Abstract
Myasthenia gravis (MG) is primarily caused by antibodies directed towards the skeletal muscle acetylcholine receptor, leading to muscle weakness. Although these antibodies may induce compromise of neuromuscular transmission by blocking acetylcholine receptor function or antigenic modulation, the predominant mechanism of injury to the neuromuscular junction is complement-mediated lysis of the postsynaptic membrane. The vast majority of data to support the role of complement derives from experimentally acquired MG (EAMG). In this article, we review studies that demonstrate the central role of complement in EAMG and MG pathogenesis along with the emerging role of complement in T- and B-cell function, as well as the potential for complement inhibitor-based therapy to treat human MG.
Keywords: autoimmunity, C5, complement, myasthenia gravis
Myasthenia gravis (MG) is a prototypic antibody-mediated autoimmune disease and among the few that strictly matches criteria defining autoimmunity [1–3]. Subclass antibodies directed against the acetylcholine receptor (AChR) have been identified that bind complement and initiate the complement cascade producing a complement-mediated lysis of the neuromuscular junction [4]. In 1977, Engel appreciated the binding of complement components to the endplates of MG patients [5–7] and experimentally acquired MG (EAMG) animals [8]. Since then, the role of complement in the initiation, progression and susceptibility of the disease has been investigated as to the structural alterations of the neuromuscular junction and the systemic changes that occur to the immune system. This review focuses on the latest evidence of complement involvement in MG, which is overwhelmingly drawn from animal models of the disease.
Complement overview
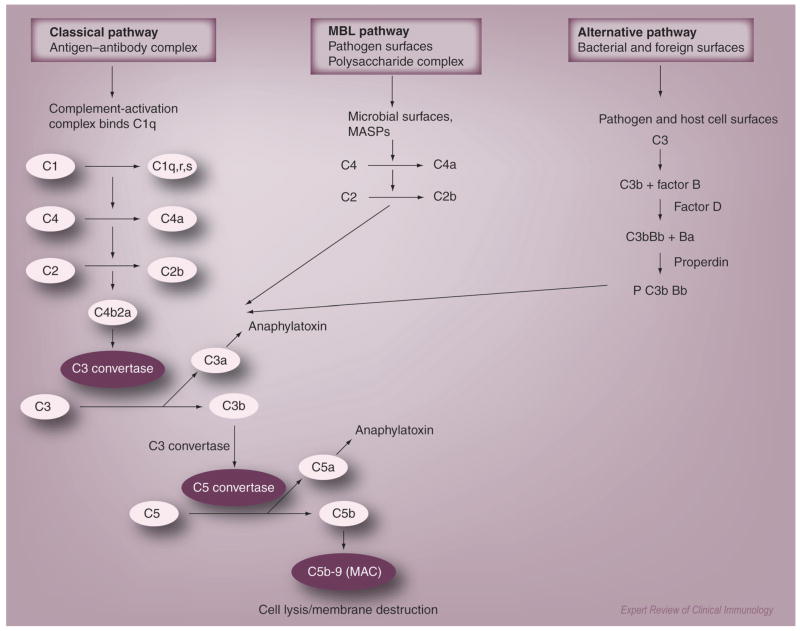
Complement is important in both innate and adaptive immunity [9,10]. Activation of the complement system protects the host against invading pathogens by distinct mechanisms, which include cell lysis of pathogens, opsonization with complement fragments, chemotaxis of inflammatory cells and formation of the membrane attack complex (MAC) [11]. In the adaptive immune response, complement is the effector system for the primary and secondary antibody responses of B cells [12,13]. Complement activation is regulated by a series of approximately 30 plasma and membrane proteins participating in classical, alternative and lectin pathways (Figure 1). The classical pathway is activated by immune complexes containing antigen and IgM or complement-fixing IgG. The alternative pathway is activated by foreign pathogens and polymeric IgA [14,15]. The lectin pathway is initiated by binding of mannose-binding lectin to microbial pathogens and may involve IgA-containing immune complexes [16,17]. Although initiated differently, all three pathways converge at the cleavage of C3 by specific convertase enzymes followed by the generation of the MAC. The existence of a new activation pathway by which generation of C5a through a coagulation pathway involving thrombin in the absence of C3 has been proposed [18]. This leads to questions such as how much cross-talk may occur between complement and systems previously thought to work in parallel or in isolation.
Figure 1. Complement regulation and activation.
Complement activation can be initiated via three pathways: classical, alternative and lectin. The classical pathway is initiated by antigen–antibody complex. The complex binds to its recognition molecule C1q and triggers the serine proteases C1r and C1s. C1s activates C2 and C4 leading to the formation of C4b2a complex. C4b2a or convertase cleaves C3 to C3b to form the C5 convertase (C3bC4bC2a). Breaking of C5 into C5a anaphylatoxin and the C6-binding fragment C5b represent the last step in the cascade. Activation of the lectin pathway is initiated by recognition of MBL and ficolins that recognize mannose on bacteria. The alternative pathway is initiated by polysaccharides on microbial surfaces. The remaining cascade is similar for all three pathways. C5 is activated and the subsequent binding of C5b to C6, C7, C8 and C9 forms the membrane attack complex. All pathways are controlled by soluble (C1 inhibitor, C4bp, factor H, vitronectin and clasterin) and cell membrane-bound (CR1, DAF, MCP and CD59) proteins.
MAC: Membrane attack complex; MASP: MBL-associated serine protease; MBL: Mannose-binding lectin.
Complement activity is modulated by regulatory proteins that prevent the cascade from progressing toward tissue damage as a result of inadvertent binding of activated complement components. In mice, the regulatory proteins, CD55 known as decay acceleration factor (DAF), and membrane inhibitor of reactive lysis (MIRL or CD59), are expressed on the skeletal muscle surface and concentrated at the neuromuscular junctions of most muscles [19,20]. Crry, the third cell surface regulator of complement, appears to be more active in the regulation of the alternative pathway. In humans, there are also three cell-associated regulators: DAF, CD59 and the membrane cofactor protein (MCP or CD46). Human DAF and CD59 behave similarly to their murine homologues, whereas the human MCP has activity similar to Crry [21]. DAF inhibits complement activation by accelerating the decay of C3 and C5 convertases [22]. CD59 prevents MAC formation at the junction by binding C8, which inhibits the attachment of C9 [23]. crry-knockout mice die in utero and, therefore, its role in mature animals has not been defined as well as the other regulators. There is emerging evidence that DAF and CD59 suppress T-cell activity where the absence of DAF or CD59 in mice was found to increase T-cell activation [24–26].
