Abstract
Older adults (age ≥65 years) now initially survive what were previously fatal critical illnesses, but long-term mortality and disability after critical illness remain high. Most studies show that the majority of deaths among older ICU survivors occur during the first 6 to 12 months after hospital discharge. Recent studies of older ICU survivors have created a new standard for longitudinal critical care outcomes studies with a systematic evaluation of pre-critical illness comorbidities and disability and detailed assessments of physical and cognitive function after hospital discharge. These studies show that after controlling for pre-morbid health, older ICU survivors experience large and persistent declines in cognitive and physical function after critical illness. Long-term health-related quality-of-life studies suggest that some older ICU survivors may accommodate to a degree of physical disability and still report good emotional and social well-being, but these studies are subject to survivorship and proxy-response bias. In order to risk-stratify older ICU survivors for long-term (6–12 month) outcomes, we will need a paradigm shift in the timing and type of predictors measured. Emerging literature suggests that the initial acuity of critical illness will be less important, whereas pre-hospitalization estimates of disability and frailty, and, in particular, measures of comorbidity, frailty, and disability near the time of hospital discharge will be essential in creating reliable long-term risk-prediction models.
Keywords: Aged, Critically Ill, Outcomes, Frailty
MeSH Keywords: Aged, Critically Ill, Outcomes, Outcome Assessment
Introduction
Older adults (age ≥65 years) comprise almost half of all intensive care admissions in developed countries, receive more intensive treatment than in the past, and survive what were previously fatal critical illnesses.1, 2 With the aging of the world’s population, the demand for critical care resources from older adults is growing rapidly.3 A large Australian and New Zealand cohort study reported a 6% annual increase in the number of adults over 80 years old admitted to ICUs between 2000 and 2005, informing predictions that adults over 80 years old will make up one out of four ICU admissions by 2015.4
While some critical illnesses constitute an acute event with minimal sequelae, we now recognize that a substantial proportion of older adults are left with marked disability and an increased risk of mortality, particularly during the first year after critical illness.5–7 While small cohort studies first reported that survival of ICU patients after hospital discharge was not affected by age,8, 9 more recent larger cohort and population-based studies show that older age is an important and independent predictor of mortality after critical illness even after controlling for the severity of critical illness and comorbidities.10–14 Understanding the long-term outcomes of older ICU survivors is important for measuring the true value of intensive care for this rapidly growing population, and fundamental to targeting appropriate rehabilitative, therapeutic, and palliative interventions that will improve survival and/or quality-of-life after critical illness.
Measuring Outcomes in Older Survivors of Critical Illness
Mortality
Historically, critical illness outcomes studies calculated long-term mortality from the time of ICU admission and included ICU patients who died in hospital.15 However, given the high hospital mortality associated with older ICU patients and a growing interest in improving outcomes for older ICU survivors, more recent studies have calculated mortality from the date of hospital discharge among those who survived intensive care.5, 16 While this latter approach allows for a clearer assessment of post-hospitalization mortality, it often prevents comparing mortality rates across old and new studies, since inclusion or exclusion of in-hospital deaths may substantially shift mortality estimates. A number of recent studies now calculate separately the short and long-term mortality of older critically-ill patients as 28-day mortality for all older ICU patients, and longer-term mortality for 28-day older ICU survivors, respectively.12, 13
The choice of control group to assess the influence of critical illness on the long-term risk of disability and death varies between studies. Comparison with age-adjusted hospitalized patients may allow for better isolation of the severity of critical illness as a risk factor,5, 11, 17 whereas comparison with the age-adjusted general population provides a better estimate of the total residual risk of death compared to the average person.5, 6, 10, 18 Survivors of critical illness should ideally be followed until the gradient of their survival curve parallels that of a relevant control group. But, the exact length of time depends upon the specific types of critically-ill patients studied and the chosen control group. Endpoints of long-term outcomes studies of older ICU survivors usually vary between 6 months and 3 years,5, 11, 19 with a majority of studies following older ICU survivors for 1 year after hospital discharge.5, 6, 8–13, 16, 17
Whether older populations are selected by country, age, intervention, or diagnosis, most studies show that the majority of deaths among older ICU survivors occur during the first 6 to 12 months after hospital discharge.5, 16, 20–22 Wunsch et al. published the largest long-term mortality study of older ICU survivors to date examining a 2.5% sample of American Medicare beneficiaries aged ≥65 years who received intensive care.5 In this study, the 6-month mortality was 14% for all older ICU survivors, 30% for older ICU survivors who received mechanical ventilation, and 26% for older ICU survivors discharged to post-acute care facilities. For those who received mechanical ventilation or who were discharged to post-acute care facilities, approximately 50% of deaths in the 3 years after hospital discharge occurred during the first 6 months.
Smaller cohort studies of older general medical and surgical ICU patients published in the past 4 years report similar mortality rates to those described by Wunsch et al (Table 1).12, 13, 16, 19, 22, 23 Differences in case-mix between cohort studies likely explain most of the variation in the reported mortalities. For example, in-hospital and long-term mortality was higher for older patients with medical or unplanned surgical ICU admissions compared to those admitted with planned surgical admissions.13
Table 1.
