Summary
Hunger is controlled by specialized neural circuits that translate homeostatic needs into motivated behaviors. These circuits are under chronic control by circulating signals of nutritional state, but their rapid dynamics on the timescale of behavior remain unknown. Here we report optical recording of the natural activity of two key cell types that control food intake, AgRP and POMC neurons, in awake behaving mice. We find unexpectedly that the sensory detection of food is sufficient to rapidly reverse the activation state of these neurons induced by energy deficit. This rapid regulation is cell-type-specific, modulated by food palatability and nutritional state, and occurs before any food is consumed. These data reveal that AgRP and POMC neurons receive real-time information about the availability of food in the external world, suggesting a primary role for these neurons in controlling appetitive behaviors such as foraging that promote the discovery of food.
Introduction
Food intake is controlled by evolutionarily hard-wired neural circuits that contain specialized neural cell types. Two cell types in the arcuate nucleus (ARC) of the hypothalamus are known to be particularly important for the control of feeding. These neurons are identified by expression of the neuropeptides Agouti-related Protein (AgRP) and Proopiomelanocortin (POMC) and have opposing functions. AgRP neurons are activated by energy deficit (Hahn et al., 1998) and promote food seeking and consumption. Optogenetic or chemogenetic activation of AgRP neurons induces voracious eating in sated mice (Aponte et al., 2011; Krashes et al., 2011), whereas inhibition or ablation of AgRP neurons results in aphagia (Gropp et al., 2005; Krashes et al., 2011; Luquet et al., 2005). These effects of AgRP neurons are mediated by release of GABA as well as two neuropeptides, AgRP and NPY, that stimulate food intake when delivered into the brain (Clark et al., 1985; Fan et al., 1997; Ollmann et al., 1997; Tong et al., 2008). POMC neurons by contrast are activated by energy surfeit and their activity inhibits food intake and promotes weight loss. These two cell types interact in part through a common set of downstream neural targets that express melanocortin receptors, which are activated by POMC and inhibited by AgRP (Fan et al., 1997; Ollmann et al., 1997; Seeley et al., 1997). Thus AgRP and POMC neurons are two intermingled, interacting neural cell types that have opposing roles in the control of feeding.
Despite intense investigation of these cells over the past 20 years, their activity dynamics during behavior remain unknown. This knowledge gap reflects the difficulty of recording cell-type-specific neural activity within heterogeneous deep brain structures such as the hypothalamus. As a result our current understanding of the regulation of AgRP and POMC neurons is based on a combination of approaches that include in vitro electrophysiology, c-fos staining, pharmacology, and genetic manipulations. These pioneering studies have revealed a dominant role for circulating hormones and nutrients in the control of these cells (Williams and Elmquist, 2012). AgRP and POMC neurons are modulated by hormones such as ghrelin and leptin (Cowley et al., 2001; Cowley et al., 2003; Nakazato et al., 2001; Pinto et al., 2004) as well as circulating nutrients (Blouet and Schwartz, 2010) in part via their metabolic effects on mitochondrial dynamics (Dietrich et al., 2013; Schneeberger et al., 2013). Together these findings have led to a generally accepted model in which AgRP and POMC neurons function as interoceptors that monitor the concentration of hormones and nutrients in the blood and then gradually adjust their activity in parallel with changes in nutritional state. This model provides a compelling explanation for how nutritional changes can be translated into counterregulatory responses but leaves unanswered the question of whether these neurons are also subject to rapid regulation on the timescale of behavior.
AgRP and POMC neurons also receive abundant synaptic input which provides the potential for more rapid modulation. However the function of this afferent input is not well understood. Fasting increases excitatory tone onto AgRP neurons (Liu et al., 2012; Yang et al., 2011), and one source of such excitatory input is neurons in the paraventricular hypothalamus (PVH) (Krashes et al., 2014). AgRP neurons also receive inhibitory input from the dorsomedial hypothalamus (DMH) among other sources (Krashes et al., 2014). POMC neurons by contrast receive inhibitory input from cells in the ARC, including neighboring AgRP neurons, as well as excitatory input from the ventromedial hypothalamus (VMH) and other regions (Cowley et al., 2001; Krashes et al., 2014; Pinto et al., 2004; Sternson et al., 2005; Vong et al., 2011). As these circuit connections have only recently been described, their regulation and function are not yet clear. An important open question regards the nature of the information that these presynaptic cells communicate to their AgRP and POMC targets.
In the present study we have used an optical approach to record the natural activity of AgRP and POMC neurons in awake behaving mice. These experiments have unexpectedly revealed that AgRP and POMC neurons are strongly regulated in vivo by the sensory detection of food. This rapid sensory regulation resets the activation state of these cells induced by food deprivation prior to the start of food consumption. This rapid regulation also contains information about the food’s hedonic properties and depends on the animal’s nutritional state. These findings reveal that AgRP and POMC neurons receive real-time information about the availability of food in the outside world, which they then use to anticipate the nutritional value of a forthcoming meal and adjust their activity in advance. This anticipatory regulation provides a mechanism to rapidly inhibit foraging upon food discovery, suggesting a primary role for these neurons in the regulation of appetitive behaviors in vivo.
Results
Optical recording of AgRP and POMC neuron activity in awake behaving mice
In order to gain deeper insight into the regulation of AgRP and POMC neurons we sought to record their natural activity during feeding behavior. To do this we used fiber photometry (Cui et al., 2013; Gunaydin et al., 2014), an approach that employs a multimode optical fiber to record the total fluorescence from a population of neurons expressing a calcium reporter for neural activity (Figure 1F). By targeting the calcium reporter to a specific cell type, this method enables optical recording of the real-time activity of a molecularly defined population of neurons within a deep brain structure. The resulting trace represents the integrated activity of the neurons defined by a genetic marker and anatomic location and therefore is particularly well-suited for use in the hypothalamus, which contains genetically separable populations of neurons with distinct functions.
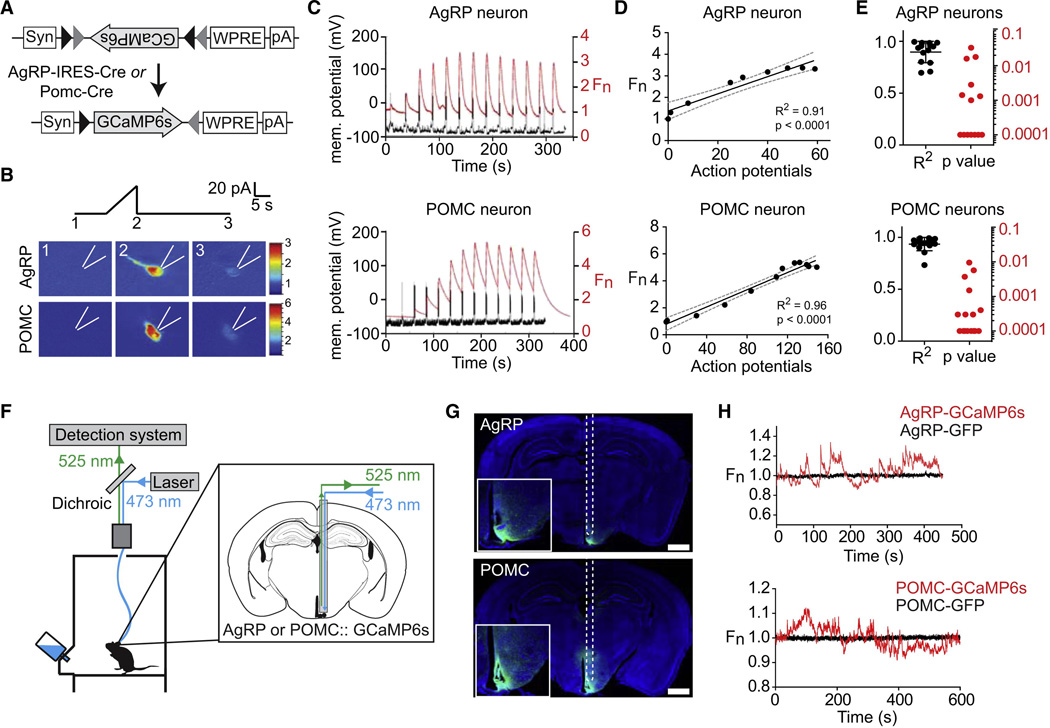
Figure 1. Optical recording of AgRP and POMC neuron activity in awake behaving mice.
