Abstract
Objectives:
To determine the prevalence of restless legs syndrome (RLS) in Saudi patients with end-stage renal disease (ESRD) on hemodialysis.
Methods:
A cross-sectional study was carried out in 3 hemodialysis centers in Jeddah, Saudi Arabia, between June 2012 and September 2013. All patients were individually interviewed and data was collected on the following demographic features, medical history, laboratory test, the International Restless Legs Syndrome Study Group questionnaire, Epworth Sleepiness Scale (ESS), and Berlin Questionnaire.
Results:
Three hundred and fifty-five patients were recruited. The prevalence of RLS among ESRD patients was 19.4%, with most patients having moderate to severe disease. The RLS was significantly associated with obstructive sleep apnea (p<0.0001) and excessive daytime sleepiness based on the ESS (p=0.009). The RLS showed no correlation with hemodialysis adequacy, chronicity, frequency per week, and hemodialysis duration per session; however, there was a weak negative relation between adequacy of hemodialysis and RLS severity. None of the comorbidities showed any association with RLS. The odds of developing RLS increased significantly with an increasing body mass index (p=0.001). Administration of aspirin (p=0.037) and anticoagulants (p=0.035) were also associated with increased risk of RLS.
Conclusion:
Restless legs syndrome is common in ESRD patients on hemodialysis, and it is an important source of sleep disruption. In addition to body mass index, Aspirin and anticoagulants may be important risk factors.
Willis-Ekbom disease or restless legs syndrome (RLS) is a sleep-related, sensorimotor, neurological disorder that primarily affects the legs.1 When severe, the disorder can also affect the arms and other parts of the body. The RLS is characterized by sore sensations in the legs and accompanied by an urge to move them, typically in the evening and night. It is worse at rest and at least temporarily relieved by activity.1,2 The prevalence of RLS in the general population ranges from 5-15%.3 It can be idiopathic in nature or secondary to conditions such as diabetes mellitus, renal failure, iron deficiency anemia, multiple sclerosis, and pregnancy. The restlessness experienced by patients with RLS can cause problems such as insomnia, excessive daytime sleepiness, poor quality of life, and depression. In its severe forms, it can develop into a chronic and devastating disorder that may require long-term treatment. The clinical management of this condition is to treat the possible causes as well as to use different drugs for symptomatic relief. However, there is no definitive cure for this condition. Patients on regular hemodialysis (HD) for the management of end-stage renal disease (ESRD) are prone to various neurological disorders, including RLS. The prevalence of RLS among HD patients is approximately 20-30%.4,5 In a Serbian study, Nikić et al6 evaluated 166 patients on HD using the International Restless Legs Syndrome Study Group (IRLSSG) criteria and found that the prevalence of RLS was 22.7%. Goffredo Filho et al7 also reported the frequency of RLS to be 14.8% among 176 Brazilian patients. With regard to our region, Salman8 reported that the prevalence of RLS was 20.3% in 123 Syrian patients on chronic HD. Finally, Al-Jahdali et al9 conducted the single local study on 227 ESRD patients on chronic hemodialysis and found that the prevalence of RLS was 50.2%. This rate was much higher than that reported previously in majority of similar studies, particularly in light of the fact that the reported percentage of RLS in the Saudi population was only 5.2%.10 In this study, our aim was to determine the prevalence of RLS among a sample of Saudi patients with ESRD maintained on regular HD, and to compare the obtained value with that previously reported. Additionally, we explored the association between the adequacy of hemodialysis and severity of RLS symptoms.
Methods
This was designed as a cross-sectional multicenter, descriptive study. Participants were recruited from HD units at 3 major general hospitals in Jeddah, Saudi Arabia between June 2012 and September 2013. These participating hospitals were King Abdulaziz University Hospital, King Abdulaziz Hospital, and King Fahd General Hospital. Ethical approval from the hospital ethical committee and written informed consent from all patients was obtained before recruitment. This study follows the principles of the Helsinki Declaration.