Autoantibodies in MG
Close to 90% of MG patients have antibodies to the AChR, binding of these antibodies to the receptor results in the failure of skeletal muscle to respond appropriately to nerve stimulation owing to antibody-induced injury of the postsynaptic muscle surface. The antibodies are produced by autosensitized B cells by a T-cell-dependent mechanism and induce neuromuscular transmission compromise by blocking the AChR, antigenic modulation or complement-mediated injury [1,2]. The subject of this review has emphasized complement mechanisms, but it is likely that two other mechanisms may be important in patients:
Antibody may bind to the AChR binding site for its ligand, acetylcholine, and although found at low concentrations, this antibody could be of clinical importance [27]. In EAMG, antibodies with such binding characteristics cause acute, severe weakness without evidence of damage to the junction [28].
Antigenic modulation is the ability of an antibody to crosslink two antigen molecules, resulting in accelerated endocytosis and degradation of the AChR [29,30]. IgG from most MG patients has been shown to accelerate the degradation rate of the AChR in vivo and in cultured muscle cells [29].
Thus far in this review, MG has been referred to as if it were a homogenous disease; however, it is not. Subgroups can be defined based on clinical findings, autoantibody profiles and pathogenesis, and further subgroup definition is sure to occur with more detailed understanding of genetic predisposition and environmental triggers. From the phenotypic perspective, the Myasthenia Gravis Foundation of America Classification [31] defines patients based on severity of weakness with class 1 patients having manifestations restricted to the ocular muscles, so-called ocular myasthenia (OM). In OM patients, the concentration of antibodies is lower, or absent, compared with patients with generalized MG, which form classes 2–5 based on worsening levels of strength. Although absolute correlation of antibody concentration and severity of weakness is not present in an individual patient, there is a tendency of higher AChR antibody concentrates being associated with greater weakness [32,33].
The low titers of AChR antibodies support the clinical impression that the neuromuscular junctions of certain ocular muscles are more susceptible to autoantibody attack. The properties that may mediate this susceptibility include antibody targets, the immune response and the safety factor of the extraocular muscle (EOM) neuromuscular junctions. Recently, a relative lack of intrinsic complement regulator has been identified in EOM [34,35] and this may prove to be a major contributor to EOM susceptibility to MG.
Approximately 10% of MG patients with generalized weakness do not have AChR antibodies. These MG patients can be divided into two groups: those with antibodies to muscle-specific kinase (MuSK) [36] and those without AChR and MuSK antibodies [37]. MuSK is essential for AChR clustering at the developing neuromuscular junction and its deficiency may lead to the complete loss of junctional ultrastructure supporting its critical role at the nerve–muscle synapse [38]. Muscle biopsies from MG patients seropositive for MuSK antibodies showed no decrease in AChR or evidence of antigen–antibody complex [39], although C3 is rarely detected at junctions of MuSK-positive patients [39,40]. MuSK antibodies have been identified as predominantly IgG4 and do not activate complement [39,40]. Animals immunized with MuSK epitopes demonstrate weakness and reduced AChR clustering [36,41–43].
Autoantibodies against other skeletal muscle proteins are detected among MG patients and are particularly common in the paraneoplastic form of MG induced by a thymoma. Titin and ryanodine receptor antibodies are present not only in thymoma-associated MG, but also in MG that develops in patients over the age of 50–60 years [44,45]. Antititin and antiryanodine receptor consist mostly of the IgG1 subtype that are capable of complement activation; however, the contribution of these autoantibodies to MG muscle dysfunction has not been fully elucidated [46].
Complement components & their roles in MG & EAMG
In human MG, the strongest evidence for complement as a pathogenic mechanism derives from identification of antibody, C3 and MAC deposition at neuromuscular junctions from MG patients [5–7]. Depletion of serum complement components, C3 and C4 is observed in patients, but their levels are not related to severity of weakness [47]. Terminal components of complements are found in sera of MG patients, but again there is a lack of correlation to the degree of weakness [48]. These observations, however, may not be surprising. The site of complement consumption – the neuromuscular junction – is square microns in area and even the summation of all these regions across all skeletal muscle is relatively small. Therefore, a reflection of significant complement consumption in the serum would not be expected. A clinical observation that plasma exchange, at times, leads to rapid, dramatic improvement also suggests that mechanisms that impair channel function may be significant in some MG patients. In summary, despite the overwhelming data discussed below regarding EAMG, the extent to which complement contributes to human disease needs to be delineated in greater detail.
A large body of evidence supports the activation of the classical pathway of complement as the major initiator of the post-synaptic membrane damage in EAMG (Figure 2). Animal models, which induce EAMG either by administration of AChR antibodies or immunization with purified AChR, support the hypothesis: complement drives pathology in mouse and rat EAMG [49,50]; agents that block, inhibit or deplete complement protect animals from EAMG [51–53]; mice with a genetic deficit in complement components are resistant or less susceptible to EAMG [54]; inability to activate complement is associated with many immunological factors (e.g., in IL-12-deficient mice) [55], antibody, C3, C9 and MAC are uniformly found at the junctions of EAMG animals; and mice deficient in cell surface regulators of complement are particularly susceptible to EAMG induced by administration of AChR antibodies [50,56,57]. The following sections discuss the function of individual complement components as they relate primarily to EAMG pathogenesis.
