Abstract
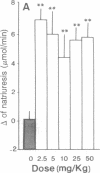
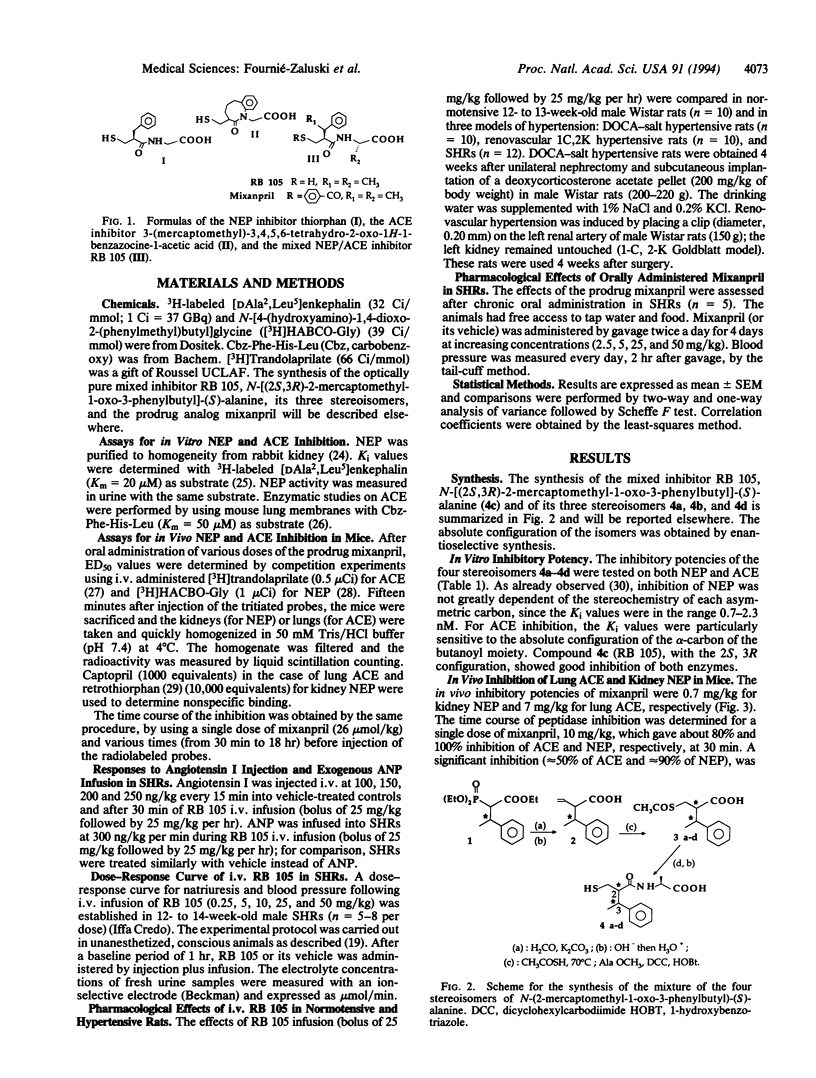
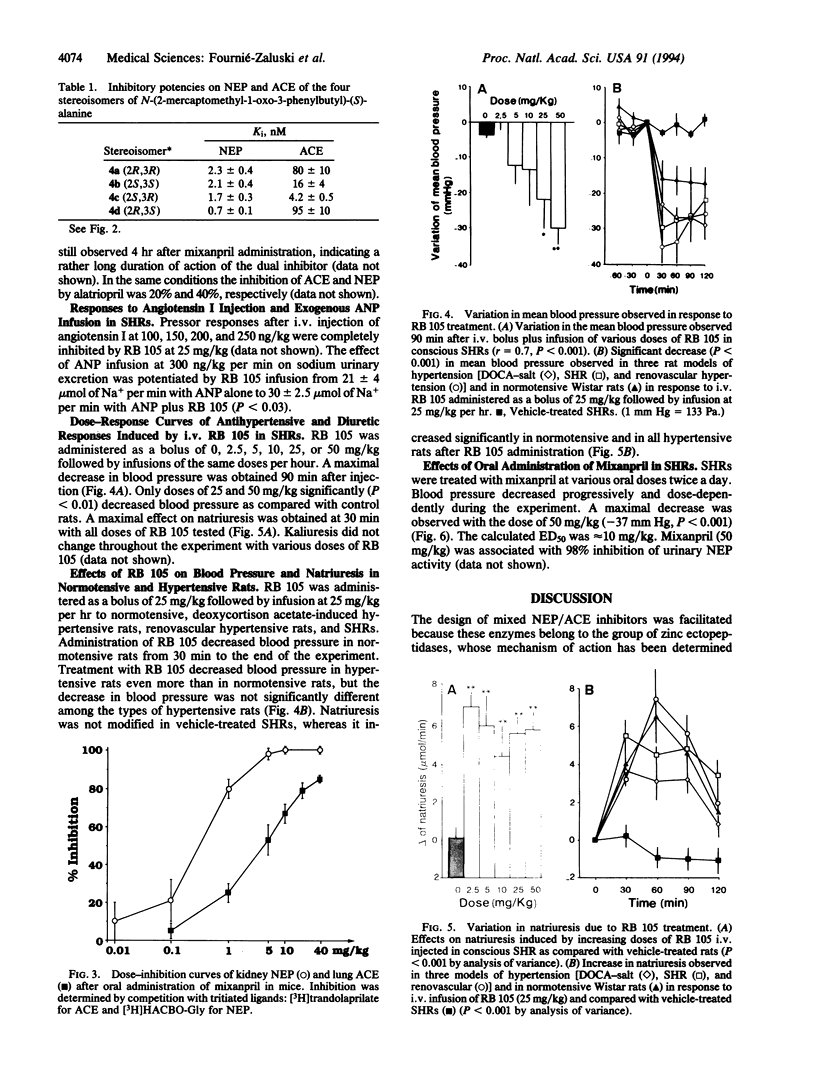
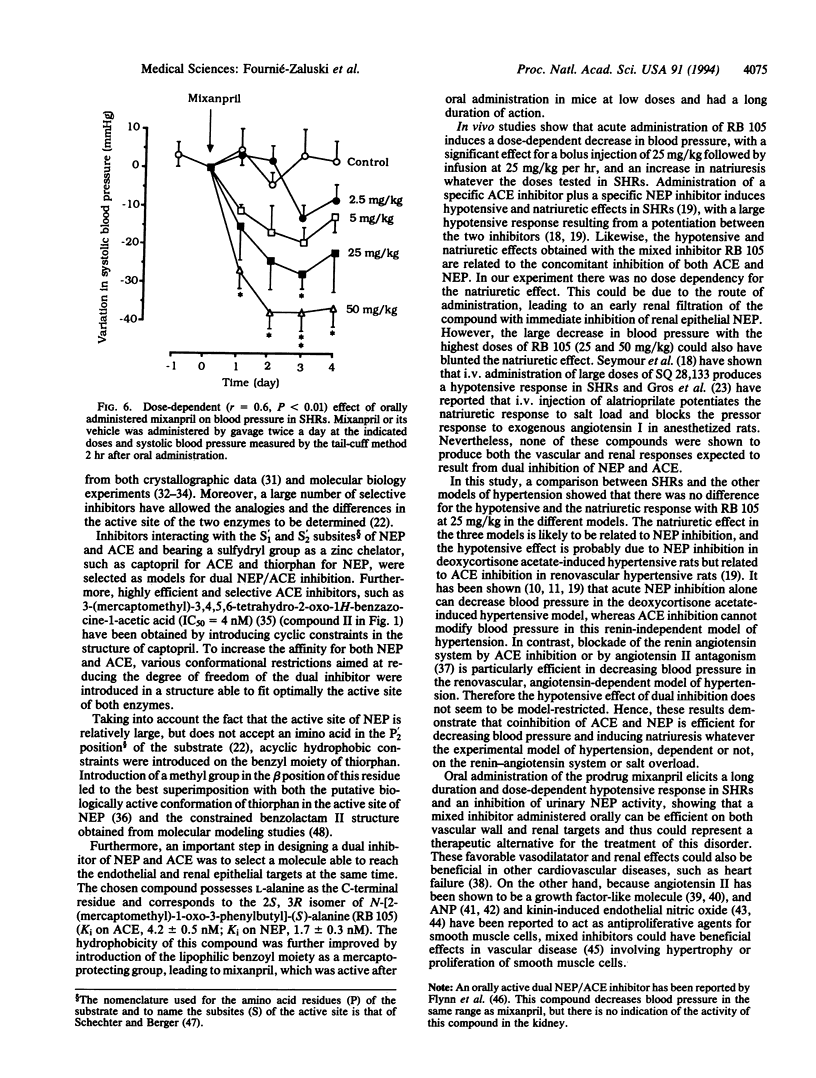
In the treatment of cardiovascular disease, it could be of therapeutic interest to associate the hypotensive effects due to the inhibition of angiotensin II formation with the diuretic and natriuretic responses induced by the protection of the endogenous atrial natriuretic peptide (ANP). Investigation of this hypothesis requires an orally active compound able to simultaneously inhibit angiotensin-converting enzyme (ACE) and neutral endopeptidase (NEP), which is involved in renal ANP metabolism. Such compounds have been rationally designed by taking into account the structural characteristics of the active site of both peptidases. Among them, RB 105, N-[(2S,3R)-2-mercaptomethyl-1-oxo-3-phenylbutyl]-(S)-alanine, inhibited NEP and ACE with Ki values of 1.7 +/- 0.3 nM and 4.2 +/- 0.5 nM, respectively. Intravenous infusion of RB 105 in conscious spontaneously hypertensive rats prevented the pressor response to exogenous angiotensin I and potentiated the natriuretic response to ANP. Infusion of RB 105, at 2.5, 5, 10, 25, and 50 mg/kg per hr decreased blood pressure dose-dependently in conscious catheterized spontaneously hypertensive rats and increased diuresis and natriuresis. Infusion of RB 105 as a bolus of 25 mg/kg followed by 25 mg/kg per hr similarly decreased blood pressure and increased natriuresis in three different models of hypertension (renovascular, deoxycorticosterone acetate-salt, and spontaneously hypertensive rats). Mixanpril, a lipophilic prodrug of RB 105 (ED50 values when given orally to mice, 0.7 mg/kg for NEP; 7 mg/kg for ACE), elicited dose-dependent hypotensive effects of long duration in spontaneously hypertensive rats after oral administration [-37 mmHg for 50 mg/kg twice a day (1 mmHg = 133 Pa) and is therefore the first dual NEP/ACE inhibitor potentially useful for clinical investigations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel R. G. Growth-regulatory properties of atrial natriuretic factor. Am J Physiol. 1992 Jun;262(6 Pt 2):F911–F918. doi: 10.1152/ajprenal.1992.262.6.F911. [DOI] [PubMed] [Google Scholar]
