Abstract
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease characterized by a loss of tolerance to multiple endogenous antigens. SLE etiology remains largely unknown, despite recent insight into the immunopathogenesis of the disease. T cells are important in the development of the disease by amplifying the immune response and contributing to organ damage. Aberrant signaling, cytokine secretion and tissue homing displayed by SLE T cells have been extensively studied and the underlying pathogenic molecular mechanisms are starting to be elucidated. T-cell targeted treatments are being explored in SLE patients. This review is an update on the T-cell abnormalities and related therapeutic options in SLE.
Keywords: Systemic lupus erythematosus, T cells, Interleukin-2 (IL-2), epigenetics, treatment
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease. Despite recent advances in our understanding of the pathogenesis, the cause of SLE remains unknown and many factors contribute to the expression of diverse clinical manifestations, including genetic, environmental, hormonal, epigenetic influences.1 A key feature of SLE is a breakdown in innate and adaptive immune responses that leads to a loss of tolerance and the production of autoantibodies. T cells play a major role in SLE by amplifying the autoimmune response once the tolerance is compromised.2 Specifically, they display aberrant cytokine secretion, tissue homing and cell signaling properties. They also contribute to the inappropriate recruitment and activation of B cells and dendritic cells at the sites of inflammation.3 Over the last three decades, the biochemical and molecular abnormalities of SLE T cells have been extensively studied in efforts to provide new diagnostic tools and therapeutic targets. In this review, we present some of the recent advances in the domain of T-cell-targeted therapies.
T cell signaling alteration
Altered CD3-TCR (T cell receptor)
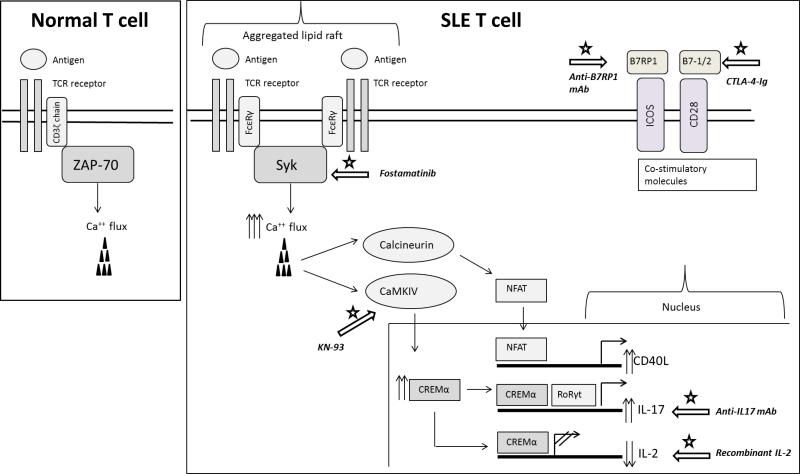
The predominant TCR is a heterodimer consisting of two transmembrane polypeptide chains, TCRα and TCRβ, covalently bound to each other. These chains are associated with the CD3 complex, which is composed of subunits δ, ε, γ and ζ. δ, ε, γ subunits are members of the immunoglobulin (Ig)-superfamily. Their C-terminal intracellular region contains an ITAM domain (immunoreceptor tyrosine-based activating motif). The ζ chain possesses a long intracytoplasmic tail that contains 3 ITAM domains. TCR signal initiation in normal individuals, after ligation of the TCR by MHC-peptide ligands, induces phosphorylation of ITAM tyrosine residues, which then recruit the tyrosine kinase ZAP-70 (ζ-chain associated protein 70). Alternatively, in SLE T cells, the CD3ζ chain levels are decreased and are replaced by an analogous protein, the FcRγ (common γ chain of the Fcε receptor) (figure 1).4 FcRγ does not partner with ZAP-70 but instead recruits Syk (spleen tyrosine kinase).5 The FcRγ-Syk interaction is 100 times stronger than the CD3ζ-ZAP interaction and this alternate TCR signaling pathway amplifies downstream early signaling events in SLE T cells, most notably leading to an enhanced calcium influx response.6 Increased tyrosine kinase phosphorylation and intracellular calcium enhances T cell signaling processes by promoting recruitment of calcineurin and translocating NFAT to the nucleus.7 Recent data emphasized that CD3ζ deficient mice develop multiorgan damage and tissue inflammation without mounting an autoantibody response.8 This suggests that restoring CD3ζ on the surface of SLE T cells could have a favorable effect on organ damage.
Figure 1. Aberrant signaling events in SLE T cells and potential therapeutic targets.
The lupus T cell is characterized by the downregulation of CD3ζ chain and its substitution by the FcRγ common chain. Following stimulation, FcRγ associates with Syk kinase, instead of ZAP-70, leading to heightened calcium responses and aberrant activation of calcineurin and CaMKIV. The increased magnitude of the early signaling events leads to a misbalance of the transcriptional machinery and results in aberrant gene expression. The white arrows with the star indicate potential therapeutic targets that are currently being considered for the management of SLE.
Kinase inhibition
The rewired TCR signaling and the ensuing downstream kinase activation may represent a promising treatment target for SLE patients. Syk inhibition corrected the calcium influx in SLE T cells and suppressed the development of lupus-related skin and kidney disease in lupus-prone mice.5, 9 Fostamatinib (R788) is a pro-drug, orally administrated, which is converted to an active Syk inhibitor form (R406). Although there are no current studies involving SLE, phase-II clinical trials have been published for other autoimmune conditions in which Syk is also aberrantly expressed. Clinical trials showed positive results in the treatment of rheumatoid arthritis in patients with inadequate response to methotrexate10, but no benefits after anti-TNFα treatment failure.11 In an open-label pilot study, fostamatinib appeared to be a promising therapeutic agent in patients with chronic immune thrombocytopenic purpura. .12
The role of Phosphoinositide-3 kinases (PI3K) has also been extensively studied in the onset of autoimmunity and SLE. PI3Ks are recruited to the TCR-complex following activation and generate phosphatidylinositol-(3, 4, 5) P3 (PIP3) from membrane phospholipids. PIP3 activates important signaling pathways implicated in T cell division, proliferation, and survival. In CD4+ T cells from a lupus mouse model and in SLE patients, PI3k activity is increased compared to a control and pharmacologic inhibition of PI3kγ or PI3kδ has been shown to reduce tissue inflammation and disease manifestations.13, 14 Therefore, these kinases could also be considered as future therapeutic targets.
