Abstract
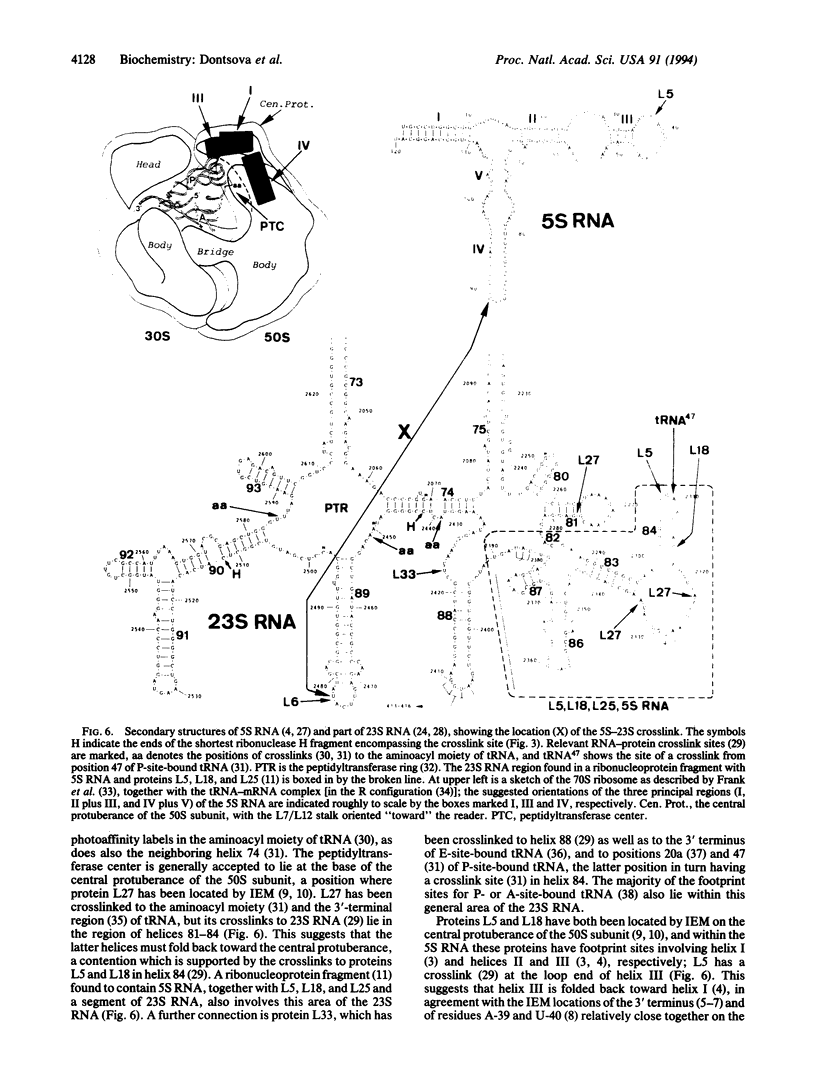
A DNA fragment containing the Escherichia coli 5S rDNA sequence linked to a T7 promoter was prepared by PCR from an M13 clone carrying the 5S-complementary sequence. The DNA was transcribed with T7 polymerase using a mixture of [alpha-32P]UTP and 4-thio-UTP, yielding a transcript in which approximately 18% of the uridine residues were randomly replaced by thiouridine. This modified 5S RNA could be reconstituted efficiently into 50S ribosomal subunits or 70S functional complexes. The reconstituted particles were irradiated at wavelengths above 300 nm, and the crosslinked ribosomal components were identified. A crosslink in high yield was reproducibly observed between the modified 5S RNA and 23S RNA, involving residue U-89 of the 5S RNA (at the loop end of helix IV) linked to nucleotide 2477 of the 23S RNA in the loop end of helix 89, immediately adjacent to the peptidyltransferase "ring." On the basis of this result, and in combination with earlier immunoelectron microscopic data, we propose a model for the orientation of the 5S RNA in the 50S subunit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Sriwidada J., Brimacombe R. RNA sequences associated with proteins L1, L9, and L5, L18, L25, in ribonucleoprotein fragments isolated from the 50-S subunit of Escherichia coli ribosomes. Eur J Biochem. 1976 Nov 15;70(2):483–492. doi: 10.1111/j.1432-1033.1976.tb11039.x. [DOI] [PubMed] [Google Scholar]
- Clark M. W., Lake J. A. Unusual rRNA-linked complex of 50S ribosomal subunits isolated from an Escherichia coli RNase III mutant. J Bacteriol. 1984 Mar;157(3):971–974. doi: 10.1128/jb.157.3.971-974.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohme F., Nierhaus K. H. Role of 5S RNA in assembly and function of the 50S subunit from Escherichia coli. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2221–2225. doi: 10.1073/pnas.73.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontsova O., Dokudovskaya S., Kopylov A., Bogdanov A., Rinke-Appel J., Jünke N., Brimacombe R. Three widely separated positions in the 16S RNA lie in or close to the ribosomal decoding region; a site-directed cross-linking study with mRNA analogues. EMBO J. 1992 Aug;11(8):3105–3116. doi: 10.1002/j.1460-2075.1992.tb05383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontsova O., Kopylov A., Brimacombe R. The location of mRNA in the ribosomal 30S initiation complex; site-directed cross-linking of