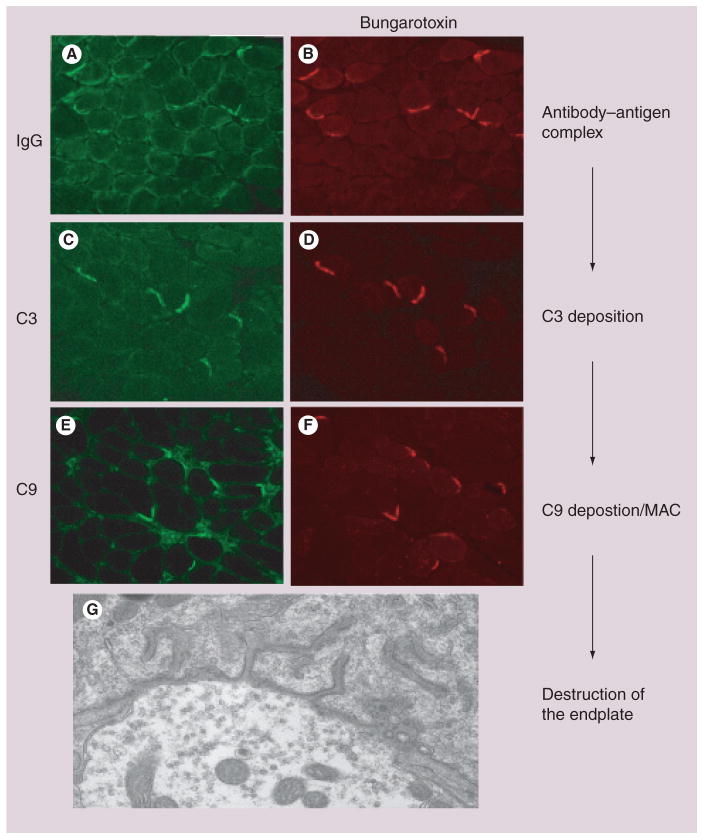
Figure 2. Complement deposition at the neuromuscular junction.
(A) Complement at the endplate begins with the binding of the antibody to the antigen on the cell surface. (B) Demonstrates the binding of IgG to the cell surface at the endplate that is marked by Texas red-bungarotoxin. (C & D) The initiation of the complement cascade occurs and allows for C3 to localize to the endplate. (E & F) The end product for the complement cascade is the deposition of the MAC, as demonstrated by C9 staining. (G) The hallmark feature of myasthenia gravis is the destruction of the endplate as shown as at the level of electron microscopy.
MAC: Membrane attack complex.
C1
A single-chain, membrane-bound glycoprotein initiates the classical complement cascade, mediates phagocytosis of C3b opsonized particles and regulates C3 and C5 activation [58]. C1q binds immune complexes and initiates the classical pathway by activating C1r and C1s thus forming the C1 complex (C1q, C1r and C1s). C1q activates production of IL-6 [59], and the forward loop allows the production of IL-6 to stimulate macrophages to produce C1q [60]. Increased gene transcript levels of IL-6 have been demonstrated in patients with MG [61] and knockout IL-6 mice are resistant to the disease [62]. Autoantibodies to C1q have been found in several autoimmune diseases. In EAMG mice, high levels of C1q antibody correlates with disease severity [63], although this was not observed in human patients. Administration of 10 μg of C1q antibody is associated with a reduction of complement deposits, decreased serum complement activity, and IL-6 production [64]. By contrast, 100 μg of C1q antibody results in an augmented response with increased anti-AChR, elevations of C3 and IgG deposition, and greater weakness [64].
The soluble recombinant form of the human complement receptor 1 (sCR1) has been shown to reduce complement-mediated tissue damage in a wide range of human acute and chronic inflammatory diseases [65]. sCR1 has also been found to reduce weight loss and weakness in passively induced EAMG [52] through its action of binding C4b and C3b and accelerating the decay of C3 and C5 convertases.
C3
Direct evidence for the involvement of the classical complement pathway in EAMG derives from the identification of IgG, C3 and MAC deposition at the neuromuscular junctions of both EAMG animals and MG patients. Mice deficient in C3 and C4 are resistant to the disease [66]. Both mouse strains produce AChR antibodies, but their production is considerably reduced in the C3-deficient strain. Despite deposition of IgG at the junction, C3 and MAC are lacking and mice do not develop significant weakness. The investigations suggests that disease could be treated effectively by inhibiting C4, thus leaving the alternative complement pathway intact.
C5
Once the C5 component is cleaved, multiple pathways are activated. C5a, as a potent anaphylatoxin and chemotaxin, enhances cell migration and adhesion, and induces release of proinflammatory cytokines [67]. C5b initiates assembly of the C5b–C9 MAC. The influence of C5 on EAMG susceptibility was analyzed on C5-sufficient and C5-deficient mice, which were otherwise genetically identical [54]. Both strains had comparable levels of serum AChR antibody. C5-intact mice show a higher incidence of disease development, death and loss of AChR, while C5 deficiency prevented EAMG development. Therefore, C5 appears critical in EAMG pathogenesis, probably through activation of the terminal C5–C9 sequence required for neuromuscular junction destruction. Blocking of C5, which does not impair function of early activated complement components, emerges with the potential for significant therapeutic benefit [68].
Membrane attack complex
Complement activation generates a number of biologically active products. These include anaphylactic peptides C3a and C5a, opsonic fragments C3b and C4b and the lytic MAC (C5b, C6, C7, C8 and C9). Any of these may contribute to the pathology of disease [69]. In murine and rat EAMG [49] produced by AChR antibody administration, activation of the terminal lytic complement complex (C5b–C9) is required for AChR destruction. The role of MAC was investigated by in vivo inhibition with anti-C6 Fab [51]. Administration of anti-C6 Fab totally inhibited in vitro serum hemolytic activity but did not obstruct previously activated components. Rats showed improvement in passively acquired EAMG weakness. Treatment with lower amounts of anti-C6 Fab also attenuated the outcome of disease but did not prevent the AChR loss. Findings suggest that targeting MAC assembly with high-affinity Fab fragments could be highly beneficial in MG.
Fcγ receptors & complement
Receptors for the Fc part of IgG can facilitate antigen presentation and activate effector mechanisms, such as antibody-dependent cellular cytotoxicity, phagocytosis and inflammatory mediators. Complement activation and C5a generation are fundamental for complexes to induce inflammation through Fcγ receptor signaling [70]. Evidence for the involvement of Fcγ receptors in EAMG pathogenesis have been studied in hypogammaglobulinemic RIIIS/J- and Fcγ RIII-knockout mice. Despite a significant B-cell deficiency, RIIIS/J mice have severe EAMG that is associated with increased lymph node cell counts, elevated anti-AChR antibodies, and C3- and C1q-conjugated circulating immune complexes. There is direct correlation between increased circulating immune complex levels and disease severity [71]. Deficiency in Fcγ RIII did not impair primary immune response to AChR but caused a reduction in serum levels of C1q and C3 circulating immune complexes. A decrease in IgG, C3 and MAC deposits at the junction correlated with resistance to EAMG [72]. An interaction between C5 and Fcγ has been proposed and this promises a therapeutic target for the treatment of inflammation and autoimmune diseases. A possible effect of an alternative pathway on amplification of the classical pathway could also be considered in future studies [73].