Lipid rafts aggregation
Lipid rafts are cholesterol-rich domains on the surface of the cell membrane which cluster the TCR and other associated signaling molecules at a high density to facilitate the formation of the immunological synapse between T cells and antigen-presenting cells. In normal T cells, lipid rafts aggregate after TCR activation. However, freshly isolated SLE T cells harbor pre-clustered lipid rafts, which contribute to the signaling alterations.15 In lupus-prone mice T cells, aggregated lipid rafts are observed prior to the development of the disease. Lipid raft clustering agents, such as the cholera toxin, exacerbate lupus pathology and disruption of the lipid rafts can delay its onset.16 Another study showed that in vitro atorvastatin treatment, a drug known to reduce cholesterol synthesis, may reverse the lipid rafts signaling associated abnormalities and normalize cytokine production in SLE T cells.17 Recently, the importance of a lipid subset, the glycosphingolipids (GSL), which are enriched in the lipid rafts has been emphasized. GSL homeostasis is severely impaired in the membrane of SLE T cells, and in vitro inhibition of GSL synthesis with glucosylceramide synthase NB-DNJ partially normalizes SLE T cells’ signaling.18
CD44-ERM/ Rho associated protein kinase (ROCK)
CD44 is a cell surface molecule involved in cell adhesion and cell migration. The CD44 gene generates numerous protein isoforms from a highly conserved gene through alternative splicing and post-translational modifications. CD44 is activated by binding to its principal ligand hyaluronic acid (HA). In order for CD44 to promote cell migration and adhesion, the phosphorylated form of the ezrin/radixin/moesin protein (ERM) needs to be recruited to the intracellular domain of CD44. ERM is phosphorylated by the rho-associated protein kinase (ROCK).
The expression levels of splice variants CD44v3 and CD44v6 are increased and correlate with disease activity in SLE patients.19 Elevated levels of HA and CD44 have been observed in damaged kidneys from SLE patients and lupus-prone mice.20, 21 Moreover, increased levels of pERM have been observed in T cells from SLE patients. A pharmacologic inhibition of ROCK decreased pERM levels, thus limiting T cells adhesion and migration and limited lupus related pathology when administered to lupus-prone mice.15, 22
Globally, these data suggest that CD44-ERM-ROCK pathway is involved in the pathogenesis of lupus nephritis by enhancing T cells migration and the adhesion. Pharmacologic inhibition of ROCK is a potentially interesting way to limit SLE related organ damages.
Interleukin-2
The role of IL-2 in peripheral tolerance
Early studies that were conducted over three decades ago demonstrated a significant defect in the production of IL-2 from in vitro activated T cells in both murine lupus models23 and humans with SLE.24, 25 IL-2 is a key T cell-derived cytokine that is mainly produced by antigen-activated T cells. It exerts its biological function via the IL-2 receptor (IL-2R) in an autocrine and/or paracrine fashion. Initially, IL-2 was thought to function primarily as a growth, survival and differentiation factor for activated T cells. IL-2 is implicated in the differentiation of both Th1 and Th2 cells (reviewed in 26) and is also involved in promoting the differentiation of effector cytolytic T cells.27 IL-2 has a unique role in promoting activation-induced cell death (AICD), an important apoptotic process that is responsible for the elimination of repeatedly activated, and potentially autoreactive, T cells.28 In vivo studies performed in il-2 −/− and il-2r −/− mice revealed an important and indispensable role of IL-2 in the induction of peripheral tolerance. Both in vitro and in vivo studies have provided evidence that IL-2 plays an important role in the development and survival of regulatory T cells (Tregs),29 and the autoimmune manifestations seen in il-2 −/− mice can be attributed to the greatly reduced numbers of Tregs in the periphery.30 Of particular interest is the finding that IL-2 may restrict the differentiation of naïve CD4+ T cells into IL-17 secreting cells (Th17 cells) in vitro.31
Therefore, treatment with IL-2 could prove beneficial for the management of diseases that are characterized by impaired Treg function and/or Th17 driven responses. However, reports suggesting that IL-2 may participate in the expansion of already differentiated Th17 cells in humans highlight the complex role of IL-2 in Th17 differentiation and maintenance and should be taken into consideration in future therapeutic attempts of Th17-based autoimmune diseases.32
IL-2 immunotherapy in autoimmunity
Infection is one of the leading causes of morbidity and mortality in patients with SLE, and IL-2 plays a central role in the proper function of the immune system of the host. Most studies agree that patients with SLE characteristically have reduced numbers and impaired function of Tregs, diminished cytotoxic activity of CD8+ T cells, and defective AICD. The inability of lupus T cells to produce adequate amounts of IL-2 following antigen-driven stimulation may account for these immunological defects and may thus contribute to the increased rates of infections in patients with SLE. Therefore, restoring IL-2 levels in patients with SLE has been considered as a potential therapeutic approach. To date, there have been some encouraging results on the use of exogenous IL-2 for the treatment of autoimmune manifestations in both murine models of autoimmunity and humans.
Daily injections of recombinant human IL-2 in neonatal il-2 −/− mice prevented the development of autoimmunity and significantly improved survival.33 Treatment of MRL/lpr mice with live vaccinia recombinant viruses expressing the human IL-2 gene also resulted in improved survival rates. Clinical symptoms, such as arthritis and kidney disease were also ameliorated in the vaccinia-treated MRL/lpr mice and autoantibody titers were decreased. Administration of IL-2 to MRL/lpr mice using an adenovirus system which secured low serum IL-2 concentrations, resulted in killing of the severely expanded IL-17 producing TCR-αβ+CD4−CD8− cells and expansion of Tregs with a profound improvement of renal and lung pathology.34 In humans, immunotherapy with recombinant human IL-2 (aldesleukin) has been approved by the FDA for the management of skin melanoma and renal cell carcinoma. Recently, administration of low-dose IL-2 has been examined as an adjunct agent for the management of active chronic graft-versus-host disease (GVHD)35 and HCV-induced vasculitis36 in two open-label, phase-I/IIa studies. Low-dose IL-2 treatment led to an increase in the percentage of peripheral functional regulatory T cells (Tregs) in patients with HCV-related vasculitis and GVHD and was associated with the amelioration of clinical symptoms. Currently, an open-label clinical trial is underway to test the efficacy and safety of low-dose IL-2 treatment in SLE.
Regulation of IL-2 in SLE and novel therapeutic targets
The molecular mechanisms responsible for the IL-2 defect in lupus have only recently been elucidated and remain the focus of intensive research. The regulation of the IL-2 gene is predominantly controlled at the transcriptional level and depends on the cooperative binding of NFAT, AP-1(c-fos/c-jun heterodimer), CREB (cAMP regulatory element binding protein) and NF-κB to cognate sites within the IL-2 promoter.37 Accumulating evidence suggests that impaired IL-2 production from lupus T cells is the result of defective transcriptional regulation. As mentioned earlier, lupus T cells are characterized by enhanced early signaling events and heightened calcium responses that lead to increased activation of calcineurin and to the eventual dephosphorylation and translocation of NFAT to nucleus.7 However, NFAT is not able to properly initiate the transcription of the IL-2 gene without the concurrent binding of AP-1, which is reported to be downregulated in SLE.38 Moreover, protein levels of the p65 activating subunit of NF-κB are downregulated in patients with SLE.39. As a result, the activation of NF-κΒ in SLE is severely diminished following in vitro stimulation of T cells, thus contributing to the defective IL-2 production. Finally, increased levels of the serine-threonine phosphatase PP2A in SLE T cells lead to the dephosphorylation and subsequent inactivation of CREB.40 Decreased IL-2 production in SLE can also be attributed to active transcriptional suppression, mediated by the binding of phosphorylated CREMα to the promoter region of the IL-2 gene.41 CREMα may also indirectly inhibit IL-2 production, by suppressing the transcription of the c-fos gene, thus contributing to the decreased levels of AP-1 in SLE.38 Collectively, the above data suggest that the IL-2 defect in SLE is the result of reduced transcriptional activity due to the decreased expression and/or activity of transcriptional activators.
