Abstract
Recently, different dehydration-based technologies have been evaluated for the purpose of cell and tissue preservation. Although some early results have been promising, they have not satisfied the requirements for large-scale applications. The long experience of using quantitative trait loci (QTLs) with the yeast Saccharomyces cerevisiae has proven to be a good model organism for studying the link between complex phenotypes and DNA variations. Here, we use QTL analysis as a tool for identifying the specific yeast traits involved in dehydration stress tolerance. Three hybrids obtained from stable haploids and sequenced in the Saccharomyces Genome Resequencing Project showed intermediate dehydration tolerance in most cases. The dehydration resistance trait of 96 segregants from each hybrid was quantified. A smooth, continuous distribution of the anhydrobiosis tolerance trait was found, suggesting that this trait is determined by multiple QTLs. Therefore, we carried out a QTL analysis to identify the determinants of this dehydration tolerance trait at the genomic level. Among the genes identified after reciprocal hemizygosity assays, RSM22, ATG18 and DBR1 had not been referenced in previous studies. We report new phenotypes for these genes using a previously validated test. Finally, our data illustrates the power of this approach in the investigation of the complex cell dehydration phenotype.
Introduction
Almost all yeast-based food industries are steadily expanding their use of active dry yeast (ADY) because of its greater genetic stability at room temperature and lower transport and storage costs. Unfortunately, most laboratory-developed industrial yeast strains, as well as strains isolated from industrial environments, have the biotechnological handicap of losing viability during the drying process [1]. Therefore, such strains are excluded from the commercial catalogues of yeast manufacturers, awaiting a breakthrough that would allow their desiccation to be optimized. In a previous study, we performed a genetic screen of the Saccharomyces cerevisiae deletion library for mutants sensitive to dehydration stress [2]. Among the genes characterized as essential for overcoming dehydration stress, only five (SIP18, STF2, GRE1, YJL144w, and NOP6) were found to have protective effects against dehydration stress when overexpressed [3, 4]. Recent studies investigating whether the response to desiccation involves regulation at the transcriptional and/or translational level detected changes in genes involved in lipid binding and synthesis, protein synthesis and mobility, and metabolism [5–9]. However, correlations were rare between these transcriptomic studies and genetic screens using the S. cerevisiae deletion library of mutants sensitive to dehydration stress [3, 10, 11]. In contrast, haploid strains overexpressing yeast genes encoding hydrophilic proteins (Stf2, Sip18, Gre1, Yjl144w, and Nop6), which are essential for overcoming dehydration stress, are tolerant of dry conditions [3, 4].
On the other hand, Rodríguez-Porrata et al.2 showed that the knockout mutants for four nuclear apoptotic-related genes with mitochondrial functions (Δaif1, Δnuc1, Δcpr3, and Δqcr7) were hyper-tolerant of dehydration stress. Most S. cerevisiae genes involved in qualitative traits related to their basic biology have been identified using recombinant DNA techniques. However, many phenotypes important to industrially appear to be quantitative traits that are determined by quantitative trait loci (QTLs), such as growth temperature, ethanol tolerance, acetic acid production, sporulation rate, sake aromatic compounds production, and nitrogen utilization [11–17]. Considering the large amount of genetic variability in industrial yeast, a characteristic as crucial as dehydration tolerance is likely controlled by multiple QTLs that cannot be identified by conventional molecular genetic approaches.
In this paper, we performed QTL analysis on 96 segregants derived from a cross between two haploid strains derivatives of two strains of wine yeast using statistical linkage analysis between dehydration tolerance characteristics and DNA marker genotype data. We functionally characterized two QTLs encompassing six genes involved in dehydration stress tolerance that contribute to the natural phenotypic variation in the paternal strains [11].
Materials and Methods
Strains and plasmids
Table 1 summarizes the yeast strains and plasmids used in this study. The RIM15, BST1, BUD27, BLM10, YFH7, FAB1, ATG18, CBT1, MRP49, RSM22, and DBR1 genes were deleted using a short-flanking homology PCR technique in which URA3 was the selectable marker (S1B Fig.) in the Mat α and Mat a versions of the WA (Hyg R), WA (Nat R), WE (Hyg R), and WE (Nat R) strains [18]. Degenerative primers (shown in S1 Table) were used to amplify the URA3 deletion module from the pNSU114 plasmid [19]. Transformants were obtained using the lithium acetate transformation protocol and selected by plating on synthetic glucose media lacking uracil [18]. URA+ transformants were selected and restreaked to obtain single colonies, for which integrations were confirmed by PCR using the primer pair URA3Fw and GENERv, a reverse primer that anneals at the downstream region of the deleted gene (S1 Table). The URA3 module was deleted from the WE, Δatg18 strain by transforming single mutant strains with the PCR DNA fragment obtained using the ATGufw-ATGurv primer pair from the atg18::URA3 locus. The transformants, which were able to grow in the presence of 5FOA and unable to grow on SC-ura medium, were further evaluated by PCR. The validated WE, Δatg18u strain was further transformed, as mentioned previously, to obtain the WE, Δatg18u, Δfab1 strain. Haploid strains with opposite mating types were crossed on yeast peptone dextrose agar (YPDA) medium supplemented with 100 μg·ml−1 hygromycin B and 200 μg·ml−1 nourseothricin sulfate. Diagnostics for isolates from individual colonies were made with the MAT locus by PCR using WA (Nat R) and WE (Hyg R) as tester strains [20]. Recombinant DNA techniques were carried out according to standard protocols [21]. The amplification reactions contained a 1x PCR buffer, 1.25 mM dNTPs, 1.0 mM MgCl2, 0.3 μM of each primer, 2 ng·μl−1 template DNA, and 3.5 U DNA Polymerase in a total volume of 100 μl. All reactions were performed using a PCR thermal cycler for 25 cycles, as follows: denaturation, 2 min at 94°C; primer annealing, 30 s at 55°C; and primer extension, 1.5 min at 68°C.
Table 1. Strains and plasmid used in the study.
