Abstract
Adipose tissue dysfunction plays a pivotal role in the development of insulin resistance in obese individuals. Cell culture studies and gain-of-function mouse models suggest that canonical Wnt proteins modulate adipose tissue expansion. However, no genetic evidence supports a role for endogenous Wnt proteins in adipose tissue dysfunction, and the role of noncanonical Wnt signaling remains largely unexplored. Here we provide evidence from human, mouse, and cell culture studies showing that Wnt5a-mediated, noncanonical Wnt signaling contributes to obesity-associated metabolic dysfunction by increasing adipose tissue inflammation. Wnt5a expression is significantly upregulated in human visceral fat compared with subcutaneous fat in obese individuals. In obese mice, Wnt5a ablation ameliorates insulin resistance, in parallel with reductions in adipose tissue inflammation. Conversely, Wnt5a overexpression in myeloid cells augments adipose tissue inflammation and leads to greater impairments in glucose homeostasis. Wnt5a ablation or overexpression did not affect fat mass or adipocyte size. Mechanistically, Wnt5a promotes the expression of proinflammatory cytokines by macrophages in a Jun NH2-terminal kinase–dependent manner, leading to defective insulin signaling in adipocytes. Exogenous interleukin-6 administration restores insulin resistance in obese Wnt5a-deficient mice, suggesting a central role for this cytokine in Wnt5a-mediated metabolic dysfunction. Taken together, these results demonstrate that noncanonical Wnt signaling contributes to obesity-induced insulin resistance independent of adipose tissue expansion.
Introduction
Obesity is a major risk factor for insulin resistance (IR), which plays a key pathogenic role in type 2 diabetes. However, the pathophysiological mechanisms that link obesity and IR are incompletely understood. In this regard, ∼15–25% of the adult obese population is resistant to the development of metabolic disease (“metabolically healthy obesity”) by mechanisms that remain ill defined (1). White adipose tissue (WAT) dysfunction is an essential hallmark of obesity-associated IR. However, different human WAT depots appear to contribute differentially to IR. Expansion of visceral WAT is strongly associated with increased metabolic risk (2–5), whereas expansion of subcutaneous fat has a very minor contribution (2–4) or, in some studies, even decreases the risk of metabolic dysfunction (5–7). Thus it has been hypothesized that visceral adipose tissue is qualitatively different than subcutaneous adipose tissue, exhibiting specific properties that are linked to a higher risk of metabolic disorders, such as increased inflammation (8,9) and defective adipogenesis (10–12). However, the specific regulatory molecules accounting for the heterogeneity among fat depots remain to be determined. A number of studies have shown that subcutaneous and visceral WAT exhibit different patterns of developmental gene expression (13–15). This has led to the hypothesis that the different developmental origins of the various fat depots contribute to its physiological, cellular, and molecular heterogeneity (16).
Wnt proteins are secreted signaling molecules that have fundamental roles during embryonic development and have been implicated in numerous critical aspects of physiology and disease in the adult (17). There are 19 Wnt family members in mammals, which frequently have overlapping or redundant functions. Wnts typically act in an autocrine/paracrine fashion and activate a number of different signaling pathways, typically classified as either canonical (β-catenin dependent) or noncanonical (β-catenin independent). In this regard, it is generally accepted that most Wnt proteins (e.g., Wnt1, Wnt3a, Wnt10b) preferentially activate β-catenin–dependent pathways, while a few Wnts (mainly Wnt5a and Wnt11) predominantly activate β-catenin–independent pathways. Wnts have fundamental roles in controlling cell proliferation, cell-fate determination, and differentiation during embryonic development and in the adult individual.
Evidence suggests that canonical Wnts play important roles in adipose tissue homeostasis by inhibiting the differentiation of adipose tissue progenitor cells (18–23). However, most of the studies published to date are based on in vitro experiments. One exception is the studies on Wnt10b, a Wnt protein that activates β-catenin–dependent Wnt signaling and has been shown to function as an inhibitor of adipogenesis. Mice that overexpress Wnt10b in adipocytes are resistant to both high-fat diet–induced and genetic obesity and exhibit improved insulin sensitivity compared with wild-type (WT) mice (22,23). While these studies demonstrate that forced overexpression of a canonical Wnt protein can block adipose tissue expansion, there is no in vivo evidence that genetic deficiency of any of the 19 endogenous Wnts can alter adipose tissue homeostasis. In addition, in contrast to the several studies that have focused on β-catenin–mediated canonical Wnt pathways in adipose tissue biology and energy homeostasis, the role of noncanonical Wnt proteins in metabolic function have not been examined previously.
Wnt5a is classified as a noncanonical Wnt protein because it predominantly activates β-catenin–independent signaling. In addition, Wnt5a is a particularly unique Wnt because cell culture studies suggest that it has a role in the modulation of the innate immune response (24–28). In the current study, we combine human, mouse, and cellular studies to provide evidence that Wnt5a-mediated noncanonical signaling promotes adipose tissue inflammation and contributes to obesity-associated IR independent of adipose tissue expansion.
Research Design and Methods
Clinical Samples
Subcutaneous and visceral adipose tissue biopsies were collected intraoperatively during planned bariatric surgery in 31 obese patients (BMI = 45 ± 1; age = 42 ± 2 years). Subcutaneous adipose tissue was collected from the lower abdominal wall and visceral tissue from the greater omentum, respectively. Patient characteristics are summarized in Supplementary Table 1. The study was approved by the Boston Medical Center Institutional Review Board, and it was conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent.
Mice
Mice with whole-body, inducible Wnt5a ablation (Wnt5a-knockout [KO] mice) were generated by crossing Wnt5a-floxed mice (29) with UBC-Cre/ERT2 mice (The Jackson Laboratory). Wnt5a deletion was induced by intraperitoneal injection of tamoxifen (75 mg/kg) for 7 consecutive days at 6 weeks of age. Tamoxifen was injected to both Cre+ mice (Wnt5a-KO) and Cre− littermates (WT controls). Mice with myeloid-restricted Wnt5a ablation (Mye-Wnt5a-KO mice) were generated by crossing Wnt5a-floxed mice with LysM-Cre mice (The Jackson Laboratory). Mice with myeloid-restricted Wnt5a overexpression (Mye-Wnt5a-TG mice) were generated by crossing LysM-Cre mice with knock-in mice carrying a Cre-inducible Wnt5a transgene. Wnt5a-KO and Mye-Wnt5a-KO mice were in a C57Bl/6J background. Mye-Wnt5a-TG mice were in a C57Bl/6J-Tyr<c-2J> (albino C57Bl/6) background. Littermate controls were used for all the experiments. Mice were maintained on a 12-h light/dark schedule and given food and water ad libitum. Mice were fed either a standard chow diet (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories) or a high-fat/high-sucrose (HFHS) diet (F1850, Bio-Serv), as indicated. The composition of the HFHS diet was 35.8% fat (primarily lard), 36.8% carbohydrate (primarily sucrose), and 20.3% protein. For the obesogenic diet feeding, 8-week-old mice were maintained on HFHS diet for 12 weeks. The Institutional Animal Care and Use Committee of Boston University approved all study procedures.
Metabolic Measurements
For glucose tolerance tests (GTTs), mice were injected intraperitoneally with 1 g glucose/kg body weight after a 16-h fast. Blood glucose levels were measured with an Accu-Chek glucometer (Roche Diagnostics) immediately before and 15, 30, 60, 90, and 120 min after glucose injection. Insulin tolerance tests (ITTs) were performed on 5-h-fasted mice injected intraperitoneally with 0.6 units/kg human insulin (Humulin R, Eli Lilly and Company). Blood glucose levels were determined as described above. Area under the curve (AUC) values were calculated with the GraphPad Prism software (GraphPad Software Inc.).
Histology
Epididymal fat samples were fixed in 10% formalin, dehydrated, and embedded in paraffin. The 5-µm-thick histological sections were stained with hematoxylin and eosin (Sigma-Aldrich) to examine tissue morphology. For quantification of crown-like structure (CLS) frequency in epididymal fat sections, CLSs were defined as necrotic-like adipocytes completely surrounded by nonadipocyte cells. At least 1,000 adipocytes per mouse were analyzed.
