SUMMARY
Hematopoietic stem cells (HSCs) are produced during embryogenesis from the floor of the dorsal aorta. The localization of HSCs is dependent upon the presence of instructive signals on the ventral side of the vessel. The nature of the extrinsic molecular signals that control the aortic hematopoietic niche is currently poorly understood. Here we demonstrate a novel requirement for FGF signaling in the specification of aortic hemogenic endothelium. Our results demonstrate that FGF signaling normally acts to repress BMP activity in the subaortic mesenchyme through transcriptional inhibition of bmp4, as well as through activation of two BMP antagonists, noggin2 and gremlin1a. Taken together, these findings demonstrate a key role for FGF signaling in establishment of the developmental HSC niche via its regulation of BMP activity in the subaortic mesenchyme. These results should help inform strategies to recapitulate the development of HSCs in vitro from pluripotent precursors.
Keywords: FGF, BMP, stem cell, definitive blood, zebrafish
INTRODUCTION
HSCs ultimately maintain all lineages of blood and immune cells throughout the lifetime of an organism. This feature underlies the long-term efficacy of bone marrow transplantation, frequently used as therapy for blood disorders including leukemia. Immune incompatibility between donor and host, insufficient number of donors, and the rarity of HSCs within many donor tissues have led to a search for alternative approaches to traditional HSC-based therapies. Recent breakthroughs using induced Pluripotent Stem Cells (iPSCs) have brought hope of in vitro derived, patient-specific HSCs, which could circumvent these issues. Despite decades of effort, it is not currently possible to generate bona fide HSCs from pluripotent precursors. The development of novel HSC-based therapeutics may thus depend upon obtaining a more precise understanding of the native molecular events that occur in vivo during HSC formation.
In all vertebrate animals examined, HSCs arise during embryogenesis from a specialized population of arterial cells localized in the ventral side of the dorsal aorta (DA) termed hemogenic endothelium 1. This endothelial-hematopoietic transition 2 appears to exist only transiently, and is characterized by changes in gene expression and shape in ventral aortic endothelial cells as HSC precursors emerge and then enter circulation 2–6. A prerequisite for HSC emergence appears to be the normal specification of arterial fate, most importantly proper formation of the DA. At the molecular level, arterial identity is governed by multiple extrinsic signals. In the zebrafish embryo, Hedgehog signals from the notochord/floor plate regulate the expression of vegfa and calcitonin in the somites, which in turn regulate expression of Notch receptors in the DA 7–11. Modulation of any of these signaling pathways alters arterial development and therefore HSC formation.
Recent studies have demonstrated that HSC formation is disrupted by defects in the Wnt1612, VegfA 13 and Bmp4 14 pathways without concomitant loss of aortic fate. Interestingly, each pathway regulates different steps of HSC development. In zebrafish, Wnt16 controls early HSC specification through its regulation of the somitic Notch ligand genes deltaC and deltaD, whose combined action is required for the Notch-dependent specification of HSCs, but not for arterial development12. More recently, it was confirmed in Xenopus that arterial fate and HSC emergence can be uncoupled based on VegfA isoforms. The short isoform controls arterial fate likely through Notch4, while HSC emergence depends on the medium/long isoforms and Notch113. Finally, Bmp4 that is localized to the sub-aortic mesenchyme is responsible for the polarization of HSC formation from the ventral side of the DA14–17. Smad1, an intracellular activator of the BMP pathway, transactivates the runx1 promoter in vitro, suggesting that Bmp4 may act directly upstream of runx118, which is required for the emergence of HSCs across vertebrate species 8,19–21. Just before the onset of definitive hematopoiesis in zebrafish, the aortic region switches from a BMP repressive to activated environment14. The mechanism of this sudden change remains unknown.
Interplay between BMP and FGF signaling pathways has been described during organogenesis. In Xenopus, FGF and BMP signaling pathways intersect in the regulation of primitive erythropoiesis where FGF inhibits Bmp4-induced erythropoiesis through the control of gata2 22. The repressive role of FGF in primitive blood is conserved across the vertebrates. For instance, in the chicken embryo, FGF signals through Fgfr2 to control erythrocyte differentiation by repressing gata1 expression in blood precursors 23. In Xenopus, FGF was shown to act on the timing of primitive hematopoiesis by holding back the onset of the molecular program that triggers primitive blood formation 24. Finally, in zebrafish, primitive erythrocyte formation depends on Fgf21, which also governs erythromyeloid precursor development, likely in concert with Fgf1 23,25,26. While several studies have established that FGF signaling represses primitive blood formation, FGF signaling acts as a positive regulator of adult HSCs. Fgf1 27 and Fgf2 28 can expand ex vivo the number of transplantable HSCs. However, this effect seems to be limited to the short-term HSC compartment in vivo and it is accompanied by an alteration of the terminal differentiation of erythrocytes, B-cells and myeloid cells 29. More recently, the role of FGF signaling in steady state conditions has been challenged and seems to be mainly required to promote mobilization and proliferation of HSCs under stress induced conditions 30,31. FGF signaling appears to have multiple roles in blood development, however, its potential role in the emergence of HSCs has not been addressed.
In this study, we have discovered a key repressive role for FGF signaling in HSC emergence through its regulation of the BMP pathway. Together with the data in the accompanying paper (Lee et al), which reveals an earlier positive role for FGF in programming the HSC lineage, these findings suggest that precise temporal inhibition as well as activation of FGF signaling may aid in vitro approaches to instruct HSC fate from pluripotent precursors.
RESULTS
FGF signaling is a negative regulator of HSC formation in the zebrafish embryo
To functionally test whether or not FGF signaling is required for definitive blood formation, we utilized transgenic zebrafish in which FGF signaling can be inducibly abrogated or enforced by heat-shock induction of a dominant-negative Fgfr1-EGFP fusion protein (hsp70:dn-fgfr1-EGFP) 32,33, or a constitutively active Fgfr1 mutant protein (hsp70:ca-fgfr1) 34, respectively. This time controlled approach allowed us to avoid mesoderm patterning defects induced by early FGF misexpression and subsequently target different developmental events according to their timing 35.
In order to identify temporal windows when FGF signaling may be involved in HSC development, we initially targeted the early stage of arterial specification by inducing the hsp:dn-fgfr1 transgene at 17 hpf (15 somite stage (ss)). At this stage, primitive blood and endothelial cells are specified and the first sign of arterial specification is detectable in the endothelial precursors that are migrating from the lateral plate mesoderm to the midline 36 to form the primitive vascular cord 37–39. Transgenic embryos were then sorted based on the expression of GFP, and GFP negative embryos were used as sibling controls. Following induction of hsp:dn-fgfr1, definitive hematopoiesis initiated normally and there was no significant difference in runx1 expression between GFP+ and GFP− animals (Supplemental Fig. 1A and B). Interestingly, arterial and endothelial differentiation were unaffected, based upon the normal expression of deltaC and kdrl, respectively (Supplemental Fig. 1C–F), suggesting that FGF signaling is not required for arterial differentiation or vascular development during the convergence of vascular precursor cells to the midline.