Recent long-term mortality studies of older general medical and surgical ICU patients
| Study Type & Author (Reference #) | Publication Year | Study Period | n | Restrictions | Ages (Years) | In-Hospital or 28-day Mortality | Only ICU-Survivorsa | Long-Term Mortality | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-months | 1-year | 2-years | 3-years | ||||||||
| Population Studies | |||||||||||
| Nielsson (13) | 2013 | 2005–2011 | 6,266 | ≥80 | - | Yes | - | - | - | - | |
| 2,332 | Medical | ≥80 | 44% | Yes | - | 25% | - | - | |||
| 2,581 | Acute Surgery | ≥80 | 40% | Yes | - | 27% | - | - | |||
| 1,353 | Elective Surgery | ≥80 | 12% | Yes | - | 12% | - | - | |||
| Wunsch (5) | 2010 | 2003 | 35,308 | ≥65 | - | Yes | 14% | 22% | 31% | 40% | |
| 2141 | MV | ≥65 | - | Yes | 30% | 39% | 49% | 58% | |||
| 33167 | No MV | ≥65 | - | 13% | 20% | 30% | 38% | ||||
| Kahn (16) | 2010 | 1997–2006 | 18,660 | Discharge to LTAC | ≥65 | - | Yes | 42% | 51% | - | - |
| Cohort Studies | |||||||||||
| Fuchs (12) | 2013 | 2001–2008 | 7,265 | ≥65 | 26% | Yes | - | 25% | - | - | |
| Baldwin (19) | 2013 | 2005–2009 | 2,536 | Medical-ICU only | ≥65 | - | Yes | 28% | - | - | - |
| Roch (22) | 2011 | 2001–2006 | 299 | ≥80 | 55% | Yes | - | 37% | 53% | - | |
| Sacanella (23) | 2011 | Not Stated | 230 | No Prior Functional or Cognitive Impairment | ≥65 | 30% | Yes | - | 21% | - | - |
|
| |||||||||||
| Range | 12–55% | Yes | 13–42% | 12–51% | 30–53% | 38–58 | |||||
ICU: Intensive Care Unit; MV: Mechanical Ventilation; LTAC: Long-Term Acute Care
Long-term mortality of ICU survivors was calculated from only those who survived to hospital discharge, and excluded patients who died in-hospital. Studies that did not calculate long-term mortality for ICU survivors instead calculated cumulative mortality that is defined as the cumulative percentage of patients dying after admission to ICU.
Regardless of the initial critical illness diagnosis, older adults who develop a need for prolonged mechanical ventilation (PMV) from critical illness are increasingly recognized to have some of the highest mortality rates among older ICU survivors.16, 24 These patients suffer from chronic critical illness (CCI), which is characterized by PMV via tracheostomy (the hallmark of CCI), and functional dependence due to some combination of profound weakness, endocrinopathy, poor nutrition, skin breakdown, and brain dysfunction.25 Several studies from the United States indicate that CCI afflicts primarily older ICU survivors; the mean (SD) age for adult patients undergoing tracheostomy for prolonged mechanical ventilation is 65 (15) years,26 and for those in specialized weaning facilities it is in the eighth decade.16, 27 The 1-year mortality of American ICU survivors age ≥65 years who are discharged to ventilator weaning facilities is 69%.16 Older age, greater pre-critical illness disability, and a higher burden of comorbidities appear to be the strongest predictors of mortality in CCI patients.24, 28–30
Disability
Inception cohort studies that empanel patients at the time of critical illness diagnosis have shown that survivors of critical illness have an enormous burden of functional and neurocognitive disabilities, regardless of age.31–33 But, the proportion of disability attributable to critical illness, versus the proportion that could be attributed to the premorbid status or disease that led to the critical illness has been difficult to determine because prospective measurements of function and cognition prior to becoming critically ill usually do not exist and retrospective reports, typically by proxy, may be subject to recall bias.34, 35
In order to estimate functional disability, cognitive impairment, and geriatric conditions attributable to critical illness in older ICU survivors, 3 recent studies cleverly linked large, nationally representative longitudinal cohort studies of older American adults with Medicare data.7, 36, 37 Barnato et al. linked Medicare Current Beneficiary Survey data with Medicare hospitalization records to assess the impact of hospitalization with mechanical ventilation on disability, compared to hospitalization without mechanical ventilation, after accounting for a prospectively assessed pre-hospitalization functional status.7 They found a 30% greater increase in ADL dependencies and worse mobility among older (mean age 76 ± 7 years) survivors of mechanical ventilation compared with survivors of hospitalization without mechanical ventilation than would have been predicted from prior functional status.7
Iwashyna et al. combined Health and Retirement Study (HRS) data which included prospectively assessed measures of cognition and disability with Medicare claims data to determine differences in cognitive impairment and physical disability among older adults who survived severe sepsis versus those hospitalized without sepsis or critical illness.36 Older survivors of severe sepsis (mean age 76 ± 9 years) had a nearly three-fold increase in new, moderate or severe cognitive impairment (6% to 17%), whereas those with non-sepsis general hospitalizations had no such change in cognitive impairment. Severe sepsis was associated with an average of 1.5 new limitations in basic or independent ADLs after hospitalization, independent of the presence or absence of premorbid functional limitations. Comparatively, those with general hospitalizations acquired, on average, only 0.5 new dependencies (p < 0.05). The large declines in cognition and physical function after severe sepsis persisted for at least 8 years, and likely resulted in a pivotal downturn in patients’ ability to live independently.36
In a separate study by Iwashyna et al., investigators again linked HRS and Medicare data to ascertain whether severe sepsis is associated with an increased risk of geriatric conditions.37 They found that low BMI, injurious falls, incontinence, and vision loss among older survivors of severe sepsis (mean age 77 ± 9 years) were more prevalent after hospitalization for severe sepsis than during the year prior, and more prevalent than in age-matched older Americans. However, a longitudinal analysis using 3 years of subjects’ data prior to hospitalization for severe sepsis demonstrated that only the prevalence of low BMI increased significantly after severe sepsis, and that the other geriatric conditions continued to develop at the same rate as prior to the hospitalization.37 This study demonstrates that outcomes studies of older ICU survivors that do not fully control for pre-critical illness health and functional trajectories might report false-positive associations between critical illness and the development of geriatric conditions.