Isolation of Stromal Vascular Fractions From Adipose Tissue
Epididymal fat pads from HFHS-fed mice were excised, minced in PBS, and digested with 1 mg/mL collagenase type 1 (Worthington Biochemical Corporation) at 37°C for 30 min. The digested fat tissue was filtered through a mesh and centrifuged at 1,000 rpm for 5 min to separate floating adipocytes from the stromal vascular fraction (SVF; pellet).
Cell Culture and Treatments
Bone marrow (BM)-derived macrophages were obtained from suspensions of femoral BM that were differentiated for 7 days in the presence of DMEM supplemented with antibiotics, 10% FBS, and 15% L929-cell conditioned medium as a source of macrophage colony-stimulating factor. Thioglycollate-elicited macrophages were obtained from the peritoneal cavity of mice 4 days after intraperitoneal injection of 1 mL of aged 4% Brewer thioglycollate broth.
Peritoneal macrophages were treated for 24 h with a 500 μmol/L saturated free fatty acid (sFFA) solution containing equimolar amounts of palmitic and myristic acids in DMEM supplemented with free fatty acid (FFA)-free BSA (Sigma-Aldrich). A 4:1 FFA:BSA ratio was used.
Gene Expression Analysis by Quantitative RT-PCR
Total RNA from tissues and cultured cells was obtained using QIAzol reagent and RNeasy Mini Kits (Qiagen). RNA (0.5–1.5 μg) was retrotranscribed with High Capacity cDNA Synthesis Kits (Life Technologies). Quantitative RT-PCR (qRT-PCR) was performed with Power SYBR Green reagent (mouse gene expression studies) or TaqMan gene expression assays (human gene expression studies) in a ViiA7 PCR system (Life Technologies). Primers for mouse gene expression studies are shown in Supplementary Table 2. TaqMan assays for human gene expression studies were from Life Technologies. Results were analyzed with the ∆∆Ct method using 36B4 expression as reference for normalization in mouse samples and GAPDH as reference for human samples.
Western Blot Analysis
Protein extracts from tissue and cultured cells were obtained using ice-cold lysis buffer (Cell Signaling Technology) supplemented with protease and phosphatase inhibitors (Roche Applied Science). Equal amounts of protein lysates were resolved by SDS-PAGE. The following antibodies were used for immunoblotting: rabbit polyclonal anti-human Wnt5a (Abcam); rat monoclonal anti-mouse Wnt5a (R&D Systems); and rabbit monoclonals anti-Akt, phospho-Akt(Ser473), phospho-Akt(Thr308), Jun NH2-terminal kinase (JNK), phospho-JNK, and GAPDH (Cell Signaling Technology).
Statistical Analysis
Data are shown as mean ± SEM unless otherwise stated. Statistical significance of differences between two groups was assessed by paired or unpaired Student t tests. Experiments with three or more groups were evaluated by one- or two-way ANOVA with post hoc Dunnet, Sidak, or Tukey multiple comparison tests. Results of GTT and ITT experiments were evaluated by two-way repeated-measures ANOVA. All statistical tests were performed using GraphPad Prism software.
Results
Differential Expression of Wnt Signaling Proteins in Visceral and Subcutaneous Fat of Obese Human Individuals
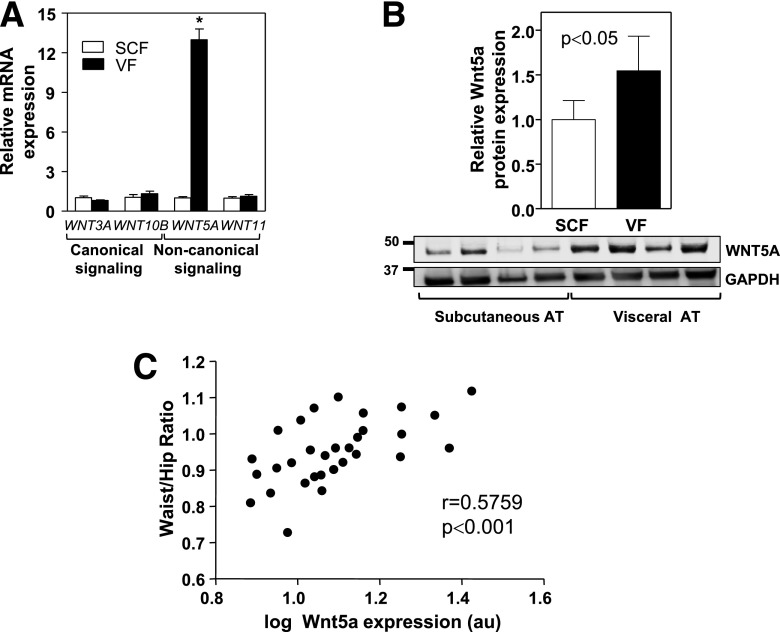
To investigate the role of Wnt signaling in adipose tissue dysfunction in humans, we evaluated the expression of different Wnt proteins in visceral and subcutaneous fat obtained from 31 obese individuals at the time of bariatric surgery. We found a marked increase of WNT5A gene expression in human visceral versus subcutaneous fat, whereas the expression of WNT11, another noncanonical Wnt, or the expression of the prototypical canonical Wnt genes WNT3A and WNT10B were comparable between both depots (Fig. 1A). WNT5A expression was also significantly elevated in human visceral fat at the protein level (Fig. 1B), and its transcript levels in this fat depot correlated positively with waist-to-hip ratio (Fig. 1C), a clinical parameter that is strongly associated with cardiometabolic risk. Overall, these data suggest the possibility that Wnt5a-mediated noncanonical signaling contributes to visceral adipose tissue dysfunction and associated metabolic impairment in obese human individuals.
Figure 1.
The expression of noncanonical Wnt signaling mediators is elevated in visceral fat of obese individuals. Visceral and subcutaneous fat samples were obtained from obese individuals at the time of bariatric surgery. A: qRT-PCR analysis of the expression of prototypical canonical (WNT3A, WNT10B) and noncanonical (WNT5A, WNT11) Wnt molecules in human fat (n = 31). *P < 0.0001, visceral vs. subcutaneous. B: Western blot analysis of the expression of WNT5A in human fat. Subcutaneous and visceral fat from 10 obese individuals were analyzed. Top: Densitometric quantification of WNT5A protein expression. Bottom: Representative immunoblot. C: WNT5A expression was analyzed by qRT-PCR and graphed as a function of patient waist-to-hip ratio (n = 31). The correlation between WNT5A mRNA expression and waist-to-hip ratio was evaluated using a Pearson test. SCF, subcutaneous fat; VF, visceral fat.
Wnt5a Ablation Inhibits Obesity-Induced Adipose Tissue Inflammation and Metabolic Dysfunction in Mice
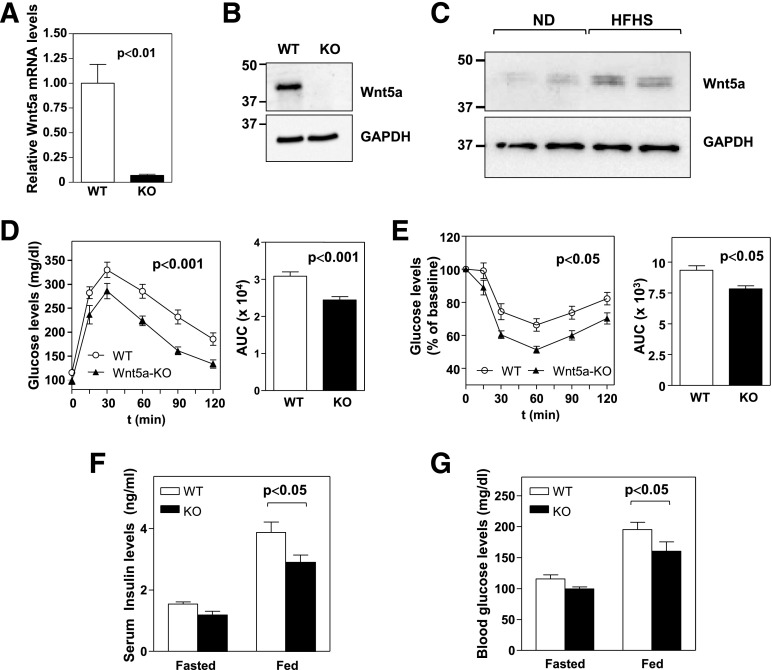
Previous attempts to evaluate the effects of endogenous Wnt5a in the adult have been limited by the perinatal lethality of conventional Wnt5a-nullizygous mice (30). To overcome this limitation, we generated whole-body inducible Wnt5a-deficient mice (Wnt5a-KO) by intercrossing Wnt5a-floxed mice (29) with mice expressing the tamoxifen-inducible Cre/ERT2 recombinase under the control of the ubiquitously expressed human ubiquitin C promoter (Supplementary Fig. 1A). qRT-PCR (Fig. 2A) and Western Blot analysis (Fig. 2B) demonstrated that Wnt5a expression was efficiently suppressed in WAT of Wnt5a-KO mice after tamoxifen administration at 6 weeks of age. These mice exhibited an apparently normal phenotype with no obvious anatomical difference with their WT littermates. When fed standard chow diet, Wnt5a-KO mice exhibited normal body weight (Supplementary Fig. 1B) and glucose homeostasis, as assessed by GTT (Supplementary Fig. 1C) and ITT (Supplementary Fig. 1D). Compared with lean mice, Wnt5a expression was upregulated in WAT of obese mice (Fig. 2C). Thus, to evaluate the role of endogenous Wnt5a in obesity-induced metabolic dysfunction, Wnt5a-KO and WT littermates were fed an obesogenic HFHS diet for 12 weeks. As expected, HFHS feeding of WT mice caused glucose intolerance and IR. However, Wnt5a ablation substantially attenuated this impairment of glucose metabolism in obese mice (Fig. 2D and E). Consistently, HFHS-fed Wnt5a-KO mice exhibited reduced insulin and glucose levels compared with WT littermates. A similar, but statistically nonsignificant, trend was observed in fasted mice (Fig. 2F and G).
