Abstract
Kidney stones develop attached to sub-epithelial plaques of calcium phosphate (CaP) crystals (termed Randall’s plaque) and/or form as a result of occlusion of the openings of the Ducts of Bellini by stone forming crystals (Randall’s plugs). These plaques and plugs eventually extrude into the urinary space, acting as a nidus for crystal overgrowth and stone formation. To better understand these regulatory mechanisms and the pathophysiology of idiopathic calcium stone disease, this review provides in-depth descriptions of the morphology and potential origins of these plaques and plugs, summarizes existing animal models of renal papillary interstitial deposits, and describes factors that are believed to regulate plaque formation and calcium overgrowth.
Based on evidence provided within this review and from the vascular calcification literature, we propose a “unified” theory of plaque formation – one similar to pathological biomineralization observed elsewhere in the body. Abnormal urinary conditions (hypercalciuria, hyperoxaluria, hypocitraturia), renal stress or trauma, and perhaps even the normal aging process leads to transformation of renal epithelial cells into an osteblastic phenotype. With this de-differentiation comes an increased production of bone specific proteins (i.e. osteopontin), a reduction in crystallization inhibitors (such as fetuin and matrix-gla-protein), and creation of matrix vesicles, which support nucleation of CaP crystals. These small deposits promote aggregation and calcification of surrounding collagen. Mineralization continues by calcification of membranous cellular degradation products and other fibers until the plaque reaches the papillary epithelium. Through the activity of matrix metalloproteinases or perhaps by brute physical force produced by the large subepithelial crystalline mass, the surface is breached and further stone growth occurs by organic matrix-associated nucleation of CaOx or by the transformation of the outer layer of CaP crystals into CaOx crystals.
Should this theory hold true, developing an understanding of the cellular mechanisms involved in progression of a small, basic interstitial plaque to that of an expanding, penetrating plaque could assist in the development of new therapies for stone prevention.
Keywords: Randall’s Plaque, Randall’s Plug, Calcium Oxalate, Hydroxyapatite, Kidney Stones, Calcification
Introduction
Nephrolithiasis is a chronic disease increasing in prevalence in both the United States and abroad. A recent analysis of US adults in the National Health and Nutrition Examination Surveys (NHANES) showed kidney stone prevalence increased from 5.2% between 1988–1994[1] to 8.4% between 2007–20102. Cost for stone disease care and treatments have also risen concomitantly, from an estimated $2 billion in 2000 to over $10 billion in 2006 (Urologic Diseases in America, National Institute of Health Publication No, 12-7865, Table 14–47, US Government Printing Office, Washington, DC; 2012). Furthermore, new epidemiological studies provide evidence that link stone formation to the development of hypertension, chronic kidney disease, and even end-stage renal disease.[2–5] Increasingly common medical conditions, such as obesity, diabetes, and metabolic syndrome, are also considered risk factors for stone formation in adult population.[3,6] Finally, stone disease may recur in 50% of individuals within the first 5 years of their first stone episode, with ensuing episodes having even higher recurrence rates.[7] Obviously, understanding and preventing stone formation is paramount to reducing recurrence and cost.
Stone formation has long been considered a passive physicochemical process resulting from increased urinary salt supersaturation and decreased crystal inhibition. Despite our understanding of these crystallization steps, medical management is considered moderately effective at best, reducing stone recurrence 30–50% over short periods of time.[8,9] Therefore, to better understand the regulatory processes and pathophysiology of idiopathic calcium stone disease, this review provides in-depth descriptions of the morphology and potential origins of Randall’s plaques and plugs, summarizes existing animal models of renal papillary interstitial deposits, and describes the known factors that are believed to regulate plaque formation and calcium overgrowth. Finally, based on evidence in the vascular calcification literature, we propose a unified theory of Randall’s plaque formation that involves epithelial cell transformation into osteoblast-like cells, formation of CaP crystals within the renal tubular epithelial basement membrane, and extension and eventual breach of these calcifications through aggregation and inflammation. Should this theory hold true, developing an understanding of the cellular mechanisms involved in progression of a small, basic interstitial plaque to that of an expanding, penetrating plaque could assist in the development of new therapies for stone prevention.
Randall’s Theory of Stone Formation
In 1937, Dr. Randall proposed that kidney stones develop on two types of pre-calculus lesions situated in the renal papillae.[10,11] Interstitial sub-epithelial deposits of calcium phosphate (CaP) and calcium carbonate, arising from pathologic conditions of renal papilla, erode through to the papillary surface forming Type I pre-calculus lesions [12] which are currently referred to as the “Randall’s plaques (RP).” He further suggested that in the case of excessive urinary supersaturation and necrosis of tubular epithelial cells, stone forming salts crystallize and deposit in the collecting ducts forming Type II lesion, [11] which may now be referred to as “Randall’s plugs.” In both cases, the lesions act as nidi for further crystal deposition, resulting in the formation of calculi in the renal pelvis or papillary ducts, respectively.
Randall’s Plaques
Since Randall proposed his theory of lesions in papillary interstitium initiating the stone formation, a number of morphological studies of renal papillae obtained from kidneys of stone formers as well as non -stone formers, have been performed. Haggitt and Pitcock [13] examined kidneys from 100 randomly selected autopsies and performed light and transmission electron microscopy (TEM) on selected specimens. They found alizarin red positive laminated spherules in the interstitium which on closer examination by TEM were found associated with collagen fibers in the interstitium as well as basement membrane of the collecting ducts. Cooke and associates [14,15] studied 62 normal kidneys and found calcification in 4, which was invariably located in the basement membrane of the loops of Henle from where it extended into the medullary interstitium. Some collecting ducts and blood vessels were also involved.
High resolution radiography of cadaveric kidneys was performed by Stoller et al.[16] They reported that 57% of the kidneys had sub-epithelial Randall’s plaques which extended deep within the papillae and were intimately associated with collecting ducts and vasa recta. With von Kossa staining, spherical CaP deposits were identified scattered in the interstitium as well as around the collecting ducts and blood vessels. Evan and associates examined renal papillae from stone patients with a variety of causes.[17] They concluded that all idiopathic calcium stones develop attached to the sub-epithelial Randall’s plaques[18] and confirmed the earlier observations of Cooke about the involvement of basement membrane of the loops of Henle in the development of Randall’s plaques. Also in their reports, they describe absence of cell injury, inflammation, interstitial fibrosis, and intratubular crystal deposition across all renal biopsies from idiopathic stone formers. Evan et al further hypothesized that interstitial crystal deposits of idiopathic stone formers migrate from the basement membrane of the loops of Henle into the surrounding interstitium and become associated with type 1 collagen, fusing into a synctium in which islands of mineral appear to float in an organic sea. [17,19] Osteopontin (OPN), [20,21] heavy chain of inter-α- inhibitor, [22,23] collagen, [13,15,24] and zinc,[25] have been identified in the organic matrix of interstitial plaques. Interestingly, all RPs do not develop into stones, as many non-stone formers’ kidneys are also plagued by plaque.[13]
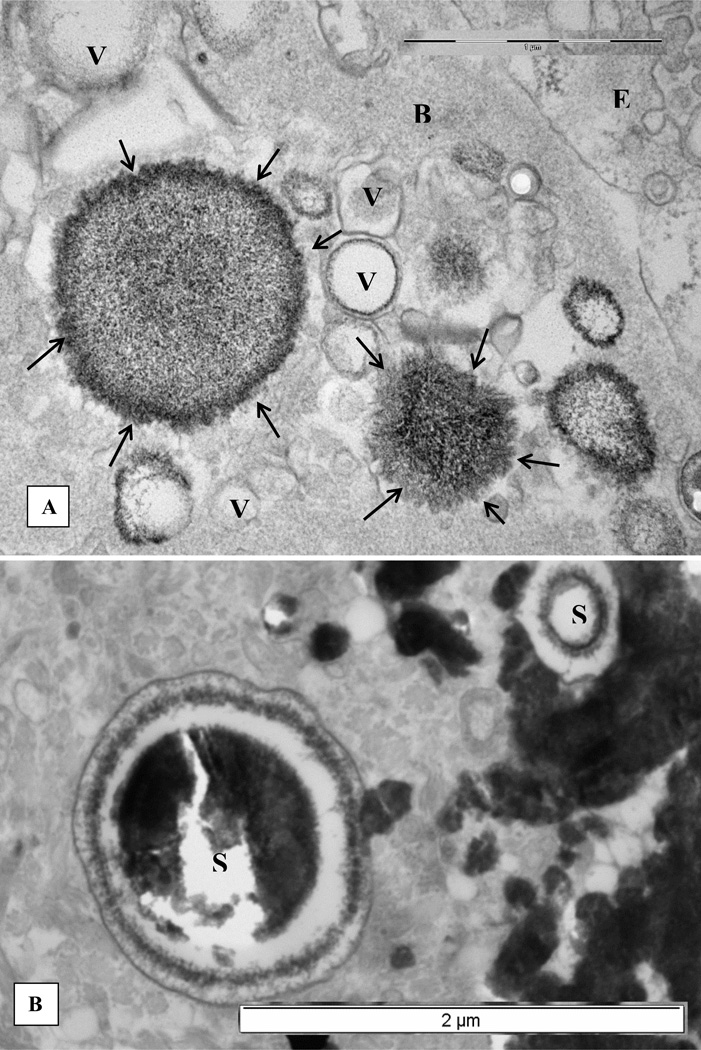
Our electron microscopic examination of the renal papillary tissue at the stone attachment site showed interstitial CaP deposits associated with membrane bound vesicles, collagen and some unidentified fibrillar material.[26] Some of the vesicles contained needle shaped electron dense objects, most probably nucleating CaP crystals (Figure 1A). There were two distinct types of calcifications. Concentrically laminated 0.5 µm to 2 µm in diameter spherical deposits were seen in the basement membrane of the renal tubules as well as the interstitium (Figure 1B). On the other hand, dark and dense deposits of elongated strands mixed with aggregating spherulitic crystals were generally located in the interstitium and under the papillary epithelium (Figure 1C, D). The outer surfaces of these deposits revealed individual calcified strands with banded pattern similar to the nearby collagen fibers (Figure 1D). Electron diffraction revealed the crystals to be hydroxyapatite (HA), with the center of the deposit being more crystalline than the periphery.