(A) FLEX AAV used to drive GCaMP6s expression. (B) Response of AgRP and POMC neurons to current ramp. Scale bar indicates GCaMP6s fluorescence normalized to 1.0 at start of the experiment (Fn). (C) Membrane potential and GCaMP6s fluorescence in response to sequential 10 pA current steps of duration 2s separated by 20s. (D) Relationship between action potential number and fluorescence for cells in panel C. (E) R-squared and p values for the linear regression of fluorescence versus action potential number for 16 POMC and 14 AgRP neurons. (F) Schematic of the fiber photometry setup. (G) Coronal section from AgRP and POMC mice showing path of optical fiber and injection site. Scale bar = 1 mm (H) Fluorescence trace during cage exploration for mice expressing GCaMP6s or GFP in AgRP neurons or POMC neurons. See also Figure S1.
We first confirmed that calcium signals from AgRP and POMC neurons correlate with changes in firing rate ex vivo. We targeted the sixth generation calcium reporter GCaMP6s (Chen et al., 2013) to AgRP and POMC neurons by stereotaxic injection of Cre-dependent AAVs into AgRP-IRES-Cre and POMC-Cre mice (Figure 1A) and then prepared acute brain slices for imaging and intracellular recording. Fluorescent cells in the ARC were identified for whole-cell patch clamp recordings and held at −70 mV in current clamp. Activation by depolarizing current ramp (0–40 pA, 10s) induced bursts in firing accompanied by increased GCaMP6s fluorescence (Figure 1B). To quantify the relationship between firing rate and fluorescence signal we applied step currents (−20 pA to +120 pA, 10 pA increments), which resulted in progressive increases in spikes and fluorescence (Figure 1C). Quantification of this response revealed a linear correlation between action potential number and GCaMP6s signal (Figure 1D–E). Thus GCaMP6s can report on activity dynamics in AgRP and POMC neurons as shown for other cell types (Chen et al., 2013).
To apply this approach in vivo, we injected AAVs expressing GCaMP6s into the ARC of the corresponding Cre mice and in the same surgery installed an optical fiber unilaterally above the ARC (Figure S1). After allowing two weeks for transgene expression, we connected mice to a photometry rig and recorded fluorescence from these cells as mice explored a feeding chamber without access to food. Baseline recordings from AgRP and POMC neurons showed dynamic fluctuations (~10–20% ΔF/F) that resembled bursts of synchronous activity observed in other cell types (Cui et al., 2013; Gunaydin et al., 2014) (Figure 1H). These dynamics were unrelated to mouse movement, unaffected by changes in ambient lighting, and absent from recordings from control mice expressing GFP in AgRP or POMC neurons (Figure 1H), indicating that they represent calcium-dependent GCaMP6s signals.
To test the sensitivity of this assay to detect changes in neural activity, we challenged mice with ghrelin, a hormone that activates AgRP neurons and inhibits POMC neurons (Cowley et al., 2003; Nakazato et al., 2001). Mice expressing GCaMP6s in either AgRP or POMC neurons were acclimated to a behavioral chamber, given an intraperitoneal injection of ghrelin, and then returned to the chamber. Ghrelin sharply increased calcium signals from AgRP neurons (ΔF/F = 71 +/− 10% at 5 min, p<0.001 compared to vehicle; Figure 2A,B and Movie S1). This increase began within seconds of injection (mean latency = 33 +/− 7s) and reached a plateau within two minutes (τ = 76 +/− 12s, where τ is the exponential time constant corresponding to the time after injection resulting in ~63.8% of the total change). In the absence of further intervention, this increase in AgRP activity was sustained for the duration of the recording (ΔF/F = 62 +/− 10% at 15 min; Figure 2B). By contrast injection of vehicle (PBS) had no effect on the activity of AgRP neurons (ΔF/F = −3 +/− 2% at 5 min Figure 2B and Movie S1).
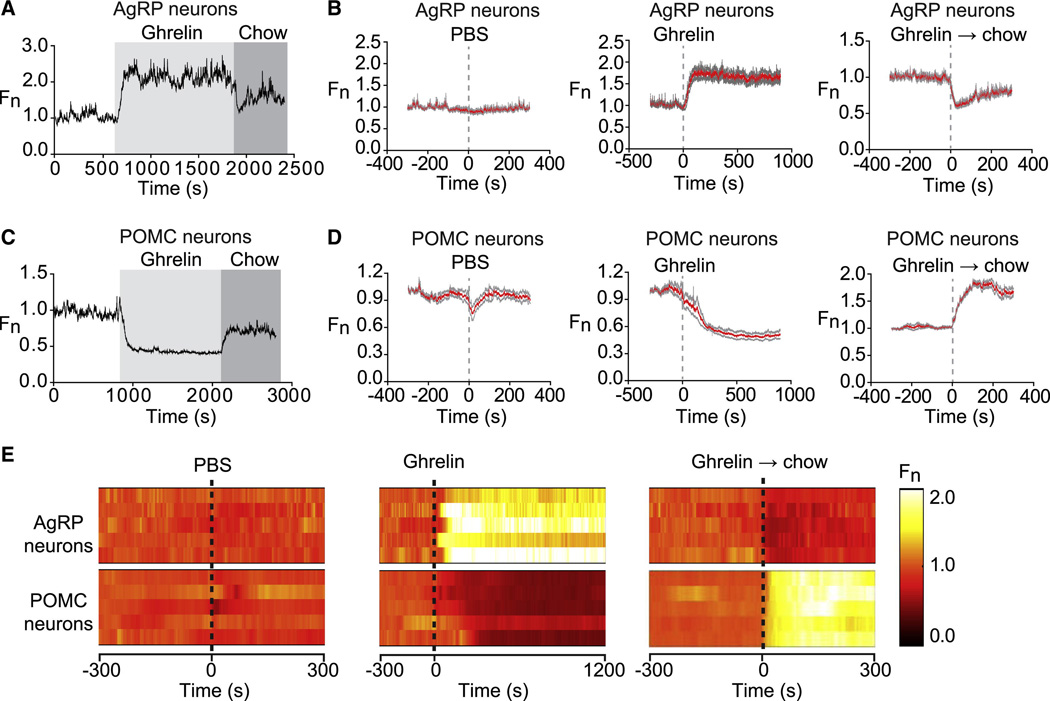
Figure 2. Ghrelin rapidly modulates AgRP and POMC neurons.
(A and C) Recordings from a mouse expressing GCaMP6s in AgRP or POMC neurons that was challenged with injection of ghrelin (light gray) followed by presentation of a pellet of chow (dark gray). (B and D). Calcium signals from AgRP and POMC neurons aligned to the time of PBS or ghrelin injection, or chow presentation to ghrelin treated mice. Red and gray indicate the mean response and standard error (AgRP, n=7; POMC, n=5). In each trial fluorescence was normalized by assigning a value of 1.0 to the median value of data points within a two minute window at −5 min before treatment. (E) Peri-event plots showing the response from a single trial of five AgRP mice and five POMC mice.