Restoration of the CD3ζ chain on the cell-surface of SLE T cells was able to restore IL-2 production back to normal levels,42 further supporting the hypothesis that IL-2 defect in SLE can be attributed to dysregulated T cell signaling. Apart from the aberrant early signaling events that characterize lupus T cells, elements in the microenvironment may also account for the diminished ability of SLE T cells to produce IL-2. The IgG fraction of serum from patients with SLE contains anti-TCR/CD3 autoantibodies that are able to induce the translocation of calcium/calmodulin kinase IV (CaMKIV) to the nucleus, resulting in the overexpression of CREMα in normal T cells.43 The importance of CaMKIV in the pathway leading to the suppression of IL-2 production in SLE is further highlighted by experiments demonstrating that overexpression of a dominant negative isoform of CaMKIV in normal T cells blocked the effect of lupus sera and abolished the CREMα binding on the IL-2 promoter.44 Inhibition of CaMKIV may represent a promising therapeutic target in SLE. Indeed, treatment of lupus-prone MRL/lpr mice with KN-93, a small molecule that inhibits CaMKIV ameliorated glomerulonephritis and skin disease.45 Recently, the role of the serine arginine protein splicing factor 2/alternative splicing factor (SF2/ASF) has been examined in the regulation of IL-2 production. SF2/ASF binds to the 3’UTR of the CD3ζ mRNA and limits the production of an alternative unstable mRNA splice variant. Therefore, by stabilizing the CD3ζ mRNA, SF2/ASF promotes the expression of CD3ζ chain on normal T cells.46 SF2/ASF expression levels are reduced in patients with SLE and are inversely correlated with disease activity. Forced expression of SF2/ASF restored IL-2 production in patients with SLE.47 The in vivo effects of the SF2/ASF defect on autoimmunity remain to be evaluated.
Epigenetics in SLE and potential therapeutic approaches
Epigenetic alterations, including DNA methylation and histone modifications, may also contribute to the pathogenetic mechanisms of SLE. Studies from the early 90s reported reduced DNA methylation in lupus T cells.48 DNA methylation is mediated by various DNA methyltransferase enzymes (DNMT). Decreased methylation may be a responsible mechanism for the increased expression of genes that are known to be involved in the pathogenesis of SLE, such as LFA-1, CD70 and PP2Ac.49-51 Reduced DNA methylation has also been associated with the overexpression of the IL-10, IL-13 and IL-17 genes in SLE.52, 53 Genome-wide-methylation analysis in T cells from SLE patients revealed persistent hypomethylation of genes involved in interferon (IFN) signaling, providing a potential mechanism for the described IFN-α signature in lupus.54 Hypomethylation of IFN-related genes was apparent in naïve, memory and regulatory T cells, suggesting that the epigenetic modification may have initially occurred in progenitor populations.55
The accessibility of transcription factors to DNA is also regulated by histone modifications. Histone proteins are highly conserved proteins and are responsible for maintaining the structure of chromatin. Post-translational alterations (acetylation, citrullination, methylation, SUMOylation, phosphorylation and/or poly-ADP-ribosylation) of histone proteins at the N-terminal tail may promote or inhibit gene transcription. Histone modifications are altered in SLE T cells. Global histone H3 and H4 hypoacetylation have been reported in CD4+ T cells from patients with active SLE.56 Moreover, IL-2 and IL-17 production are controlled at an epigenetic level through histone acetylation and methylation. In SLE T cells, a misbalance of these processes may be involved in altered IL-2 expression and increased IL-17 production. Reduced acetylation of histone H3 at lysine 18 (H3K18) together with increased methylation of histone H3 at lysine 27 (H3K27) characterizes the wholelength of the IL-2 gene in lupus and may lead to reduced transcriptional activity.57 Conversely, the IL-17A gene in lupus T cells is characterized by increased H3K18 acetylation and reduced H3K27 methylation, promoting enhanced transcription of the IL-17A gene and further highlighting the importance of gene-specific modifications in SLE T cells.53
Because epigenetic alterations gradually accumulate over the course of the disease, targeting DNA methylation and/or histone modifications may present a promising therapeutic approach in SLE. Ex vivo pre-treatment of MLR/lpr splenocytes with deacetylase inhibitors, such as Trichostatin A (TSA) and suberonylanilide hydroxamic acid (SAHA) resulted in downregulation of IL-12, IFN-γ, IL-6, and IL-10 protein levels following stimulation with concanavalin A.58 Although treatment of normal human T cells with TSA in vitro resulted in signaling aberrations reminiscent of those that have been described for human SLE, results from experiments in mice treated with TSA have been favorable. MRL/lpr mice treated with either TSA or SAHA display significantly reduced proteinuria and glomerulonephritis.59
Aberrant microRNA (miRNA) expression with potentially pathogenetic repercussions has been described in both murine and human lupus. miRNA are short, non-coding RNA sequences that bind to their target mRNA and regulate gene expression either by inhibiting translation or by promoting mRNA degradation. miRNA have emerged over the past few years as significant contributors to a variety of human diseases such as cancer and autoimmunity. Several miRNA sequences have been reported to be either upregulated or downregulated in lupus T cells and many studies have been conducted in an attempt to elucidate the role of these aberrantly expressed miRNA sequences in the pathogenesis of SLE. miR146A, a negative regulator of the type I IFN pathway has been reported to be downregulated in patients with SLE and was inversely correlated with disease activity.60 In vitro reconstitution of miR-146A in PBMCs from patients with SLE was associated with a reduction in the expression of certain IFN-inducible genes, indicating that miR146A may present a promising therapeutic target in SLE. Low levels of miR-31 in T cells from patients with SLE have been associated with IL-2 defect in SLE T cells.61 On the other hand, miR-21 is upregulated in SLE patients and may be implicated in the mechanisms leading to DNA hypomethylations.62 In vivo silencing of miR-21 ameliorated autoimmune splenomegaly in lupus-prone B6.Sle123 mice.63 Furthermore, silencing miR-21 in human lupus T cells in vitro reduced IL-10 production, suppressed the activation-induced up-regulation of CD40L and reduced the capacity of T cells to drive the maturation of B cells.64 Recent work has demonstrated that the anti-inflammatory effects of methylprednisolone may be mediated by the up-regulation of miR-98 and downregulation of miR-155 and the subsequent suppression of predicted targets.65, 66
Principal T cells subtypes involved in SLE pathogenesis
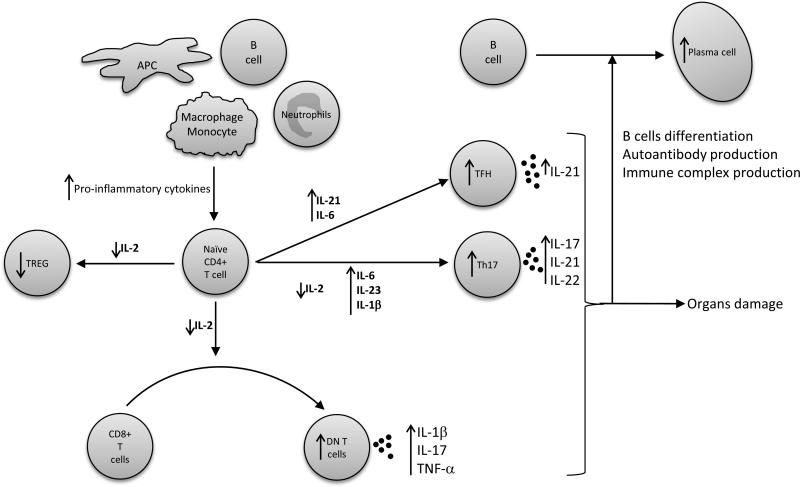
T cells subsets distribution is altered in SLE patients compared to healthy control. This is notably due to a decreased IL-2 production by T cells, and also to the presence of increased pro-inflammatory cytokines in the serum of SLE patients (figure 2). The most important T cells subtypes implicated in SLE pathogenesis are described below.