Figure 2.
Wnt5a ablation attenuates obesity-induced IR in mice. (A) qRT-PCR and (B) Western blot analysis of Wnt5a levels in epididymal WAT of Wnt5a-KO mice and WT littermates (n = 4 per genotype). C: Western blot analysis of Wnt5a protein levels in normal-diet–fed and obese HFHS-fed C57Bl/6J mice. D–G: Wnt5a-KO mice and WT littermates (n = 7–10 per genotype) were fed an HFHS diet for 12 weeks. D: GTT and AUC analysis. E: ITT and AUC analysis. F: Serum insulin levels and (G) blood glucose levels in mice fasted 16 h or fed HFHS diet ad libitum. ND, normal diet.
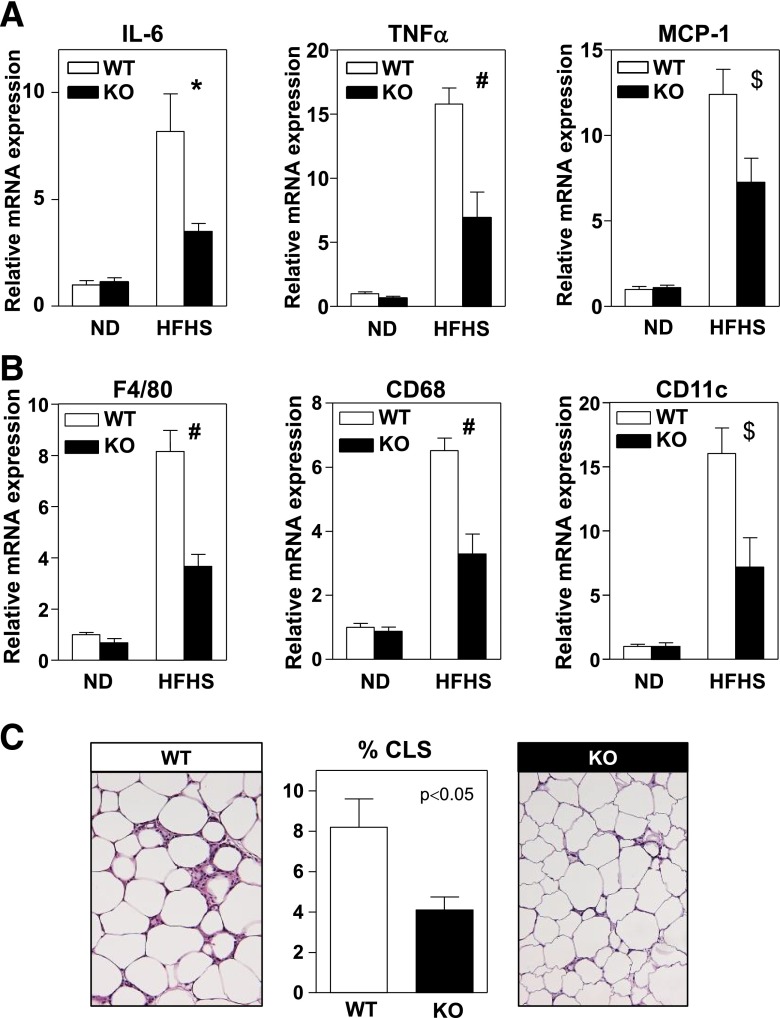
Previous cell culture studies have suggested that Wnt signaling can modulate two of the main cellular processes that are typically deregulated in dysfunctional WAT, namely, adipogenesis and inflammation. Therefore, we evaluated these cellular processes in WAT of Wnt5a-KO mice fed an HFHS diet. No differences were observed in total body weight (Supplementary Fig. 2A), percentage body fat assessed by MRI (Supplementary Fig. 2B), or weight of epididymal, perirenal, and mesenteric fat depots (Supplementary Fig. 2C–E) between Wnt5a-KO mice and WT controls, suggesting that Wnt5a does not affect adipogenesis. Consistent with this interpretation, no differences could be detected in adipocyte size (Supplementary Fig. 2F) or adipose tissue expression of the adipogenic transcription factors Pparγ and Cebpα or the adipocyte marker genes Glut4, Lpl, and Apn between WT and Wnt5a-KO mice fed HFHS diet (Supplementary Fig. 2G). In contrast, epididymal WAT of obese Wnt5a-KO mice exhibited lower expression of the proinflammatory cytokines tumor necrosis factor-α (TNF-α), CCL2/MCP-1, and interleukin (IL)-6 (Fig. 3A), suggesting that Wnt5a controls WAT inflammation. Supporting this notion, reduced expression of the macrophage-specific transcripts F4/80 and CD68 was found in the epididymal fat of Wnt5a-KO mice, as well as lower mRNA levels of CD11c, a marker of proinflammatory adipose tissue macrophages (Fig. 3B). The frequency of CLSs, clusters of macrophage content that are a major histological feature of inflamed adipose tissue (31), was lower in histological sections of obese Wnt5a-KO WAT (Fig. 3C). These data suggest that Wnt5a contributes to obesity-associated WAT inflammation but not to defective adipogenesis and fat pad expansion.
Figure 3.
Wnt5a ablation inhibits obesity-induced adipose tissue inflammation in mice. Wnt5a-KO mice and WT littermates (n = 4–7 per group) were fed standard chow or HFHS diet for 12 weeks. A: qRT-PCR analysis of the transcript levels of IL-6, TNF-α, and CCL2/MCP-1 in epididymal WAT. B: qRT-PCR analysis of the expression of various macrophage-specific transcripts in epididymal WAT. C: Quantification of the amount of CLS in epididymal WAT of HFHS-fed mice. At least 1,000 adipocytes per mouse were analyzed. Representative images of hematoxylin/eosin-stained histological sections are shown. *P < 0.05; #P < 0.001; $P < 0.01, KO vs. WT. ND, normal diet.
