Abstract
Purpose
The difficulties related to treatment of deep surgical site infection involve formation of biofilm on the surface of synthetic material. It is considered that in treatment of infections involving formation of biofilm, concomitant therapy shall be applied covering anti-inflammatory drugs. The purpose of the work was to assess the impact of diclofenac and ibuprofen on bacterial biofilm formation on the surface of monofilament polypropylene mesh.
Materials and methods
The study involved 70 strains of Staphylococcus aureus and 70 strains of Escherichia coli isolated from different patients and those which differ with chromosomal DNA pattern within the species. The assessment of the impact of non-steroidal anti-inflammatory drugs (NSAIDs) on biofilm formation was carried out with the use of qualitative method (TTC reduction), quantitative (tenfold serial dilution) and with the use of scanning electron microscope (SEM).
Results
In the qualitative assessment, after incubation in the medium containing NSAIDs statistically significant growth of S. aureus strain amount and E. coli which poorly make up biofilm was stated. Quantitative examination indicated characteristic decrease of the number of colony forming units in 1 ml of the suspension isolated from bacterial biofilm formed as a result of incubation of isolates in the medium with the addition of examined NSAIDs in comparison to biofilm from control regimen. In the examination with the use of SEM it was stated that the effect of isolates incubation in the medium with NSAIDs was decrease of the number of bacteria adjacent to the biomaterial surface.
Conclusions
Diclofenac and ibuprofen in the concentration obtained in the serum limit the formation of biofilm by S. aureus and E. coli.
Keywords: Biofilm, Diclofenac, Ibuprofen, Polypropylene mesh, Hernia
Introduction
One of the most serious complications related to application of biomaterials in hernia surgery is deep surgical site infection (DSSI) covering the implanted material. The main etiological factors for DSSI include Staphylococcus aureus and Escherichia coli [1, 2]. Inefficiency of preservative methods for treating the complications leads to the situation where, often, the only way for treating it is removal of infected synthetic material [1, 3, 4]. It is considered that difficulties regarding treatment of DSSI may be connected with the formation of bacterial biofilm on the surface of implanted biomaterial [2, 5].
Taking into account the role of biofilm in DSSI pathogenesis, it is necessary to change the hitherto strategy of therapeutic proceeding. In the opinion of Chen and Wen [6] treatment of infections involving formation of biofilm shall include administration of antibiotics together with anti-inflammatory drugs and agents which are active against bacteria in the biofilm.
It was demonstrated that non-steroidal anti-inflammatory drugs such as acetylsalicylic acid or diclofenac characterize with bactericidal reaction on bacteria in the planktonic form [7, 8]. It was also shown that acetylsalicylic acid decreases the number of live cells in biofilm formed by E. coli, Pseudomonas aeruginosa and Candida albicans on the abiotic surface [9], and diclofenac changes the structure of biofilm of S. epidermidis [10]. Until now, the impact of diclofenac and ibuprofen, drugs applied in pre- and post-surgical analgesia [11–14], on formation of biofilm by S. aureus and E. coli on the surface of implants used in hernia surgery, has not been studied.
The purpose of the work was to assess the impact of diclofenac and ibuprofen on formation of biofilm on the surface of monofilament polypropylene mesh by clinical isolates of S. aureus and E. coli.
Materials and methods
Bacteria strains
For the purposes of examination, 70 strains of S. aureus and 70 strains of E. coli from the collection of the Department of Microbiology of the Ludwik Rydygier College of Medicine of the Nicolaus Copernicus University in Toruń, isolated in the years 2008–2009 from wound smears and pus samples collected from various patients hospitalized at the Departments of: General and Endocrine Surgery, General and Vascular Surgery and General Surgery and Transplantology of the No. 1 Dr. A. Jurasz University Hospital in Bydgoszcz.
Affinity to the species of S. aureus isolates was determined based on morphology of colony on Columbia Agar with the addition of 5 % sheep blood (Becton–Dickinson, Sparks, USA), in the presence of clumping factor and/or free coagulase and in the event verification was required; the results of reactions of ID32 Staph (bioMérieux, Lyon, France) biochemical tests were taken into account. Identification of E. coli strains was carried out based on colony morphology on MacConkey Agar medium (Becton–Dickinson, Sparks, USA) and the results of ID32 E test (bioMérieux, Lyon, France). ID32 Staph and ID32 E tests were carried out in accordance with the manufacturer’s recommendations and read after 24 h of incubation in aerobic atmosphere at the temperature of 37 °C in ATB Expression system (bioMérieux, Lyon, France) with the use of data base in V 2.8.8 version.