Figure 2. T cells differentiation and cytokines aberration in SLE patients.
The cytokines produced by macrophages, antigen-presenting cells (APC), B cells, neutrophils and activated T cells, along with the decreased IL-2, influence the development of naïve CD4+ T cells in SLE patients. These alterations favor the development of Th17, TCRab+CD4-CD8 double negative (DN) and T follicular helper (TFH) T cells, whilst inhibiting regulatory T cells (TREG) differentiation. Collectively, these abnormalities enhance B cell maturation and differentiation, antibody production and immune complex formation, and also promote organ damage. Arrows indicate increased/decreased cytokines or cell subset in SLE compared to control.
T helper (Th) 17 cells
Th17 cells represent a subset of CD4+ T helper cells that are developmentally distinct from Th1 and Th2 subsets. They are characterized by the expression of the orphan nuclear factor receptor RORγt, the expression of the cytokines IL-17A, IL-17F and IL-22, and by the inflammatory chemokine receptor CCR6.67, 68
IL-17 promotes inflammation and tissue damage in the context of SLE by recruiting neutrophils and monocytes, facilitating T-cell tissue infiltration and promoting antibody production.69 This cytokine has been shown to be increased in the serum of SLE patients compared to healthy controls.70-72 However, a correlation between IL-17 levels and disease activity, dsDNA levels, C-reactive protein or sedimentation rate has not been established.70, 72 Therefore, IL-17 is not a suitable biomarker of SLE disease activity. Of note, Th17 cell are not the only source of IL-17 in SLE patients, as TCR-αβ+CD4-CD8- double negative (DN) T cells from peripheral blood and kidney biopsies have been shown to secrete high amount of IL-17.73 Moreover, neutrophils from lupus skin lesion biopsy also demonstrated the ability to produce IL-17.74
Some reports show that the percentage of IL-17A producing T cells is higher in peripheral blood and among inflammatory infiltrate in skin and kidneys affected by SLE.73, 75, 76 Recent studies in mice and in humans argued that IL-17A expression is not sufficient to define pathogenic Th17 cells.77, 78 IL-23 plays a central role in inducing Th17 cells differentiation with pathogenic properties, and pathogenic Th17 cells express high level of IL-23 receptor (il-23r)77 and produce high amount of IFNα and GM-CSF.79, 80 In contrast, non-pathogenic Th17 cells develop in the absence of IL-23/il-23r and express high levels of IL-10 in addition to IL-17. These cells are supposed to have an immunosuppressive effect. So far, no studies have evaluated the pathogenic potential of Th17 cells in the onset of SLE.
A number of molecular alterations contributing to increased IL-17 production have been observed in SLE patients. Studies have reported an increased ROCK activity in T cells, which leads to enhanced binding of IRF4 to the IL-17 promoter.22, 81 CREMα expression is increased in SLE patients and enhances IL-17 production by binding to its promoter and by diminishing IL-2 production.53 Recently, Koga et al. emphasized the importance of the CaMKIV in the development of Th17 differentiation. This kinase phosphorylates CREMα and activates the AKT/mTOR pathway, which in turn leads to IL-17 transcription.82
IL-17 inhibition
Currently, there are no available data on the effect of IL-17 blockade by monoclonal antibodies in SLE. Secukinumab and ixekizumab are two monoclonal antibodies directed toward IL-17A. Phase-II clinical trials in psoriasis, ankylosing spondylitis and psoriatic arthritis showed good clinical results for both drugs without major safety concerns.83-85 Brodalumab is a monoclonal antibody directed against IL-17 receptor A, which significantly improved psoriasis in a phase-II clinical trial.86
Ustekinumab is a monoclonal antibody directed toward the p40 subunit common to IL-12 and IL-23, thus targeting the IL-23/IL-17 immune axis. It is already approved for the treatment of psoriasis and phase-III clinical trials are underway in the onset of psoriatic arthritis. There are currently no data available for SLE; however administration of ustekinumab improved cutaneous lupus in some cases.87, 88
Experimental models showed that CaMKIV inhibition may be a potential therapeutic target. Pharmocologic inhibition of CaMKIV in mice models and silencing CaMKIV in T cells from SLE patients inhibited IL-17 production.85
Double negative (DN) T cells
A small subpopulation of T cells lack the expression of CD4 and CD8 surface markers in healthy individuals. These cells express the phenotype TCR-αβ+CD4−CD8− and are called DN T cells. In SLE patients and lupus prone-mice, DN T cells are expanded in the peripheral blood and in disease affected kidneys.73, 75 These cells produce inflammatory cytokines like IL-1β, IFN-γ and IL-17.89 They also induce antibody production through promoting B cell differentiation.90, 91 The origin of DN T cells remains unclear. There is evidence showing that expanded DN T cells in SLE derivate from CD8+ T cells, through downregulation of the CD8 receptor after TCR stimulation.89 Regulatory mechanisms leading to DN T cells generation involve the transcription factor CREMα, which is expressed at increased level in SLE, and acts as a trans-repressor of the CD8b promoter.92, 93 In a recent study, Mizui et al. used an inducible IL-2-recombinant adeno-associated virus to restore continuous systemic IL-2 production in an MRL/lpr lupus mouse model. This treatment decreased the number of DN T cells and effectively reduced lupus-related organs’ inflammation.34
Regulatory T cells (Tregs)
Tregs are implicated in the maintenance of peripheral immune tolerance and the prevention of autoimmune disease by suppressing autoreactive effector cells. Human Tregs represent about 2% of CD4+ T cells and are characterized by the constitutive expression of CD25(IL-2 receptor α), low level of CD127 (IL-7 receptor α) and the expression of the transcription factor forkhead box P3 (FoxP3).94
Studies in SLE patients suggest a dysregulation of Tregs. Due to a lack of specific markers, data assessing the number of Tregs in the peripheral blood of SLE patients are conflictive. While some reports highlighted a decreased number of Tregs95-97, others showed no difference98-100 compared to healthy controls. Some authors emphasized that the suppressive function of Tregs may be impaired in SLE patients96-98 or that SLE effector cells may be resistant to Treg-induced suppression.101 Treg dysregulation may be a result of altered IL-2 production in SLE patients. This is supported by recent studies in lupus-prone mice, where restoring IL-2 production through CaMKIV inhibition enhanced the generation of Tregs along with diminished organ inflammation and damage.45, 102
T follicular helper (TFH) cells
TFH cells belong to a recently identified subset of T helper cells. They are activated by antigens in the T cell zone of the lymphoid tissues. After activation they migrate to the outer edge of the B cells’ follicle in order to encounter cognate B cells and support their differentiation. These cells are CD4+ T cells, that express CXCR5, programmed cell death protein 1(PD-1), the surface Inducible T-cell Co-stimulator (ICOS) and the CD40 ligand. The main transcription factor of TFH is B cell lymphoma 6 (BCL-6), and its signature cytokine IL-21, which is essential for promoting B cell somatic hypermutation, Ig isotype switching and plasma cell generation. IL-21 also induces TFH cell differentiation in an autocrine fashion.103 Accordingly, an exaggerated TFH response would be a candidate driver of autoimmunity.
