cells, determined on the basis of c-fos immunoreactivity, were colocalized to ChEF-expressing neurons in mice tested in Box B (N=5/6 per group; t(9) = 3.60, P=0.006). Quantification specifically reflects non-astrocytic labeling in the granular A subregion of RSC, the area most directly targeted during optical stimulation. ** indicates P<0.01; error bars represent s.e.m.
Figure S3. Infusion of CNQX/TTX significantly reduced endogenous c-fos expression in hippocampal area CA1 90min after LED stimulation (Related to Fig. 4). A) Cell counts of c-fos (+) cells confirm that CNQX/TTX had significantly reduced hippocampal activation at the time of LED stimulation. CA1 N=5/6 per group; 2-tailed t-test, t(9) = 2.63, P=0.027; scale bar = 50μM; B) Merged 20x confocal images from CA1 show endogenous c-fos expression (green) overlapping with ChEF-tdTomato (red) and DAPI counterstain (blue) in mice infused with CNQX/TTX (bot) or vehicle (top); C) Correct bilateral placement of micro-injectors was histologically confirmed for all experimental animals. Representative image counterstained with DAPI shows the entry site of an injector tip indicated by the arrow. Scale bar = 250μM. * indicates P<0.05; error bars represent s.e.m.
Table S1 (Related to Figure 2). Statistical output of ANOVA tests and post-hoc analysis performed on data shown in in Figure 2B. Red values indicate P < 0.05, indicating a statistically significant difference.
Table S2 (Related to Experimental Procedures and Fig. 4). Summary of additional statistical information based on fos-CatFISH regional mRNA overlap data presented in Figure 4B. For each region of interest, post-hoc tests provide a comparison between “observed overlap” and “overlap expected by chance,” (intronic/DAPI * cyto/DAPI). These comparisons do not account for any underlying differences in network behavior that may intrinsically differentiate sensory from associative areas. Therefore, the comparisons in Table S2 should be interpreted separately from the main group differences shown in Figure 4B. Red values indicate P < 0.05, indicating a statistically significant difference.