POMC neurons showed the opposite response, with ghrelin injection rapidly and potently inhibiting POMC activity (τ = 160 +/− 17s; ΔF/F = −49 +/− 4% at 15 min, p=0.001 compared to vehicle; Figure 2C,D and Movie S2). Interestingly vehicle injection alone produced a small but reversible drop in POMC activity (Figure 2D and Movie S2). This transient decline in POMC activity was consistently observed following animal handling, suggesting that POMC but not AgRP neurons receive an inhibitory stress regulated input.
We next tested the effect of food on the response to ghrelin. Our prediction based on the known nutritional regulation of these cells was that food consumption would gradually inhibit AgRP neurons and activate POMC neurons as animals transitioned from hunger to satiety. To test this we challenged mice with ghrelin and then 20 minutes later presented them with a pellet of chow. Unexpectedly we found that food presentation alone rapidly reversed much of the effect of ghrelin treatment (ΔF/F = −29 +/− 3% at 2 min for AgRP neurons and ΔF/F = 80 +/− 3% at 2 min for POMC neurons; Figure 2). This response began immediately upon placing food in the cage and was complete within seconds (τ = 12 +/− 2s for AgRP neurons; τ = 44 +/− 3s for POMC neurons). All animals tested showed this response to food presentation (traces for ten mice are shown in Figure 2E), suggesting that it represents a general mechanism that regulates the activity of these neurons in vivo.
Food detection reverses the effects of fasting on AgRP and POMC activity
The regulation of AgRP and POMC neurons by sensory detection of food has not previously been described. To investigate this phenomenon under more physiologic conditions, we fasted mice overnight and then presented a pellet of chow. As observed for ghrelin-treated animals, food presentation to fasted mice strongly inhibited AgRP neurons (ΔF/F = −37 +/− 4%, at 5 min, p<0.001 compared to object) and activated POMC neurons (ΔF/F = 38 +/− 5% at 5 min, p<0.001 compared to object; Figure 3 and Movies S3,S4). These responses began the moment that food was presented and were rapidly complete (τ = 20 +/− 4s for AgRP neurons and τ = 42 +/− 18s for POMC neurons). To quantify the extent to which these changes required food consumption, we analyzed video data to estimate the moment at which the first bite of food was consumed in each trial and then aligned calcium traces to this event. This revealed that most of the activity changes in these neurons were already complete by the time food intake was initiated (96 +/− 6% complete before feeding in AgRP neurons, 85 +/− 5% in POMC neurons; Figure 3H,I). Thus the response of AgRP and POMC neurons to food is triggered primarily by food detection rather food consumption. Of note, these stereotyped responses to food presentation were consistently observed in the first trial of each mouse (Figure 3G), indicating that this effect does not require prior training.
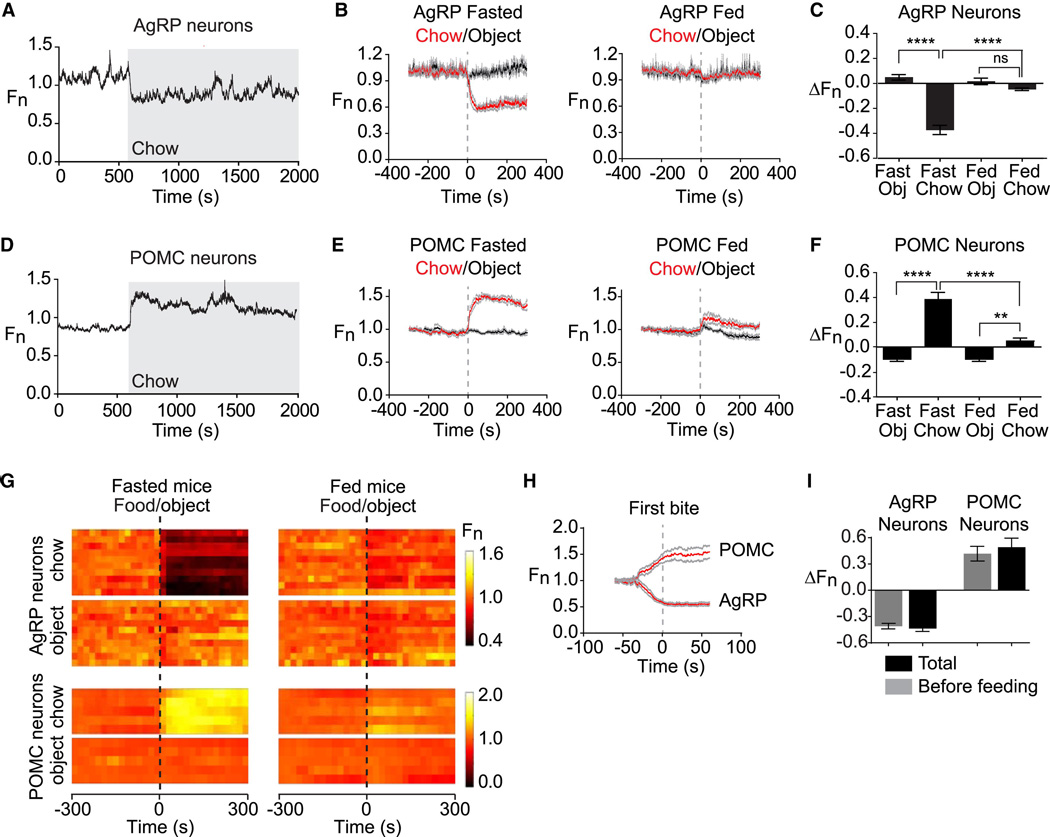
Figure 3. Sensory detection of food rapidly regulates AgRP and POMC neurons.
(A and D) Recordings from fasted mice expressing GCaMP6s in AgRP or POMC neurons presented with a pellet of chow (gray). (B and E) Plots of calcium signals from AgRP and POMC neurons aligned to the time of presentation of a pellet of chow (red) or inedible object (black). Mice were either subjected to an overnight fast (left) or fed ad libitum (right) prior to experiment. Gray indicates standard error (AgRP, n=10; POMC, n=5). (C and F) Quantification of fluorescence changes 5 min after event, as indicated. (G) Peri-event plots aligned to the time of event. Each row is a single trial of a different mouse. (H) Calcium signals aligned to the initiation of feeding for AgRP and POMC neurons. (I) Quantification of change in fluorescence occurs before feeding is initiated versus the total change in the trial. * p<0.05. ** p<0.01,*** p<0.001,**** p<0.0001.
We investigated the determinants of this rapid response to food discovery. Presentation of an inedible object (a rubber stopper similar in size to a piece of chow) had little effect on the activity of AgRP neurons (ΔF/F = 4.9 +/− 2.2%) and induced a small change in POMC neurons in the opposite direction of food (ΔF/F = −10 +/− 2%). Thus the response of these neurons to food presentation is food-specific (Figure 3 and Movies S3,S4). The sensitivity of these cells to food presentation also depended on nutritional state, as AgRP neurons from ad libitum fed mice showed no response to food presentation (ΔF/F = −4.7 +/− 1.0%, p=0.21 compared to object) whereas POMC neurons from ad libitum fed mice showed a greatly diminished response (ΔF/F = 4.7 +/− 2.4%, p=0.01 compared to object; Figure 3E,F). Thus conditions that reflect energy deficit, such as fasting or ghrelin treatment, potentiate the response of AgRP and POMC neurons to food detection.