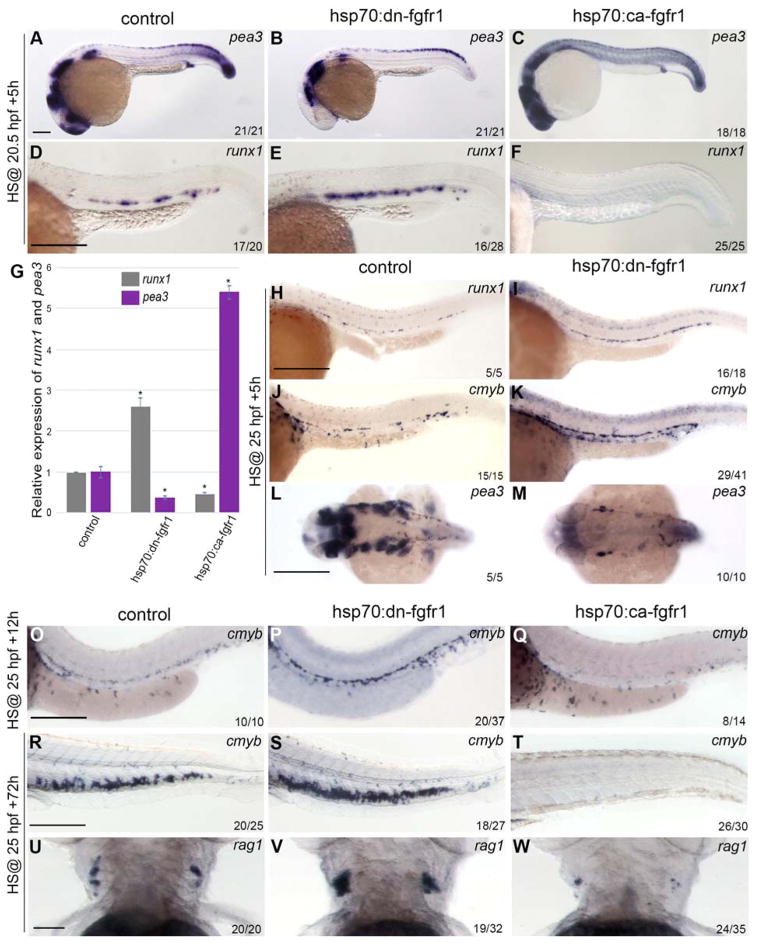
To investigate possible later requirements for FGF signaling in HSC development, embryos were heat-shocked at 20.5 hpf (23 ss), just prior to when runx1 expression along the aortic floor marks initiation of the definitive HSC program. To verify loss of FGF signaling, we analyzed the expression of pea3, a direct transcriptional target 40 of FGF signaling. In embryos heat-shocked at 20.5 hpf, pea3 expression decreased, and was accompanied by an increase in runx1 expression (Figure 1, A, B and D, E). At the stage when the heatshock was performed, the aortic region contains the precursors of the HSCs that will specifically express runx1 from 23hpf, but also primitive blood that can be distinguished from HSC based on gata1 expression. The effect of the modulation of FGF signaling is restricted to the HSCs as shown by the absence of alteration of gata1 expression in transgenic embryos (Supplemental Fig. 2 A). EMPs, bipotent precursors that arise in the posterior blood island 41, are not affected upon FGF modulation (Supplemental Fig. 2 A). In converse experiments, where FGF signaling was enforced via 20.5 hpf induction of the hsp:ca-fgf1 transgene, embryos exhibited embryo-wide upregulation of pea3 expression and a substantial decrease in runx1 expression along the aortic floor (Figure 1, C and F). Despite strong GFP expression at 5 hours post-heatshock (hpHS), hsp:dn-fgfr1 embryos displayed only a slight decrease in pea3 expression, leading to the conclusion that cellular turnover of the truncated receptor may outpace the turnover of GFP. To address this, pea3 expression was analyzed in hsp:dn-fgfr1 embryos from 23 hpf to 27 hpf. At 2 hpHS, pea3 was nearly absent in GFP+ embryos. Expression of pea3 gradually returned to normal around 6 hpHS (Supplemental Fig. 2 B). Effects on runx1 expression followed this trend. Between 2–4 hpHS a greater proportion of hsp:dn-fgfr1 embryos showed stronger upregulation of runx1 than when analyzed between 5–6 hpHS (Supplemental Fig. 2C). The effects of Fgf modulation on runx1 expression were confirmed by quantitative PCR using cDNAs from the dissected trunks of hsp:dn-fgfr1 and hsp:ca-fgfr1 embryos (Figure 1G).
Figure 1. FGF signaling represses HSC formation and maintenance.
(A–F) Embryos were heatshocked at 20.5 hpf and analyzed at 26 hpf. (A–C) pea3 expression is downregulated in hsp:dn-fgfr1 (B) and upregulated in hsp70:ca-fgfr1 embryos (C) compared to controls (A). (D–F) Aortic expression of runx1 is enhanced in hsp70:dn-fgfr1 (E) and depleted in hsp70:ca-fgfr1 embryos (F) compared to controls (D). (G) Quantification of runx1 and pea3 mRNA expression in dissected trunks normalized to ef1α. Expression of each gene was set to 1 in the control. Data are shown as average ± s.d. values; p<0.001. (H–M) Embryos were heatshocked at 25 hpf and analyzed at 30 hpf. (H–I) runx1 expression is increased in hsp70:dn-fgfr1 embryos (I) compared with controls (H). Similar results are seen with cmyb expression in control (J) and hsp70:dn-fgfr1 embryos (K). (L and M) Dorsal view of pea3 expression in the head shows downregulation of pea3 upon depletion of FGF signaling. (O–W) Embryos were heatshocked at 25hpf and analyzed 12h and 72hpHS. (O–Q) cmyb expression is more intense in the hsp70:dn-fgfr1 embryos (P) compared to control embryos (O). Conversely, embryos in which FGF signaling is increased, display a drastic diminution of cmyb expression (Q). (R–S) Comparison of cmyb expression in the CHT of heatshocked transgenic embryos and controls. The augmentation of HSC numbers detected at 26hpf upon FGF modulation is maintained in the CHT of the hsp70:dn-fgfr1 embryos while the CHT of the hsp70:ca-fgfr1 embryos are devoid of cmyb cells. (U–W) Effect of FGF signal alteration on T-cells. FGF ablation (V) or augmentation (W) has opposite effects on rag1+ cells. Scale bars: (A) 50um, (D, H) 200um, (L, O, R) 250um, (U) 50um.