- Aubry M., Berteloot A., Beaumont A., Roques B. P., Crine P. The use of a monoclonal antibody for the rapid purification of kidney neutral endopeptidase ("enkephalinase") solubilized in octyl glucoside. Biochem Cell Biol. 1987 Apr;65(4):398–404. doi: 10.1139/o87-050. [DOI] [PubMed] [Google Scholar]
- Bateman R. C., Jr, Jackson D., Slaughter C. A., Unnithan S., Chai Y. G., Moomaw C., Hersh L. B. Identification of the active-site arginine in rat neutral endopeptidase 24.11 (enkephalinase) as arginine 102 and analysis of a glutamine 102 mutant. J Biol Chem. 1989 Apr 15;264(11):6151–6157. [PubMed] [Google Scholar]
- Beaumont A., Le Moual H., Boileau G., Crine P., Roques B. P. Evidence that both arginine 102 and arginine 747 are involved in substrate binding to neutral endopeptidase (EC 3.4.24.11). J Biol Chem. 1991 Jan 5;266(1):214–220. [PubMed] [Google Scholar]
- Berk B. C., Vekshtein V., Gordon H. M., Tsuda T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989 Apr;13(4):305–314. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- Burnier M., Ganslmayer M., Perret F., Porchet M., Kosoglou T., Gould A., Nussberger J., Waeber B., Brunner H. R. Effects of SCH 34826, an orally active inhibitor of atrial natriuretic peptide degradation, in healthy volunteers. Clin Pharmacol Ther. 1991 Aug;50(2):181–191. doi: 10.1038/clpt.1991.123. [DOI] [PubMed] [Google Scholar]
- Cumin F., Vellaud V., Corvol P., Alhenc-Gelas F. Evidence for a single active site in the human angiotensin I-converting enzyme from inhibitor binding studies with [3H] RU 44 403: role of chloride. Biochem Biophys Res Commun. 1989 Sep 15;163(2):718–725. doi: 10.1016/0006-291x(89)92282-1. [DOI] [PubMed] [Google Scholar]
- Daemen M. J., Lombardi D. M., Bosman F. T., Schwartz S. M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991 Feb;68(2):450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- Danilewicz J. C., Barclay P. L., Barnish I. T., Brown D., Campbell S. F., James K., Samuels G. M., Terrett N. K., Wythes M. J. UK-69,578, a novel inhibitor of EC 3.4.24.11 which increases endogenous ANF levels and is natriuretic and diuretic. Biochem Biophys Res Commun. 1989 Oct 16;164(1):58–65. doi: 10.1016/0006-291x(89)91682-3. [DOI] [PubMed] [Google Scholar]
