Abstract
Importance
Persistent pain is highly prevalent, costly, and frequently disabling in later life.
Objective
To describe barriers to the management of persistent pain among older adults, summarize current management approaches, including pharmacologic and nonpharmacologic modalities; present rehabilitative approaches; and highlight aspects of the patient-physician relationship that can help to improve treatment outcomes. This review is relevant for physicians who seek an age-appropriate approach to delivering pain care for the older adult.
Evidence Acquisition
Search of MEDLINE and the Cochrane database from January 1990 through May 2014, using the search terms older adults, senior, ages 65 and above, elderly, and aged along with non-cancer pain, chronic pain, persistent pain, pain management, intractable pain, and refractory pain to identify English-language peer-reviewed systematic reviews, meta-analyses, Cochrane reviews, consensus statements, and guidelines relevant to the management of persistent pain in older adults.
Findings
Of the 92 identified studies, 35 evaluated pharmacologic interventions, whereas 57 examined nonpharmacologic modalities; the majority (n = 50) focused on older adults with osteoarthritis. This evidence base supports a stepwise approach with acetaminophen as first-line therapy. If treatment goals are not met, a trial of a topical nonsteroidal anti-inflammatory drug, tramadol, or both is recommended. Oral nonsteroidal anti-inflammatory drugs are not recommended for long-term use. Careful surveillance to monitor for toxicity and efficacy is critical, given that advancing age increases risk for adverse effects. A multimodal approach is strongly recommended–emphasizing a combination of both pharmacologic and nonpharmacologic treatments to include physical and occupational rehabilitation, as well as cognitive-behavioral and movement-based interventions. An integrated pain management approach is ideally achieved by cultivating a strong therapeutic alliance between the older patient and the physician.
Conclusions and Relevance
Treatment planning for persistent pain in later life requires a clear understanding of the patient's treatment goals and expectations, comorbidities, and cognitive and functional status, as well as coordinating community resources and family support when available. A combination of pharmacologic, nonpharmacologic, and rehabilitative approaches in addition to a strong therapeutic alliance between the patient and physician is essential in setting, adjusting, and achieving realistic goals of therapy.
The Patient's Story
Mrs L is a 90-year-old widow who has experienced persistent pain for more than 30 years. Her medical conditions include osteoarthritis, vertebral compression fractures, depression, insomnia, and hypothyroidism. She first developed left arm and leg pain in the 1970s because of a schwannoma at C6-7. She underwent 2 operations, which were successful in removing the spinal cord tumor but left her with persistent bilateral leg pain as well as lower extremity spasticity. She has had numerous falls in recent years; at least one was caused by polypharmacy, and another resulted in a wrist fracture.
Mrs L has tried many analgesics, including opioid and non-opioid medications, but these provided minimal relief or had to be stopped because of adverse effects. She has also tried antidepressants of various classes for depression and pain and benzodiazepines for insomnia, all of which were unhelpful. Baclofen and tizandidine proved ineffective for her lower extremity spasticity. She also failed to benefit from treatments not involving oral medications, including trigger point corticosteroid/local anesthetic injections, acupuncture, physical therapy, self-hypnosis, and psychotherapy, among others.
On presentation to Dr O (her geriatrician), Mrs L reported leg, shoulder, and hand pain. She also reported depressed mood, hopelessness, and passive suicidal ideation. Herpain/antidepressant regimen consisted of methadone (10 mg twice daily); hydromorphone (1-2 mg every 3 hours as needed); gabapentin (1200 mg 3 times daily); venlafaxine extended release (75 mg twice daily); and diclofenac gel as needed.
Mrs L was dependent in all basic and most instrumental activities of daily living (ADL) and lived at home with 24-hour care. Her home attendant reported that Mrs L was able to use her walker for short distances but was mostly wheelchair bound. Her2 daughters reside several hundred miles away but telephone her regularly and visit whenever possible.
On examination, Mrs L had atrophy and weakness of all extremities, a flexion contracture of the left hand, and left-sided foot drag. She required a 1-person assist to stand. Her Montreal Cognitive Assessment (MoCA) score was 20/29 (she did not attempt the cube copy), a score consistent with mild cognitive impairment. She performed most poorly on the 3 tests of executive functioning. Like the Mini Mental State Examination (for which users must now pay), the MoCA has a maximum possible score of 30. A score of 26 or greater on the MoCA is considered normal.
Perspectives
Mrs L: I can't remember a time without pain since my neck surgery. I know it's not going to go away, but I would like to have less pain. I would also really like to be able to go the bathroom by myself but right now I can't.
Dr O: Trying to maintain her function and minimize her risk for falls/injury was high on my list of goals. I wasn't sure I had much to offer in terms of pain management, as she had already tried almost every treatment I could think of.
Putting Mrs L's Care Into Context
Persistent pain is highly prevalent, costly, and frequently disabling in later life.1-6 It is most often attributable to musculoskeletal causes,7 usually involves multiple sites,8 and rarely occurs in the absence of other comorbidities.9 Based on her history and physical examination, Mrs L likely has a mixed pain disorder, ie, a combination of both nociceptive (osteoarthritis, compression fractures) and neuropathic (surgery for schwannoma) pain. Persistent pain is similar to other geriatric syndromes in that it results from accumulated impairments in multiple systems. As with other geriatric syndromes, diagnostic workups frequently fail to contribute useful information,10,11 but interventions can be effective even in the absence of a firm diagnosis.12 Consistent with other geriatric syndromes, persistent pain in older adults frequently develops via a multifactorial pathway, resulting in adverse sequelae6,13 that include poor self-reported health, decreased quality of life, and significant disability that often occurs and is attributable to falls, depression or anxiety, sleep impairment, and decreased socialization.14-16 Older patients who experience persistent pain should be routinely assessed for its effect on quality of life, gait, mood, and functioning. Dr O seeks to maintain Mrs L's present level of independence, however compromised, and if possible to restore some of the functional ability she has lost. At the same time, Dr O aims to reduce her pain, minimizing the risks of medication-related morbidity (eg, falls, delirium). Dr O's goals mirror those of the patient, ie, pain reduction, improvement in ADL functioning, and preservation of independence.
We describe barriers to the management of persistent pain among older adults, summarize pharmacologic and nonpharmacologic approaches for its management, present rehabilitative modalities that are important to consider, and highlight aspects of the patient-physician relationship that can help to improve outcomes for patients like Mrs L and others who present on the spectrum of persistent pain.
Methods
We searched MEDLINE and the Cochrane database for English-language reports of human studies published from January 1, 1990, through May 31, 2014, using the search terms older adults, senior, ages 65 and above, elderly, and aged, along with non-cancer pain, chronic pain, persistent pain, pain management, intractable pain, and refractory pain (search results are provided in the eAppendix in the Supplement). Given the paucity of randomized controlled trials focusing on older adults with persistent pain, we also included reviews, published guidelines, and consensus statements. We synthesized and graded the evidence of the retained intervention studies using a standard approach.17 Our recommendations are based on this evidence and our clinical experience.
Of the 92 studies, 35 provided results on various pharmacologic interventions to include randomized controlled trials (n = 11), meta-analyses (n = 8), reviews or guidelines (n = 4), and other types of study designs, eg, pilot or open-label studies (n = 9); 3 focused on safety outcomes. Of the 57 studies that evaluated nonpharmacologic interventions, most were randomized controlled trials (n = 26), followed by meta-analyses (n = 9) and nonrandomized pilot studies (n = 11); 11 used other types of designs (eg, crossover studies). Osteoarthritis was the target pain condition in 50 studies, 29 enrolled individuals with persistent pain attributable to a noncancer cause, 7 focused on neuropathic pain disorders, and the remaining 6 enrolled older patients with other pain problems (eg, back pain).
