Abstract
Background
Hyperprolactinemia (HPL) is a common side effect of antipsychotic medications. Recent reports suggest that aripiprazole can ameliorate antipsychotic-induced HPL, but results are inconsistent and the single available systematic review only considered five studies.
Aim
Conduct an updated meta-analysis of all randomized controlled trials (RCTs) about the efficacy and safety of aripiprazole as an adjunctive treatment for antipsychotic-induced hyperprolactinemia.
Methods
English and Chinese databases were searched for RCTs about the use of aripiprazole in treating antipsychotic-induced HPL published by January 20, 2015. Studies were selected using pre-defined inclusion and exclusion criteria. The Cochrane Risk of Bias tool was used to evaluate risk of biases, the Cochrane GRADE measure was used to assess the quality of evidence, and Review Manager 5.3 software was used for data analysis.
Results
A total of 21 studies, 19 of which were conducted in mainland China, were included in the analysis. Meta-analysis of data from 8 of the studies with a pooled sample of 604 individuals found that compared to the control condition adjunctive aripiprazole significantly increased the proportion of participants who experienced HPL recovery (risk ratio [RR]=19.2, 95%CI=11.0-33.5). The proportion who experienced any adverse effect during follow-up did not differ between the two groups, but the aripiprazole group was more likely to report somnolence (RR=2.76, 95%CI=1.34-5.69) and headaches (RR=2.31, 95%CI=1.08-4.92). High-dose aripiprazole (>5mg/day) was more effective than low-dose (<5mg/day) aripiprazole (RR=30.0, 95%CI=10.2-120.7 v. RR=15.1, 95%CI=8.1-28.1), but this difference was not statistically significant. The risk of bias in the studies was rated as ‘high’ in 6 of the studies and ‘unclear’ in 15 studies, and the quality of evidence was rated as ‘high’ for only 7 of the 57 outcome measures assessed.
Conclusions
This study systematically reviewed and evaluated all relevant RCTs and found that adjunctive aripiprazole is effective and safe to use in the treatment of antipsychotic-induced HPL. However, the low quality of some of the studies, the incomplete methodological information provided for most of the studies, and the relatively short follow-up time of the studies raises question about the validity of the results. Further work that resolves these methodological and reporting issues is needed.
Keywords: aripiprazole, hyperprolactinemia, randomized controlled trial, meta-analysis
Abstract
背景
高泌乳素血症是抗精神病药物治疗中一种常见的不良反应。近年来有报道提示阿立哌唑能减轻其他抗精神病药所致的高泌乳素血症,但不同研究的结果不尽一致。虽然已有一篇相关系统综述,但是只纳入了5项研究。
目标
对有关阿立哌唑辅助治疗其他抗精神病药物所致高泌乳素血症的有效性和安全性的所有随机对照研究进行meta 分析。
方法
检索国内外常用数据库中2015年1月20日前发表的所有关于阿立哌唑治疗其他抗精神病药物所致高泌乳血症的随机对照研究。按照预先规定的纳入标准及排除标准筛选相关研究。根据Cochrane偏移风险评估工具对纳入研究偏移风险进行评价,采用Cochrane GRADE评估证据质量,使用Review Manager 5.3和R3.1.1软件进行数据分析。
结果
共纳入21 项随机对照研究,其中在中国大陆开展的研究有19 项。对21项研究中的8项研究共604例样本进行meta 分析,发现与对照组相比,阿立哌唑辅助治疗后,泌乳素水平恢复的患者比例显著增加(RR=19.2,95%CI =11.0-33.5)。两组患者在随访中出现不良反应的总的比例没有差异,但阿立哌唑组报告嗜睡(RR=2.76,95%CI=1.34-5.69)和 头痛(RR=2.31,95%CI=1.08-4.92)的比例相对高。高剂量阿立哌唑(>5mg /d)比低剂量(<5mg /d)更有效(RR=30.0,95%CI=10.2-120.7 比RR=15.1,95%CI=8.1-28.1),但这种差异无统计学意义。这些研究中有6 项被评为“ 高” 偏移风险,而其他15项的偏移风险“ 不清楚”。对57 个研究结果的证据水平评估显示只有7个是“ 高”质量的。
结论
本研究系统地回顾和评估了所有相关的随机对照研究,发现阿立哌唑辅助治疗其他抗精神病药物所致的高泌乳素血症是安全有效的。然而,一些研究的质量较低,大多数研究的方法学信息不完善,研究中随访时间相对较短,这些都会影响研究结果的有效性。需要进一步工作以解决上述方法学和研究报告方面的问题。
中文全文
本文全文中文版从2015年4月8日起在http://dx.doi.org/10.11919/j.issn.1002-0829.215014可供免费阅览下载
1. Background
Hyperprolactinemia (HPL) is a common and severe side effect of using antipsychotic medications.[1] Both typical antipsychotic medications (e.g., chlorpromazine, perphenazine, sulpiride, and haloperidol) and atypical antipsychotic medications (e.g., risperidone, paliperidone, and amisulpride) can increase prolactin levels, especially among females. The reported occurrence of HPL among individuals taking antipsychotic medication ranges from 42 to 89%.[2,3,4,5] HPL is associated with both short-term and long-term physical and psychological problems, including sexual dysfunction, amenorrhea, osteoporosis, the metabolic syndrome, depression, and anxiety.[2,6,7] There have also been reports that HPL can increase the risk of breast cancer and prostate cancer,[8,9] and exacerbate auto-immune diseases.[10] Problems related to HPL can decrease patient adherence to treatment with antipsychotic medications and, thus, lead to fluctuations of psychotic symptoms.[11]
Aripiprazole is a partial dopamine D2 receptor agonist which has been reported to improve antipsychoticsinduced HPL.[12, 13,14,15, 16,17] Several clinical trials specifically focused on assessing the efficacy and safety of aripiprazole in treating antipsychotic-induced HPL[18,19,20,21, 22,23] have had inconsistent findings. Some found that adjunctive treatment with aripiprazole was well tolerated and effective in reducing prolactin levels,[20,24] while others reported increased insomnia, headaches, and sedation after the use of adjunctive aripiprazole.[18] Two studies[25,26] reported that aripiprazole was effective at low doses, but another study did not support this finding.[27] The single available meta-analysis on this topic[28] reported the aripiprazole is effective and safe, but these results were based on pooling results from only five studies. This review aims to identify and pool results of all previous randomized controlled trials to summarize the current state of knowledge about the efficacy and safety of aripiprazole in the treatment of antipsychotic-induced HPL.
2. Methods
2.1. Search strategy
We searched the following databases for studies published by January 20, 2015: Pubmed, EMBASE, The Cochrane Library, EBSCO, Chinese National Knowledge Infrastructure (CNKI), Chongqing VIP database for Chinese Technical Periodicals, WANFANG DATA, Chinese Biological Medical Literature Database, Taiwan Electronic Periodical Services, and ClinicalTrials.gov using keywords ‘aripiprazole’, ‘hyperprolactinemia’,‘prolactin abnormal’, ‘randomized controlled trial’, ‘controlled clinical trial’, ‘randomized,placebo’, ‘drug therapy’, and ‘randomly, trial’. Proprietary names for aripiprazole in Chinese were also included as the Chinese search terms. Various Boolean combinations of these keywords were used to search for articles; reference lists of included articles were hand-checked for further relevant studies; and experts in the field were asked about ongoing studies.
2.2. Inclusion and exclusion criteria
All reports of randomized controlled trails (RCTs) about treating antipsychotic-induced HPL among individuals with schizophrenia with aripiprazole were screened using the following inclusion criteria: (a) diagnosis of schizophrenia was based on criteria specified by the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders,[29] the World Health Organization’s International Classification of Diseases,[30] or the Chinese Society of Psychiatry’s Chinese Classification of Mental Disorders;[31] (b) HPL confirmed using blood tests; (c) comparison of aripiprazole to placebo or to no treatment; (d) reported data on sample size, number of HPL cases,and on serum prolactin levels before and after treatment. Studies published in either English or Chinese were considered. Observational studies, anthropologic studies, review articles, research protocols, case reports, and duplicated reports were excluded.