Isolates were stored at the temperature of −70 °C in brain heart infusion (Brain Heart Infusion, BHI, Becton–Dickinson, Sparks, USA) with the addition of 15 % glycerol (POCH S.A., Gliwice, Poland). For the purposes of examination, strains coming from different patients and those which differ with chromosomal DNA pattern within the species were used, what was verified in the initial tests with the use of pulsed-field gel electrophoresis.
Assessment of the impact of diclofenac and ibuprofen on biofilm formation
The examination was performed with the use of qualitative method [15, 16], quantitative method [17] and with the use of scanning electron microscope (SEM) [18]. The impact of diclofenac (Diclofenac, Sigma, Steinheim, Germany) with the concentration of 1 μg ml−1 and ibuprofen (Ibuprofen, Sigma, Steinheim, Germany) with the concentration of 20 μg ml−1 on biofilm formation on the surface of monofilament polypropylene mesh (Tricomed S.A., Łódź, Poland), biomaterial applied most often in hernia surgery, was assessed [2], while deciding on the concentrations of NSAIDs, values reached by diclofenac and ibuprofen in human blood serum were taken into account [19, 20].
Bacteria suspension with the density of 0.5 per McFarland scale was prepared in 4 ml of trypticase soy broth (TSB, Becton–Dickinson, Sparks, USA) with diclofenac, ibuprofen and TSB medium without non-steroidal anti-inflammatory drug being the control regimen. In the mixtures obtained, sterile fragments of biomaterial with the dimensions of 2 × 1 cm were placed and incubated 72 h at the temperature of 37 °C in aerobic atmosphere, replacing the medium (with diclofenac, ibuprofen or without drug, respectively) with the sterile one, every 24 h.
In the examination applying qualitative method [15, 16] the change of color of the mesh surface that occurs as a result of reduction of colorless 2,3,5-triphenyl tetrazolium chloride (TTC) to red formazan carried out by metabolically active microbes was evaluated. After 72-h incubation, implant fragments were washed with 0.9 % phosphate buffered saline solution (PBS, BTL, Łódź, Poland) with pH 7.2 and was placed in 4 ml of sterile TSB medium with the addition of 20 μl of 1 % 2,3,5-triphenyltetrazolium chloride solution (TTC, POCH S.A., Gliwice, Poland). The samples were incubated for 24 h at the temperature of 37 °C in aerobic atmosphere and then biofilm formation was assessed according to assumed scale: 0—no biofilm formation (or fragment of sterile mesh), 1—poor biofilm formation (slight pink dotting of the mesh), 2—strong biofilm formation (solid pink coloring of the entire mesh), 3—very strong biofilm formation (red coloring of the mesh) (Fig. 1). The examination for each strain was carried out in threefold repetition.
Fig. 1.
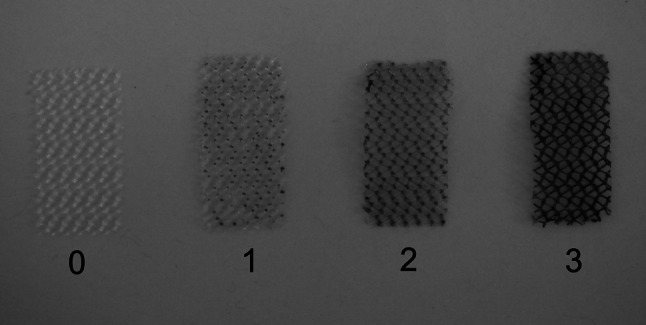
Evaluation of biofilm formation using qualitative method (explanation in text)
In the quantitative examination [17] fragments of monofilament polypropylene mesh, after 72-h incubation were washed with PBS with pH 7.2 and shaken out (1,100 rpm) in 1 ml of 0.5 % saponin solution (Fluka, Steinheim, Germany) for 2 min [21]. Serial tenfold dilutions of suspension obtained were prepared and inoculated in the amount of 100 μl in three Petri dishes with trypticase soy agar (TSA, Becton–Dickinson, Sparks, USA) for each dilution in every conditions that is with diclofenac, ibuprofen or without the drugs (control regimen). After 24-h incubation at the temperature of 37 °C in aerobic atmosphere, the result (mean from three measurements for each dilution) was presented in the form of number of colony forming units per one millilitre of the suspension (CFU ml−1).