Barriers to Managing Pain in Later Life
Barriers to managing persistent pain specific to geriatric populations include age-related physiologic changes resulting in altered drug absorption and decreased renal excretion; sensory and cognitive impairments; polypharmacy; and multimorbidity, particularly involving chronic conditions such as cognitive impairment, gait disorders, and kidney, lung, and cardiovascular disease.18 Other barriers include a limited evidence base to guide management of pain in the older adult,18 physician concerns about the potential for treatment-related harm,19 as well as older adults' beliefs about pain and pain treatments.20 Additional attitudinal, access, and system-level barriers have been identified that also contribute to undertreated pain in vulnerable populations, including the elderly.21
This patient presents with mild cognitive impairment and significant deficits in executive functioning, the latter suggested by her poor performance on the MoCA and clinical history. Executive dysfunction is a term that encompasses a broad range of regulatory behaviors directed by the frontal lobe, including planning, organizing, problem-solving, and decision making.22 As described below, even milder forms of cognitive impairment that include executive dysfunction can be a barrier to managing pain.
Specific Management Approaches
Considering the complexity of managing the care of older patients with persistent pain, these individuals are most likely to benefit from a multidisciplinary team, including a geriatrician, rheumatologist, physical medicine and rehabilitation physician, physical and occupational therapists, and a psychiatrist or psychologist when appropriate. Two guidelines and several consensus statements provide useful information regarding the assessment and management of pain in later life.13,23-26 There is broad consensus around the need to intervene aggressively using a multimodal approach that includes both pharmacologic and nonpharmacologic treatments. This approach is supported by the fact that pain relief constitutes one of the most commonly endorsed goals of older adults.27
Collaborative care approaches have been found to be effective. One randomized clinical trial found that a collaborative multi-component intervention that included physician and patient education, activation, and symptom monitoring in targeted primary care patients with persistent pain was associated with significant improvement in pain-related disability, pain intensity, and depressive symptoms scores during a 12-month period.28
Frequently prescribed or recommended pharmacologic, non-pharmacologic, and rehabilitative approaches for managing pain in older adults are presented below. Box 1 and Box 2 summarize key aspects of the general approach to managing the care of older patients with persistent pain and highlight important considerations regarding its management. A review of interventional approaches for persistent pain in older adults is beyond the scope of this review but has been presented elsewhere.13
Box 1. Key Points Regarding Overall Approach to Management of Persistent Pain in the Older Adult.
Determine patient's comorbidities, cognitive and functional status, treatment goals and expectations, and socialand family supports prior to initiating treatment
Intervene using a multimodal approach, including pharmacologic and nonpharmacologic treatments as well as physical and occupational rehabilitation modalities
Develop and enrich therapeutic alliance between patient and physician (physician must respond promptly and reliably to patient calls and provide backup coverage when away; consider all patient input seriously; encourage hope without overpromising therapeutic success)
Be willing to revisit previously used pharmacologic and nonpharmacologic treatment modalities with indicated modifications
Involve and engage caregivers and seek out other resources (eg, community-based programs) that can help to reinforce treatment adherence and maintain treatment gains
Reinforce positive outcomes at each visit
Box 2. Key Points Regarding Pharmacologic and Nonpharmacologic Approaches.
Link potential treatment benefits with important patient goals (eg, increased ability to perform activities of daily living)
Use medication combinations (in which each analgesic works by a different mechanism) to enhance analgesic effectiveness
Acetaminophen remains first-line pharmacologic treatment for older adults with mild-to-moderate pain
Avoid long-term use of oral nonsteroidal anti-inflammatory drugs, given their significant cardiovascular, gastrointestinal, and renal risks
Trial of opioid is appropriate for patients not responsive to first-line therapies and who continue to experience significant functional impairment due to pain
Consider serotonin-norepinephrine reuptake inhibitors or selective serotonin reuptake inhibitors in patients with comorbid depression and pain
Implement surveillance plan (ie, efficacy, tolerability, adherence) with each new treatment
Physical activity (including physical therapy, exercise, or other movement-based programs such as tai chi) constitutes a core component of managing persistent pain in older patients
Educate older patients about safety and efficacy of cognitive behavioral and movement-based therapies and identify local practitioners or agencies that provide them
Determine whether treatment goals are being met; if goals are not met, medication should be tapered and discontinued, physical and occupational therapy prescription modified, or both
Pharmacologic Approaches
Mrs L: I am taking a lot of pain medication. If I miss a dose, I really start feeling the pain. When you walk around after taking medication that has morphine in it (or is stronger than morphine), you feel like a zombie.
Dr O: I tried to adjust her pain regimen earlier this year. Whether real or perceived, she had significant side effects. Her daughters and caregivers report that when she is on a number of pain medications, her cognitive function plummets; she is far less functional.
Multiple failed attempts to treat an older patient's pain in the past complicate future treatment choices. Physicians and patients must guard against an attitude of therapeutic nihilism, ie, the conviction that further treatments are not likely to yield benefit. In this case, Dr O appears committed and earnest in trying to identify a regimen that will reduce Mrs L's pain, even if that involves persuading her to revisit medications that did not help in the past. The hesitation to revisit or try different medications in this case appears to be coming from Mrs L, who has been disappointed by the results of previous attempts and finds it difficult to believe that meaningful pain relief is achievable. Negative treatment expectations–which often occur as a consequence of multiple failed analgesic trials in the past– have been shown to significantly reduce analgesic efficacy in experimental studies.29,30 Thus, negative beliefs that patients maintain about the likelihood of future therapeutic success may serve as a target for intervention efforts.29,30
The challenge for Dr O is how to instill in this patient a hopeful and expectantmind-set justified by an adequate evidence base, with-out minimizing her pain or the realistic difficulty of addressing her pain. Strategies that Dr O can use to counter Mrs L's negative treatment expectations include (1) remaining readily available to respond to questions and concerns with each intervention, (2) providing an effective backup or “rescue” pain medication during each new analgesic trial, and (3) taking the patient's reports of pain seriously, while at the same time refraining from promises about the results of treatment. Dr O can also make clear that she will not abandon her patient, emotionally or otherwise. Linking potential treatment benefits with an important patient goal (eg, increased ability to perform ADL because of reductions in pain) may also prove helpful. Last, recommending the use of nonpharmacologic treatments that emphasize a positive attitude, for example by encouraging a patient to reconnect with longstanding artistic or religious interests, may also help to neutralize the patient's feeling of hopelessness.31,32
Table 1 shows that many commonly administered analgesic medications can reduce pain.13,25,26,33-52 There are currently no specific dosing guidelines for older patients. Given that older age is associated with greater incidence of treatment-related adverse effects,26 we recommend starting at the lowest possible dose and titrating up based on tolerability and efficacy. This does not mean that physicians should “start low and stay low,” which contributes to potential undertreatment.53 Table 1 also provides information about recommended starting doses and frequency of administration.
