Abstract
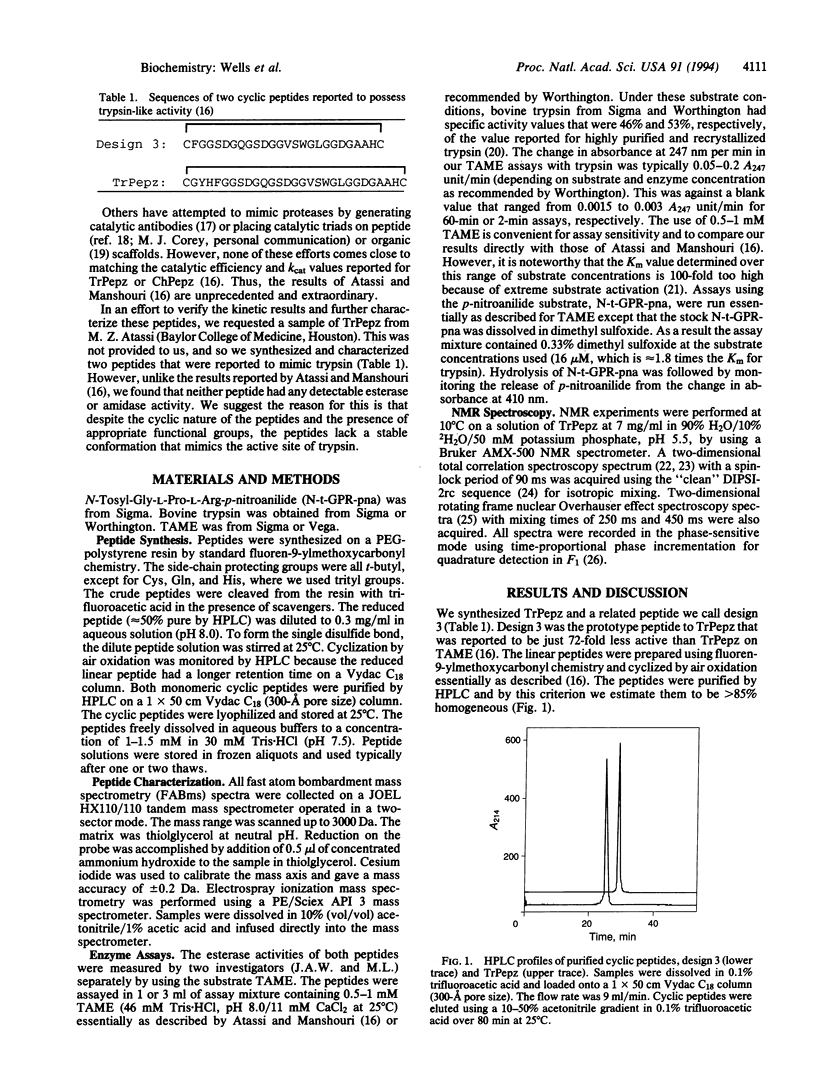
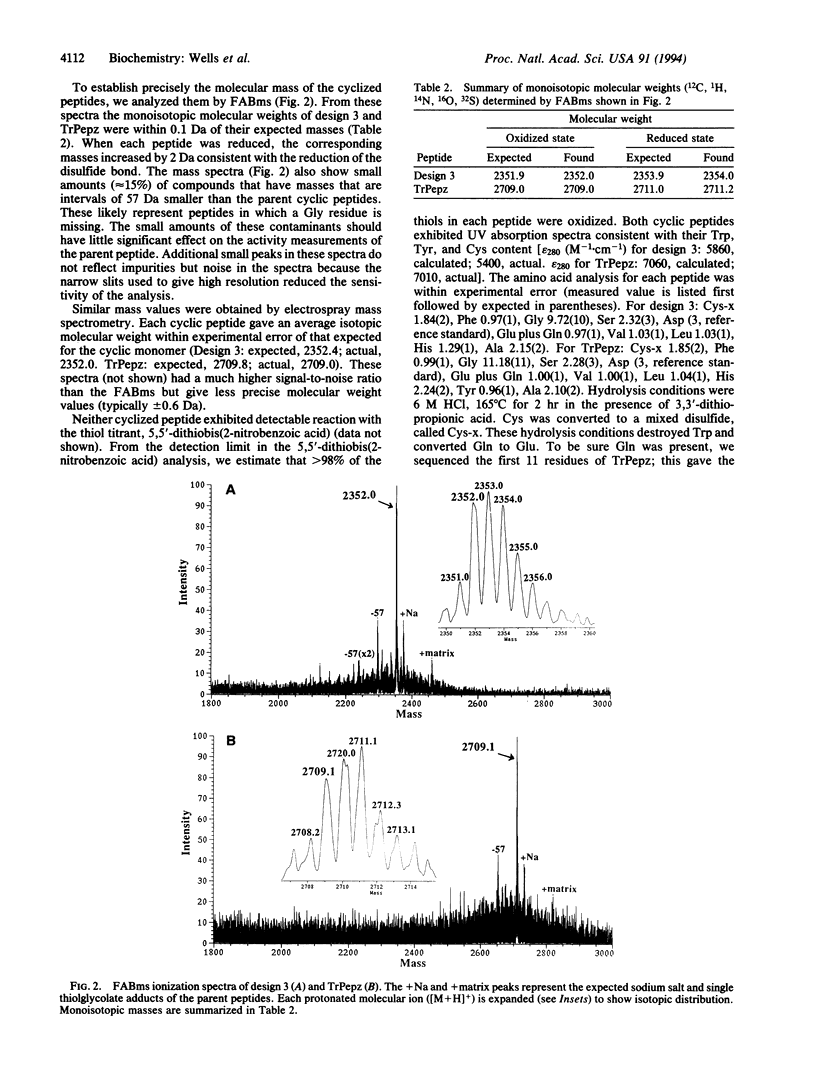
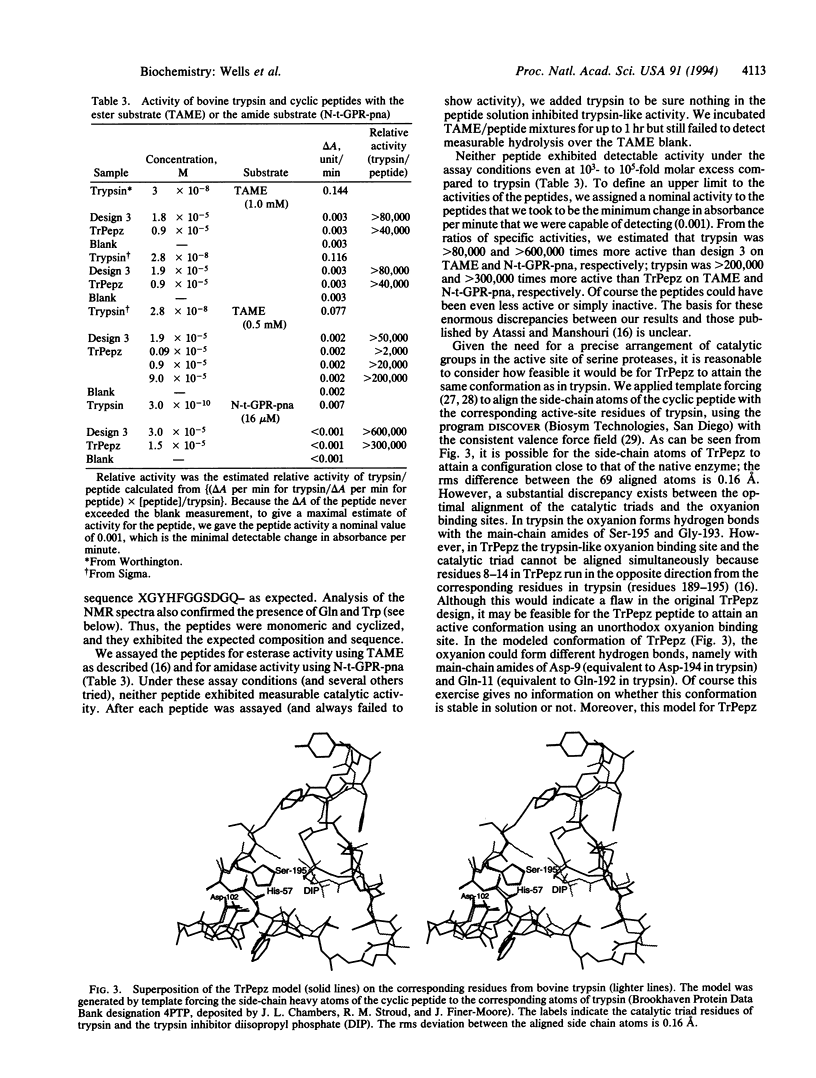
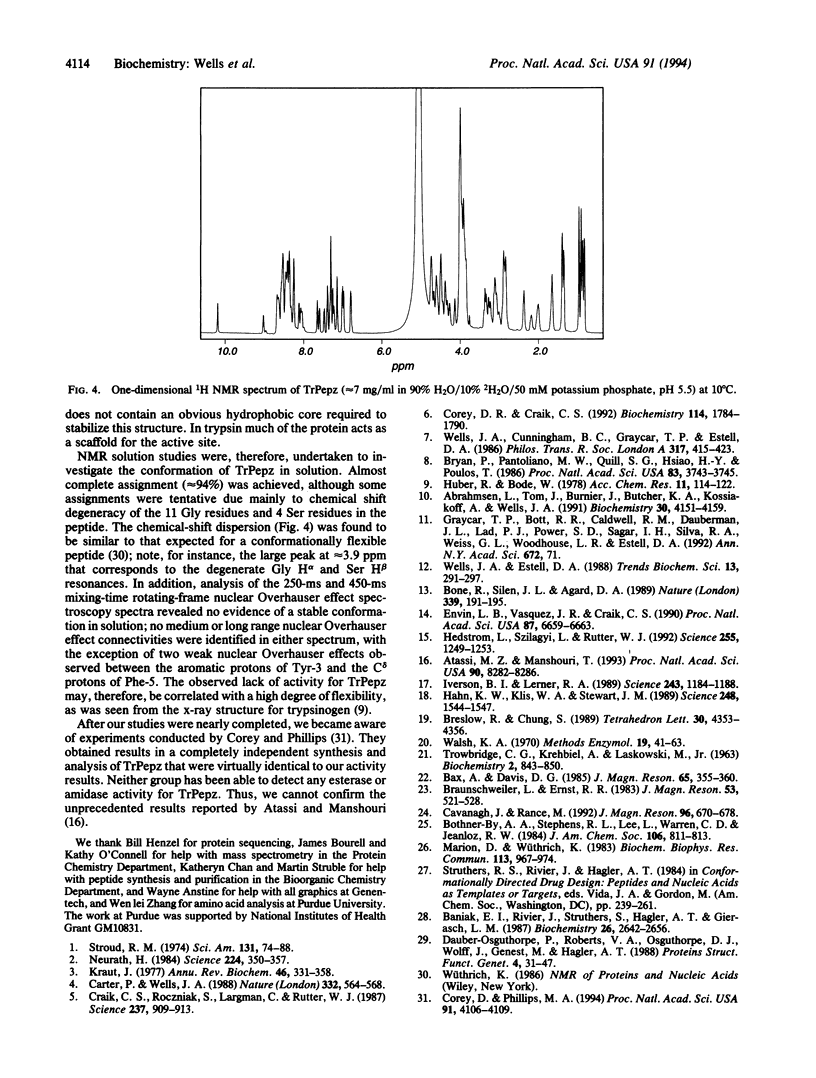
Recently, a 29-residue cyclic peptide was synthesized (TrPepz) that was reported to possess nearly the same catalytic activity and specificity as the pancreatic serine protease, trypsin, for hydrolysis of a small ester substrate, N-tosyl-L-arginine methyl ester (TAME), and small and large peptides [Atassi, M. Z. & Manshouri, T. (1993) Proc. Natl. Acad Sci. USA 90, 8282-8286]. To study these results we have resynthesized TrPepz and a related cyclic peptide reported to possess some trypsin-like activity. The authenticity of each peptide was confirmed by mass spectrometry, peptide sequencing, compositional analysis, and 1H NMR spectroscopy. However, neither peptide exhibited any detectable esterase activity or amidase activity under a variety of conditions tested. Molecular modeling studies indicated it was possible for TrPepz to be nearly superimposed upon the active site of trypsin. However, NMR experiments showed the structure of the cyclic peptide to be disordered. Thus, we were unable to confirm the results of Atassi and Manshouri. Our results are consistent with the view that serine protease activity depends not only on the presence of catalytic groups but also on their precise and stable alignment.