Participation was sought from all ESRD stable patients regularly attending HD units between 0700 and 2300 hours. Patients who were confused, demented, and unwilling or unable to participate were excluded from the study. Each patient was personally interviewed. The administered questionnaire collected data on the following: demographic features; comorbidities; hemodialysis-related data, includes underlying cause of ESRD, frequency of hemodialysis per week, duration of each hemodialysis session, and hemodialysis adequacy using urea reduction ratio (URR);11 medications; Epworth Sleepiness Scale (ESS) score to assess daytime sleepiness;12 International Restless Legs Syndrome Study Group criteria to diagnose restless leg syndrome;2 severity of RLS using the Restless Legs Syndrome Rating Scale;13 and Berlin Sleep Questionnaire (BQ) to assess the risk of sleep apnea.14 In addition, we also recorded the results of laboratory tests, including serum levels of hemoglobin, calcium, creatinine, and urea before and after hemodialysis, glycated hemoglobin, and iron store (ferritin).
Statistical analysis
Data analysis was carried out using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA) version 21. Data were summarized by calculating the mean ± standard deviation for quantitative data and by frequencies and percentages (%) for the categorical variables. The results were stratified according to presence (RLS+) and absence (RLS-) of RLS. Two independent samples t tests, or its nonparametric equivalent Mann-Whitney test was used, as appropriate, to compare the 2 groups. Chi square test, or Fisher exact test was used, as needed, to investigate the association between RLS and the various clinical and laboratory factors. Results were considered statistically significant if the p-value was less than 0.05. The predictive power of the likelihood and odds ratio (OR) of RLS for different risk factors was estimated using the logistic regression models, while controlling for the following confounding variables: age, gender, body mass index, smoking, traditional predictors of RLS, and comorbidities that can disturb sleep, including anemia, diabetes, hypertension, chronic obstructive pulmonary disease, and daytime sleepiness. Odds ratios (OR) were reported along with the 95% confidence intervals (CI) and p-values.
Results
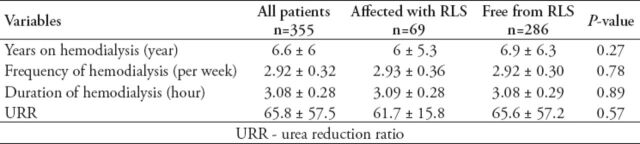
In all, 355 patients were recruited. The mean patient age was 48.5±15.4 years; mean BMI, 25.18±6.88 kg/m2; and percentage of male subjects, 61%. Most patients underwent hemodialysis at least 3 times a week for at least 3 hours per session over 78.88±72.35 months in average (Table 1). The prevalence of RLS among the 355 ESRD patients recruited was 19.4%. Most RLS patients (91%) experienced moderate to severe symptoms, with a mean severity score of 19.07±9.02 according to the RLS rating scale. The BMI differed significantly in both RLS-affected (RLS+) and RLS-free (RLS-) groups (p=0.004). Furthermore, the correlation of obesity with RLS was significant (r=0.22, p<0.0001). There is a 5% increased risk of RLS per unit increase in BMI (kg/m2) (OR=1.054; CI=1.017, 1.094; p=0.004). The RLS+ and RLS- groups did not show any significant difference in the values of the parameters used to analyze the hemodialysis adequacy, including URR, number of years on hemodialysis, frequency of hemodialysis per week, and duration of hemodialysis (Table 2). However, a borderline significant correlation (r=-0.23, p=0.053) was noted between hemodialysis adequacy in terms of URR and the severity score of RLS.
Table 1.
Clinical and demographic profile for all patients stratified according to the presence or absence of restless legs syndrome (RLS).
Table 2.
Hemodialysis-related data for all patients stratified according to the presence or absence of restless legs syndrome (RLS).