The involvement of TFH cells in the development of SLE came first from mice models.104 For example, Sanroque lupus mice spontaneously develop a lupus-like pathology with anti-dsDNA, immune-complexes, and glomerulonephritis. They exhibit germinal center formation, excessive TFH numbers, and increased expression of ICOS and IL-21. The underlying mechanism involves a recessive mutation that disrupts a repressor of ICOS expression.105 Human studies are limited by reduced access to secondary lymphoid organ samples. Most human studies focus on the so-called “blood TFH cells”, which are CD4+ T characterized by the expression of CXCR5 and are phenotypically and functionally considered as TFH circulating counterparts.106 Some studies showed expanded circulating TFH107, 108 and increased serum levels of IL-21109 in SLE patients. However, as of now, no study has evaluated this cell type in tissues affected by SLE or in lymph nodes from SLE patients.
Targeting T cell co-stimulatory molecules
Targeting CTLA-4
Full activation of T cells is a two-step process that requires the binding of the antigen to the TCR followed by the engagement of the co-stimulatory T cell surface molecule CD28 with CD80 or CD86 that are expressed on the surface of antigen-presenting cells. Following T-cell activation, CTLA-4, a CD28 homologue with inhibitory properties, is induced on the surface of T cells. It recognizes both CD80 and CD86 with a higher affinity than CD28, and thus leads to the termination of the activation process. Abatacept (CTLA4-Ig) is a human recombinant fusion protein that consists of the Fc region of an IgG1 fused to the extracellular domain of CTLA-4. It has been approved by the FDA for the treatment of RA and juvenile idiopathic arthritis. Belatacept is another human recombinant fusion protein consisting of a modified extracellular domain of human CTLA-4 linked to the Fc portion of a human IgG1, which has recently been approved for patients undergoing kidney transplantation. The role of abatacept in SLE has been evaluated in two multicenter double-blind placebo-controlled clinical trials. The first one included a total of 175 patients with “non-life-threatening” SLE with polyarthritis, discoid lesions, or serositis.110 Although the primary end point of the study was not met, a post hoc analysis indicated that SLE patients with polyarthritis benefited from treatment with abatacept. The increased percentage of serious adverse events, however, raised concerns on the safety of abatacept in patients with SLE. The second study evaluated the efficacy and safety of intravenous abatacept injections in 298 patients with lupus nephritis in addition to treatment with mycophenolate mofetil and corticosteroids.111 In this study, abatecept was well tolerated in patients with active class III or IV lupus nephritis. Even though the primary end point was not met, a decrease of 20-30% in mean urinary protein-to-creatinine ratio as well as a favorable effect on anti-dsDNA autoantibody titers and complement levels was observed in the abatacept-treated group. Currently, an ongoing clinical trial is assessing the safety and efficacy of abatacept in addition to cyclophosphamide in patients with lupus nephritis compared to treatment with standard cyclophosphamide regimen alone.
Targeting the CD40-CD40L interaction
CD40L is a member of the tumor necrosis factor superfamily and is mainly expressed on CD4+ T cells shortly after activation. Interaction of CD40L-expressing T cells with CD40 on B cells leads to B-cell proliferation, Ig isotype switching and generation of memory B cells. CD40L is overexpressed on the cell-surface of both B- and T-cells in patients with SLE and in murine models of lupus.112-114 CD40L blockade in lupus-prone mice had contradictory results. Even though anti-CD40L treatment prevented the development of nephritis in NZB/W F1 mice,115 it accelerated renal disease in the MRL/lpr model.116 Nevertheless, anti-CD40L treatment has been evaluated in patients with SLE with two different monoclonal antibodies, IDEC-131 and BG9588. In the first double-blind placebo-controlled clinical trial, the IDEC-131 mAb was tested in 85 patients with mild-to-moderately active SLE, but no efficacy was demonstrated compared to the placebo group.117 The second study attempted to evaluate the efficacy and safety of the BG9588 mAb in patients with biopsy-proven lupus glomerulonephritis. Treatment with BG9588 resulted in 50% reduction of proteinuria, with no worsening of renal function. Anti-dsDNA autoantibody titers were also decreased and serum C3 complement levels significantly increased. However, the study was prematurely terminated due to the increased incidence of catastrophic thromboembolic events,118 thus precluding for the moment any further consideration on the use of anti-CD40L mAbs for the treatment of SLE.