Complement regulatory proteins
Complement activation at the neuromuscular junction is the primary effector mechanism in MG pathogenesis. Based on identification of complement deposition at the neuromuscular junction and the expression of cell surface complement regulators on muscle fiber, the influence of complement regulators on MG severity could be expected [19,74].
Two groups have evaluated the role of CD59 and DAF in passive transfer EAMG, and both support that the loss of cell surface complement regulators is protective [50,56,57]. Wild-type mice do not show significant weakness while mice deficient in DAF become significantly weak to moribund and have greater complement deposition and AChR loss at the neuromuscular junctions. CD59 deficiency triggered very mild disease in one study [57], whereas the other group did not identify significant differences in severity between CD59- and DAF-deficient mice [50]. The differences may have been related to specific experimental conditions, such as AChR antibody doses to induce disease. By contrast, in both studies, mice deficient in both regulators developed a severe disease. In a study of active EAMG, CD59-deficient mice had decreased serum anti-AChR IgG1, IgG2b and complement levels. IL-2 production and recall responses to AChR were also reduced. The data challenge the current paradigm that CD59 is solely involved in MAC regulation, and suggest a role in antigen-driven T-cell and B-cell activation [75].
As mentioned previously, the EOM are particularly susceptible to MG [76], and murine EOM have been found to have low expression levels of the cell surface regulators compared with other skeletal muscles and when EAMG was induced [34,35]. Coupling studies that indicate that deficiency of complement regulators increase EAMG disease severity and that EOM has low levels of these regulators would suggest that EOM is susceptible to MG because of intrinsic low levels of local complement inhibition. Such a conceptualization is supported by observations of varying expression levels of regulatory proteins in parts of the nervous system affecting susceptibility to complement-mediated injury [77].
Complement & cytokine regulation
Both MG and EAMG are antibody mediated and T-cell dependent. Complement represents an important component of the innate response and may impact disease pathogenesis at various levels. It is not only identified as an instructor for the humoral immune response [78] but also plays a role in modulation of antigen presentation, T-cell activation and determination of the Th1/Th2 cell responses [10,79]. This intensive crosstalk between innate and adaptive immunity shapes the response, not only in MG but in many other autoimmune diseases [80].
Cytokines released by T-helper-cell subsets regulate humoral responses and are critical for the activation of AChR-specific T and B cells [81]. Studies of cytokine-deficient mice or treatments with peptides, antibodies and receptor antagonists provide considerable evidence for their role in EAMG pathogenesis. Both destructive and protective effects are described for a variety of cytokines.
Administration of anti-TNF-α antibodies results in a lower incidence of EAMG, and in delayed onset of disease and mild muscle weakness. Mild signs are accompanied by lower AChR-specific lymphocyte proliferation, downregulated IFN-γ, IL-10 and upregulated TGF-β. Anti-inflammatory treatment lowers the levels of complement-fixing antibodies (IgG, IgG2a and IgG2b) and decreases the affinity of anti-AChR IgG. Treatment with anti-TNF-α antibodies can suppress the induction and development of EAMG [82]. Comparable results were obtained with experimental treatment with human recombinant IL-1 receptor antagonist (IL-1ra). IL-1ra treatment during ongoing EAMG reduced symptoms of disease [83]. IL-1ra-mediated suppression results in suppression of IFN-γ, TNF-α, IL-1β, IL-2, IL-6, C3 and anti-AChR IgG1 [83].
IL-6-deficient mice are resistant to development of EAMG-induced weakness [62] and show significant reduction in anti-AChR antibodies (IgG, IgG2b and IgG2c) and germinal center formation. Decreased antigen-specific proliferative responses, suppressed levels of IFN-γ, IL-10 and serum complement C3 are also observed. By contrast, IL-4 has a protective function in EAMG [84]. B6 mice genetically deficient in IL-4 develop long-lasting muscle weakness after a single immunization with non-murine AChR, while wild-type mice require repeated immunization. Mice develop chronic self-reactive antibodies and their CD4+ T cells respond not only to the AChR for immunization, but also to mouse AChR-subunit peptides. Regulatory mechanisms that involve IL-4 appear to prevent the development of a chronic autoimmune response.
Possible role for complement in MG thymus
The initial trigger of MG is not known, but extensive literature points towards the thymus as a site of disease initiation given its central role in immune tolerance [85,86] The mechanism for autosensitization of the helper T cells appears to fall to binding of antibody to the AChR expressed myoid and epithelial cells followed by complement-mediated lysis of the myoid cells with subsequent generation of germinal centers [87]. Early-onset MG patients demonstrate evidence of complement activation in thymus with C1q, C3b and C9 deposition. An absence of cell surface regulators of complement on myoid and epithelial cells appears to be a further contributor to susceptibility to antibody attack [87]. MuSK antibody-specific MG patients showed fewer germinal centers in the thymus, however, demonstrated similar numbers of cellular infiltrates, suggesting that similar complement-related mechanisms could be active in this subgroup of patients [85]. However, the complement-mediated lysis of the myoid cells would not be expected in the MuSK-positive patients with the noncomplement binding IgG4 subclass antibodies.
Therapeutic approaches to MG by regulation of complement
Complement inhibitor-based therapeutics are now coming into clinical use [88]. As alluded to previously, EAMG has been inhibited by administration of complement inhibitors [50–52,68]. Administration of anti-C1q [64], anti-C6 [51] or sCR1 have been shown to protect rats against EAMG. Administration of an antimouse C5 monoclonal antibody protected CD59-deficient mice from passive EAMG in the absence of CD59, the complement regulator that protects self-cells against endogenous C5b-mediated injury [50]. Anti-C5 antibody also is protective in passive EAMG of rats [68].
Expert commentary
Complement activation generates a number of biologically active products. These include anaphylactic peptides C3a and C5a, opsonic fragments C3b and C4b and the lytic MAC. Activation and regulation of complement consist of a complex set of reactions and feedback check points where any of the components may contribute to the MG pathology. The pattern of complement deposition at the junction is a hallmark of ultrastructural destruction. Efficient therapy that focuses on complement-mediated destruction is still years away owing to the intricate effects that complement has in both the innate and adaptive immune system. Treatment must act to deter the complement deposition without harm or extensive manipulation to the adaptive immune system.