These 3 studies have elevated the standard for longitudinal critical-care outcomes studies by systematically evaluating pre-critical illness comorbidities and disability, using a control group, and performing detailed assessments of physical and cognitive function after hospital discharge.7, 36, 37 Controlling for premorbid physical and neurocognitive function is particularly important for determining disability attributable to critical illness in older adults because the prevalence and severity of physical limitations and cognitive impairment in the general population increases with age, particularly during the last 2 to 4 years of life.38, 39
Health-Related Quality-of-Life
The complexity and heterogeneity of physical, psychological, cognitive, and social deficits in the aftermath of critical illness have led investigators to use health-related quality-of-life (HRQOL) as a patient-centered global outcome assessment of these deficits.31, 40 The SF-36 and Euro-QoL5D are the most commonly used surveys, and are well validated for critical illness survivors.41 Studies published over the past 20 years focusing on long-term follow-up for HRQOL in older ICU survivors show discrepant results due to differences in case-mix, HRQOL surveys used, and the duration of follow-up.42
Determining the deficits in HRQOL attributable to critical illness is particularly difficult. Prospective measurements of HRQOL prior to critical illness generally do not exist, making it difficult to determine exactly what proportion of these outcomes are new and attributable to critical illness. Furthermore, while retrospective reports of physical disability and cognition by proxy have been shown to be valid in certain instances,43, 44 serious doubts have been raised about the accuracy of retrospective and proxy assessments of HRQOL,35 especially regarding psychological function.45 Some assessments of physical function and cognition can be assessed from proxy observations if a patient is too debilitated to actively participate (e.g. Katz ADLs 46 or the Informant Questionnaire on Cognitive Decline in the Elderly (IQ-CODE)47), but determining HRQOL requires subjective responses from a patient which can be difficult or impossible if that patient has cognitive dysfunction. For example, in a 1-year cohort study of patients receiving PMV (mean age 55 ± 16 years), ADLs were assessed in all patients, but one third of patients were too debilitated to complete the Euro-QoL5D HRQOL survey themselves so only proxy assessments of HRQOL were used in the analysis.30
Several recent studies that have measured HRQOL in ICU survivors older than 75 or 80 years report worse physical but good emotional well-being and social function scores 1 to 2 years later.22, 48–51 These studies are single-center cohort studies with <300 participants, and are subject to survivorship or proxy-response bias in three ways: (1) measuring HRQOL in 1-year survivors of critical illness ignores the likely poor HRQOL of the many ICU survivors who die during the first year after critical illness;22, 48, 50 (2) reporting the HRQOL of only older ICU survivors who are capable of completing these surveys ignores the likely poor HRQOL of those who survive but are too disabled to complete them;49–51 and (3) collectively analyzing both proxy reports of HRQOL for patients who cannot complete them and reports by patients who complete their own HRQOL survey potentially introduces proxy-response bias.22 Despite these limitations, these studies add to a body of research that suggests that at least some older long-term survivors of critical illness accommodate to a degree of physical disability and still report good emotional and social well-being.42, 52
Predicting Outcomes in Older Survivors of Critical Illness
Severity of Critical Illness
Existing ICU risk-stratification models that predict in-hospital mortality rely heavily on physiologic variables during the first 24 hours after ICU admission.53–55 The Acute Physiologic and Chronic Health Evaluation (APACHE) II score has been shown to not independently predict 1-year mortality in ICU patients older than 75 or 80 years,20, 21 and the Simplified Acute Physiology Score (SAPS) II score at ICU admission does not independently predict 6-month mortality in older ICU survivors.19 These results are not surprising given that these models were specifically derived to predict in-hospital mortality, and not longer-term mortality. Still, these studies suggest that measures of the initial severity of critical illness may be less important than other measures of health when predicting outcomes for older ICU survivors.
Burden of Comorbidity
Several studies have shown that the pre-existing burden of comorbidity is one of the most important predictors of mortality after critical illness in older adults. The Charlson Comorbidity Index score56,57 was found to contribute more to predicting long-term mortality in ICU survivors than the APACHE-II score, ventilator days, vasopressor use, use of renal replacement therapy, peak number of organ failures, and gender.14 Among older ICU survivors, the Charlson Comorbidity Index score contributed more to a 6-month mortality prediction model than age, SAPS-II score, or use of mechanical ventilation.19 Since comorbidities may be acquired from critical illness (e.g. renal insufficiency after surviving septic shock), future studies should examine whether measuring comorbidities just prior to hospital discharge captures better the burden of comorbidity for older ICU survivors.
Disability
Studies have found that disability or a need for skilled-care (a surrogate marker of disability) both prior to and immediately following hospitalization for critical illness are strong independent predictors of 6 and 12-month disability and mortality in older ICU survivors.4, 19, 20, 23, 28, 58 One recent study of octogenarian ICU patients did not find functional status prior to admission to be associated with long-term mortality,22 but a high prevalence of functional limitations and pre-existing fatal diseases in the cohort were thought to have suppressed this association.59 Retrospective assessments of pre-hospitalization disability with Katz ADLs from either the subject or proxy have been shown to have predictive validity,60 but more detailed assessments of function have not yet been validated for such retrospective use (e.g. the Barthel Index61). Sacanella et al. recently showed that lower Barthel index scores (i.e., greater disability) at hospital discharge independently predicted partial versus full functional recovery among 1-year older ICU survivors.23 These findings suggest that the degree of disability at hospital discharge may help differentiate between those who most need post-ICU rehabilitative interventions and those who are most likely to recover on their own.