Before 26–27 hpf, the close proximity of primitive erythrocytes within the DA and posterior cardinal vein (PCV) to the floor of the DA 42 makes it difficult to distinguish them from emerging HSCs since each lineage shares expression of early hematopoietic markers. We therefore shifted the heat-shock regimen to 25 hpf and fixed at 30 hpf. By this time, erythroid precursors have entered circulation, which allows visualization of the hemogenic endothelial markers cmyb by whole-mount in situ hybridization (WISH). Induced hsp70:dn-fgfr1 embryos showed elevated expression of both runx1 and cmyb in the DA (Figure 1 H–K, Supplemental Fig. 2 D) within the period during which the FGF transcriptional target pea3 is still downregulated (Figure 1 L and M). In mammals, HSCs leave the DA region quickly after their emergence to seed the fetal liver and the thymus. In zebrafish, a similar shift occurs: runx1+/cmyb+ cells migrate from the DA to the caudal hematopoietic tissue (CHT) and the thymus 43–45. To ascertain whether the expanded pool of runx1+/cmyb+ cells are HSCs, FGF signaling was modulated at 25hpf and the effect on HSCs was monitored in the DA, the CHT and the thymus at 36hpf (25hpf + 12h), 3 (25hpf + 48h), and 4dpf (25hpf + 72h)(Fig. 1. O–W, Supplemental Fig. 2 E and F). In induced hsp70:dn-fgfr1 embryos, cmyb expression is still expanded in the DA 12hpHS (Fig. 1 O and P). Conversely, embryos in which FGF signaling was enforced are devoid of cmyb cells in the DA (Fig. 1 O and Q). Similarly, at 48 and 72hpHS, hsp70:dn-fgfr1 embryos showed a more robust expression of runx1 and cmyb in the CHT while hsp70:ca-fgfr1 embryos showed a drastic decrease of the runx1+ and cmyb+ cells (Supplemental Fig. 2 E, Fig. 1 R–T). Quantitative PCR analysis of dissected CHT confirmed that runx1, cmyb and CD41 levels of expression vary according to the modulation of FGF signaling (Supplemental Fig. 2 F). T-cells are thought to be the first functional derivatives of HSCs. They are first detected around 3 dpf and by day4, rag1 expression becomes robust in the thymus. The effect of FGF signaling modulation at 25hpf also affects the number of thymic rag1+ (Fig. 1 U–W). Importantly, the increase in the number of rag1+ cells was observed only in hsp70:dn-fgfr1 embryos whose blood circulation was unaffected.
Taken together, these results demonstrate that FGF signaling is important in the establishment of hemogenic endothelium, acting to repress the specification of HSC fate from the aortic floor.
Fgf10a represses HSC formation possibly by acting on fgfr2 and fgfr3
To identify the cell types that may mediate the effects of FGF signaling on HSC emergence, we examined localization of Fgf receptor expression at 20.5 hpf and at 24 hpf (Supplemental Fig. 3). Fgfr1a, fgfr 1b, and fgfr 4 were not detected in the tissues surrounding the DA at either time point (Supplemental Fig. 3 A–H and Q–T). Fgfr2 showed strong expression in the pronephric ducts, the hypochord, and the neural tube at 20.5 hpf (Supplemental Fig. 3I and J). In contrast to fgfr2, fgfr3 transcripts were detected in the somites at 20.5 hpf (Supplemental Fig. 3 M and N). At 24 hpf, fgfr3 is expressed throughout the trunk, whereas fgfr2 expression is restricted to the neural tube, the pronephric ducts, the hypochord, and in cells surrounding the axial vasculature (Supplemental Fig. 3K, L and O, P). The localization of each receptor suggests that the effects of FGF modulation on HSC formation may act through fgfr2 and/or fgfr3. However, morpholino knockdown of these receptors failed to phenocopy the increase in runx1 and cmyb expression observed in the hsp70:dn-fgfr1 transgenic line. Loss of either receptor led to the absence of runx1 expression in the DA at 26 hpf (Supplemental Fig. 4). The discrepancy between the phenotype observed in morphants and that observed in hsp70:dn-fgfr1 embryos suggests that fgfr2 and fgfr3 may be required at earlier stages of mesoderm or vascular development.
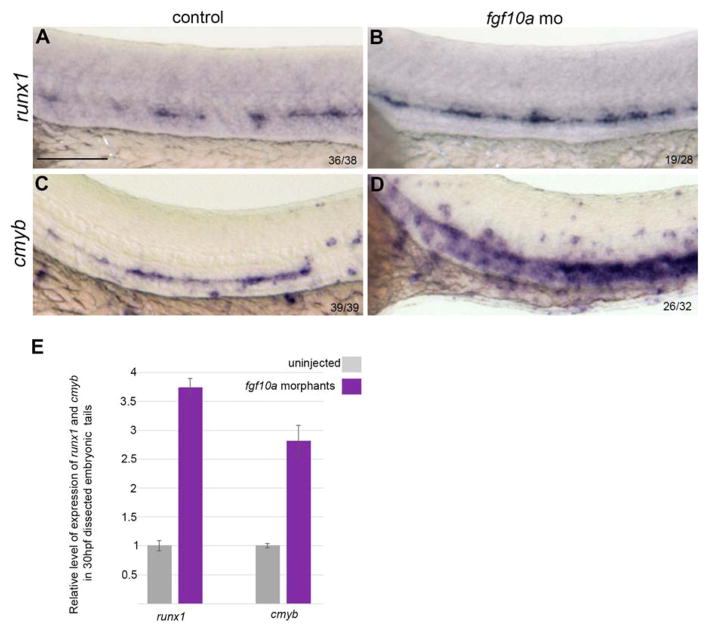
In zebrafish, 27 Fgf ligands have been identified 46. At the stage of the heatshock, fgf10a is expressed throughout the trunk 47 which made it a good candidate. To analyze its potential role in HSC specification, knockdown experiments were carried out using a splice-blocking morpholino (Figure 2). Our LOF experiments showed that depletion of fgf10a gives a similar phenotype than the phenotype observed in the hsp70:dn-fgfr1 embryos. At 30hpf, in morphant embryos, runx1 and cmyb expression are extended along the entire DA (Figure 2 A–D and E).
Figure 2. Loss of fgf10a mimics the effect of FGF ablation.
(A–B) runx1 expression is expanded along the entire DA in the morphant embryos. Similarly, loss of Fgf10a significantly increases cmyb expression in the aortic region (C–D). Comparison of the relative level of expression of runx1 and cmyb by qPCR in control and morphant embryos (E). Scale bar: 150um.
Taken together, our results confirm that FGF signaling acts as a negative regulator of definitive hematopoiesis. This function is mediated by fgf10a which likely signals through fgfr2 and/or fgfr3.
FGF acts independently of the Notch and Vegf pathways
In both mouse and zebrafish, Notch signaling is required for aortic and HSC specification 9,21,48,49. Since fgfr2 is expressed in the aortic region and fgfr3 in the somites, it is possible that the effects of FGF signaling on HSC emergence could be due in part to effects on the Notch pathway. In zebrafish, enforced expression of the Notch intra cellular domain (NICD) throughout the embryo is sufficient to generate an excess of HSCs 21. Similarly in mice, genetic depletion of COUP-TFII, which normally represses Notch in the PCV, leads to the formation of ectopic hematopoietic clusters in the PCV 50. We therefore investigated whether the modulation of HSC number observed in the DA following loss or gain of FGF signaling might be due to effects on the Notch pathway. Transgenic hsp70:dn-fgfr1 or hsp70:ca-fgfr1 embryos were heatshocked at 20.5 hpf, fixed at 25 hpf, then assayed for Notch-related vascular and arterial gene markers by WISH (Supplemental Fig. 5 A–O). Following either loss or gain of FGF function, the integrity of the vascular system was unaffected, as indicated by normal kdrl expression (Supplemental Fig. 5 A–C). Aortic markers, including gridlock (a target of the Vegf pathway 9) (Supplemental Fig. 3 D–F), notch1b (Supplemental Fig. 5 G–I), deltaC (Supplemental Fig. 5 J–L), and ephrinb2a (a target of the Notch pathway 48) (Supplemental Fig. 5 M–O) were unchanged following modulation of FGF signaling. These results indicate that the effects of FGF signaling on HSC fate are not dependent upon downstream Notch signaling events. To test the converse, that is whether or not FGF signaling requirements are downstream of Notch, Notch signaling was blocked using DAPM, a small chemical inhibitor of NICD release from Notch receptors. If the increase in HSC number following FGF inhibition acts downstream of Notch, blockade of Notch signaling in the same temporal window as FGF inhibition should not prevent runx1 upregulation in the DA. In accord with this hypothesis, hsp70:dn-fgfr1 embryos treated with DAPM maintained strong expression of runx1 in the DA (Supplemental Fig. 5 P–U). These results demonstrate that the increase in HSC number observed in absence of FGF signaling acts in a dominant manner with respect to loss of Notch signaling. Taken together, our studies on the interaction of Notch and FGF suggest that the effects of FGF on HSC fate either occur independently or downstream of the roles of the Notch/Vegf signaling axis during arterial development and HSC formation.