Five-year view
There can be little argument that the primary means by which AChR antibodies produce neuromuscular transmission compromise in EAMG is through their activation of complement, and it is likely that human MG complement-mediated injury is also a significant contributor to disease pathology. The authors foresee three major paths of investigation: pilot clinical trials of complement inhibitors in MG; refinement of complement inhibitors for various autoimmune disorders, including MG, as the best-defined antibody-mediated disease; and characterization of complement in adaptive immunity.
Clinical trials
At present, eculizumab is the only complement inhibitor approved for use in humans and its only indication is paroxysmal nocturnal hemoglobinuria [88]. Given the promise of complement inhibition shown in EAMG, it is likely that clinicians and industry will organize themselves to evaluate efficacy and safety of exulizumab for MG. The challenge will be how to design a trial. Animal studies would suggest that patients with severe disease would be most likely to benefit; however, such patients must receive the standard-of-care therapies of plasma exchange or intravenous immunoglobulin coupled with corticosteroids. It is hard to imagine that add-on therapy with a complement inhibitor would clearly demonstrate a difference to placebo. Choosing to evaluate patients with milder disease for short term could be a more fruitful approach as a proof of concept. If long-term complement inhibition will be considered, then safety issues will be of significant concern.
Improvement of complement inhibitors
The treatment of MG may take two paths. In the past few years, there has been an appreciation for the role of complement inhibitors in the autoimmune diseases, and several groups have reported beneficial results of complement inhibitors in reducing severity of EAMG [64,68,89]. Certainly, the ability for these reagents to treat MG will rely on the overall effect that these may have on the systemic immune system. The significance to the field of MG may change the focus of the therapeutic agents that have been suggested for intervention.
Of particular importance is a recent study that defined the ability of IgG4 to exchange Fab arms with other IgG classes [90]. In a monkey model of EAMG, the exchange of Fab arms between the IgG1, complement-fixing subclass, with the poorly complement-fixing IgG4 class led to moderation of disease severity [90]. The preliminary result of the IgG4 effect is interesting since a shift of AChR antibodies to an IgG4 subtype could lead to moderation of disease and serve as a therapeutic modality.
Complement & adaptive immunity
Thus far not investigated in EAMG or MG is increasing evidence that complement components and the complement regulatory proteins function in modulating T-cell activity [26]. Engineered deficiency of cell surface-complement inhibitors enhance T-cell responsiveness and secretion of certain cytokines. Given the enthusiasm that this review has reflected in complement inhibition for EAMG and by extension for MG, it will be important to define how modulation of complement activity will influence the primary autoimmune disease. The critical question will be assuring that systemic complement inhibition does not inadvertently activate the underlying autoimmune disease.
Key issues.
Myasthenia gravis (MG) is a chronic, autoimmune disease caused by antibodies directed primarily against the acetylcholine receptor at the neuromuscular junction. Immunosuppression and immunomodulation are effective but poorly tolerated treatments.
A plethora of data supports that complement serves as the primary effector of damage to the neuromuscular junction in experimental models of MG. Evidence is not as strong in human MG, and blockade of receptor function may also occur.
Approximately a third of patients without acetylcholine receptor antibodies have antibodies directed against the muscle-specific kinase concentrated at the neuromuscular junction. These antibodies appear to be pathogenic but present information does not indicate that they activate complement.
Preclinical studies indicate that complement inhibitors are effective in moderating the severity of experimental MG in rodents. The availability of inhibitors of complement for human use offers the possibility of a new class of agents for treatment of MG.
Footnotes
For reprint orders, please contact: reprints@future-drugs.com
Financial & competing interests disclosure
Supported by grants from the National Institutes of Health, (R24EY014837 and R01EY013238 to HJK). Disclose any financial interests. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Linda L Kusner, Email: lkusner@slu.edu, Department of Neurology & Psychiatry, Saint Louis University, 1438 South Grand Blvd, St Louis, MO 63104, USA, Tel.: +1 314 977 4849, Fax: +1 314 977 4876.
Henry J Kaminski, Email: hkaminsk@slu.edu, Department of Neurology & Psychiatry, Saint Louis University, 1438 South Grand Blvd, St Louis, MO 63104, USA, Tel.: +1 314 977 4849, Fax: +1 314 977 4876.
Jindrich Soltys, Email: jsoltys@slu.edu, Department of Neurology & Psychiatry, Saint Louis University, 1438 South Grand Blvd, St Louis, MO 63104, USA, Tel.: +1 314 977, 4849 Fax: +1 314 977 4876.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1•.Lindstrom J. Acetylcholine receptors and myasthenia. Muscle Nerve. 2000;23:453–477. doi: 10.1002/(sici)1097-4598(200004)23:4<453::aid-mus3>3.0.co;2-o. Excellent review of myasthenia gravis and the primary autoantigen. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357(9274):2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 3.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen FC, Rodgaard A, Djurup R, Somnier F, Gammeltoft S. A triple antibody assay for the quantitation of plasma IgG subclass antibodies to acetylcholine receptors in patients with myasthenia gravis. J Immunol Methods. 1985;83(2):249–258. doi: 10.1016/0022-1759(85)90247-9. [DOI] [PubMed] [Google Scholar]
- 5•.Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis. Ultrastructure and light microscopic localization and electrophysiological correlations. Mayo Clin Proc. 1977;52:267–280. Described complement component deposition at human neuromuscular junctions. [PubMed] [Google Scholar]