Frailty
Frailty is a measurable clinical phenotype in which there is an increase in an individual’s vulnerability for developing increased disability and/or mortality when exposed to a stressor.62 In essence, frailty is a measure of physiologic age, and while it is correlated with chronological age, it is not inevitably present in all older adults.63 There is a consensus that physical frailty is characterized by a loss of physiologic reserve with declines in muscle mass, metabolic rate, energy expenditure, strength, and endurance.64 These deficits that typically take years to accumulate in the outpatient geriatric population, rapidly develop or worsen in older ICU patients.65
Several measures of frailty predict morbidity and mortality in community-dwelling older adults, independent of comorbidities and disability.64 Fried and colleagues developed what is perhaps the most widely adopted measure of physical frailty based upon 5 possible components (>10% weight loss in the past year, weak hand-grip strength, slow walk speed, reduced baseline physical activity, and feelings of exhaustion) that mark an underlying physiological state of multisystem energy dysregulation. Traditionally, community-dwelling older adults who have 1–2 or ≥3 components are considered intermediate-frail or frail, respectively.62 Another commonly used frailty index is the Rockwood Clinical Frailty Scale (CFS), a well-validated 9-point assessment tool which incorporates multi-morbidity and dementia in the frailty assessment, and scoring is based on clinical judgment.66
Two studies have prospectively measured frailty and outcomes in older ICU survivors with notable differences in the timing and type of frailty measurements made.67, 68 Bagshaw et al. measured the CFS at ICU admission in 421 adults age ≥ 50 years across 6 Canadian hospitals. Defining frailty as a CFS score >4, they found that frailty predicted both in-hospital and 1-year mortality independent of other important demographic and clinical variables. Compared with non-frail survivors, frail survivors were significantly more likely to be discharged with new disability (71% versus 52%), and were more likely to die within 1 year (48% versus 25%).67
Baldwin et al. measured Fried’s frailty index in 22 older (age ≥65 years) medical-ICU survivors of mechanical ventilation just prior to discharge from a single tertiary-care hospital in the United States.68 They found in unadjusted analyses that each 1-point increase in Fried’s frailty score was associated with a 3-fold increase in 6-month mortality (RR: 3.0, 95% CI 1.4–6.3). The small sample size of the study precluded multivariable analyses, but age, pre-existing disability, Charlson Comorbidity Index score, APACHE II score, and chronic critical illness status explained only 45% of the variance of frailty. Given that easily measured and important dimensions of physical health in older ICU survivors explain less than half the variance in Fried’s frailty score, frailty likely represents a previously unmeasured phenotype of interest in older ICU survivors.
There are advantages and disadvantages to using either the Rockwood or Fried Frailty index either at ICU admission or just prior to hospital discharge. The Rockwood index has an inherent element of informed subjectivity, but using the index at ICU admission may quickly provide more accurate prognostication and identify a vulnerable population that is less likely to have long-term benefit from intensive care. The Fried index takes more time to measure just prior to hospital discharge, but the components of Fried’s frailty have potential to be in and of themselves targets for post-ICU rehabilitative and therapeutic interventions aimed at treating ICU-acquired debilitation. For example, ICU survivors who are weak or slow may benefit from novel exercise interventions (e.g. bedside cycle ergometry69 or diaphragmatic strength training70, 71) or pharmacologic therapies (e.g. myostatin agonists to decrease and prevent muscle loss72 or vitamin D supplementation to improve muscle function73), and those who have weight loss and exhaustion may benefit from protein-calorie supplementation.74
Assessing Older ICU Survivors for Post-ICU Care
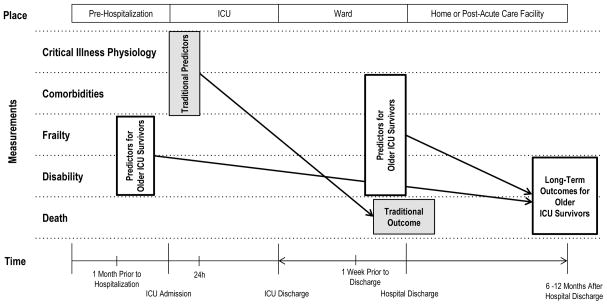
Despite ICU-based interventions that have decreased disability after critical illness (e.g. sedation minimization and early mobilization75), the number of older ICU survivors with significant disability and high 6-month mortality is increasing.5, 7, 76, 77 Now more than ever, we need reliable 6–12 month disability and mortality prediction models for this rapidly growing population of older adults so that we can risk-stratify them for post-acute care interventions aimed at improving their quality-of-life and/or survival. To do this, we should shift the primary focus of our measurements away from the physiologic variables used in existing ICU risk-stratification models designed to predict in-hospital mortality,54, 55 and instead direct our attention toward estimates of pre-critical illness disability and frailty and direct measurements of cormorbidity, disability, and frailty just prior to hospital discharge (Figure 1). Indeed, Baldwin et al. retrospectively derived and externally validated an accurate 6-month mortality prediction model for older medical-ICU survivors using some of these measurements.19
Figure 1.
Existing ICU risk-stratification models* designed to predict in-hospital or 28-day mortality rely heavily on physiologic variables around the time of ICU admission. Recent literature suggests that pre-hospitalization estimates of disability and frailty, and in particular measures of comorbidity, frailty, and disability near the time of hospital discharge, may be the most important predictors of long-term (6–12 month) disability and mortality for older ICU survivors. *Acute Physiologic and Chronic Health Evaluation (APACHE), Simplified Acute Physiologic Score (SAPS)
Several studies already offer guidance with assessing prognosis for older ICU survivors (Table 3). First, older ICU survivors with a low burden of comorbidities and little to no disability at hospital discharge have generally good outcomes.19, 23, 48, 50, 51 Second, older ICU survivors who were autonomous prior to critical illness and disabled at hospital discharge may still be able to recover and may be appropriate candidates for post-ICU rehabilitation that may speed that recovery.23 Third, older ICU survivors of mechanical ventilation with pre-critical illness ADL disabilities and signs of frailty (e.g. low BMI or weight loss), a high number of ADL disabilities or a need for skilled-care at the time of hospital discharge, and a Do-Not-Resuscitate (DNR) preference have a particularly high 6-month mortality rate.5, 19, 78 These more chronically debilitated older patients should have their goals of care addressed, and if appropriate, be offered hospice or home-hospice services prior to hospital discharge.79 Many older ICU survivors who have mild to moderate degrees of comorbidity, disability, and frailty are particularly susceptible to permanent disability and death in the first year after critical illness. Future research aimed at understanding how these variables interact will be fundamental to risk-stratifying and identifying these patients for appropriate post-ICU care.