Targeting the ICOS-B7RP1 pathway
ICOS is a transmembrane glycoprotein that is structurally and functionally related to CD28 and is expressed on activated T cells. ICOS binds to its ligand B7-related protein-1 (B7RP1) that is constitutively expressed on B cells and on activated dendritic cells and monocytes. The ICOS-B7RP1 engagement plays an important role in T cell-B cell interaction. It promotes germinal center formation and B cell differentiation and drives T cell dependent antibody production and Ig isotype switching. The ICOS-B7RP1 pathway appears to be directly implicated in the process of generating memory B cells and plasma cells in SLE.119 Moreover, ICOS is found to be overexpressed on the cell surface of CD4+ and CD8+ T cells and promotes the production of anti-dsDNA autoantibodies and total IgG in patients with SLE.120 Therefore, inhibiting the ICOS-B7RP1 pathway represents a possible therapeutic target for human SLE and, so far, results from murine lupus models treated with a monoclonal antibody directed against ICOS-B7 homologous protein (B7h) have been encouraging. Treatment of NZB/NZW F1 lupus-prone mice with an anti-B7h mAb before the onset of renal disease delayed the onset of proteinuria and prolonged survival. When anti-B7h mAb was administered after the onset of proteinuria, it managed to delay disease progression and improved renal pathology.121 A phase Ib, double-blind, placebo-controlled, dose-escalating study assessing the safety and tolerability of a B7RP1 mAb (AMG 557) in patients with stable SLE has been completed and the results are being analyzed.
Conclusions
SLE is a multifactorial and complex autoimmune disease with diverse clinical manifestations and is characterized by various cellular and molecular aberrations. Traditional management of patients with SLE relies on the use of corticosteroids and immunosuppressive agents, such as hydroxycloroquine, azathioprine, cyclophosphamide, methotrexate and, more recently, mycophenolate mofetil. However, these treatments are often accompanied by significant side effects. Moreover, patients with refractory SLE do not adequately respond to conventional immunosuppressive agents, thus making the need for developing newer therapeutic strategies mandatory. T cells have emerged as central players in the pathogenesis of SLE. Even though our understanding still remains incomplete, significant progress has been made over the past years in identifying the biochemical aberrations that characterize the lupus T cell in an attempt to elucidate the pathogenic mechanisms underlying SLE. During this process, new and promising therapeutic targets have been identified. Biologic agents and small-molecule drugs are being developed and the currently expanding field of epigenetics is expected to further enhance our knowledge of gene-regulation. These new insights may culminate in the development of safer and more effective treatments.
Acknowledgments
Funding
The work performed in the authors’ laboratory was supported by National Institutes of Health (grant numbers PO1 AI065687, RO1 AI49954, and RO1 42269). This work was also supported by a SICPA foundation grant (to D.C.).
Footnotes
Disclosures
The authors have no financial conflicts of interest
References
- 1.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nature reviews Immunology. 2001;1:147–53. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Yuan J, Pan Y, et al. T cell CD4770LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clinical immunology. 2009;132:362–70. doi: 10.1016/j.clim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis and rheumatism. 2001;44:1114–21. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan S, Juang YT, Chowdhury B, et al. Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells. Journal of immunology. 2008;181:8145–52. doi: 10.4049/jimmunol.181.11.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. The Journal of clinical investigation. 1998;101:1448–57. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyttaris VC, Wang Y, Juang YT, Weinstein A, Tsokos GC. Increased levels of NF-ATc2 differentially regulate CD154 and IL-2 genes in T cells from patients with systemic lupus erythematosus. Journal of immunology. 2007;178:1960–6. doi: 10.4049/jimmunol.178.3.1960. [DOI] [PubMed] [Google Scholar]
- 8.Deng GM, Beltran J, Chen C, Terhorst C, Tsokos GC. T cell CD3zeta deficiency enables multiorgan tissue inflammation. Journal of immunology. 2013;191:3563–7. doi: 10.4049/jimmunol.1300634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng GM, Liu L, Bahjat FR, Pine PR, Tsokos GC. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis and rheumatism. 2010;62:2086–92. doi: 10.1002/art.27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. The New England journal of medicine. 2010;363:1303–12. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Kavanaugh A, Weinblatt ME, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis: a three-month randomized, placebo-controlled, phase II study in patients with active rheumatoid arthritis that did not respond to biologic agents. Arthritis and rheumatism. 2011;63:337–45. doi: 10.1002/art.30114. [DOI] [PubMed] [Google Scholar]
- 12.Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113:3154–60. doi: 10.1182/blood-2008-07-166439. [DOI] [PubMed] [Google Scholar]
- 13.Suarez-Fueyo A, Rojas JM, Cariaga AE, et al. Inhibition of PI3Kdelta Reduces Kidney Infiltration by Macrophages and Ameliorates Systemic Lupus in the Mouse. Journal of immunology. 2014 doi: 10.4049/jimmunol.1400350. [DOI] [PubMed] [Google Scholar]
- 14.Barber DF, Bartolome A, Hernandez C, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nature medicine. 2005;11:933–5. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Harada T, Juang YT, et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. Journal of immunology. 2007;178:1938–47. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 16.Deng GM, Tsokos GC. Cholera toxin B accelerates disease progression in lupus-prone mice by promoting lipid raft aggregation. Journal of immunology. 2008;181:4019–26. doi: 10.4049/jimmunol.181.6.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jury EC, Isenberg DA, Mauri C, Ehrenstein MR. Atorvastatin restores Lck expression and lipid raft-associated signaling in T cells from patients with systemic lupus erythematosus. Journal of immunology. 2006;177:7416–22. doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 18.McDonald G, Deepak S, Miguel L, et al. Normalizing glycosphingolipids restores function in CD4+ T cells from lupus patients. The Journal of clinical investigation. 2014;124:712–24. doi: 10.1172/JCI69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crispin JC, Keenan BT, Finnell MD, et al. Expression of CD44 variant isoforms CD44v3 and CD44v6 is increased on T cells from patients with systemic lupus erythematosus and is correlated with disease activity. Arthritis and rheumatism. 2010;62:1431–7. doi: 10.1002/art.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yung S, Chan TM. Pathophysiology of the peritoneal membrane during peritoneal dialysis: the role of hyaluronan. Journal of biomedicine & biotechnology. 2011;2011:180594. doi: 10.1155/2011/180594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen RA, Bayliss G, Crispin JC, et al. T cells and in situ cryoglobulin deposition in the pathogenesis of lupus nephritis. Clinical immunology. 2008;128:1–7. doi: 10.1016/j.clim.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. The Journal of clinical investigation. 2010;120:3280–95. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman A, Theofilopoulos AN, Weiner R, Katz DH, Dixon FJ. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. The Journal of experimental medicine. 1981;154:791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcocer-Varela J, Alarcon-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. The Journal of clinical investigation. 1982;69:1388–92. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). Journal of immunology. 1983;130:2651–5. [PubMed] [Google Scholar]