Randomly selected fragments of biomaterials after 72-h incubation in TSB medium (with examined NSAIDs or without drugs) with the suspension of S. aureus and E. coli strains with the density of 0.5 per McFarland scale were subject to assessment with the use of SEM. In order to do it, the samples were washed with PBS with pH 7.2 and preserved in 2.5 % solution of glutaraldehyde (POCH S.A., Gliwice, Poland) in 0.1 M phosphate buffer with pH 7.4 for 48 h at the temperature of 4 °C. After preservation, biomaterials were rinsed twice for 20 min in room temperature in phosphate buffer and then dehydrated in ethanol with increasing concentration 30, 50, 70, 80, and 96 % for 10 min and twice in 99.8 % ethanol (POCH S.A., Gliwice, Poland) for 30 min. After dehydration, the samples were put in the solution of hexamethyldisilazane (HMDS, Polysciences GmbH, Baden-Wurttenberg, Germany) for 45 min and then dried in room temperature. Dried material was then placed on copper tables and sputter-coated with gold in an atmosphere of argon in an ionic coater (Fine Coater, JCF-1200, JEOL, Tokyo, Japan), which was then moved to an SEM column (JSM-5310LV, JEOL, Tokyo, Japan) and tests were carried out with the voltage of 20 kV [18]. The results were analyzed and registered with the use of NSS Version 3.0 programme (Thermo Fisher Scientific Inc, Waltham, USA).
Statistical analysis
Statistical analysis was performed using the Statistica 10.0 software package (StatSoft Poland). The interaction of variables in the qualitative assessment of the impact of NSAIDs on biofilm formation was assessed using non-parametric χ 2 test. To describe quantitative variables whose distribution was different from the standard distribution, the following were used: median (Me), first and third quartile (Q1, Q3) and, additionally, mean (M) and standard deviation (SD). The statistical analysis of variables in the quantitative study into the effects of NSAIDs was performed using the non-parametric ANOVA Friedman test for dependent samples using Dunn’s post hoc test to assess differences between individual groups, i.e., strains from the control group and strains incubated in medium containing the tested drugs. Test probability of p ≤ 0.05 was considered as statistically significant.
Results
Qualitative assessment
The results of the examination on the impact of diclofenac and ibuprofen on formation of biofilm with the use of qualitative method are presented in Table 1. All the examined strains of S. aureus and E coli formed biofilm on the surface of monofilament polypropylene mesh. After incubation of S. aureus isolates in the medium with the addition of NSAIDs, it was stated that the percentage of strains which strongly formed biofilm decreased from 22.9 % (control regimen) to 2.9 % (diclofenac and ibuprofen). However, the percentage of strains which poorly formed biofilm increased from 1.4 % (control regimen) to 10.0 % (diclofenac) and 14.2 % (ibuprofen). Differences in biofilm formation by strains of S. aureus after incubation in the mediums containing the examined NSAIDs in comparison to control regiment were statistically significant (p < 0.0001, df = 4).
Table 1.
The impact of NSAIDs on biofilm formation by S. aureus (n = 70) and E. coli (n = 70) strains on the surface of monofilament polypropylene mesh—qualitative method of assessment
| Biofilm formation | S. aureus | E. coli | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Diclofenac | Ibuprofen | Control | Diclofenac | Ibuprofen | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Poor | 1 | 1.4 | 7 | 10.0 | 10 | 14.2 | 7 | 10.0 | 22 | 31.4 | 18 | 25.7 |
| Strong | 53 | 75.7 | 61 | 87.1 | 58 | 82.9 | 51 | 72.9 | 40 | 57.2 | 46 | 65.7 |
| Very strong | 16 | 22.9 | 2 | 2.9 | 2 | 2.9 | 12 | 17.1 | 8 | 11.4 | 6 | 8.6 |
N number of strains, % percentage of strains
As a result of incubation of E. coli in the mediums containing NSAIDs decrease of percentage of strains which strongly formed biofilm was noted down from 17.1 % (control regimen) to 11.4 % (diclofenac) and 8.6 % (ibuprofen). However, the percentage of strains which poorly formed biofilm increased from 10.0 % (control regimen) to 25.7 % (ibuprofen) and 31.4 % (diclofenac). Differences in biofilm formation by strains of E. coli after incubation in the mediums with examined NSAIDs in comparison to control regimens were statistically significant (p = 0.0246, df = 4).
