Abstract
Lupus-like autoimmunity in (NZB x NZW)F1 mice is frequently marked by the development of a severe and fatal renal disease. Genes from both NZB and NZW parents are required for the full expression of disease. We applied a mapping technique based on polymorphism in simple sequence repeats to the analysis of (NZB x NZW)F1 x NZW backcross mice to determine the NZB genetic contribution to disease. The results show that a single NZB locus or tightly linked group of loci on the distal part of chromosome 4 provides the strongest association with renal disease and death. This locus, designated here as nba-1 (New Zealand Black autoimmunity), lies distal to the locus elp-1, 60-70 centimorgans from the centromere. It is of interest that a gene encoding a receptor for tumor necrosis factor maps to the vicinity of this disease-associated gene.
Full text
PDF
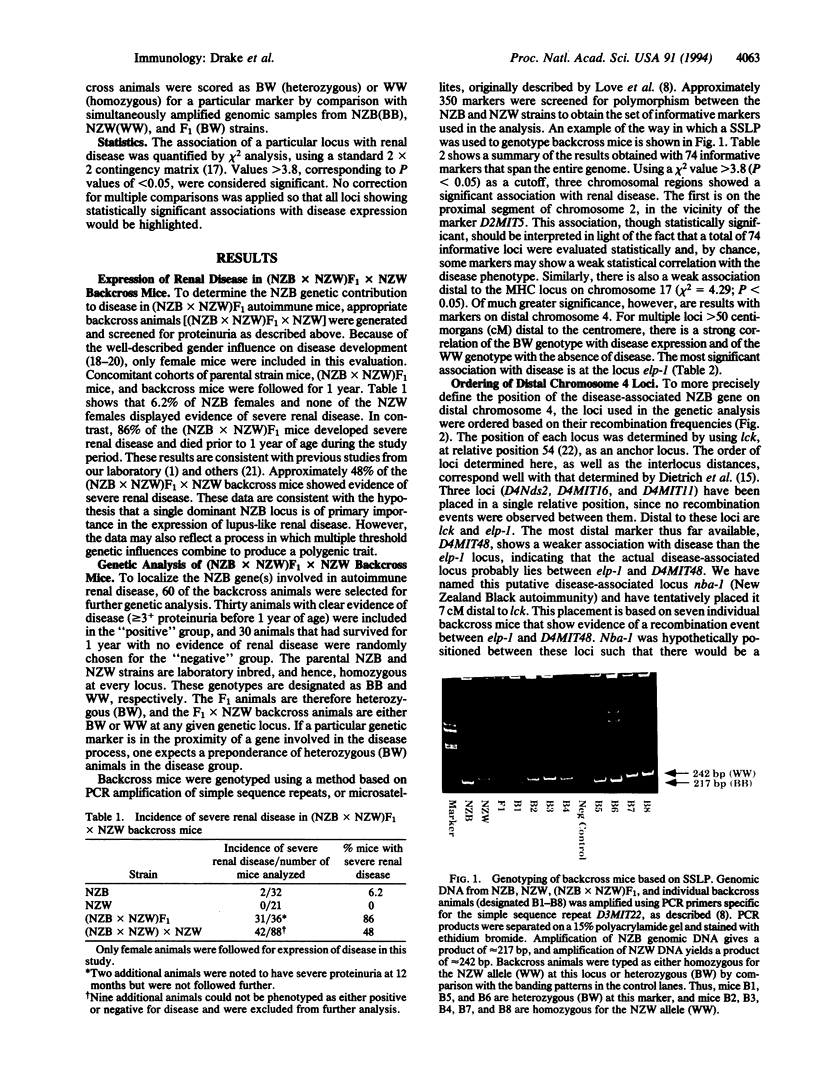
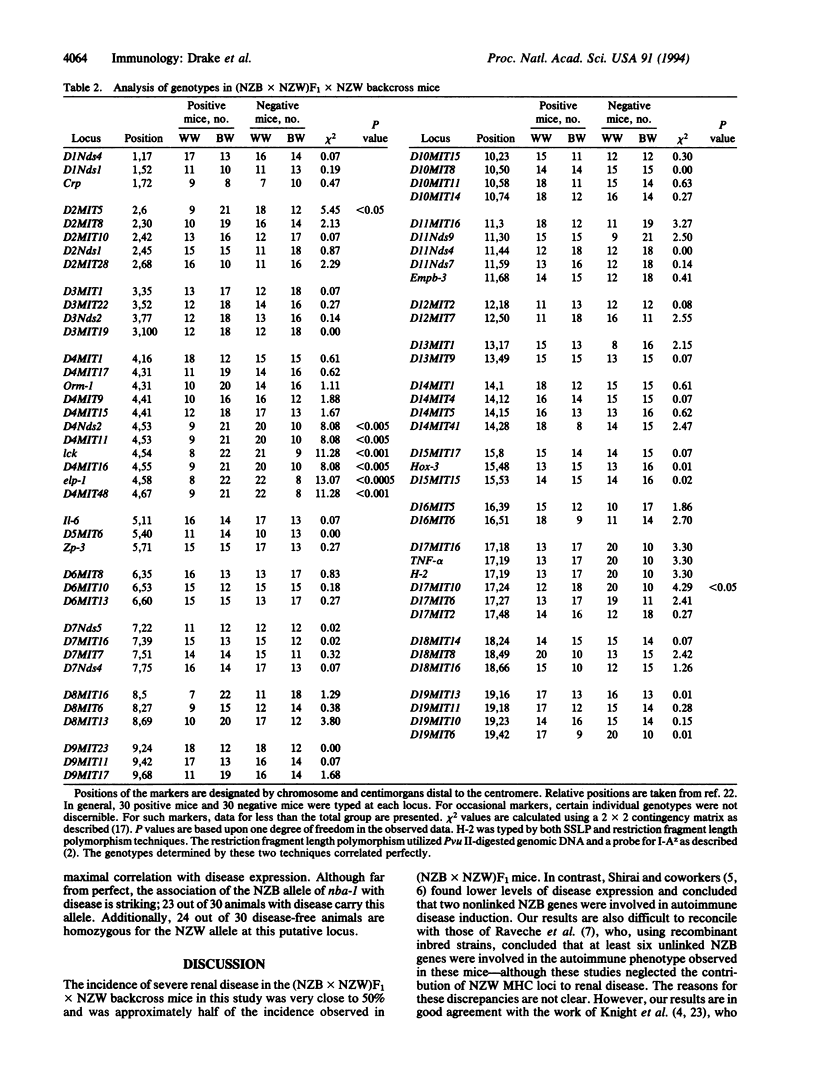


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott C. M., Blank R., Eppig J. T., Friedman J. M., Huppi K. E., Jackson I., Mock B. A., Stoye J., Wiseman R. Mouse chromosome 4. Mamm Genome. 1992;3(Spec No):S55–S64. doi: 10.1007/BF00648422. [DOI] [PubMed] [Google Scholar]
- Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitman T. J., Hearne C. M., McAleer M. A., Todd J. A. Mononucleotide repeats are an abundant source of length variants in mouse genomic DNA. Mamm Genome. 1991;1(4):206–210. doi: 10.1007/BF00352326. [DOI] [PubMed] [Google Scholar]
- Babcock S. K., Appel V. B., Schiff M., Palmer E., Kotzin B. L. Genetic analysis of the imperfect association of H-2 haplotype with lupus-like autoimmune disease. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7552–7555. doi: 10.1073/pnas.86.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B. L., Bearer E., Ansari A., Dorshkind K., Gershwin M. E. The BM12 mutation and autoantibodies to dsDNA in NZB.H-2bm12 mice. J Immunol. 1990 Jul 1;145(1):94–101. [PubMed] [Google Scholar]
- Cornall R. J., Aitman T. J., Hearne C. M., Todd J. A. The generation of a library of PCR-analyzed microsatellite variants for genetic mapping of the mouse genome. Genomics. 1991 Aug;10(4):874–881. doi: 10.1016/0888-7543(91)90175-e. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C., Ranges G. E., Greenspan J. S., Wofsy D. Chronic therapy with recombinant tumor necrosis factor-alpha in autoimmune NZB/NZW F1 mice. Clin Immunol Immunopathol. 1989 Sep;52(3):421–434. doi: 10.1016/0090-1229(89)90157-8. [DOI] [PubMed] [Google Scholar]