- 26.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Current opinion in immunology. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–61. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 29.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–17. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 31.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 33.Klebb G, Autenrieth IB, Haber H, et al. Interleukin-2 is indispensable for development of immunological self-tolerance. Clinical immunology and immunopathology. 1996;81:282–6. doi: 10.1006/clin.1996.0190. [DOI] [PubMed] [Google Scholar]
- 34.Mizui M, Koga T, Lieberman LA, et al. IL-2 Protects Lupus-Prone Mice from Multiple End-Organ Damage by Limiting CD4-CD8-IL-17-Producing T Cells. Journal of immunology. 2014;193:2168–77. doi: 10.4049/jimmunol.1400977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. The New England journal of medicine. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. The New England journal of medicine. 2011;365:2067–77. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- 37.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Current opinion in immunology. 1995;7:333–42. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 38.Kyttaris VC, Juang YT, Tenbrock K, Weinstein A, Tsokos GC. Cyclic adenosine 5′-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. Journal of immunology. 2004;173:3557–63. doi: 10.4049/jimmunol.173.5.3557. [DOI] [PubMed] [Google Scholar]
- 39.Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. Journal of immunology. 1999;163:1682–9. [PubMed] [Google Scholar]
- 40.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. The Journal of clinical investigation. 2005;115:3193–204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. Journal of immunology. 2001;166:4216–22. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 42.Nambiar MP, Fisher CU, Warke VG, et al. Reconstitution of deficient T cell receptor zeta chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2003;48:1948–55. doi: 10.1002/art.11072. [DOI] [PubMed] [Google Scholar]
- 43.Juang YT, Wang Y, Solomou EE, et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. The Journal of clinical investigation. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichinose K, Juang YT, Crispin JC, Kis-Toth K, Tsokos GC. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis and rheumatism. 2011;63:523–9. doi: 10.1002/art.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koga T, Mizui M, Yoshida N, et al. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3 regulatory T cells in MRL/lpr mice. Autoimmunity. 2014:1–6. doi: 10.3109/08916934.2014.915954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulton VR, Tsokos GC. Alternative splicing factor/splicing factor 2 regulates the expression of the zeta subunit of the human T cell receptor-associated CD3 complex. The Journal of biological chemistry. 2010;285:12490–6. doi: 10.1074/jbc.M109.091660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moulton VR, Grammatikos AP, Fitzgerald LM, Tsokos GC. Splicing factor SF2/ASF rescues IL-2 production in T cells from systemic lupus erythematosus patients by activating IL-2 transcription. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1845–50. doi: 10.1073/pnas.1214207110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis and rheumatism. 1990;33:1665–73. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- 49.Sunahori K, Juang YT, Tsokos GC. Methylation status of CpG islands flanking a cAMP response element motif on the protein phosphatase 2Ac alpha promoter determines CREB binding and activity. Journal of immunology. 2009;182:1500–8. doi: 10.4049/jimmunol.182.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sunahori K, Juang YT, Kyttaris VC, Tsokos GC. Promoter hypomethylation results in increased expression of protein phosphatase 2A in T cells from patients with systemic lupus erythematosus. Journal of immunology. 2011;186:4508–17. doi: 10.4049/jimmunol.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Qiu X, Luo Y, et al. Histone modifications and methyl-CpG-binding domain protein levels at the TNFSF7 (CD70) promoter in SLE CD4+ T cells. Lupus. 2011;20:1365–71. doi: 10.1177/0961203311413412. [DOI] [PubMed] [Google Scholar]
- 52.Zhao M, Tang J, Gao F, et al. Hypomethylation of IL10 and IL13 promoters in CD4+ T cells of patients with systemic lupus erythematosus. Journal of biomedicine & biotechnology. 2010;2010:931018. doi: 10.1155/2010/931018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rauen T, Hedrich CM, Juang YT, Tenbrock K, Tsokos GC. cAMP-responsive element modulator (CREM)alpha protein induces interleukin 17A expression and mediates epigenetic alterations at the interleukin-17A gene locus in patients with systemic lupus erythematosus. The Journal of biological chemistry. 2011;286:43437–46. doi: 10.1074/jbc.M111.299313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coit P, Jeffries M, Altorok N, et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naive CD4+ T cells from lupus patients. Journal of autoimmunity. 2013;43:78–84. doi: 10.1016/j.jaut.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Absher DM, Li X, Waite LL, et al. Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS genetics. 2013;9:e1003678. doi: 10.1371/journal.pgen.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu N, Qiu X, Luo Y, et al. Abnormal histone modification patterns in lupus CD4+ T cells. The Journal of rheumatology. 2008;35:804–10. [PubMed] [Google Scholar]
- 57.Hedrich CM, Rauen T, Tsokos GC. cAMP-responsive element modulator (CREM)alpha protein signaling mediates epigenetic remodeling of the human interleukin-2 gene: implications in systemic lupus erythematosus. The Journal of biological chemistry. 2011;286:43429–36. doi: 10.1074/jbc.M111.299339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. The Journal of clinical investigation. 2003;111:539–52. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reilly CM, Mishra N, Miller JM, et al. Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. Journal of immunology. 2004;173:4171–8. doi: 10.4049/jimmunol.173.6.4171. [DOI] [PubMed] [Google Scholar]
- 60.Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis and rheumatism. 2009;60:1065–75. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 61.Fan W, Liang D, Tang Y, et al. Identification of microRNA-31 as a novel regulator contributing to impaired interleukin-2 production in T cells from patients with systemic lupus erythematosus. Arthritis and rheumatism. 2012;64:3715–25. doi: 10.1002/art.34596. [DOI] [PubMed] [Google Scholar]
- 62.Pan W, Zhu S, Yuan M, et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. Journal of immunology. 2010;184:6773–81. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- 63.Garchow BG, Bartulos Encinas O, Leung YT, et al. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO molecular medicine. 2011;3:605–15. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stagakis E, Bertsias G, Verginis P, et al. Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Annals of the rheumatic diseases. 2011;70:1496–506. doi: 10.1136/ard.2010.139857. [DOI] [PubMed] [Google Scholar]
- 65.Davis TE, Kis-Toth K, Tsokos GC. A114: Methylprednisolone-Induced Inhibition of miR-155 Expression Increases SOCS1-Driven Suppression of Cytokine Signaling. Arthritis & rheumatology. 2014;66(Suppl 11):S151. [Google Scholar]
- 66.Davis TE, Kis-Toth K, Szanto A, Tsokos GC. Glucocorticoids suppress T cell function by up-regulating microRNA-98. Arthritis and rheumatism. 2013;65:1882–90. doi: 10.1002/art.37966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 68.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. The New England journal of medicine. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 69.Apostolidis SA, Crispin JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20:120–4. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 70.Cheng F, Guo Z, Xu H, Yan D, Li Q. Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2009;68:604–6. doi: 10.1136/ard.2008.097089. [DOI] [PubMed] [Google Scholar]
- 71.Chen XQ, Yu YC, Deng HH, et al. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. Journal of clinical immunology. 2010;30:221–5. doi: 10.1007/s10875-009-9365-x. [DOI] [PubMed] [Google Scholar]
- 72.Zhao XF, Pan HF, Yuan H, et al. Increased serum interleukin 17 in patients with systemic lupus erythematosus. Molecular biology reports. 2010;37:81–5. doi: 10.1007/s11033-009-9533-3. [DOI] [PubMed] [Google Scholar]