| Strain | Relevant characteristics | References |
|---|---|---|
| BY4742 | MATα, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0 | [22] |
| DBVPG6044 (WA Hyg R) | MATa, ho::HygMX, ura3::KanMX | [23] |
| DBVPG6044 (WA Nat R) | MATα, ho::NatMX, ura3::KanMX | [23] |
| DBVPG6765 (WE Hyg R) | MATa, ho::HygMX, ura3::KanMX | [23] |
| DBVPG6765 (WE Nat R) | MATα, ho::NatMX, ura3::KanMX | [23] |
| Y12 (SA Hyg R) | MATa, ho::HygMX, ura3::KanMX | [23] |
| YPS128 (NA Hyg R) | MATa, ho::HygMX, ura3::KanMX | [23] |
| WE/NA | WE Nat R/NA Hyg R | [11] |
| WE/WA | WE Nat R/ WA Hyg R | [11] |
| WA/WE | WA Nat R/ WE Hyg R | This work |
| WE/SA | WE Nat R/ SA Hyg R | [11] |
| 96 spores WE/NA | F1 from WE Nat R/NA Hyg R | [11] |
| 96 spores WE/WA | F1 from WE Nat R/ WA Hyg R | [11] |
| 96 spores WE/SA | F1 from WE Nat R/ SA Hyg R | [11] |
| WA, Δrim15 | MATα, ho::NatMX, rim15::URA3 | This work |
| WA, Δbst1 | MATα, ho::NatMX, bst1::URA3 | This work |
| WA, Δbud27 | MATα, ho::NatMX, bud27::URA3 | This work |
| WA, Δblm10 | MATα, ho::NatMX, blm10::URA3 | This work |
| WA, Δyfh7 | MATα, ho::NatMX, yfh7::URA3 | This work |
| WA, Δfab1 | MATα, ho::NatMX, fab1::URA3 | This work |
| WA, Δatg18 | MATα, ho::NatMX, atg18::URA3 | This work |
| WA, Δcbt1 | MATα, ho::NatMX, cbt1::URA3 | This work |
| WA, Δmrp49 | MATα, ho::NatMX, mrp49::URA3 | This work |
| WA, Δrsm22 | MATα, ho::NatMX, rsm22::URA3 | This work |
| WA, Δdbr1 | MATα, ho::NatMX, dbr1::URA3 | This work |
| WE, Δrim15 | MATα, ho::NatMX, rim15::URA3 | This work |
| WE, Δbst1 | MATα, ho::NatMX, bst1::URA3 | This work |
| WE, Δbud27 | MATα, ho::NatMX, bud27::URA3 | This work |
| WE, Δblm10 | MATα, ho::NatMX, blm10::URA3 | This work |
| WE, Δyfh7 | MATα, ho::NatMX, yfh7::URA3 | This work |
| WE, Δfab1 | MATα, ho::NatMX, fab1::URA3 | This work |
| WE, Δatg18 | MATα, ho::NatMX, atg18::URA3 | This work |
| WE, Δrpl2a | MATα, ho::NatMX, rpl2a::URA3 | This work |
| WE, Δcbt1 | MATα, ho::NatMX, cbt11::URA3 | This work |
| WE, Δmrp49 | MATα, ho::NatMX, mrp49::URA3 | This work |
| WE, Δrsm22 | MATα, ho::NatMX, rsm22::URA3 | This work |
| WE, Δdbr1 | MATα, ho::NatMX, dbr1::URA3 | This work |
| WE, Δatg18u | MATa, ho::HygMX, atg18::ura3 | This work |
| WE, Δatg18u, Δfab1 | MATa, ho::HygMX, atg18::ura3, fab1::URA3 | This work |
| WA/Δrim15 WE | WA Hyg R/WE, Δrim15 | This work |
| WA/Δbst1 WE | WA Hyg R/WE, Δbst1 | This work |
| WA/Δbud27 WE | WA Hyg R/WE, Δbud27 | This work |
| WA/Δblm10 WE | WA Hyg R/WE, Δblm10 | This work |
| WA/Δyfh7 WE | WA Hyg R/WE, Δyfh7 | This work |
| WA/Δfab1 WE | WA Hyg R/WE, Δfab1 | This work |
| WA/Δatg18 WE | WA Hyg R/WE, Δatg18 | This work |
| WA/Δrpl2a WE | WA Hyg R/WE, Δrpl2a | This work |
| WA/Δcbt1 WE | WA Hyg R/WE, Δcbt1 | This work |
| WA/Δmrp49 WE | WA Hyg R/WE, Δmrp49 | This work |
| WA/Δrsm22 WE | WA Hyg R/WE, Δrsm22 | This work |
| WA/Δdbr1 WE | WA Hyg R/WE, Δdbr1 | This work |
| WE/Δrim15 WA | WE Hyg R/WA, Δrim15 | This work |
| WE/Δbst1 WA | WE Hyg R/WA, Δbst1 | This work |
| WE/Δblm10 WA | WE Hyg R/WA, Δblm10 | This work |
| WE/Δyfh7 WA | WE Hyg R/WA, Δyfh7 | This work |
| WE/Δfab1 WA | WE Hyg R/WA, Δfab1 | This work |
| WE/Δatg18 WA | WE Hyg R/WA, Δatg18 | This work |
| WE/Δcbt1 WA | WE Hyg R/WA, Δcbt1 | This work |
| WE/Δmrp49 WA | WE Hyg R/WA, Δmrp49 | This work |
| WE/Δrsm22 WA | WE Hyg R/WA, Δrsm22 | This work |
| WE/Δdbr1 WA | WE Hyg R/WA, Δdbr1 | This work |
| WA/Δatg18u we, Δfab1 we | WA Nat R/WE, Δatg18u, Δfab1 | This work |
| Plasmid | ||
| pNSU114 | [24] |
Growth conditions and desiccation-rehydration process
Yeast strains were grown in shake flasks at 150 rpm in SC medium containing 0.17% yeast nitrogen base, 2% glucose, 0.5% (NH4)2SO4, and 25 mg·l−1 uracil. The desiccation-rehydration process and yeast viability assays were performed as previously described [25].
Linkage analysis
Linkage analysis was performed using the rQTL software, and the LOD score was calculated using a normal model [11, 26, 27]. Briefly, the significance of a QTL was determined from permutations. For each trait and cross, we permuted the phenotype values within tetrads 1,000 times and recorded the maximum LOD score each time. A QTL was considered significant if its LOD score was greater than the 0.05 tail of the 1,000 permuted LOD scores.
RNA isolation and cDNA synthesis
The total RNA was obtained from: WE, WA, WEΔatg18, WEΔfab1, WAΔatg18, WAΔfab1, and WA/Δatg18u WE, Δfab1 WE yeast cells using a RNA Kit according to the manufacturer’s protocol. The RNA was resuspended in 100 μL RNase-free water. The DNase I RNAase free kit was used to remove the 16 genomic DNA from the RNA preparations. The RNA was quantified with a spectrophotometer at an absorbance of 260 nm and tested for purity (by the A260/280 ratio) and integrity by denaturing gel electrophoresis. The first strand of cDNA was reverse transcribed from 1 μg total RNA from each sample using a First Strand cDNA Synthesis Kit according to the manufacturer’s protocol. An identical reaction without the reverse transcription was performed to verify the absence of genomic DNA. The cDNA was subsequently amplified by PCR using yeast strain specific couple of primers forward-reverse for: ATG18, FAB1, ALG9 and TAF10 genes (S1 Table).
Real-time RT-PCR
Quantitative PCR for ATG18 and FAB1, was carried out using a Real Time qPCR kit according to the manufacturer's protocol and was analysed on a Real-Time PCR Detection System. The thermal cycling was composed of an initial step at 50°C for 2 min followed by a polymerase activation step at 95°C for 10 min and a cycling step with the following conditions: 40 cycles of denaturation at 95°C for 15 s, annealing at 63°C for 1 min, and extension at 72°C for 1 min. Oligonucleotides of varying lengths produce dissociation peaks at different melting temperatures. Therefore, at the end of the PCR cycles, the PCR products were analysed using a heat dissociation protocol to confirm that a single PCR product was detected by the SYBR Green dye. The fluorescence data was acquired at the 72°C step. The threshold cycle (Ct) was calculated using a software to indicate significant fluorescence signals above the noise during the early cycles of amplification. The software calculated copy numbers for the target samples from the Ct using interpolation from the standard curve. The relative levels of expression of the target genes were measured using ALG9 and TAF10 mRNA as an internal control and calculated according to the 2−ΔΔC T method [28].
Microscopy
Cultures of strains harbouring the GFP-tagged genes were grown to the stationary phase in SC medium. The cells were washed with 1× PBS buffer (pH 7.4) and fixed in 70% ethanol for 10 min at room temperature. Fluorescence was viewed using a fluorescence microscope. A digital camera and a software were used for image acquisition.
Statistical analysis
To determine the statistical significance of data the results were analysed by one-way ANOVA, the Shapiro-Wilk test and the Scheffé test were carried out using a statistical software package. Statistical significance was set at p<0.001.