Table 1. Efficacy and Safety Data and Guideline Recommendations Regarding Frequently Prescribed Pharmacologic Treatments for Persistent Pain in Older Adults.
| Treatment | Efficacy | Level of Evidencea | Safety Issues | Guideline Recommendations | Recommended Starting Dose(s)b |
|---|---|---|---|---|---|
| Pain Medications | |||||
| Acetaminophen/paracetamol | Reduces pain relative to placebo (effect size, 0.21 [95% CI, 0.02-0.41])33; inferior when compared with oral NSAIDs for pain reduction, stiffness, and physical functioning34 | Ia | Acetaminophen toxicity remains leading cause of acute liver failure in United States; unintentional overdose is leading cause of acetaminophen-induced hepatotoxicity35 | Recommended as first-line therapy, given excellent overall safety profile13,25,26 | 325 mg every 4 h; maximum daily dose, 3250 mg |
| Oral NSAIDs | Reduce pain (effect size, 0.32 [95% CI, 0.24-0.39]) and functional disability (effect size, 0.29 [95% CI, 0.18-0.40]) relative to placebo36 | Ia | Established gastrointestinal, renal, and cardiovascular toxicity; gastrointestinal bleeding risk increases with age; patients taking long-term therapy should be monitored closely for gastrointestinal, renal, and cardiovascular adverse effects13,26 | Should be used with caution and for the shortest time possible, given related risks; use only when other therapies have failed or continuing therapeutic goals not met13,25,26 | Naproxen sodium: 220 mg every 12 h Ibuprofen: 200 mg every 8 h Diclofenac extended release: 100 mg every 12 h Celecoxib: 100 mg every 12 h |
| Topical NSAIDs | Reduce pain (effect size, 0.41 [95% CI, 0.14-0.68]); improve physical function (effect size, 0.44 [95% CI, 0.16-0.71]) and stiffness (effect size, 0.43 [95% CI, 0.15-0.70]) relative to placebo37; equivalent to oral NSAIDs in terms of pain reduction at 1 y38 | Ib | Generally well tolerated, given lower systemic absorption; however, safety of topical NSAIDs in patients taking anticoagulation therapy or with renal impairment remains unknown39 | Consider as alternative to oral NSAIDs, particularly if pain is localized13,25,26 | Volataren gel/diclofenac gel: apply 4 g to affected area every 6 h; maximum daily dose, 32 g |
| Tramadol | Reduces pain relative to placebo (visual analog score at day 7, P = .002; day 14, P = .01). At day 14, mean decrease in pain intensity of 2.43 cm in tramadol group compared with 1.55 cm in placebo group (VAS, 0-10 cm). No difference between groups for functional index score40 | Ib | Adverse effects include constipation, nausea/vomiting, dizziness, headache, somnolence41 Potential drug interactions and increased risk of seizures or serotonin syndrome when used with antidepressants, especially monoamine oxidase inhibitors, serotonin reuptake inhibitors, and tricyclic antidepressants.42 | Monitor for adverse effects25,26 | 50 mg every night, then 25-50 mg immediate release every 6 h; maximum daily dose, 400 mg |
| Opioids | Reduce pain (effect size, 0.56; P < .001) and functional disability (effect size, 0.43; P < .002) relative to placebo43 | Ia | Established risk of fall or fracture44,45; increased risk of hospitalization relative to nonselective NSAIDs45; constipation and other adverse effects (eg, lethargy, nausea/vomiting) constitute major causes of treatment failure46 | Consider for use in older patients with moderate-to-severe pain or with substantial impairments in physical functioning or quality of life that have failed other treatments13,25,26 | Oxycodone: 2.5 mg every night, then 2.5-5.0 mg every 4-6 h Hydrodocone: 2.5 mg every night, then 2.5-5.0 mg every 4-6 h |
| Adjuvant Medications | |||||
| Tricyclic antidepressants | Amitriptyline reduces pain relative to placebo in patients with diabetic neuropathy (67% of patients reported moderate or greater pain relief)47 | Ib | Adverse effects (particularly anticholinergic and noradrenergic) limit use; QTc prolongation risk requires ECG monitoring; monitoring serum levels is recommended given substantial potential for toxicity at higher doses13,26 | Tertiary tricyclics (eg, amitriptyline, doxepin) should be avoided because of high incidence of adverse effects25,26 | Nortriptyline: 25 mg every night to start; maximum daily dose, 200 mg (if comorbid depression is present and depending on serum level) |
| Anticonvulsants | Pregabalin and gabapentin both reduce pain relative to placebo among patients with diabetic neuropathy, 25% average pain reduction for patients taking pregabalin,48 whereas 52% of patients treated with gabapentin reported moderate or greater pain relief47 | Ib | Adverse effect profile can limit use in older patients (eg, sedation, dizziness, peripheral edema); dose adjustment of gabapentin and pregabalin necessary in those with renal impairment13,26,49 | Recommended for use in older patients with neuropathic pain13,25,26 | Pregabalin: 50 mg every night, then 50 mg every 8 h; maximum daily dose, 300 mg Gabapentin: 100 mg every night, then 100 mg every 8 h; maximum daily dose, 3600 mg |
| Serotonin-norepinephrine reuptake inhibitors | Duloxetine reduces diabetic neuropathic pain (50% reduction in average pain score achieved by ≈50% of duloxetine-treated vs 26% of placebo-treated patients)50; duloxetine also superior to placebo for pain reduction and improved physical functioning in patients with knee osteoarthritis51 | Ib | Generally well tolerated, but adverse effects include hyponatremia, dizziness, abdominal pain, and nausea13,26 | Recommended for use in older patients with neuropathic pain13,25,26 | Duloxetine: 20 mg daily; maximum daily dose, 60 mg Venlafaxine: 37.5 mg daily; maximum daily dose, 300 mg (if comorbid depression is present) |
| Serotonin reuptake inhibitors | Did not identify any studies that met age criterion | NA | NA | Not recommended for use as analgesic13 | NA |
| Topical lidocaine | Among patients with osteoarthritis of the knee, at least 50% improvement in symptom severity reported by 40% for pain, by 40% for stiffness, and by 38% for increased physical functioning52 | IIb | Generally well tolerated; most commonly reported adverse effect is headache52 | Consider for use in older patients with localized neuropathic pain13,25,26 | Apply patch daily to affected area for 12-h period |
Abbreviations: ECG, electrocardiography; NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug; VAS, visual analog scale.
Level of evidence ratings: Ia, evidence from meta-analysis of randomized controlled trials; Ib, evidence from at least 1 randomized controlled trial; IIa, evidence from at least 1 controlled study without randomization; IIb, evidence from at least 1 type of quasi-experimental study; II, evidence from nonexperimental studies, such as comparative studies, correlation studies, and case-control studies.17
Dosing recommendations based on American Geriatrics Society clinical guideline26 and the author's clinical experience. Maximal ceiling doses are reported when present.
Because of its favorable safety profile, acetaminophen/paracetamol remains a first-line therapy for older adults with mild-to-moderate pain.13,26 Although the overall safety profile of the medication is excellent,26 acetaminophen toxicity remains the leading cause of acute liver failure in the United States.35 Given that acetaminophen is present in more than 600 over-the-counter and prescription products aimed at (and heavily advertised commercially for) treating conditions such as pain, fever, and the aches and pains associated with colds and flu, it is not surprising that unintentional over-dose is the leading cause of acetaminophen-induced hepatotoxicity.54 In addition, both geriatric pain management guidelines13,26 recommend that oral nonsteroidal anti-inflammatory drugs (NSAIDs) be used with caution and for the shortest time possible, given their significant cardiovascular, gastrointestinal, and renal risks (Table 1). A trial of an opioid, ideally prescribed by a single physician for continuity purposes, is reasonable in the older patient with pain that has not responded to other treatments or when significant pain-related functional impairments are present despite treatment. Risks associated with opioid use need to be weighed against the deleterious consequences of untreated or partially treated pain, which include negative emotional states, functional impairment, and decreased physical functioning.55-57 A careful surveillance plan is strongly recommended, however, to determine whether treatment goals are being met. If the goals are not met, the medication should be tapered and discontinued.13,26
Although the short-term efficacy of opioids has been established,43 data supporting their long-term use are lacking. An emerging body of evidence has documented that opioid therapy for chronic noncancer pain in older adults is associated with significant risks, including falls and fall-related injuries, hospitalization, and all-cause mortality.58-61 A recent study evaluating Medicare beneficiaries found that opioid prescribing by multiple physicians is common and associated with higher rates of hospital admissions related to opioid use.62 Given these findings, educating older patients about the risks of obtaining opioid prescriptions from multiple physicians is prudent.62
Individual use of opioids has increased dramatically during the past 15 years in all age groups63 and has been associated with increases in fatal overdoses, drug diversion, and opioid abuse or misuse.64,65 Efforts to reduce risks from opioids in older patients with persistent pain include the use of screening tools, eg, the SOAPP (Screener and Opioid Assessment for People with Pain), that can help physicians assess risk for the likelihood of patient misuse or abuse of opioids, as well as guide decisions about the extent of monitoring needed if a trial of opioids is undertaken.66 Such monitoring might extend to biological testing, eg, urine toxicology screens. Prior to prescribing opioid analgesics to older patients, physicians should be satisfied with arrangements for safe storage of the medication. As a rule, prescription of opioids for older patients with cognitive impairment should not be undertaken unless the administration of medication can be supervised by a responsible third party. Efforts to mitigate these risks at the system level include the development and implementation of prescription drug monitoring programs, ie, electronic databases that collect and distribute data on controlled substance prescribing and that are now operational in 48 states.67
In the case of Mrs L, the treatment of mood symptoms and the management of chronic pain are deeply interrelated. Dual-action antidepressants, such as the serotonin-norepinephrine reuptake inhibitors venlafaxine and duloxetine, provide a broad spectrum of neurotransmitter enhancement at moderate to high doses and for this reason are thought to have analgesic effects superior to those of traditional selective serotonin reuptake inhibitors.68 Tricyclic antidepressants have also been used to target both depression and pain and may additionally aid in sleep induction.69 However, anticholinergic adverse effects frequently limit their use in older patients.13,26 Reduction or remission of depression might favorably influence motivation to socialize or engage in physical therapy and other non-pharmacologic pain treatments.