- 73.Crispin JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. Journal of immunology. 2008;181:8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. Journal of immunology. 2011;187:538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. Journal of immunology. 2009;183:3160–9. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Ito S, Chino Y, et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clinical and experimental immunology. 2010;159:1–10. doi: 10.1111/j.1365-2249.2009.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nature immunology. 2012;13:991–9. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–8. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 80.Ramesh R, Kozhaya L, McKevitt K, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. The Journal of experimental medicine. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isgro J, Gupta S, Jacek E, et al. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2013;65:1592–602. doi: 10.1002/art.37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koga T, Hedrich CM, Mizui M, et al. CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. The Journal of clinical investigation. 2014;124:2234–45. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. The New England journal of medicine. 2012;366:1190–9. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 84.Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1705–13. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 85.McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Annals of the rheumatic diseases. 2014;73:349–56. doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 86.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. The New England journal of medicine. 2012;366:1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 87.Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. 2012;21:1007–10. doi: 10.1177/0961203312441982. [DOI] [PubMed] [Google Scholar]
- 88.Dahl C, Johansen C, Kragballe K, Olesen AB. Ustekinumab in the treatment of refractory chronic cutaneous lupus erythematosus: a case report. Acta dermato-venereologica. 2013;93:368–9. doi: 10.2340/00015555-1467. [DOI] [PubMed] [Google Scholar]
- 89.Crispin JC, Tsokos GC. Human TCR-alpha beta+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. Journal of immunology. 2009;183:4675–81. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7020–4. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. Journal of immunology. 1989;143:103–12. [PubMed] [Google Scholar]
- 92.Hedrich CM, Rauen T, Crispin JC, et al. cAMP-responsive element modulator alpha (CREMalpha) trans-represses the transmembrane glycoprotein CD8 and contributes to the generation of CD3+CD4-CD8- T cells in health and disease. The Journal of biological chemistry. 2013;288:31880–7. doi: 10.1074/jbc.M113.508655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hedrich CM, Crispin JC, Rauen T, et al. cAMP responsive element modulator (CREM) alpha mediates chromatin remodeling of CD8 during the generation of CD3+ CD4- CD8- T cells. The Journal of biological chemistry. 2014;289:2361–70. doi: 10.1074/jbc.M113.523605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nature reviews Immunology. 2011;11:119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. Journal of autoimmunity. 2003;21:273–6. doi: 10.1016/s0896-8411(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 96.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. Journal of immunology. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 97.Barath S, Aleksza M, Tarr T, Sipka S, Szegedi G, Kiss E. Measurement of natural (CD4+CD25high) and inducible (CD4+IL-10+) regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2007;16:489–96. doi: 10.1177/0961203307080226. [DOI] [PubMed] [Google Scholar]
- 98.Venigalla RK, Tretter T, Krienke S, et al. Reduced CD4+,CD25- T cell sensitivity to the suppressive function of CD4+,CD25high,CD127 −/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis and rheumatism. 2008;58:2120–30. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 99.Yates J, Whittington A, Mitchell P, Lechler RI, Lightstone L, Lombardi G. Natural regulatory T cells: number and function are normal in the majority of patients with lupus nephritis. Clinical and experimental immunology. 2008;153:44–55. doi: 10.1111/j.1365-2249.2008.03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Golding A, Hasni S, Illei G, Shevach EM. The percentage of FoxP3+Helios+ Treg cells correlates positively with disease activity in systemic lupus erythematosus. Arthritis and rheumatism. 2013;65:2898–906. doi: 10.1002/art.38119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vargas-Rojas MI, Crispin JC, Richaud-Patin Y, Alcocer-Varela J. Quantitative and qualitative normal regulatory T cells are not capable of inducing suppression in SLE patients due to T-cell resistance. Lupus. 2008;17:289–94. doi: 10.1177/0961203307088307. [DOI] [PubMed] [Google Scholar]
- 102.Koga T, Ichinose K, Mizui M, Crispin JC, Tsokos GC. Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. Journal of immunology. 2012;189:3490–6. doi: 10.4049/jimmunol.1201785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nature reviews Immunology. 2013;13:412–26. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X, Ing S, Fraser A, et al. Follicular helper T cells: new insights into mechanisms of autoimmune diseases. The Ochsner journal. 2013;13:131–9. [PMC free article] [PubMed] [Google Scholar]
- 105.Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–8. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 106.Schmitt N, Ueno H. Blood Tfh cells come with colors. Immunity. 2013;39:629–30. doi: 10.1016/j.immuni.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feng X, Wang D, Chen J, et al. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PloS one. 2012;7:e51982. doi: 10.1371/journal.pone.0051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 109.Terrier B, Costedoat-Chalumeau N, Garrido M, et al. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. The Journal of rheumatology. 2012;39:1819–28. doi: 10.3899/jrheum.120468. [DOI] [PubMed] [Google Scholar]
- 110.Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2010;62:3077–87. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 111.Furie R, Nicholls K, Cheng TT, et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis & rheumatology. 2014;66:379–89. doi: 10.1002/art.38260. [DOI] [PubMed] [Google Scholar]
- 112.Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. The Journal of clinical investigation. 1996;97:2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blossom S, Chu EB, Weigle WO, Gilbert KM. CD40 ligand expressed on B cells in the BXSB mouse model of systemic lupus erythematosus. Journal of immunology. 1997;159:4580–6. [PubMed] [Google Scholar]
- 114.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. The Journal of clinical investigation. 1996;98:826–37. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Early GS, Zhao W, Burns CM. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. Journal of immunology. 1996;157:3159–64. [PubMed] [Google Scholar]
- 116.Russell JQ, Mooney T, Cohen PL, MacPherson B, Noelle RJ, Budd RC. Anti-CD40L accelerates renal disease and adenopathy in MRL-lpr mice in parallel with decreased thymocyte apoptosis. Journal of immunology. 1998;161:729–39. [PubMed] [Google Scholar]
- 117.Kalunian KC, Davis JC, Jr., Merrill JT, Totoritis MC, Wofsy D, Group I-LS Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis and rheumatism. 2002;46:3251–8. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 118.Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis and rheumatism. 2003;48:719–27. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 119.Hutloff A, Buchner K, Reiter K, et al. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis and rheumatism. 2004;50:3211–20. doi: 10.1002/art.20519. [DOI] [PubMed] [Google Scholar]
- 120.Yang JH, Zhang J, Cai Q, et al. Expression and function of inducible costimulator on peripheral blood T cells in patients with systemic lupus erythematosus. Rheumatology. 2005;44:1245–54. doi: 10.1093/rheumatology/keh724. [DOI] [PubMed] [Google Scholar]
- 121.Iwai H, Abe M, Hirose S, et al. Involvement of inducible costimulator-B7 homologous protein costimulatory pathway in murine lupus nephritis. Journal of immunology. 2003;171:2848–54. doi: 10.4049/jimmunol.171.6.2848. [DOI] [PubMed] [Google Scholar]