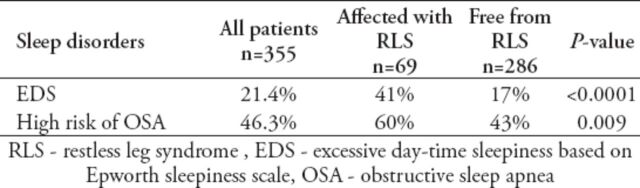
None of the comorbid diseases, including diabetes mellitus (in 35% of the patients) and anemia (in 84% of the patients), showed any association with RLS, even when the coexistence was controlled. On comparison between the patients with RLS and those without, no difference was noted in the serum levels of hemoglobin, ferritin, calcium, and phosphorus and the renal profile. With regard to the coexistence of other sleep disorders, 21.4% of our sample had excessive daytime sleepiness and 46.3% were at a high risk of obstructive sleep apnea (OSA) according to the Berlin questionnaire. These percentages were significantly higher in the RLS+ group than in the RLS group with p<0.0001 for EDS, and 0.009 for OSA risk. Moreover, the high risk for OSA was found to be a significant predictor of RLS, since the OR for RLS was almost double in high-risk OSA patients (OR = 1.99; CI: 1.16-3.40, p=0.013). This relationship persisted even after controlling for confounders such as age, gender, and smoking (Table 3).
Table 3.
Relation of RLS affected (RLS+) and RLS free (RLS-) patients with other sleep disorders.
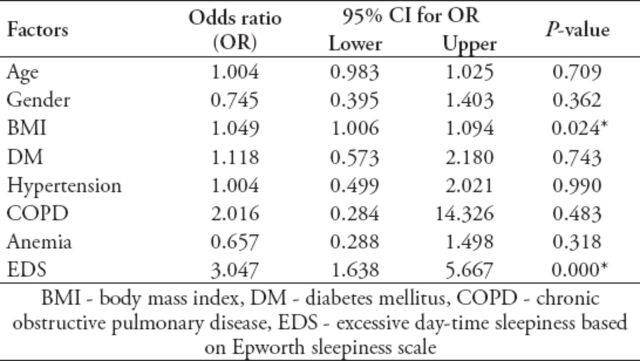
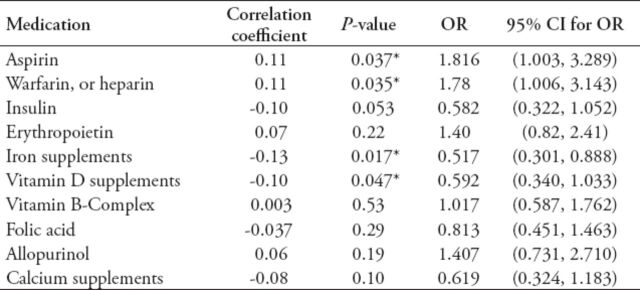
Multivariate logistic regression was used to study the likelihood of RLS for different risk factors controlling for confounding variables. The ORs were estimated and are reported along with the 95% CI and p-values in Table 4. Consistent with the findings of the previous analysis, BMI and excessive daytime sleepiness continued to remain, as expected, the important predictors for RLS. Although OSA was a strong predictor for RLS, it was not included in the multivariate model due to its significant correlation with EDS, where one sufficed. A high percentage of patients received supplements such as vitamin B complex (62%), iron supplements (66%), folic acid (72%), and calcium (81%). The correlation status between medications and risk of RLS, as expressed in terms of correlation coefficient (r) and OR, are summarized in Table 5.
Table 4.
Multivariate logistic regression analysis for independent predictors of RLS.
Table 5.
Drugs used by both restless leg syndrome (RLS) affected and RLS free groups, and the correlation coefficient for each drug with its significance level, as well as odds ratio (OR) with its confidence interval (CI).
Administration of aspirin (p=0.037) and anticoagulant drugs (p=0.035) were found to be associated with increased risk of RLS. Patients who were taking iron supplements were approximately 50% less likely to get RLS than those not taking them (OR=0.517, CI: 0.301-0.888). Similarly, those on vitamin D supplements were less likely to be RLS+ (OR=0.592, CI: 0.340-1.033).