Figure 1.
Electron microscopic examination of renal interstitial deposits of the CaP (For details please refer to [26]. A. Membrane bound vesicles associated with the basement membrane (B) of an epithelial cell (E). Some vesicles appear empty (V) while others contain electron dense material with needle shaped crystals of apatite on their periphery (arrows). B. Edge of a dense calcium phosphate deposit showing two large laminated spherical (S) bodies of apatite crystals. C, collagen. C. Calcium phosphate deposits as seen by scanning electron microscopy. Spherical apatite bodies (S) of different sizes are aggregated together with fibrous material (arrows) of various thicknesses. Needle shaped apatite crystals are sticking out on the surface of the spherical bodies similar to what is seen in Figure 1B by transmission electron microscopy. D. Another area of the dense calcium phosphate deposit shows an elongated calcified body with distinct bands (arrows). Nearby collagen fibers (C) demonstrate similar banding. V, vesicles.
Randall’s Plugs
Cystine, brushite, CaP stones in primary hyperparathyroidism, and CaOx stones of primary hyperoxaluria and post-bariatric surgery have all been found attached to Randall’s plugs.[17,27,28] The plugs form, in part, as a result of higher supersaturation with respect to the precipitating salt[10,12,13] and are generally associated with renal tubular injury and focal inflammation as indicated by Randall.[10] A recent study [27] has provided newer evidence that even some idiopathic CaOx stones may develop on Randall’s plugs formed as a result of intratubular deposits of CaP.
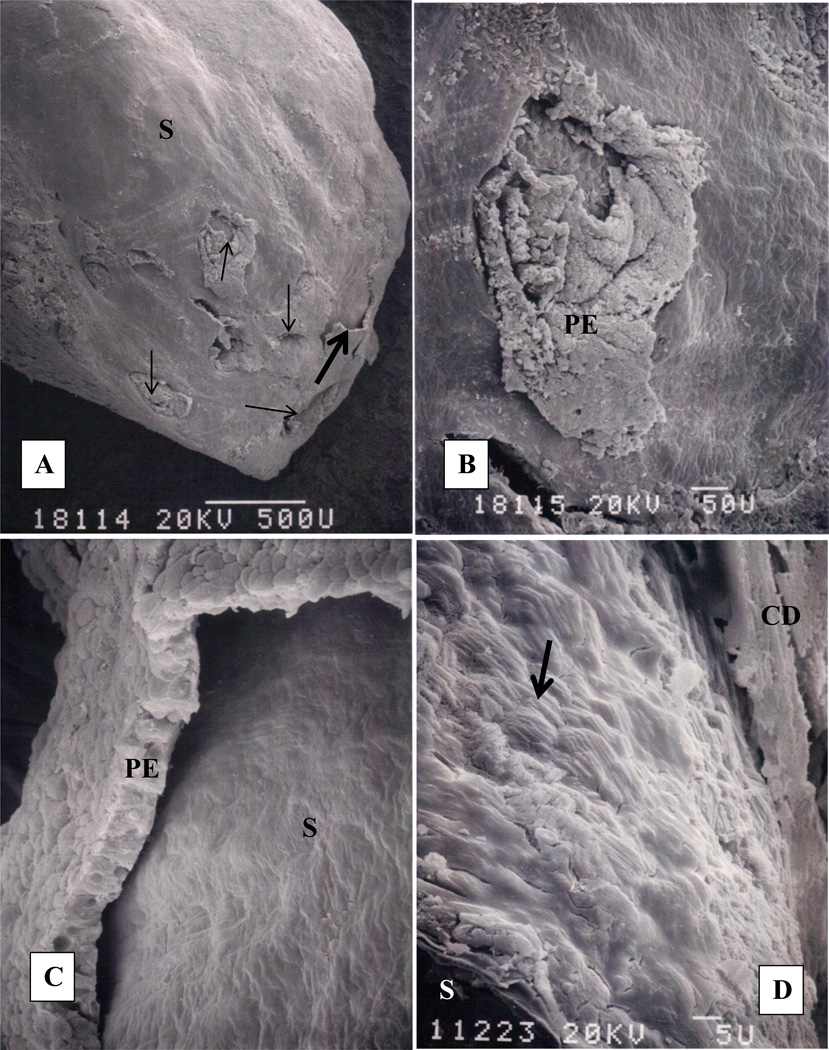
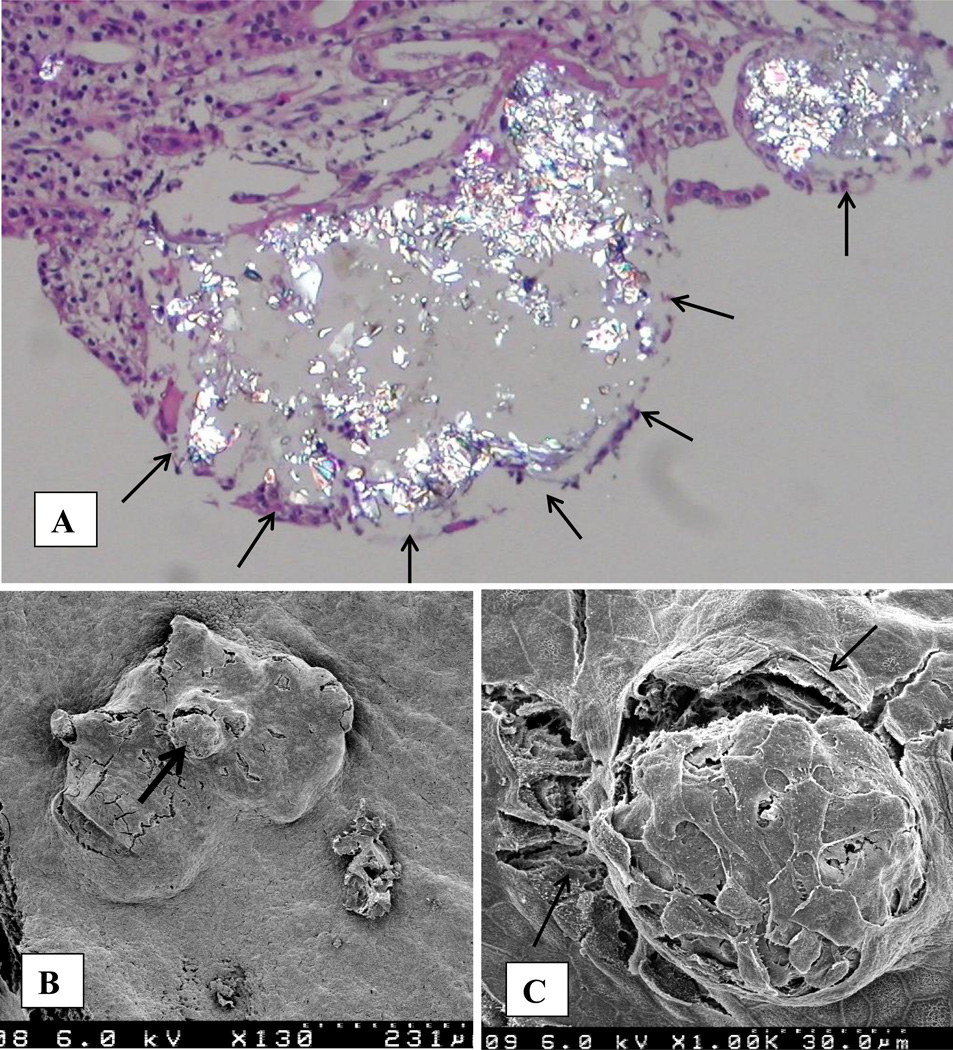
Randall’s plugs, consisting of CaOx crystals, were seen in the terminal collecting ducts of a patient with primary hyperoxaluria and bilateral renal calculi. [28] Following removal of several stones from the renal pelvis and calices, calculi bearing papillae were resected, processed and examined by light, scanning (SEM), and transmission electron microscopic (TEM) techniques. Stone composition was determined by x-ray diffraction and energy dispersive x-ray microanalysis. All stones consisted of CaOx mixed with small amount of apatite. Seven stones were extracted from the collecting ducts. A large stone was found completely lodged inside the papilla, in a duct of Bellini growing finger like extensions into the collecting ducts. The stone appeared as a bulge at the papillary tip (Figure 2A). Openings of the ducts of Bellini were deformed (Figure 2B). Papillary surface epithelium was mostly sloughed off revealing the basement membrane and underlying stone (Figure 2C, D). Removal of the stone from the duct left an exposed basement membrane. Stone itself was covered with epithelial degradation products. Examination of the fractured stones extracted from the collecting ducts showed concentric layers of plate-like crystals of CaOx monohydrate around a central nucleus (Figure 3A). One of the stone however showed concentric rings originating from a nucleus situated on the periphery (Figure 3B) indicating that stone may have developed attached to the tubular surface.
Figure 2.
Renal papilla of a patient with primary hyperoxaluria examined by scanning electron microscopy (modified from Khan, Finlayson and Hackett [28]). A. Papillary tip with a calcium oxalate stone inside. The stone appears as a protuberance on the papillary surface (S). Papilla is mostly denuded of surface epithelium which can be seen sloughing off (thick arrow) the underlying basement membrane. Openings of the ducts of Bellini (thin arrows) are completely obliterated. B. High magnification view of an obliterated opening, PE, papillary epithelium. C. High magnification of the sloughing papillary epithelium (PE) exposing the bulging stone (S) underneath. D. Surface of the underlying stone still covered with organic material. Tips of the plate-like calcium oxalate monohydrate crystals are clearly visible (arrow). Fractured stone (S) surface is in the left lower corner while surface of the damaged collecting duct epithelium (CD) is seen on the right upper corner.