Food quality influences the magnitude of the response
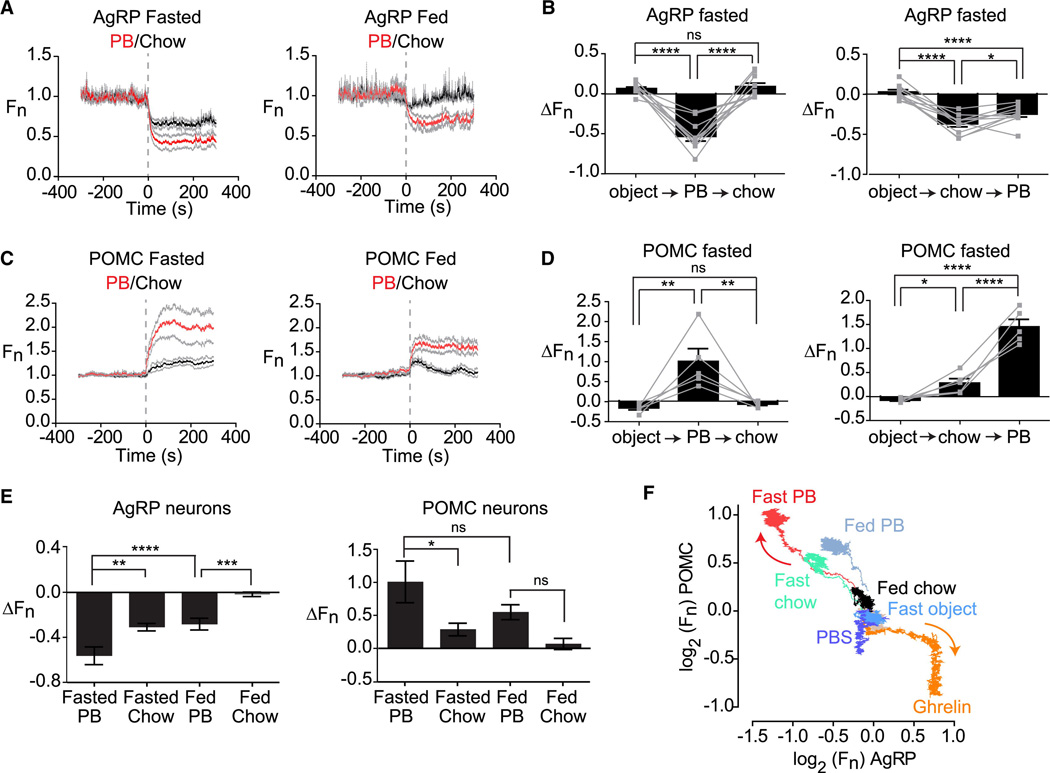
We considered the possibility that the response of AgRP and POMC neurons to food presentation depends on the food’s hedonic properties. In this regard sensory cues associated with palatable or energy dense foods trigger activation of brain regions involved in reward, but how this hedonic information is integrated with homeostatic signals remains poorly understood. To investigate this we first measured the response to peanut butter, an energy dense food that mice will eat in preference to chow and is considered rewarding. Mice were fasted overnight, acclimated to a behavioral chamber, and then presented with either pellet of chow or a dollop of peanut butter. Presentation of peanut butter strongly inhibited AgRP neurons (ΔF/F = −54 +/− 6% at 5 min; Figure 4A) and activated POMC neurons (ΔF/F = 101 +/− 31% at 5 min; Figure 4C). These responses began immediately upon food presentation (Movies S5 and S6) and were complete in less than one minute (τ = 23 +/− 6s for AgRP and τ = 29 +/− 6s for POMC). The responses to peanut butter were significantly larger than the responses to chow (Figure 4E) and indeed were comparable in magnitude (but opposite in sign) to the effect of injection with pharmacologic doses of ghrelin (Figure 4F), which to our knowledge is the strongest known stimulus that modulates these cells.
Figure 4. Food palatability determines the magnitude of the response to food detection.
(A and C) Calcium signals from AgRP and POMC neurons in fasted and fed mice aligned to the time of presentation of peanut butter or chow. (B and D) Fluorescence change of AgRP and POMC neurons upon sequential presentation of an inedible object, chow, and peanut butter in fasted mice. (E) Quantification of responses of AgRP and POMC neurons 5 min after food presentation. (F) Plot showing the response of AgRP and POMC neurons over 5 min to different foods and pharmacologic treatments in the context of varying nutritional states. All traces start at the origin (0,0) and emanate outward. Arrows indicate the direction of movement. See also Figure S2.
A defining feature of palatable foods is that animals will consume them in the absence of hunger because they are intrinsically rewarding (e.g. eating dessert after a meal). We therefore tested whether AgRP and POMC neurons from ad libitum fed mice, which show little or no response to chow (Figure 3), would nonetheless respond to the presentation of peanut butter. Indeed we found that presentation of peanut butter to ad libitum fed mice strongly inhibited AgRP neurons (ΔF/F = −24% +/− 4%, at 5 min, p<0.001 compared to chow) and activated POMC neurons (ΔF/F = 55 +/− 11%, at 5 min, p=0.14 compared to chow; Figure 4A,C). Thus more palatable food can modulate these neurons even in the absence of signals of energy deficit.
To further probe this relationship, we tested whether the response of these neurons to different foods depended on the order in which they were presented. Mice were fasted overnight and then sequentially presented with an inedible object, peanut butter, or chow in randomized order at 10 minute intervals. We then calculated the change in activity that occurred following each of these presentations. This revealed that presentation of peanut butter could completely block the subsequent neural response to presentation of chow (Figure 4B,D). By contrast, presentation of chow had no effect on the response to peanut butter in POMC neurons (Figure 4D) and only partially diminished the response in AgRP neurons (Figure 4B). The asymmetry in the response to these two foods is consistent with their differential effects in fasted and fed mice.
To extend these findings we tested a chocolate, a second food that is commonly used in rodent studies of reward. We found that presentation of chocolate (Hershey Kiss) to fasted mice inhibited AgRP neurons to a greater extent than chow (Figure S2A). Like peanut butter, chocolate also elicited a response in AgRP neurons from ad libitum fed mice that are unresponsive to chow (Figure S2B). Sequential presentation experiments revealed that chocolate could block the neural response to subsequent presentation of chow, but not vice versa, similar to our observations with peanut butter (Figure S2D,E). Although chocolate was a novel food for these animals, we observed responses to chocolate presentation in the first trial, indicating mice could identify it as food without prior experience. However the speed of the response to chocolate increased during subsequent tests, suggesting involvement of a learning process as well (τ = 40 +/− 8s in trial 1 versus 17 +/− 2s in trial 4, p<0.01; Figure S2C). Collectively these data show that the rapid sensory regulation of AgRP and POMC neurons contains information about the hedonic properties of the food that has been detected.
Food accessibility modulates the response to food discovery
Most of the response of AgRP and POMC neurons to food presentation occurred before food intake was initiated (Figure 3H,I). We therefore wondered whether food consumption played any role in this response. To test this mice were fasted overnight and then presented with peanut butter in a container that allowed the food to be seen and smelled but not consumed (Figure 5A). Presentation of this inaccessible peanut butter rapidly activated POMC neurons (ΔF/F = 43 +/− 9% after 2 min; τ = 31 +/− 8s) and inhibited AgRP neurons (ΔF/F = −39 +/− 4% after 2 min; τ = 21 +/− 4s; Figure 5B,C). Similar responses were observed in mice pretreated with ghrelin (Figure S3A,B). These responses occurred as quickly as the response to accessible food, but were somewhat smaller in magnitude (Figure S3C,D) and the response of POMC neurons was less durable (Figure 5B,C). This indicates that food accessibility can modulate the strength of the response to food presentation.