FGF signaling does not affect dorsal polarization of the DA
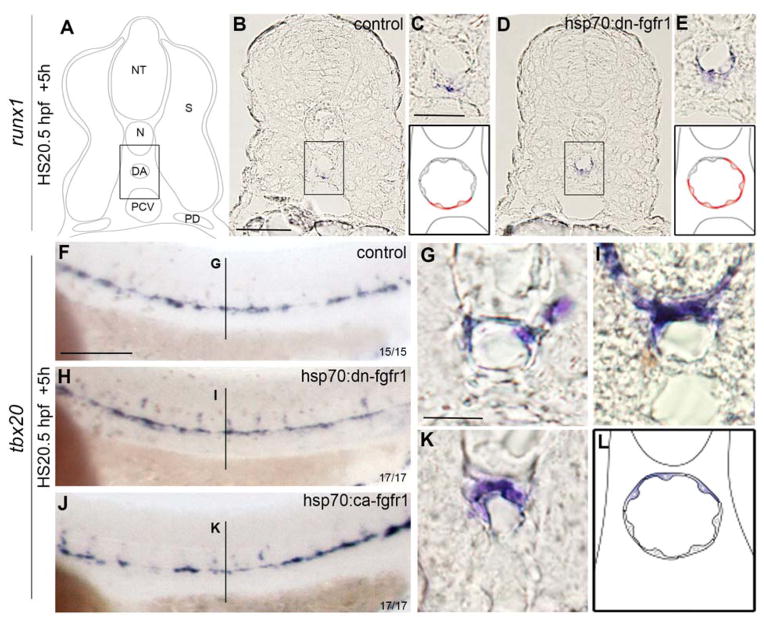
Since the increase in HSC marker expression in the DA is not a result of overactivation of the Vegf or Notch signaling pathways, we reasoned that it may be due to an increase in the number of runx1+ cells in the DA or the surrounding mesenchyme. We thus examined runx1 expression in transverse sections following induction of the hsp70:dn-fgfr1 transgene at 20.5 hpf. In WT controls, rare runx1+ cells were visible only in the floor of the DA (Figure 3 A–C). Following loss of FGF signaling, the expression of runx1 in the DA was expanded beyond the floor region to the roof of the aorta (Figure 3 D–E). Cells expressing runx1 were never detected in the surrounding mesenchyme or neighboring PCV, suggesting that ectopic runx1+ cells must transit through an arterial precursor. Since runx1 is normally expressed only in the aortic floor, the ectopic appearance of runx1+ cells in the aortic roof may indicate that FGF signaling is involved in DA polarization. In mice and zebrafish, DA polarization depends upon opposing morphogen gradients; dorsal identity is established by Hedgehog secretion from the notochord whereas ventral identity relies upon BMP production from ventral domains 14–16. We examined the expression of tbx20, a transcription factor regulated by Hedgehog signaling8,14,51, that distinguishes the dorsal side of the DA. Neither activation nor inhibition of FGF signaling had any effect on tbx20 expression (Figure 3 F, H and J). Examination of transverse sections confirmed that only cells in the roof of the DA and in the developing intersomitic vessels expressed tbx20, indicating that dorsal polarization is not affected by FGF modulation (Figure 3 G, I, K and L).
Figure 3. Loss of FGF signaling expands runx1 dorsally without affecting dorsal polarization of the DA.
(A) Schematic representation of a trunk section of 26hpf embryos. (B) Aortic localization of runx1+ cells in control (B and C) and hsp70:dn-fgfr1 (D and E) embryos. Expression of tbx20 in control (F and G), hsp70:dn-fgfr1 (H and I), and hsp70:ca-fgfr1 (J and K) embryos. The black line (G, I and K) denotes where sections were made. (L) Schematic representation of tbx20 expression in the roof of the DA. DA: dorsal aorta, N: notochord, NT: neural tube, PCV: posterior cardinal vein; PD: pronephric duct, S: somite. Scale bars: (B) 30um, (F) 200um, (G) 20um.
FGF signaling controls HSC formation by modulating BMP activity
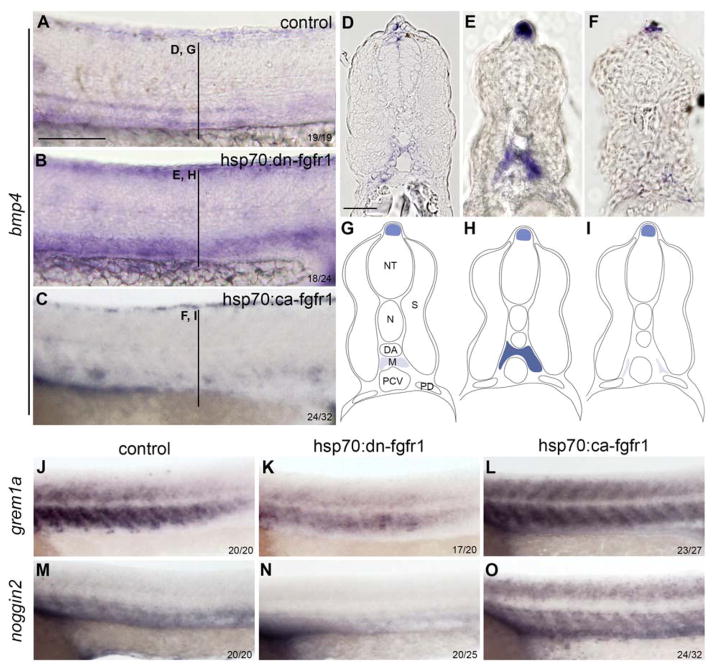
We next investigated whether FGF regulates the ventral polarization of the DA by modulating BMP activity. In zebrafish, we previously demonstrated that bmp4 is required for the emergence and maintenance of HSCs 14. Whereas bmp4 is normally expressed in the mesenchyme underlying the DA (Figure 4A, D and G), bmp4 expression was upregulated in the aortic region in the absence of FGF (Figure 4B, E and H). Conversely, in embryos with FGF overactivation, bmp4 was absent from the aortic region (Figure 4C, F and I), supporting the idea that FGF signaling may regulate HSC formation via its effects on the BMP pathway.
Figure 4. FGF signaling regulates Bmp4 expression, as well as noggin2 and gremlin1a, two BMP antagonists.
(A–C) bmp4 expression is altered following manipulation of FGF signaling. bmp4 levels of expression are increased in hsp70:dn-fgfr1 embryos (B and E) and decreased in hsp70:ca-fgfr1 embryos (C and F). (D–F) Transverse sections of embryos from WISH samples (A–C). (G–I) Schematic representing bmp4 expression in control (G), hsp70:dn-fgfr1 (H), and hsp70:ca-fgfr1 (I) embryos. (J–O) gremlin1a (J–L) and noggin2 (M–O) expression is reduced in hsp70:dn-fgfr1 embryos (K and N), and enhanced in hsp70:ca-fgfr1 embryos (L and O). M: mesenchyme. Scale bars: (A) 200um, (D) 50um.