Figure 3.
Scanning electron microscopic examination of the fractured surfaces of the ductal stones. A. Stone is plugging the opening of a duct of Bellini. A nearby terminal collecting duct (CD) is on the right. Stone shows concentrically arranged layers of calcium oxalate monohydrate crystals around an acentric nucleus (N). Stone appears to have grown attached to tubular surface. B. A calcium oxalate monohydrate stone which was found free in the collecting duct. Crystals are arranged in concentric layers around a central nucleus (N).
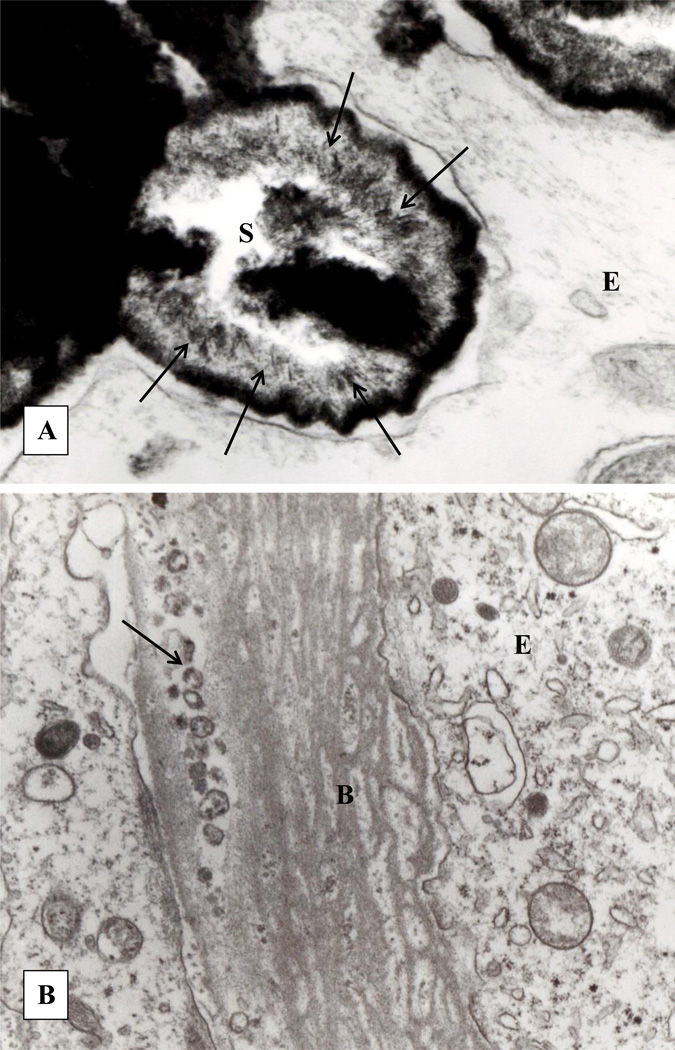
Light microscopic examination of the kidneys with CaOx plugs revealed interstitial fibrosis, focal tubular epithelial hyperplasia, interstitial calcification and intratubular deposits of birefringent CaOx crystals. The matrix of CaOx crystals was PAS and alcian blue positive.[28] Transmission electron microscopy showed an enlarged interstitium, with myofibroblasts, abundant collagen, fibrillar material as well as electron dense deposits of CaP. Membranous vesicles and laminated spherulites (Figure 4A) with needle shaped apatite crystals were present on the periphery of the large interstitial CaP deposits. There was thickening and layering of the basement membrane of the tubular epithelium (Figure 4B).
Figure 4.
Transmission electron microscopic analyses of the renal papilla with CaOx plug. A. Calcium phosphate deposit present in the interstitium shows a spherical internally laminated body of apatite crystals (S). Individual needle shaped crystal are arranged on the periphery (arrows). B. Highly laminated basement membrane of tubular epithelium. Membrane bound vesicles and other cellular degradation products (arrows) are seen within the epithelial (E) laminated basement membrane (B).
Current Theories about the Origin of Randall’s Plaques and Plugs
Randall’s Plaque
There are two theories about the origin of Randall’s plaques, both of which are based upon morphological examination of kidneys from a variety of stone formers. Evan and associates concluded that Randall’s plaques begin in the basement membrane of the thin limbs of the Loop of Henle.[24,29,30] Stoller et al. on the other hand reported the plaques reaching deep into the papilla into basement membrane of the vasa recta and proposed a pathway involving the vascular system, [31] somewhat similar to the formation of calcified plaques in arteries and capillaries. They have argued that vascular theory of the plaque formation is supported by renal papillary physiology as well as association between stone formation and vascular diseases with renal involvement.[32] The laminar blood flow changes to turbulent flow at the tip of the renal papilla. There is a more than a 10 fold increase in osmolality and decrease in oxygen-carrying capacity between renal cortex and papillary tip. Results of epidemiological studies also provided evidence for an association between stone formation and cardiovascular diseases, including hypertension, myocardial infarction, diabetes, chronic kidney disease, metabolic syndrome.[3,6,32] Many of the co-morbidities not only lead to stone disease but are also triggered by it. Stone formation is a risk factor for development of hypertension and stone formers have higher prevalence of diabetes mellitus. Some hypertensive and diabetic patients are at a greater risk for the formation of kidneys stones. All vascular diseases with renal connection trigger production of reactive oxygen species and development of oxidative stress.[3,33,34]
Randall’s Plug
Randall’s plugs are crystalline deposits in the ducts of Bellini that clog the ducts and their openings into the renal pelvis. Plugging is a tubular event and plugs are generally considered to be formed as a result of excessive urinary supersaturation with respect to the stone salts. Urinary pH appears to play a significant role.[17] Cystinurics develop cystine stones over cystine plugs.[35] Similarly patients with primary hyperoxaluria develop CaOx stones in addition to CaOx plugs.[17,28] In cases where urine is supersaturated with respect to more than one salt, plugs as well as stones contain more than one type of mineral.[17]
Intuitively, plugging should also depend upon the inner diameter of the ducts as well as urinary flow through them. The inner diameter of the inner medullary collecting ducts ranges between 35–60µm and of the ducts of Bellini 60 to 100 µm.[36] As a result, single crystals are too small to be able to occlude the tubules [37] and would require attachment to the tubular wall [38,39] followed by growth and/or aggregation with other crystals.[36] It has been suggested that in the collecting ducts urine flows in discreet boluses propelled by the peristaltic waves.[40] Such a movement would result in significant turbulence increasing the possibility of crystal contact and aggregation.[41] Retention of the crystals for plugging may also be assisted by the fact that the opening of the ducts of Bellini into the renal pelvis are narrower than the luminal diameter the ducts. [39]
Animal Models
To our knowledge, there is no animal model in which CaOx stones are formed attached to Randall’s plaques. However, NHERF-1(sodium-hydrogen exchanger regulatory factor-1) and THP (Tamm Horsfall Protein) null mice produce interstitial apatite plaques in the renal papillae, similar to that of human plaque.[42] NHERF null mice are hypercalciuric and hyperphosphaturic, producing small interstitial CaP deposits associated with both the loops of Henle and inner collecting ducts. Smaller crystals are also seen in the basement membrane of their loops of Henle. 88% of the 15 month old THP-null mice produce numerous large deposits in the renal papillary interstitium. The deposits consist of HA, surround both the thin loops of Henle as well as inner medullary collecting ducts but are not associated with their basement membrane. The mice are hypercalciuric and hypocitraturic and their urine is supersaturated with respect to brushite, a type of CaP.[43] Higher urinary excretion of calcium and/or phosphate do not appear to be the direct cause of interstitial deposition of CaP, in the NHERF-1 or THP null mice. If this were the case, crystals would have formed primarily in the tubular lumens as has been seen in experimentally induced hypercalciuria and hyperoxaluria in rats.[44–47]
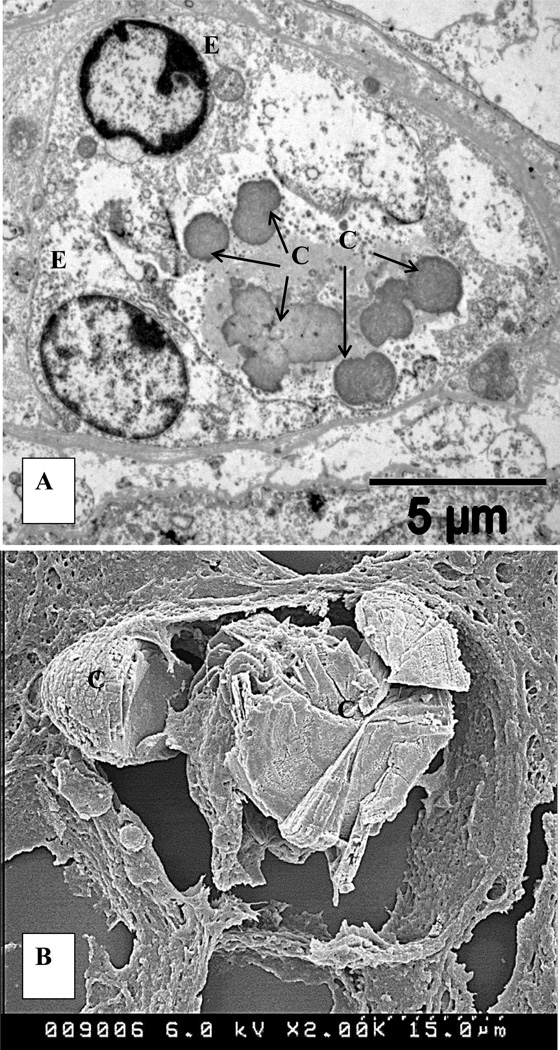
The type IIa sodium dependent phosphate co- transporter (Npt2a) null mice are also hypercalciuric and hyperphosphaturic. They produce both intratubular and interstitial deposits of CaP crystals.[48–50] However, these interstitial deposits are not similar to the interstitial plaques seen in stone patients as they appear to start within the renal tubular lumen and eventually become relocated into the interstitium (Figure 5).[51]
Figure 5.