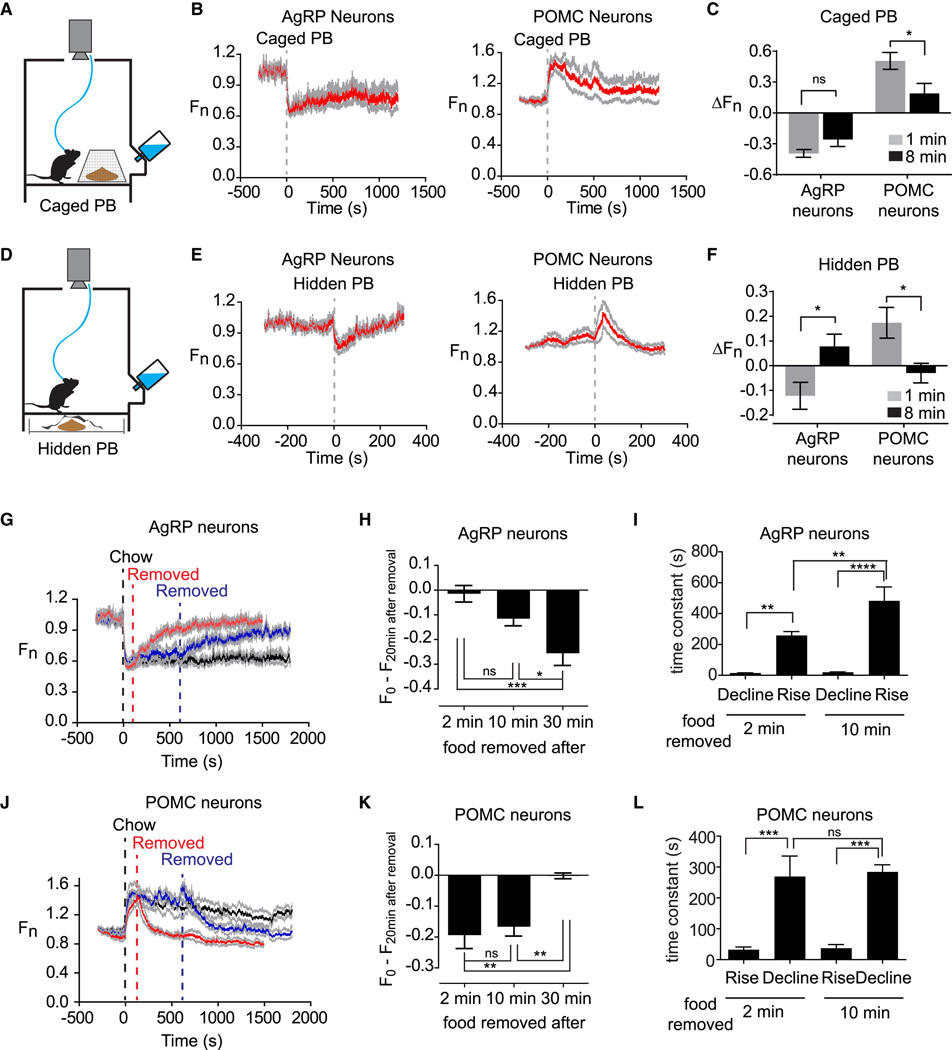
Figure 5. The response to food detection depends on food accessibility and is reversible.
(A) Schematic of caged peanut butter. (B) Calcium signals aligned to the time of presentation of a caged peanut butter. (C) Change in fluorescence in 1 and 8 min after caged peanut butter presentation. (D) Schematic of hidden peanut butter. (E) Calcium signals aligned to the time of presentation of a hidden peanut butter. (F) Change in fluorescence in 1 and 8 min after hidden peanut butter presentation. (G and J) Chow was presented at time 0, and then food was removed at 2 min (red), 10 min (blue) or not removed (black). (H and K) Recovery in fluorescence 20 min after food removal for experiments in which food was removed after 2, 10, or 30 min. (I and L) Time constant for the response to upon food presentation and food removal after 2 and 10 min. See also Figure S3.
To further dissect this effect we tested whether an isolated sensory cue could modulate the activity of these two cell types. As mice rely heavily on the sense of smell, we tested whether the smell of peanut butter could elicit an activity change in AgRP and POMC neurons. Mice were fasted overnight and then exposed to peanut butter placed underneath the cage in a covered container so that it could be smelled but not seen or accessed (Figure 5D). We found that this “hidden peanut butter” rapidly modulated AgRP and POMC neurons in a way that resembled food presentation (ΔF/F = −12 +/− 5% after 1 min in AgRP neurons and ΔF/F = 17 +/− 6% after 1 min in POMC neurons; Figure 5E,F). However this effect was much smaller in magnitude and transient, with neural activity returning to baseline within eight minutes (ΔF/F = 8.3 +/− 4.5% after 8 min in AgRP neurons and ΔF/F = −3.0 +/− 4.0% after 8 min in POMC neurons; Figure 5F and S3). Together these data suggest that food-associated sensory cues can modulate these two cell types, but that the magnitude and durability of this response depends on the extent to which these cues are interpreted as representing access to food.
Food removal reverses the effects of food presentation
The response of AgRP and POMC neurons to food presentation is consistent with a model in which these neurons anticipate the change in their activity that will occur after food consumption and then enact this change in advance, taking into account factors such as the food’s energy density, the food’s accessibility, and the animal’s nutritional state. A prediction of this model is that the response to food presentation should be reversed if the food is removed before it can be consumed. To test this mice were fasted overnight, presented with accessible chow, and then the food was removed after either a 2, 10, or 30 minute interval. As predicted we found that food removal reversed the effects of food presentation, resulting activation of AgRP neurons and inhibition of POMC neurons (Figure 5G, J; for clarity only data after 2 and 10 min removal are shown). The magnitude and kinetics of this reversal depended on the duration that mice were given food access. For example, mice given access to food for 30 minutes showed a smaller reversal of AgRP and POMC neuron activity following food removal than mice given access to food for 2 or 10 minutes (Figure 5H, K). Extended food access also slowed the response to food removal in AgRP but not POMC neurons (Figure 5I, L). These findings are consistent with food consumption during the feeding interval partially resetting the activation state of these neurons.
The response to food removal exhibited hysteresis, occurring approximately ten-fold more slowly than the initial response to food presentation (Figure 5I, L). This asymmetry was was observed after only 2 min food access in both AgRP (τ =15 +/− 1s versus 258 +/− 26s, p<0.0001) and POMC neurons (τ =19 +/− 3s versus 269 +/− 66s, p=0.03) and therefore was unlikely to be caused by the post-ingestive effects of food consumption. Rather this suggests that the circuit interprets the sensory detection of food in such a way that food removal induces a more gradual change than food discovery.
Neural dynamics within feeding bouts
We have focused on the initial response of AgRP and POMC neurons to food presentation, because this response is much larger than the fluctuations in the activity of these neurons that occur during feeding (Figure 3A,D). However we considered the possibility that these smaller intrameal dynamics might also be correlated with components of behavior. To test this we switched to a system in which mice were fed a liquid diet (vanilla Ensure) via a lickometer, so that we could align individual feeding bouts to photometry data with millisecond precision.
Mice were transitioned from a solid to liquid diet over several days, then fasted overnight and tested in an one hour trial. Licks were aligned to photometry traces, and individual feeding bouts defined as clusters of licks separated from their nearest neighbor by >20 seconds. This resulted in identification of an average of 17 +/− 2 feeding bouts in each one hour trial, with each bout lasting an average of 17 +/− 3 seconds and containing 53 +/− 10 licks. The start of each bout in a representative trial is indicated by gray lines in Figure 6A, B.
