Abstract
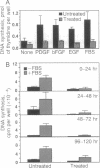
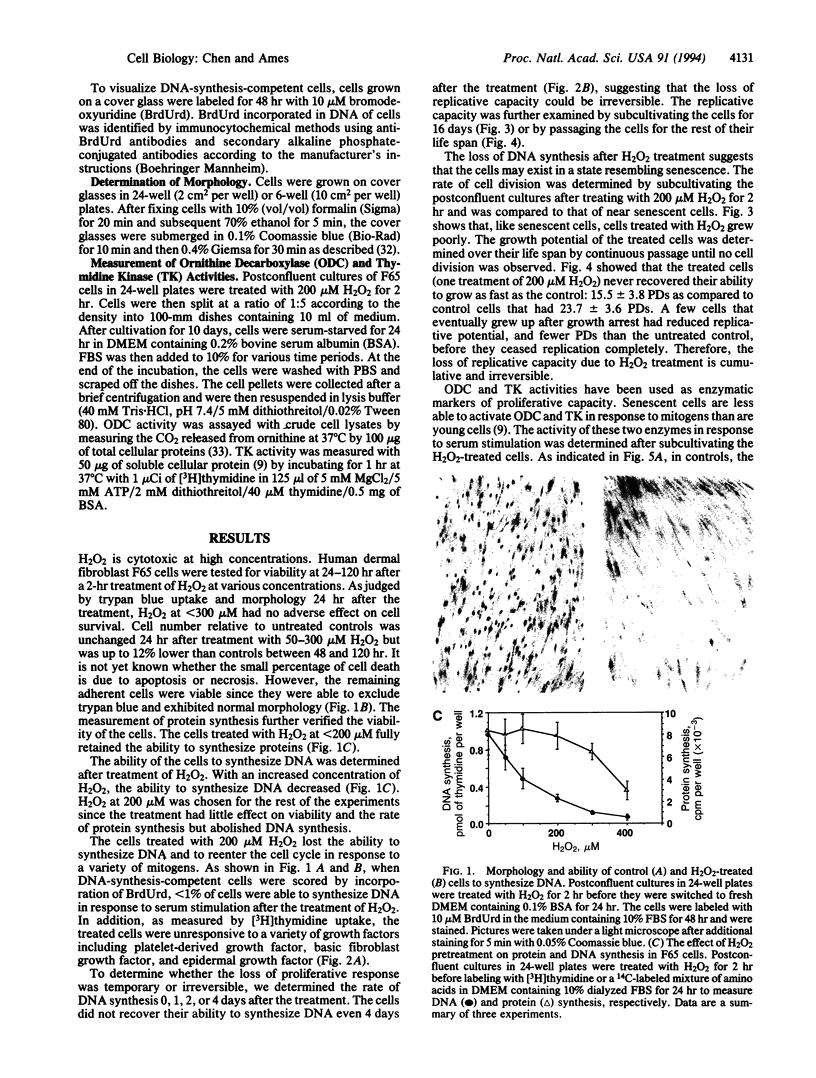
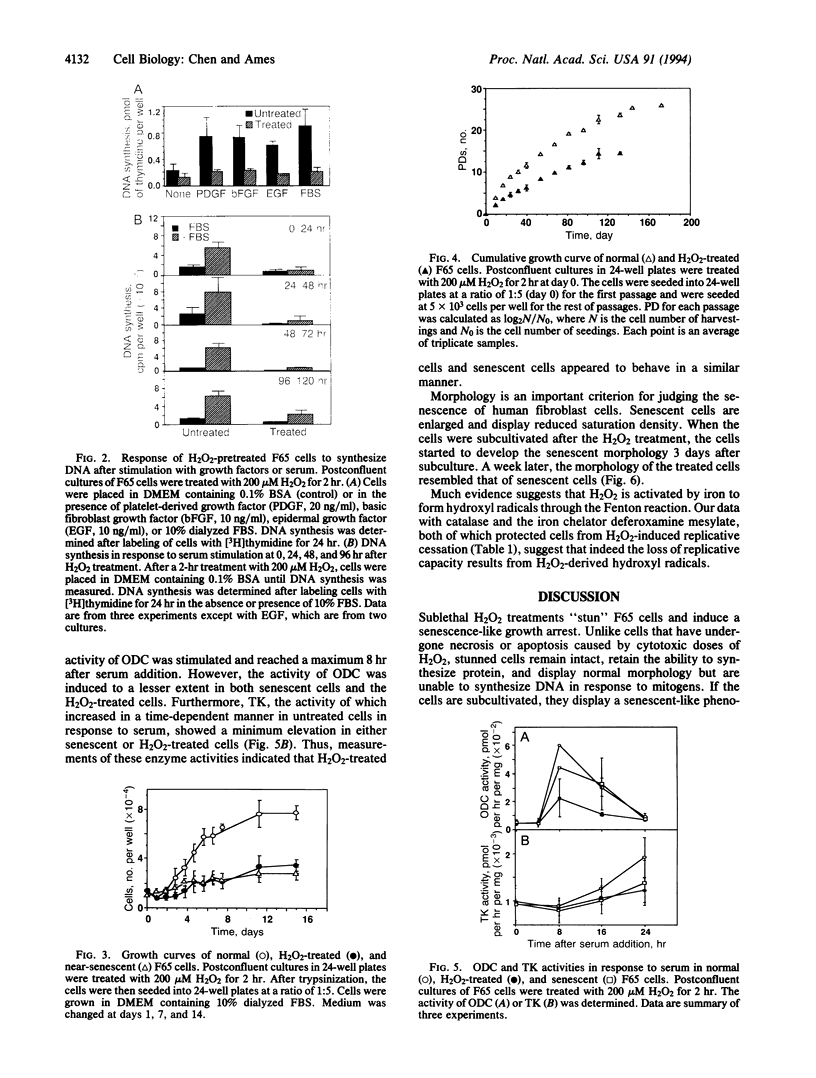
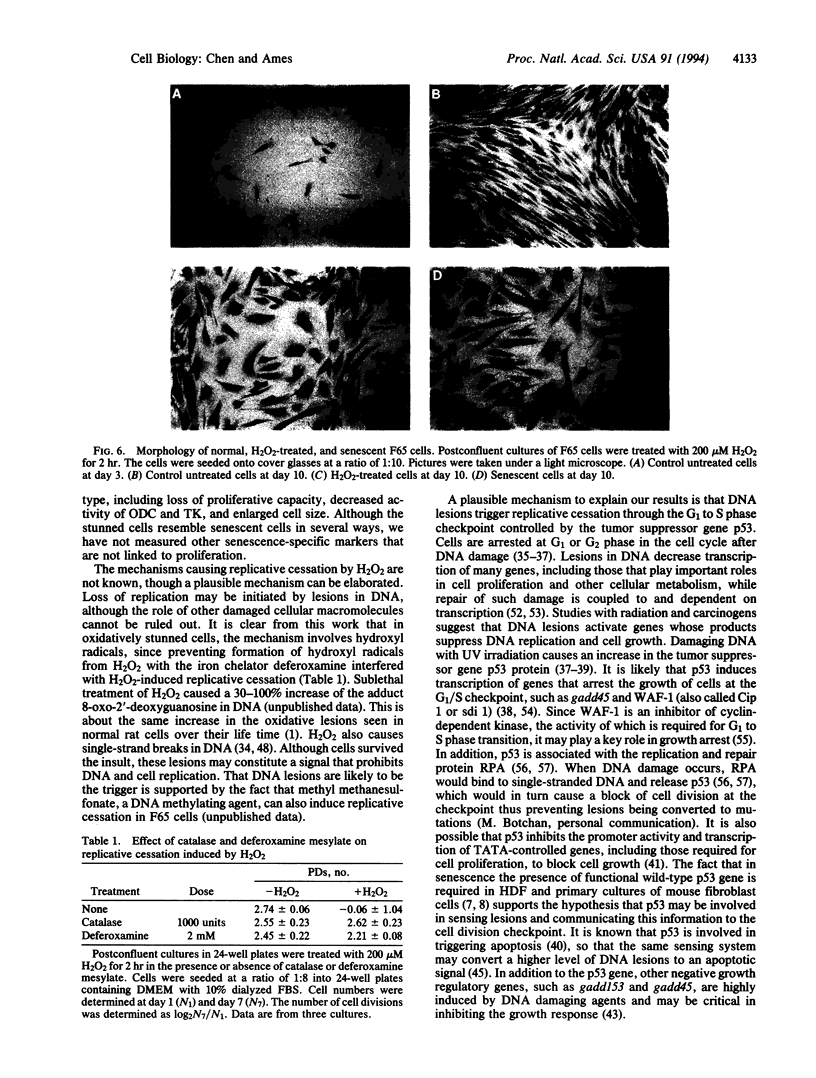
Human diploid fibroblast cells lose replicative potential after a certain number of population doublings. We use this experimental system to investigate the role of oxidative damage in cellular aging. Treating cells with H2O2 at < 300 microM did not affect the viability of the majority of cells when judged by morphology, trypan blue exclusion, and protein synthesis. However, the treatment caused a dose-dependent inhibition of DNA synthesis. After a 2-hr treatment with 200 microM H2O2, the cells failed to respond to a stimulus of serum, platelet-derived growth factor, basic fibroblast growth factor, or epidermal growth factor by synthesizing DNA, and the loss of response could not be recovered by 4 days. Subcultivation showed that, as in senescent cells, division of the treated cells was inhibited. The life-time cumulative growth curve showed that the loss of replication due to H2O2 treatment was cumulative and irreversible. The H2O2 treatment decreased the number of the population doublings in the rest of the life span by 35.3 +/- 10.3%. Enzymatic assays indicated that, like the cells in their senescent state, the treated cells were less able to activate ornithine decarboxylase and thymidine kinase. Furthermore, subcultivation after the H2O2 treatment showed that the cells developed the morphology of senescent cells. In conclusion, sublethal treatment of H2O2 "stunned" F65 cells and caused the cells to enter a state resembling senescence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Chemical carcinogenesis: too many rodent carcinogens. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7772–7776. doi: 10.1073/pnas.87.19.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Gold L. S. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990 Aug 31;249(4972):970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K., Gold L. S. DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. Environ Health Perspect. 1993 Dec;101 (Suppl 5):35–44. doi: 10.1289/ehp.93101s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angello J. C., Pendergrass W. R., Norwood T. H., Prothero J. Cell enlargement: one possible mechanism underlying cellular senescence. J Cell Physiol. 1989 Aug;140(2):288–294. doi: 10.1002/jcp.1041400214. [DOI] [PubMed] [Google Scholar]