CaP crystal deposits in kidneys of Npt2a null mice. A. Transmission electron micrograph of a cross section of a renal tubule. Spherules of CaP crystals, (C), approximately 1µm across, are present in the tubular lumen surrounded by renal epithelium (E). B. Scanning electron microscopy shows large, concentrically laminated deposit of CaP crystals, completely occluding the tubular lumen and relocating into the interstitium (For details please refer to Khan and Glenton [48].
Experimentally induced hypercalciuria and hyperoxaluria lead to the deposition of CaP and/or CaOx in the tubular lumens including terminal collecting ducts and ducts of Bellini.[44–46,48,52] The deposits plugging the ducts are similar to the Randall’s plugs. Some of the ductal deposits appear to have been formed freely in the lumens while the others attached to the tubular epithelium. At times, CaOx crystal deposits completely occluded the ducts of Bellini of the hyperoxaluric rats and their renal papillary tip appeared nodular. Microscopic analyses showed layers of CaOx crystals attached to the basement membrane totally devoid of a covering of tubular epithelium.[53] Transmission electron microscopy of the demineralized crystals showed crystal matrix interacting with the basement membrane helping in crystal attachment. We have identified osteopontin as the major component of the organic matrix of the CaOx crystals and stones.[54]
Kidneys of both the stone formers and normal individuals produce Randall’s plaques, but only some of these plaques break through the surface epithelium and develop into the nidus for stone formation. Animal models of nephrolithiasis may provide some insight into this phenomenon. As discussed above, intratubular deposits of CaOx present in the subepithelial collecting ducts at the papillary tips break through the epithelium and expose their contents to the pelvic urine. The deposits appeared as nodules or bulges on the papillary surface (Figure 6). Close examination showed small nodules with intact but stretched epithelium separating at cell junctions while large nodules had their epithelium sloughed off exposing the basement membrane. Papillary epithelium around openings of ducts of Bellini with plugs of CaOx crystals also appeared mechanically disrupted with cells separating from each other. Subepithelial deposits of CaP, the Randall’s plaque, may similarly become exposed to the pelvic urine which is generally metastable with respect to CaOx and would promote heterogeneous nucleation of CaOx over a compatible surface. Further deposition and overgrowth of CaOx crystals would eventually lead to the formation of a CaOx stone attached to the renal papillary tip.[38,39,44]
Figure 6.
CaOx crystal deposits in the papillary collecting ducts of a male rat receiving hydroxyl-L-proline in the diet (For details please refer to Khan and Glenton [44]. A. Light microscopic examination of CaOx crystal deposit in papillary collecting duct. Deposit of birefringent crystals, present in the outer papillary collecting ducts, appear as bulges, protruding into the renal pelvis, and are covered with a thin epithelial layer (arrows). B. Scanning electron microscopic view of the papillary surface with CaOx deposit under the papillary surface. The deposit is protruding into the renal pelvis. Papillary epithelium shows signs of stretching and cracks. The area pointed by the arrow is shown at higher magnification in Fig 6C. C. Papillary epithelium over the deposit surface appears stretched and show signs of developing cracks (arrows).
Calcium Phosphate as a Calcium Oxalate Nidus and Transition
In addition to being a constituent of the Randall’s plaque, hydroxyapatite is a common constituent of most idiopathic CaOx stones, is frequently found at the CaOx crystal nucleation sites deep within a stone, [55] and acts as suitable nucleator of CaOx in vitro.[56,57] Similarly, CaOx crystals have been found to support the nucleation of CaP crystals. [57] In vivo animal models as well as in vitro studies have provided the evidence that transition from one crystal type to another depends upon the urinary environment.[48,58–60] Hydroxyapatite and struvite (St) crystals coated the surface of the foreign body implanted into the bladder of a rat on a normal diet. Induction of hyperoxaluria in these rats, which was associated with lower urinary pH, generated a layer of CaOx crystals growing over the CaP/St. Reversing the hyperoxaluria led to the deposition of a layer of CaP/St over CaOx.[55,60] Both, the pH and supersaturation /activity product with respect to CaOx and CaP change as tubular fluid courses through the renal tubules. [61–64] A driving force for CaP precipitation is present in the thin loops of Henle while that for CaOx exists in the collecting ducts and the pelvic urine. Precipitation of CaP within the interstitium would require medullary alkalinization involving K+ depletion and activation of H+/K+ ATPase.[65] Dissolution of HA of the plaque at lower urinary pH that exists in the calyceal and pelvic urine would create localized areas of higher calcium concentration and therefore CaOx supersaturation promoting CaOx growing over CaP.[63]
CaOx and CaP crystals found in both urine and kidney stones are coated with phospholipids, Tamm Horsfall Protein, albumin and a mix of organic matrix macromolecules such as osteopontin, inter-α-inhibitor, and urinary prothrombin fragment-1.[66–68] These macromolecule are present on mineral externally as well as internally, existing within stones as concentric tree-ring like layers of crystalline deposits[55] and within Randall’s plaque at the sites of stone attachment, making epitaxial nucleation of one crystal over another unlikely[20]. Despite their production by epithelial cells to aid in crystal clearance, these macromolecules may by themselves promote the nucleation of CaOx as has been shown in vitro[69–71] and may inhibit CaP dissolution. In vitro studies were performed to determine the possibility of HA replacement by CaOx monohydrate.[72] Large single HA crystals as well as bone pieces (mimic for Randall’s plaque) were incubated in 0.25 mM, 0.5 mM and 1.00 mM oxalate solution with pH between 4.5 to 7.5 with the hypothesis that dissolution of HA will release calcium which will bind with the available oxalate to form CaOx crystals. Precipitation of CaOx monohydrate on HA crystals required very acidic pH and or high oxalate. CaOx precipitation on bone was seen even at a physiological oxalate concentration of 0.25 mM oxalate and pH of 5.0. Higher oxalate concentration of 0.5 mM was required for precipitation at pH of 6.00. Demineralization of bone produced a gel like layer on the surface. This layer may have produced physical hindrance for diffusion of calcium resulting in its accumulation near the bone surface and increasing local supersaturation and precipitation of CaOx as has previously been suggested.[63]
Vascular Calcification
Years ago, vascular calcification, or the deposition of apatite in the medial or intimal layers of the vessel walls, was considered as an irreversible degenerative process that occurred by a passive, unregulated, physicochemical mechanism. Over the last 10–15 years, a large body of literature has replaced this passive theory to a central theory that describes vascular calcification as an active, regulated process in which vascular smooth cells (VSMC) acquire osteogenic phenotype.[73–75] Exposure of VSMC to elevated levels of calcium and phosphate triggers osteogenic transformation of VSMC, [76–79] which involves an increased expression of osteoblast specific genes and a decrease in smooth muscle cell markers.[80,81] Bone morphogenetic proteins, BMP 2 and BMP 4, and Wnt signaling pathways are activated through up-regulation of transcription factor, Runt-related transcription factor 2 (RUNX2)/ msh homeobox 2 (MSX-2). The cells then produce matrix proteins, and crystallization starts in membrane bound matrix vesicles produced by the viable transformed vascular smooth muscle cells or apoptotic bodies produced on their death.[79,82,83] The vesicles are similar in composition to the matrix vesicles derived from chondrocytes and provide sites for the nucleation of calcium phosphate (CaP) crystals.[77] Once mineralized, the crystals extrude through the limiting membrane of the vesicles, and help mineralize the nearby extracellular matrix which sustains calcification. In addition to abnormal mineral metabolism, oxidative stress, inflammation and aberrant crystallization inhibition play a significant role in vascular calcification. Reactive oxygen species are likely involved in the VSMC transformation to osteogenic phenotype by regulating RUNX-2 transcription factor.[84,85] Advanced glycation end-products commonly seen in blood and arteries of diabetic patients and older individuals can promote vascular calcification mediated by NADPH oxidase induced reactive oxygen species.[86] Cytokines such as IL (interleukin)-1β, IL-6, IL-8, (TNF) tumor necrosis factor-α, TGF (transforming growth factor)-β produced by macrophages induce transformation of VSMCs.[87] Inflammatory cells also produce proteolytic enzymes such as metalloproteinases (MMP)-2 and -9 which degrade matrix and promote calcification.[88–91]
Calcification of VSMC is inhibited by matrix Gla protein (MGP), pyrophosphate, osteopontin (OPN) and Fetuin-A.[80] MGP is a vitamin K-dependent protein functioning primarily as an inhibitor of vascular calcification. [92] MGP also regulates BMP-2 activity.[93] Mutations in the MGP gene lead to Keutel Syndrome, a disorder associated with extensive soft tissue and vascular calcification.[94] MGP knockout mice die within two months as a result of arterial calcification and blood vessel rupture, [95] while restoration of MGP in these mice prevents arterial calcification.[96] Polymorphism of MGP may play a role in vascular calcification, [97] and has shown an association with myocardial infarction.[98] Fetuin A, a member of cystatin family of protease inhibitors, is a serum protein, produced by the liver and specifically enriched in mineralized tissues.[99–101] Irrespective of its origin and posttranslational modifications, fetuin-A prevents precipitation of hydroxyapatite in vitro.[102] In vivo, serum fetuin A levels are lower in patients with chronic kidney disease,[103] and ectopic calcification is seen in fetuin A −/− mice.[101]
Regulation of Randall’s Plaque Formation
Results of a number of clinical and experimental investigations suggest that reactive oxygen species (ROS) may be involved in the pathogenesis of idiopathic stone disease, [33,104,105] and are likely a link between stone formation and hypertension, diabetes, metabolic syndrome, and chronic kidney diseases [3]. Urine from CaOx stone patients showed significantly higher N-acetyl-β-glucoseaminidase (NAG), β-galactosidase, α-glutathione S-transferase (α-GST), malondialdehyde (MDA) and thiobarbituric acid-reactive substances (TBARS) [104], biomarkers of injury and production of ROS. Urinary 8-hydroxydeoxyguanosine(8-OHdG), a marker of oxidative damage of DNA, was also increased in stone patients and positively correlated with tubular damage as assessed by urinary excretion of NAG [106]. Antioxidant deficit was common in recurrent idiopathic stone formers and was unrelated to the presence or absence of stones. [107]
Exposure of the renal epithelial cells to oxalate and/or CaOx/CaP crystals leads to the production of reactive oxygen species, [108–116] with the activation of NADPH oxidase, [117–122] through the involvement of renin angiotensin aldosterone pathway.[118,123] Renal epithelial cells under oxidative stress may become osteogenic [124] as happens to vascular smooth muscle cells during vascular calcification.[78] The production of OPN,[125,126] MGP,[127,128], collagen, fibronectin, osteonectin and fetuin, [129] by renal epithelial cells of rats with experimentally induced CaOx nephrolithiasis are indicative of such a transition. The presence of OPN, osteocalcin, fibronectin, and collagen [130] in stone matrices also suggests their increased production and excretion into the urine. Renal crystals in a CaOx stone patient were also associated with bone sialoprotein (BSP).[131] Epithelial to mesenchymal transition[132] as well as endothelial to mesenchymal transition[133,134] are regularly seen in the diseased kidneys. Mesenchymal stromal cells have the ability to differentiate into osteoblast. Interestingly, perivascular cells or pericytes showed heavy staining for MGP in kidneys of hyperoxaluric rats.[127] Stone patients excrete lower amounts of fetuin-A,[135] and more BMP-2.[136] Single nucleotide polymorphism of MGP gene is associated with CaOx kidney stones disease in the Japanese [137] and Chinese populations.[138]
Further evidence for epithelial cells becoming osteogenic comes from animal models and in vitro tissue culture studies. Basal levels of bone-related factors, such as bone morphogenetic protein 2 (BMP2), Runx2, Osterix, and OPN are higher in genetic hypercalciuric rats that produce intrarenal CaP deposits than the normal control rats.[139] Knock down of vitamin D receptor in the genetic hypercalciuric rats reduced the bone-related factors as well as amount of CaP deposition in the rat kidneys. Vitamin D treatment of the primary culture of renal tubular epithelial cells from the kidneys of genetic hypercalciuric rats increased the gene and protein expression of the bone related factors as well as intracellular calcium phosphate deposits.