Last, despite the favorable effect sizes for most medications listed in Table 1, important limitations of the evidence include the short-term nature of the studies (most were 12 weeks or less) and enrollment of “young-old” participants (ie, persons in their 60s or 70s) lacking significant comorbidity. The long-term safety and efficacy of frequently prescribed analgesic medications among older adults remain to be determined.
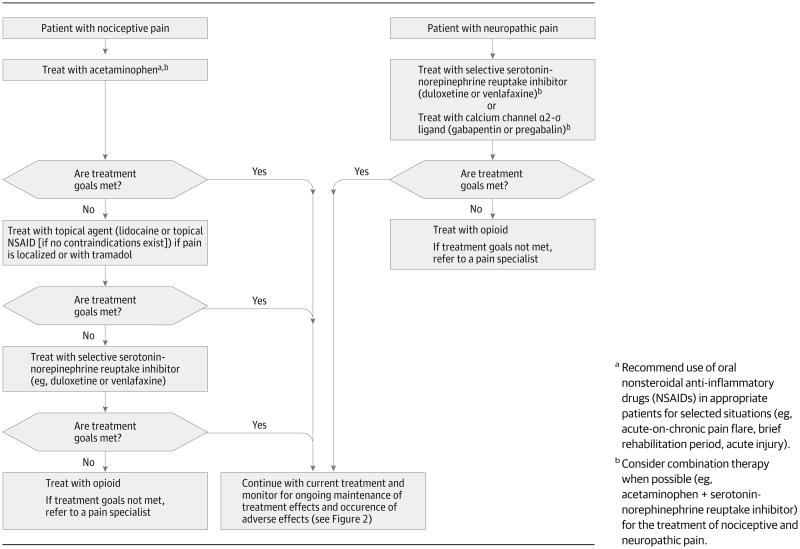
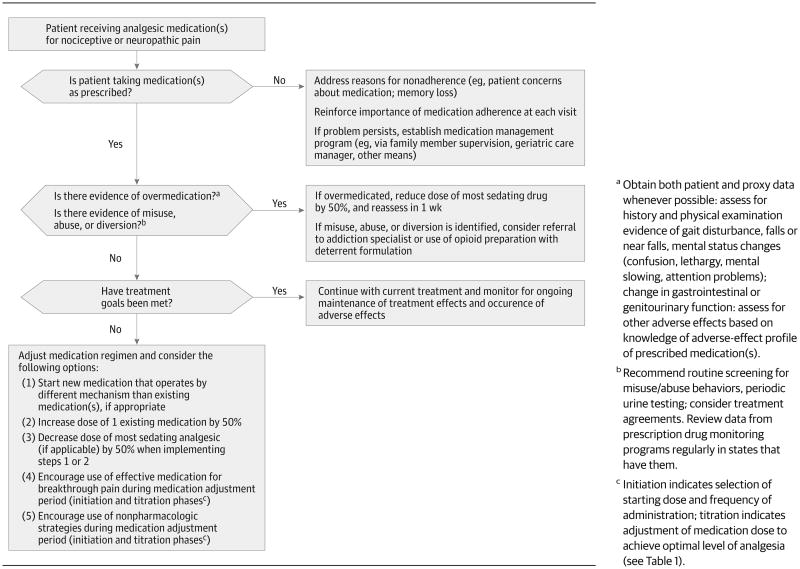
Figure 1 shows 2 treatment algorithms for older patients with persistent pain who are not currently receiving pharmacologic treatment (one for those with nociceptive pain and the other for those with neuropathic pain.) Although they have not been tested in formal evaluation studies, these approaches represent the collective clinical experience of the authors and are consonant with guidelines for the treatment of persistent pain in older adults13,26 and for adults with neuropathic pain.70 For example, for nociceptive pain, as experienced by the majority of older patients with osteoarthritis, a stepwise approach is recommended, starting with acetaminophen and, if treatments goals are not met, attempting trials of topical therapies, tramadol, or both. A trial of an opioid therapy is appropriate for older patients who do not respond to first-line therapies and for those who continue to experience significant functional impairments due to pain. Oral NSAIDs should be used with caution as a long-term therapy. Regardless of pain etiology, the use of medication combinations (in which each analgesic works by a different mechanism) is recommended because of enhanced analgesic effectiveness, often with less toxicity than is seen with use of a single agent at higher doses.26,71,72 In the present case, Mrs L is already using long- and short-acting opioids, an anticonvulsant, and an antidepressant; she is also using a topical NSAID on an as-needed basis. Despite these interventions, she reports poorly controlled pain and associated functional impairments. Figure 2 provides an approach to decision making for such patients. Assessing adherence to medication(s), development of toxicity, and achievement of therapeutic goals are key concepts in medication monitoring and adjustment and are particularly salient for older patients with persistent pain.
Figure 1. Treatment Algorithms for Nociceptive and Neuropathic Pain Disorders in Older Adults.
Figure 2. Approach for Monitoring Pain Management and Adjusting Medication for Older Adults Already Taking Analgesic Medications.
It is important to note that analgesic adverse effects occur frequently in older patients, whether the individual is receiving a first prescription or a change in medication dose or frequency of administration. Moreover, adverse effects increase in the setting of multi-morbidity, polypharmacy, and physiologic vulnerability18 and often lead patients to stop taking their pain medicationsaltogether.46,71 After initiating a new (or making a change in) analgesic therapy, careful surveillance in the form of follow-up telephone calls or e-mail is strongly recommended throughout the initiation and titration phases of treatment13,26 to address adverse effects and decrease the risk of treatment discontinuation.46 The period of surveillance will vary based on both medication and patient factors as described below. Aside from obvious toxicity or intolerance, no single algorithm currently exists to determine how much time physicians should allot before determining that a given analgesic medication is effective. Factors important to consider include the properties of the drug (eg, speed of onset of action, half-life, degree of protein-binding), individual patient characteristics (eg, severity and duration of pain, comorbidities that might predispose the patient to drug toxicity, potential for effect-enhancing or effect-dampening interactions with other analgesics taken simultaneously, protein binding capacity), and treatment goals (eg, pain reduction, improved mood, enhanced mobility). For example, the effects of oral NSAIDs and antidepressants typically require the establishment of minimum serum levels, a process consuming variable lengths of time, in contrast to some opioids that might produce pain relief more rapidly. Also, patients with more chronic or intractable pain should be given drugtrials of longer duration to avoid the premature abandonment of potentially helpful agents.