- Bayreuther K., Rodemann H. P., Hommel R., Dittmann K., Albiez M., Francz P. I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benn P. A. Specific chromosome aberrations in senescent fibroblast cell lines derived from human embryos. Am J Hum Genet. 1976 Sep;28(5):465–473. [PMC free article] [PubMed] [Google Scholar]
- Catania J., Fairweather D. S. DNA methylation and cellular ageing. Mutat Res. 1991 Mar-Nov;256(2-6):283–293. doi: 10.1016/0921-8734(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Cerutti P., Larsson R., Krupitza G., Muehlematter D., Crawford D., Amstad P. Pathophysiological mechanisms of active oxygen. Mutat Res. 1989 Sep;214(1):81–88. doi: 10.1016/0027-5107(89)90200-5. [DOI] [PubMed] [Google Scholar]
- Chang Z. F., Chen K. Y. Regulation of ornithine decarboxylase and other cell cycle-dependent genes during senescence of IMR-90 human diploid fibroblasts. J Biol Chem. 1988 Aug 15;263(23):11431–11435. [PubMed] [Google Scholar]
- Chen K. Y., Chang Z. F., Pang J. H., He G. S., Liu A. Y. Polyamine metabolism and cell-cycle-dependent gene expression in IMR-90 human diploid fibroblasts during senescence in culture. Exp Gerontol. 1989;24(5-6):523–537. doi: 10.1016/0531-5565(89)90058-2. [DOI] [PubMed] [Google Scholar]
- Dice J. F. Altered intracellular protein degradation in aging: a possible cause of proliferative arrest. Exp Gerontol. 1989;24(5-6):451–459. doi: 10.1016/0531-5565(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Drescher-Lincoln C. K., Smith J. R. Inhibition of DNA synthesis in proliferating human diploid fibroblasts by fusion with senescent cytoplasts. Exp Cell Res. 1983 Apr 1;144(2):455–462. doi: 10.1016/0014-4827(83)90424-x. [DOI] [PubMed] [Google Scholar]
- Dutta A., Ruppert J. M., Aster J. C., Winchester E. Inhibition of DNA replication factor RPA by p53. Nature. 1993 Sep 2;365(6441):79–82. doi: 10.1038/365079a0. [DOI] [PubMed] [Google Scholar]
- Fiorani M., Fumo M., Sestili P., Cattabeni F., Cantoni O. Inhibition of Chinese hamster ovary cell DNA synthesis by hydrogen peroxide. Chem Biol Interact. 1990;76(1):129–139. doi: 10.1016/0009-2797(90)90039-p. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille J. J., Joenje H. Cell culture models for oxidative stress: superoxide and hydrogen peroxide versus normobaric hyperoxia. Mutat Res. 1992 Sep;275(3-6):405–414. doi: 10.1016/0921-8734(92)90043-o. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Hanawalt P., Mellon I. Stranded in an active gene. Curr Biol. 1993 Jan;3(1):67–69. doi: 10.1016/0960-9822(93)90156-i. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Harvey M., Sands A. T., Weiss R. S., Hegi M. E., Wiseman R. W., Pantazis P., Giovanella B. C., Tainsky M. A., Bradley A., Donehower L. A. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene. 1993 Sep;8(9):2457–2467. [PubMed] [Google Scholar]