MDCK cells were grown for up to 60 days in monolayers, directly on the plastic dish or dish coated with collagen gel. [140,141] After 21 days small blisters or domes started to appear in the monolayers. After 30 days, the domes became more visible. Microscopic examination showed the presence of crystalline material which appeared as fused spherulites with needle shaped crystals. The crystals were identified as calcium phosphate using x-ray microanalyses and micro-infrared spectroscopy. In another experiment, MDCK cells grown in agar produced spherical colonies in which layers of epithelial cells with their apical surface on the outside, enclosed the CaP crystal inside.[141,142] CaP deposition in the MDCK colonies developed in agar was inhibited by alendronate.[142]
Both physiological and ectopic calcification start through heterogeneous nucleation of crystals in the so-called matrix vesicles or similar entities, and propagate in a scaffolding of collagen fibers. [83,143,144] The formation of intratubular CaP deposits in the female mice on AIN-76, a semipurified diet, starts in the vesicles budding off the proximal tubular epithelial cells.[52] Intratubular CaP deposits in Npt2a null mice are also intimately associated with membrane bound vesicles (MV) budding from the renal epithelium.[51] We have provided evidence that membrane fragments as well as membrane bound vesicles may also be involved in the formation of kidney stones. Lipids and membranes are present in the matrices of CaOx kidney stones.[145] Phospholipids of membranous vesicles obtained from renal tubular brush border are excellent nucleators of crystals, [146,147] even at physiological concentrations.[64] Membranous profiles were seen tightly bound to the ghosts of crystals. Matrices of CaOx crystals induced in vitro in human urine also contained membrane fragments and lipids.[148] Crystals in the Randall’s plaques were seen associated with both the collagen fibers as well as MVs.[26] Collagen fibers appeared calcified and vesicles contained crystals. It was concluded that crystal deposition in renal papillae may have started with membrane vesicle induced nucleation, and grew by addition of crystals on the periphery within a collagen framework.
A Unified Theory on Pathogenesis of Randall’s Plaque and Plug Formation
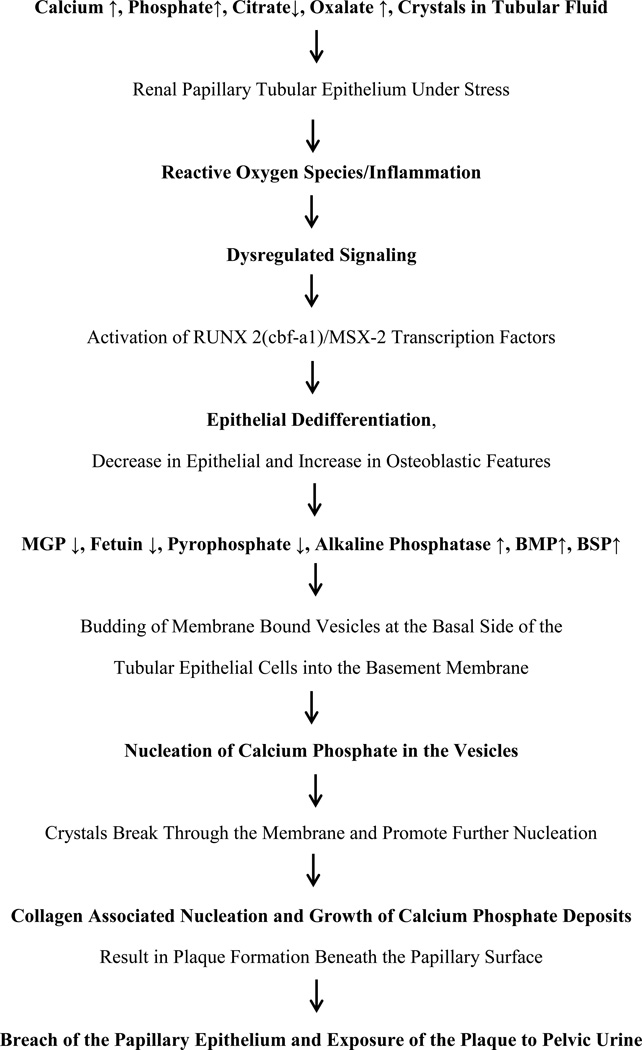
Based upon the currently described clinical and experimental data, we propose a “unified” theory for the formation of plaques and plugs (Figure 5). Renal epithelial cells of stone formers become stressed when challenged by increased urinary excretion of calcium/oxalate/phosphate and/or decrease in the production of functional crystallization inhibitors or perhaps with renal insults, trauma, or even normal aging. The abnormal urinary conditions of higher supersaturations and lower crystallization inhibitory potential produces oxidative stress leading to de-differentiation of renal epithelial cells into osteoblast like cells and the formation of CaP crystals in the renal tubular epithelial basement membrane. Transformation involves increased expression of osteoblast specific genes and decrease in epithelial cell markers. Signaling pathways become activated through upregulation of transcription factor, RUNX2/MSX-2. Bone associated proteins such as bone morphogenetic proteins (BMPs), bone sialoprotein (BSP), alkaline phosphatase are expressed. Initial crystal formation begins inside the membrane bound vesicles produced by the transformed epithelial cells. Small spherical units of CaP grow from less than a micron to a few microns in diameter and spread out beyond the basement membrane. They start to aggregate and form larger deposits or plaques which increase in size by further addition of crystals on the periphery. Additional expansion leads to fusion of the aggregated spherical units and loss of their individual identity in the interior of the deposits, while spherical units are still visible on the periphery of the growing plaque. During the outward growth the crystals come in contact with the collagen fibers and membranous degradation products which also calcify. Thus the extension of plaque into the interstitium and beyond is through outward growth by the addition of crystals at the periphery through aggregation and calcification of the collagen and membranous vesicles. Interstitial crystal deposition may produce localized inflammation and fibrosis, providing substrate for further calcification until the front reaches the papillary surface epithelium. Papillary surface epithelium is most likely breached through the activity of matrix metalloproteinases and physical force produced by the deposition of the large crystalline mass underneath. Once the surface epithelium loses its integrity the plaque is exposed to the pelvic urine which is generally metastable with respect to CaOx and also contains urinary proteins such as THP, not available in the renal interstitium. Urinary macromolecules deposit on the plaque surface. Surface layers of HA are replaced by CaOx through demineralization of CaP and mineralization of CaOx. Alternatively, or in addition to, the CaOx crystals directly nucleate on the organic matrix covering the plaque. Stone growth is through aggregating deposition of CaOx crystals on the periphery.
As discussed above, Randall’s plug formation appears to be dependent upon the supersaturation of the tubular fluid and pelvic urine. Once the plug starts to develop, urinary movement through the duct and into the pelvis will be disturbed, providing longer retention time behind the developing plug. This should promote crystal formation, aggregation and retention, and is perhaps responsible for the formation of free unattached stones in the collecting ducts, somewhat similar to the stones formed in the bladder.