Quantitative assessment
The results of the examination on the impact of diclofenac and ibuprofen on formation of biofilm with the use of quantitative method are presented in Table 2. As a result of incubation of S. aureus strains in mediums containing tested NSAIDs, a decrease in the median of CFU ml−1 isolated from biofilm was noted from 42.0 × 105 (control regimen) to 10.2 × 105 (diclofenac) and 9.6 × 105 (ibuprofen). Differences in the values obtained were statistically significant (p < 0.0001, df = 2). However, as a result of incubation of E. coli bacilli in mediums containing diclofenac or ibuprofen, a decrease in the median of CFU ml−1 isolated from biofilm was noted from 21.8 × 105 (control regimen) to 7.9 × 105 (diclofenac) and 7.4 × 105 (ibuprofen). Differences in the values obtained were statistically significant (p < 0.0001, df = 2).
Table 2.
The impact of NSAIDs on biofilm formation by strains of S. aureus (n = 70) and E. coli (n = 70) on the surface of the monofilament polypropylene mesh—quantitative assessment (abbreviations in the table are explained in the text)
| ×105 CFU ml−1 | S. aureus | E. coli | ||
|---|---|---|---|---|
| Me (Q1–Q3) | M (SD) | Me (Q1–Q3) | M (SD) | |
| Control | 42.0 (19.4–69.0) | 53.3 (42.1) | 21.8 (13.4–50.0) | 37.2 (45.2) |
| Diclofenac | 10.2 (5.1–18.2) | 15.8 (17.4) | 7.9 (3.0–14.7) | 17.9 (41.1) |
| Ibuprofen | 9.6 (4.6–25.0) | 20.7 (37.8) | 7.4 (3.0–16.3) | 21.8 (51.7) |
From biofilm formed both, by strains of S. aureus as well as E. coli on the surface of monofilament polypropylene mesh after incubation in mediums with examined NSAIDs, significantly less CFU ml−1 were obtained than from biofilm from control regimen (p < 0.001) (Table 2). No statistically significant difference was stated in the number of CFU ml−1 isolated from biofilm formed after incubation of bacteria in the mediums containing diclofenac and ibuprofen.
SEM
The effect of incubation of strains of S. aureus and E coli in mediums with the addition of examined NSAIDs was the decrease of the number of bacteria adjacent to the surface of monofilament polypropylene mesh (Figs. 2, 3) in relation to control regimen (Figs. 4, 5). Single bacteria clusters were observed (Figs. 6, 7).
Fig. 2.
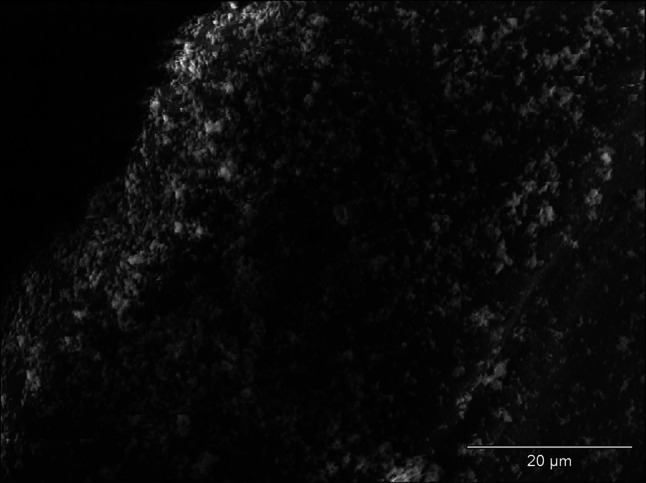
Decrease of S. aureus adhesion to the surface of monofilament polypropylene mesh after incubation in the medium with diclofenac; SEM—resolution of ×1,500
Fig. 3.
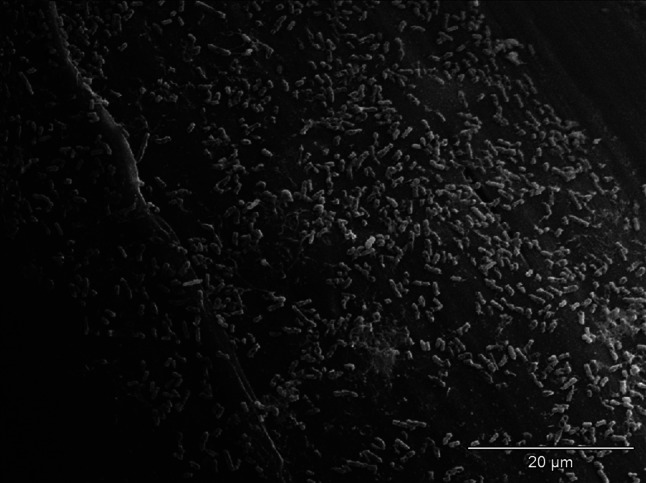
Decrease of E. coli adhesion to surface of monofilament polypropylene mesh after incubation in the medium with ibuprofen; SEM—resolution of ×1,500
Fig. 4.