2.3. Screening of articles
All search results were imported into Endnote X5 software. Two authors (MM and LW) independently screened titles and abstracts after eliminating duplicates. The full text of the remaining articles were screened according to the above inclusion and exclusion criteria. When the two authors disagreed about the inclusion of an article and were unable to agree after discussing the article, a third author (LC) made the final determination.
2.4. Evaluation of risk of bias
Two authors (LW and MM) assessed the risk of bias for all included articles using the Cochrane Risk of Bias tool (ROB) [32] which considers seven specific items: sequence generation (randomization); allocation concealment; blinding of participants and treating clinicians about group assignment; blinding of evaluators of outcomes about group assignment; incomplete data (attrition and exclusions); selective outcome reporting; and other biases (including study-specific biases or concerns about fraudulent results). Each aspect was rated as ‘low risk of bias’, ‘high risk of bias’, or ‘unclear’ if insufficient information was provided in the article to make a determination. A third author’s (LC) opinion was sought when the two raters disagreed. We also evaluated the quality and level of evidence of each of the 21 included studies using the Cochrane collaboration’s GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) software which assesses the limitations of the design,consistency of results, indirect evidence,precision of results, publication bias, and effect size for each outcome.[32, 33] The level of evidence was rated as high, medium, low, or very low.
2.5. Outcome measures
The primary outcome is the proportion of individuals whose prolactin levels returned to the normal range after adjunctive treatment with aripiprazole, that is, HPL recovery. Secondary outcomes are prolactin levels after the aripiprazole treatment, occurrence of adverse events based on use of the Treatment Emergent Symptom Scale (TESS),[34] and improvement of psychotic symptoms.
2.6. Data extraction
For each included study, two authors (LW and MM) independently extracted data using a pre-designed data extraction form including names of authors, publication year, sample size, number of outcome events,age of participants, and antipsychotics used. Discrepancies were checked by a third author (ZS).
2.7. Analysis
Based on the results of a previous study about risk of bias,[35] the overall risk of bias for each of the 21 studies was classified as ‘low’ if the ratings were ‘low’ for all seven items on the ROB tool,‘unclear’ if any item is rated as ‘unclear’ and all other items are rated as ‘low’,and ‘high’ if any of the items are rated as ‘high’. The kappa statistic was used to measure the interrater agreement between the two independent raters for the ratings of each item and for the overall rating.[36] Review Manager (RevMan 5.3) and R 3.1.1 were used to estimate pooled standard mean difference (SMD) for continuous measures and risk ratios (RR) for categorical measures. Heterogeneity was measured using I2.[37] When I2 is less than 50% and p>0.10,the results were considered homogeneous and the fixed-effect model was used; when I2 is greater than 50% and less than 75%,results were considered heterogeneous and the random-effect model was used. If I2 is 75% or greater, we conducted sensitivity analysis to identify potential contributors to heterogeneity; if I2 remained 75% or greater after removing outliers we only provided descriptive results without pooling estimates. Subgroup analysis was conducted to explore the effect of the type of antipsychotic used and of the dosage of adjunctive aripiprazole on the outcome. A funnel plot was used to evaluate publication bias.[32]
3. Results
3.1. Characteristics of included studies
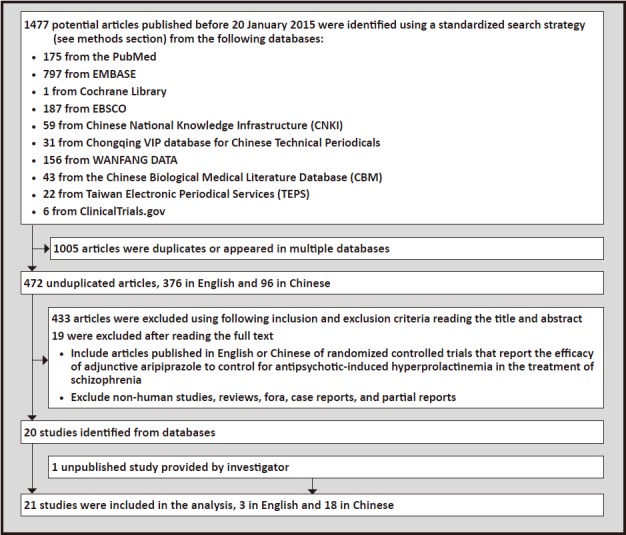
The identification of articles included in the analysis is shown in Figure 1. Using the search strategy,we found a total of 1477 references to articles in the 10 databases. Many of the references appeared in multiple databases; after removing these duplicates and studies that were reported in more than one article 472 unduplicated articles remained. Reading the title and abstract of these unduplicated articles identified 433 that did not meet our inclusion and exclusion criteria and reading the full text identified an additional 19 that did not meet our criteria. This left 20 articles; [18,19,20,21,24,27,38,39,40,41,42,43,44,45,46,47,48,49,50,51] data from one additional unpublished study[52] was provided by the investigator (available on request). These 21 articles, 3 in English and 18 in Chinese,were included in the subsequent analyses.
Figure 1. Identification of included studies.
The characteristics of these 21 studies are shown in Table 1.
Table 1.
Characteristics of the included randomized controlled trials
| study ID | blinding, type of control |
gender of participants |
age range |
N | duration of trial (weeks) |
primary antipsychotic medication(s) used |
daily dosage of aripiprazole |
|---|---|---|---|---|---|---|---|
| Xu 2006[24] | single-blind, placebo-control |
female | 18-35 | 60 | 6 | risperidone, sulpiride |
5mg |
| Shim 2007[18] | double-blind, placebo-control |
both genders | 18-45 | 54 | 8 | haloperidol | 15mg-30mg |
| Zhang 2008[38] | single-blind, placebo-control |
both genders | 25-52 | 60 | 6 | perphenazine | 5mg |
| Ji 2008[39] | single-blind, placebo-control |
female | 18-35 | 117 | 6 | risperidone | 5mg |
| Jin 2008[40] | blinding not specified, placebo-control |
both genders | 18-52 | 80 | 6 | chlorpromazine | 5mg |
| Chen 2009[20] | double-blind, placebo-control |
male | 18-50 | 72 | 8 | risperidone | 5mg |
| Kane 2009[19] | double-blind, placebo-control |
both genders | >18 | 252 | 16 | risperidone, quetiapine |
10mg |
| Wang 2009[41] | single-blind, placebo-control |
female | not specified |
60 | 6 | haloperidol | 5mg |
| Song 2009[42] | single-blind, placebo-control |
both genders | 18-35 | 140 | 6 | sulpiride | 5mg |
| Chen 2010[21] | single-blind, placebo-control |
male | not specified |
60 | 8 | sulpiride | 5mg |
| Liu (L) 2011[43] | blinding not specified, placebo-control |
both genders | 18-45 | 86 | 4 | risperidone | 5mg-10mg |
| Liu (Z) 2011[44] | blinding not specified, no-treatment control |
both genders | 18-70 | 180 | 26 | risperidone, clozapine, perphenazine, chlorpromazine |
5mg |
| Sun 2011[45] | blinding not specified, no-treatment control |
female | 18-45 | 56 | 12 | olanzapine | 10mg |
| Xue 2012[46] | single-blind, placebo-control |
male | not specified |
68 | 6 | risperidone | 5mg |
| Zhou 2012[47] | single-blind, placebo-control |
female | 18-45 | 60 | 12 | risperidone | 10mg |
| Zhu 2012[48] | single-blind, placebo-control |
female | 18-60 | 65 | 8 | risperidone | 5mg |
| Wu 2013[49] | blinding not specified, no-treatment control |
both genders | >60 | 63 | 12 | risperidone, sulpiride, perphenazine, chlorpromazine |
5mg |
| Guo 2013[50] | blinding not specified, no-treatment control |
female | 18-45 | 86 | 12 | risperidone, sulpiride, perphenazine, chlorpromazine |
5mg |
| Chen 2014[27] | double-blind, placebo-control |
both genders | 18-45 | 116 | 8 | risperidone | 20mg |
| Pan 2014[51] | blinding not specified, placebo-control |
female | 18-52 | 58 | 6 | risperidone, sulpiride |
10mg |
| Qiao 2015[52] | blinding not specified, no-treatment control |
female | 18-45 | 60 | 8 | risperidone, paliperidone |
5mg |
(a) Time of publication: all the articles were published between 2006 and 2015.