Concluding Remarks
Most of the information about the plaques and plugs has been obtained by examination of the stone bearing kidneys, long after the completion of the process of stone formation. Thus we have a better picture of what kidneys look like at the end of stone formation than how did the stone develop. Plaque, plug and stone formation occur over time and in many stages. Experimental animal model and tissue culture studies have provided significant information and insight into the pathogenesis. But in the absence of an animal model in which stones develop attached to a plaque or plug, where formation and growth of the stones could be examined over time and can be experimentally tested, it is difficult to establish the precise sequence of events. For example, one can only hypothesize the exact location of the initial site of crystal formation in the papillary interstitium. Just because hydroxyapatite is seen at a site such as basement membrane of the collecting ducts or loops of Henle or in the vicinity of vasa recta does not necessarily indicate that crystal deposition started there. Calcification could have started elsewhere in the papillary interstitium and grew from there expanding through and consuming the available substrate. Some of the interstitial calcification, particularly in cases with Randall’s plugs, is very likely a result of the tubular occlusion rather than a contributory factor.
Randall’s plaque formation and its transformation into a stone nidus occur in at least four distinct phases. First there is the initial crystal deposition in the papillary interstitium which is followed by the 2nd phase of growth and expansion. In the 3rd phase epithelium covering the plaque is breached followed by the 4th phase in which outer layer of hydroxyapatite is replaced by CaOx or CaOx nucleate on the crystal associated organic matrix. Plaques and interstitial calcification are common in the renal papillae. But only a small number of plaques progress to the next step of becoming exposed to the pelvic urine and promote stone formation. This situation is somewhat similar to atherosclerosis where only some of the atherosclerotic lesions transform into dangerous vulnerable plaques.[149] Thus developing an understanding of the mechanisms involved in progression of the basic interstitial plaque to vulnerable and dangerous plaque open to the pelvic urine should be a goal of researchers so that new therapies can be developed to prevent stone recurrence. It has been postulated that soft tissue mineralization may be reversed through mineral dissolution and phagocytosis, [101] and the same may be true for the formation of plaque associated kidney stones.
Figure 7.
Potential steps in the development of Randall’s Plaques as described in the text. RUNX2, runt-related transcription factor 2; cbf-a1, core binding factor subunit alpha-1; MSX-2, Msh homebox 2; MGP, matrix gla protein; OPN, osteopontin; BMP, bone morphogenetic proteins; BSP, bone sialoprotein.
Acknowledgements
Research supported by NIH grant #RO1 DK092311 and by an AUA Foundation Rising Star in Urology Research Award in conjunction with Astellas Global Development, Inc.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Rule AD, Roger VL, Melton LJ, 3rd, Bergstralh EJ, Li X, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010;21:1641–1644. doi: 10.1681/ASN.2010030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40:95–112. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jungers P, Joly D, Barbey F, Choukroun G, Daudon M. ESRD caused by nephrolithiasis: prevalence, mechanisms, and prevention. Am J Kidney Dis. 2004;44:799–805. [PubMed] [Google Scholar]
- 5.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens. 2008;21:257–264. doi: 10.1038/ajh.2007.62. [DOI] [PubMed] [Google Scholar]
- 7.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77–84. doi: 10.1056/NEJMoa010369. [DOI] [PubMed] [Google Scholar]
- 8.Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Rev Urol. 2011;13:34–38. [PMC free article] [PubMed] [Google Scholar]
- 9.Raynal G, Petit J, Saint F. Which efficiency index for urinary stones treatment? Urol Res. 2009;37:237–239. doi: 10.1007/s00240-009-0200-x. [DOI] [PubMed] [Google Scholar]
- 10.Randall A. Papillary pathology as a precursor of primary renal calculus. Journal of Urology. 1940;44:580–589. [Google Scholar]
- 11.Randall A. The etiology of primary renal calculus. International Abstract of Surgery. 1940;71:209–240. [Google Scholar]
- 12.Randall A. The Origin and Growth of Renal Calculi. Ann Surg. 1937;105:1009–1027. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggit RC, Pitcock JA. Renal medullary calcification: a light and electron microscopic study. The Journal of Urology. 1971;106:342–347. doi: 10.1016/s0022-5347(17)61284-9. [DOI] [PubMed] [Google Scholar]
- 14.Weller RO, Nester B, Cooke SAR. Calcification in the human renal papilla: an electron microscope study. Journal of Pathology. 1971;107:211–216. doi: 10.1002/path.1711070308. [DOI] [PubMed] [Google Scholar]
- 15.Cooke SAR. The site of calcification in the human renal papilla. British Journal of Surgery. 1970;57:890–897. doi: 10.1002/bjs.1800571205. [DOI] [PubMed] [Google Scholar]
- 16.Stoller ML, Low RK, Shami GS, McCormick VD, Kerschmann RL. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall's plaque formation. J Urol. 1996;156:1263–1266. doi: 10.1016/s0022-5347(01)65565-4. [DOI] [PubMed] [Google Scholar]
- 17.Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller NL, Gillen DL, Williams JC, Jr, Evan AP, Bledsoe SB, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall's plaque. BJU Int. 2009;103:966–971. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evan AP, Lingeman JE, Coe FL, Worcester EM. Role of interstitial apatite plaque in the pathogenesis of the common calcium oxalate stone. Semin Nephrol. 2008;28:111–119. doi: 10.1016/j.semnephrol.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2009 doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evan AP, Coe FL, Rittling SR, Bledsoe SM, Shao Y, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int. 2005;68:145–154. doi: 10.1111/j.1523-1755.2005.00388.x. [DOI] [PubMed] [Google Scholar]
- 22.Evan AP, Bledsoe S, Worcester EM, Coe FL, Lingeman JE, et al. Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int. 2007;72:1503–1511. doi: 10.1038/sj.ki.5002569. [DOI] [PubMed] [Google Scholar]
- 23.Evan A, Lingeman J, Coe FL, Worcester E. Randall's plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69:1313–1318. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 24.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpentier X, Bazin D, Combes C, Mazouyes A, Rouziere S, et al. High Zn content of Randall's plaque: A mu-X-ray fluorescence investigation. J Trace Elem Med Biol 2011. 2011 doi: 10.1016/j.jtemb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Khan SR, Rodriguez DE, Gower LB, Monga M. Association of Randall plaque with collagen fibers and membrane vesicles. J Urol. 2012;187:1094–1100. doi: 10.1016/j.juro.2011.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linnes MP, Krambeck AE, Cornell L, Williams JC, Jr, Korinek M, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013 doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SR, Finlayson B, Hackett R. Renal papillary changes in patient with calcium oxalate lithiasis. Urology. 1984;23:194–199. doi: 10.1016/0090-4295(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 29.Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2010;25:831–841. doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res. 2010;38:147–160. doi: 10.1007/s00240-010-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoller ML, Meng MV, Abrahams HM, Kane JP. The primary stone event: a new hypothesis involving a vascular etiology. J Urol. 2004;171:1920–1924. doi: 10.1097/01.ju.0000120291.90839.49. [DOI] [PubMed] [Google Scholar]
- 32.Bagga HS, Chi T, Miller J, Stoller ML. New insights into the pathogenesis of renal calculi. Urol Clin North Am. 2013;40:1–12. doi: 10.1016/j.ucl.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol. 2013;189:803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan SR. Stress oxidative: nephrolithiasis and chronic kidney diseases. Minerva Med. 2013;104:23–30. [PubMed] [Google Scholar]
- 35.Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 36.Kok DJ, Khan SR. Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int. 1994;46:847–854. doi: 10.1038/ki.1994.341. [DOI] [PubMed] [Google Scholar]
- 37.Finlayson B, Reid F. The expectation of free and fixed particles in urinary stone disease. Invest Urol. 1978;15:442–448. [PubMed] [Google Scholar]
- 38.Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995;23:71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 39.Khan SR. Experimental calcium oxalate nephrolithiasis and the formation of human urinary stones. Scanning Microsc. 1995;9:89–100. discussion 100–101. [PubMed] [Google Scholar]
- 40.Reinking LN, Schmidt-Nielsen B. Peristaltic flow of urine in the renal capillary collecting ducts of hamsters. Kidney Int. 1981;20:55–60. doi: 10.1038/ki.1981.104. [DOI] [PubMed] [Google Scholar]
- 41.Khan SR, Hackett RL. Retention of calcium oxalate crystals in renal tubules. Scanning Microsc. 1991;5:707–711. discussion 711–702. [PubMed] [Google Scholar]
- 42.Evan AP, Weinman EJ, Wu XR, Lingeman JE, Worcester EM, et al. Comparison of the pathology of interstitial plaque in human ICSF stone patients to NHERF-1 and THP-null mice. Urol Res. 2010;38:439–452. doi: 10.1007/s00240-010-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, et al. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol. 2010;299:F469–F478. doi: 10.1152/ajprenal.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006;70:914–923. doi: 10.1038/sj.ki.5001699. [DOI] [PubMed] [Google Scholar]