- Hearne C. M., McAleer M. A., Love J. M., Aitman T. J., Cornall R. J., Ghosh S., Knight A. M., Prins J. B., Todd J. A. Additional microsatellite markers for mouse genome mapping. Mamm Genome. 1991;1(4):273–282. doi: 10.1007/BF00352339. [DOI] [PubMed] [Google Scholar]
- Hirose S., Ueda G., Noguchi K., Okada T., Sekigawa I., Sato H., Shirai T. Requirement of H-2 heterozygosity for autoimmunity in (NZB X NZW)F1 hybrid mice. Eur J Immunol. 1986 Dec;16(12):1631–1633. doi: 10.1002/eji.1830161226. [DOI] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., McDevitt H. O. Tumour necrosis factor-alpha in murine autoimmune 'lupus' nephritis. Nature. 1988 Jan 28;331(6154):356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Acha-Orbea H., Blankenstein T. A polymorphic microsatellite in the tumor necrosis factor alpha promoter identifies an allele unique to the NZW mouse strain. J Exp Med. 1990 Jun 1;171(6):2141–2146. doi: 10.1084/jem.171.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D. Genes determining autoimmune disease in New Zealand mice. J Clin Lab Immunol. 1981 May;5(3):165–170. [PubMed] [Google Scholar]
- Knight J. G., Adams D. D., Purves H. D. The genetic contribution of the NZB mouse to the renal disease of the NZB x NZW hybrid. Clin Exp Immunol. 1977 May;28(2):352–358. [PMC free article] [PubMed] [Google Scholar]
- Knight J. G., Adams D. D. Three genes for lupus nephritis in NZB x NZW mice. J Exp Med. 1978 Jun 1;147(6):1653–1660. doi: 10.1084/jem.147.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno A., Yoshida H., Sekita K., Maruyama N., Ozaki S., Hirose S., Shirai T. Genetic regulation of the class conversion of dsDNA-specific antibodies in (NZB X NZW)F1 hybrid. Immunogenetics. 1983;18(5):513–524. doi: 10.1007/BF00364392. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Palmer E. The contribution of NZW genes to lupus-like disease in (NZB x NZW)F1 mice. J Exp Med. 1987 May 1;165(5):1237–1251. doi: 10.1084/jem.165.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J. M., Knight A. M., McAleer M. A., Todd J. A. Towards construction of a high resolution map of the mouse genome using PCR-analysed microsatellites. Nucleic Acids Res. 1990 Jul 25;18(14):4123–4130. doi: 10.1093/nar/18.14.