- Holliday R. A re-examination of the effects of ionizing radiation on lifespan and transformation of human diploid fibroblasts. Mutat Res. 1991 Mar-Nov;256(2-6):295–302. doi: 10.1016/0921-8734(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. A death in the life of p53. Nature. 1993 Apr 29;362(6423):786–787. doi: 10.1038/362786a0. [DOI] [PubMed] [Google Scholar]
- Li R., Botchan M. R. The acidic transcriptional activation domains of VP16 and p53 bind the cellular replication protein A and stimulate in vitro BPV-1 DNA replication. Cell. 1993 Jun 18;73(6):1207–1221. doi: 10.1016/0092-8674(93)90649-b. [DOI] [PubMed] [Google Scholar]
- Luce M. C., Bunn C. L. Decreased accuracy of protein synthesis in extracts from aging human diploid fibroblasts. Exp Gerontol. 1989;24(2):113–125. doi: 10.1016/0531-5565(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Lumpkin C. K., Jr, McClung J. K., Pereira-Smith O. M., Smith J. R. Existence of high abundance antiproliferative mRNA's in senescent human diploid fibroblasts. Science. 1986 Apr 18;232(4748):393–395. doi: 10.1126/science.2421407. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Marumo M., Hayashi S. Ornithine decarboxylase antizyme in kidneys of male and female mice. Biochem J. 1988 Sep 1;254(2):367–372. doi: 10.1042/bj2540367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose K., Shibanuma M., Kikuchi K., Kageyama H., Sakiyama S., Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991 Oct 1;201(1):99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- Okada A. A., Dice J. F. Altered degradation of intracellular proteins in aging human fibroblasts. Mech Ageing Dev. 1984 Aug;26(2-3):341–356. doi: 10.1016/0047-6374(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Peacocke M., Campisi J. Cellular senescence: a reflection of normal growth control, differentiation, or aging? J Cell Biochem. 1991 Feb;45(2):147–155. doi: 10.1002/jcb.240450205. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Vogelstein B. Tumour suppressor genes. No room at the p53 inn. Nature. 1993 Sep 2;365(6441):17–18. doi: 10.1038/365017a0. [DOI] [PubMed] [Google Scholar]