- 45.Khan SR, Glenton PA. Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol. 2010;184:1189–1196. doi: 10.1016/j.juro.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res. 2010;38:429–438. doi: 10.1007/s00240-010-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2000;57:550–560. doi: 10.1046/j.1523-1755.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- 48.Khan SR, Glenton PA. Calcium oxalate crystal deposition in kidneys of hypercalciuric mice with disrupted type IIa sodium-phosphate cotransporter. Am J Physiol Renal Physiol. 2008;294:F1109–F1115. doi: 10.1152/ajprenal.00620.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chau H, El-Maadawy S, McKee MD, Tenenhouse HS. Renal calcification in mice homozygous for the disrupted type IIa Na/Pi cotransporter gene Npt2. J Bone Miner Res. 2003;18:644–657. doi: 10.1359/jbmr.2003.18.4.644. [DOI] [PubMed] [Google Scholar]
- 50.Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, et al. Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am J Physiol Renal Physiol. 2006;290:F838–F843. doi: 10.1152/ajprenal.00374.2005. [DOI] [PubMed] [Google Scholar]
- 51.Khan SR, Canales BK. Ultrastructural investigation of crystal deposits in Npt2a knockout mice: are they similar to human Randall's plaques? J Urol. 2011;186:1107–1113. doi: 10.1016/j.juro.2011.04.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen HT, Woodard JC. Intranephronic calculosis in rats: an ultrastructural study. Am J Pathol. 1980;100:39–56. [PMC free article] [PubMed] [Google Scholar]
- 53.Khan SR, Finlayson B, Hackett RL. Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. Am J Pathol. 1982;107:59–69. [PMC free article] [PubMed] [Google Scholar]
- 54.McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995;10:1913–1929. doi: 10.1002/jbmr.5650101211. [DOI] [PubMed] [Google Scholar]
- 55.Khan SR. Calcium phosphate/calcium oxalate crystal association in urinary stones: implications for heterogeneous nucleation of calcium oxalate. J Urol. 1997;157:376–383. [PubMed] [Google Scholar]
- 56.Meyer JL, Bergert JH, Smith LH. Epitaxial relationships in urolithiasis: the calcium oxalate monohydrate-hydroxyapatite system. Clin Sci Mol Med. 1975;49:369–374. doi: 10.1042/cs0490369. [DOI] [PubMed] [Google Scholar]
- 57.Achilles W, Jockel U, Schaper A, Burk M, Riedmiller H. In vitro formation of "urinary stones": generation of spherulites of calcium phosphate in gel and overgrowth with calcium oxalate using a new flow model of crystallization. Scanning Microsc. 1995;9:577–585. [PubMed] [Google Scholar]
- 58.Khan SR, Finlayson B, Hackett RL. MICROSTRUCTURE OF CALCIUM-OXALATE FOREIGN-BODY STONES PRODUCED IN RAT BLADDER. Urological Research. 1984;12:54–54. [Google Scholar]
- 59.Khan SR, Finlayson B, Thomas WC, Jr, Hackett RL. Relationship between experimentally induced crystalluria and relative supersaturation of various stone salts in rats. Urol Res. 1984;12:271–273. doi: 10.1007/BF00258033. [DOI] [PubMed] [Google Scholar]
- 60.Khan SR, Hackett RL. Urolithogenesis of mixed foreign body stones. J Urol. 1987;138:1321–1328. doi: 10.1016/s0022-5347(17)43592-0. [DOI] [PubMed] [Google Scholar]
- 61.Asplin JR, Mandel NS, Coe FL. Evidence of calcium phosphate supersaturation in the loop of Henle. Am J Physiol. 1996;270:F604–F613. doi: 10.1152/ajprenal.1996.270.4.F604. [DOI] [PubMed] [Google Scholar]
- 62.Hojgaard I, Fornander AM, Nilsson MA, Tiselius HG. The effect of pH changes on the crystallization of calcium salts in solutions with an ion composition corresponding to that in the distal tubule. Urol Res. 1999;27:409–416. doi: 10.1007/s002400050129. [DOI] [PubMed] [Google Scholar]
- 63.Tiselius HG. A hypothesis of calcium stone formation: an interpretation of stone research during the past decades. Urol Res. 2011;39:231–243. doi: 10.1007/s00240-010-0349-3. [DOI] [PubMed] [Google Scholar]
- 64.Fasano JM, Khan SR. Intratubular crystallization of calcium oxalate in the presence of membrane vesicles: an in vitro study. Kidney Int. 2001;59:169–178. doi: 10.1046/j.1523-1755.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- 65.Halperin ML, Cheema Dhadli S, Kamel KS. Physiology of acid-base balance: links with kidney stone prevention. Semin Nephrol. 2006;26:441–446. doi: 10.1016/j.semnephrol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Atmani F, Glenton PA, Khan SR. Identification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in the urine of healthy and stone forming subjects. Urological Research. 1998;26:201–207. doi: 10.1007/s002400050047. [DOI] [PubMed] [Google Scholar]
- 67.Maslamani S, Glenton PA, Khan SR. Changes in urine macromolecular composition during processing. J Urol. 2000;164:230–236. [PubMed] [Google Scholar]
- 68.Ryall RL, Chauvet MC, Grover PK. Intracrystalline proteins and urolithiasis: a comparison of the protein content and ultrastructure of urinary calcium oxalate monohydrate and dihydrate crystals. BJU Int. 2005;96:654–663. doi: 10.1111/j.1464-410X.2005.05701.x. [DOI] [PubMed] [Google Scholar]
- 69.Kohri K, Yasui T, Okada A, Hirose M, Hamamoto S, et al. Biomolecular mechanism of urinary stone formation involving osteopontin. Urol Res. 2012;40:623–637. doi: 10.1007/s00240-012-0514-y. [DOI] [PubMed] [Google Scholar]
- 70.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 71.Ryall RL. Macromolecules and urolithiasis: parallels and paradoxes. Nephron Physiol. 2004;98:37–42. doi: 10.1159/000080262. [DOI] [PubMed] [Google Scholar]
- 72.Sethman I, Grohe B, Kleebe H-J. Replacement of hydroxyapatite by whewellite: implications for kidney-stone formation. Mineralogical Magazine. 2014;78:91–100. [Google Scholar]
- 73.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 74.Shanahan CM. Vascular calcification. Curr Opin Nephrol Hypertens. 2005;14:361–367. doi: 10.1097/01.mnh.0000172723.52499.38. [DOI] [PubMed] [Google Scholar]
- 75.Briet M, Burns KD. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci (Lond) 2012;123:399–416. doi: 10.1042/CS20120074. [DOI] [PubMed] [Google Scholar]
- 76.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 77.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–e12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 78.Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends Cardiovasc Med. 2012;22:133–137. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial. 2007;20:103–109. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 81.Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- 82.Schoppet M, Shroff RC, Hofbauer LC, Shanahan CM. Exploring the biology of vascular calcification in chronic kidney disease: what's circulating? Kidney Int. 2008;73:384–390. doi: 10.1038/sj.ki.5002696. [DOI] [PubMed] [Google Scholar]
- 83.Murshed M, McKee MD. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr Opin Nephrol Hypertens. 2010;19:359–365. doi: 10.1097/MNH.0b013e3283393a2b. [DOI] [PubMed] [Google Scholar]
- 84.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun Y, Byon CH, Yuan K, Chen J, Mao X, et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tada Y, Yano S, Yamaguchi T, Okazaki K, Ogawa N, et al. Advanced glycation end products-induced vascular calcification is mediated by oxidative stress: functional roles of NAD(P)H-oxidase. Horm Metab Res. 2013;45:267–272. doi: 10.1055/s-0032-1329965. [DOI] [PubMed] [Google Scholar]
- 87.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, et al. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Irwin CL, Guzman RJ. Matrix metalloproteinases in medial arterial calcification: potential mechanisms and actions. Vascular. 2009;17(Suppl 1):S40–S44. doi: 10.2310/6670.2008.00086. [DOI] [PubMed] [Google Scholar]
- 89.Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–773. doi: 10.1016/j.ajpath.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, et al. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vyavahare N, Jones PL, Tallapragada S, Levy RJ. Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol. 2000;157:885–893. doi: 10.1016/S0002-9440(10)64602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 93.Shanahan CM, Proudfoot D, Farzaneh-Far A, Weissberg PL. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr. 1998;8:357–375. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 94.Meier M, Weng LP, Alexandrakis E, Ruschoff J, Goeckenjan G. Tracheobronchial stenosis in Keutel syndrome. Eur Respir J. 2001;17:566–569. doi: 10.1183/09031936.01.17305660. [DOI] [PubMed] [Google Scholar]
- 95.Luo G, Ducy P, McKee M, Pinero G, Loyer E, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 96.Murshed M, Schinke T, McKee M, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farzaneh-Far A, Davies JD, Braam LA, Spronk HM, Proudfoot D, et al. A polymorphism of the human matrix gamma-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J Biol Chem. 2001;276:32466–32473. doi: 10.1074/jbc.M104909200. [DOI] [PubMed] [Google Scholar]
- 98.Herrmann SM, Whatling C, Brand E, Nicaud V, Gariepy J, et al. Polymorphisms of the human matrix gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20:2386–2393. doi: 10.1161/01.atv.20.11.2386. [DOI] [PubMed] [Google Scholar]
- 99.Herrmann M, Kinkeldey A, Jahnen-Dechent W. Fetuin-A function in systemic mineral metabolism. Trends Cardiovasc Med. 2012;22:197–201. doi: 10.1016/j.tcm.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Jahnen-Dechent W, Heiss A, Schafer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ Res. 2011;108:1494–1509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 101.Jahnen-Dechent W, Schafer C, Ketteler M, McKee MD. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med (Berl) 2008;86:379–389. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 102.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, et al. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 103.Ketteler M, Wanner C, Metzger T, Bongartz P, Westenfeld R, et al. Deficiencies of calcium-regulatory proteins in dialysis patients: a novel concept of cardiovascular calcification in uremia. Kidney Int Suppl. 2003:S84–S87. doi: 10.1046/j.1523-1755.63.s84.21.x. [DOI] [PubMed] [Google Scholar]