(b) Location of study: nineteen studies[20, 39, 41, 45, 47, 21, 24, 27, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52] are from mainland China,one[18] from the Republic of Korea,and one[19] from the United States.
(c) Gender of participants: nine studies[24, 39, 41, 45, 47, 48, 50, 51, 52] only included females,three studies[20, 21, 46] only included males,and the remaining nine studies[18, 19, 27, 28, 40, 42, 43, 44, 49] included both men and women.
(d) Age of participants: three studies[21, 41, 46] did not specify the age of participants; the remaining eightteen studies[18, 19, 20, 24, 27, 38, 39, 40, 42, 43, 44, 45, 47, 48, 49, 50, 51, 52] were conducted in adults 18 years of age or older - one of these studies[49] was limited to elderly individuals.
(e) Type of control group: five studies[44, 45, 49, 50, 52] provided no treatment to individuals in the control group and sixteen studies[18, 19, 20, 21, 24, 27, 38, 39, 40, 41, 42, 43, 48, 51] used placebo controls.
(f) Duration of follow-up: one study[43] followed patients for 4 weeks,eight studies[24, 38, 39, 40, 41, 42, 46, 51] for 6 weeks,six studies[18, 20, 21, 27, 48, 52] for 8 weeks, four studies[45, 47, 49, 50] for 12 weeks,one study[19] for 16 weeks,and one study[44] for 26 weeks.
(g) Type of blinding: four studies[18, 19, 20, 27] were double-blind,nine studies[21, 24, 38, 39, 41, 42, 46, 47, 48] were single-blind,and the reports for eight studies[40, 43, 44, 45, 49, 50, 51, 52] did not provide information about blinding.
(h) Type of antipsychotic medication: seven studies[19, 24, 44, 49, 50, 51, 52] included patients with HPL induced by different types of antipsychotic medication; the remaining fourteen studies only included patients with HPL induced by a single type of antipsychotic medication (seven[20, 27, 39, 43, 46, 47, 48] only considered risperidoneinduced HPL,two[18, 41] haloperidol-induced HPL, two[21, 42] sulpiride-induced HPL, one[40] chlorpromazine-induced HPL, one[38] perphenazine-induced HPL, and one[45] olanzapine-induced HPL). All participants in the studies were using a single antipsychotic medication prior to the study.
(i) Dosage of aripiprazole: fourteen studies[20, 21, 24, 38, 39, 40, 41, 42, 44, 46, 48, 49, 50, 51, 52] used 5mg/day and seven studies[18, 19, 27, 43, 45, 47, 51] used 10mg/day or higher.
3.2. Risk of bias
The results of the assessment of risk of bias in the 21 studies is shown in Table 2. Only five studies[24, 27, 39, 42, 46] explicitly stated that the evaluators were blinded and only seven studies[20, 24, 38, 39, 40, 42, 51] described the method of randomization. The overall assessment of risk of bias in the 21 studies was based on the results of the 7 items in the ROB tool. Six studies[43, 44, 45, 49, 50, 52] were classified as being at high-risk for bias; five[44, 45, 49, 50, 52] because of failure to blind participants or personnel (studies using no-treatment controls) and two[43, 45] because of selective reporting. The risk of bias in the remaining 15 studies[18, 19, 20, 21, 24, 27, 38, 39, 40, 41, 42, 46, 47, 48, 51] was classified as ‘unclear’,primarily because none of the reports of the studies provided sufficient information to code the ROB ‘concealment of allocation item’ and several reports did not provide information about other ROB items. None of the studies was classified as being at low-risk of bias. The inter-rater reliability of the two independent coders’ assessment of overall risk of bias in the studies was acceptable (kappa=0.62),but one of the seven items of the ROB tool,the item about blinding of participants and providers,had poor inter-rater reliability (kappa=0.30), suggesting that the included papers provided conflicting or confusing information about the blinding of treating clinicians.
Table 2.
Evaluation of risk of bias in the included studies based on the seven items in the Cochrane Risk of Bias (ROB) tool[29]
| Study ID | random sequence generation |
allocation concealment |
blinding of participants and providers |
blinding of outcome assessment |
incomplete outcome data |
selective reporting |
other biasesa |
OVERALL RISK OF BIASb |
|---|---|---|---|---|---|---|---|---|
| Xu 2006[24] | low | unclear | low | low | low | low | low | Unclear |
| Shim 2007[18] | unclear | unclear | unclear | unclear | low | low | low | Unclear |
| Zhang 2008[38] | low | unclear | low | low | low | low | low | Unclear |
| Ji 2008[39] | low | unclear | low | low | low | low | low | Unclear |
| Jin 2008[40] | low | unclear | unclear | unclear | low | low | low | Unclear |
| Chen 2009[20] | low | unclear | low | unclear | low | low | low | Unclear |
| Kane 2009[19] | unclear | unclear | unclear | unclear | low | low | low | Unclear |
| Wang 2009[41] | unclear | unclear | low | unclear | low | low | low | Unclear |
| Song 2009[42] | low | unclear | low | low | low | low | low | Unclear |
| Chen 2010[21] | unclear | unclear | low | unclear | low | low | low | Unclear |
| Liu (L) 2011[43] | unclear | unclear | unclear | unclear | unclear | high | low | High |
| Liu (Z) 2011[44] | unclear | unclear | high | unclear | low | low | low | High |
| Sun 2011[45] | unclear | unclear | high | unclear | low | high | low | High |
| Xue 2012[46] | unclear | unclear | low | low | low | low | low | Unclear |
| Zhou 2012[47] | unclear | unclear | low | unclear | low | low | low | Unclear |
| Zhu 2012[48] | unclear | unclear | low | unclear | low | low | low | Unclear |
| Wu 2013[49] | unclear | unclear | high | unclear | unclear | low | low | High |
| Guo 2013[50] | unclear | unclear | high | unclear | low | low | low | High |
| Chen 2014[27] | unclear | unclear | low | low | low | low | low | Unclear |
| Pan 2014[51] | low | unclear | unclear | unclear | unclear | low | low | Unclear |
| Qiao 2015[52] | unclear | unclear | high | unclear | low | low | low | High |
| kappac | 1.00 | 0.62 | 0.30 | 0.79 | 1.00 | 1.00 | 1.00 | 0.62 |
a Other biases considered include study-specific biases or concerns about fraudulent results
b If any of seven items are coded high-risk of bias the overall study is classified as high-risk, if all seven items are coded as low-risk the overall study is classified as low-risk; all other studies (i.e., those with some items coded a ‘unclear’ and no items coded as high-risk) are classified as ‘unclear’
c Kappa values for inter-rater reliability of the two independent coders who assessed for each item for the 21 studies
3.3. Findings from meta-analyses
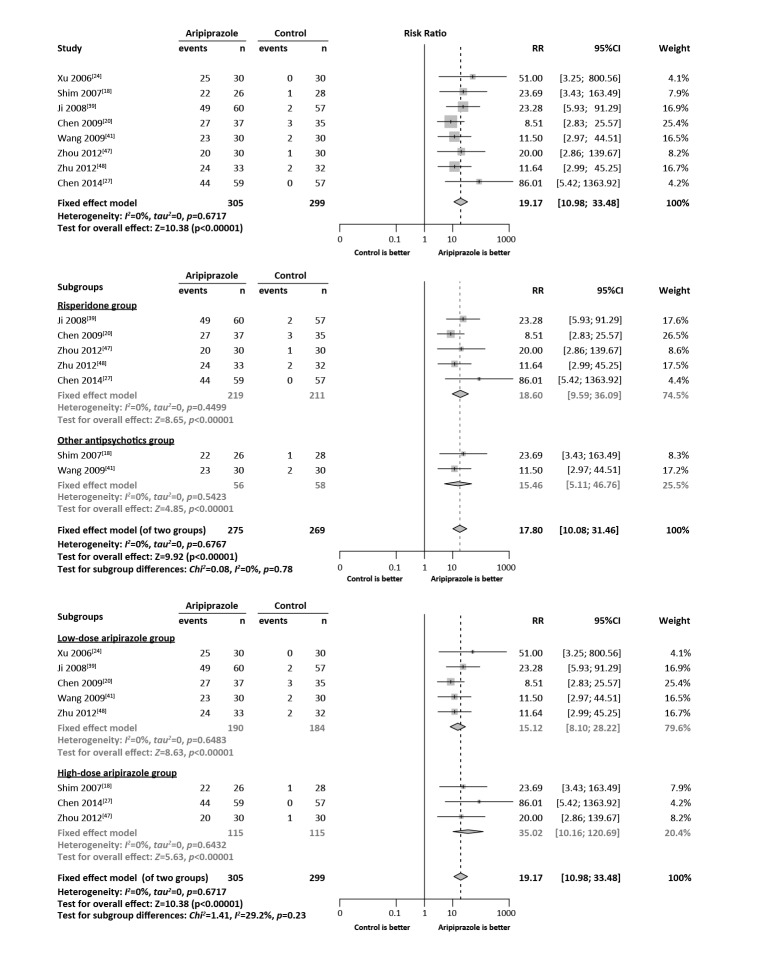
The results of the meta-analysis for the primary outcome are shown in the Forest plot in Figure 2 and those for the various secondary outcomes are shown in Table 3.