Table 3.
Characteristics associated with disability and/or death in older ICU survivors
| Characteristic or Exposure | Measurement | Comment | Supporting Studies |
|---|---|---|---|
| Age | Independent predictor in more recent larger cohort studies, but not in older smaller cohort studies | 10–14 | |
| Pre-Existing Disability | Admission from skilled-care facility, Katz ADLs, Barthel Index | Pre-hospitalization estimates of disability using more detailed surveys than the Katz ADLs need validation (e.g. Barthel Index). | 4, 19, 20, 28, 58 |
| Pre-Existing Frailty | CFS | Subjective measure that quickly identifies at risk patients | 67 |
| Severe Sepsis | Persistent physical disability and neurocognitive impairment for up to 8 years after treatment of the initial infection | 36 | |
| Medical or Unplanned Surgical ICU Admission | 13 | ||
| Use of Mechanical Ventilation | 5, 16 | ||
| Chronic Critical Illness | PMV via tracheostomy ≥ 10 days | Highest reported mortality among older ICU survivors | 16, 24 |
| Burden of Comorbidity | High Charlson Comorbidity Score | 14, 19 | |
| DNR Preference | DNR order at hospital discharge | DNR decision reflects a patient preference, and may also reflect a severity of chronic illness and frailty not captured with other measurements | 19 |
| Disability at Hospital Discharge | Discharge to skilled-care facility, Katz ADLs, Barthel Index | Less disability is predictive of full-functional recovery among 1-year older ICU survivors (23) | 5, 16, 19, 23, 28 |
| Frailty at Hospital Discharge | CFS or Fried’s Index | Fried’s frailty measurements identify deficits that may be targets for post-ICU interventions | 19, 68 |
ADL: Activities of Daily Living; CFS: Clinical Frailty Scale; PMV: Prolonged Mechanical Ventilation; DNR: Do-Not-Resuscitate
Conclusion
Outcomes in older survivors of critical illness vary widely as a function of the interaction between the acute critical illness, comorbid disease, pre-and post-critical illness functional status, and physiologic reserve. For most older ICU survivors, the most severe disability and highest mortality occur during the first 6 to 12 months after hospital discharge, suggesting that there may be a window for future studies and potential interventions to improve outcomes. While existing ICU risk-stratification models that predict in-hospital mortality rely heavily on physiologic variables around the time of ICU admission,54, 55 future prognostic models for older ICU survivors will have to incorporate measures of disability, comorbidity, and frailty both before and immediately after the critical illness in order to more accurately risk-stratify and identity patients most suitable for post-ICU palliative, rehabilitative, and therapeutic interventions that may improve survival and/or quality-of-life after critical illness.
Table 2.
Recent long-term disability studies in older survivors of critical illness that control for pre-critical illness function
| Author (Reference #) | Publication Year | Study Period | n | Mean (SD) Age in years | Pre and Post-Critical Illness Measurements | Interval Between Measurements | Duration of Follow-up After Critical Illness | Primary Finding |
|---|---|---|---|---|---|---|---|---|
| Barnato (7) | 2011 | 1996–2003 | 26,072 | 76 (7) | Mobility and ADL disabilities | 3 mo | 1 yr | 30% greater increase in ADL disabilities and worse mobility among MV survivors compared to those hospitalized without MV than would be predicted from pre-hospitalization functional status. |
| Iwashyna (36) | 2010 | 1998–2005 | 1,194 | 76 (9) | Cognitive function and ADL + iADL disabilities | 2 yr | up to 8 yr | Severe sepsis was associated independently with new moderate to from 6% to 17%) and 1.5 new ADL or iADL limitations on average. |
| Iwashyna (37) | 2012 | 1998–2006 | 623* | 77 (9) | Geriatric Conditions† | 2 yr | 2 yr | Severe sepsis was associated with increased rates of only a subset of geriatric conditions, not all. Examining longitudinal trends in geriatric conditions for 3 years prior to severe sepsis, only low BMI increased significantly after severe sepsis. Failing to measure pre-illness levels and trajectories may led to false- positive associations with impairments after severe sepsis. |
MV: Mechanical Ventilation; ADL and iADL: basic Activities of Daily Living and independent Activities of Daily Living; BMI: Body Mass Index
sepsis hospitalizations (516 individuals)
Falls, incontinence, low body mass index, poor vision, poor hearing, severe pain
Key Messages.
The majority of deaths in older ICU survivors occur during the first 6–12 months after hospital discharge.
Recent studies of older ICU survivors have created a new standard for critical care outcomes studies by carefully controlling for pre-morbid health, using a control group, and obtaining detailed assessments of physical and cognitive function after hospital discharge. They have shown that older ICU survivors experience large and persistent declines in cognitive and physical function after critical illness.
Estimations of disability and frailty prior to the onset of critical illness and direct measurements of cormorbidity, disability, and frailty just prior to hospital discharge will be essential in creating reliable 6–12 month risk-prediction models for older ICU survivors.
Acknowledgments
Sources of Support: Dr. Baldwin is supported by the National Institutes of Health [KL2 TR000081, and by a Loan Repayment Grant from the National Institute on Aging].
The author is grateful for input and editorial assistance from Darryl Abrams, MD, and David J. Lederer, MD, MS.