- 104.Huang HS, Ma MC, Chen CF, Chen J. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology. 2003;62:1123–1128. doi: 10.1016/s0090-4295(03)00764-7. [DOI] [PubMed] [Google Scholar]
- 105.Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res. 2005;33:65–69. doi: 10.1007/s00240-004-0444-4. [DOI] [PubMed] [Google Scholar]
- 106.Boonla C, Wunsuwan R, Tungsanga K, Tosukhowong P. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res. 2007;35:185–191. doi: 10.1007/s00240-007-0098-0. [DOI] [PubMed] [Google Scholar]
- 107.Schwille PO, Manoharan M, Schmiedl A. Is idiopathic recurrent calcium urolithiasis in males a cellular disease? Laboratory findings in plasma, urine and erythrocytes, emphasizing the absence and presence of stones, oxidative and mineral metabolism: an observational study. Clin Chem Lab Med. 2005;43:590–600. doi: 10.1515/CCLM.2005.103. [DOI] [PubMed] [Google Scholar]
- 108.Escobar C, Byer KJ, Khaskheli H, Khan SR. Apatite induced renal epithelial injury: insight into the pathogenesis of kidney stones. J Urol. 2008;180:379–387. doi: 10.1016/j.juro.2008.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grewal JS, Tsai JY, Glenton PA, Byer KJ, Khan SR. Oxalate toxicity induces an immunosuppressive lipocalin, alpha 1-microglobulin in LLC-PK cells via intermediating reactive oxygen species. Journal of the American Society of Nephrology. 2002;13:493A–493A. [Google Scholar]
- 110.Grewal JS, Tsai JY, Khan SR. Oxalate-inducible AMBP gene and its regulatory mechanism in renal tubular epithelial cells. Biochem J. 2005;387:609–616. doi: 10.1042/BJ20041465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Habibzadegah-Tari P, Byer KG, Khan SR. Reactive oxygen species mediated calcium oxalate crystal-induced expression of MCP-1 in HK-2 cells. Urol Res. 2006;34:26–36. doi: 10.1007/s00240-005-0007-3. [DOI] [PubMed] [Google Scholar]
- 112.Hatanaka Y, Umekawa T, Kurita T, Khan SR. Angiotensin II type 1 receptor blockade prevents calcification in ethylene glycol treated rat kidney - Relation to kidney inflammation. Journal of the American Society of Nephrology. 2002;13:576A–576A. [Google Scholar]
- 113.Thamilselvan S, Byer KJ, Hackett RL, Khan SR. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol. 2000;164:224–229. [PubMed] [Google Scholar]
- 114.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157:1059–1063. [PubMed] [Google Scholar]
- 115.Umekawa T, Kurita T, Khan SR. Calcium oxalate monorydrateand, brushite and oxalate ions stimulate MCP-1 production in NRK 52E cells. Journal of Urology. 2002;167:258–258. [Google Scholar]
- 116.Aihara K, Byer KJ, Khan SR. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int. 2003;64:1283–1291. doi: 10.1046/j.1523-1755.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- 117.Joshi S, Saylor BT, Wang W, Peck AB, Khan SR. Apocynin-treatment reverses hyperoxaluria induced changes in NADPH oxidase system expression in rat kidneys: a transcriptional study. PLoS One. 2012;7:e47738. doi: 10.1371/journal.pone.0047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zuo J, Khan A, Glenton PA, Khan SR. Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-L-proline-induced hyperoxaluria in the male Sprague-Dawley rats. Nephrol Dial Transplant. 2011;26:1785–1796. doi: 10.1093/ndt/gfr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Umekawa T, Tsuji H, Uemura H, Khan SR. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int. 2009;104:115–120. doi: 10.1111/j.1464-410X.2009.08374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Umekawa T, Byer K, Uemura H, Khan SR. Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant. 2005;20:870–878. doi: 10.1093/ndt/gfh750. [DOI] [PubMed] [Google Scholar]
- 121.Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant. 2003;18:664–669. doi: 10.1093/ndt/gfg140. [DOI] [PubMed] [Google Scholar]
- 122.Khan SR, Khan A, Byer KJ. Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals. Nephrol Dial Transplant. 2011;26:1778–1785. doi: 10.1093/ndt/gfq692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Umekawa T, Hatanaka Y, Kurita T, Khan SR. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol. 2004;15:635–644. doi: 10.1097/01.asn.0000113321.49771.2d. [DOI] [PubMed] [Google Scholar]
- 124.Gambaro G, D'Angelo A, Fabris A, Tosetto E, Anglani F, et al. Crystals, Randall's plaques and renal stones: do bone and atherosclerosis teach us something? J Nephrol. 2004;17:774–777. [PubMed] [Google Scholar]
- 125.Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA. Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol. 2002;168:1173–1181. doi: 10.1016/S0022-5347(05)64621-6. [DOI] [PubMed] [Google Scholar]
- 126.Gokhale JA, Glenton PA, Khan SR. Localization of tamm-horsfall protein and osteopontin in a rat nephrolithiasis model. Nephron. 1996;73:456–461. doi: 10.1159/000189110. [DOI] [PubMed] [Google Scholar]
- 127.Khan A, Wang W, Khan SR. Calcium oxalate nephrolithiasis and expression of matrix GLA protein in the kidneys. World J Urol. 2013 doi: 10.1007/s00345-013-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yasui T, Fujita K, Sasaki S, Sato M, Sugimoto M, et al. Expression of bone matrix proteins in urolithiasis model rats. Urol Res. 1999;27:255–261. doi: 10.1007/s002400050119. [DOI] [PubMed] [Google Scholar]
- 129.Khan SR, Joshi S, Wang W, Peck AB. Regulation of Macromolecular Modulators of Urinary Stone Formation by Reactive Oxygen Species: Transcriptional Study in an Animal Model of Hyperoxaluria. Am J Physiol Renal Physiol. 2014 doi: 10.1152/ajprenal.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Canales BK, Anderson L, Higgins L, Frethem C, Ressler A, et al. Proteomic analysis of a matrix stone: a case report. Urol Res. 2009;37:323–329. doi: 10.1007/s00240-009-0213-5. [DOI] [PubMed] [Google Scholar]
- 131.Kumar V, Farell G, Yu S, Harrington S, Fitzpatrick L, et al. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med. 2006;54:412–424. doi: 10.2310/6650.2006.06021. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 133.Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, et al. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shoshani O, Zipori D. Transition of endothelium to cartilage and bone. Cell Stem Cell. 2011;8:10–11. doi: 10.1016/j.stem.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Stejskal D, Karpisek M, Vrtal R, Student V, Solichova P, et al. Urine fetuin-A values in relation to the presence of urolithiasis. BJU Int. 2008;101:1151–1154. doi: 10.1111/j.1464-410X.2007.07432.x. [DOI] [PubMed] [Google Scholar]
- 136.Salama RH, Alghasham A, Mostafa MS, El-Moniem AE. Bone morphogenetic protein-2 will be a novel biochemical marker in urinary tract infections and stone formation. Clin Biochem. 2012;45:766–769. doi: 10.1016/j.clinbiochem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 137.Gao B, Yasui T, Itoh Y, Tozawa K, Hayashi Y, et al. A polymorphism of matrix Gla protein gene is associated with kidney stones. J Urol. 2007;177:2361–2365. doi: 10.1016/j.juro.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 138.Lu X, Gao B, Liu Z, Tian X, Mao X, et al. A polymorphism of matrix Gla protein gene is associated with kidney stone in the Chinese Han population. Gene. 2012;511:127–130. doi: 10.1016/j.gene.2012.09.112. [DOI] [PubMed] [Google Scholar]
- 139.Jia Z, Wang S, Tang J, He D, Cui L, et al. Does crystal deposition in genetic hypercalciuric rat kidney tissue share similarities with bone formation? Urology. 2014;83:509, e507–e509, e514. doi: 10.1016/j.urology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 140.Kageyama S, Ohtawara Y, Fujita K, Watanabe T, Ushiyama T, et al. Microlith formation in vitro by Madin Darby canine kidney (MDCK) cells. Int J Urol. 1996;3:23–26. doi: 10.1111/j.1442-2042.1996.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 141.Naito Y, Ohtawara Y, Kageyama S, Nakano M, Ichiyama A, et al. Morphological analysis of renal cell culture models of calcium phosphate stone formation. Urol Res. 1997;25:59–65. doi: 10.1007/BF00941907. [DOI] [PubMed] [Google Scholar]
- 142.Senzaki H, Yasui T, Okada A, Ito Y, Tozawa K, et al. Alendronate inhibits urinary calcium microlith formation in a three-dimensional culture model. Urol Res. 2004;32:223–228. doi: 10.1007/s00240-004-0409-7. [DOI] [PubMed] [Google Scholar]
- 143.Habibovic P, Bassett DC, Doillon CJ, Gerard C, McKee MD, et al. Collagen biomineralization in vivo by sustained release of inorganic phosphate ions. Adv Mater. 2010;22:1858–1862. doi: 10.1002/adma.200902778. [DOI] [PubMed] [Google Scholar]
- 144.Golub EE. Biomineralization and matrix vesicles in biology and pathology. Semin Immunopathol. 2010 doi: 10.1007/s00281-010-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Khan SR, Atmani F, Glenton P, Hou Z, Talham DR, et al. Lipids and membranes in the organic matrix of urinary calcific crystals and stones. Calcif Tissue Int. 1996;59:357–365. doi: 10.1007/s002239900140. [DOI] [PubMed] [Google Scholar]
- 146.Khan SR, Glenton PA, Backov R, Talham DR. Presence of lipids in urine, crystals and stones: Implications for the formation of kidney stones. Kidney International. 2002;62:2062–2072. doi: 10.1046/j.1523-1755.2002.00676.x. [DOI] [PubMed] [Google Scholar]
- 147.Khan SR, Shevock PN, Hackett RL. INVITRO PRECIPITATION OF CALCIUM-OXALATE IN THE PRESENCE OF WHOLE MATRIX OR LIPID COMPONENTS OF THE URINARY STONES. Journal of Urology. 1988;139:418–422. doi: 10.1016/s0022-5347(17)42447-5. [DOI] [PubMed] [Google Scholar]
- 148.Khan SR, Maslamani SA, Atmani F, Glenton PA, Opalko FJ, et al. Membranes and their constituents as promoters of calcium oxalate crystal formation in human urine. Calcif Tissue Int. 2000;66:90–96. doi: 10.1007/s002230010019. [DOI] [PubMed] [Google Scholar]
- 149.Silva Marques J, Pinto FJ. The vulnerable plaque: Current concepts and future perspectives on coronary morphology, composition and wall stress imaging. Rev Port Cardiol. 2014 doi: 10.1016/j.repc.2013.07.017. [DOI] [PubMed] [Google Scholar]