- Devault A., Nault C., Zollinger M., Fournie-Zaluski M. C., Roques B. P., Crine P., Boileau G. Expression of neutral endopeptidase (enkephalinase) in heterologous COS-1 cells. Characterization of the recombinant enzyme and evidence for a glutamic acid residue at the active site. J Biol Chem. 1988 Mar 15;263(8):4033–4040. [PubMed] [Google Scholar]
- Dussaule J. C., Grangé J. D., Wolf J. P., Lecomte J. M., Gros C., Schwartz J. C., Bodin F., Ardaillou R. Effect of sinorphan, an enkephalinase inhibitor, on plasma atrial natriuretic factor and sodium urinary excretion in cirrhotic patients with ascites. J Clin Endocrinol Metab. 1991 Mar;72(3):653–659. doi: 10.1210/jcem-72-3-653. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989 Feb;3(2):145–151. [PubMed] [Google Scholar]
- Farhy R. D., Ho K. L., Carretero O. A., Scicli A. G. Kinins mediate the antiproliferative effect of ramipril in rat carotid artery. Biochem Biophys Res Commun. 1992 Jan 15;182(1):283–288. doi: 10.1016/s0006-291x(05)80142-1. [DOI] [PubMed] [Google Scholar]
- Flynn G. A., Beight D. W., Mehdi S., Koehl J. R., Giroux E. L., French J. F., Hake P. W., Dage R. C. Application of a conformationally restricted Phe-Leu dipeptide mimetic to the design of a combined inhibitor of angiotensin I-converting enzyme and neutral endopeptidase 24.11. J Med Chem. 1993 Aug 6;36(16):2420–2423. doi: 10.1021/jm00068a022. [DOI] [PubMed] [Google Scholar]
- Fournie-Zaluski M. C., Lucas E., Waksman G., Roques B. P. Differences in the structural requirements for selective interaction with neutral metalloendopeptidase (enkephalinase) or angiotensin-converting enzyme. Molecular investigation by use of new thiol inhibitors. Eur J Biochem. 1984 Mar 1;139(2):267–274. doi: 10.1111/j.1432-1033.1984.tb08003.x. [DOI] [PubMed] [Google Scholar]
- Fournié-Zaluski M. C., Lucas-Soroca E., Devin J., Roques B. P. 1H NMR configurational correlation for retro-inverso dipeptides: application to the determination of the absolute configuration of "enkephalinase" inhibitors. Relationships between stereochemistry and enzyme recognition. J Med Chem. 1986 May;29(5):751–757. doi: 10.1021/jm00155a027. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. M., Cushman D. W., Tung R., Cheung H. S., Wang F. L., Delaney N. G. Rat brain enkephalinase: characterization of the active site using mercaptopropanoyl amino acid inhibitors, and comparison with angiotensin-converting enzyme. Life Sci. 1983;33 (Suppl 1):113–116. doi: 10.1016/0024-3205(83)90457-5. [DOI] [PubMed] [Google Scholar]
- Gros C., Noël N., Souque A., Schwartz J. C., Danvy D., Plaquevent J. C., Duhamel L., Duhamel P., Lecomte J. M., Bralet J. Mixed inhibitors of angiotensin-converting enzyme (EC 3.4.15.1) and enkephalinase (EC 3.4.24.11): rational design, properties, and potential cardiovascular applications of glycopril and alatriopril. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4210–4214. doi: 10.1073/pnas.88.10.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Pratt R. E., Dzau V. J. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest. 1990 Nov;86(5):1690–1697. doi: 10.1172/JCI114893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Stephenson S. L. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988 May 9;232(1):1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- Kromer E. P., Elsner D., Kahles H. W., Riegger G. A. Effects of atriopeptidase inhibitor UK 79300 on left ventricular hydraulic load in patients with congestive heart failure. Am J Hypertens. 1991 May;4(5 Pt 1):460–463. doi: 10.1093/ajh/4.5.460. [DOI] [PubMed] [Google Scholar]
- Llorens C., Malfroy B., Schwartz J. C., Gacel G., Roques B. P., Roy J., Morgat J. L., Javoy-Agid F., Agid Y. Enkephalin dipeptidyl carboxypeptidase (enkephalinase) activity: selective radioassay, properties, and regional distribution in human brain. J Neurochem. 1982 Oct;39(4):1081–1089. doi: 10.1111/j.1471-4159.1982.tb11500.x. [DOI] [PubMed] [Google Scholar]
- Mourlon-Le Grand M. C., Poitevin P., Benessiano J., Duriez M., Michel J. B., Levy B. I. Effect of a nonhypotensive long-term infusion of ANP on the mechanical and structural properties of the arterial wall in Wistar-Kyoto and spontaneously hypertensive rats. Arterioscler Thromb. 1993 May;13(5):640–650. doi: 10.1161/01.atv.13.5.640. [DOI] [PubMed] [Google Scholar]