Nonpharmacologic Approaches
Mrs L: I've tried everything, including physical therapy and acupuncture, but they just have not been helpful. If I distract myself by reading or shopping, that seems to help some.
Dr O: I definitely think about nonpharmacologic approaches in these patients. I have tried a number of times, without success, to get her to contact the local integrative medicine clinic to learn nonpharmacologic techniques that may help her better manage pain.
Interest in the role of nonpharmacologic approaches for managing pain in older adults is increasing.73 Reasons for this interest include patient and physician concerns about the potential for drug-related adverse events and physician concerns about drug-drug interactions in the setting of polypharmacy.18 Many nonpharmacologic approaches involve cognitive techniques (eg, distraction), behavioral techniques (eg, goal setting, exercise), or both, that constitute well-established methods for treating pain.25 Existing evidence suggests that these therapies are safe, can reduce pain, and in many cases improve functioning (Table 2).13,17,25,26,74-82 However, almost all studies of nonpharmacologic interventions conducted to date have been short-term (less than 6 months). The long-term efficacy of nonpharmacologic interventions and the ability of older adults to sustain their use over time remain inadequately determined. However, even temporary relief may offer an opportunity for development of positive expectations and commitments that can be reinforced by the physician.
Table 2. Efficacy and Safety Data and Guideline Recommendations Regarding Common Nonpharmacologic Treatments for Persistent Pain in Older Adults.
| Treatment | Efficacy | Level of Evidencea | Safety Issues | Guideline Recommendations |
|---|---|---|---|---|
| General nonpharmacologic approaches | ||||
| Cognitive-behavioral therapy | Reduces pain (effect size, 0.47; P < .01) and improves physical functioning (effect size, 0.15, P < .05)74 | IIb | Did not report on any safety issues74 | Recommended for use by older patients, provided therapy is delivered by a professional13,25,26 |
| Acupuncture | Reduces pain (standardized mean difference, −0.35 [95% CI, −0.14 to −0.55]) and functional disability (−0.35 [95% CI, −0.15 to −0.56]) relative to sham controls75 | Ia | No serious adverse events reported; minor adverse events include bruising and bleeding at needle insertion sites75 | Consider use in older patients as an adjunctive therapy13,25,26 |
| Mindfulness meditation | Reduces pain and disability and improved psychological function among patients with chronic back pain but not relative to attention control group76 | Ib | No adverse events reported76 | Limited/weak evidence supporting use13,25,26 |
| Massage | Reduces pain (effect size, 0.96) and stiffness (effect size, 0.31) and improves functioning (effect size, 0.74) relative to attention control group in patients with osteoarthritis77 | Ib | No serious adverse events reported77 | Consider use in older patients as an adjunctive therapy13,25,26 |
| Self-management education programs | Reduces pain (effect size, −0.06 [95% CI, −0.02 to −0.10]) and improves functioning (−0.06 [95% CI, −0.02 to −0.10]) relative to controls in older patients with osteoarthritis78 | Ia | No serious adverse events reported78 | Recommended that older adults participate in programs by US25,26 (but not British13) guideline |
| Movement-based approaches | ||||
| Exercise | Reduces pain relative to usual care or attention control, effect size range, 0.25 to 2.75 in older patients with osteoarthritis of the knee79; improves physical functioning and self efficacy in older patients with osteoarthritis80 | Ib | Did not report on safety issues79,80 | Strong recommendation that physical activity program be considered; exercise program should involve strengthening, flexibility, endurance, and balance strategies13,25,26 |
| Tai chi | Reduces pain (standardized mean difference, −0.86 [95% CI, −1.19 to −0.39]), physical disability (standardized mean difference, −0.86 [95% CI, −1.20 to −0.53]), and joint stiffness (standardized mean difference, −0.53 [95% CI, −0.99 to −0.08]) among patients with osteoarthritis81 | IIb | No serious adverse events reported; minor adverse events include muscle soreness and increased joint pain81 | Consider for use in older patients, if delivered appropriately13 |
| Yoga | Reduces pain and improved physical function in pretest vs posttest comparisons82 among older patients with osteoarthritis of diverse joints | III | None reported82 | Consider for use in older patients, if delivered appropriately13 |
Level of evidence ratings: Ia, evidence from meta-analysis of randomized controlled trials; Ib, evidence from at least 1 randomized controlled trial; IIa, evidence from at least 1 controlled study without randomization; IIb, evidence from at least 1 type of quasi-experimental study; II, evidence from nonexperimental studies, such as comparative studies, correlation studies, and case-control studies; and III, evidence from nonexperimental descriptive studies.17
Mrs L's pain treatment regimen (like that of many older patients) consists predominantly, if not exclusively, of pharmacotherapies. At the time of her visit to Dr O, she is taking 3 scheduled and 2 as-needed pain medications. Dr O's focus on trying to engage Mrs L in the use of nonpharmacologic approaches is clearly appropriate. Communicating to older patients that using nondrug as well as drug therapies is the standard of care can be productive, particularly with individuals reluctant to engage in nonpharmacologic treatments. Recommending a specific nonpharmacologic modality will depend on its availability and affordability (eg, Medicare does not cover many of the approaches listed in Table 2), patient preferences, and the physician's ability to accurately describe its benefits and risks. Because there are no head-to-head comparisons evaluating the nonpharmacologic approaches shown in Table 2, recommending a modality that is accessible and affordable is prudent. Given Mrs L's level of functional deficits and goal of improving her ability to toilet independently, directing her to a movement-based therapy could prove rewarding.
Motivating the older patient with comorbid pain and depression to engage in a particular treatment is often difficult, particularly if the patient holds out little hope of future benefit. Although factors influencing the motivational level of any individual patient are complex, certain factors, if addressed, may help to improve treatment outcomes. For example, when discussing a management plan, the physician could explain the benefits of the recommended intervention(s), reemphasizing the benefits at each subsequent visit. Similarly, the physician can reinforce patient successes at each visit. The management program can be tailored to the individual's preferences and abilities; preferably, the patient has identified aspects of the program that are most desirable, realistic, and feasible, thus increasing likelihood of adherence. To motivate patients to adhere to a management program, the environment in which the older patient engages in a therapeutic activity should be appropriate and accessible. To reinforce treatment initiation and maintenance, the physician can leverage social supports in the form of family members, home attendants, and community-based agencies.
In her interview Dr O emphasized MrsL's executive function deficits as a challenge in her care. Mrs L's executive dysfunction represents one partial explanation for the failure of some prior nondrug interventions, most of which required substantial levels of cognitive effort and motivation. Managing pain by redirecting one's attention, suppressing ruminative thoughts about pain and countering feelings of hopelessness, or even exercising requires an ability to self-regulate, essentially an executive function.83 Deficits in executive functioning are therefore likely to limit an individual's ability to engage in behavioral techniques, such as distraction or redirection, that could help to moderate pain.
Problem-solving therapy84 is a form of cognitive-behavioral therapy that teaches individuals how to address and solve problems encountered in everyday life and improves outcomes among older adults with comorbid arthritis and depression85 as well as those with depression and executive dysfunction.86 Problem-solving therapy may prove especially effective in the treatment of persistent pain, by redirecting cognitive focus away from the pain.87 Problem-solving therapy appears to hold particular promise for older patients, who, like Mrs L, have the “triad” of persistent pain, depression, and executive dysfunction.
Because Mrs L has acknowledged the benefits of distraction as a way of managing her pain, efforts to encourage her engagement in pleasurable activities (eg, reading, listening to music, shopping for clothes) are indicated. Educating her paid and family caregivers to reinforce and support her engagement in these activities is indicated.