- 6.Nakano S, Engel AG. Myasthenia gravis: quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 1993;43(6):1167–1172. doi: 10.1212/wnl.43.6.1167. [DOI] [PubMed] [Google Scholar]
- 7.Sahashi K, Engel AG, Lambert EH, Howard FM., Jr Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol. 1980;39(2):160–172. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Engel AG, Sakakibara H, Sahashi K, Lindstrom J, Lambert EH, Lennon VA. Passively transferred experimental autoimmune myasthenia gravis. Sequential and quantitative study of the motor end-plate fine structure and ultrastructural localization of immune complexes (IgG and C3), and of the acetylcholine receptor. Neurology. 1979;29(2):179–188. doi: 10.1212/wnl.29.2.179. [DOI] [PubMed] [Google Scholar]
- 9.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7(1):9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 10••.Morgan BP, Marchbank KJ, Longhi MP, Harris CL, Gallimore AM. Complement: central to innate immunity and bridging to adaptive responses. Immunol Lett. 2005;97(2):171–179. doi: 10.1016/j.imlet.2004.11.010. Comprehensive review of complement relationships with adaptive immunity. [DOI] [PubMed] [Google Scholar]
- 11.Kohl J. Self, non-self, and danger: a complementary view. Adv Exp Med Biol. 2006;586:71–94. doi: 10.1007/0-387-34134-X_6. [DOI] [PubMed] [Google Scholar]
- 12.Boackle SA, Holers VM. Role of complement in the development of autoimmunity. Curr Dir Autoimmun. 2003;6:154–168. doi: 10.1159/000066860. [DOI] [PubMed] [Google Scholar]
- 13.Blank M, Schoenfeld Y. B cell targeted therapy in autoimmunity. J Autoimmun. 2007;28(2–3):62–68. doi: 10.1016/j.jaut.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Austen KF, Feeron DT. A molecular basis of activation of the alternative pathway of human complement. Adv Exp Med Biol. 1979;120B:3–17. [PubMed] [Google Scholar]
- 15.Bogers WM, Stad RK, van Es LA, Daham MR. Complement enhances the clearence of large-sized soluble IgA aggregates in rats. Eur J Immunol. 1991;21(5):1093–1099. doi: 10.1002/eji.1830210502. [DOI] [PubMed] [Google Scholar]
- 16.Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol. 2001;167(5):2861–2868. doi: 10.4049/jimmunol.167.5.2861. [DOI] [PubMed] [Google Scholar]
- 17.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386(6624):506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 18.Huber-Lang M, Sarma JV, Zetoune FS, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 19.Navenot JM, Villanova M, Lucas-Heron B, Malandrini A, Blanchard D, Louboutin JP. Expression of CD59, a regulator of the membrane attack complex of complement, on human skeletal muscle fibers. Muscle Nerve. 1997;20(1):92–96. doi: 10.1002/(sici)1097-4598(199701)20:1<92::aid-mus12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Louboutin JP, Navenot JM, Rouger K, Blanchard D. S-protein is expressed in necrotic fibers in Duchenne muscular dystrophy and polymyositis. Muscle Nerve. 2003;27(5):575–581. doi: 10.1002/mus.10360. [DOI] [PubMed] [Google Scholar]
- 21.Lublin DM, Liszewski MK, Post TW, et al. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita T, Inoue T, Ogawa K, Iida K, Tamura N. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J Exp Med. 1987;166(5):1221–1228. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meri S, Morgan BP, Davies A, et al. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Miwa T, Hilliard B, et al. The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med. 2005;201(4):567–577. doi: 10.1084/jem.20040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeger PS, Lalli PN, Lin F, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longhi MP, Harris CL, Morgan BP, Gallimore AM. Holding T cells in check – a new role for complement regulators? Trends Immunol. 2006;27(2):102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Whiting PJ, Cooper J, Lindstrom JM. Antibodies in sera from patients with myasthenia gravis do not bind to nicotinic acetylcholine receptors from human brain. J Neuroimmunol. 1987;16(2):205–213. doi: 10.1016/0165-5728(87)90075-0. [DOI] [PubMed] [Google Scholar]
- 28.Gomez CM, Richman DP. Anti-acetylcholine receptor antibodies directed against the α-bungarotoxin binding site induce a unique form of experimental myasthenia. Proc Natl Acad Sci USA. 1983;80(13):4089–4093. doi: 10.1073/pnas.80.13.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978;298:1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- 30.Lindstrom J, Einarson B. Antigenic modulation and receptor loss in experimental autoimmune myasthenia gravis. Muscle Nerve. 1979;2:173–179. doi: 10.1002/mus.880020304. [DOI] [PubMed] [Google Scholar]
- 31.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 32.Howard FJ, Lennon V, Finley J, Matsumoto J, Elveback L. Clinical correlations of antibodies that bind, block, or modulate human acetylcholine receptors in myasthenia gravis. Ann NY Acad Sci. 1987;505:526–538. doi: 10.1111/j.1749-6632.1987.tb51321.x. [DOI] [PubMed] [Google Scholar]
- 33.Limburg PC, The TH, Hummel-Tappel E, Oosterhuis HJ. Anti-acetylcholine receptor antibodies in myasthenia gravis. I Relation to clinical parameters in 250 patients. J Neurol Sci. 1983;58:357–370. doi: 10.1016/0022-510x(83)90095-3. [DOI] [PubMed] [Google Scholar]
- 34.Kaminski HJ, Li Z, Richmonds C, Lin F, Medof ME. Complement regulators in extraocular muscle and experimental autoimmune myasthenia gravis. Exp Neurol. 2004;189(2):333–342. doi: 10.1016/j.expneurol.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Porter JD, Khanna S, Kaminski HJ, et al. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc Natl Acad Sci USA. 2001;98:12062–12067. doi: 10.1073/pnas.211257298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. doi: 10.1038/85520. First to describe muscle-specific kinase as a new autoantigen in myasthenia gravis. [DOI] [PubMed] [Google Scholar]
- 37.Vincent A, Bowen J, Newsom-Davis J, McConville J. Seronegative generalised myasthenia gravis: clinical features, antibodies, and their targets. Lancet Neurol. 2003;2(2):99–106. doi: 10.1016/s1474-4422(03)00306-5. [DOI] [PubMed] [Google Scholar]