References
- 1.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA. Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006 Apr;34(4):1016–24. doi: 10.1097/01.CCM.0000206105.05626.15. Epub 2006/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 2.Lerolle N, Trinquart L, Bornstain C, Tadie JM, Imbert A, Diehl JL, et al. Increased intensity of treatment and decreased mortality in elderly patients in an intensive care unit over a decade. Crit Care Med. 2010 Jan;38(1):59–64. doi: 10.1097/CCM.0b013e3181b088ec. Epub 2009/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000 Dec 6;284(21):2762–70. doi: 10.1001/jama.284.21.2762. Epub 2000/12/06. eng. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Webb SA, Delaney A, George C, Pilcher D, Hart GK, et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Critical care (London, England) 2009;13(2):R45. doi: 10.1186/cc7768. Epub 2009/04/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010 Mar 3;303(9):849–56. doi: 10.1001/jama.2010.216. Epub 2010/03/04. eng. [DOI] [PubMed] [Google Scholar]
- 6.Brinkman S, de Jonge E, Abu-Hanna A, Arbous MS, de Lange DW, de Keizer NF. Mortality after hospital discharge in ICU patients. Critical care medicine. 2013 May;41(5):1229–36. doi: 10.1097/CCM.0b013e31827ca4e1. Epub 2013/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 7.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011 Apr 15;183(8):1037–42. doi: 10.1164/rccm.201002-0301OC. Epub 2010/11/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelluri L, Pinsky MR, Donahoe MP, Grenvik A. Long-term outcome of critically ill elderly patients requiring intensive care. JAMA. 1993 Jun 23–30;269(24):3119–23. Epub 1993/06/23. eng. [PubMed] [Google Scholar]
- 9.Rockwood K, Noseworthy TW, Gibney RT, Konopad E, Shustack A, Stollery D, et al. One-year outcome of elderly and young patients admitted to intensive care units. Crit Care Med. 1993 May;21(5):687–91. doi: 10.1097/00003246-199305000-00011. Epub 1993/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 10.Williams TA, Dobb GJ, Finn JC, Knuiman MW, Geelhoed E, Lee KY, et al. Determinants of long-term survival after intensive care. Crit Care Med. 2008 May;36(5):1523–30. doi: 10.1097/CCM.0b013e318170a405. Epub 2008/04/25. eng. [DOI] [PubMed] [Google Scholar]
- 11.Keenan SP, Dodek P, Chan K, Hogg RS, Craib KJ, Anis AH, et al. Intensive care unit admission has minimal impact on long-term mortality. Crit Care Med. 2002 Mar;30(3):501–7. doi: 10.1097/00003246-200203000-00002. Epub 2002/05/07. eng. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs L, Chronaki CE, Park S, Novack V, Baumfeld Y, Scott D, et al. ICU admission characteristics and mortality rates among elderly and very elderly patients. Intensive care medicine. 2012 Oct;38(10):1654–61. doi: 10.1007/s00134-012-2629-6. Epub 2012/07/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsson MS, Christiansen CF, Johansen MB, Rasmussen BS, Tonnesen E, Norgaard M. Mortality in elderly ICU patients: a cohort study. Acta anaesthesiologica Scandinavica. 2013 Oct 13; doi: 10.1111/aas.12211. Epub 2013/10/15. Eng. [DOI] [PubMed] [Google Scholar]
- 14.Ho KM, Knuiman M, Finn J, Webb SA. Estimating long-term survival of critically ill patients: the PREDICT model. PLoS One. 2008;3(9):e3226. doi: 10.1371/journal.pone.0003226. Epub 2008/09/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams TA, Dobb GJ, Finn JC, Webb SA. Long-term survival from intensive care: a review. Intensive Care Med. 2005 Oct;31(10):1306–15. doi: 10.1007/s00134-005-2744-8. Epub 2005/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010 Jun 9;303(22):2253–9. doi: 10.1001/jama.2010.761. Epub 2010/06/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan PS, Nallamothu BK, Krumholz HM, Spertus JA, Li Y, Hammill BG, et al. Long-term outcomes in elderly survivors of in-hospital cardiac arrest. The New England journal of medicine. 2013 Mar 14;368(11):1019–26. doi: 10.1056/NEJMoa1200657. Epub 2013/03/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JC, Plenderleith L, Ridley SA. Long-term survival following intensive care: subgroup analysis and comparison with the general population. Anaesthesia. 2003 Jul;58(7):637–42. doi: 10.1046/j.1365-2044.2003.03205.x. Epub 2003/06/07. eng. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin MR, Narain WR, Wunsch H, Schluger NW, Cooke JT, Maurer MS, et al. A prognostic model for 6-month mortality in elderly survivors of critical illness. Chest. 2013 Apr;143(4):910–9. doi: 10.1378/chest.12-1668. Epub 2013/05/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boumendil A, Maury E, Reinhard I, Luquel L, Offenstadt G, Guidet B. Prognosis of patients aged 80 years and over admitted in medical intensive care unit. Intensive Care Med. 2004 Apr;30(4):647–54. doi: 10.1007/s00134-003-2150-z. Epub 2004/02/27. eng. [DOI] [PubMed] [Google Scholar]
- 21.Somme D, Maillet JM, Gisselbrecht M, Novara A, Ract C, Fagon JY. Critically ill old and the oldest-old patients in intensive care: short- and long-term outcomes. Intensive Care Med. 2003 Dec;29(12):2137–43. doi: 10.1007/s00134-003-1929-2. Epub 2003/11/14. eng. [DOI] [PubMed] [Google Scholar]
- 22.Roch A, Wiramus S, Pauly V, Forel JM, Guervilly C, Gainnier M, et al. Long-term outcome in medical patients aged 80 or over following admission to an intensive care unit. Crit Care. 2011;15(1):R36. doi: 10.1186/cc9984. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacanella E, Perez-Castejon JM, Nicolas JM, Masanes F, Navarro M, Castro P, et al. Functional status and quality of life 12 months after discharge from a medical ICU in healthy elderly patients: a prospective observational study. Critical care (London, England) 2011;15(2):R105. doi: 10.1186/cc10121. Epub 2011/03/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dermot Frengley J, Sansone GR, Shakya K, Kaner RJ. Prolonged Mechanical Ventilation in 540 Seriously Ill Older Adults: Effects of Increasing Age on Clinical Outcomes and Survival. J Am Geriatr Soc. 2014 Jan 9; doi: 10.1111/jgs.12597. Epub 2014/01/11. Eng. [DOI] [PubMed] [Google Scholar]