Figure 2. Forest plots comparing the proportion of subjects who recovered from hyperprolactinemia (HPL) at the end of the trial between the aripiprazole group and the control group.
Table 3.
Summary of subgroup meta-analysis and GRADEa assessments of quality of data about different outcome measures comparing adjunctive treatment with aripiprazole with placebo (or blank control) in patients with schizophrenia treated with other antipsychotic medications
| Outcomes | number of studies (pooled sample) |
test for heterogeneity |
analytic model |
test for overall effect |
RR /SMDb |
95%CI of RR/SMDb |
GRADEa | ||
|---|---|---|---|---|---|---|---|---|---|
| I2 | P | I2 | P | ||||||
| Prolactin level normalization | 8 (604) | 0% | 0.67 | fixed | 10.38 | <0.001 | 19.17 | 10.98-33.48 | moderate |
| Risperidone group | 5 (430) | 0% | 0.45 | fixed | 8.65 | <0.001 | 18.60 | 9.59-36.09 | high |
| Other antipsychotic group | 2 (114) | 0% | 0.54 | fixed | 4.85 | <0.001 | 15.46 | 5.11-46.76 | low |
| Low-dose aripiprazole group | 5 (374) | 0% | 0.65 | fixed | 8.53 | <0.001 | 15.12 | 8.10-28.22 | moderate |
| High-dose aripiprazole group | 3 (230) | 0% | 0.64 | fixed | 5.63 | <0.001 | 35.02 | 10.16-120.69 | moderate |
| Any adverse events | 12 (963) | 0% | 1.00 | fixed | 0.84 | 0.40 | 1.16 | 0.82-1.64 | high |
| Risperidone group | 5 (444) | 0% | 1.00 | fixed | 0.78 | 0.44 | 1.21 | 0.75-1.98 | high |
| Other antipsychotic group | 5 (400) | 0% | 1.00 | fixed | 0.33 | 0.74 | 1.10 | 0.62-1.95 | moderate |
| Low-dose aripiprazole group | 9 (698) | 0% | 1.00 | fixed | 0.50 | 0.62 | 1.11 | 0.74-1.67 | moderate |
| High-dose aripiprazole group | 4 (321) | 0% | 1.00 | fixed | 0.68 | 0.50 | 1.23 | 0.68-2.23 | high |
| Insomnia | 14 (948) | 5% | 0.39 | fixed | 1.32 | 0.19 | 0.77 | 0.52-1.14 | low |
| Risperidone group | 4 (336) | 0% | 0.91 | fixed | 1.44 | 0.15 | 0.51 | 0.20-1.28 | moderate |
| Other antipsychotic group | 5 (314) | 51% | 0.24 | random | 0.46 | 0.64 | 0.80 | 0.31-2.05 | very low |
| Low-dose aripiprazole group | 12 (835) | 0% | 0.74 | fixed | 2.19 | 0.03 | 0.60 | 0.39-0.95 | very low |
| High-dose aripiprazole group | 3 (169) | 0% | 0.39 | fixed | 1.32 | 0.19 | 1.69 | 0.77-3.67 | moderate |
| Headache | 9 (611) | 0% | 0.71 | fixed | 2.16 | 0.03 | 2.31 | 1.08-4.92 | low |
| Risperidone group | 3 (268) | 0% | 0.66 | fixed | 0.30 | 0.77 | 0.84 | 0.27-2.63 | moderate |
| Other antipsychotic group | 4 (254) | 0% | 0.90 | fixed | 2.55 | 0.01 | 6.68 | 1.55-28.77 | low |
| Low-dose aripiprazole group | 7 (498) | 0% | 0.71 | fixed | 1.04 | 0.30 | 1.58 | 0.67-3.73 | low |
| High-dose aripiprazole group | 3 (169) | 25% | 0.26 | fixed | 1.77 | 0.08 | 3.42 | 0.88-13.36 | moderate |
| Sedation | 2 (113) | 0% | 0.39 | fixed | 0.25 | 0.80 | 0.86 | 0.27-2.80 | moderate |
| Risperidone group | 1(59) | --- | --- | fixed | 0.66 | 0.51 | 2.90 | 0.12-68.50 | low |
| Other antipsychotic group | 1(54) | --- | --- | fixed | 0.64 | 0.52 | 0.65 | 0.17-2.44 | low |
| High-dose aripiprazole group | 2(113) | 0% | 0.39 | fixed | 0.25 | 0.80 | 0.86 | 0.27-2.80 | low |
| Dry mouth | 3 (185) | 0% | 0.80 | fixed | 0.78 | 0.43 | 1.35 | 0.63-2.90 | high |
| Risperidone group | 2(128) | 0% | 0.54 | fixed | 0.28 | 0.78 | 1.21 | 0.31-4.67 | low |
| Other antipsychotic group | 1 (44) | --- | --- | fixed | 0.78 | 0.44 | 1.44 | 0.58-3.58 | low |
| Low-dose aripiprazole group | 2 (128) | 0% | 0.54 | fixed | 0.28 | 0.78 | 1.21 | 0.31-4.67 | low |
| High-dose aripiprazole group | 1 (44) | --- | --- | fixed | 0.78 | 0.44 | 1.44 | 0.58-3.58 | low |
| Fatigue | 2 (140) | 0% | 0.32 | fixed | 0.72 | 0.47 | 1.61 | 0.44-5.86 | moderate |
| Other antipsychotic group | 2 (140) | 0% | 0.32 | fixed | 0.72 | 0.47 | 1.61 | 0.44-5.86 | moderate |
| Low-dose aripiprazole group | 1 (86) | --- | --- | fixed | 0.00 | 1.00 | 1.00 | 0.21-4.68 | low |
| High-dose aripiprazole group | 1 (54) | --- | --- | fixed | 1.10 | 0.27 | 5.37 | 0.27-106.88 | moderate |
| Somnolence | 9 (654) | 0% | 0.87 | fixed | 2.76 | 0.006 | 2.76 | 1.34-5.69 | low |
| Risperidone group | 3(250) | 0% | 0.73 | fixed | 2.08 | 0.04 | 6.13 | 1.11-33.94 | moderate |
| Other antipsychotic group | 3 (200) | 0% | 0.96 | fixed | 1.43 | 0.15 | 3.67 | 0.62-21.85 | low |
| Low-dose aripiprazole group | 8 (586) | 0% | 0.80 | fixed | 2.70 | 0.007 | 2.85 | 1.33-6.10 | low |
| High-dose aripiprazole group | 1 (58) | --- | --- | fixed | 0.58 | 0.56 | 2.00 | 0.19-20.86 | low |
| Anxiety and depressive symptoms | 2 (126) | 32% | 0.23 | fixed | 0.26 | 0.79 | 1.15 | 0.40-3.35 | moderate |
| Risperidone group | 1 (72) | --- | --- | fixed | 0.44 | 0.66 | 0.76 | 0.22-2.59 | moderate |
| Other antipsychotic group | 1 (54) | --- | --- | fixed | 1.10 | 0.27 | 5.37 | 0.27-106.88 | moderate |
| Low-dose aripiprazole group | 1 (72) | --- | --- | fixed | 0.44 | 0.66 | 0.76 | 0.22-2.59 | moderate |
| High-dose aripiprazole group | 1 (54) | --- | --- | fixed | 1.10 | 0.27 | 5.37 | 0.27-106.88 | moderate |
| Extrapyramidal symptoms | 3 (218) | 0% | 0.92 | fixed | 0.40 | 0.69 | 1.18 | 0.53-2.60 | low |
| Risperidone group | 1 (72) | --- | --- | fixed | 0.08 | 0.93 | 0.95 | 0.26-3.94 | low |
| Low-dose aripiprazole group | 3 (218) | 0% | 0.92 | fixed | 0.40 | 0.69 | 1.18 | 0.53-2.60 | low |
| Lost to follow-up during study | 7 (561) | 0% | 0.63 | fixed | 0.72 | 0.47 | 1.24 | 0.69-2.22 | moderate |
| Risperidone group | 3 (305) | 0% | 0.68 | fixed | 0.54 | 0.59 | 0.82 | 0.39-1.71 | moderate |
| Other antipsychotic group | 2 (110) | 0% | 0.76 | fixed | 1.48 | 0.14 | 3.77 | 0.65-21.96 | low |
| Low-dose aripiprazole group | 4 (335) | 0% | 0.52 | fixed | 0.10 | 0.92 | 0.97 | 0.48-1.95 | low |
| High-dose aripiprazole group | 3 (226) | 0% | 0.63 | fixed | 1.34 | 0.18 | 2.10 | 0.71-6.23 | moderate |
| Improved psychotic symptoms | 16 (1157) | 1% | 0.44 | fixed | 0.37 | 0.71 | -0.02 | -0.14-0.09 | moderate |
| Risperidone group | 5 (438) | 11% | 0.35 | fixed | 0.40 | 0.69 | -0.04 | -0.23-0.15 | high |
| Other antipsychotic group | 8 (510) | 0% | 0.47 | fixed | 0.53 | 0.60 | -0.05 | -0.22-0.13 | low |
| Low-dose aripiprazole group | 13 (931) | 15% | 0.29 | fixed | 0.49 | 0.63 | -0.03 | -0.16-0.10 | moderate |
| High-dose aripiprazole group | 3 (226) | 0% | 0.64 | fixed | 0.14 | 0.89 | 0.02 | -0.24-0.28 | high |
a use of Cochrane collaboration’s GRADE software (Grades of Recommendation, Assessment, Development, and Evaluation)[33] to assess quality of evidence for each outcome
b pooled standard mean difference (SMD) is used to compare continuous measures and risk ratio (RR) for categorical measures
3.3.1. HPL recovery after treatment with aripiprazole