- Rattan S. I. Protein synthesis and the components of protein synthetic machinery during cellular aging. Mutat Res. 1991 Mar-Nov;256(2-6):115–125. doi: 10.1016/0921-8734(91)90005-v. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993 Apr 2;260(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Growth-factor-inducible gene expression in senescent human fibroblasts. Exp Gerontol. 1989;24(5-6):515–522. doi: 10.1016/0531-5565(89)90057-0. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Pereira-Smith O. M., Wright W. E. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991 Sep;196(1):33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Kuroki T., Nose K. Stimulation by hydrogen peroxide of DNA synthesis, competence family gene expression and phosphorylation of a specific protein in quiescent Balb/3T3 cells. Oncogene. 1990 Jul;5(7):1025–1032. [PubMed] [Google Scholar]
- Smith J. R., Lincoln D. W., 2nd Aging of cells in culture. Int Rev Cytol. 1984;89:151–177. doi: 10.1016/s0074-7696(08)61303-0. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. V., Holliday R. Chromosome changes during the in vitro ageing of MRC-5 human fibroblasts. Exp Cell Res. 1975 Nov;96(1):1–6. doi: 10.1016/s0014-4827(75)80029-2. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Different drugs arrest cells at a number of distinct stages in G2. Nature. 1975 Mar 20;254(5497):245–247. doi: 10.1038/254245a0. [DOI] [PubMed] [Google Scholar]
- Venkatachalam S., Denissenko M. F., Alvi N., Wani A. A. Rapid activation of apoptosis in human promyelocytic leukemic cells by (+/-)-anti-benzo[a]pyrene diol epoxide induced DNA damage. Biochem Biophys Res Commun. 1993 Dec 15;197(2):722–729. doi: 10.1006/bbrc.1993.2539. [DOI] [PubMed] [Google Scholar]
- Wang E., Tomaszewski G. Granular presence of terminin is the marker to distinguish between the senescent and quiescent states. J Cell Physiol. 1991 Jun;147(3):514–522. doi: 10.1002/jcp.1041470318. [DOI] [PubMed] [Google Scholar]
- Wistrom C., Villeponteau B. Cloning and expression of SAG: a novel marker of cellular senescence. Exp Cell Res. 1992 Apr;199(2):355–362. doi: 10.1016/0014-4827(92)90445-e. [DOI] [PubMed] [Google Scholar]
- Won K. A., Xiong Y., Beach D., Gilman M. Z. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]
- Zhan Q., Carrier F., Fornace A. J., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993 Jul;13(7):4242–4250. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]