- Olins G. M., Krieter P. A., Trapani A. J., Spear K. L., Bovy P. R. Specific inhibitors of endopeptidase 24.11 inhibit the metabolism of atrial natriuretic peptides in vitro and in vivo. Mol Cell Endocrinol. 1989 Feb;61(2):201–208. doi: 10.1016/0303-7207(89)90131-7. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Cushman D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977 Apr 22;196(4288):441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- Pham I., Gonzalez W., el Amrani A. I., Fournié-Zaluski M. C., Philippe M., Laboulandine I., Roques B. P., Michel J. B. Effects of converting enzyme inhibitor and neutral endopeptidase inhibitor on blood pressure and renal function in experimental hypertension. J Pharmacol Exp Ther. 1993 Jun;265(3):1339–1347. [PubMed] [Google Scholar]
- Pham I., el Amrani A. I., Fournié-Zaluski M. C., Corvol P., Roques B., Michel J. B. Effects of the selective neutral endopeptidase inhibitor, retrothiorphan, on renal function and blood pressure in conscious normotensive Wistar and hypertensive DOCA-salt rats. J Cardiovasc Pharmacol. 1992 Dec;20(6):847–857. doi: 10.1097/00005344-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Piquilloud Y., Reinharz A., Roth M. Studies on the angiotensin converting enzyme with different substrates. Biochim Biophys Acta. 1970 Apr 22;206(1):136–142. doi: 10.1016/0005-2744(70)90090-2. [DOI] [PubMed] [Google Scholar]
- Richards M., Espiner E., Frampton C., Ikram H., Yandle T., Sopwith M., Cussans N. Inhibition of endopeptidase EC 24.11 in humans. Renal and endocrine effects. Hypertension. 1990 Sep;16(3):269–276. doi: 10.1161/01.hyp.16.3.269. [DOI] [PubMed] [Google Scholar]
- Roderick S. L., Fournie-Zaluski M. C., Roques B. P., Matthews B. W. Thiorphan and retro-thiorphan display equivalent interactions when bound to crystalline thermolysin. Biochemistry. 1989 Feb 21;28(4):1493–1497. doi: 10.1021/bi00430a011. [DOI] [PubMed] [Google Scholar]
- Roques B. P., Lucas-Soroca E., Chaillet P., Costentin J., Fournié-Zaluski M. C. Complete differentiation between enkephalinase and angiotensin-converting enzyme inhibition by retro-thiorphan. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3178–3182. doi: 10.1073/pnas.80.11.