mRNA analogues carrying several photo-reactive labels simultaneously on either side of the AUG start codon. EMBO J. 1991 Sep;10(9):2613–2620. doi: 10.1002/j.1460-2075.1991.tb07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstafieva A. G., Shatsky I. N., Bogdanov A. A., Vasiliev V. D. Topography of RNA in the ribosome: location of the 5 S RNA residues A39 and U40 on the central protuberance of the 50 S subunit. FEBS Lett. 1985 Jun 3;185(1):57–62. doi: 10.1016/0014-5793(85)80740-7. [DOI] [PubMed] [Google Scholar]
- Favre A., Bezerra R., Hajnsdorf E., Lemaigre Dubreuil Y., Expert-Bezançon A. Substitution of uridine in vivo by the intrinsic photoactivable probe 4-thiouridine in Escherichia coli RNA. Its use for E. coli ribosome structural analysis. Eur J Biochem. 1986 Nov 3;160(3):441–449. doi: 10.1111/j.1432-1033.1986.tb10060.x. [DOI] [PubMed] [Google Scholar]
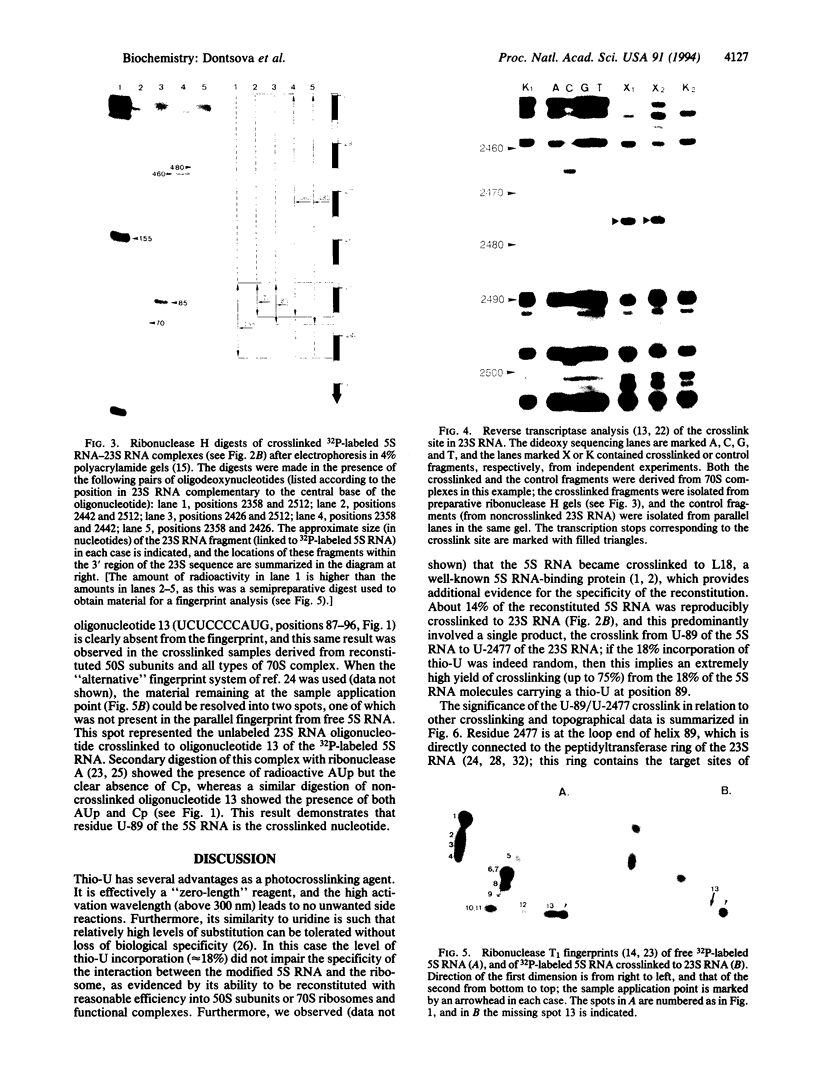
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Frank J., Penczek P., Grassucci R., Srivastava S. Three-dimensional reconstruction of the 70S Escherichia coli ribosome in ice: the distribution of ribosomal RNA. J Cell Biol. 1991 Nov;115(3):597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. A., Noller H. F. Structures of complexes of 5S RNA with ribosomal proteins L5, L18 and L25 from Escherichia coli: identification of kethoxal-reactive sites on the 5S RNA. J Mol Biol. 1979 Aug 25;132(4):637–648. doi: 10.1016/0022-2836(79)90379-6. [DOI] [PubMed] [Google Scholar]
- Golden B. L., Ramakrishnan V., White S. W. Ribosomal protein L6: structural evidence of gene duplication from a primitive RNA binding protein. EMBO J. 1993 Dec 15;12(13):4901–4908. doi: 10.1002/j.1460-2075.1993.tb06184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber P. W., Wool I. G. Nuclease protection analysis of ribonucleoprotein complexes: use of the cytotoxic ribonuclease alpha-sarcin to determine the binding sites for Escherichia coli ribosomal proteins L5, L18, and L25 on 5S rRNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):322–326. doi: 10.1073/pnas.81.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietzke R., Nierhaus K. H. Total reconstitution of 70S ribosomes from Escherichia coli. Methods Enzymol. 1988;164:278–283. doi: 10.1016/s0076-6879(88)64049-3. [DOI] [PubMed] [Google Scholar]