Eleven studies[18, 19, 20, 24, 27, 39, 41, 47, 48, 51, 52] with a pooled sample of 974 individuals provided information on the proportion of participants whose serum prolactin returned to the normal range by the end of follow-up. These studies were quite heterogeneous (I2=83%), so a random-effect model was used to generate the pooled estimates. Compared to the control group,individuals in the aripiprazole group were more likely to have normal prolactin levels by the end of the follow-up (pooled RR=8.81, 95%CI=3.66-21.23). Sensitivity analyses found that after excluding three outlier studies (Kane,[19] Pan,[51] and Qiao[52]), there was little heterogeneity (I2=0%) in the remaining eight studies. As shown in the Forest plot in Figure 2, using the fixed effect model on the results from the pooled sample of 604 individuals in these eight heterogeneous studies resulted in a pooled RR of 19.17 (95%CI=10.98-33.48).
3.3.2. Comparison of serum prolactin levels at the end of the study
A total of 19 studies[20, 21, 24, 27, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52] reported data on serum prolactin levels at the end of the trial. However, results from seven studies[24, 27, 40, 48, 49, 51, 52] were not included due to the non-normal distribution of the results. The pooled sample size from the remaining 12 studies[20, 21, 38, 39, 41, 42, 43, 44, 45, 46, 47, 50] was 1016. The sample size for these 12 studies varied from 56[45] to 180[44] and all of them reported statistically significant lower serum prolactin levels in the aripiprazole group compared to the control group at the end of follow-up. But these studies were quite heterogeneous (I2=94%) and sensitivity analysis did not identify a subset of results that were heterogeneous,so we did not pool the results in a meta-analysis.
3.3.3. Comparison of the occurrence of adverse events
Twelve studies[21, 24, 27, 38, 39, 40, 41, 42, 47, 48, 51] with a pooled sample of 962 individuals reported a total of 115 adverse events. No statistically significant differences were found in the proportion of participants who experienced an adverse event between the aripiprazole and control group (RR=1.16, 95%CI=0.82-1.64).
Reported adverse events included insomnia, somnolence, sedation, dry mouth, fatigue, anxious or depressive symptoms, extrapyramidal symptoms, and psychotic symptoms. Meta-analysis revealed no statistically significant differences in the occurrence of adverse events between the treatment and control group except for somnolence and headache: somnolence was more commonly reported in the aripiprazole group (RR=2.76, 95%CI=1.34-5.69) and headaches were also more commonly reported in the aripiprazole group (RR=2.31, 95%CI=1.08-4.92).
3.3.4. Loss to follow-up
Seven studies[18, 20, 27, 39, 45, 50, 52] with a pooled sample of 561 individuals reported that 41 individuals (7.3%) were lost to follow-up during the trial. Meta-analysis did not find any differences between the treatment and control group in the proportion of enrolled participants that were lost to follow-up (RR=1.24, 95%CI=0.69-2.22).
3.3.5. Comparison of improvement of psychotic symptoms
Fifteen studies[18, 20, 21, 27, 38, 39, 40, 41, 42, 45, 46, 48, 49, 50, 52] with a pooled sample of 1157 individuals assessed changes in the severity of psychotic symptoms during the trial using the Positive and Negative Syndrome Scale (PANSS)[53] or the Brief Psychiatric Rating Scale (BPRS).[54] The metaanalysis comparing the standardized mean difference in the final scale scores between the two groups found no significant difference in the improvement of psychotic symptoms between the aripiprazole groups and control groups (SMD=-0.02, 95%CI=-0.14~0.09).
3.4. Subgroup analysis
3.4.1. Analysis stratified by types of antipsychotics
Risperidone was the most commonly used medication in the studies, so we compared results of studies that only used risperidone with the results of studies that used other types of antipsychotic medications; studies that included participants that used different antipsychotic medications were not included in this analysis. As shown in Figure 2 and in Table 3, the results comparing five studies that reported the primary outcome (HPL recovery) in individuals using risperidone was not significantly different from that of the two studies reporting the primary outcome that used other antipsychotic medications (in this case, haloperidol) (RR=18.60 v. RR=15.46; χ2=0.08, p=0.78).
The meta-analysis of results of the secondary outcomes of interest stratified by type of antipsychotic medication used are shown in Table 3. There were only two statistically significant differences in the prevalence of these secondary outcomes in individuals who did or did not use adjunctive aripiprazole: among individuals taking other antipsychotic medications (i.e., not risperidone), those using adjunctive aripiprazole were more likely to report headaches than those in the control group; and among individuals taking risperidone, those using adjunctive aripiprazole were more likely to report somnolence than those in the control group. Comparison of the risk ratios for these secondary outcomes of the risperidone group versus those of the other antipsychotic group only found one significant difference: this risk ratio of headaches in the risperidone group (RR=0.84) was significantly lower than that for the other antipsychotic group (RR=6.68) (χ2=4.80, p=0.03).
3.4.2. Analysis stratified by dosage of aripiprazole
We also stratified studies into a low-dose group (i.e., daily dosage of aripiprazole <5mg) and a high-dose group (i.e., daily dosage of aripiprazole >5mg). As shown in the Forest plot and in Table 3, the main outcome (HPL recovery) was significantly more common at both dosages of aripiprazole than in individuals in the corresponding control groups. The risk ratio for the high-dose group was more than double that of the low-dose group (35.0 v. 15.1), but this did not reach statistical significance (χ2=1.41, p=0.23) due to the wide confidence intervals around these estimates of the RR. Assessment of the secondary outcomes in each of these groups only identified two outcomes that were significantly different in individuals who did or did not take adjunctive aripiprazole: compared to controls, individuals taking low-dose aripiprazole were significantly less likely to report insomnia and significantly more likely to report somnolence. There were no significant differences in the risk ratios for any of these secondary outcomes between the low-dose group and the high-dose group.