Results
Variation in dehydration stress tolerance in recombinant yeast populations
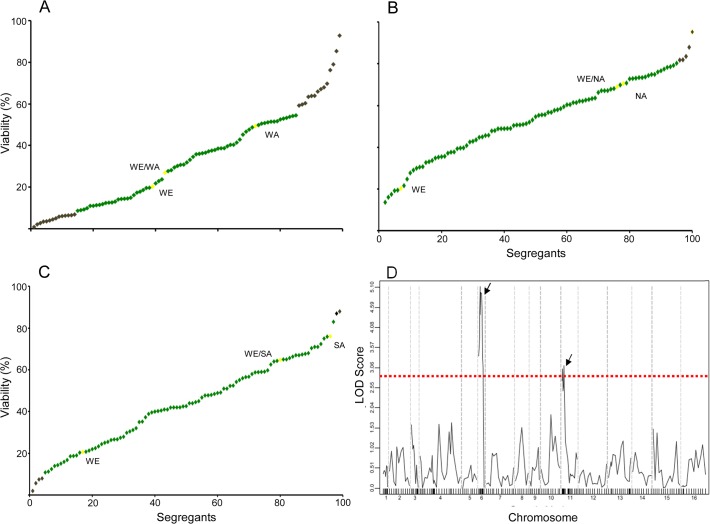
Using a colony-counting assay, desiccation tolerance was assessed for a set of three recombinant populations of 96 segregants generated from a cross of divergent S. cerevisiae isolates (WE [Wine European] x WA [West African], WE x NA [North American], and WE x SA [Sake]) previously described (S1A Fig.) [11]. The mean CFU (colony-forming units) per ml value for survival after rehydration was calculated, taking into account the viability before drying (Fig. 1A-C). The W value obtained from the Shapiro-Wilk test carried out with the three sets of segregants were lower than 0.5, therefore, for an α level of 0.05, the phenotypic distributions of segregants did not show a normal distribution, suggesting a polygenic contribution to cellular desiccation tolerance (Fig. 1A-C). The highest number of transgressive segregants (24%) was observed in the cross between the low dehydration stress-resistant strains WE (20.3%) and WA (49.4%) (Fig. 1A). However, when the highly sensitive WE strain was crossed with the resistant SA and NA strains (75.9% and 70.5%, respectively), approximately 5.5% of segregants exceeded the phenotypic range of their parents by at least 2 SD, criteria previously used to name these segregants as transgressive, Fig. 1B-C [29]. By running a linkage analysis using ~200 previously reported genotype markers, we evaluated whether the different genotypes correlated with the viability trend observed in the WE/WA strain segregants [11]. Only the genetic markers Y034W, BST1, FRS2, RPN11, ROG3, TRP3, and FAS1 showed significant differences (p<0.005). The same analysis performed for the segregants from the WE/NA and WE/SA strains did not show any correlation between genomic region and cell viability.
Fig 1. Viability rate variation after dehydration stress.
Viability rate values are shown on the y-axis for the 96 ranked segregants of the WE x WA cross (A), WE x NA cross (B), and WE x SA cross (C). Dots indicate segregants with transgressive phenotypes (exceeding two parental standard deviations, black), parental and hybrid strains (yellow), and segregants within the phenotypic range of the parental strains (green). D) Linkage analysis for dehydration stress tolerance from WE/WA segregants. The chromosomes are displayed on the x-axis, and LOD viability values, according to each molecular marker across the 16 yeast chromosomes, are displayed on the y-axis. The significant LOD score threshold is indicated by a red line and was determined by a permutation test. The significant QTLs are indicated by arrows.
Identification of QTLs involved in dehydration tolerance
To identify the QTL intervals responsible for natural phenotypic variations in dehydration stress, linkage analysis was performed based on the cellular viability after stress induction and the genotypes of the 96 F1 segregants [11, 24]. In total, two significant regions were mapped using the marker regression model and permutation method in the WE x WA cross, allowing the identification of 15 candidate genes (Fig. 1D; Table 2). A region in chromosome XI (from 37 to 137 kb) with a peak LOD score of 3.10 was identified and after further inspections, we identified seven candidate genes (CBT1, YKT6, FAS1, MRP49, RSM22, DBR1 and AVT3) within this QTL. In the second QTL (Chr VI, LOD 5.1), eight candidate genes (RIM15, BST1, BUD27, BLM10, YFH7, FAB1, ATG18 and ROG3) were identified between 65 KB and 196 KB. After a sequence alignment, only 11 of the genes encompassed by either QTL interval (RIM15, BST1, BUD27, BLM10, YFH7, FAB1, ATG18, CBT1, MRP49, RSM22 and DBR1) contained single-nucleotide polymorphisms (SNPs) (Table 2). Furthermore, the SNPs did not create premature stop codons in the coding sequence of the WE and WA strains. Among these genes, only BUD27, FAB1, and CBT1 were found to be necessary for the yeast to overcome desiccation stress [3, 10, 27].
Table 2. The position in the genome, significance value, genes in the respective regions and the differences in the amino acid sequence for each gene in WE strain versus WA are described.
| Chromosome | QTL's | Position (cM) | LOD | Gene / Position | Position of amino acid change WA allele→WE allele |
|---|---|---|---|---|---|
| VI | Y034w | 65 | 3.85 | RIM15 /69.11 | 161 E → K; 240 S→G; 249 E→D; 251 T→S; 366 T→S; 399 V→A; 771 R→P; 1020 T→I; 1022 C→Y |
| BST1 | 84 | 5.11 | BST1 /84.14 | 202 A→T; 221 N→D; 253 A→P; 432 N→D; 438 K→M; 506 Q→L; 610K→R; 636S→W; 849 D→V | |
| BUD27 /90.9 | 32 Δ→E; 33D→Y; 75 S→F; 177 E→G; 182 D→E | ||||
| HTX10 | 111 | 4.95 | BLM10 /123.47 | 99 Q→R; 220 T→A; 258 G→A; 729 S→N; 759 I→V; 791 N→D; 902 C→Y; 1102 R→K; 1315 G→S; 1444 D→N; 1586 P→A; 1592 R→C; 1698 T→A; 1782 G→D; 1861 D→Y; 1900 I→V; 1971 M→I | |
| ARS605 | 136 | 4.93 | - | ||
| RPN11 | 153 | 4.50 | YFH7 /159.29 | 109 V→I; 138 A→T; 149 V→A | |
| YFR016c | 180 | 3.32 | FAB1 /184.50 | 120 S→N; 126 N→S; 333 A→S; 583 Δ→N; 1273 N→D; 1300 Y→H; 1524 G→E; 1604 R→M; 1780 P→S; 1878 I→M; 1882 S→A; 1884 Q→Δ | |
| ATG18 /194.81 | 195 N→S | ||||
| ROG3 | 196 | 2.40 | - | ||
| XI | TRP3 | 37 | 2.72 | CBT1 /47.15 | 29 S→G; 109 T→A |
| ARS1103 | 58 | 3.03 | - | ||
| YKT6 | 75 | 2.46 | - | ||
| FAS1 | 103 | 2.58 | - | ||
| TP05 | 121 | 3.10 | MRP49 /133.72 | 131 G→R | |
| PIR1 | 142 | 2.34 | RSM22 /159.45 | 228 E→K; 474 D→S; 619 S→N | |
| DBR1 /167.61 | 223 Q→R; 286 K→E; 325 N→D | ||||
| AVT3 | 173 | 1.16 | - |
Allele without mismatch (-).