Myeloid-Restricted Ablation of Wnt5a Reduces Adipose Tissue Inflammation and Improves Glucose Metabolism in Obese Mice
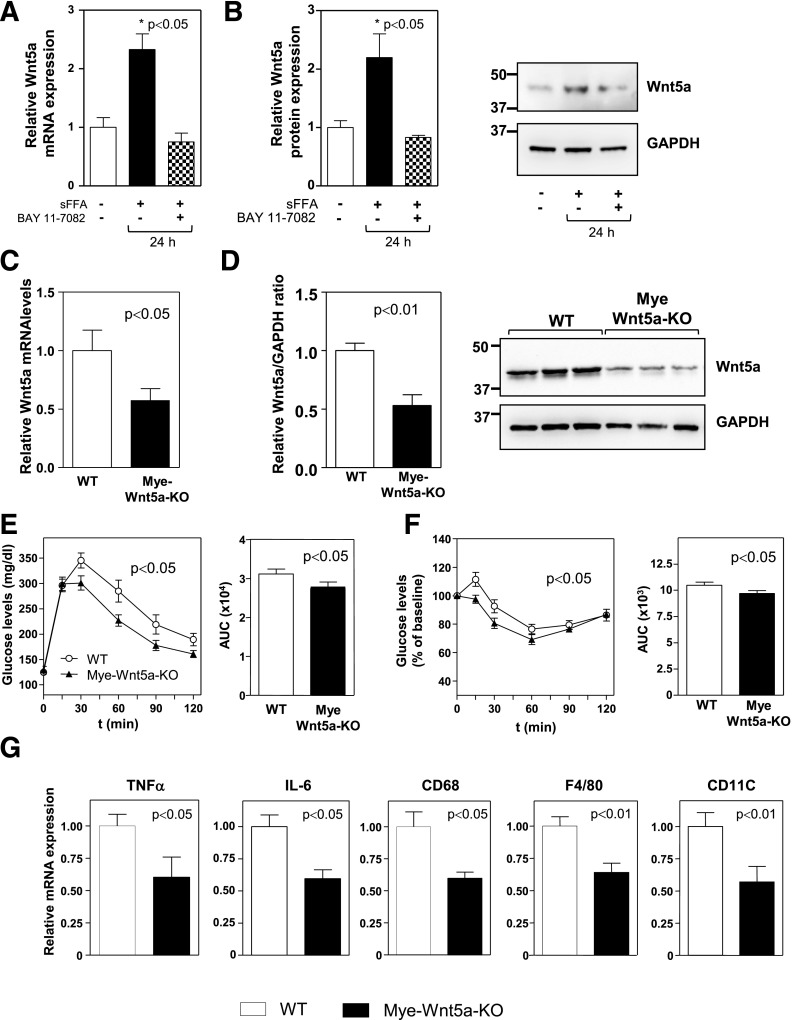
Although Wnt5a can be expressed by a variety of cell types, evidence demonstrates that macrophages are a significant source of this protein in adult tissues (25,27,28,32,33). Furthermore, inflammatory stimuli upregulate Wnt5a expression, at least in part, via nuclear factor-κB–mediated transcriptional activation through a conserved binding site in both human WNT5A and mouse Wnt5a genes (25,34,35). Emerging studies suggest that sFFAs promote inflammatory responses in adipose tissue macrophages (36). In this regard, treatment of primary macrophages with a mixture of sFFAs was found to significantly increase the expression of Wnt5a at the levels of transcript and protein. Under these conditions, Wnt5a upregulation was blocked by pretreatment with a pharmacological inhibitor of nuclear factor-κB signaling (Fig. 4A and B).
Figure 4.
Myeloid-restricted Wnt5a-KO ablation is sufficient to attenuate IR and adipose tissue inflammation in obese mice. Thioglycollate-induced peritoneal macrophages were isolated from C57Bl/6J mice, and Wnt5a expression was evaluated by qRT-PCR (A) or Western blot (B). Graphs show the average of three independent experiments. SVFs of epididymal WAT were collected by enzymatic digestion from HFHS-fed Mye-Wnt5a-KO mice and WT littermates (n = 6 per genotype) and analyzed by qRT-PCR (C) and Western blot (D). E–G: Mye-Wnt5a-KO mice and WT littermates (n = 11 per genotype) were fed an HFHS diet for 12 weeks. E: GTT and corresponding AUC analysis. F: ITT and corresponding AUC analysis. G: qRT-PCR analysis of gene expression in epididymal WAT. Asterisk indicates the experimental group corresponding to the P value shown in the panel.
Based on these observations, the role of macrophage-derived Wnt5a in obesity-associated metabolic dysfunction was evaluated by generating mice deficient in Wnt5a specifically in myeloid cells (Mye-Wnt5a-KO mice). Mye-Wnt5a-KO mice were obtained by intercrossing Wnt5a-floxed mice with lysozyme M-Cre (LysM-Cre) mice. In this model, Wnt5a levels were reduced by ∼80% in cultured primary macrophages derived from BM (data not shown) and by ∼50% in the SVF of epididymal WAT of HFHS-fed Mye-Wnt5a-KO mice (Fig. 4C and D), demonstrating that myeloid cells are a significant, but not exclusive, source of Wnt5a in WAT. Consistent with the partial ablation of Wnt5a expression, HFHS-fed Mye-Wnt5a-KO mice exhibited, in general, a milder phenotype than whole-body Wnt5a-KO mice. However, myeloid-restricted inactivation of Wnt5a was sufficient to significantly improve glucose metabolism in obese mice, as assessed by GTT and ITT experiments (Fig. 4E and F), without affecting body weight (data not shown). In addition, obese Mye-Wnt5a-KO mice exhibited significant reductions in the expression of the proinflammatory cytokines TNF-α and IL-6 and the macrophage markers F4/80, CD68, and CD11c in WAT (Fig. 4G). These data suggest that while Wnt5a expression is not exclusively restricted to macrophages, macrophage-derived Wnt5a significantly contributes to WAT inflammation and impaired glucose metabolism under conditions of metabolic stress.
Myeloid-Restricted Overexpression of Wnt5a Increases Adipose Tissue Inflammation and Worsens Glucose Metabolism in Obese Mice
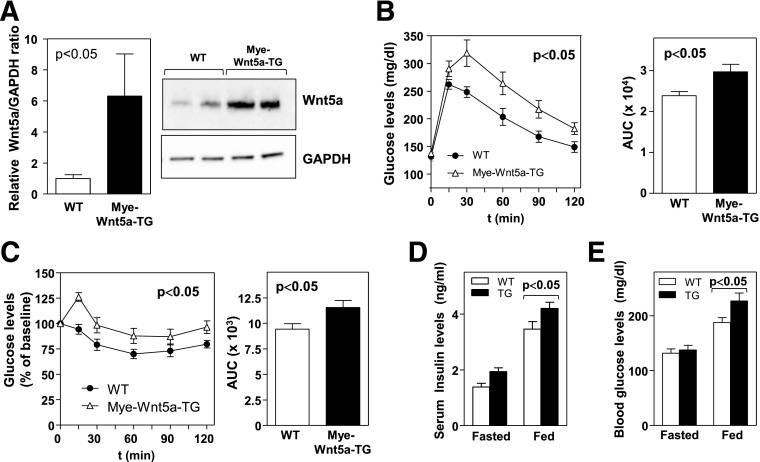
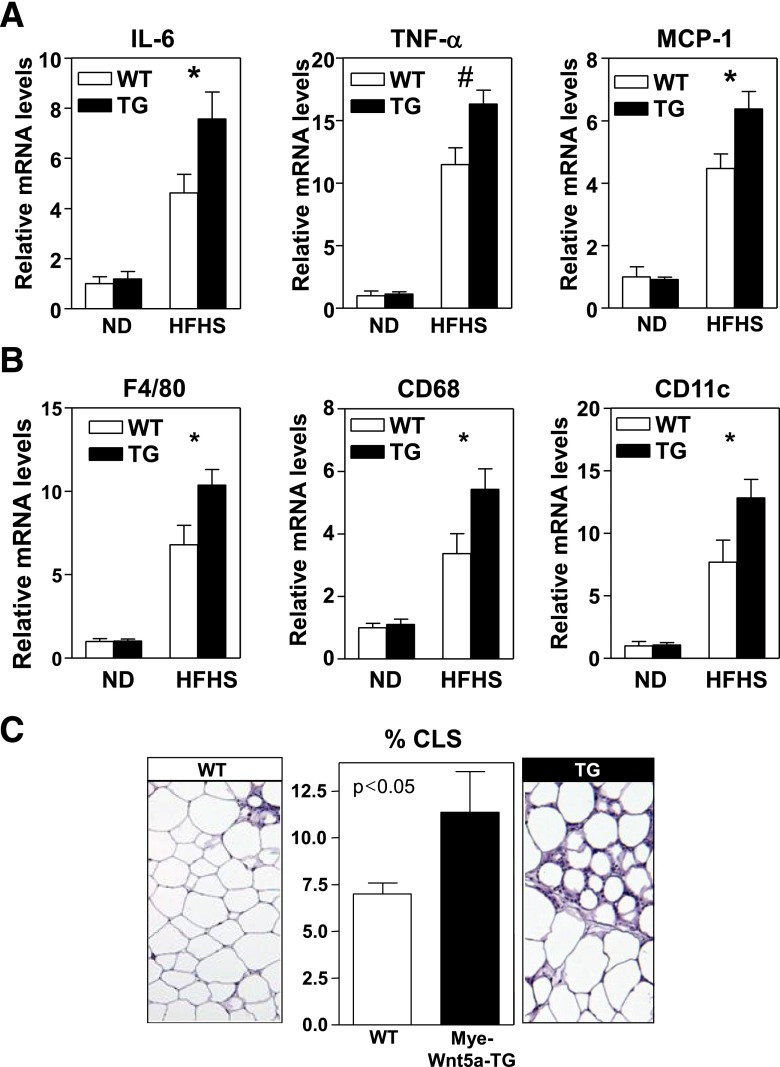
Having demonstrated the role of endogenous Wnt5a in obesity-associated metabolic dysfunction, we next evaluated the effects of Wnt5a gain-of-function. Mice were generated that overexpress Wnt5a in myeloid cells (Mye-Wnt5a-TG) by intercrossing LysM-Cre mice with knock-in mice carrying a Cre-inducible Wnt5a transgene (Supplementary Fig. 3A). Mye-Wnt5a-TG mice exhibited an approximately sixfold increase in Wnt5a transcript and protein levels in epididymal WAT (Fig. 5A and data not shown). No differences in body weight (Supplementary Fig. 3B) or glucose homeostasis (Supplementary Fig. 3C and D) were observed in mice fed standard chow diet. However, Mye-Wnt5a-TG mice fed HFHS exhibited increased glucose intolerance (Fig. 5B) and IR (Fig. 5C), as well as increased serum insulin and blood glucose levels in the fed state (Fig. 5D and E). Under these conditions, the transgenic mice displayed augmented WAT inflammation, as revealed by the increased expression of the proinflammatory