3.5. Quality of the level of evidence in the meta-analyses
This analysis investigated a total of 12 outcomes (57 when including the 45 subgroup analyses stratified by type of antipsychotic medication and by dosage of aripiprazole) about the level of prolactin, the occurrence of adverse events, non-compliance,and improvement of psychotic symptoms. Table 3 shows the GRADE assessment of the level of evidence for these outcomes. As shown in Table 3, based on the GRADE measure, the quality of evidence was classified as ‘high’ for 7 (12.3%) of the 57 outcomes, ‘medium’ for 24 (42.1% ) outcomes, ‘low’ for 24 (42.1%) outcomes,and ‘very low’ for 2 (3.5%) outcomes.
3.6. Risk of publication bias
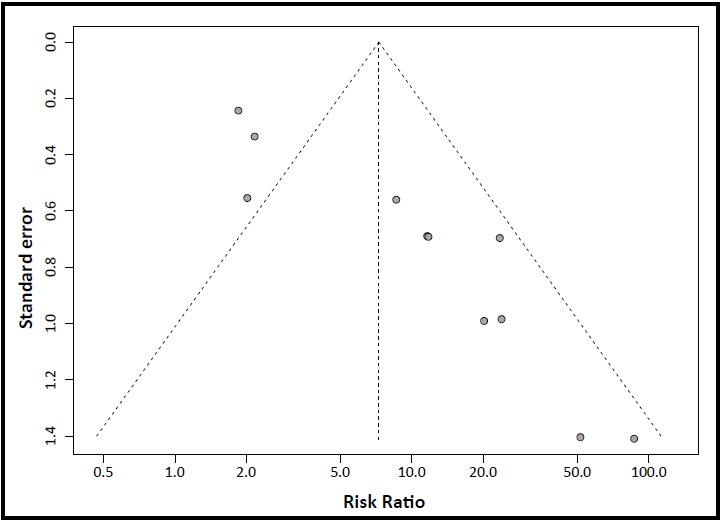
Figure 3 shows the funnel plot used to assess the possibility of publication bias in the eleven studies[18, 19, 20, 24, 27, 39, 41, 47, 48, 51, 52] that reported the proportion of participants whose serum prolactin levels returned to the normal range by the end of the follow-up (HPL recovery). As shown in the figure, smaller studies tended to report larger risk ratios in favor of aripiprazole. Egger’s test results indicate a statistically significant level of bias (Egger’s bias parameter=3.17, 95%CI=2.20-4.15, p<0.001).
Figure 3. Funnel plot of potential publication bias in 11 studies that report the proportion of participants whose serum prolactin returned to the normal range by the end of follow-up. (Note: the sample size and results for two studies are almost identical, so only 10 points appear in the plot.).
4. Discussion
4.1. Main findings
Extensive screening of English-language and Chinese-language databases identified 21 RCTs about the use of aripiprazole in the treatment of antipsychotic-induced hyperprolactinemia (HPL).
Meta-analysis of the 11 studies that reported the proportion of participants whose serum prolactin returned to the normal range at the time of follow-up (i.e., HPL recovery) indicated that both low-dose (<5mg/ day) and high-dose (>5mg/day) adjunctive treatment with aripiprazole can effectively treat antipsychotic-induced HPL. Two of the studies[18, 24] also reported recovered menstrual cycle and disappearance of spontaneous lactation in the aripiprazole group but not the control group. These findings are consistent with results from the previous meta-analysis[28] on this topic, which pooled results from five studies. There is on-going controversy about the appropriate dose of aripiprazole to treat antipsychotic-induced HPL;[18, 55] our study found a much higher risk ratio among individuals treated with high-dose aripiprazole than those treated with low-dose aripiprazole (35 v. 15),but the difference was not statistically significant,so more studies with larger samples will be needed to resolve this issue. Previous studies[56, 57, 58] have reported that HPL is a relatively common condition among individuals taking risperidone; our study found that aripiprazole was equally effective in the treatment of risperidoneinduced HPL and HPL induced by other antipsychotic medications.
Aripiprazole is a partial dopamine D2 receptor agonist,[59] but we found no evidence that it exacerbates existing psychotic symptoms. The occurrence of any adverse event during the follow-up period was similar in the aripiprazole and control groups, but - consistent with previously documented side-effects of aripiprazole[60, 61]—analysis of each specific adverse event found that somnolence was significantly more common in the aripiprazole group than in the control group, particularly in individuals taking low-dose aripiprazole.[59] We also found that individuals taking adjunctive aripiprazole were more likely to report headaches than those in the control group,especially if they were using antipsychotic medications other than risperidone.
There were substantial concerns about the quality of the data provided by the identified RCTs. Only a minority of the reports provided sufficient information to assess the method of randomization, allocation concealment,and blinding of the outcome measure, so risk of bias (assessed using the Cochrane ROB tool) was classified as ‘uncertain’ in 15 of the 21 studies. The remaining 6 studies were classified as ‘high-risk’ of bias because of failure to blind the outcome assessment or because of selective reporting. Based on the Cochrane GRADE measure of the quality of evidence supporting the results for the 12 full-sample meta-analyses and the 45 subgroup meta-analyses, only 7 of the 57 outcomes had ‘high-quality’ evidence. And there was a suggestion of publication bias,with smaller studies reporting greater treatment effects of aripiprazole. Clearly, conducting RCTs about a topic of interest - usually considered the ‘gold standard’ for informing evidencebased clinical medicine[62, 63] — is not enough to ensure high-quality data. Rigorous adherence to the reporting requirements specified in the CONSORT statement[64] and appropriate management of the issues discussed in the CONSORT statement when designing an RCT are essential to generating the high-quality data needed to inform clinical practice.
4.2. Limitations
In addition to concerns about the potential risk of bias and the quality of the evidence provided for the reported meta-analyses, there was substantial heterogeneity between the results of the studies. Partly due to this heterogeneity, only 8 of the 21 identified RCTs contributed data to the pooled sample used in the meta-analysis to assess the main outcome - recovery from hyperprolactinemia. This heterogeneity may be due to differences in the characteristics of participants, in the organization of the trials, or in the method of assessing the primary and secondary outcome measures between the different studies, but it may also mean that the results are inherently unstable.
Another issue is the duration of treatment. In clinical practice it is probable that aripiprazole will need to be taken continuously with antipsychotic medication to reduce the occurrence of HPL. The current studies only assess the effectiveness and safety of aripiprazole over relatively short follow-up periods, so long-term follow-up studies will be needed before aripiprazole can become a recommended treatment for antipsychoticinduced hyperprolactinemia.
4.3. Implications
The current study systematically reviewed and evaluated all available RCTs about the use of aripiprazole to treat antipsychotic-induced HPL. We found that adjunctive aripiprazole is effective and safe to use in the treatment of antipsychotic-induced HPL and that adjunctive aripiprazole is associated with increase reports of somnolence and headaches. However the potential for bias in the included studies was either ‘high’ or ‘uncertain’ and the level of evidence for most of the assessed outcomes was rated as ‘moderate’ or ‘low’. Moreover, the appropriate dose of aripiprazole and the long-term effectiveness and safety of this treatment remain uncertain. Further work that resolves these methodological and reporting issues will be needed before a definitive conclusion about the usefulness and safety of adjunctive aripiprazole in the management of antipsychotic-induced hyperprolactinemia is justified.
Acknowledgments
We thank the translators and reviewers of this analysis for their useful comments.
Biographies

Meiling Meng graduated from Jining Medical College with a degree in psychiatry and mental health. She is currently a masters’ student and is in her third-year residency at the Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine. Her main research interest is evidence-based psychiatry.

Wei Li graduated with a Bachelor’s degree of Medicine from Hebei Medical University in 2012. He is currently a PhD Candidate at Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine. His main research interests include basic and clinical aspects of generalized anxiety disorder and other anxiety disorders and the conduct of systematic reviews.
Funding Statement
This study was funded by the Science and Technology Commission of the Shanghai Municipality (13dz2260500) and by the Shanghai Health System Leadership in Health Research Program (XBR2011005).