- 38.Chevessier F, Faraut B, Ravel-Chapuis A, et al. Pathophysiological characterization of congenital myasthenic syndromes: the example of mutations in the MUSK gene. J Soc Biol. 2005;199(1):61–77. doi: 10.1051/jbio:2005008. [DOI] [PubMed] [Google Scholar]
- 39.Shiraishi H, Motomura M, Yoshimura T, et al. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol. 2005;57(2):289–293. doi: 10.1002/ana.20341. [DOI] [PubMed] [Google Scholar]
- 40.McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55(4):580–584. doi: 10.1002/ana.20061. [DOI] [PubMed] [Google Scholar]
- 41.Jha S, Xu K, Maruta T, et al. Myasthenia gravis induced in mice by immunization with the recombinant extracellular domain of rat muscle-specific kinase (MuSK) J Neuroimmunol. 2006;175(1–2):107–117. doi: 10.1016/j.jneuroim.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 42.Shigemoto K, Kubo S, Maruyama N, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest. 2006;116(4):1016–1024. doi: 10.1172/JCI21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrugia ME, Bonifati DM, Clover L, Cossins J, Beeson D, Vincent A. Effect of sera from AChR-antibody negative myasthenia gravis patients on AChR and MuSK in cell cultures. J Neuroimmunol. 2007;185(1–2):136–144. doi: 10.1016/j.jneuroim.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Mygland Å, Vincent A, Newsom-Davis J, et al. Autoantibodies in thymoma-associated myasthenia gravis with myositis or neuromyotonia. Arch Neurol. 2000;57:527–531. doi: 10.1001/archneur.57.4.527. [DOI] [PubMed] [Google Scholar]
- 45.Skeie GO, Mygland Å, Aarli JA, Gilhus NE. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99–104. doi: 10.3109/08916939509001933. [DOI] [PubMed] [Google Scholar]
- 46.Romi F, Skeie GO, Vedeler C, Aarli JA, Zorzato F, Gilhus NE. Complement activation by titin and ryanodine receptor autoantibodies in myasthenia gravis. A study of IgG subclasses and clinical correlations. J Neuroimmunol. 2000;111(1–2):169–176. doi: 10.1016/s0165-5728(00)00394-5. [DOI] [PubMed] [Google Scholar]
- 47.Romi F, Kristoffersen EK, Aarli JA, Gilhus NE. The role of complement in myasthenia gravis: serological evidence of complement consumption in vivo. J Neuroimmunol. 2005;158(1–2):191–194. doi: 10.1016/j.jneuroim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Barohn RJ, Brey RL. Soluble terminal complement components in human myasthenia gravis. Clin Neurol Neurosurg. 1993;95(4):285–290. doi: 10.1016/0303-8467(93)90103-n. [DOI] [PubMed] [Google Scholar]
- 49•.Chamberlain-Banoub J, Neal JW, Mizuno M, Harris CL, Morgan BP. Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin Exp Immunol. 2006;146(2):278–286. doi: 10.1111/j.1365-2249.2006.03198.x. Beautiful study describing complement and complement regulators in experimentally acquired myasthenia gravis (EAMG) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Morgan BP, Chamberlain-Banoub J, Neal JW, Song W, Mizuno M, Harris CL. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol. 2006;146(2):294–302. doi: 10.1111/j.1365-2249.2006.03205.x. Beautiful study describing complement and complement regulators in EAMG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biesecker G, Gomez CM. Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol. 1989;142(8):2654–2659. [PubMed] [Google Scholar]
- 52.Piddlesden SJ, Jiang S, Levin JL, Vincent A, Morgan BP. Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol. 1996;71(1–2):173–177. doi: 10.1016/s0165-5728(96)00144-0. [DOI] [PubMed] [Google Scholar]
- 53.Lennon VA, Seybold ME, Lindstrom JM, Cochrane C, Ulevitch R. Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med. 1978;147(4):973–983. doi: 10.1084/jem.147.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christadoss P. C5 gene influences the development of murine myasthenia gravis. J Immunol. 1988;140:2589–2592. [PubMed] [Google Scholar]
- 55.Karachunski P, Ostlie N, Monfardini C, Conti-Fine B. Absence of IFN-γ or IL-12 has different effects on experimental myasthenia gravis in C57BL/6 mice. J Immunol. 2000;164:5236–5244. doi: 10.4049/jimmunol.164.10.5236. [DOI] [PubMed] [Google Scholar]
- 56•.Lin F, Kaminski H, Conti-Fine B, Wang W, Richmonds C, Medof M. Enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J Clin Invest. 2002;110:1269–1274. doi: 10.1172/JCI16086. First study demonstrating that cell surface regulators of complement influence disease severity of EAMG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaminski HJ, Kusner LL, Richmonds C, Medof ME, Lin F. Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp Neurol. 2006;202(2):287–293. doi: 10.1016/j.expneurol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Reid KB, Colomb M, Petry F, Loos M. Complement component C1 and the collectins – first-line defense molecules in innate and acquired immunity. Trends Immunol. 2002;23(3):115–117. doi: 10.1016/s1471-4906(01)02164-0. [DOI] [PubMed] [Google Scholar]
- 59.van den Berg RH, Faber-Krol MC, Sim RB, Daha MR. The first subcomponent of complement, C1q, triggers the production of IL-8, IL-6, and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J Immunol. 1998;161(12):6924–6930. [PubMed] [Google Scholar]
- 60.Faust D, Loos M. In vitro modulation of C1q mRNA expression and secretion by interleukin-1, interleukin-6, and interferon-γ in resident and stimulated murine peritoneal macrophages. Immunobiology. 2002;206(4):368–376. doi: 10.1078/0171-2985-00187. [DOI] [PubMed] [Google Scholar]
- 61.Matusevicius D, Navikas V, Palasik W, Pirskanen R, Fredrikson S, Link H. Tumor necrosis factor-α, lymphotoxin, interleukin (IL)-6, IL-10, IL-12 and perforin mRNA expression in mononuclear cells in response to acetylcholine receptor is augmented in myasthenia gravis. J Neuroimmunol. 1996;71(1–2):191–198. doi: 10.1016/s0165-5728(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 62•.Deng C, Goluszko E, Tuzun E, Yang H, Christadoss P. Resistance to experimental autoimmune myasthenia gravis in IL-6-deficient mice is associated with reduced germinal center formation and C3 production. J Immunol. 2002;169(2):1077–1083. doi: 10.4049/jimmunol.169.2.1077. Cytokine influence on EAMG disease severity as determined by complement cascade. [DOI] [PubMed] [Google Scholar]