- Lim V., Venclovas C., Spirin A., Brimacombe R., Mitchell P., Müller F. How are tRNAs and mRNA arranged in the ribosome? An attempt to correlate the stereochemistry of the tRNA-mRNA interaction with constraints imposed by the ribosomal topography. Nucleic Acids Res. 1992 Jun 11;20(11):2627–2637. doi: 10.1093/nar/20.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombie W. R., Heiner C., Kelley J. M., Fitzgerald M. G., Gocayne J. D. Rapid and reliable fluorescent cycle sequencing of double-stranded templates. DNA Seq. 1992;2(5):289–296. doi: 10.3109/10425179209030961. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Schueler D., Brimacombe R. Selective isolation and detailed analysis of intra-RNA cross-links induced in the large ribosomal subunit of E. coli: a model for the tertiary structure of the tRNA binding domain in 23S RNA. Nucleic Acids Res. 1990 Aug 11;18(15):4325–4333. doi: 10.1093/nar/18.15.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Stade K., Osswald M., Brimacombe R. Site-directed cross-linking studies on the E. coli tRNA-ribosome complex: determination of sites labelled with an aromatic azide attached to the variable loop or aminoacyl group of tRNA. Nucleic Acids Res. 1993 Feb 25;21(4):887–896. doi: 10.1093/nar/21.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Osswald M., Greuer B., Brimacombe R. Localization of a series of RNA-protein cross-link sites in the 23S and 5S ribosomal RNA from Escherichia coli, induced by treatment of 50S subunits with three different bifunctional reagents. Nucleic Acids Res. 1990 Dec 11;18(23):6755–6760. doi: 10.1093/nar/18.23.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkowinski J., Gornicki P. Neighbourhood of the central fold of the tRNA molecule bound to the E. coli ribosome--affinity labeling studies with modified tRNAs carrying photoreactive probes attached to the dihydrouridine loop. Nucleic Acids Res. 1991 Feb 25;19(4):801–808. doi: 10.1093/nar/19.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinberger H. J., Geigenmüller U., Wedde M., Nierhaus K. H. Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol. 1988;164:658–670. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- Rinke-Appel J., Jünke N., Stade K., Brimacombe R. The path of mRNA through the Escherichia coli ribosome; site-directed cross-linking of mRNA analogues carrying a photo-reactive label at various points 3' to the decoding site. EMBO J. 1991 Aug;10(8):2195–2202. doi: 10.1002/j.1460-2075.1991.tb07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatsky I. N., Evstafieva A. G., Bystrova T. F., Bogdanov A. A., Vasiliev V. D. Topography of RNA in the ribosome: location of the 3'-end of 5 S RNA on the central protuberance of the 50 S subunit. FEBS Lett. 1980 Nov 17;121(1):97–100. doi: 10.1016/0014-5793(80)81274-9. [DOI] [PubMed] [Google Scholar]