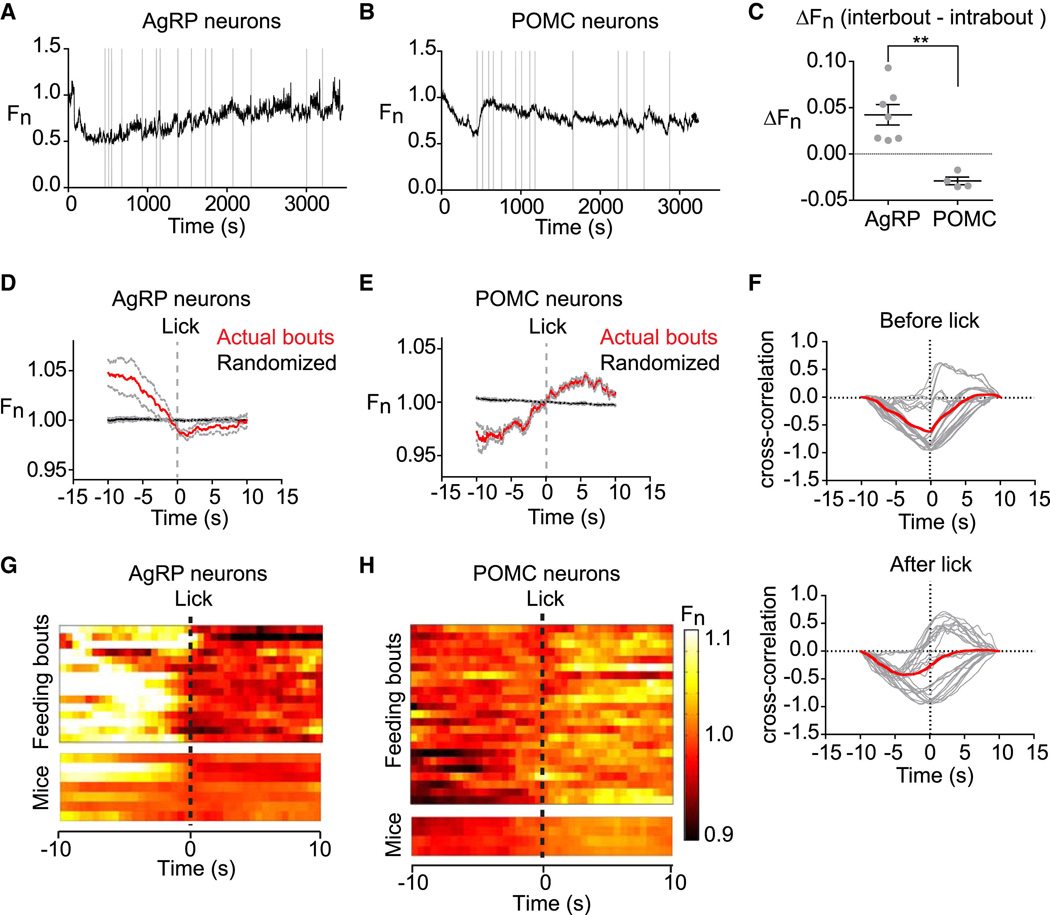
Figure 6. Intrameal dynamics of AgRP and POMC neurons.
(A, and B) Traces of AgRP and POMC activity in mice during consumption of a liquid diet. Licks that mark initiation of a feeding bout are shown in gray. (C) Difference in average fluorescence between periods of feeding (intrabout) and intermeal intervals (interbout) for each mouse. (D, E) Calcium signals from AgRP and POMC neurons aligned to the moment of the first lick that initiates a feeding bout. Data from actual feeding bouts shown in red; data from simulated randomly generated feeding bouts in black. (F) Cross-correlation plots showing the correlation between activity of AgRP and POMC neurons before and after licking. Red is mean, gray is 28 individual comparisons between AgRP (n=7) and POMC (n=4) mice. (G and H) Peri-event plots showing the activity of AgRP and POMC neurons aligned to the start of feeding bouts. The top plot shows all of the bouts for one trial of a mouse. The bottom plot shows the average response across all bouts for 7 AgRP and 4 POMC mice.
We compared the average activity of these neurons during active feeding (intrabout) versus intermeal intervals (interbout), by calculating the difference in fluorescence between these stages (interbout - intrabout). This revealed that POMC neurons were more active during feeding whereas AgRP neurons were less active (ΔFn = 0.042 +/− 0.011 for AgRP versus ΔFn = −0.029 +/− 0.004 for POMC, p = 0.001; Figure 6C). To investigate the dynamics underlying these differences, we aligned each feeding bout so that the start of the bout (first lick) corresponded to time zero and then analyzed a ten-second window flanking this moment. We found that AgRP and POMC neurons showed a consistent pattern of activity that predicted the onset of each meal. AgRP neurons declined in activity until the moment of the first lick and then their activity flattened (Figure 6D) whereas POMC neurons increased in activity prior to and throughout the start of feeding (Figure 6E). Cross-correlation analysis between AgRP and POMC showed that there was an inverse correlation between the activity of these two cell types that reached a peak at approximately time zero (Figure 6F). These effects were tightly linked to behavioral state, as they were robust to changes in the definition of a feeding bout (e.g. changes in the minimum intermeal interval) yet were completely absent when the data was reanalyzed using randomly generated feeding bouts (Figure 6D,E black). Remarkably, these intrameal anticipatory changes in AgRP and POMC activity appear to recapitulate, on a smaller scale, the dramatic changes in activity that occur in these neurons in response to food presentation.
Dynamics of AgRP projections to the PVH
AgRP neurons project broadly to brain regions involved in the control of food intake in a primarily one-to-one configuration (Betley et al., 2013). Optogenetic experiments have identified AgRP projections to the PVH as being particularly important for the control of feeding (Atasoy et al., 2012). As fiber photometry enables direct monitoring of axonal calcium transients (Gunaydin et al., 2014), we sought to record the activity of these key AgRP (ARC → PVH) projections during behavior.
AAVs expressing Cre-dependent GCaMP6s were delivered to the ARC of AgRP-IRESCre mice and in the same surgery an optical fiber was implanted ipsilaterally in the PVH (Figure 7A). Photometry recordings four weeks after surgery revealed spontaneous synchronous activity in these projections (Figure 7B) that resembled calcium dynamics observed in AgRP cell bodies (Figure 1H), albeit somewhat smaller in magnitude. Intraperitoneal injection of ghrelin but not vehicle induced a rapid increase in calcium signals in these projections (ΔF/F = 17 +/− 5% for ghrelin versus −9 +/− 3% for PBS at 5 min, p=0.02; Figure S4), indicating that they are appropriately regulated by hormonal signals.
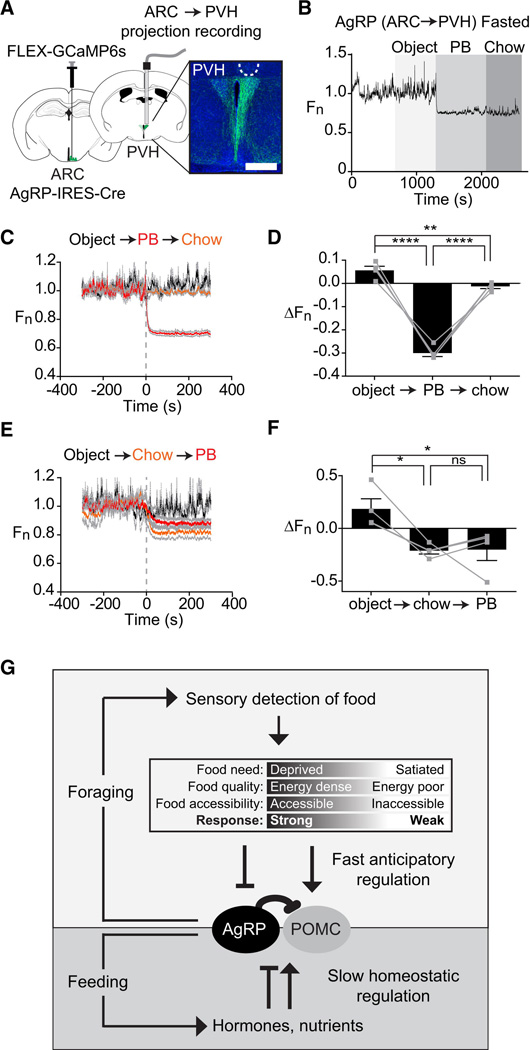
Figure 7. Natural dynamics of AgRP projections to the PVH.