Footnotes
Conflict of interest: Authors report no conflict of interest related to this manuscript.
References
- 1.Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia:mechanisms clinical features and management. Drugs. 2004;64(20): 2291–2314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- 2.Halbreich U, Kahn LS. Hyperprolactinemia and schizophrenia:mechanisms and clinical aspects. J Psychiatr Pract. 2003;9(5): 344–353. doi: 10.1097/00131746-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Melkersson K. Differences in prolactin elevation and relatedsymptoms of atypical antipsychotics in schizophrenicpatients. J Clin Psychiatry. 2005;66(6): 761–767. doi: 10.4088/jcp.v66n0614. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen E, Kroken RA, Abaza M, Olberg H, Jørgensen H. Antipsychotic-induced hyperprolactinemia: a cross-sectionalsurvey. J Clin Psychopharmacol. 2008;28(6): 686–690. doi: 10.1097/JCP.0b013e31818ba5d8. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZM, Xiang YT, Rong F, Correll CU, Ungvari GS, Wang Y, et al. Frequency of hyperprolactinemia and its associationswith demographic and clinical characteristics andantipsychotic medications in psychiatric inpatients in China. Perspect Psychiatr Care. 2014;50(4): 257–263. doi: 10.1111/ppc.12050. [DOI] [PubMed] [Google Scholar]
- 6.Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia:mechanisms consequences and management. Clin Endocrinol (Oxf) 2011;74(2): 141–147. doi: 10.1111/j.1365-2265.2010.03814.x. [DOI] [PubMed] [Google Scholar]
- 7.Byerly M, Suppes T, Tran QV, Baker RA. Clinical implicationsof antipsychotic-induced hyperprolactinemia in patientswith schizophrenia spectrum or bipolar spectrum disorders:recent developments and current perspectives. J ClinPsychopharmacol. 2007;27(6): 639–661. doi: 10.1097/jcp.0b013e31815ac4e5. [DOI] [PubMed] [Google Scholar]
- 8.Cookson J, Hodgson R, Wildgust HJ. Prolactin hyperprolactinaemia and antipsychotic treatment: a review and lessons for treatment of early psychosis. J Psychopharmacol. 2012;26(5 Suppl): 42–51. doi: 10.1177/0269881112442016. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto T, De Hert M, Carison HE, Manu P, Correll CU. Osteoporosis and fracture risk in people with schizophrenia. Curr Opin Psychiatry. 2012;25(5): 415–429. doi: 10.1097/YCO.0b013e328355e1ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krysiak R, Kedzia A, Okopien B. Unfavorable effects of hyperprolactinemia in autoimmune endocrine disorders. Neuro Endocrinol Lett. 2012;33(3): 298–300. [PubMed] [Google Scholar]
- 11.Inder WJ, Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N Z J Psychiatry. 2011;45(10): 830–837. doi: 10.3109/00048674.2011.589044. [DOI] [PubMed] [Google Scholar]
- 12.Rainka MM, Capote HA, Ross CA, Gengo FM. Attenuation of risperidone-induced hyperprolactinemia with the addition of aripiprazole. J Clin Pharm Ther. 2009;34(5): 595–598. doi: 10.1111/j.1365-2710.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin SK, Chen CK. Reversal of antipsychotic-induced hyperprolactinemia,weight gain, and dyslipidemia by aripiprazole:A case report. J Clin Psychiatry. 2006;67(8): 1307. doi: 10.4088/jcp.v67n0821a. [DOI] [PubMed] [Google Scholar]
- 14.Chen JX, Su YA, Wang SL, Bian QT, Liu YH, Wang N, et al. Aripiprazole treatment of risperidone-induced hyperprolactinemia. J Clin Psychiatry. 2009;70(7): 1058–1059. doi: 10.4088/JCP.08l04671. [DOI] [PubMed] [Google Scholar]
- 15.Wahl R, Ostroff R. Reversal of symptomatichyperprolactinemia by aripiprazole. Am J Psychiatry. 2005;162(8): 1542–1543. doi: 10.1176/appi.ajp.162.8.1542-a. [DOI] [PubMed] [Google Scholar]
- 16.Mir A, Shivakumar K, Williamson RJ, McAllister V, O’Keane V, Aitchison KJ. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol. 2008;22(3): 244–253. doi: 10.1177/0269881107082901. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz RA, Weinstein B. Resolution of haloperidolinduced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5): 524–525. doi: 10.1097/JCP.0b013e31814f4d5d. [DOI] [PubMed] [Google Scholar]
- 18.Shim JC, Shin JG, Kelly DL, Jung DU, Seo YS, Liu KH. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebocontrolled trial. Am J Psychiatry. 2007;164(9): 1404–1410. doi: 10.1176/appi.ajp.2007.06071075. [DOI] [PubMed] [Google Scholar]
- 19.Kane JM, Correll CU, Goff DC, Kirkpatrick B, Marder SR, Vester-Blokland E, et al. A multicenter, randomized, doubleblind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10): 1348–1357. doi: 10.4088/JCP.09m05154yel. [DOI] [PubMed] [Google Scholar]
- 20.Chen HZ, Niu FR, Qian MC, Yu BR, Shen XH, Yang SG, et al. The effect of Aripiprazole plus risperidone on the level of hyperprolactinemia of male patients with schizophrenia. Zhong Hua Jing Shen Ke Za Zhi. 2009;42(4): 224–227. Chinese. [Google Scholar]
- 21.Chen LJ, Zhuo ZM, Zhuang H. A study of aripiprazole in sulpiride induced male hyperprolactinemia. Lin Chuang Jing Shen Yi Xue Za Zhi. 2010;20(5): 304–305. Chinese. [Google Scholar]
- 22.Xie J. Aripiprazole on the treatment of hyperprolactinemia for schizophrenia induced byamisulpride. Qiqihaer Yi Xue Yuan Xue Bao. 2013;34(16): 2350–2352. Chinese. [Google Scholar]
- 23.Liang J, Yan J, Zhang XY. Randomized, double-blind, placebocontrolled study of aripiprazole reducing the elevation of prolactin induced by paliperidone in the treatment of schizophrenia. Zhongguo Xin Yao Za Zhi. 2014;11: 1300–1303, 1310. Chinese. [Google Scholar]
- 24.Xu LP, Ji JY, Shi H, Zhai FL, Zhang B, Shao YQ, et al. A control study of aripiprazole in the treatment of hyperprolactinemia by antipsychotics. Zhongguo Xing Wei Yi Xue Ke Xue. 2006;15(18): 718–720. doi: 10.3760/cma.j.issn.1674-6554.2006.08.019. Chinese. [DOI] [Google Scholar]
- 25.Liu ZB. Study of lactation and amenorrhea induced by lowdose aripiprazole treatment with antipsychotics. Sichuan Jing Shen Wei Sheng. 2009;22(1): 46–47. Chinese. [Google Scholar]
- 26.Sun J, Zhang W. 60 cases of hyperprolactinemia induced by small doses of aripiprazole treatment with risperidone. Zhongguo Bao Jian Ying Yang. 2014;24(4): 2242–2243. Chinese. [Google Scholar]
- 27.Chen JX, Zhang RZ, Li W, Liu YH, Jiang LY, Bian QT, et al. Adjunctive treatment of risperidone-induced hyperprolactinemia with aripiprazole: a randomized doubleblind placebo-controlled study. Zhongguo Xin Yao Za Zhi. 2014;7: 811–814. Chinese. [Google Scholar]
- 28.Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: metaanalysis of randomized controlled trials. PLoS One. 2013;8(8): e70179. doi: 10.1371/journal.pone.0070179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frances A, Pincus HA, First MB. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington: American Psychiatric association; 1994. [Google Scholar]
- 30.Liu P, Xu YX, translators. World Health Organization. ICD-10 Diagnostic Criteria of Classification of Mental and Behavioral Disorders. Beijing: People’s Medical Publishing House; 1995. [Google Scholar]
- 31.Psychiatry branch of Chinese Medical Society. CCMD-3 Chinese Classification and Diagnostic Criteria for Mental Disorders, 3rdedition. Jinan: Shandong Science and Technology Press; 2001. [Google Scholar]