Discussion
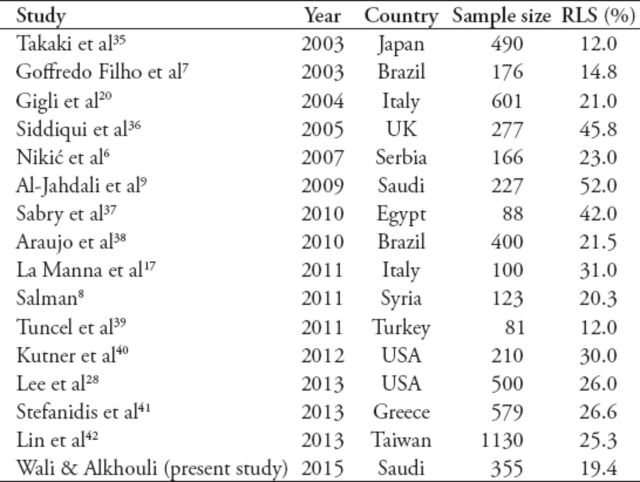
Our study showed that RLS is a common disorder affecting almost one-fifth of ESRD patients on HD. In addition, our findings showed that RLS had no gender predominance in ESRD patients, and that it was manifested as moderate to severe disease in most of such patients. The prevalence of RLS noted in this study was similar to that reported in many other studies, but extremely lower than that indicated in the local report (52.8%).9 As shown in Table 6, the variability in the reported prevalence of RLS in the literature may be due to genetic factors, heterogeneity of patient populations studied, and the differences in the diagnostic criteria adopted in these studies.15 However, the remarkable disparity in the reported prevalence of RLS between our study and that of Al-Jahdali et al9 may be due to the heterogeneity of the patient populations studied (it is worth declaring that their study population was recruited from 2 different centers than ours). The mean age group in our study population was 10 years younger than in their study, since it is well known that the prevalence of RLS increases with ageing.16 Another factor is that there were more diabetic patients in the their study (n=119; 52.4%)than in ours (n=62; 35%), in which may contribute to this discrepancy in RLS prevalence. The absence of gender difference in our study is in contrast with previous studies, which showed female predominance,9,17 but is in agreement with others.18 The pathogenesis of primary RLS seems to be related to disturbances in the central dopaminergic system and iron metabolism, in addition to genetic factors.19 The RLS pathogenesis in patients with ESRD is not well understood. Iron deficiency in ESRD may contribute to RLS, both by the induction of anemia and by the alternation in the dopamine metabolism in the central nervous system.6,20 The RLS may also be secondary to uremia-related peripheral neuropathy and/or diabetes mellitus as the underlying cause for renal failure. High serum calcium concentration was also reported to be a possible contributor to the pathophysiology of RLS.6 In our report, anemia, iron deficiency (based on serum ferritin), and calcium levels were not found to be significant risk factors for RLS (Table 5). Although previous reports have shown that diabetes mellitus and other comorbidities may be independent etiologies of RLS, these conditions were not found to be risk factors for RLS in the uremic patients enrolled in this study.20-23
Table 6.
Comparison of the prevalence of restless legs syndrome (RLS) diagnosed by International Restless Legs Syndrome Study Group in patients with end-stage renal disease, as reported in previous studies.
Interestingly, obesity was found to be an important risk factor for RLS. There was a 5% increased risk of RLS associated with a unit increase in BMI (kg/m2), (OR=1.054; CI=1.017- 1.094, p=0.004). In fact, the odds of developing RLS for obese patients were triple that in normoweight patients (Table 1). This was in contrast with a previous study that reported no such association between BMI and RLS in hemodialysis patients.18 Our finding is consistent with those of epidemiological studies that indicated BMI as a risk factor associated with a higher likelihood of RLS.24-26 Gao et al24 conducted a large cohort study and found that the OR for RLS were 1.42 (95% CI: 1.3-1.6; p<0.0001) for participants with BMI at 23-30 kg/m2 and 1.60 (95% CI: 1.5-1.8, p<0.0001) for highest versus lowest waist circumference.24 Thus, independent of all other confounders, obesity and abdominal adiposity were associated with increased likelihood of RLS.24 This relationship may be due to a decrease in number of dopamine receptors in the brain of obese subjects, leading to increased risk of RLS.27 Similar to previous studies,9,26 the current study showed that RLS was associated with other sleep disorders and was an important source of sleep disturbance. It also revealed that excessive daytime sleepiness (41%, p<0.0001), and sleep apnea symptoms (60%, p=0.009) were more prevalent in RLS patients.