Since BMP signaling activity is tightly regulated by several antagonists 52, we also examined their expression at the time of heat shock. At 20.5 hpf, the DA is surrounded by several BMP antagonists, including chordin from the pronephric ducts, as well as noggin1, noggin2 53, and gremlin1a 54. Although chordin is an important regulator of primitive hematopoiesis 55, it is dispensable for HSC formation 14. We therefore focused on noggin1, noggin2, and gremlin1a. In WT embryos, noggin1 is barely detected in the ventral side of the somite at 24hpf, while gremlin1a and noggin2 are strongly expressed in the sclerotome (Figure 4J, M) 53,54. In induced hsp70:dn-fgfr1 embryos, both gremlin1a and noggin2 were markedly downregulated (Figure 4K, N and Supplemental Fig. 6). In contrast, when FGF signaling was enforced there was substantial upregulation of sclerotomal gremlin1a and noggin2 (Figure 4L, O and Supplemental Fig. 6). Augmentation of FGF activity was also observed to induce ectopic expression of gremlin1a and noggin2 in the most dorsal compartment of the somite (Figure 4L, O). Together, these results demonstrate that FGF signaling represses bmp4 expression directly, and concomitantly induces expression of the BMP inhibitors noggin2 and gremlin1a in the neighboring somite.
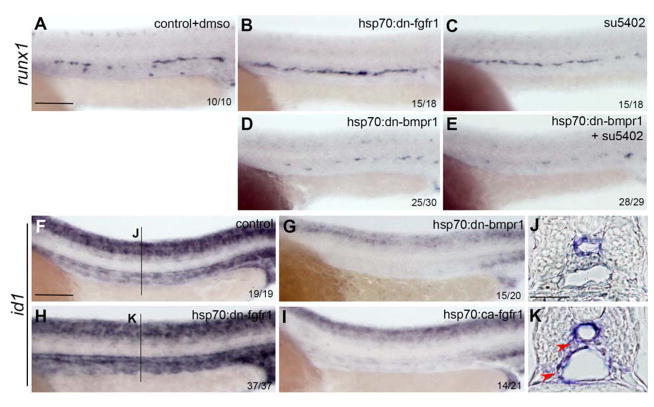
To further analyze how FGF and BMP signaling interact, we tested whether inhibition of BMP signaling in FGF depleted embryos would affect runx1 expression. Inhibition of FGF signaling, either using the hsp:dn-fgfr1 trangenic animals or a small chemical inhibitor su5402, increases runx1 expression in the DA (Figure 5A, B, C), while blockage of BMP signaling abrogates runx1 expression (Figure 5D). Inhibition of FGF signaling in a BMP-repressed environment could not rescue HSC production, supporting the idea that FGF acts upstream of the BMP pathway (Figure 5E).
Figure 5. Epistatic analysis of BMP and FGF signaling interaction.
(A–C) runx1 expression is increased in hsp70:dn-fgfr1 embryos (B) and embryos treated with su5402 (C) compared to controls (A). Overexpression of hsp70:dn-bmpr1 impairs emergence of HSCs (D) compared to control (A) or FGF inhibited embryos (B and C). HSC emergence is not rescued in hsp70:dn-bmpr1 embryos following blockade of FGF signaling using su5402 (E). (F–P) id1 expression is reduced upon inhibition of BMP signaling (G) as well as augmentation of FGF signaling (K), compared to control embryos (F). In the absence of FGF signaling, id1 expression is increased in the vasculature and in some cells surrounding the vessels (N, and K, red arrows). Scale bars: (A, F) 100um, (J) 30um.
To further examine the epistasis between the FGF and BMP pathways, we sought a molecular marker of BMP activity. The transcriptional repressor id1 is a known target of BMP signaling 56, and its targeted deletion in the mouse embryo impairs hematopoiesis by affecting the proliferation and the self-renewal of HSCs 57. In zebrafish, id1 is expressed in developing neural tissue, somites and axial vasculature (Figure 5F); this expression is largely ablated following inhibition of BMP signaling (Figure 5G). Inhibition of FGF signaling leads to an increase in id1 expression in the vasculature (Figure 5 F, H), which becomes more apparent in transverse sections (Figure 5 J, K). Conversely, stimulation of FGF significantly decreases id1 expression (Figure 5 I). Together, these results further demonstrate that the FGF signaling pathway acts upstream of BMP signaling to regulate HSC emergence.
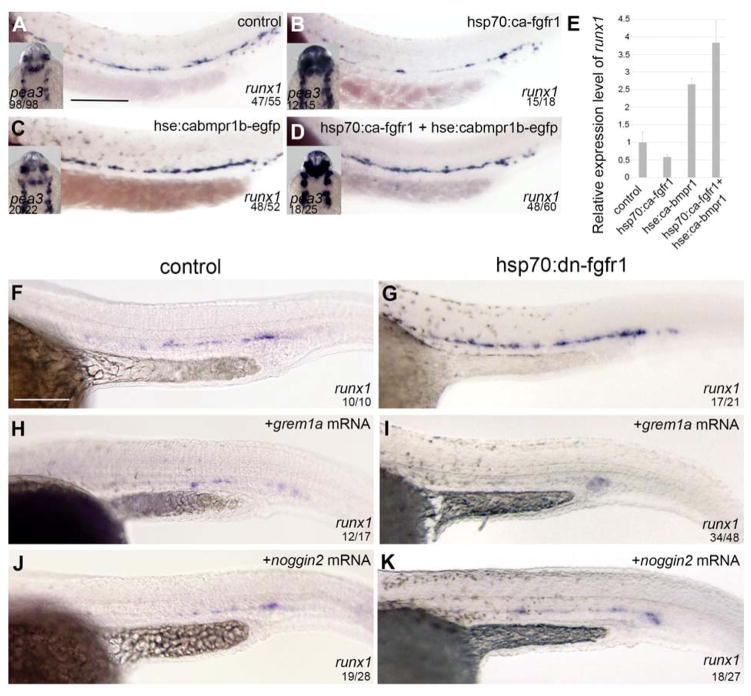
Finally, we performed genetic rescue experiments to determine if enforced BMP signaling could rescue loss of HSCs in hsp70:ca-fgfr1 animals. Enforced activity of the BMP pathway was achieved following induction of a constitutively active bmp receptor 1b (hse:ca-bmpr1b) construct in transient transgenic animals, as previously described 58. Compared to WT siblings, hsp70:ca-fgfr1 animals induced at 20.5 hpf showed loss of HSCs accompanied by an increase of pea3 expression (Figure 6A, B and E). Induction of the hse:ca-bmpr1b transgene alone showed a robust increase in runx1 expression without affecting pea3 (Figure 6C and E). As predicted by our results above, enforced activity of BMP signaling could rescue HSC development in hsp70:ca-fgfr1 animals (Figure 6D and E).
Figure 6. Ectopic activation of BMP signaling rescues runx1 expression in an activated FGF background.
(A–D) runx1 expression increases in the DA following activation of the BMP pathway (C), compared to controls (A). runx1 expression in hsp70:ca-fgfr1 (B) embryos is rescued by activation of a hse:cabmpr1b transgene (D). Quantitative analysis of runx1 expression (E). pea3 expression in the head (inserts, A–D) is upregulated upon FGF activation. (F–K) runx1 expression is impaired upon overexpression of either gremlin1a (H) or noggin2 (J) compared to control (F). Inhibition of FGF signaling fails to rescue runx1 expression when gremlin1a (I) and noggin2 (K) are overexpressed. Scale bars: (A, F) 200um.