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama N., Furukawa F., Nakai Y., Sasaki Y., Ohta K., Ozaki S., Hirose S., Shirai T. Genetic studies of autoimmunity in New Zealand mice. IV. Contribution of NZB and NZW genes to the spontaneous occurrence of retroviral gp70 immune complexes in (NZB X NZW)F1 hybrid and the correlation to renal disease. J Immunol. 1983 Feb;130(2):740–746. [PubMed] [Google Scholar]
- Papoian R., Pillarisetty R., Talal N. Immunological regulation of spontaneous antibodies to DNA and RNA. II. Sequential switch from IgM to IgG in NZB/NZW F1 mice. Immunology. 1977 Jan;32(1):75–79. [PMC free article] [PubMed] [Google Scholar]
- Prins J. B., Todd J. A., Rodrigues N. R., Ghosh S., Hogarth P. M., Wicker L. S., Gaffney E., Podolin P. L., Fischer P. A., Sirotina A. Linkage on chromosome 3 of autoimmune diabetes and defective Fc receptor for IgG in NOD mice. Science. 1993 Apr 30;260(5108):695–698. doi: 10.1126/science.8480181. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S., Oh J., Zhong L. T., Yang J., Bitler C. M., Butcher L. L., Bredesen D. E. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993 Jul 16;261(5119):345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Raveche E. S., Novotny E. A., Hansen C. T., Tjio J. H., Steinberg A. D. Genetic studies in NZB mice. V. Recombinant inbred lines demonstrate that separate genes control autoimmune phenotype. J Exp Med. 1981 May 1;153(5):1187–1197. doi: 10.1084/jem.153.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Tite J. P., Ruddle N. H. DNA fragmentation: manifestation of target cell destruction mediated by cytotoxic T-cell lines, lymphotoxin-secreting helper T-cell clones, and cell-free lymphotoxin-containing supernatant. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1881–1885. doi: 10.1073/pnas.83.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Hay F. C. Changes in immunoglobulin class and subclass of anti-DNA antibodies with increasing age in N/ZBW F1 hybrid mice. Clin Exp Immunol. 1976 Nov;26(2):363–370. [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Aitman T. J., Cornall R. J., Ghosh S., Hall J. R., Hearne C. M., Knight A. M., Love J. M., McAleer M. A., Prins J. B. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991 Jun 13;351(6327):542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]