- 25.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010 Aug 15;182(4):446–54. doi: 10.1164/rccm.201002-0210CI. Epub 2010/05/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993–2002. Crit Care Med. 2004 Nov;32(11):2219–26. doi: 10.1097/01.ccm.0000145232.46143.40. Epub 2005/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 27.Scheinhorn DJ, Hassenpflug MS, Votto JJ, Chao DC, Epstein SK, Doig GS, et al. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007 Jan;131(1):85–93. doi: 10.1378/chest.06-1081. Epub 2007/01/16. eng. [DOI] [PubMed] [Google Scholar]
- 28.Carson SS, Bach PB, Brzozowski L, Leff A. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Am J Respir Crit Care Med. 1999 May;159(5 Pt 1):1568–73. doi: 10.1164/ajrccm.159.5.9809002. Epub 1999/05/06. eng. [DOI] [PubMed] [Google Scholar]
- 29.Carson SS, Kahn JM, Hough CL, Seeley EJ, White DB, Douglas IS, et al. A multicenter mortality prediction model for patients receiving prolonged mechanical ventilation. Crit Care Med. 2012 Apr;40(4):1171–6. doi: 10.1097/CCM.0b013e3182387d43. Epub 2011/11/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010 Aug 3;153(3):167–75. doi: 10.1059/0003-4819-153-3-201008030-00007. Epub 2010/08/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011 Feb;39(2):371–9. doi: 10.1097/CCM.0b013e3181fd66e5. Epub 2010/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 32.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011 Apr 7;364(14):1293–304. doi: 10.1056/NEJMoa1011802. Epub 2011/04/08. eng. [DOI] [PubMed] [Google Scholar]
- 33.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013 Oct 3;369(14):1306–16. doi: 10.1056/NEJMoa1301372. Epub 2013/10/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lum TY, Lin WC, Kane RL. Use of proxy respondents and accuracy of minimum data set assessments of activities of daily living. The journals of gerontology Series A, Biological sciences and medical sciences. 2005 May;60(5):654–9. doi: 10.1093/gerona/60.5.654. Epub 2005/06/24. eng. [DOI] [PubMed] [Google Scholar]
- 35.Scales DC, Tansey CM, Matte A, Herridge MS. Difference in reported pre-morbid health-related quality of life between ARDS survivors and their substitute decision makers. Intensive Care Med. 2006 Nov;32(11):1826–31. doi: 10.1007/s00134-006-0333-0. Epub 2006/09/08. eng. [DOI] [PubMed] [Google Scholar]
- 36.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA: the journal of the American Medical Association. 2010 Oct 27;304(16):1787–94. doi: 10.1001/jama.2010.1553. Epub 2010/10/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. American journal of respiratory and critical care medicine. 2012 Apr 15;185(8):835–41. doi: 10.1164/rccm.201109-1660OC. Epub 2012/02/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AK, Walter LC, Miao Y, Boscardin WJ, Covinsky KE. Disability during the last two years of life. JAMA internal medicine. 2013 Sep 9;173(16):1506–13. doi: 10.1001/jamainternmed.2013.8738. Epub 2013/07/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychosomatic medicine. 2007 Feb-Mar;69(2):131–7. doi: 10.1097/PSY.0b013e31803130ae. Epub 2007/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 40.Hofhuis JG, van Stel HF, Schrijvers AJ, Rommes JH, Bakker J, Spronk PE. Health-related quality of life in critically ill patients: how to score and what is the clinical impact? Current opinion in critical care. 2009 Oct;15(5):425–30. doi: 10.1097/MCC.0b013e32833079e4. Epub 2009/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 41.Angus DC, Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003 Mar;29(3):368–77. doi: 10.1007/s00134-002-1624-8. Epub 2003/01/22. eng. [DOI] [PubMed] [Google Scholar]
- 42.Hennessy D, Juzwishin K, Yergens D, Noseworthy T, Doig C. Outcomes of elderly survivors of intensive care: a review of the literature. Chest. 2005 May;127(5):1764–74. doi: 10.1378/chest.127.5.1764. Epub 2005/05/13. eng. [DOI] [PubMed] [Google Scholar]
- 43.Pol MC, Buurman BM, de Vos R, de Rooij SE. Patient and proxy rating agreements on activities of daily living and the instrumental activities of daily living of acutely hospitalized older adults. J Am Geriatr Soc. 2011 Aug;59(8):1554–6. doi: 10.1111/j.1532-5415.2011.03514.x. Epub 2011/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 44.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. International psychogeriatrics / IPA. 2004 Sep;16(3):275–93. doi: 10.1017/s1041610204000390. Epub 2004/11/24. eng. [DOI] [PubMed] [Google Scholar]
- 45.Rogers J, Ridley S, Chrispin P, Scotton H, Lloyd D. Reliability of the next of kins’ estimates of critically ill patients’ quality of life. Anaesthesia. 1997 Dec;52(12):1137–43. doi: 10.1111/j.1365-2044.1997.240-az0374.x. Epub 1998/03/05. eng. [DOI] [PubMed] [Google Scholar]
- 46.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The indx of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963 Sep 21;185:914–9. doi: 10.1001/jama.1963.03060120024016. Epub 1963/09/21. eng. [DOI] [PubMed] [Google Scholar]
- 47.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychological medicine. 1989 Nov;19(4):1015–22. doi: 10.1017/s0033291700005742. Epub 1989/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 48.de Rooij SE, Govers AC, Korevaar JC, Giesbers AW, Levi M, de Jonge E. Cognitive, functional, and quality-of-life outcomes of patients aged 80 and older who survived at least 1 year after planned or unplanned surgery or medical intensive care treatment. J Am Geriatr Soc. 2008 May;56(5):816–22. doi: 10.1111/j.1532-5415.2008.01671.x. Epub 2008/04/04. eng. [DOI] [PubMed] [Google Scholar]