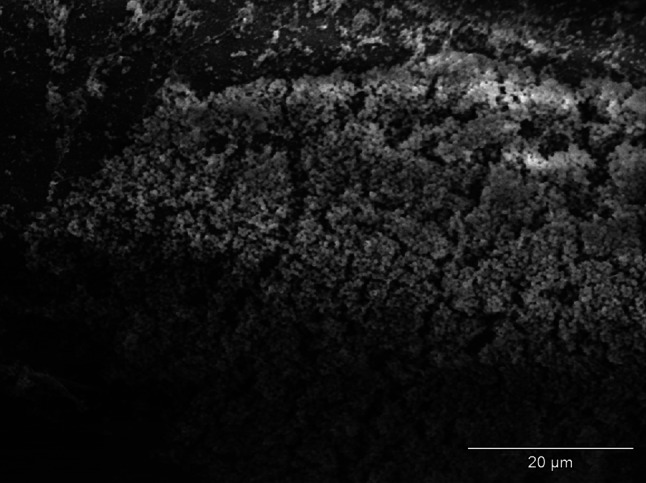
Biofilm of S. aureus on the surface of monofilament polypropylene mesh (control regimen); SEM—resolution of ×1,500
Fig. 5.
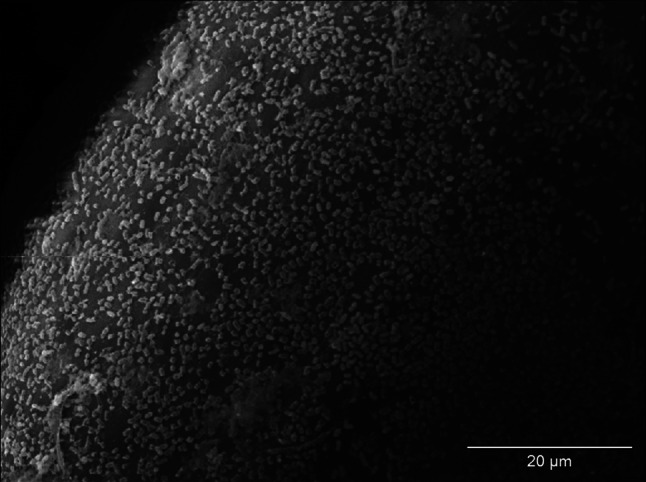
Biofilm of E. coli on the surface of monofilament polypropylene mesh (control regimen); SEM—resolution of ×1,500
Fig. 6.

Decrease of S. aureus adhesion to the surface of monofilament polypropylene mesh after incubation in the medium with ibuprofen; visible single bacteria clusters; SEM—resolution of ×1,500
Fig. 7.
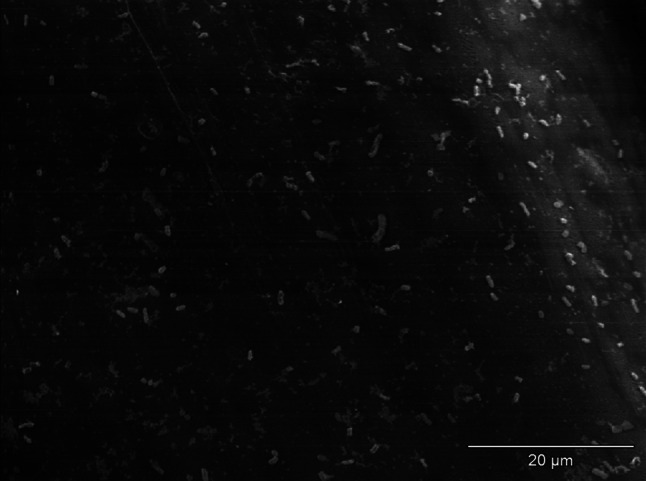
Decrease of E. coli adhesion to surface of monofilament polypropylene mesh after incubation in the medium with diclofenac; visible single bacteria clusters; SEM—resolution of ×1,500
Discussion
In the work, it was shown that diclofenac and ibuprofen in the concentration obtained by the drugs in human blood serum inhibit biofilm formation by S. aureus and E. coli on the surface of monofilament polypropylene mesh.