Dr O also identified social isolation as an important problem for Mrs L. Reinforcing the importance of socialization as a pain management technique to both the patient and her caregivers also seems promising.88 This recommendation could take the form of encouraging more frequent communication with her daughters and grandchildren by way of telephone or Skype.89 Dr O could also search the Eldercare Locator,90 which provides information about local social support services for older adults to find programs (eg, Internet chat groups for seniors91) that may provide benefit. Last, encouraging participation in group-based activities (eg, chair yoga, music appreciation classes) at a senior center or other agency might help to reduce her social isolation, pain, and functional impairment.92
Rehabilitative Approaches
Mrs L: My balance is poor, I can take a few steps and then all of a sudden my leg will drag.
Dr O: Her walking is poor; she is at high risk for a recurrent fall and associated injury.
Addressing function and fall risk is critically important for all older patients, particularly for those with persistent pain.93 Rehabilitative therapies, including physical therapy and occupational therapy, can help Mrs L maintain and possibly enhance her current functional status. Geriatric pain management guidelines13,25 recommend that all older patients with persistent pain adopt physical activity regimens that include strengthening, flexibility, balance, and endurance exercises.94-96Given Mrs L's significant mobility impairments, she likely qualifies for home-based physical and occupational therapy. Physical and occupational therapists can help patients like Mrs L to implement individualized home-based treatment programs.97 Although Mrs L stated that prior physical therapy courses were not helpful, home-based98 services directed at improving her safety and mobility could potentially facilitate involvement in out-of-home activities; these services might indirectly yield dividends such as more time spent socializing with family and friends. In support of this approach, a recent clinical review highlights the critical role physical therapists can play in developing “function-enhancing interventions” in older adults with mobility limitations.99
In addition, physical therapists can conduct an inventory of existing equipment (eg, does the patient have a properly functioning walker?) and make recommendations about new assistive/mobility devices.100 Occupational therapists can directly observe a patient's ADL functioning in the home and make recommendations about assistive devices that may help to improve ADL functioning. Both paid and family caregivers, especially of older adults with cognitive impairment,101 can reinforce patients' ongoing use of rehabilitative techniques and should be engaged and empowered to do so.101 Last, physical and occupational therapists can also train caregivers to reinforce concepts learned during treatment sessions, including coaching on fall risk, safety, body mechanics, and pacing.
Pain and the Patient-Physician Relationship
Mrs L: The doctors I like recognize I have a problem that is not going to go away and don't sugar coat things.
Dr O: I view my practice as a resource for Mrs L, as a place where she can call for help and get reassurance when needed, and receive guidance in how to take her pain medications so she does not end up back in the hospital.
The patient-physician relationship lies at the center of treatment for patients with persistent pain. Therapeutic alliance, defined most simply as a constructive collaboration between the patient and physician,102 has been impressively enduring in the case of Mrs L and Dr O, surviving the failure of prior attempts to alleviate Mrs L's pain. Although few studies have addressed the specific contribution of the therapeutic alliance to treatment outcomes in patients with pain,103 a positive patient-physician relationship has been associated with improved treatment outcomes among patients receiving general104 and rehabilitative105 medical care. Devoting the time to establish mutually agreed-on treatment goals is an important step in building a therapeutic alliance.106 Other core elements of the therapeutic alliance include (1) setting realistic expectations about what can and cannot be accomplished, taking into account such immutable factors as patient age, etiology, and duration of the pain; (2) availability of the physician for advice, reassurance, and support during pain flares; (3) tenacity and commitment on the part of both physician and patient; (4) mutual respect; and (5) a reciprocal bond generated by both parties' having an emotional investment in the outcomes of treatment.106-109
Conclusions
Formulating an effective treatment plan for older patients with persistent pain requires a clear understanding of their comorbidities, cognitive and functional status, treatment goals and expectations, and resources, including both social and family supports (Box 1). A multimodal approach that includes both pharmacologic and nonpharmacologic interventions is recommended and supported by the literature. The use of 2 or more analgesic medications with complementary mechanisms of action, as opposed to higher doses of a single pain medication, may lead to greater relief of pain with less toxicity (Box 2). Referring patients for specific nonpharmacologic modalities that are accessible and affordable is recommended. Although prior rehabilitation interventions were not successful in relieving Mrs L's pain, a fresh approach that involves seeking out home-based physical therapy and occupational therapy services, encouraging the use of nonpharmacologic approaches, and engaging paid and family caregivers in these efforts should be presented to the patient as a comprehensive new trial. Reinforcement of the regimen based on methods of managing executive dysfunction, including cues for steps in treatment; rehearsing choices, planning, and sequencing; encouraging self-monitoring; and problem-solving with the physician (and family members) may yield additional benefits with respect to relief of pain that the patient expressly desires. In addition, the physician should convey a conviction that the efforts of the patient herself matter, and that the energy she invests in active participation in treatment–for example, in rehabilitation exercises–will be rewarded, if not in guaranteed reductions in her pain level, then at least in better functioning or improvements in other important outcomes. Last, cultivating an enduring, trusting therapeutic alliance in which mutual treatment goals are established may be one of the most rewarding aspects of delivering (for the physician) and receiving (for the older patient) longitudinal pain care.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by funding from The SCAN Foundation. Dr Makris was supported by a National Institute on Aging GEMSSTAR grant (R03AG040653) and a pilot grant award from the Cornell Translational Research Institute for Pain in Later Life (P30AG022845); she is currently supported by the Rheumatology Research Foundation/ASP Junior Career Development Award in Geriatric Medicine and by Center for Translational Medicine, National Institutes of Health/National Center for Advancing Translational Sciences grants KL2TR001103 and UL1TR001105. Dr Gurland is supported by the Columbia University Stroud Center. Dr Reid is supported by grants from the National Institute on Aging (P30AG022845) and Agency for Healthcare Quality and Research (R01HS020648). Dr Abrams and Dr Reid receive support from the Howard and Phyllis Schwartz Philanthropic Fund.
Role of the Funders/Sponsors: The funders/sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Reid reported serving as a consultant to Endo Pharmaceuticals. No other authors reported disclosures.
Care of the Aging Patient Series: Authors interested in contributing Care of the Aging Patient articles may contact the section editor Dr Livingston at edward.livingston@jamanetwork.org.
Care of the Aging Patient: From Evidence to Action is produced and edited at the University of California, San Francisco, by Kenneth Covinsky, MD, Louise Walter, MD, Louise Aronson, MD, MFA, and Anna Chang, MD; Amy J. Markowitz, JD, is managing editor.