(A) Schematic showing infection of cell bodies in the ARC and installation of optical fiber in the PVH. Scale bar = 0.5 mm (B) Recording from PVH of a fasted mouse presented sequentially with an inedible object, peanut butter, and chow. (C and E) Calcium signals from from PVH of mice presented sequentially with an inedible object, peanut butter, and chow. (D and F) Quantification of calcium signals five minutes after event. (n=4 mice). (G) Model for regulation of AgRP and POMC neurons by homeostatic and sensory information. See also Figure S4.
We next tested the effect of food presentation. Mice were fasted overnight and then presented with either an inedible object, chow, or peanut butter. Presentation of either chow or peanut butter rapidly and potently inhibited calcium dynamics in AgRP (ARC → PVH) projections (ΔF/F = −30 +/− 2% for peanut butter versus −21 +/− 3% for chow at 5 min) whereas presentation of an inedible object had no effect (Figure 7C,E). Of note, peanut butter almost completely eliminated detectable synchronous activity in PVH axons (Figure 7B, C), suggesting that palatable food presentation is particularly potent in suppressing the activity of this pathway. Assays utilizing sequential food presentation revealed a pattern of responses in PVH projections that closely resembled responses observed in AgRP cell bodies (Figure 7D, F). Likewise chow presentation partially reversed the activation of these PVH projections by ghrelin (Figure S4). Thus the activity of AgRP (ARC → PVH) projections is regulated by ghrelin and food presentation in a way that mirrors the population response in the ARC.
Discussion
It has been known for more than 75 years that the hypothalamus plays a critical role in the control of food intake (Hetherington and Ranson, 1939), yet the dynamics of the hypothalamic circuits that give rise to feeding behavior have remained a mystery. Here we have used an optical approach to record the natural dynamics of the two most widely-studied cell types that control feeding, AgRP and POMC neurons, in awake behaving mice. These experiments have revealed unexpectedly that these neurons are potently regulated by the sensory detection of food. This rapid regulation resets the activation state of AgRP and POMC neurons induced by orexigenic signals such as ghrelin or fasting. The magnitude and robustness of this response suggests that it is a primary mechanism that controls the activity of these neurons in vivo. The speed of this response suggests that it is likely mediated by neural input. The dependence on food palatability suggests that this response contains information about the food’s hedonic properties or energy content, possibly through a learned association with smells or other sensory cues. Collectively, these findings reveal that AgRP and POMC neurons receive real-time information about the availability of food in the external world, which they then integrate with homeostatic signals arising from the body (Figure 7G). This demonstrates a more complex and dynamic role for these circuits in the control of feeding behavior than is currently appreciated.
Sensory feedback enables rapid inhibition of appetitive processes
The rapid sensory regulation of AgRP and POMC neurons is counterintuitive, since it appears to “short circuit” their well-established function as interoceptive sensors of nutritional state. In this model energy deficit activates AgRP neurons and inhibits POMC neurons, thereby generating a motivational drive that promotes food intake and is only relieved when energy stores are replenished. An assumption of this model is that internal signals generated during feeding (e.g. accumulation of circulating nutrients or hormones) are responsible for resetting the activation state of these neurons and thereby reducing the drive to eat.
Our data by contrast show that food detection alone rapidly resets the activity of these two cell types and that this resetting precedes the onset of actual food consumption. This is surprising in light of the fact that stimulation of AgRP neurons is sufficient to promote food intake (Aponte et al., 2011; Krashes et al., 2011). However, our data also show that if food is removed before it can be consumed, then these neurons revert to their activity level prior to food presentation (Figure 5G,J). We have likewise found that inaccessible food induces smaller and less durable changes in AgRP and POMC neuron activity (Figure 5C,F). Together these findings suggest that food detection modulates AgRP and POMC neurons in a way that anticipates the change in their activity that will occur following food consumption, taking into account factors such as the food’s energy density, perceived accessibility, and the nutritional state of the mouse (Figure 7G).
What is the purpose of this anticipatory regulation? We propose that it represents a mechanism to rapidly inhibit foraging and other appetitive behaviors once food has been discovered (Figure 7G). In this regard activation of AgRP neurons induces not only food consumption but also motivational processes that drive food obtainment, including dramatic foraging behavior and a willingness to work for food (Atasoy et al., 2012; Krashes et al., 2011). These appetitive processes are blocked by food discovery as part of the natural transition from foraging to feeding, but the mechanisms by which this transition is regulated have not been described. Our data show that food discovery results in rapid feedback inhibition of AgRP neurons themselves, rather than some downstream circuit element, which provides a direct mechanism to inhibit foraging once food has been obtained. The fact that this feedback occurs at the level of AgRP neurons is surprising and suggests that the activity of these neurons is particularly important for generating the motivation to search for food relative to other aspects of feeding behavior.
Models for AgRP driven food consumption
The natural dynamics of AgRP neuron activity are consistent with a primary function for these neurons in regulating appetitive behaviors that promote food discovery. Yet multiple lines of evidence have suggested a role for these neurons in controlling food consumption as well. We discuss below two possible mechanisms by which AgRP neurons could drive food intake that are consistent with our data.
Subpopulations of AgRP neurons with specialized functions
A limitation of fiber photometry is that it measures the average activity of a population of a neurons, which can mask heterogeneity in the responses of individual cells. AgRP neurons that project to different downstream targets differ in their ability to induce food intake and in their expression of the leptin receptor (Atasoy et al., 2012; Betley et al., 2013; Wu et al., 2012). It is therefore unlikely that all AgRP neurons show identical responses to stimuli such as hormone challenge or food presentation. One possibility is that a subset of AgRP neurons are activated, rather than inhibited, by food presentation, and that this subpopulation of AgRP neurons is responsible for driving food consumption. Testing this possibility will require measuring the single-cell dynamics of AgRP neurons during behavior, using approaches such as optogenetic phototagging combined with in vivo recording (Lima et al., 2009) or fluorescence microendoscopic imaging (Ziv et al., 2013).
While future experiments are likely to uncover additional heterogeneity in these cells, three observations argue against this heterogeneity being the primary explanation for how AgRP activity drives food consumption. First, the magnitude of the decrease in AgRP calcium dynamics that we observe following food presentation, particularly for palatable foods (Figure 4F), is inconsistent with a major subset of these neurons having the opposite regulation. Therefore if some AgRP neurons are activated during feeding, they must represent a minority of the population. Second, our analysis of AgRP dynamics during individual feeding bouts reveals that AgRP activity declines immediately preceding meal initiation and then is relatively flat during the course of food intake (Figure 6D). These intrameal dynamics are not what would be predicted for a neuron whose activity directly drives food consumption. Third, and most importantly, we have shown that food presentation potently inhibits AgRP projections to the PVH (Figure 7). Optogenetic experiments have strongly implicated these ARC → PVH projections in the control of food intake (Atasoy et al., 2012; Betley et al., 2013). The fact that these PVH projections show the same activity pattern as the population as a whole argues that projection-specific dynamics are unlikely to be the primary explanation for how these neurons can drive feeding.
Learning mediated by AgRP activity
An alternative possibility is that AgRP neurons drive food consumption indirectly via a learning process. In this regard we have shown that the inhibition of AgRP activity following food discovery is contingent on subsequent food intake, since this inhibition is reversed if the food is removed before it can be consumed (Figure 5G). If AgRP activity has negative motivational valence (analogous to the unpleasant sensation of hunger), then this might enable animals to learn the consequences of failing to eat after obtaining food. In this model food discovery would temporarily relieve the sensation of hunger, but animals would learn through experience that this sensation returns if the food is not consumed. Over time this would result in appetitive and consummatory aspects of feeding becoming linked in sequence, so that food discovery is always followed by food intake, even though AgRP activity itself would largely be extinguished before the onset of feeding. Alternative models based on negative reinforcement and learning are also conceivable, and untangling these possibilities will be an important area for future investigation.