In our study, similar to some studies8,9,23 and unlike that reported by Gigli et al,20 the 2 groups (RLS+ and RLS-) did not differ significantly as far as adequacy of HD, the chronicity in years, frequency of hemodialysis per week, and duration of hemodialysis sessions (Table 2). On the other hand, there was a borderline significant correlation (r=-0.23, p=0.053) between hemodialysis adequacy and severity score of RLS. This indicates a weak negative relation between adequacy of HD and RLS severity.
Furthermore, factors that were found to be associated with reduced risk of RLS were iron and vitamin D supplements (Table 5). Patients taking these supplements, approximately 50% for iron and 40% for vitamin D, were less likely to develop RLS than others (OR=0.517, CI: 0.301- 0.888 for iron supplements) and (OR=0.592, CI: 0.340-1.033 for vitamin D supplements). Patients on medications for diabetes were also approximately 40% less likely to have RLS, but the association was barely significant (p=0.053). This is not surprising since both iron deficiency and diabetes are predisposing factors for RLS.6,21,22 Although no evidence was obtained to show that controlling diabetes would improve RLS symptoms, previous reports support the efficacy of iron replacement in treating RLS.29,30 Moreover, the fact that two-thirds of the patients were actually on iron supplements may have led to an underestimation of the prevalence of RLS in our study. On the other hand, the association between vitamin D and RLS has been reported in a previous study.31 Balaban et al31 compared the vitamin D levels of RLS patients and matched them with those in controls to explore the link between the vitamin D levels and presence/absence and severity of RLS. The mean serum 25-hydroxyvitamin D levels were 7.31 ± 4.63 ng/mL in patients with RLS, and 12.31 ± 5.27 ng/mL in control subjects (p=0.001). They also reported a significant inverse correlation between the vitamin D level and disease severity of RLS (p=0.01, r=-0.47).31 In addition, hemodialysis patients with RLS were found to experience increased muscle atrophy compared to RLS free patients.32 This is actually in keeping with the findings reported in vitamin D deficiency patients, which may reinforce the relationship between vitamin D deficiency and RLS.33 More recently, Wali et al34 reported that vitamin D supplementation improves the severity of RLS symptoms and advocated that vitamin D deficiency is a potential risk factor for RLS.34
Interestingly, the administration of Aspirin (p=0.037) and anticoagulant drugs (p=0.035) were found to be associated with increased risk of RLS. This relationship is probably reported for the first time. These medications may actually contribute to the pathogenesis of RLS and play an important role as new risk factors for RLS in ESRD patients. However, a possible mechanism is by causing iron deficiency anemia, which is a potential side effect of these medications. Further confirmatory studies are needed.
This study was limited by the small sample size of RLS+ group (69 patients), which may affect the power of detecting a real association between RLS and other factors. This could be partly due to a small number of patients in each group, whereby the power for the statistical tests to detect real differences or association with RLS.
Finally, health care providers dealing with renal patients should be alert to this common sleep disorder that may actually worsen the quality of life of this already drained population. It would be worth conducting a survey on the awareness of such physicians regarding sleep disorders in general and RLS in particular and its implementation on patient care.
In conclusion, RLS is common in ESRD patients on HD and it is an important source of sleep disruption. In addition, medications and obesity are important predictors for RLS.
Acknowledgment
The authors would like to acknowledge Ms. Walaa M. Abuzahra for editing, reviewing, and typing this manuscript. Acknowledgment is also forwarded to Prof. Saad Alshohaib for facilitating access to the hemodialysis centers involved and Dr. Ibrahim Zakaria for his valuable help in data collection.
Footnotes
Related Articles
Elsayed NM, Hamed ST, El-Khatib MM, El-Shehaby AM. The relation between dual energy x-ray absorptiometry measurement of body fat composition and plasma ghrelin in patients with end-stage renal disease. Saudi Med J 2009; 30: 109-115.
Tayyem RF, Mrayyan MT. Malnutrition, and anthropometric and biochemical abnormalities in end-stage renal disease patients. Saudi Med J 2007; 28: 1575-1581.
Tayyem RF, Ahmad IM. Prevalence of malnutrition among end-stage renal disease patients in Jordanian Hospitals. Saudi Med J 2006; 27: 1928-1930.