References
- 1.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89(2-3):127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 2.Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248–1252. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 3.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17(3):417–431. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 5.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 6.Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154(12):2649–2657. doi: 10.1016/j.pain.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Buchman AS, Shah RC, Leurgans SE, Boyle PA, Wilson RS, Bennett DA. Musculoskeletal pain and incident disability in community-dwelling older adults. Arthritis Care Res (Hoboken) 2010;62(9):1287–1293. doi: 10.1002/acr.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitson HE, Landerman LR, Newman AB, Fried LP, Pieper CF, Cohen HJ. Chronic medical conditions and the sex-based disparity in disability: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65(12):1325–1331. doi: 10.1093/gerona/glq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou R, Qaseem A, Snow V, et al. Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824–835. doi: 10.1016/j.pain.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Abdulla A, Adams N, Bone M, et al. British Geriatric Society. Guidance on the management of pain in older people. Age Ageing. 2013;42(suppl 1):i1–i57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 14.Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51(8):1092–1098. doi: 10.1046/j.1532-5415.2003.51357.x. [DOI] [PubMed] [Google Scholar]
- 15.Leveille SG, Ling S, Hochberg MC, et al. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med. 2001;135(12):1038–1046. doi: 10.7326/0003-4819-135-12-200112180-00007. [DOI] [PubMed] [Google Scholar]
- 16.Leveille SG, Bean J, Ngo L, McMullen W, Guralnik JM. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain. 2007;128(1-2):69–77. doi: 10.1016/j.pain.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318(7183):593–596. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: identifying the research gaps and methods to address them. Pain Med. 2011;12(9):1336–1357. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitz A, Moore AA, Papaleontiou M, Granieri E, Turner BJ, Reid MC. Primary care providers' perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC Geriatr. 2011;11:35. doi: 10.1186/1471-2318-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thielke S, Sale J, Reid MC. Aging: are these 4 pain myths complicating care? J Fam Pract. 2012;61(11):666–670. [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor AL, Gostin LO, Pagonis KA. Ensuring effective pain treatment: a national and global perspective. JAMA. 2008;299(1):89–91. doi: 10.1001/jama.2007.25. [DOI] [PubMed] [Google Scholar]
- 22.Luria AR. Higher Cortical Functions in Man. Oxford, England: Basic Books; 1966. [Google Scholar]
- 23.Hadjistavropoulos T, Herr K, Turk DC, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. 2007;23(1 suppl):S1–S43. doi: 10.1097/AJP.0b013e31802be869. [DOI] [PubMed] [Google Scholar]
- 24.Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8(4):287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 25.AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50(6 suppl):S205–S224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 26.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 27.Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171(20):1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301(12):1242–1252. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 29.Bingel U, Wanigasekera V, Wiech K, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 30.Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. The effect of treatment history on therapeutic outcome: an experimental approach. JAMA Intern Med. 2013;173(15):1468–1469. doi: 10.1001/jamainternmed.2013.6705. [DOI] [PubMed] [Google Scholar]
- 31.Johnson CM, Sullivan-Marx EM. Art therapy: using the creative process for healing and hope among African American older adults. Geriatr Nurs. 2006;27(5):309–316. doi: 10.1016/j.gerinurse.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Hicks TJ., Jr Spirituality and the elderly: nursing implications with nursing home residents. Geriatr Nurs. 1999;20(3):144–146. doi: 10.1016/s0197-4572(99)70006-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63(8):901–907. doi: 10.1136/ard.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;1(1):CD004257. doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson AM, Polson J, Fontana RJ, et al. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 36.Bjordal JM, Ljunggren AE, Klovning A, Slørdal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;329(7478):1317. doi: 10.1136/bmj.38273.626655.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886] BMC Musculoskelet Disord. 2005;6(1):44. doi: 10.1186/1471-2474-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underwood M, Ashby D, Cross P, et al. TOIB Study Team. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomised controlled trial and patient preference study. BMJ. 2008;336(7636):138–142. doi: 10.1136/bmj.39399.656331.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makris UE, Kohler MJ, Fraenkel L. Adverse effects of topical nonsteroidal anti inflammatory drugs in older adults with osteoarthritis: a systematic literature review. J Rheumatol. 2010;37(6):1236–1243. doi: 10.3899/jrheum.090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malonne H, Coffiner M, Sonet B, Sereno A, Vanderbist F. Efficacy and tolerability of sustained-release tramadol in the treatment of symptomatic osteoarthritis of the hip or knee: a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2004;26(11):1774–1782. doi: 10.1016/j.clinthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Malonne H, Coffiner M, Fontaine D, et al. Long-term tolerability of tramadol LP, a new once-daily formulation, in patients with osteoarthritis or low back pain. J Clin Pharm Ther. 2005;30(2):113–120. doi: 10.1111/j.1365-2710.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 42.Sansone RA, Sansone LA. Tramadol: seizures, serotonin syndrome, and coadministered antidepressants. Psychiatry (Edgmont) 2009;6(4):17–21. [PMC free article] [PubMed] [Google Scholar]
- 43.Papaleontiou M, Henderson CR, Jr, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(7):1353–1369. doi: 10.1111/j.1532-5415.2010.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25(4):310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170(22):1968–1976. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 46.Reid MC, Henderson CR, Jr, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11(7):1063–1071. doi: 10.1111/j.1526-4637.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159(16):1931–1937. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 48.Boyle J, Eriksson MEV, Gribble L, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451–2458. doi: 10.2337/dc12-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fine PG. Treatment guidelines for the pharmacological management of pain in older persons. Pain Med. 2012;13(suppl 2):S57–S66. doi: 10.1111/j.1526-4637.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Burch F, Codding C, Patel N, Sheldon E. Lidocaine patch 5% improves pain, stiffness, and physical function in osteoarthritis pain patients: a prospective, multicenter, open-label effectiveness trial. Osteoarthritis Cartilage. 2004;12(3):253–255. doi: 10.1016/j.joca.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Hanlon JT, Backonja M, Weiner D, Argoff C. Evolving pharmacological management of persistent pain in older persons. Pain Med. 2009;10(6):959–961. doi: 10.1111/j.1526-4637.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 54.Shega JW, Morrissey MB, Reid MC. From Publication to Practice: An Interdisciplinary Look at Labeling Changes for Acetaminophen and the Implications for Patient Care. [Accessed July 21, 2014];LoPDF website. 2011 http://www.lopdf.net/preview/wXPKBoFu5y_YgnBowdaQG8s66T0OaRYmAOFX6YSB3ek,/An-interdisciplinary-look-at-labeling-changes-for.html?query=OTC-Acetaminophen-Products-Use-in-Children.
- 55.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 56.Leveille SG, Fried L, Guralnik JM. Disabling symptoms: what do older women report? J Gen Intern Med. 2002;17(10):766–773. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60(6):793–797. doi: 10.1093/gerona/60.6.793. [DOI] [PubMed] [Google Scholar]