Neural input into AgRP and POMC neurons communicates the discovery of food
AgRP and POMC neurons receive abundant neural input and indeed the activation of AgRP neurons by fasting is mediated primarily by increased excitatory tone (Liu et al., 2012; Yang et al., 2011). Yet most studies of these cells have focused on the role of hormones and nutrients, and the role of this afferent neural input has remained unclear. Our data indicate that one function of this neural input is to communicate to AgRP and POMC neurons the discovery of food. This is appealing because it demonstrates a function for this synaptic input that extends beyond merely serving as a redundant source of homeostatic information. The fact that the strength of this neural input varies depending on the hedonic properties of the detected food suggests that, at some level, the upstream circuit encodes an association between sensory information and the food’s nutritional content (i.e. a “food memory”). Identification of the neural substrate of this association may provide an entry point into the study of the maladaptive associations between sensory cues and food that develop in some eating disorders. As several cell types that provide input into AgRP neurons have recently been identified (Krashes et al., 2014), it should be possible to elucidate this afferent pathway using modern circuit mapping techniques.
Information processing by arcuate feeding circuits
Feeding is influenced by diverse types of signals including sensory, hedonic, homeostatic, and visceral cues. A longstanding question has been whether there exists a site in the brain where the neural circuits that sense these signals converge, thereby enabling integration of this information into a single decision to eat or not to eat (Friedman, 2014). The arcuate nucleus in this model is traditionally viewed as a sensor for homeostatic cues, which it then relays to higher centers where more complex integration occurs. This viewpoint is encapsulated in the fact that AgRP and POMC neurons are often described as “first order” neurons, analogous to primary sensory afferent neurons such as rods and cones in the visual system.
A complication for this model as mentioned previously is that AgRP and POMC neurons are strongly regulated by neural input and therefore are not merely sensors of circulating nutritional signals. However absent an understanding of the function of this afferent input it has not been possible to assemble a complete picture of the role of these cells. The discovery that this input contains information about the sensory and hedonic properties of food reveals that these long-studied neurons themselves integrate multiple types of food-related information and indeed may represent a key convergence point in the feeding circuit. The further application of new methods for recording cell-type-specific neural activity is likely to provide additional insight into how this complex integration is achieved.
Experimental Procedures
Experimental protocols were approved by the University of California, San Francisco IACUC following the National Institutes of Health guidelines for the Care and Use of Laboratory Animals
Stereotaxic surgery
Recombinant AAV expressing GCaMP6s (AAV1.Syn.Flex.GCaMP6s) was purchased from the Penn Vector Core. AAV was stereotaxically injected into the ARC of AgRP-IRES-Cre and POMC-Cre mice. In the same surgery a photometry cannula was implanted unilaterally in either the ARC or PVH. Mice were allowed 2–4 weeks for viral expression and recovery from surgery before behavioral testing.
Slice electrophysiology and calcium imaging
Acute hypothalamic slices were prepared from 8- to 15-week-old AgRP-IRES-Cre and POMC-Cre mice expressing AAV GCaMP6s for 2–4 weeks. Fluorescent cells in the ARC were identified for whole-cell patch clamp recordings, and cells were activated using step currents (10 pA, 2 s) from −20 pA to +120 pA or ramp currents (0–40 pA, 10 s) injected under current clamp mode. Calcium imaging was performed simultaneously using a digital CCD camera mounted on an Olympus BX51 microscope.
Immunohistochemistry
Mice were transcardially perfused with PBS followed by formalin. Brains were postfixed overnight in formalin and placed in 30% sucrose for 2 days. Free floating sections (40 μm) were prepared with a cryostat, blocked (3% BSA, 2% NGS and 0.1% Triton-X in PBS for 2 h), and then incubated with primary antibody (chicken anti-GFP, Abcam, ab13970, 1:1000) overnight at 4°C. Samples were washed, incu bated with secondary antibody (goat antichicken Alexa 488 secondary antibody; Invitrogen, 1:500) for 2h at room temperature, mounted and imaged.
Fiber photometry
A rig for performing fiber photometry recordings was constructed following basic specifications previously described (Gunaydin et al., 2014). All experiments were performed in behavioral chambers (Coulbourn Instruments, Habitest Modular System) and video recorded using infrared cameras installed above each cage. Experiments were performed at the beginning of the dark cycle (CT12 – CT14) to control for circadian factors and performed in a dark environment with illumination of red or infrared light. Mice were acclimated to the behavioral chamber for at least 15 min prior to the beginning of each testing session. For hormone challenge, ghrelin (60 µg/mouse) or vehicle (PBS) was delivered by intraperitoneal injection in a total volume of 200 µL. For food presentation experiments, mice were exposed in their home cage prior to testing to both peanut butter and the rubber stopper in order to remove any effects of novelty. Mice were not exposed to chocolate prior to testing. Liquid diet experiments were performed using a behavioral chamber equipped with an optical lickometer (Coulbourn Instruments). Mice were habituated to a liquid diet (Ensure vanilla flavor) for 3 days prior to the experiment. Mice were then fasted overnight, acclimated to the behavioral chamber for 15 min, and then a bottle filled with liquid diet was plugged into the lickometer system and the trial was run for 1h. Photometry data were subjected to minimal processing consisting of only autofluorescence background subtraction and within trial fluorescence normalization.
Statistics
Values are reported as mean +/− SEM in the figures and text. P values for pairwise comparison were performed using a two-tailed Student’s t-test. p values for comparisons across multiple groups were corrected using the Holm-Sidak method in Prism. * p<0.05. ** p<0.01,*** p<0.001,**** p<0.0001.
Supplementary Material
Article Highlights.
Sensory detection of food rapidly inhibits AgRP and activates POMC neurons
Rapid sensory feedback occurs before any food is consumed
The magnitude of neuronal response depends on food palatability and nutritional state
AgRP/POMC neurons may play a primary role in driving food discovery
Acknowledgements
Z.A.K. is a New York Stem Cell Foundation – Robertson Investigator and acknowledges additional support from the Rita Allen Foundation, the McKnight Foundation, the Alfred P. Sloan Foundation, a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation, the Esther A. & Joseph Klingenstein Foundation, the Program for Breakthrough Biomedical Research, and the UCSF Diabetes Center Obesity Pilot program (U01 DK089541). This work was supported by NIH R00-DK083531 (Z.A.K.). We thank Jennifer Garrison, Nirao Shah, Kevan Shokat, and members of the Knight lab for comments on the manuscript, and Anthony Lee and Scott Owen for technical advice on photometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Y.C. and Z.A.K. designed the experiments, analyzed the data, and wrote the paper. Y.C. built the photometry rig, wrote the programs for data analysis, and performed the photometry experiments. Y.L. assisted with PVH projection experiments and histology. T.K. and Y.L. performed slice physiology experiments.
Supplemental Information: Supplemental information includes Extended Experimental Procedures, four figures, and six movies and can be found with this article online at
References
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behavioural brain research. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117:2435–2442. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich MO, Liu ZW, Horvath TL. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell. 2013;155:188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Friedman J. 20 YEARS OF LEPTIN: Leptin at 20: an overview. The Journal of endocrinology. 2014;223:T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature neuroscience. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature neuroscience. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Hetherington AW, Ranson SW. Experimental hypothalamico-hypophyseal obesity in the rat. Proceedings of the Society for Experimental Biology and Medicine. 1939;41:465–466. [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PloS one. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH -->arcuate nucleus microcircuits and their reorganization by fasting. Nature neuroscience. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature neuroscience. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nature neuroscience. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.