- 63.Tuzun E, Saini SS, Ghosh S, Rowin J, Meriggioli MN, Christadoss P. Predictive value of serum anti-C1q antibody levels in experimental autoimmune myasthenia gravis. Neuromuscul Disord. 2006;16(2):137–143. doi: 10.1016/j.nmd.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Tuzun E, Li J, Saini SS, Yang H, Christadoss P. Pros and cons of treating murine myasthenia gravis with anti-C1q antibody. J Neuroimmunol. 2007;182(1–2):167–176. doi: 10.1016/j.jneuroim.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Kirschfink M. Targeting complement in therapy. Immunol Rev. 2001;180:177–189. doi: 10.1034/j.1600-065x.2001.1800116.x. [DOI] [PubMed] [Google Scholar]
- 66.Tuzun E, Scott BG, Goluszko E, Higgs S, Christadoss P. Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J Immunol. 2003;171(7):3847–3854. doi: 10.4049/jimmunol.171.7.3847. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y. Complementary therapies for inflammation. Nat Biotech. 2006;24(10):1224–1226. doi: 10.1038/nbt1006-1224. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y, Gong B, Lin F, Rother RP, Medof ME, Kaminski HJ. Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J Immunol. 2007;179:8562–8567. doi: 10.4049/jimmunol.179.12.8562. [DOI] [PubMed] [Google Scholar]
- 69.Morgan BP, Gasque P, Singhrao SK, Piddlesden SJ. Role of complement in inflammation and injury in the nervous system. Exp Clin Immunogenet. 1997;14(1):19–23. [PubMed] [Google Scholar]
- 70.Schmidt RE, Gessner JE. Fc receptors and their interaction with complement in autoimmunity. Immunol Lett. 2005;100(1):56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 71.Tuzun E, Scott BG, Yang H, et al. Circulating immune complexes augment severity of antibody-mediated myasthenia gravis in hypogammaglobulinemic RIIIS/J mice. J Immunol. 2004;172(9):5743–5752. doi: 10.4049/jimmunol.172.9.5743. [DOI] [PubMed] [Google Scholar]
- 72.Tuzun E, Saini SS, Yang H, Alagappan D, Higgs S, Christadoss P. Genetic evidence for the involvement of Fcγ receptor III in experimental autoimmune myasthenia gravis pathogenesis. J Neuroimmunol. 2006;174(1–2):157–167. doi: 10.1016/j.jneuroim.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 73.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol. 2004;138(3):439–446. doi: 10.1111/j.1365-2249.2004.02627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Louboutin JP, Navenot JM, Villanova M, Rouger K, Merlini L, Fardeau M. X-linked vacuolated myopathy: membrane attack complex deposition on the surface membrane of injured muscle fibers is not accompanied by S-protein. Muscle Nerve. 1998;21(7):932–935. doi: 10.1002/(sici)1097-4598(199807)21:7<932::aid-mus11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 75.Tuzun E, Saini SS, Morgan BP, Christadoss P. Complement regulator CD59 deficiency fails to augment susceptibility to actively induced experimental autoimmune myasthenia gravis. J Neuroimmunol. 2006;181:29–33. doi: 10.1016/j.jneuroim.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Kusner LL, Puwanant A, Kaminski HJ. Ocular myasthenia: diagnosis, treatment, and pathogenesis. Neurologist. 2006;12(5):231–239. doi: 10.1097/01.nrl.0000240856.03505.b5. [DOI] [PubMed] [Google Scholar]
- 77.Tang H, Brimijoin S. Complement regulatory proteins and selective vulnerability of neurons to lysis on exposure to acetylcholinesterase antibody. J Neuroimmunol. 2001;115(1–2):53–63. doi: 10.1016/s0165-5728(01)00249-1. [DOI] [PubMed] [Google Scholar]
- 78.Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004;41(2–3):141–146. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Nielsen CH, Hegedus L, Rieneck K, Moeller AC, Leslie RG, Bendtzen K. Production of interleukin (IL)-5 and IL-10 accompanies T helper cell type 1 (Th1) cytokine responses to a major thyroid self-antigen, thyroglobulin, in health and autoimmune thyroid disease. Clin Exp Immunol. 2007;147(2):287–295. doi: 10.1111/j.1365-2249.2006.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molina H. Complement and immunity. Rheum Dis Clin North Am. 2004;30(1):1–18. doi: 10.1016/S0889-857X(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 81.Poea-Guyon S, Christadoss P, Le Panse R, et al. Effects of cytokines on acetylcholine receptor expression: implications for myasthenia gravis. J Immunol. 2005;174(10):5941–5949. doi: 10.4049/jimmunol.174.10.5941. [DOI] [PubMed] [Google Scholar]
- 82.Duan RS, Wang HB, Yang JS, Scallon B, Link H, Xiao BG. Anti-TNF-α antibodies suppress the development of experimental autoimmune myasthenia gravis. J Autoimmun. 2002;19(4):169–174. doi: 10.1006/jaut.2002.0618. [DOI] [PubMed] [Google Scholar]
- 83.Yang H, Tuzun E, Alagappan D, et al. IL-1 receptor antagonist-mediated therapeutic effect in murine myasthenia gravis is associated with suppressed serum proinflammatory cytokines, C3, and anti-acetylcholine receptor IgG1. J Immunol. 2005;175(3):2018–2025. doi: 10.4049/jimmunol.175.3.2018. [DOI] [PubMed] [Google Scholar]
- 84.Ostlie N, Milani M, Wang W, Okita D, Conti-Fine BM. Absence of IL-4 facilitates the development of chronic autoimmune myasthenia gravis in C57BL/6 mice. J Immunol. 2003;170(1):604–612. doi: 10.4049/jimmunol.170.1.604. [DOI] [PubMed] [Google Scholar]
- 85.Leite MI, Strobel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57(3):444–448. doi: 10.1002/ana.20386. [DOI] [PubMed] [Google Scholar]
- 86.Shiono H, Roxanis I, Zhang W, et al. Scenarios for autoimmunization of T and B cells in myasthenia gravis. Ann NY Acad Sci. 2003;998:237–256. doi: 10.1196/annals.1254.026. [DOI] [PubMed] [Google Scholar]
- 87.Leite MI, Jones M, Strobel P, et al. Myasthenia gravis thymus: complement vulnerability of epithelial and myoid cells, complement attack on them, and correlations with autoantibody status. Am J Pathol. 2007;171(3):893–905. doi: 10.2353/ajpath.2007.070240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. doi: 10.1056/NEJMoa061648. Complement inhibitor proves to be dramatically effective in paroxysmal nocturnal hemoglobinuria. [DOI] [PubMed] [Google Scholar]
- 89.Hepburn NJ, Chamberlain-Banoub JL, Williams AS, Morgan BP, Harris CL. Prevention of experimental autoimmune myasthenia gravis by rat Crry-Ig: a model agent for long-term complement inhibition in vivo. Mol Immunol. 2008;45(2):395–405. doi: 10.1016/j.molimm.2007.06.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]