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques B. P., Noble F., Daugé V., Fournié-Zaluski M. C., Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993 Mar;45(1):87–146. [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Seymour A. A., Asaad M. M., Lanoce V. M., Langenbacher K. M., Fennell S. A., Rogers W. L. Systemic hemodynamics, renal function and hormonal levels during inhibition of neutral endopeptidase 3.4.24.11 and angiotensin-converting enzyme in conscious dogs with pacing-induced heart failure. J Pharmacol Exp Ther. 1993 Aug;266(2):872–883. [PubMed] [Google Scholar]
- Seymour A. A., Swerdel J. N., Abboa-Offei B. Antihypertensive activity during inhibition of neutral endopeptidase and angiotensin converting enzyme. J Cardiovasc Pharmacol. 1991 Mar;17(3):456–465. doi: 10.1097/00005344-199103000-00015. [DOI] [PubMed] [Google Scholar]
- Sybertz E. J. SCH 34826: an overview of its profile as a neutral endopeptidase inhibitor and ANF potentiator. Clin Nephrol. 1991 Oct;36(4):187–191. [PubMed] [Google Scholar]
- Ura N., Carretero O. A., Erdös E. G. Role of renal endopeptidase 24.11 in kinin metabolism in vitro and in vivo. Kidney Int. 1987 Oct;32(4):507–513. doi: 10.1038/ki.1987.239. [DOI] [PubMed] [Google Scholar]
- Waksman G., Bouboutou R., Devin J., Besselievre R., Fournie-Zaluski M. C., Roques B. P. Binding of the bidentate inhibitor [3H]HACBO-Gly to the rat brain neutral endopeptidase "enkephalinase". Biochem Biophys Res Commun. 1985 Aug 30;131(1):262–268. doi: 10.1016/0006-291x(85)91797-8. [DOI] [PubMed] [Google Scholar]
- Watthey J. W., Gavin T., Desai M. Bicyclic lactam inhibitors of angiotensin converting enzyme. J Med Chem. 1984 Jun;27(6):816–818. doi: 10.1021/jm00372a022. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Chiu A. T., Duncia J. V., Carini D. J., Wexler R. R., Johnson A. L., Timmermans P. B. Nonpeptide angiotensin II receptor antagonists. IX. Antihypertensive activity in rats of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther. 1990 Feb;252(2):726–732. [PubMed] [Google Scholar]
- Wyvratt M. J., Patchett A. A. Recent developments in the design of angiotensin-converting enzyme inhibitors. Med Res Rev. 1985 Oct-Dec;5(4):483–531. doi: 10.1002/med.2610050405. [DOI] [PubMed] [Google Scholar]