cytokines IL-6, TNF-α, and CCL2/MCP-1 (Fig. 6A) and the macrophage markers F4/80, CD68, and CD11c (Fig. 6B). Furthermore, obese Mye-Wnt5a-TG mice exhibited an increased frequency of CLSs (Fig. 6C) compared with WT littermates. While this gain-of-function model provides further support that Wnt5a plays a role in obesity-linked metabolic dysfunction through the control of adipose tissue inflammation, no differences were observed in body weight (Supplementary Fig. 4A), fat mass (Supplementary Fig. 4B–E), adipocyte size (Supplementary Fig. 4F), or the expression of adipogenesis regulators and adipocyte markers (Supplementary Fig. 4G) when comparing obese WT and Mye-Wnt5a-TG mice. This indicates that Wnt5a overexpression does not affect adipogenesis.
Figure 5.
Myeloid-restricted overexpression of Wnt5a promotes obesity-induced IR. Mye-Wnt5a-TG and WT mice (n = 8–10 per genotype) were fed an HFHS diet for 12 weeks. A: Western blot analysis of Wnt5a protein levels in epididymal WAT. Four independent pools of two mice per genotype were analyzed. Left: Densitometric quantification of the Wnt5a/GAPDH ratio. Right: Representative immunoblot. B: GTT and corresponding AUC analysis. C: ITT and corresponding AUC analysis. D: Serum insulin levels and (E) blood glucose levels in mice fasted 16 h or fed HFHS diet ad libitum.
Figure 6.
Myeloid-restricted overexpression of Wnt5a promotes obesity-induced adipose tissue inflammation. Mye-Wnt5a-TG and WT mice (n = 4–9 mice per group) were fed a normal diet or HFHS diet for 12 weeks. A: qRT-PCR analysis of the transcript levels of IL-6, TNF-α, and CCL2/MCP-1 in epididymal WAT. B: qRT-PCR analysis of the expression of various macrophage-specific transcripts in epididymal WAT. C: Quantification of the amount of CLS in epididymal WAT of HFHS-fed mice. At least 1,000 adipocytes per mouse were analyzed. Representative images of hematoxylin/eosin-stained histological sections are shown. *P < 0.05; #P < 0.01, TG vs. WT. ND, normal diet.
Wnt5a-Induced Inflammation Contributes to Adipose Tissue IR
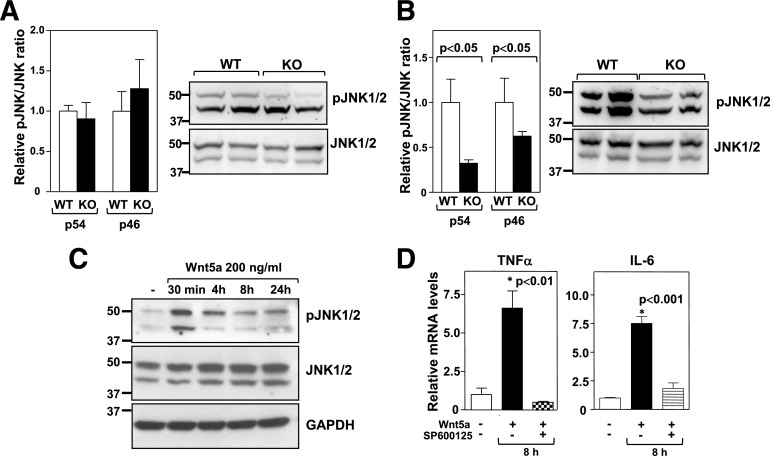
Wnt5a-induced noncanonical signaling is often mediated by JNK activation (37–41). Thus the effect of Wnt5a ablation on JNK signaling in WAT of obese mice was analyzed. While Wnt5a-KO and WT mice exhibited similar degrees of JNK phosphorylation in total WAT (Fig. 7A), Wnt5a ablation led to a substantial decrease in JNK phosphorylation within the SVF of this tissue (Fig. 7B). Although the phosphorylation of both p54 and p46 JNK isoforms was inhibited, Wnt5a ablation led to a greater reduction of p54 phosphorylation. Macrophages are the most abundant immune cell in the SVF of WAT, and macrophage JNK signaling has recently been shown to be a major regulator of adipose tissue inflammation (42). Consistent with these findings, recombinant Wnt5a was found to induce JNK activation in cultured BM-derived macrophages (Fig. 7C). Interestingly, consistent with our observation in WAT-SVF, Wnt5a seemed to have a more pronounced effect on phospho-p54 levels in cultured macrophages. Furthermore, recombinant Wnt5a promoted the expression of TNF-α and IL-6 in macrophages in a JNK-dependent manner, as revealed by experiments with the JNK pharmacological inhibitor SP600125 (Fig. 7D). In contrast, recombinant Wnt5a did not affect the expression of these proinflammatory cytokines in cultured 3T3-L1 adipocytes (data not shown). Overall, these results suggest that Wnt5a promotes adipose tissue inflammation via JNK signaling in macrophages.
Figure 7.
Wnt5a induces JNK signaling and proinflammatory cytokine expression in macrophages. JNK1/2 phosphorylation was evaluated by Western blot analysis in whole epididymal WAT (A) or SVFs (B) obtained from Wnt5a-KO and WT mice fed an HFHS diet for 12 weeks. Four mice per genotype were analyzed. Left: Densitometric quantification of the phospho-JNK/JNK ratio. Right: Representative immunoblots. C: JNK1/2 phosphorylation was evaluated by Western blot analysis in BM-derived macrophages treated with 200 ng/mL recombinant Wnt5a. A representative immunoblot is shown. D: BM-derived macrophages were treated with 200 ng/mL recombinant Wnt5a protein for 8 h in the absence or presence of 10 μmol/L SP600125, and TNF-α and IL-6 expression was evaluated by qRT-PCR. The graphs show the average of three independent experiments. Asterisk indicates the experimental group corresponding to the P value shown in the panel. pJNK, phospho-JNK.