One of the methods of evaluation of the impact of non-steroidal anti-inflammatory drugs on biofilm formation was a qualitative assessment of colorless TTC reduction that occurs regardless of the type, shape and color of the biomaterial [16]. However, due to the subjective nature of the qualitative method resulting from the use of pre-arranged scale for TTC reduction, the results obtained by this method were compared with the results of the quantitative examination. To investigate the effect of diclofenac and ibuprofen on biofilm formation, SEM was also used as it allows, among others, to carry out assessment of the morphology of biofilm-forming bacteria and to observe biofilm structure. However, its use is limited to samples of small sizes [22]. It should also be borne in mind that biofilm never covers the entire surface of the mesh and there may be areas without biofilm [23]. The evaluation of effects of tested drugs on biofilm formation using SEM confirmed, in spite of its shortcomings, the results of studies using TTC and the quantitative method.
Own studies indicated differentiation between the impacts of NSAIDs on biofilm formation within a species. Moreover, there were differences in the ability to form biofilm among individual strains of S. aureus species and E. coli. species. Results of own studies confirm the validity of the use of two groups composed of multiple strains of the same species reflecting the presence of a given feature in the population of microorganisms.
In own examinations it was stated that diclofenac and ibuprofen limit the biofilm formation by the strains of S. aureus and E. coli on the surface of monofilament polypropylene mesh. Similar impact of ibuprofen on biofilm formation by S. pneumoniae and E. coli on the polystyrene surface was noted by other researchers [24, 25]. It was also shown that diclofenac and salicylic acid inhibit formation of biofilm by S. epidermidis, S. pneumoniae, H. influenzae and P. aeruginosa [26, 27].
The mechanism of NSAIDs impact on biofilm formation has not been fully explained. It is considered that it shall be connected with inhibition of bacterial adhesion. Diclofenac, ibuprofen and salicylic acid limit bacteria adhesion to abiotic surfaces, including polymers applied in medicine [27–29]. Decrease of adhesion and biofilm formation may be the result of the inhibiting impact of NSAIDs on production of agents which play essential roles in the processes and the modification of the surface properties of the bacterial cell. It was demonstrated that salicylic acid reduces production of extracellular polysaccharide, teichoic acids and proteins by S. epidermidis [27, 28], while incubation of E. coli strains with ibuprofen change their hydrophobicity and ibuprofen in sub-inhibitory concentration inhibit the production of fimbriae by E. coli [25].
Another possible explanation for inhibition of bacteria biofilm formation by NSAIDs is their impact on the process by quorum sensing system. In the opinion of Ulusoy and Bosgelmez-Tinaz [30] diclofenac, ibuprofen, ketoprofen, naproxen and piroxicam may limit biofilm formation by P. aeruginosa by means of decreasing the production of virulence factors dependant from quorum sensing system. While Bryers and contributors [31] indicated that salicylic acid limits biofilm formation by P. aeruginosa by means of inhibition of quorum sensing las system. Until now, it has not been demonstrated for NSAIDs to decrease biofilm formation by S. aureus and E. coli using quorum sensing systems.
The impact of NSAIDs on adhesion and bacterial biofilm formation leads to the situation in which, in the opinion of some researchers, administration of the drugs in post-surgical period may lead to decrease of the infection risk [29]. Taking into account the fact that NSAIDs act synergistically with antibiotics [32], application of the drugs in perioperative period may increase the effectiveness of antibiotics applied for prophylactic purposes.
Conclusions
Diclofenac and ibuprofen in the concentration obtained in serum limit biofilm formation by S. aureus and E. coli on the surface of monofilament polypropylene mesh.
Acknowledgments
The authors would like to thank Professor Eugenia Gospodarek, Head of the Department of Microbiology of the Ludwik Rydygier College of Medicine of the Nicolaus Copernicus University in Toruń for making it possible to carry out research. The work is co-financed from the resources of the European Social Fund and National Budget in terms of Integrated Operational Programms of Regional Development, Measure 2.6. ‘Regional Innovation Strategies and Knowledge Transfer’, by the European Union in terms of European Social Fund - ‘Nicolaus Copernicus University College of Medicine development programme’ and from the resources of Nicolaus Copernicus University (UMK) 06/2009 grant.