- 49.Tabah A, Philippart F, Timsit JF, Willems V, Francais A, Leplege A, et al. Quality of life in patients aged 80 or over after ICU discharge. Crit Care. 2010;14(1):R2. doi: 10.1186/cc8231. Epub 2010/01/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroder MA, Poulsen JB, Perner A. Acceptable long-term outcome in elderly intensive care unit patients. Danish medical bulletin. 2011 Jul;58(7):A4297. Epub 2011/07/05. eng. [PubMed] [Google Scholar]
- 51.Hofhuis JG, van Stel HF, Schrijvers AJ, Rommes JH, Spronk PE. Changes of health-related quality of life in critically ill octogenarians: a follow-up study. Chest. 2011 Dec;140(6):1473–83. doi: 10.1378/chest.10-0803. Epub 2011/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 52.Herridge MS. Long-term outcomes after critical illness: past, present, future. Curr Opin Crit Care. 2007 Oct;13(5):473–5. doi: 10.1097/MCC.0b013e3282eff3af. Epub 2007/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006 May;34(5):1297–310. doi: 10.1097/01.CCM.0000215112.84523.F0. Epub 2006/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 54.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993 Dec 22–29;270(24):2957–63. doi: 10.1001/jama.270.24.2957. Epub 1993/12/22. eng. [DOI] [PubMed] [Google Scholar]
- 55.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–29. Epub 1985/10/01. eng. [PubMed] [Google Scholar]
- 56.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. Epub 1987/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 57.Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005 Mar;20(1):12–9. doi: 10.1016/j.jcrc.2004.09.007. Epub 2005/07/15. eng. [DOI] [PubMed] [Google Scholar]
- 58.Ginde AA, Moss M, Shapiro NI, Schwartz RS. Impact of older age and nursing home residence on clinical outcomes of US emergency department visits for severe sepsis. Journal of critical care. 2013 Oct;28(5):606–11. doi: 10.1016/j.jcrc.2013.03.018. Epub 2013/05/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDermid RC, Bagshaw SM. Octogenarians in the ICU: are you ever too old? Crit Care. 2011;15(1):125. doi: 10.1186/cc10018. Epub 2011/03/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Covinsky KE, Palmer RM, Counsell SR, Pine ZM, Walter LC, Chren MM. Functional status before hospitalization in acutely ill older adults: validity and clinical importance of retrospective reports. J Am Geriatr Soc. 2000 Feb;48(2):164–9. doi: 10.1111/j.1532-5415.2000.tb03907.x. Epub 2000/02/22. eng. [DOI] [PubMed] [Google Scholar]
- 61.Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Maryland state medical journal. 1965 Feb;14:61–5. Epub 1965/02/01. eng. [PubMed] [Google Scholar]
- 62.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. Epub 2001/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 63.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC geriatrics. 2002 Feb 27;2:1. doi: 10.1186/1471-2318-2-1. Epub 2002/03/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013 Jun;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022. Epub 2013/06/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. 2011;15(1):301. doi: 10.1186/cc9297. Epub 2011/02/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005 Aug 30;173(5):489–95. doi: 10.1503/cmaj.050051. Epub 2005/09/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2014 Feb 4;186(2):E95–102. doi: 10.1503/cmaj.130639. Epub 2013/11/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldwin MR, Reid MC, Westlake AA, Rowe JW, Granieri EC, Wunsch H, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014 Jan 6; doi: 10.1016/j.jcrc.2013.12.019. Epub 2014/02/25. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Needham DM, Truong AD, Fan E. Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009 Oct;37(10 Suppl):S436–41. doi: 10.1097/CCM.0b013e3181b6fa29. Epub 2010/02/06. eng. [DOI] [PubMed] [Google Scholar]
- 70.Aznar-Lain S, Webster AL, Canete S, San Juan AF, Lopez Mojares LM, Perez M, et al. Effects of inspiratory muscle training on exercise capacity and spontaneous physical activity in elderly subjects: a randomized controlled pilot trial. International journal of sports medicine. 2007 Dec;28(12):1025–9. doi: 10.1055/s-2007-965077. Epub 2007/05/31. eng. [DOI] [PubMed] [Google Scholar]
- 71.Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15(2):R84. doi: 10.1186/cc10081. Epub 2011/03/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci U S A. 2008 Mar 18;105(11):4318–22. doi: 10.1073/pnas.0709144105. Epub 2008/03/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2011 Dec;59(12):2291–300. doi: 10.1111/j.1532-5415.2011.03733.x. Epub 2011/12/23. eng. [DOI] [PubMed] [Google Scholar]
- 74.Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. The Cochrane database of systematic reviews. 2009;(2):CD003288. doi: 10.1002/14651858.CD003288.pub3. Epub 2009/04/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009 May 30;373(9678):1874–82. doi: 10.1016/S0140-6736(09)60658-9. Epub 2009/05/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit Care Med. 2005 Mar;33(3):574–9. doi: 10.1097/01.ccm.0000155992.21174.31. Epub 2005/03/09. eng. [DOI] [PubMed] [Google Scholar]
- 77.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. Journal of the American Geriatrics Society. 2012 Jun;60(6):1070–7. doi: 10.1111/j.1532-5415.2012.03989.x. Epub 2012/05/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagshaw SM, McDermid RC. The role of frailty in outcomes from critical illness. Curr Opin Crit Care. 2013 Oct;19(5):496–503. doi: 10.1097/MCC.0b013e328364d570. Epub 2013/09/03. eng. [DOI] [PubMed] [Google Scholar]
- 79.Baldwin MR, Wunsch H, Reyfman PA, Narain WR, Blinderman CD, Schluger NW, et al. High Burden of Palliative Needs among Older Intensive Care Unit Survivors Transferred to Post-Acute Care Facilities. A Single-Center Study. Ann Am Thorac Soc. 2013 Oct;10(5):458–65. doi: 10.1513/AnnalsATS.201303-039OC. Epub 2013/08/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]