In this experiment, BMP signaling was enforced at the receptor level, bypassing therefore any potential effect of the Bmp antagonists noggin2 and gremlin1a. In hsp:ca-fgfr1 embryos, their expression levels are elevated suggesting that they may reinforce the BMP repressive environment in the DA. According to this hypothesis, overexpression of noggin2 or gremlin1a following inhibition of FGF signaling should prevent runx1 increase. Overexpression of noggin2 and gremlin1a were achieved by mRNA injection into hsp70:dn-fgfr1 embryos and analyzed for runx1 expression. As predicted, both noggin2 and gremlin1a represses HSC formation when injected in control embryos (Figure 6. F, H and J). Similar results were obtained when noggin2 and gremlin1a were overexpressed in hsp:dn-fgfr1 embryos (Figure 6. G, I and K), confirming that both antagonists are acting downstream of FGF signaling and upstream of bmp4/bmpr1.
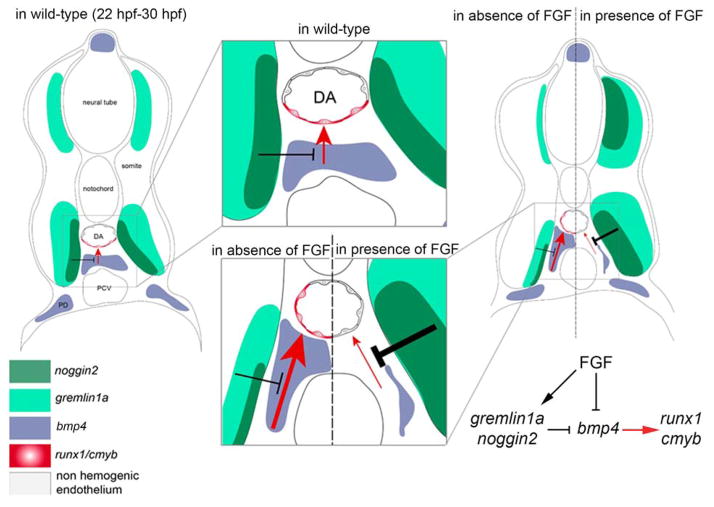
Collectively, our results indicate that FGF signaling controls the emergence of HSCs by modulating the activity of BMP signaling in the aortic region. The inhibition of BMP signaling by FGF acts at two levels, first by repressing the transcription of bmp4 in the subaortic mesenchyme, and second by increasing the expression of BMP antagonists in the neighboring somite. These results suggest that the level of FGF signaling controls the capacity of the aortic microenvironment to support or repress the formation of HSCs (Figure 7).
Figure 7. Role of FGF signaling in the formation of HSCs.
Model for the regulation of HSC emergence by FGF signaling.
DISCUSSION
Despite FGF signaling playing key roles in the formation of mesoderm and the vascular system, no previous studies have examined potential roles for FGF in HSC development. Studies in adult mice have demonstrated that HSCs express Fgfr1, and that provision of Fgf1 ex vivo can stimulate HSC expansion 27. More recent work, however, has demonstrated that Fgfr1 is not required for the normal homeostasis of adult HSCs, but rather in hematopoietic recovery following injury via irradiation or chemotherapy by stimulating HSC proliferation 30. FGF signaling may therefore be important in regulating the number of adult HSCs.
A current bottleneck in the field of regenerative medicine is the inability to instruct HSC fate in vitro from pluripotent precursors, including iPSCs. This is due, at least in part, to an incomplete understanding of the native factors that are required to specify HSCs during embryonic development. In this study, we have demonstrated a novel requirement for FGF signaling in the generation of HSCs. We show that FGF represses the emergence and maintenance of HSCs in the DA by blocking BMP signals that originate from the aortic mesenchyme. This negative role is in contrast to the role that FGF signaling has in adult hematopoiesis, where it promotes HSC amplification. Along with the results of the companion paper (Lee et al.,), it is now apparent that the FGF pathway is required at multiple stages of development to properly specify HSC fate.
HSCs originate from arterial precursors, which depend upon the Notch and Vegf signaling pathways for their specification and differentiation 7,9,11,21,48,49,59. Unlike the results of Lee et al., where early FGF signaling (14–17 hpf) is required within the somite to bridge the Wnt16-mediated expression of the Notch ligand deltaC, the subsequent FGF signaling requirement (22–30 hpf) for HSC emergence lies downstream of Notch function. Neither Notch nor Vegf dependent gene expression programs were affected following modulation of FGF activity after 20.5 hpf. Moreover, the combined inhibition of Notch and FGF signaling failed to decrease runx1 expression in the DA, indicating that FGF acts downstream of the Notch pathway in HSC emergence. Interestingly, while FGF inhibition increased the number of HSCs emerging from the DA, we did not observe ectopic runx1+ cells outside of the DA, suggesting that FGF signaling affects only arterial precursors previously specified by Notch signaling.
Following the induced repression of FGF signaling, runx1 expression expands dorsally within the DA without affecting dorsal identity, as defined by the normal expression of tbx20. This finding suggests that the expression domains of runx1 and tbx20 are not mutually exclusive, confirming our previous report 14. Interestingly, the expanded pool of runx1+/cmyb+ cells behave as normal HSC. Ectopically induced HSCs have the capacity to migrate from the DA, seed the different hematopoietic organs, and differentiate into T-cells in the embryos showing normal blood flow 2 to 3 days after heatshock.
The proper establishment of ventral aortic identity, in contrast to the dorsal identity, appears to depend upon the FGF pathway. FGF signaling controls the ventral polarization of the DA by restricting bmp4 expression in the mesenchyme around the aortic endothelium. The timing and location of bmp4 expression in the sub-aortic mesenchyme is conserved among several classes of vertebrates 14,15,17,60. Interestingly, clusters of blood cells emerge from both the dorsal and ventral side of the DA in mouse embryos 61. However, the adult reconstituting potential is restricted to the ventral clusters 62, supporting the idea that the ventral mesenchyme provides the cues critical to conferring stem cell potential. This hypothesis is supported by previous findings, where early resection of ventral mesenchyme led to loss of aortic runx1 expression and hematopoietic cluster formation 3. Our analysis of fgfr2 and fgfr3 expression patterns showed that while fgfr3 is mainly found in the somitic tissue at the time of HSC emergence, fgfr2 transcripts are detected in the few cells surrounding the axial vasculature corresponding to the territory of expression of bmp4. This tissue localization suggests that fgfr2 may mediate the effect observed on bmp4 expression when FGF signaling is modulated. Fgf10a, whose loss gives rise to a similar hematopoietic phenotype to that observed in the hsp:dn-fgfr1, was shown to preferentially interact with fgfr2 63, supporting the existence of an axis involving fgf10a/fgfr2/bmp4 to play the role of a switch that triggers the aortic blood program. However we cannot rule out that other Fgf ligands may be involved in the regulation of HSC specification from the hemogenic endothelium.