Conflict of interest
A. R. declares no conflict of interest. S. D. declares no conflict of interest. K. G. declares no conflict of interest.
References
- 1.Stremitzer S, Bachleitner-Hofmann T, Gradl B, Gruenbeck M, Bachleitner-Hofmann B, Mittlboeck M, Bergmann M. Mesh graft infection following abdominal hernia repair: risk factor evaluation and strategies of mesh graft preservation. A retrospective analysis of 476 operations. World J Surg. 2010;34:1702–1709. doi: 10.1007/s00268-010-0543-z. [DOI] [PubMed] [Google Scholar]
- 2.Engelsman AF, van der Mei HC, Ploeg RJ, Busscher HJ. The phenomenon of infection with abdominal wall reconstruction. Biomaterials. 2007;28:2314–2327. doi: 10.1016/j.biomaterials.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Tolino MJ, Tripoloni DE, Ratto R, Garcia MI. Infections associated with prosthetic repairs of abdominal wall hernias: pathology, management and results. Hernia. 2009;13:631–637. doi: 10.1007/s10029-009-0541-y. [DOI] [PubMed] [Google Scholar]
- 4.Jezupors A, Mihelsons M. The analysis of infection after polypropylene mesh repair of abdominal wall hernia. World J Surg. 2006;30:2270–2278. doi: 10.1007/s00268-006-0130-5. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez VM, Abi-Haidar YE, Itani KM. Mesh infection in ventral incisional hernia repair: incidence, contributing factors, and treatment. Surg Infect (Larchmt) 2011;12:205–2010. doi: 10.1089/sur.2011.033. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Wen YM. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3:66–73. doi: 10.4248/IJOS11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta NK, Mazumdar K, Baek MW, Kim DJ, Na YR, Park SH, Lee HK, Lee BH, Park JH. In vitro efficacy of diclofenac against Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 2008;27:315–319. doi: 10.1007/s10096-007-0439-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang WH, Wong WM, Dailidiene D, Berg DE, Gu Q, Lai KC, Lam SK, Wong BC. Aspirin inhibits the growth of Helicobacter pylori and enhances its susceptibility to antimicrobial agents. Gut. 2003;52:490–495. doi: 10.1136/gut.52.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Bakri AG, Othman G, Bustanji Y. The assessment of the antibacterial and antifungal activities of aspirin, EDTA and aspirin-EDTA combination and their effectiveness as antibiofilm agents. J Appl Microbiol. 2009;107:280–286. doi: 10.1111/j.1365-2672.2009.04205.x. [DOI] [PubMed] [Google Scholar]
- 10.Perilli R, Marziano ML, Formisano G, Caiazza S, Scorcia G, Baldassarri L. Alteration of organized structure of biofilm formed by Staphylococcus epidermidis on soft contact lenses. J Biomed Mater Res. 2000;49:53–57. doi: 10.1002/(SICI)1097-4636(200001)49:1<53::AID-JBM7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Hasani A, Maloku H, Sallahu F, Gashi V, Ozgen SU. Preemptive analgesia with midazolam and diclofenac for hernia repair pain. Hernia. 2011;15:267–272. doi: 10.1007/s10029-010-0772-y. [DOI] [PubMed] [Google Scholar]
- 12.Mixter CG, Meeker LD, Gavin TJ. Preemptive pain control in patients having laparoscopic hernia repair: a comparison of ketorolac and ibuprofen. Arch Surg. 1998;133:432–437. doi: 10.1001/archsurg.133.4.432. [DOI] [PubMed] [Google Scholar]
- 13.Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Syst Rev. 2009;2:CD004768. doi: 10.1002/14651858.CD004768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derry CJ, Derry S, Moore RA, McQuay HJ. Single dose oral ibuprofen for acute postoperative pain in adults. Cochrane Database of Syst Rev. 2009;3:CD001548. doi: 10.1002/14651858.CD001548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallimore B, Gagnon RF, Subang R, Richards GK. Natural history of chronic Staphylococcus epidermidis foreign body infection in a mouse model. J Infect Dis. 1991;164:1220–1223. doi: 10.1093/infdis/164.6.1220. [DOI] [PubMed] [Google Scholar]
- 16.Różalska B, Sadowska B, Więckowska M, Rudnicka W. Detection of bacterial biofilm on medical biomaterials. Med Dosw Mikrobiol. 1998;50:115–122. [PubMed] [Google Scholar]