JNK signaling has been previously shown to modulate insulin sensitivity in WAT (43,44). Therefore, we evaluated whether Wnt5a ablation affects insulin signaling in this tissue. As shown in Supplementary Fig. 5A, HFHS feeding suppressed insulin-induced Akt phosphorylation in WAT of WT mice, but not that of Wnt5a-KO mice. Conversely, Wnt5a overexpression in myeloid cells further impaired insulin signaling in WAT of obese mice (Supplementary Fig. 5B). In contrast, in vitro studies showed that treatment with recombinant Wnt5a protein does not affect insulin-stimulated Akt activation in cultured 3T3-L1 adipocytes (Supplementary Fig. 6A), thus suggesting that an indirect mechanism contributes to the effects of Wnt5a ablation on WAT insulin signaling in vivo.
Since it has been reported that macrophage-mediated inflammation suppresses insulin actions in adipocytes (45–47), factors secreted by Wnt5a-treated macrophages were tested for their ability to inhibit insulin-induced Akt activation in cultured adipocytes. In this experiment, 3T3-L1 adipocytes were treated with the conditioned medium of macrophages exposed to recombinant Wnt5a or vehicle. The Wnt5a/macrophage conditioned media significantly inhibited insulin-stimulated Akt phosphorylation in 3T3-L1 adipocytes (Supplementary Fig. 6B). Notably, this Wnt5a-induced inhibitory effect was lost in the conditioned medium of macrophages treated with the JNK pharmacological inhibitor. These data suggest that Wnt5a contributes to IR in WAT in an indirect manner, i.e., by promoting JNK-dependent macrophage proinflammatory activation.
IL-6 Has a Pivotal Role in Mediating the Effects of Wnt5a on Obesity-Induced Metabolic Dysfunction
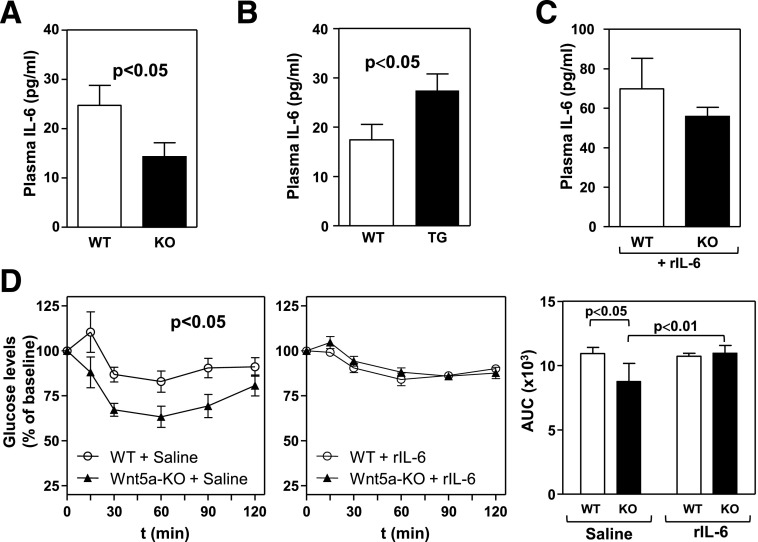
Adipose tissue dysfunction contributes to systemic metabolic dysfunction by generating a low-grade systemic chronic inflammatory response. Therefore, we next evaluated the effects of Wnt5a on blood levels of TNF-α and IL-6, two cytokines that are widely accepted markers of systemic inflammation and are modulated by Wnt5a in WAT of obese mice. Plasma levels of TNF-α were undetectable or very low in most samples regardless of Wnt5a genotype (data not shown). In contrast, Wnt5a ablation significantly reduced circulating levels of IL-6 in HFHS-fed mice (Fig. 8A). Conversely, plasma IL-6 levels were increased in Mye-Wnt5a-TG mice compared with WT controls (Fig. 8B), suggesting a central role for this cytokine in the effects of Wnt5a on obesity-induced metabolic dysfunction. To test this possibility, we evaluated whether increasing circulating IL-6 levels was sufficient to restore IR in obese Wnt5a-KO mice. Wnt5a-KO mice and WT littermates were fed HFHS diet for 12 weeks and then infused with a low dose of recombinant IL-6 (5 ng/kg/day) for 5 days via subcutaneous osmotic pumps. This treatment resulted in increased plasma levels of IL-6, which were comparable between WT and Wnt5a-KO mice after infusion (Fig. 8C). Notably, IL-6 delivery was sufficient to restore IR in HFHS-fed Wnt5a-KO mice to levels comparable to WT mice, while having a negligible effect on this parameter in WT mice (Fig. 8D). Overall, these data support a causal role for increased IL-6 secretion by WAT in Wnt5a-induced systemic IR. Further supporting this notion, we found that impaired insulin signaling in 3T3-L1 adipocytes treated with the conditioned medium of Wnt5a-stimulated macrophages was improved to levels comparable to control cells by the addition of an IL-6–neutralizing antibody (Supplementary Fig. 6C).
Figure 8.
Increased circulating IL-6 levels mediate IR in obese Wnt5a-deficient mice. A and B: Plasma levels of IL-6 were measured by ELISA in Wnt5a-KO mice, Wnt5a-TG mice, and WT littermates (n = 7–9 per genotype) fed an HFHS diet for 12 weeks. C: Plasma levels of IL-6 in HFHS-fed Wnt5a-KO mice and WT littermates after 5 days of exogenous IL-6 delivery (n = 5 per genotype). D: ITT and corresponding AUC analysis of HFHS-fed mice after 5 days of saline or exogenous IL-6 delivery (n = 5). rIL-6, recombinant IL-6.
Discussion
Visceral adiposity is strongly associated with IR and associated metabolic dysfunction in humans. However, the mechanisms underlying this association remain relatively ill defined from a molecular perspective. Several studies have shown that visceral and subcutaneous adipose tissue exhibit different expression patterns for many developmental genes (13–15). However, the role of most of these developmental genes in obesity-associated metabolic dysfunction has not been evaluated in detail using mouse genetic models. In this study, we focused on Wnt proteins, master regulators of embryonic development, because no studies have examined the role of endogenous Wnt signaling proteins in metabolic control using targeted gene ablation approaches. In the present work, we analyzed two different loss-of-function mouse models and one gain-of-function model to evaluate in vivo the role of Wnt5a-mediated noncanonical Wnt signaling in obesity-associated metabolic dysfunction. We show that noncanonical Wnt5a signaling plays an essential role in obesity-induced WAT inflammation and metabolic dysfunction, and that it is sufficient to promote IR under conditions of overnutrition. These findings provide causal evidence and mechanistic insight supporting the observations of recent human studies that found an association between increased circulating Wnt5a levels and IR (48,49).
Our results demonstrate that Wnt5a promotes IR in obese mice via heightened macrophage-mediated WAT inflammation. We show that whole-body ablation of Wnt5a prevents obesity-induced IR and decreases macrophage content and proinflammatory cytokine expression in WAT. Although to a lesser extent than whole-body Wnt5a deficiency, myeloid- restricted ablation of Wnt5a is sufficient to attenuate obesity-induced WAT inflammation and systemic IR in spite of leading to a partial inhibition of Wnt5a gene expression in WAT. These data demonstrate that myeloid cells are a significant, but not exclusive, source of Wnt5a in WAT. Consistently, previous studies have reported Wnt5a expression in endothelial cells (50) and mesenchymal stem cells (51), two other cell types relatively abundant in WAT. Regardless of other potential cellular sources of Wnt5a, our observations support a model where macrophage-derived Wnt5a plays a central role in WAT inflammation by promoting proinflammatory activation of adipose tissue macrophages in an autocrine/paracrine manner. Consistent with this notion, myeloid-specific overexpression of Wnt5a leads to greater WAT inflammation in obese mice. These data are also consistent with the widely accepted notion that Wnt proteins signal in a local fashion, mostly via autocrine/paracrine mechanisms, due to their strong interactions with the cell membrane and the extracellular matrix. Interestingly, we also found that sFFAs upregulate Wnt5a expression in macrophages, thus suggesting that Wnt5a signaling may be particularly relevant in the context of CLSs, which are comprised of lipid-scavenging macrophages surrounding free lipid droplets of dead adipocytes (31). Supporting this notion, we found that Wnt5a deficiency is associated with fewer CLSs, whereas transgenic overexpression of Wnt5a promotes CLS formation.