Dissection of the QTLs associated with stress tolerance
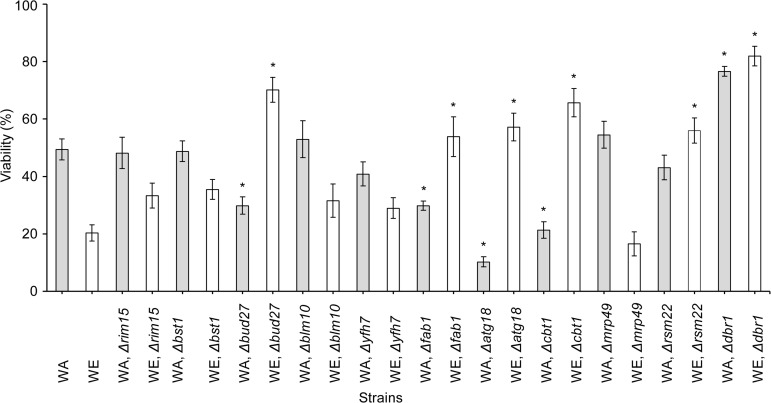
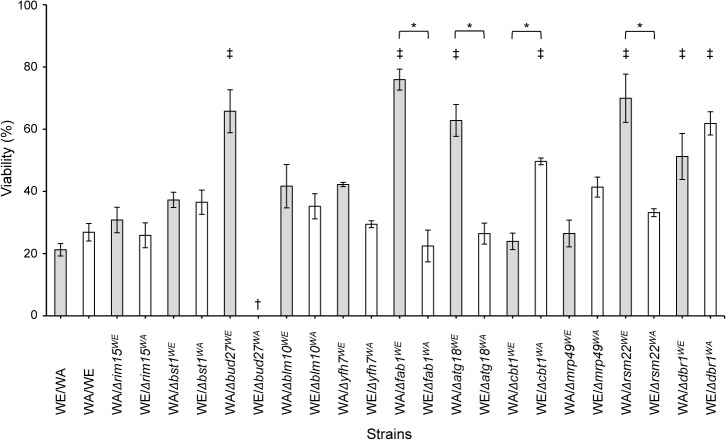
To identify causative genes within the mapped QTL intervals on chromosomes VI and XI, we generated a set of haploid strains with deletions in the candidate genes (Table 1). Then, their desiccation tolerance capacity was assessed (Fig. 2). After rehydration, four strains (WA, Δbud27; WA, Δfab1; WA, Δatg18; and WA, Δcbt1) exhibited a similar reduction in cell viability values, which were ~20% lower than in the WA strain (49%). Surprisingly, the same set of gene deletions in the WE genetic background showed the opposite effect, with viability values ~30% higher than the WT. In addition, both versions of the Δdbr1 strain showed significantly higher viability values after dehydration stress compared with the WT WA and WE strains (20% and 80%, respectively). Furthermore, the WE, Δrsm22 strain displayed 30% higher viability than its reference strain, whereas the WA, Δrsm22 strain had similar viability to the WA strain. The viabilities of the Δrim15, Δbst1, Δblm10, Δyfh7, and Δmrp49 strains were not significantly different from the WT strains, WA and WE, suggesting that these genes are not involved in desiccation-rehydration stress resistance. Therefore, two-thirds of the WE mutants enhanced dehydration stress tolerance, suggesting that the BUD27 WE, FAB1 WE, ATG18 WE, CBT1 WE, and RSM22 WE alleles have a detrimental effect on the ability of the WE strain to overcome this type of stress. To confirm the impact of these alleles on dehydration stress, we used a reciprocal hemizygosity analysis (S1B Fig.) [29]. A set of isogenic hybrid strains was developed by crossing the haploid knockout strains with the complementary WA (Nat R) or WE (Hyg R) strain [e.g., WA (Nat R) x WE Δrim15 (Hyg R) or WA Δrim15 (Hyg R) x WE (Nat R), Table 1]. The desiccation tolerance of the hemizygous strains was measured (Fig. 3). The WA/Δbud27 WE strain showed ~40% higher viability than the WA/WE strain, which correlated with the increased viability of the WE, Δbud27 strain after stress induction, suggesting an adverse effect of the BUD27 WE allele on stress resistance. Additionally, the WE/Δbud27 WA strain could not be obtained, suggesting a certain level of incompatibility between the BUD27 WE allele and the WA genetic background. After dehydration stress induction, the hybrid strains carrying FAB1 WA, ATG18 WA, CBT1 WE, and RSM22 WA showed viability values nearly 30% higher than the hybrids carrying FAB1 WE, ATG18 WE, CBT1 WA, and RSM22 WE and the reference strains. The detrimental effects of the FAB1 WE, ATG18 WE, CBT1 WA, and RSM22 WE alleles on overcoming dehydration stress were concomitant with the enhanced viability values obtained for the WE, Δfab1, WE, Δatg18, WA, Δcbt1, and WE, Δrsm22 strains (Fig. 2). Furthermore, hybrids carrying either the DBR1 WE or DBR1 WA allele exhibited 30% higher viability than the heterozygous strains (Fig. 3). From the cell viability results for the WA, Δdbr1, WE, Δdbr1 and heterozygous strains, a correlation can be assumed between the increasing number of DBR1 allele copies per cell and the decreasing desiccation survival rate. The desiccation tolerances of a collection of 4,850 viable mutant haploid strains (BY4742) were previously assessed [3, 30]. For the genes above, only the Δrsm22 and Δdbr1 strains (BY4742 background) exhibited significantly higher viability values after stress induction (73% and 77%, respectively) compared with the BY4742 strain. The viability of the Δrim15, Δbst1, Δbud27, Δyfh7, Δfab1, Δatg18, and Δcbt1 strains did not significantly differ from the reference strain (34%) [2]. However, the BY4742, Δmrp49 strain showed 20% viability, which contrasts with the unchanging viability values for the WA, Δmrp49 and WE, Δmrp49 strains. These results confirm that RSM22 WE, which has 98% sequence identity to the RSM22 BY4742, DBR1 WA, DBR1 WE, and DBR1 BY4742 gene products, has a detrimental effect on dehydration stress tolerance.
Fig 2. Effect of knockout haploid strains on yeast viability after DRS.
The scale of viability (%) indicates the percentage of experimental values for the different strains. The values shown are means of n = 3 independent samples ± SD. *Significant differences (p≤0.01) between knockout and its own parental strains.
Fig 3. Hybrid viability after DRS.
The scale of viability (%) indicates the percentage of experimental values for the different strains.
The values shown are the means of at least n = 3 independent samples ± SD. † Non-viable strain. *Significant differences at p≤0.01 between hemizygous strains. ‡ Significant differences at p≤0.01 between the hemizygous and reference strains.