Our attempts to mimic the hsp70:dn-fgfr1 phenotype by knocking down fgfr2 and fgfr3 failed and morphant embryos showed a drastic decrease of runx1 expression at 26hpf. Knowing that Fgf receptors can heterodimerise 64 and that both fgfr2 and fgfr3 are expressed early on in the forming somites 65,66, it is possible that fgfr2 and fgfr3 may be required in the somites to promote HSC specification in concert with fgfr1 and fgfr4. To better understand how and when each receptor mediates the activity of the FGF signaling, new tools offering time control and tissue specificity have to be developed.
Our previous studies demonstrated that bmp4 is crucial for HSC formation in zebrafish14. Attempts to locally increase Bmp4 activity using the zebrafish mutant in chordin, a Bmp antagonist, failed to increase runx1 expression in the DA 14, suggesting that bmp4 alone is insufficient for HSC formation, or that other Bmp antagonists regulate HSC emergence. Our current findings indicate that the regulation of BMP signaling by FGF acts at multiple levels. First, enforced expression of a constitutively active Bmp receptor1 rescued runx1 expression in hsp70:ca-fgfr1 embryos, indicating that BMP signaling is sufficient to trigger the definitive hematopoietic program in the DA. This result, along with the finding that combined inhibition of FGF and BMP failed to generate HSCs, indicates that FGF signaling acts genetically upstream of BMP. In addition, inhibition of FGF function substantially increases bmp4 expression in the subaortic mesenchyme and prevents expression of two BMP antagonists, noggin2 and gremlin1a, in the surrounding somites. Here we report that overexpression of either noggin2 or gremlin1a is enough to prevent runx1 upregulation in the absence of FGF. This indicates that local increases of bmp4 should be accompanied by inhibition of the bmp4 antagonists, gremlin1a and noggin2, to trigger the definitive blood program. Collectively, these results indicate that ablation of FGF signaling intensifies the effects of BMP signaling in the DA on the hematopoietic program by both enhancing the expression of Bmp4 directly and repressing the expression of local BMP antagonists.
In conclusion, we show that the FGF signaling pathway is a negative regulator of HSC emergence through its control of bmp4 function underlying the aortic floor. FGF signaling may thus provide a missing link in what regulates the developmental switch from a BMP repressive to supportive environment that is linked to the emergence of HSCs from ventral aortic endothelium. These findings suggest that careful modulation of the FGF/BMP signaling axis may be important in the instruction of HSC fate in regenerative medicine approaches.
EXPERIMENTAL PROCEDURES
Zebrafish strains
Wild type AB* and transgenic lines, hsp70:dn-fgfr1 (Tg(hsp70l:dnfgfr-EGFP)pd1) 33, hsp70:ca-fgfr1 (Tg(hsp70l:Xla.fgfr1, cryaa:DsRed)pd3) 34 and hsp70:dn-bmpr1 tg(hsp70l:dn-bmpr1-EGFP) 67, were maintained and stage as previously described in 68. All animal work was carried out according to UK Home Office and UCSD IACUC regulations and the under the appropriate project license.
Heatshock conditions
Embryos were heatshocked by transferring them into prewarm E3 medium for 30 mins at either 39°C for hsp70:dn-fgfr1, hsp70:ca-fgfr1, hsp70:ca-fgfr1 injected with HSE:ca-bmpr1b- EGFP construct 58 or 43°C for hsp70:dn-bmpr1 then transferred to 28°C until fixation. Transgenic embryos were selected based on their reporter expression or by genotyping as previously described in 69.
Chemical treatments
Small molecule inhibitors were resuspended in DMSO and diluted in E3 medium. Su5402 (Calbiochem) was used at 5μM, and DAPM (Calbiochem) at 100μM 70. Control embryos were treated with the corresponding volumes of DMSO added to E3 medium just after heatshock.
WISH
Embryos were fixed in fresh PFA4%, dehydrated in EtOH and assayed for WISH as described in 8. RNA probes were labelled with Digoxigenin (Roche) and detected using an anti-Dig antibody (Roche). Embryos were stained using a solution of NBT/BCIP (Roche).
Wax sectioning
Embryos were dehydrated in EtOH 100% O/N, transferred in Xylene for 30mins then embedded in wax. Blocks containing stained embryos were sectioned at 10 or 4 μm using a microtome (Leica). Sections were transferred on glass slides, incubated at 37°C O/N. Wax was removed in xylene, and EtOH 100%, 70%, 50% and rehydrated in PBS. Slides were mounted and imaged. For the FGF receptors, representative embryos were selected and transversally sectioned using a razor blade. Slices of embryo were then soaked in glycerol and imaged.
Transient transgenesis and injection experiments
20pg of HSE:ca-bmpr1b-EGFP transgenesis construct 58 combined to 25pg of transposase mRNA were injected in one cell stage of AB* or hsp70:ca-fgfr1 embryos. As a negative control, the construct was injected without transposase.
One-cell stage embryos were injected with morpholino solution diluted in water. Injected and uninjected embryos were incubated at 28°C until fixation. Sequences and working concentrations available in Supplemental Table 1.
Real Time PCR
Total RNA was isolated from dissected trunk embryos, 5h after heat shock using the RNAeasy Micro Kit (Qiagen). Single heads of hsp70:ca-fgfr1 were used for genotyping while corresponding trunks were kept individually on dry ice. Positive trunks were then pooled and processed as other samples with Superscript III Reverse Transcriptase (Invitrogen). qPCR were performed with Sybr Green (Applied Biosystems) and analyzed by the comparative method (ΔΔCt) with ef1a house-keeping gene as internal control. Statistical analysis was performed using t-test. Primer sequences available in Supplemental Table 1.
Statistical analysis
All the experiments presented in this study were performed at least three times. Data were collected from independent experiments and are given as the mean +/− s.d. Student’s t-test was used for statistical comparisons and p< 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to D. Kimelman and K. Poss for sharing transgenic lines and the hse:ca-bmpr1b-EGFP trangenesis contruct. We thank L. Zon, P. Crozier, I. Kobayashi, S. Wilson, M. Tada and K. Yamasu for sharing in situ probes. We are grateful to Emerald Butko and Maggie Walmsley for critical reading of the manuscript. This work was supported by the UK Medical Research Council (R.P) and the National Institutes of Health (D.T).
Footnotes
Author contributions
C.P. led the study, conducted the experiments, analyzed the data, and wrote the paper; T.P. and F.C.S. conducted experiments, analyzed the data, and edited the manuscript; Y.L. analyzed the data; D.T. and R.P. supervised the study and edited the manuscript.