Previous cell culture studies using recombinant Wnt5a protein or forced overexpression of Wnt5a have reached conflicting conclusions regarding the role of Wnt5a in inflammation. While some reports support that Wnt5a has a proinflammatory activity in monocytes/macrophages (24–27) and endothelial cells (52), other studies suggest that Wnt5a induces the formation of tolerogenic/immunosuppressive dendritic cells (53,54) and macrophages (55). To shed light on the roles of Wnt5a, our study used Wnt5a gain- and loss-of-function strains, representing the first evaluation of noncanonical Wnt signaling in an inflammatory process in vivo. These data support the proinflammatory actions of Wnt5a. Interestingly, some studies have reported that canonical Wnt signaling has anti-inflammatory effects in various settings (56–59), thus suggesting opposing roles for canonical and noncanonical Wnt signaling in immune regulation.
Signaling by Wnt proteins is modulated by a large number of secreted inhibitors. We recently reported that one of these secreted inhibitors, secreted frizzled related protein 5 (Sfrp5), can function as an anti-inflammatory adipokine, which is expressed in the adipose tissue of lean mice but downregulated in severely obese mice (60). Sfrp5-deficient mice exhibit impaired insulin sensitivity and increased adipose tissue inflammation compared with WT mice when fed a prolonged HFHS diet. Sfrp5 had been previously shown to bind and inhibit noncanonical Wnt proteins (61). Based on these data, it was hypothesized that the anti-inflammatory activities of Sfrp5 are mediated, at least in part, by inhibition of noncanonical Wnt signaling. However, this hypothesis and the anti-inflammatory actions of Sfrp5 have been challenged by a study of mice harboring a hypomorphic allele of Sfrp5 derived from a genomic N-ethyl-N-nitrosourea–mutagenesis approach (62). Our study sheds light onto this controversy by demonstrating the proinflammatory effects of noncanonical Wnt signaling in obese adipose tissue and further supports the existence of a Sfrp5/Wnt5a regulatory system that modulates WAT inflammation in obesity. Also consistent with this hypothesis, a recent study found that Wnt5a expression in WAT is significantly reduced in adiponectin-overexpressing mice (63). Thus it is possible that suppression of Wnt5a is a common mechanism by which both Sfrp5 and adiponectin exert their anti-inflammatory activities.
Adipogenesis is one of the main driving forces of adipose tissue expansion in response to excessive caloric intake. Canonical Wnts have been reported to inhibit adipogenesis (18–23). However, the role of noncanonical Wnt signaling in this cellular process is more controversial. Several in vitro studies have previously investigated the role of Wnt5a in adipogenesis, with conflicting results (33,64–66). A study with heterozygous, germ-line Wnt5a-KO mice showed that partial deficiency of Wnt5a leads to increased number of adipocytes in the BM, and mechanistic experiments suggested that this antiadipogenic effect of Wnt5a was secondary to Nemo-like kinase–mediated peroxisome proliferator–activated receptor-γ (PPARγ) repression in mesenchymal stem cells (67). In marked contrast, other studies show that Wnt5a upregulates PPARγ expression in cultured preadipocytes (65) and promotes adipogenesis (64,65). Furthermore, the same Wnt5a/Nemo-like-kinase pathway is reported to inhibit antiadipogenic β-catenin signaling (68), which further suggests a proadipogenic role of Wnt5a. However, none of these studies evaluated the role of Wnt5a in WAT adipogenesis in vivo. Here we show that neither Wnt5a ablation nor overexpression affect body weight, body fat mass, adipocyte size, expression of PPARγ, or expression of various adipocyte markers in WAT of obese mice. These results suggest that Wnt5a does not affect the adipogenic expansion of WAT in mice.
Noncanonical Wnt signaling comprises an array of frequently overlapping pathways with two main branches: the planar cell polarity or Wnt/JNK pathway and the Wnt/Ca2+ pathway. JNK signaling is particularly relevant in the setting of obesity-induced adipose tissue inflammation and IR (42–44,69). Thus we hypothesized that the proinflammatory effects of Wnt5a in WAT are mediated by exacerbated JNK signaling. In this regard, we found that recombinant Wnt5a activates JNK signaling and promotes the expression of the proinflammatory cytokines TNF-α and IL-6 in a JNK-dependent manner in cultured macrophages, consistent with a previous report (60). Previous studies have shown that JNK1 deficiency in nonhematopoietic cells reduces adiposity in mice (69). In contrast, deficient macrophage JNK signaling protects against obesity-induced WAT inflammation and IR without any change in adiposity (42,69). Similarly, we found that Wnt5a ablation reduces WAT inflammation and improves systemic glucose homeostasis, without any effects in body weight or fat mass. These phenotypic similarities between macrophage JNK-deficient models and Wnt5a-deficient mice strongly support our hypothesis that Wnt5a promotes WAT inflammation and associated metabolic dysfunction via increased JNK signaling in macrophages. Overall, these data suggest the existence of a Wnt5a/JNK signaling axis that controls the inflammatory microenvironment of the adipose tissue without affecting fat pad expansion.
Adipose tissue inflammation contributes to systemic metabolic dysfunction, at least in part, by generating a low-grade systemic chronic inflammatory response. Plasma IL-6 is a widely accepted marker of systemic inflammation in obese individuals. Although the role of IL-6 in metabolism is complex and studies with IL-6-deficient mice have given rise to inconsistent data in different studies (70–73), gain- and loss-of-function studies with recombinant IL-6 or IL-6–neutralizing antibodies strongly suggest that IL-6 promotes IR in vivo (74,75). Furthermore, elevated circulating IL-6 levels are associated with IR (76–78) and are predictive for the development of type 2 diabetes in humans (79). A number of previous in vitro studies have shown that Wnt5a induces IL-6 expression in several cell types (26,52,54,60). Consistent with these findings, we observed that recombinant Wnt5a increases IL-6 expression in macrophages and that improved insulin sensitivity in obese Wnt5a-KO mice is paralleled by lower plasma levels of IL-6. Conversely, Wnt5a overexpression led to increased plasma IL-6 levels. These data suggested that IL-6 plays a pivotal role in mediating the effects of Wnt5a in obesity-induced IR. Supporting this notion, increases in circulating IL-6 levels via recombinant IL-6 delivery restored systemic IR in obese Wnt5a-KO mice, while this level of IL-6 had no statistically significant effect in WT mice.
Finally, we provide human data suggesting that Wnt5a-mediated noncanonical signaling contributes to visceral adipose tissue dysfunction in obese individuals. We found that transcript expression of the prototypical Wnt genes WNT3A and WNT10B was comparable between depots, thus suggesting that canonical Wnt signaling does not contribute to the metabolic dysfunction that is attributed to visceral adipose tissue. In contrast, WNT5A expression was significantly higher in visceral fat. Interestingly, human obesity is associated with increased JNK signaling in visceral fat, but not in subcutaneous fat (80). In addition, indexes of visceral adiposity have been shown to be associated with increased circulating levels of IL-6, but not of other proinflammatory cytokines such as TNF-α (81,82). Based on these findings, it is tempting to speculate that Wnt5a is a major contributor to exacerbated JNK signaling and increased IL-6 production in visceral fat, which likely plays an important role in the increased fat inflammation and metabolic dysfunction typically seen in individuals with visceral obesity.
Taken together, our studies suggest that Wnt5a-induced noncanonical signaling contributes to the development of adipose tissue inflammation and the metabolic complications associated with obesity. Wnt5a has these effects independently of adipogenesis, adipocyte hypertrophy, or adipose tissue expansion. Future studies are warranted to assess the therapeutic potential of acute Wnt5a-blocking strategies in the setting of obesity and visceral adipose tissue dysfunction.
Supplementary Material
Article Information
Funding. This work was supported by a postgraduate studies grant sponsored by the Ramón Areces Foundation (to J.J.F.); American Heart Association Postdoctoral Fellowship grant 12POST11780028 (to M.G.F.); a pilot and feasibility grant from the Boston Nutrition Obesity Research Center (P30DK046200, to T.A.); a pilot grant from the Clinical and Translational Science Institute at Boston University (National Institutes of Health [NIH] UL1-RR-025771, to T.A.); NIH grants HL-081587, HL-1145675, and HL-084213 (to N.G.); and NIH grants HL-081587, HL-116591, and HL-120160 (to K.W.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.J.F. conceived the study, analyzed the data, wrote the manuscript, and performed most of the experiments. M.A.Z. contributed to the experiments and analyzed gene expression in mouse and human samples. D.T.-M.N. and M.G.F. contributed to the experiments and collected and analyzed human samples. T.A. contributed to the experiments and contributed to mouse adipose tissue studies. T.P.Y. generated and provided mouse strains. N.G. collected and analyzed human samples. K.W. conceived the study, analyzed the data, and wrote the manuscript. All authors read and approved the manuscript. K.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-1164/-/DC1.