The ATG18 WE allele compromises vacuole function
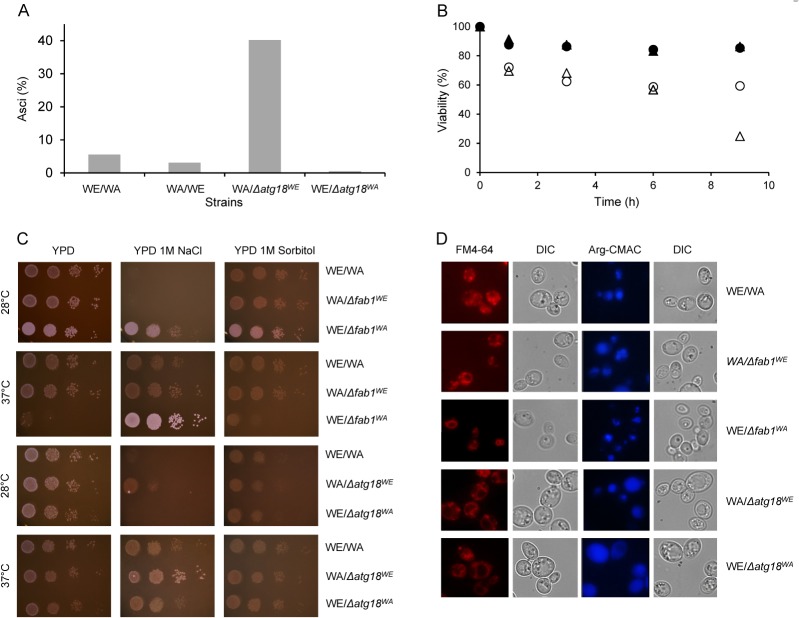
Atg18 is a key component in retrograde membrane trafficking from the vacuole to the Golgi apparatus via the endosome and is also an apparent effector and modulator of phosphatidylinositol (3,5)-bisphosphate [PtdIns(3,5)P 2] [31]. It should be noted that the vacuole is responsible for amino acid storage and therefore represents the cellular reserve of nitrogen and phosphate. When yeast cells are exposed to starvation conditions, such as upon entrance into the stationary phase or during sporulation, vacuolar hydrolases are upregulated to obtain recycled nutrients through the turnover of macromolecules [32]. It follows then that malfunctions in the nutrient storage or recycling machinery are likely to compromise cell viability. Homozygous diploid Δatg18 is defective in autophagy prior to vacuole fusion of autophagosomes, causing the development of cell sensitivity to nitrogen starvation and non-sporulating cells [33]. The hybrid carrying ATG18 WA showed 35% higher asci formation than the WE (Nat R)/WA (Hyg R) and WA (Nat R)/WE (Hyg R) strains, at 7% and 3%, respectively. However, the hybrid carrying ATG18 WE showed the lowest asci formation, at 0.5% of the total cells (Fig. 4A). The wild-type and hemizygous strains were first grown to the mid-log phase and then shifted to nitrogen starvation conditions, and their viability was determined over time (Fig. 4B). The hybrid strains survived nine days of nitrogen starvation with no significant decrease in viability. In contrast, the number of viable cells for the hybrid carrying ATG18 WE and the hybrid carrying ATG18 WA decreased by up to 60% and 20%, respectively, over the same time period. Additionally, Δatg18 cells exhibited phenotypic defects, including non-acidic and conspicuous vacuoles and the loss of osmotic stress tolerance [34]. To determine putative changes in vacuole morphologies, samples of aerated wild-type, WA/Δatg18 WE, and WE/Δatg18 WA cells in the stationary phase were analysed by fluorescence microscopy using FM4-64 and the blue fluorescent dye Arg-CMAC, which accumulates in acidic vacuoles (Fig. 4D). Both Δatg18 hemizygous strains had larger vacuoles than the WE/WA cells, but the hybrid carrying ATG18 WE showed abnormal vacuolar acidification compared with the hybrid carrying ATG18 WA and the WE/WA strains. To assess the consequences of the ATG18 WE allele, the osmotic sensitivity was tested when the cells were grown on media containing 1 M NaCl or 1 M sorbitol at 28°C and 37°C (Fig. 4C). On the 1 M NaCl plates, the hybrid carrying ATG18 WA showed better growth performance at 37°C and 28°C relative to the hybrid carrying ATG18 WE. No significant growth differences were exhibited between hybrids for the other serial dilutions grown on YPD and 1 M sorbitol at 37°C and 28°C. The data indicates that ATG18 WE may not provide adequate nutrient storage to tolerate starvation conditions, thereby inducing both low cell viability under nitrogen starvation conditions and impaired asci formation. The ATG18 WE allele was more sensitive to osmotic stress at high temperatures than the ATG18 WA allele, which correlated with the differences in dehydration tolerance observed for these alleles. Furthermore, the ionic osmotic sensitivity showed by the hybrids carrying either the ATG18 WA or the ATG18 WE allele reverted to a resistant phenotype when the cells were grown at a high temperature.
Fig 4. Phenotypic characterization of ATG18 and FAB1 alleles.
A) Hemizygous diploid Δatg18 cells showed different sporulation patterns. After 48 hours on 1% K-acetate, the counted asci were expressed as a percentage of total cells. B) Effect of nitrogen starvation on cell viability of the Δatg18 strains. The hybrid WE (Nat R)/WA (Hyg R) (●), WA (Nat R)/WE (Hyg R) (▲), WE/ATG18 WA (○), and WA/ATG18 WE (Δ) strains were grown until the mid-log phase in SD and then moved to SD-N. Aliquots were collected and plated on YPD at the indicated times. The scale of viability (%) indicates the percentage of viable cells for the different strains against the time in starvation medium. Values are the mean of triplicate measurements, and the standard deviation was less than 15%. C) FAB1 WA and ATG18 WE rescue cells from ionic-hyperosmotic stress at 37°C. Serial dilutions of heterozygous and hemizygous strain cells were plated on YPD medium, YPD medium containing 1 M NaCl, and 1 M sorbitol and grown at the indicated temperatures. D) Hemizygous cells show vacuole fragmentation and vacuole acidification deficiency. Each pair of image columns show phase microscopy of the same field, which shows cells stained with FM4-64 to visualize vacuole membrane, pH vacuolar dye cell blue Arg-CMAC, and the differential interference contrast (DIC) images.
The FAB1 WE allele enhances osmotic ionic stress tolerance
Retrograde membrane traffic from the vacuole to the Golgi apparatus via the endosome depends on PtdIns(3,5)P 2.[35, 36]. The kinase FAB1p generates PtdIns(3,5)P 2 via phosphatidylinositol (3)-phosphate phosphorylation [37, 38]. Abnormal levels of PtdIns(3,5)P 2 were observed in Δatg18 yeast cells, suggesting that Atg18 is an inhibitor of the Fab1 kinase [39]. Yamamoto et al. [34] suggested that fab1 mutations in yeast cells cause aberrant chromosome segregation, defects in cell surface integrity, and deficiencies in vacuole morphology and function. To determine the incidence of FAB1 alleles in vacuole activity, WA/Δfab1 WE and WE/Δfab1 WA cells were grown on medium containing 1 M NaCl or 1 M sorbitol at 28°C and 37°C (Fig. 4C). The hybrid carrying FAB1 WE grew on 1 M NaCl at 28°C, whereas the hybrid carrying FAB1 WA and the WE/WA strain did not. However, all of the strains grew similarly on 1 M sorbitol. At 37°C, the hybrid carrying FAB1 WE was osmoremediated on 1 M NaCl but was not recovered on 1 M sorbitol. The data indicates that ionic osmotic stress rescues the growth of FAB1 WE hemizygous cells at this non-permissive temperature. The vacuolar morphology and activity of hybrid-carrying FAB1 WA or FAB WE in the stationary phase were analysed using FM4-64 and Arg-CMAC dyes, respectively (Fig. 4D). The vacuolar acidity Arg-CMAC dye profile of the hemizygote cells was similar to that of the reference cells. However, Arg-CMAC and FM4-64 staining revealed vacuolar fragmentation in the hybrid carrying FAB1 WE, which contrasts with the single large vacuole per cell observed in both the hybrid carrying FAB1 WA and the WE/WA strain. The FAB1 WE allele is more sensitive than the FAB1 WA allele to osmotic stress at high temperatures, which correlates with the differences in dehydration tolerance observed for these alleles. Alternatively, an isogenic strain was developed by crossing the haploid double knockout strain WE, Δatg18u, Δfab1 with the complementary WA (Nat R) strain (Table 1). The WA/Δatg18u we, Δfab1 we strain showed ~60% higher viability than the WA/WE strain, which was correlated with the increase in viability of the WE, Δatg18u, Δfab1 strain after dehydration stress, which showed 65% viability (data not shown). Surprisingly, the double knockout WA, Δatg18u, Δfab1 strain could not be obtained. To exclude putative artificial regulatory effect of the deletions over the genes ATG18 or FAB1, which are in the same chromosome at a distance of 3.5 kb, we quantified their expression in samples from WA; WE; WA, Δfab1; WA, Δatg18; WE, Δfab1; WE, Δatg18 and WA/Δatg18u we, Δfab1 strains (S2 Fig.). Our data showed no statistically significant differences between the controls and the strain samples in the expression of any of the tested genes.