- 58.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979–1986. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 59.Buckeridge D, Huang A, Hanley J, et al. Risk of injury associated with opioid use in older adults. J Am Geriatr Soc. 2010;58(9):1664–1670. doi: 10.1111/j.1532-5415.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- 60.Miller M, Stürmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59(3):430–438. doi: 10.1111/j.1532-5415.2011.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331–342. doi: 10.1016/j.amjopharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. doi: 10.1136/bmj.g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olfson M, Wang S, Iza M, Crystal S, Blanco C. National trends in the office-based prescription of schedule II opioids. J Clin Psychiatry. 2013;74(9):932–939. doi: 10.4088/JCP.13m08349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beletsky L, Rich JD, Walley AY. Prevention of fatal opioid overdose. JAMA. 2012;308(18):1863–1864. doi: 10.1001/jama.2012.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inciardi JA, Surratt HL, Cicero TJ, Beard RA. Prescription opioid abuse and diversion in an urban community: the results of an ultrarapid assessment. Pain Med. 2009;10(3):537–548. doi: 10.1111/j.1526-4637.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112(1-2):65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 67.National Alliance for Model State Drug Laws (NAMSDL). Status of State Prescription Drug Monitoring Programs (PDMPs) [Accessed June 9, 2014];NAMSDL website. 2014 http://www.namsdl.org/library/1E4808C8-1372-636C-DD0293F829471A7E/
- 68.Trivedi MH. The link between depression and physical symptoms. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 1):12–16. [PMC free article] [PubMed] [Google Scholar]
- 69.Maizels M, McCarberg B. Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician. 2005;71(3):483–490. [PubMed] [Google Scholar]
- 70.Dworkin RH, O'Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varrassi G, Müller-Schwefe G, Pergolizzi J, et al. Pharmacological treatment of chronic pain–the need for CHANGE. Curr Med Res Opin. 2010;26(5):1231–1245. doi: 10.1185/03007991003689175. [DOI] [PubMed] [Google Scholar]
- 72.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 73.Park J, Hughes AK. Nonpharmacological approaches to the management of chronic pain in community-dwelling older adults: a review of empirical evidence. J Am Geriatr Soc. 2012;60(3):555–568. doi: 10.1111/j.1532-5415.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- 74.Lunde LH, Nordhus IH, Pallesen S. The effectiveness of cognitive and behavioural treatment of chronic pain in the elderly: a quantitative review. J Clin Psychol Med Settings. 2009;16(3):254–262. doi: 10.1007/s10880-009-9162-y. [DOI] [PubMed] [Google Scholar]
- 75.Manheimer E, Linde K, Lao L, Bouter LM, Berman BM. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. 2007;146(12):868–877. doi: 10.7326/0003-4819-146-12-200706190-00008. [DOI] [PubMed] [Google Scholar]
- 76.Morone NE, Rollman BL, Moore CG, Li Q, Weiner DK. A mind-body program for older adults with chronic low back pain: results of a pilot study. Pain Med. 2009;10(8):1395–1407. doi: 10.1111/j.1526-4637.2009.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perlman AI, Sabina A, Williams AL, Njike VY, Katz DL. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533–2538. doi: 10.1001/archinte.166.22.2533. [DOI] [PubMed] [Google Scholar]
- 78.Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143(6):427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 79.Focht BC. Effectiveness of exercise interventions in reducing pain symptoms among older adults with knee osteoarthritis: a review. J Aging Phys Act. 2006;14(2):212–235. doi: 10.1123/japa.14.2.212. [DOI] [PubMed] [Google Scholar]
- 80.Levy SS, Macera CA, Hootman JM, et al. Evaluation of a multi-component group exercise program for adults with arthritis: Fitness and Exercise for People with Arthritis (FEPA) Disabil Health J. 2012;5(4):305–311. doi: 10.1016/j.dhjo.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Kang JW, Lee MS, Posadzki P, Ernst E. T'ai chi for the treatment of osteoarthritis: a systematic review and meta-analysis. BMJ Open. 2011;1(1):e000035. doi: 10.1136/bmjopen-2010-000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park J, McCaffrey R, Dunn D, Goodman R. Managing osteoarthritis: comparisons of chair yoga, Reiki, and education (pilot study) Holist Nurs Pract. 2011;25(6):316–326. doi: 10.1097/HNP.0b013e318232c5f9. [DOI] [PubMed] [Google Scholar]
- 83.Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: regulatory depletion patterns. J Pers Soc Psychol. 1998;74(3):774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- 84.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist. 2008;48(3):311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 85.Lin EH, Katon W, Von Korff M, et al. IMPACT Investigators. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290(18):2428–2429. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 86.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011;68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karp JF, Rollman BL, Reynolds CF, III, et al. Addressing both depression and pain in late life: the methodology of the ADAPT study. Pain Med. 2012;13(3):405–418. doi: 10.1111/j.1526-4637.2011.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroenke CH, Kwan ML, Neugut AI, et al. Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat. 2013;139(2):515–527. doi: 10.1007/s10549-013-2477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gueldner SH, Smith CA, Neal M, et al. Patterns of telephone use among nursing home residents. J Geronol Nurs. 2001;27(5):35–41. doi: 10.3928/0098-9134-20010501-09. [DOI] [PubMed] [Google Scholar]
- 90.Eldercare Locator. US Administration on Aging website. [Accessed July 25, 2014]; http://www.eldercare.gov/Eldercare.NET/Public/Index.aspx.
- 91.Nimrod G. Seniors' online communities: a quantitative content analysis. Gerontologist. 2010;50(3):382–392. doi: 10.1093/geront/gnp141. [DOI] [PubMed] [Google Scholar]
- 92.Tobias KR, Lama SD, Parker SJ, Henderson CR, Jr, Nickerson AJ, Reid MC. Meeting the public health challenge of pain in later life: what role can senior centers play? Pain Manag Nurs. doi: 10.1016/j.pmn.2013.07.013. published online October 19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214–2221. doi: 10.1001/jama.2009.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jamtvedt G, Dahm KT, Christie A, et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys Ther. 2008;88(1):123–136. doi: 10.2522/ptj.20070043. [DOI] [PubMed] [Google Scholar]
- 95.Walsh NE, Mitchell HL, Reeves BC, Hurley MV. Integrated exercise and self-management programmes in osteoarthritis of the hip and knee: a systematic review of effectiveness. Phys Ther Rev. 2006;11(4):289–297. [Google Scholar]
- 96.Hasegawa R, Islam MM, Nasu E, et al. Effects of combined balance and resistance exercise on reducing knee pain in community-dwelling older adults. Phys Occup Ther Geriatr. 2010;28(1):44–56. [Google Scholar]
- 97.Whitney SL, Marchetti GF, Ellis JL, Otis L. Improvements in balance in older adults engaged in a specialized home care falls prevention program. J Geriatr Phys Ther. 2013;36(1):3–12. doi: 10.1519/JPT.0b013e3182550ea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jerant AF, von Friederichs-Fitzwater MM, Moore M. Patients' perceived barriers to active self-management of chronic conditions. Patient Educ Couns. 2005;57(3):300–307. doi: 10.1016/j.pec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Brown CJ, Flood KL. Mobility limitation in the older patient: a clinical review. JAMA. 2013;310(11):1168–1177. doi: 10.1001/jama.2013.276566. [DOI] [PubMed] [Google Scholar]
- 100.Steultjens EM, Dekker J, Bouter LM, Jellema S, Bakker EB, van den Ende CH. Occupational therapy for community dwelling elderly people: a systematic review. Age Ageing. 2004;33(5):453–460. doi: 10.1093/ageing/afh174. [DOI] [PubMed] [Google Scholar]
- 101.Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ. 2000;321(7269):1107–1111. doi: 10.1136/bmj.321.7269.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bordin ES. The generalizability of the psychoanalytic concept of the working alliance. Psychother Theor Res Pract. 1979;16(3):252–260. [Google Scholar]
- 103.Ferreira PH, Ferreira ML, Maher CG, Refshauge KM, Latimer J, Adams RD. The therapeutic alliance between clinicians and patients predicts outcome in chronic low back pain. Phys Ther. 2013;93(4):470–478. doi: 10.2522/ptj.20120137. [DOI] [PubMed] [Google Scholar]
- 104.Thom DH, Kravitz RL, Bell RA, Krupat E, Azari R. Patient trust in the physician: relationship to patient requests. Fam Pract. 2002;19(5):476–483. doi: 10.1093/fampra/19.5.476. [DOI] [PubMed] [Google Scholar]
- 105.Schönberger M, Humle F, Teasdale TW. Subjective outcome of brain injury rehabilitation in relation to the therapeutic working alliance, client compliance and awareness. Brain Inj. 2006;20(12):1271–1282. doi: 10.1080/02699050601049395. [DOI] [PubMed] [Google Scholar]
- 106.Frantsve LM, Kerns RD. Patient-provider interactions in the management of chronic pain: current findings within the context of shared medical decision making. Pain Med. 2007;8(1):25–35. doi: 10.1111/j.1526-4637.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 107.Dorflinger L, Kerns RD, Auerbach SM. Providers' roles in enhancing patients' adherence to pain self management. Transl Behav Med. 2013;3(1):39–46. doi: 10.1007/s13142-012-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nordin C, Gard G, Fjellman-Wiklund A. Being in an exchange process: experiences of patient participation in multimodal pain rehabilitation. J Rehabil Med. 2013;45(6):580–586. doi: 10.2340/16501977-1136. [DOI] [PubMed] [Google Scholar]
- 109.Teh CF, Karp JF, Kleinman A, Reynolds CF, Iii, Weiner DK, Cleary PD. Older people's experiences of patient-centered treatment for chronic pain: a qualitative study. Pain Med. 2009;10(3):521–530. doi: 10.1111/j.1526-4637.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.