References
- 1.Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010;21:38–43 [DOI] [PubMed] [Google Scholar]
- 2.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 2012;308:1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000;23:465–471 [DOI] [PubMed] [Google Scholar]
- 4.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011;96:E1756–E1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009;32:1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snijder MB, Visser M, Dekker JM, et al.; Health ABC Study . Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005;48:301–308 [DOI] [PubMed] [Google Scholar]
- 8.Farb MG, Ganley-Leal L, Mott M, et al. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol 2012;32:467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010;299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 10.Adams M, Montague CT, Prins JB, et al. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest 1997;100:3149–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol 2002;282:R1286–R1296 [DOI] [PubMed] [Google Scholar]
- 12.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 2006;55:2571–2578 [DOI] [PubMed] [Google Scholar]
- 13.Gesta S, Blüher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 2006;103:6676–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Gesta S, Lee KY, Tran TT, Saadatirad P, Kahn CR. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 2010;18:872–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vohl M-C, Sladek R, Robitaille J, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res 2004;12:1217–1222 [DOI] [PubMed] [Google Scholar]
- 16.Gesta S, Tseng Y-H, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007;131:242–256 [DOI] [PubMed] [Google Scholar]
- 17.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781–810 [DOI] [PubMed] [Google Scholar]
- 18.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009;20:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002;277:30998–31004 [DOI] [PubMed] [Google Scholar]
- 20.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science 2000;289:950–953 [DOI] [PubMed] [Google Scholar]
- 21.Cawthorn WP, Bree AJ, Yao Y, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 2012;50:477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes 2007;56:295–303 [DOI] [PubMed] [Google Scholar]
- 23.Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem 2004;279:35503–35509 [DOI] [PubMed] [Google Scholar]
- 24.Halleskog C, Dijksterhuis JP, Kilander MBC, et al. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation 2012;9:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal A, Ehlers S, Lauber J, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006;108:965–973 [DOI] [PubMed] [Google Scholar]
- 26.Rauner M, Stein N, Winzer M, et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J Bone Miner Res 2012;27:575–585 [DOI] [PubMed] [Google Scholar]
- 27.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol 2008;28:504–510 [DOI] [PubMed] [Google Scholar]
- 28.Pukrop T, Klemm F, Hagemann T, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A 2006;103:5454–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 2012;338:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999;126:1211–1223 [DOI] [PubMed] [Google Scholar]
- 31.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 32.Zhao C, Ma H, Bu X, Wang W, Zhang N. SFRP5 inhibits gastric epithelial cell migration induced by macrophage-derived Wnt5a. Carcinogenesis 2013;34:146–152 [DOI] [PubMed] [Google Scholar]
- 33.Bilkovski R, Schulte DM, Oberhauser F, et al. Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans. Int J Obes (Lond) 2011;35:1450–1454 [DOI] [PubMed] [Google Scholar]
- 34.Katoh M, Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med 2009;23:763–769 [DOI] [PubMed] [Google Scholar]
- 35.Ge XP, Gan YH, Zhang CG, et al. Requirement of the NF-κB pathway for induction of Wnt-5A by interleukin-1β in condylar chondrocytes of the temporomandibular joint: functional crosstalk between the Wnt-5A and NF-κB signaling pathways. Osteoarthritis Cartilage 2011;19:111–117 [DOI] [PubMed] [Google Scholar]
- 36.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab 2012;15:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda K, Kobayashi Y, Udagawa N, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 2012;18:405–412 [DOI] [PubMed] [Google Scholar]
- 38.Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem 2008;283:27973–27981 [DOI] [PubMed] [Google Scholar]
- 39.Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003;8:645–654 [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka H, Moriguchi T, Masuyama N, et al. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 2002;3:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 2007;12:779–792 [DOI] [PubMed] [Google Scholar]
- 42.Han MS, Jung DY, Morel C, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013;339:218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 44.Sabio G, Das M, Mora A, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008;322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab 2007;292:E166–E174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005;25:2062–2068 [DOI] [PubMed] [Google Scholar]
- 47.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun 2006;341:507–514 [DOI] [PubMed] [Google Scholar]
- 48.Prats-Puig A, Soriano-Rodríguez P, Carreras-Badosa G, et al. Balanced duo of anti-inflammatory SFRP5 and proinflammatory WNT5A in children. Pediatr Res 2014;75:793–797 [DOI] [PubMed] [Google Scholar]
- 49.Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Activation of noncanonical Wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab 2014;99:E1407–E1417 [DOI] [PubMed] [Google Scholar]
- 50.Masckauchán TNH, Agalliu D, Vorontchikhina M, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell 2006;17:5163–5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilkovski R, Schulte DM, Oberhauser F, et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J Biol Chem 2010;285:6170–6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Kim J, Kim DW, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol 2010;185:1274–1282 [DOI] [PubMed] [Google Scholar]
- 53.Oderup C, LaJevic M, Butcher EC. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J Immunol 2013;190:6126–6134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valencia J, Hernández-López C, Martínez VG, et al. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J Immunol 2011;187:4129–4139 [DOI] [PubMed] [Google Scholar]
- 55.Bergenfelz C, Medrek C, Ekström E, et al. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol 2012;188:5448–5458 [DOI] [PubMed] [Google Scholar]
- 56.Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol 2011;90:553–559 [DOI] [PubMed] [Google Scholar]
- 57.Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp Hematol 2009;37:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manicassamy S, Reizis B, Ravindran R, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 2010;329:849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neumann J, Schaale K, Farhat K, et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J 2010;24:4599–4612 [DOI] [PubMed] [Google Scholar]
- 60.Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 2010;329:454–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev 2008;22:3050–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori H, Prestwich TC, Reid MA, et al. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest 2012;122:2405–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wada N, Hashinaga T, Otabe S, et al. Selective modulation of Wnt ligands and their receptors in adipose tissue by chronic hyperadiponectinemia. PLoS ONE 2013;8:e67712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishizuka M, Koyanagi A, Osada S, Imagawa M. Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett 2008;582:3201–3205 [DOI] [PubMed] [Google Scholar]
- 65.van Tienen FHJ, Laeremans H, van der Kallen CJH, Smeets HJM. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun 2009;387:207–211 [DOI] [PubMed] [Google Scholar]
- 66.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 2011;46:R65–R72 [DOI] [PubMed] [Google Scholar]
- 67.Takada I, Mihara M, Suzawa M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol 2007;9:1273–1285 [DOI] [PubMed] [Google Scholar]
- 68.Ishitani T, Kishida S, Hyodo-Miura J, et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol 2003;23:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solinas G, Vilcu C, Neels JG, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 2007;6:386–397 [DOI] [PubMed] [Google Scholar]
- 70.Wallenius V, Wallenius K, Ahrén B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002;8:75–79 [DOI] [PubMed] [Google Scholar]
- 71.Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab 2004;287:E182–E187 [DOI] [PubMed] [Google Scholar]
- 72.Park EJ, Lee JH, Yu G-Y, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010;140:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 2014;15:423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 2003;52:2784–2789 [DOI] [PubMed] [Google Scholar]
- 75.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 2005;146:3417–3427 [DOI] [PubMed] [Google Scholar]
- 76.Bastard JP, Maachi M, Van Nhieu JT, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 2002;87:2084–2089 [DOI] [PubMed] [Google Scholar]
- 77.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745–E751 [DOI] [PubMed] [Google Scholar]
- 78.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 2001;9:414–417 [DOI] [PubMed] [Google Scholar]
- 79.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 80.Bashan N, Dorfman K, Tarnovscki T, et al. Mitogen-activated protein kinases, inhibitory-kappaB kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology 2007;148:2955–2962 [DOI] [PubMed] [Google Scholar]
- 81.Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després J-P. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab 2008;93:1931–1938 [DOI] [PubMed] [Google Scholar]
- 82.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract 2005;69:29–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.