The CBT1 and RSM22 alleles do not show respiratory deficiencies
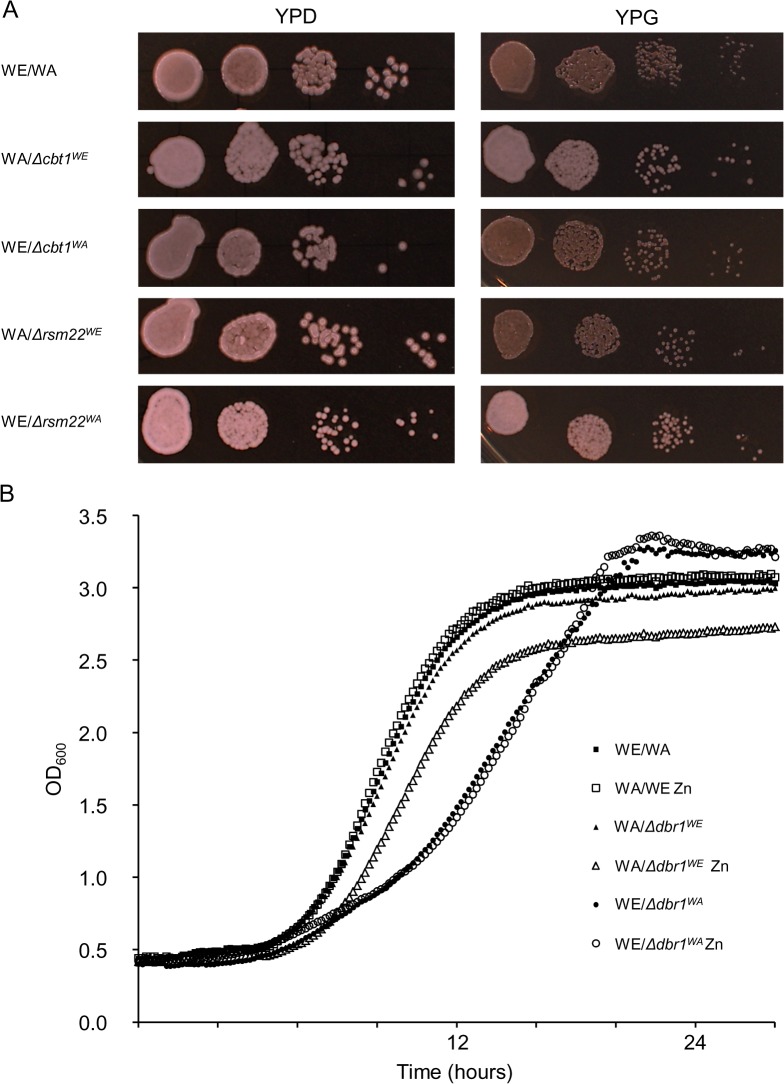
From a gene pool identified after a large-scale functional analysis of respiratory-deficient yeast, the mutant Δcbt1 and Δrsm22 strains showed impaired respiratory performance [39]. The mitochondrial small ribosomal subunit protein Rsm22 participates in mitochondrial mRNA translation, and Cbt1 is involved in mt mRNA stabilization. Both of these proteins are essential for respiratory growth. To assess the putative effects of these alleles on respiration activity, serial dilutions of the wild-type, WA/Δcbt1 WE, WE/Δcbt1 WA, WA/Δrsm22 WE, and WE/Δrsm22 WA strains were plated on YPD and YPG media and incubated at 28°C for 24 h and 48 h. No significant differences in growth were observed between the different hybrids on YPG medium with glycerol as the respiratory carbon source (Fig. 5A), suggesting that the CBT1 and RSM22 alleles do not significantly affect the respiratory activity of hybrid cells. Therefore, both the hybrid carrying CBT1 WE and the hybrid carrying RSM22 WA enhance dehydration tolerance with no apparent variation in cellular respiration.
Fig 5. Phenotypic characterization of CBT1, RSM22, and DBR1 alleles.
A) CBT1 and RSM22 alleles did not show respiratory deficiency. Serial dilutions of heterozygous and hemizygous strain cells were plated on YPD medium and YPG medium containing 2% glycerol, which were grown at 28°C for one and two days, respectively. B) The hybrid carrying DBR1 WA shows defective competitive fitness. Optical density at 600 nm (OD600) was monitored every 10 min as a growth measure at 28°C of the strains in SD medium and SD medium containing 3.5 mM ZnCl2.
The DBR1 WA allele provides competitive disadvantages to yeast cells
The RNA lariat debranching enzyme Dbr1p is involved in intron turnover, which is required for efficient Ty1 transposition [40]. The phenotypes already described for the Δdbr1 strain include decreasing competitive fitness and lower resistance to zinc deficiency. [41, 42]. We aimed to ascertain the growth performance of the Δdbr1 hemizygous strains in minimal medium and minimal medium supplemented with 1 μM, 3.5 mM, or 7 mM zinc dichloride (Fig. 5B shows the growth with 3.5 mM ZnCl2). Hybrids carrying DBR1 WA and DBR1 WE exhibited doubling times (DT) that were 5.8 min and 67.7 min higher, respectively, than the WE/WA strain. Both the hybrid carrying DBR1 WE and the reference strain showed similar DT in media with or without Zn, but the hybrid carrying DBR1 WA exhibited a 24.8 min higher DT in the presence of Zn than when grown in minimal medium alone.
Discussion
Most of the genetic determinants of dehydration tolerance in yeast are still unknown. In this paper, two dehydration-tolerant QTLs were identified using a segregating population. By analysing strains with deleted genes in each QTL and by reciprocal hemizygosity assays, six genes have been confirmed to affect the capacity of yeast cells to survive dehydration and rehydration, namely the BUD27, FAB1, and ATG18 genes mapped to QTLs on chromosome VI and the CBT1, RSM22, and DBR1 genes in QTLs on chromosome XI. Furthermore, their phenotypic effects have been estimated. The genes ATG18, RSM22, and DBR1 were not found to be necessary for desiccation tolerance in yeast cells [3, 10]. The fact that the genes mapped in our results do not fully coincide with previous genetic studies carried out with the S. cerevisiae deletion libraries of mutants sensitive to dehydration stress may indicate that different cellular mechanisms for overcoming stress imposition were caused by dissimilar selective forces exerted during the evolution of the yeast strains, or because the mutations present in the laboratory strains used for these studies are the effectors of these particular phenotypes [43–46]. Therefore, small discrepancies among the genes associated with cell dehydration tolerance from different studies support the idea that different allelic combinations exert different effects.