Competing financial interests
The authors declare no competing financial interests
References
- 1.Swiers G, Rode C, Azzoni E, de Bruijn MF. A short history of hemogenic endothelium. Blood cells, molecules & diseases. 2013;51:206–212. doi: 10.1016/j.bcmd.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 3.Richard C, et al. Endothelio-mesenchymal interaction controls runx1 expression and modulates the notch pathway to initiate aortic hematopoiesis. Dev Cell. 2013;24:600–611. doi: 10.1016/j.devcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 7.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 8.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Rowlinson JM, Gering M. Hey2 acts upstream of Notch in hematopoietic stem cell specification in zebrafish embryos. Blood. 2010;116:2046–2056. doi: 10.1182/blood-2009-11-252635. [DOI] [PubMed] [Google Scholar]
- 10.Nicoli S, Tobia C, Gualandi L, De Sena G, Presta M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood. 2008;111:4965–4972. doi: 10.1182/blood-2007-10-118166. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson RN, et al. Hedgehog signaling via a calcitonin receptor-like receptor can induce arterial differentiation independently of VEGF signaling in zebrafish. Blood. 2012;120:477–488. doi: 10.1182/blood-2011-10-383729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements WK, et al. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474:220–224. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung A, et al. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev Cell. 2013;24:144–158. doi: 10.1016/j.devcel.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkinson RN, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durand C, et al. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters M, et al. Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development. 2009;136:2613–2621. doi: 10.1242/dev.034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suonpaa P, et al. Development of early PCLP1-expressing haematopoietic cells within the avian dorsal aorta. Scandinavian journal of immunology. 2005;62:218–223. doi: 10.1111/j.1365-3083.2005.01655.x. [DOI] [PubMed] [Google Scholar]
- 18.Pimanda JE, et al. The SCL transcriptional network and BMP signaling pathway interact to regulate RUNX1 activity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:840–845. doi: 10.1073/pnas.0607196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 20.North T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 21.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes & development. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu RH, et al. Opposite effects of FGF and BMP-4 on embryonic blood formation: roles of PV.1 and GATA-2. Developmental biology. 1999;208:352–361. doi: 10.1006/dbio.1999.9205. [DOI] [PubMed] [Google Scholar]
- 23.Nakazawa F, Nagai H, Shin M, Sheng G. Negative regulation of primitive hematopoiesis by the FGF signaling pathway. Blood. 2006;108:3335–3343. doi: 10.1182/blood-2006-05-021386. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley M, Cleaver D, Patient R. Fibroblast growth factor controls the timing of Scl, Lmo2, and Runx1 expression during embryonic blood development. Blood. 2008;111:1157–1166. doi: 10.1182/blood-2007-03-081323. [DOI] [PubMed] [Google Scholar]
- 25.Songhet P, Adzic D, Reibe S, Rohr KB. fgf1 is required for normal differentiation of erythrocytes in zebrafish primitive hematopoiesis. Developmental dynamics: an official publication of the American Association of Anatomists. 2007;236:633–643. doi: 10.1002/dvdy.21056. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi H, et al. Fgf21 is essential for haematopoiesis in zebrafish. EMBO reports. 2006;7:649–654. doi: 10.1038/sj.embor.7400685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Haan G, et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell. 2003;4:241–251. doi: 10.1016/s1534-5807(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 28.Yeoh JS, et al. Fibroblast growth factor-1 and -2 preserve long-term repopulating ability of hematopoietic stem cells in serum-free cultures. Stem cells. 2006;24:1564–1572. doi: 10.1634/stemcells.2005-0439. [DOI] [PubMed] [Google Scholar]
- 29.Buono M, Visigalli I, Bergamasco R, Biffi A, Cosma MP. Sulfatase modifying factor 1-mediated fibroblast growth factor signaling primes hematopoietic multilineage development. The Journal of experimental medicine. 2010;207:1647–1660. doi: 10.1084/jem.20091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao M, et al. FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood. 2012;120:1831–1842. doi: 10.1182/blood-2011-11-393991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itkin T, et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood. 2012;120:1843–1855. doi: 10.1182/blood-2011-11-394692. [DOI] [PubMed] [Google Scholar]
- 32.Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- 34.Marques SR, Lee Y, Poss KD, Yelon D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Developmental biology. 2008;321:397–406. doi: 10.1016/j.ydbio.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Patient R, Liu F. Hematopoietic stem cell development and regulatory signaling in zebrafish. Biochimica et biophysica acta. 2013;1830:2370–2374. doi: 10.1016/j.bbagen.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Current biology: CB. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Developmental biology. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. [DOI] [PubMed] [Google Scholar]
- 38.Herbert SP, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 40.Roehl H, Nusslein-Volhard C. Zebrafish pea3 and erm are general targets of FGF8 signaling. Current biology: CB. 2001;11:503–507. doi: 10.1016/s0960-9822(01)00143-9. [DOI] [PubMed] [Google Scholar]
- 41.Bertrand JY, et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iida A, et al. Metalloprotease-dependent onset of blood circulation in zebrafish. Current biology: CB. 2010;20:1110–1116. doi: 10.1016/j.cub.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Murayama E, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 45.Kissa K, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 46.Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biological & pharmaceutical bulletin. 2007;30:1819–1825. doi: 10.1248/bpb.30.1819. [DOI] [PubMed] [Google Scholar]
- 47.Thisse B, et al. Spatial and temporal expression of the zebrafish genome by large-scale in situ hybridization screening. Methods in cell biology. 2004;77:505–519. doi: 10.1016/s0091-679x(04)77027-2. [DOI] [PubMed] [Google Scholar]
- 48.Lawson ND, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 49.Bigas A, D’Altri T, Espinosa L. The Notch pathway in hematopoietic stem cells. Current topics in microbiology and immunology. 2012;360:1–18. doi: 10.1007/82_2012_229. [DOI] [PubMed] [Google Scholar]
- 50.You LR, et al. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 51.Szeto DP, Griffin KJ, Kimelman D. HrT is required for cardiovascular development in zebrafish. Development. 2002;129:5093–5101. doi: 10.1242/dev.129.21.5093. [DOI] [PubMed] [Google Scholar]
- 52.Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends in cell biology. 2010;20:244–256. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Developmental biology. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- 54.Nicoli S, Gilardelli CN, Pozzoli O, Presta M, Cotelli F. Regulated expression pattern of gremlin during zebrafish development. Gene expression patterns: GEP. 2005;5:539–544. doi: 10.1016/j.modgep.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Lieschke GJ, et al. Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Developmental biology. 2002;246:274–295. doi: 10.1006/dbio.2002.0657. [DOI] [PubMed] [Google Scholar]
- 56.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. The Journal of biological chemistry. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 57.Jankovic V, et al. Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1260–1265. doi: 10.1073/pnas.0607894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Row RH, Kimelman D. Bmp inhibition is necessary for post-gastrulation patterning and morphogenesis of the zebrafish tailbud. Developmental biology. 2009;329:55–63. doi: 10.1016/j.ydbio.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. BioEssays: news and reviews in molecular, cellular and developmental biology. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 60.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96:1591–1593. [PubMed] [Google Scholar]
- 61.de Bruijn MF, et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 62.Taoudi S, Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC. FGFs, their receptors, and human limb malformations: clinical and molecular correlations. American journal of medical genetics. 2002;112:266–278. doi: 10.1002/ajmg.10775. [DOI] [PubMed] [Google Scholar]
- 64.Ueno H, Gunn M, Dell K, Tseng A, Jr, Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. The Journal of biological chemistry. 1992;267:1470–1476. [PubMed] [Google Scholar]
- 65.Tonou-Fujimori N, et al. Expression of the FGF receptor 2 gene (fgfr2) during embryogenesis in the zebrafish Danio rerio. Mechanisms of development. 2002;119 (Suppl 1):S173–178. doi: 10.1016/s0925-4773(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 66.Groves JA, Hammond CL, Hughes SM. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development. 2005;132:4211–4222. doi: 10.1242/dev.01958. [DOI] [PubMed] [Google Scholar]
- 67.Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- 68.Westerfield M. The Zebrafish Book; A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) 2. University of Oregon Press; Eugene: 1993. 300P (Book. [Google Scholar]
- 69.Gonzalez-Quevedo R, Lee Y, Poss KD, Wilkinson DG. Neuronal regulation of the spatial patterning of neurogenesis. Dev Cell. 2010;18:136–147. doi: 10.1016/j.devcel.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sacilotto N, et al. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11893–11898. doi: 10.1073/pnas.1300805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.