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahmsén L., Tom J., Burnier J., Butcher K. A., Kossiakoff A., Wells J. A. Engineering subtilisin and its substrates for efficient ligation of peptide bonds in aqueous solution. Biochemistry. 1991 Apr 30;30(17):4151–4159. doi: 10.1021/bi00231a007. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Manshouri T. Design of peptide enzymes (pepzymes): surface-simulation synthetic peptides that mimic the chymotrypsin and trypsin active sites exhibit the activity and specificity of the respective enzyme. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8282–8286. doi: 10.1073/pnas.90.17.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniak E. L., 2nd, Rivier J. E., Struthers R. S., Hagler A. T., Gierasch L. M. Nuclear magnetic resonance analysis and conformational characterization of a cyclic decapeptide antagonist of gonadotropin-releasing hormone. Biochemistry. 1987 May 5;26(9):2642–2656. doi: 10.1021/bi00383a036. [DOI] [PubMed] [Google Scholar]
- Bone R., Silen J. L., Agard D. A. Structural plasticity broadens the specificity of an engineered protease. Nature. 1989 May 18;339(6221):191–195. doi: 10.1038/339191a0. [DOI] [PubMed] [Google Scholar]
- Bryan P., Pantoliano M. W., Quill S. G., Hsiao H. Y., Poulos T. Site-directed mutagenesis and the role of the oxyanion hole in subtilisin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3743–3745. doi: 10.1073/pnas.83.11.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P., Wells J. A. Dissecting the catalytic triad of a serine protease. Nature. 1988 Apr 7;332(6164):564–568. doi: 10.1038/332564a0. [DOI] [PubMed] [Google Scholar]