The nitrogen-deficient sporulation medium contains acetate as a carbon source to promote high levels of respiration, which induce sporulation in diploid yeast strains. In S. cerevisiae, the Δatg18 mutant is defective in sporulation but does not exhibit impaired vacuolar acidification [33]. The sequences of the ATG18 WA and ATG18 WE alleles revealed seven non-identical nucleotides. However, only one point mutation at nucleotide 584, from G to A, causes a single amino acid change of a serine to an asparagine residue (S195N; Table 2). Multiple sequence alignment of the WE and WA ATG18 alleles with 25 sequences of the ATG18 gene from different S. cerevisiae strains annotated in the Saccharomyces Genome Database (SGD), as well as the Atg18 sequence characterized in this study, showed that the S residue is present in 16 genes, the N in eight genes, and the R in only one. This residue is located in the N-terminal region before the two WD40 domains and within a patch of highly conserved residues in Atg18 from Pichia pastoris, Schizosaccharomyces pombe, and H. sapiens [47]. The immediate response of yeast cells to osmotic challenge involves the release of calcium from the vacuole and the formation of fragmented vacuoles [48]. Our results suggest that the FAB1 WE allele principally affects vacuolar morphology, which might allow the hybrid carrying FAB1 WE to adapt quickly to ionic stress. However, 1 M sorbitol osmotic stress at 37°C is lethal to these cells when the WE/WA strain and the hybrid carrying FAB1 WA are adapted. The FAB1 WA and FAB1 WE allele sequences revealed 15 non-identical nucleotides, producing differences in 12 residues (Table 2); however, only the N1273D and Y1300H mutations are located in a region of conserved residues within the Zn-finger domain [49]. Furthermore, none of these residues have a high identity ratio among the Fab1 sequences from the 28 S. cerevisiae strains (SGD). Fab1 governs vacuole homeostasis by generating PtdIns(3,5)P 2 on the vacuolar membrane. Atg18 colocalizes with Fab1, and its deletion causes an abnormal elevation in the levels of PtdIns(3,5)P 2, which suggests that Atg18 is also a negative regulator of the Fab1 kinase pathway [31]. The hybrid carrying FAB1 WA and the hybrid carrying ATG18 WE exhibit an osmotic pressure-dependent growth phenotype (Fig. 4C), indicating that the genes are essential for growth only at high temperatures in the presence of osmotic ionic stress. At the permissive temperature, the hybrids carrying FAB1 WA and the hybrid carrying ATG18 WE exhibited extremely defective growth. These phenotypes are comparable to the ones exhibited by some of the temperature-sensitive isolated vacuolar protein sorting (vps) mutants, which require one or more vacuolar functions at the permissive temperature that cannot be provided at 37°C by other vacuolar components in these mutant cells [50].
The DBR1 gene is conserved in humans (hDBR1) and maintains the same function in both human and yeast cells [51]. Among other phenotypes of the Δdbr1 strain, decreases in competitive fitness and Zn deficiency stress resistance have been previously described [41–42]. The growth fitness of a strain with the DBR1 WE allele is affected and this strain is less sensitive to Zn stress than the DBR1 WA allele, for which the opposite effect on growth is observed. The DBR1 WA allele had K286 and N325 residues in the putative HMM domain, replacing E286 and D325, respectively (Table 2), which are 100% conserved in other Dbr1 peptides deduced from the genomic sequences of 26 different S. cerevisiae strains (SGD). The deduced sequence of Cbt1WA showed two residue differences with Cbt1WE, S29G, and T109A. In addition, three mutations were observed between the deduced peptide sequences of the RSM22 WA and RSM22 WE genes: E228K, D474S, and S619N (Table 2). These mutations do not affect the respiratory capacity of the different strains, thus enabling the separation of dehydration stress tolerance from respiration capacity. However, the above-mentioned variations in the sporulation efficiency of the ATG18 hemizygous strains are not due to a pleotropic effect of the RSM22 or CBT1 alleles that affects cellular respiration.
The genetic approach used in this study, with a population of 96 segregants, allowed the detection of yeast dehydration resistance QTLs. The RSM22 and ATG18 genes enclosed within these QTLs that provide dehydration tolerance to the cell were not referenced in previous studies. Additionally, a detrimental effect on dehydration stress tolerance was shown to be provided by DBR1 gene products. Our results further the understanding that dehydration stress tolerance is not a phenotype that results from the individual addition of independent genes. Furthermore, the monogenic approach is not suitable for summarizing all of the epistatic effects driven by a group of alleles. Currently, the successful long-term storage of living cells is of critical importance, but the frequently contradictory results associated with complex eukaryotic cells make the application of a simpler model system desirable. There are a number of advantages, including ease of growth and modification and well-characterized cell physiology, genetics and biochemistry, which make yeast cells the model of choice for anhydrobiotic engineering.
Supporting Information
A) Production of F1 population [52]. B) Haploid strains were disrupted for the identified genes (e.g., ATG18) using URA3 and used to develop heterozygous diploid strains by reciprocal hemizygous crossover.
(TIF)
Data represent mean relative expression ± SD (y axis, Log2 values) of each individual gene (show at the bottom) before dehydration of different strains. Genes ALG9 and TAF10 were simultaneously used as constitutive reference genes as determined by the geNorm algorithm [53]. Relative expression was calculated using REST-MCS v2 software [54].
(TIF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grant AGL2012-40018 from the Spanish Ministerio de Ciencia e Innovación. The authors thank Rovira i Virgili University for the doctoral fellowships FI-DGR 2012, AGAUR to GL. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dupont S, Rapoport A, Gervais P, Beney L. Survival kit of Saccharomyces cerevisiae for anhydrobiosis. Appl Microbiol Biotechnol. 2014;98: 8821–8834. 10.1007/s00253-014-6028-5 [DOI] [PubMed] [Google Scholar]
- 2. Rodríguez-Porrata B, Carmona-Gutierrez D, López-Martínez G, Reisenbichler A, Bauer M, Frank M, et al. Yeast cell death during drying and rehydration process In Indrid Schmid, INTECH, UCLA editors. Flow Cytometry, Vol 1; 2012. pp. 119–132. [Google Scholar]
- 3. Rodríguez-Porrata B, Carmona-Gutierrez D, Reisenbichler A, Bauer M, López G, Escoté X, et al. Sip18 hydrophilin prevents yeast cell death during desiccation stress. J Appl Microbiol. 2012;112: 512–525. 10.1111/j.1365-2672.2011.05219.x [DOI] [PubMed] [Google Scholar]
- 4. López-Martínez G, Rodríguez-Porrata B, Margalef-Català M, Cordero-Otero R. The STF2p hydrophilin from Saccharomyces cerevisiae is required for dehydration stress tolerance. PLoS One. 2012;7: 3 Available: http://www.plosone.org/article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novo M, Beltran G, Rozes N, Guillamon JM, Sokol S, Leberre V, et al. Early transcriptional response of wine yeast after rehydration: osmotic shock and metabolic activation. FEMS Yeast Res. 2007;7: 304–316. [DOI] [PubMed] [Google Scholar]
- 6. Singh J, Kumar D, Ramakrishnan N, Singhal V, Jervis J, Garst JF, et al. Transcriptional response of Saccharomyces cerevisiae to desiccation and rehydration. Appl Environ Microbiol. 2005;71: 8752–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miermont A, Waharte F, Hu S, McClean MN, Bottani S, León L: et al. Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc Natl Acad Sci USA. 2013;110: 5725–5730. 10.1073/pnas.1215367110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossignol T, Postaire O, Storaï J, Blondin B. Analysis of the genomic response of a wine yeast to rehydration and inoculation. Appl Microbiol Biotechnol. 2006;71: 699–712. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura T, Mizukami-Murata S, Ando A, Murata Y, Takagi H, Shima J. Changes in gene expression of commercial baker's yeast during an air-drying process that simulates dried yeast production. J Biosci Bioeng. 2008;106: 405–408. 10.1263/jbb.106.405 [DOI] [PubMed] [Google Scholar]
- 10. Ratnakumar S, Hesketh A, Gkargkas K, Wilson M, Rash BM, Hayes A, et al. Phenomic and transcriptomic analyses reveal that autophagy plays a major role in desiccation tolerance in Saccharomyces cerevisiae . Mol Biosyst. 2011;7: 139–149. 10.1039/c0mb00114g [DOI] [PubMed] [Google Scholar]