References
- 1.American Academy of Sleep Medicine. The International Classification of Sleep Disorders. 2nd ed. Chicago (IL): American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14:82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kawauchi A, Inoue Y, Hashimoto T, Tachibana N, Shirakawa S, Mizutani Y, et al. Restless legs syndrome in hemodialysis patients: health-related quality of life and laboratory data analysis. Clin Nephrol. 2006;66:440–446. doi: 10.5414/cnp66440. [DOI] [PubMed] [Google Scholar]
- 6.Nikić PM, Andrić BR, Stojanović-Stanojević M, Dordević V, Petrović D, Stojimirović BB. Restless legs syndrome prevalence in patients on chronic hemodialysis in central Serbia. Vojnosanit Pregl. 2007;64:129–134. doi: 10.2298/vsp0702129n. [DOI] [PubMed] [Google Scholar]
- 7.Goffredo Filho GS, Gorini CC, Purysko AS, Silva HC, Elias IE. Restless legs syndrome in patients on chronic hemodialysis in a Brazilian city: frequency, biochemical findings and comorbidities. Arq Neuropsiquiatr. 2003;61:723–727. doi: 10.1590/s0004-282x2003000500004. [DOI] [PubMed] [Google Scholar]
- 8.Salman SM. Restless legs syndrome in patients on hemodialysis. Saudi J Kidney Dis Transpl. 2011;22:368–372. [PubMed] [Google Scholar]
- 9.Al-Jahdali HH, Al-Qadhi WA, Khogeer HA, Al-Hejaili FF, Al-Ghamdi SM, Al Sayyari AA. Restless legs syndrome in patients on dialysis. Saudi J Kidney Dis Transpl. 2009;20:378–385. [PubMed] [Google Scholar]
- 10.BaHammam A, Al-shahrani K, Al-zahrani S, Al-shammari A, Al-Amri N, Sharif M. The prevalence of restless legs syndrome in adult Saudis attending primary health care. Gen Hosp Psychiatry. 2011;33:102–106. doi: 10.1016/j.genhosppsych.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Sherman RA, Cody RP, Rogers ME, Solanchick JC. Accuracy of the urea reduction ratio in predicting dialysis delivery. Kidney Int. 1995;47:319–321. doi: 10.1038/ki.1995.41. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20:844–949. doi: 10.1093/sleep/20.10.844. [DOI] [PubMed] [Google Scholar]
- 13.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. International Restless Legs Syndrome Study Group. Sleep Med. 2003;4:121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi N, Chung SA, Gibbs A, Shapiro C. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:39–45. doi: 10.1007/s11325-007-0125-y. [DOI] [PubMed] [Google Scholar]
- 15.Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–1006. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 16.Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 17.La Manna G, Pizza F, Persici E, et al. Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant. 2011;26:1976–1983. doi: 10.1093/ndt/gfq681. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Kwon M, Lim S, Kim S, Lee J, Nam H. Restless Legs Syndrome in Patients on Hemodialysis: Symptom Severity and Risk Factors. J Clin Neurol. 2008;4:153–157. doi: 10.3988/jcn.2008.4.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto M, Miyamoto T, Iwanami M, Suzuki K, Hirata K. Pathophysiology of restless legs syndrome. Brain Nerve. 2009;61:523–532. [PubMed] [Google Scholar]
- 20.Gigli GL, Adorati M, Dolso P, Piani A, Valente M, Brotini S, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5:309–315. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Winter AC, Berger K, Glynn RJ, Buring JE, Gaziano JM, Schürks M, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in men. Am J Med. 2013;126:228–235. doi: 10.1016/j.amjmed.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter AC, Schürks M, Glynn RJ, Buring JE, Gaziano JM, Berger K, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in women. Am J Med. 2013;126:220–227. doi: 10.1016/j.amjmed.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collado-Seidel V, Kohnen R, Samtleben W, Hillebrand GF, Oertel WH, Trenkwalder C. Clinical and biochemical findings in uremic patients with and without restless legs syndrome. Am J Kidney Dis. 1998;31:324–328. doi: 10.1053/ajkd.1998.v31.pm9469505. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009;72:1255–1261. doi: 10.1212/01.wnl.0000345673.35676.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–554. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Choi C, Shin K, Yi H, Park M, Cho N, et al. Prevalence of restless legs syndrome and associated factors in the Korean adult population: the Korean Health and Genome Study. Psychiatry Clin Neurosci. 2005;59:350–353. doi: 10.1111/j.1440-1819.2005.01381.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Nicholl DD, Ahmed SB, et al. The prevalence of restless legs syndrome across the full spectrum of kidney disease. J Clin Sleep Med. 2013;9:455–459. doi: 10.5664/jcsm.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, O’Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10:973–975. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Sloand JA, Shelly MA, Feigin A, Bernstein P, Monk RD. A double-blind, placebo-controlled trial of intravenous iron dextran therapy in patients with ESRD and restless legs syndrome. Am J Kidney Dis. 2004;43:663–670. doi: 10.1053/j.ajkd.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Balaban H, Yıldız ÖK, Çil G, Şentürk İA, Erselcan T, Bolayır E, et al. Serum 25-hydroxyvitamin D levels in restless legs syndrome patients. Sleep Med. 2012;13:953–957. doi: 10.1016/j.sleep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Giannaki CD, Sakkas GK, Karatzaferi C, Hadjigeorgiou GM, Lavdas E, Liakopoulos V, et al. Evidence of increased muscle atrophy and impaired quality of life parameters in patients with uremic restless legs syndrome. PLoS One. 2011;6:e25180. doi: 10.1371/journal.pone.0025180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceglia L. Vitamin D and Its Role in Skeletal Muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–633. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wali S, Shukr A, Boudal A, Alsaiari A, Krayem A. The effect of vitamin D supplements on the severity of restless legs syndrome. Sleep & Breathing. 2014;23 doi: 10.1007/s11325-014-1049-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Takaki J, Nishi T, Nangaku M, Shimoyama H, Inada T, Matsuyama N, et al. Clinical and psychological aspects of restless legs syndrome in uremic patients on hemodialysis. Am J Kidney Dis. 2003;41:833–839. doi: 10.1016/s0272-6386(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui S, Kavanagh D, Traynor J, Mak M, Deighan C, Geddes C. Risk factors for restless legs syndrome in dialysis patients. Nephron. 2005;101:c155–c160. doi: 10.1159/000087073. [DOI] [PubMed] [Google Scholar]
- 37.Sabry AA, Abo-Zenah H, Wafa E, Mahmoud K, El-Dahshan K, Hassan A, et al. Sleep disorders in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21:300–305. [PubMed] [Google Scholar]
- 38.Araujo SM, de Bruin VM, Nepomuceno LA, Maximo ML, Daher Ede F, Correia Ferrer DP, et al. Restless legs syndrome in end-stage renal disease: Clinical characteristics and associated morbidities. Sleep Med. 2010;11:785–790. doi: 10.1016/j.sleep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Tuncel DL, Orhan FO, Sayarlioglu H, Isık IO, Utku U, Dinc A. Restless legs syndrome in hemodialysis patients: association with depression and quality of life. Sleep Breath. 2011;15:311–315. doi: 10.1007/s11325-010-0382-z. [DOI] [PubMed] [Google Scholar]
- 40.Kutner NG, Zhang R, Szczech LA, Bliwise DL. Restless legs syndrome reported by incident hemodialysis patients: is treatment time of day relevant? Nephrology. 2012;17:783–784. doi: 10.1111/j.1440-1797.2012.01629.x. [DOI] [PubMed] [Google Scholar]
- 41.Stefanidis I, Vanias A, Dardiotis E, Giannaki CD, Gourli P, Papadopoulou D, et al. Restless legs syndrome in hemodialysis patients: an epidemiologic survey in Greece. Sleep Med. 2013;14:1381–1386. doi: 10.1016/j.sleep.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Lin CH, Wu VC, Li WY, Sy HN, Wu SL, Chang CC, et al. Restless legs syndrome in end-stage renal disease: a multicenter study in Taiwan. Eur J Neurol. 2013;20:1025–1031. doi: 10.1111/ene.12095. [DOI] [PubMed] [Google Scholar]