- 17.Saygun O, Agalar C, Aydinuraz K, Agalar F, Daphan C, Saygun M, Ceken S, Akkus A, Denkbas EB. Gold and gold-palladium coated polypropylene grafts in a S. epidermidis wound infection model. J Surg Res. 2006;131:73–79. doi: 10.1016/j.jss.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Araujo JC, Téran FC, Oliveira RA, Nour EA, Montenegro MA, Campos JR, Vazoller RF. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc (Tokyo) 2003;52:429–433. doi: 10.1093/jmicro/52.4.429. [DOI] [PubMed] [Google Scholar]
- 19.Lissy M, Stiff DD, Kowalski MM, Moore KA. Single-dose pharmacokinetic study of rapidly dispersing diclofenac potassium formulations in healthy volunteers. Curr Med Res Opin. 2009;25:2423–2428. doi: 10.1185/03007990903158513. [DOI] [PubMed] [Google Scholar]
- 20.Lötsch J, Muth-Selbach U, Tegeder I, Brune K, Geisslinger G. Simultaneous fitting of R- and S-ibuprofen plasma concentrations after oral administration of the racemate. Br J Clin Pharmacol. 2001;52:387–398. doi: 10.1046/j.1365-2125.2001.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiecińska-Piróg J, Bogiel T, Gospodarek E. Evaluation of biofilm formation by Proteus mirabilis strains on the surface of different biomaterials by two methods. Med Dosw Mikrobiol. 2011;63:131–138. [PubMed] [Google Scholar]
- 22.Grin I, Schwarz H, Linke D. Electron microscopy techniques to study bacterial adhesion. Adv Exp Med Biol. 2011;715:257–269. doi: 10.1007/978-94-007-0940-9_16. [DOI] [PubMed] [Google Scholar]
- 23.Engelsman AF, van der Mei HC, Busscher HJ, Ploeg RJ. Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br J Surg. 2008;95:1051–1059. doi: 10.1002/bjs.6154. [DOI] [PubMed] [Google Scholar]
- 24.del Prado G, Ruiz V, Naves P, Rodríguez-Cerrato V, Soriano F, del Carmen Ponte M. Biofilm formation by Streptococcus pneumoniae strains and effects of human serum albumin, ibuprofen, N-acetyl-l-cysteine, amoxicillin, erythromycin, and levofloxacin. Diagn Microbiol Infect Dis. 2010;67:311–318. doi: 10.1016/j.diagmicrobio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Naves P, del Prado G, Huelves L, Rodríguez-Cerrato V, Ruiz V, Ponte MC, Soriano F. Effects of human serum albumin, ibuprofen and N-acetyl-l-cysteine against biofilm formation by pathogenic Escherichia coli strains. J Hosp Infect. 2010;76:165–170. doi: 10.1016/j.jhin.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Bandara BM, Sankaridurg PR, Willcox MD. Non-steroidal anti inflammatory agents decrease bacterial colonisation of contact lenses and prevent adhesion to human corneal epithelial cells. Curr Eye Res. 2004;29:245–251. doi: 10.1080/02713680490516729. [DOI] [PubMed] [Google Scholar]
- 27.Muller E, Al-Attar J, Wolff AG, Farber BF. Mechanism of salicylate-mediated inhibition of biofilm in Staphylococcus epidermidis. J Infect Dis. 1998;177:501–503. doi: 10.1086/517386. [DOI] [PubMed] [Google Scholar]
- 28.Farber BF, Wolff AG. The use of nonsteroidal antiinflammatory drugs to prevent adherence of Staphylococcus epidermidis to medical polymers. J Infect Dis. 1992;166:861–865. doi: 10.1093/infdis/166.4.861. [DOI] [PubMed] [Google Scholar]
- 29.Demirag MK, Esen S, Zivalioglu M, Leblebicioglu H, Keceligil HT. The effect of aspirin on adherence of slime-producing, coagulase-negative staphylococci to vascular grafts. Ann Vasc Surg. 2007;21:464–467. doi: 10.1016/j.avsg.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Ulusoy S, Bosgelmez-Tinaz G. Nonsterodial anti-inflammatory drugs reduce the production of quorum sensing regulated virulence factors and swarming motility in human pathogen Pseudomonas aeruginosa. Drug Res (Stuttg) 2013;63:409–413. doi: 10.1055/s-0033-1343430. [DOI] [PubMed] [Google Scholar]
- 31.Bryers JD, Jarvis RA, Lebo J, Prudencio A, Kyriakides TR, Uhrich K. Biodegradation of poly (anhydride-esters) into non-steroidal anti-inflammatory drugs and their effect on Pseudomonas aeruginosa biofilms in vitro and on the foreign-body response in vivo. Biomaterials. 2006;27:5039–5048. doi: 10.1016/j.biomaterials.2006.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutta NK, Mazumdar K, Park JH. In vitro synergistic effect of gentamicin with the anti-inflammatory agent diclofenac against Listeria monocytogenes. Lett Appl Microbiol. 2009;48:783–785. doi: 10.1111/j.1472-765X.2009.02588.x. [DOI] [PubMed] [Google Scholar]