- 32.JPT CHH, Green S. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] 2011. [Google Scholar]
- 33.Guyatt GH, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650): 924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health. DHEW Publ ADM. 1976: 223–244. [Google Scholar]
- 35.Hartling L, Hamm MP, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. Testing the risk of bias tool showed low reliability between individual reviewers and across consensus assessments of reviewer pairs. J Clin Epidemiol. 2013;66(9): 973–981. doi: 10.1016/j.jclinepi.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Li CB, He YL, Zhang MY. The rational application of consistency test methods. Shanghai Arch Psychiatry. 2000;4: 228–230,232. [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414): 557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B, Wang L, Xu LP, Shi JA, Sun J. Clinical study of aripiprazole treatment on hyperprolactinemia induced by perphenazine. Shen Jing Ji Bing Yu Jing Shen Wei Sheng. 2008;8(5): 375–376. Chinese. [Google Scholar]
- 39.Ji JY, Song ZX, Xu LP, Sun J, Shi JA, Zhao HJ, Wang HL. Aripiprazole in treatment of female schizophrenics with risperidone induced hyperprolactinemia. Zhong Hua Jing Shen Ke Za Zhi. 2008;41(3): 169–171. doi: 10.3321/j.issn:1006-7884.2008.03.011. Chinese. [DOI] [Google Scholar]
- 40.Jin JF, Cao YQ, Xu LP, Song ZY, Shao YQ. Clinical studies on improving hyperprolactinemia induced by chlorpromazine with small doses of aripiprazole. Jing Shen Yi Xue Za Zhi. 2008;21(6): 455–456. doi: 10.3969/j.issn.1009-7201.2008.06.022. Chinese. [DOI] [Google Scholar]
- 41.Wang L, Zhang B, Xu LP, Shi JA, Shao YQ. A clinical study on aripiprzole in the treatment of female hyperprolactinemia by haloperidol. Zhongguo Jian Kang Xin Li Xue Za Zhi. 2009;17(2): 194–195. Chinese. [Google Scholar]
- 42.Song ZX, Chen Q, Xu LP, Cai ZK, Ji JY, Wang WH, et al. 70 cases of aripiprazole treatment on hyperprolactinemia induced by sulpiride. Yi Yao Dao Bao. 2009;28(4): 479–481. doi: 10.3870/yydb.2009.04.030. Chinese. [DOI] [Google Scholar]
- 43.Liu L, Qi SG, Dong XH, Jiang DZ, Cui FW, Pan QH. A placebo- controlled trial of adjunctive treatment with aripiprazole for risperidone-induced hyperprolactinemia. Zhongguo Jian Kang Xin Li Xue Za Zhi. 2011;19(11): 1288–1290. Chinese. [Google Scholar]
- 44.Liu ZB, Cao B, Jiao F, Li DC, Chen HB, Liu W. A control study of aripiprazole in the treatment for antipsychotics-induced hyperprolactinemia. Zhongguo Yi Yuan Yao Xue Za Zhi. 2011;31(10): 843–846. Chinese. [Google Scholar]
- 45.Xun W, Zhang JL, Kang MX. Improving of Combined aripiprazole treatment on weight gain and prolactin increased levels induced by olanzapine treatment on female patients with schizophrenia. Sichuan Jing Shen Wei Sheng. 2011;24(2): 98–100. doi: 10.3969/j.issn.1007-3256.2011.02.013. Chinese. [DOI] [Google Scholar]
- 46.Xue L, Zhou LJ, Tan WZ, Zhou YF, Hu JW, Huang ZM. Efficacy analysis of Aripiprazole treatment on hyperprolactinemia induced by risperidone in 68 male patients. Jilin Yi Xue. 2012;33(1): 107–108. doi: 10.3969/j.issn.1004-0412.2012.01.063. Chinese. [DOI] [Google Scholar]
- 47.Zhou HS, Li B, Liu J, Wang HL. The efficacy and safety of aripiprazole in the treatment of female hyperprolactinemia caused by risperidone. Zhongguo Jian Kang Xin Li Xue Za Zhi. 2012;20(8): 1129–1130. Chinese. [Google Scholar]
- 48.Zhu JX, Yin JB. Clinical case-control study of aripiprazole treatment on hyperprolactinemia induced by risperidone in female patients. Zhongguo She Qu Yi Shi (Yi Xue Zhuan Ye) 2012;14(1): 124–125. doi: 10.3969/j.issn.1007-614x.2012.01.115. Chinese. [DOI] [Google Scholar]
- 49.Wu HL, Lin DD, Li Y, Xu YJ. [Influence of aripiprazole on hyperprolactinemia caused by antipsychotics in elderly patients with schizophrenia] Lin Chuang Jing Shen Yi Xue Za Zhi. 2013;23(4): 263–264. Chinese. [Google Scholar]
- 50.Guo JH, Cao CA, Liao CP, Xu YM, Liu YY. [A control study on aripiprazole in the treatment of hyperprolactinaemaia by antipsychotics] Zhongguo Jian Kang Xin Li Xue Za Zhi. 2013;21(4): 487–489. Chinese. [Google Scholar]
- 51.Pan JY, Wei YL. [Clinical observation of aripiprazole orally disintegrating tablets on weight gain and prolactin increase caused by risperidone and sulpiride] You Jiang Yi Xue. 2014;42(1): 48–50, 53. doi: 10.3969/j.issn.1003-1383.2014.01.014. Chinese. [DOI] [Google Scholar]
- 52.Qiao Y, Sheng JH, Li CB, Guo Q, Wen H, Zhu SY. [Addon effects of low dose aripiprazole in resolving hyperprolactinemia induced by risperidone or paliperidone] 2015 doi: 10.1016/j.psychres.2015.12.033. Unpublished Article. Chinese. (This study was registered on the China Clinical Trial Register ( http://www.chictr.org/en/proj/search.aspx) on 8 January 2014. The protocol can be accessed by entering the registration number for the study (ChiCTR-TRC-14004186) on this website and the results of the study, that were used in this paper, are available by contacting the first author of the protocol.) [DOI] [PubMed] [Google Scholar]
- 53.Lewis AO, Paul MR. Informant questionnaire for the positive and negative syndrome scale (IQ-PANSS) Muti-Health Systems Inc; 1999. [Google Scholar]
- 54.Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale. Schizophr Bull. 1986;13: 594–602. [Google Scholar]
- 55.Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2): 248–259. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 56.Duan DX, Cui GM. [The clinical observation and follow-up on Risperidone-induced hyperprolactinemia] Shi Yong Yi Xue Za Zhi. 2010;26(17): 3215–3216. doi: 10.3969/j.issn.1006-5725.2010.17.062. Chinese. [DOI] [Google Scholar]
- 57.Wang ZW, Zhang SP, Zhou TX, Chen YD, Liu RS. [Effect of risperidone on life quality in schizophrenics: Meta-analysis] Lin Chuang Jing Shen Yi Xue Za Zhi. 2004;14(5): 270–272. Chinese. [Google Scholar]
- 58.Shah SK. A comparative study of sexual dysfunction in schizophrenia patients taking aripiprazole versus risperidone. Kathmandu Univ Med J (KUMJ) 2013;11(42): 121–125. doi: 10.3126/kumj.v11i2.12486. [DOI] [PubMed] [Google Scholar]
- 59.Zhu ZQ, Zhang MY. [Progress of the study on the third generation antipsychotics aripiprazole] Zhongguo Yi Yuan Yong Yao Ping Jia Yu Fen Xi. 2005;5(2): 121–123. Chinese. [Google Scholar]
- 60.Huang WW, Jiang DG. [New antipsychotic aripiprazole] Shanghai Arch Psychiatry. 2003;15(5): 307–308. doi: 10.3969/j.issn.1002-0829.2003.05.020. Chinese. [DOI] [Google Scholar]
- 61.Shen YC. [Psychiatry] Beijing: People’s Medical Publishing House; 2009. pp. 528–529. Chinese. [Google Scholar]
- 62.Prato GP, Pagliaro U, Buti J, Rotundo R, Newman MG. Evaluation of the literature: evidence assessment tools for clinicians. J Evid Based Dent Pract. 2013;13(4): 130–141.. doi: 10.1016/j.jebdp.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Atkins D. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454): 1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134: 663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]