- Corey D. R., Phillips M. A. Cyclic peptides as proteases: a reevaluation. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4106–4109. doi: 10.1073/pnas.91.10.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Largman C., Rutter W. J. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987 Aug 21;237(4817):909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- Dauber-Osguthorpe P., Roberts V. A., Osguthorpe D. J., Wolff J., Genest M., Hagler A. T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins. 1988;4(1):31–47. doi: 10.1002/prot.340040106. [DOI] [PubMed] [Google Scholar]
- Evnin L. B., Vásquez J. R., Craik C. S. Substrate specificity of trypsin investigated by using a genetic selection. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6659–6663. doi: 10.1073/pnas.87.17.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graycar T. P., Bott R. R., Caldwell R. M., Dauberman J. L., Lad P. J., Power S. D., Sagar I. H., Silva R. A., Weiss G. L., Woodhouse L. R. Altering the proteolytic activity of subtilisin through protein engineering. Ann N Y Acad Sci. 1992 Nov 30;672:71–79. doi: 10.1111/j.1749-6632.1992.tb35605.x. [DOI] [PubMed] [Google Scholar]
- Hahn K. W., Klis W. A., Stewart J. M. Design and synthesis of a peptide having chymotrypsin-like esterase activity. Science. 1990 Jun 22;248(4962):1544–1547. doi: 10.1126/science.2360048. [DOI] [PubMed] [Google Scholar]
- Hedstrom L., Szilagyi L., Rutter W. J. Converting trypsin to chymotrypsin: the role of surface loops. Science. 1992 Mar 6;255(5049):1249–1253. doi: 10.1126/science.1546324. [DOI] [PubMed] [Google Scholar]
- Iverson B. L., Lerner R. A. Sequence-specific peptide cleavage catalyzed by an antibody. Science. 1989 Mar 3;243(4895):1184–1188. doi: 10.1126/science.2922606. [DOI] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Marion D., Wüthrich K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem Biophys Res Commun. 1983 Jun 29;113(3):967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- Neurath H. Evolution of proteolytic enzymes. Science. 1984 Apr 27;224(4647):350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- Stroud R. M. A family of protein-cutting proteins. Sci Am. 1974 Jul;231(1):74–88. doi: 10.1038/scientificamerican0774-74. [DOI] [PubMed] [Google Scholar]
- TROWBRIDGE C. G., KREHBIEL A., LASKOWSKI M., Jr SUBSTRATE ACTIVATION OF TRYPSIN. Biochemistry. 1963 Jul-Aug;2:843–850. doi: 10.1021/bi00904a037. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Estell D. A. Subtilisin--an enzyme designed to be engineered. Trends Biochem Sci. 1988 Aug;13(8):291–297. doi: 10.1016/0968-0004(88)90121-1. [DOI] [PubMed] [Google Scholar]