- Spierer P., Zimmermann R. A. Stoichiometry, cooperativity, and stability of interactions between 5S RNA and proteins L5, L18, and L25 from the 50S ribosomal subunit of Escherichia coli. Biochemistry. 1978 Jun 27;17(13):2474–2479. doi: 10.1021/bi00606a002. [DOI] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G., Kuechler E., Barta A. Photo-affinity labelling at the peptidyl transferase centre reveals two different positions for the A- and P-sites in domain V of 23S rRNA. EMBO J. 1988 Dec 1;7(12):3949–3955. doi: 10.1002/j.1460-2075.1988.tb03281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiege W., Zwieb C., Brimacombe R. Precise localisation of three intra-RNA cross-links in 23S RNA and one in 5S RNA, induced by treatment of Escherichia coli 50S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1982 Nov 25;10(22):7211–7229. doi: 10.1093/nar/10.22.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöffler-Meilicke M., Stöffler G., Odom O. W., Zinn A., Kramer G., Hardesty B. Localization of 3' ends of 5S and 23S rRNAs in reconstituted subunits of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5538–5542. doi: 10.1073/pnas.78.9.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate W., Greuer B., Brimacombe R. Codon recognition in polypeptide chain termination: site directed crosslinking of termination codon to Escherichia coli release factor 2. Nucleic Acids Res. 1990 Nov 25;18(22):6537–6544. doi: 10.1093/nar/18.22.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. The importance of highly conserved nucleotides in the binding region of chloramphenicol at the peptidyl transfer centre of Escherichia coli 23S ribosomal RNA. EMBO J. 1988 Nov;7(11):3577–3587. doi: 10.1002/j.1460-2075.1988.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. A micromethod for base analysis of 32P-labeled oligoribonulcleotides. Anal Biochem. 1977 Nov;83(1):222–227. doi: 10.1016/0003-2697(77)90530-9. [DOI] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Scheffer P., Sylvers L. A., Wintermeyer W., Zimmermann R. A. Topography of the E site on the Escherichia coli ribosome. EMBO J. 1993 Feb;12(2):617–623. doi: 10.1002/j.1460-2075.1993.tb05694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]