- 11. Cubillos FA, Billi E, Zörgö E, Parts L, Fargier P, Omholt S, et al. Assessing the complex architecture of polygenic traits in diverged yeast populations. Mol Ecol. 2011;20: 1401–1413. 10.1111/j.1365-294X.2011.05005.x [DOI] [PubMed] [Google Scholar]
- 12. Yang Y, Foulquié-Moreno MR, Clement L, Erdei E, Tanghe A, Schaerlaekens K, et al. QTL analysis of high thermotolerance with superior and downgraded parental yeast strains reveals new minor QTLs and converges on novel causative alleles involved in RNA processing. PLoS Gnet. 2013;9: 8 Available: http://www.plosgenetics.org/article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu XH, Wang MH, Tan T, Li JR, Yang H, Leach L, et al. Genetic dissection of ethanol tolerance in the budding yeast Saccharomyces cerevisiae . Genetics. 2007;175: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marullo P, Aigle M, Bely M, Masneuf-Pomarède I, Durrens P, Dubourdieu D, et al. Single QTL mapping and nucleotide-level resolution of a physiologic trait in wine Saccharomyces cerevisiae strains. FEMS Yeast Res. 2007;7: 941–952. [DOI] [PubMed] [Google Scholar]
- 15. Deutschbauer AM, Davis RW. Quantitative trait loci mapped to single-nucleotide resolution in yeast. Nat Genet. 2005;37: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 16. Katou T, Namise M, Kitagaki H, Akao T, Shimoi H. QTL mapping of sake brewing characteristics of yeast. J Biosci Bioeng. 2009;107: 383–393. 10.1016/j.jbiosc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 17. Ambroset C, Petit M, Brion C, Sanchez I, Delobel P, Guerín C, et al. Deciphering the molecular basis of wine yeast fermentation traits using a combined genetic and genomic approach. G3 (Bethesda). 2011;1: 263–281. 10.1534/g3.111.000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16: 339–346. [DOI] [PubMed] [Google Scholar]
- 19. Louis EJ, Borts RH. A complete set of marked telomeres in Saccharomyces cerevisiae for physical mapping and cloning. Genetics. 1995;139: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huxley C, Green ED, Dunham I. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 1990;6: 236 [DOI] [PubMed] [Google Scholar]
- 21. Sambrook J, Russell DW. Molecular Cloning, A Laboratory Manual. New York, Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 22. Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14: 115–132. [DOI] [PubMed] [Google Scholar]
- 23. Cubillos FA, Louis EJ, Liti G. Generation of a large set of genetically tractable haploid and diploid Saccharomyces strains. FEMS Yeast Res. 2009;9: 1217–1225. 10.1111/j.1567-1364.2009.00583.x [DOI] [PubMed] [Google Scholar]
- 24. Louis EJ, Borts RH. A complete set of marked telomeres in Saccharomyces cerevisiae for physical mapping and cloning. Genetics. 1995;139: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodríguez-Porrata B, López-Martínez G, Redón M, Sancho M, Mas A, Rozès N, et al. Enhancing yeast cell viability after dehydration by modification of the lipid profile. W J Microbiol Biotech. 2011;27: 75–83. [Google Scholar]
- 26. Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19: 889–890. [DOI] [PubMed] [Google Scholar]
- 27. Salinas F, Cubillos FA, Soto D, Garcia V, Bergström A, Warringer J, et al. (2012) The genetic basis of natural variation in oenological traits in Saccharomyces cerevisiae . PLoS One. 2012;7: 11 Available: http://www.plosone.org/article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 29. Marullo P, Bely M, Masneuf-Pomarède I, Pons M, Aigle M. Breeding strategies for combining fermentative qualities and reducing off-flavor production in a wine yeast model. FEMS Yeast Res. 2006;6: 268–279. [DOI] [PubMed] [Google Scholar]
- 30. Shima J, Ando A, Takagi H. Possible roles of vacuolar H(+)-ATPase and mitochondrial function in tolerance to air-drying stress revealed by genome-wide screening of Saccharomyces cerevisiae deletion strains. Yeast. 2008;25: 179–190. 10.1002/yea.1577 [DOI] [PubMed] [Google Scholar]
- 31. Efe JA, Botelho RJ, Emr SD. Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate. Mol Biol Cell. 2007;18: 4232–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function, and biogenesis. Microbiol Mol Biol Rev. 1990;54: 266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barth H, Meiling-Wesse K, Epple UD, Thumm M. Autophagy and the cytoplasm to vacuole targeting pathway both require Aut10p. FEBS Lett. 2001;508: 23–28. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995;6: 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gary JD, Sato TK, Stefan CJ, Bonangelino CJ, Weisman LS, Emr SD. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell. 2002;13: 1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dove SK, McEwen RK, Mayes A, Hughes DC, Beggs JD, Michell RH. Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr Biol. 2002;12: 885–893. [DOI] [PubMed] [Google Scholar]
- 37. Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dove SK, Piper RC, McEwen RK, Yu JW, King MC, Hughes DC, et al. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23: 1922–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Merz S, Westermann B. Genome-wide deletion mutant analysis reveals genes required for respiratory growth, mitochondrial genome maintenance and mitochondrial protein synthesis in Saccharomyces cerevisiae . Genome Biol. 2009;10: R95 Available: http://genomebiology.com. 10.1186/gb-2009-10-9-r95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chapman KB, Boeke JD. Isolation and characterization of the gene encoding yeast debranching enzyme. Cell. 1991;65: 483–492. [DOI] [PubMed] [Google Scholar]
- 41. Breslow DK, Cameron DM, Collins SR, Schuldiner M, Stewart-Ornstein J, Newman HW, et al. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods. 2008;5: 711–718. 10.1038/nmeth.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. North M, Steffen J, Loguinov AV, Zimmerman GR, Vulpe CD, Eide DJ. Genome-wide functional profiling identifies genes and processes important for zinc-limited growth of Saccharomyces cerevisiae . PLoS Genet. 2012;8: e1002699 Available: http://www.plosgenetics.org/article. 10.1371/journal.pgen.1002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40: 584–591. 10.1038/ng.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maher B. Personal genomes: The case of the missing heritability. Nature. 2008;456: 18–21. 10.1038/456018a [DOI] [PubMed] [Google Scholar]
- 45. Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40: 575–583. 10.1038/ng.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Romano GH, Gurvich Y, Lavi O, Ulitsky I, Shamir R, Kupiec. Different sets of QTLs influence fitness variation in yeast. Mol Syst Biol. 2010;6: 346 10.1038/msb.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guan J, Stromhaug PE, George MD, Habibzadegah-Tari P, Bevan A, Dunn WA Jr, et al. Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris . Mol Biol Cell. 2001;12: 3821–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li SC, Kane PM. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim Biophys Acta. 2009;1793: 650–663. 10.1016/j.bbamcr.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shaw JD, Hama H, Sohrabi F, DeWald DB, Wendland B. PtdIns(3,5)P2 is required for delivery of endocytic cargo into the multivesicular body. Traffic. 2003;4: 479–490. [DOI] [PubMed] [Google Scholar]
- 50. Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62: 230–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim JW, Kim HC, Kim GM, Yang JM, Boeke JD, Nam K. Human RNA lariat debranching enzyme cDNA complements the phenotypes of Saccharomyces cerevisiae dbr1 and Schizosaccharomyces pombe dbr1 mutants. Nucleic Acids Res. 2000;28: 3666–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liti G, Carter DM, Moses AM, Warringer J, Parts L. Population genomics of domestic and wild yeasts. Nature. 2009;19: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3: RESEARCH00340034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30: e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Production of F1 population [52]. B) Haploid strains were disrupted for the identified genes (e.g., ATG18) using URA3 and used to develop heterozygous diploid strains by reciprocal hemizygous crossover.
(TIF)
Data represent mean relative expression ± SD (y axis, Log2 values) of each individual gene (show at the bottom) before dehydration of different strains. Genes ALG9 and TAF10 were simultaneously used as constitutive reference genes as determined by the geNorm algorithm [53]. Relative expression was calculated using REST-MCS v2 software [54].
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.