Abstract
Objectives
Epidemiological evidence linking diet, one of the most important modifiable lifestyle factors, and risk of Alzheimer’s disease (AD) is rapidly increasing. However, there is little or no evidence for a direct association between dietary nutrients and brain biomarkers of AD. This study identifies nutrient patterns associated with major brain AD biomarkers in a cohort of clinically and cognitively normal (NL) individuals at risk for AD.
Design
Cross-sectional study.
Setting
Manhattan (broader area).
Participants
Fifty-two NL individuals (age 54+12 y, 70% women, Clinical Dementia Rating=0, MMSE>27, neuropsychological test performance within norms by age and education) with complete dietary information and cross-sectional, 3D T1-weighted Magnetic Resonance Imaging (MRI; gray matter volumes, GMV, a marker of brain atrophy), 11C-Pittsburgh compound-B (PiB; a marker of fibrillar amyloid-β, Aβ) and 18F-fluorodeoxyglucose (FDG; a marker of glucose metabolism, METglc) Positron Emission Tomography (PET) scans were examined.
Measurements
Dietary intake of 35 nutrients associated with cognitive function and AD was assessed using the Harvard/Willet Food Frequency Questionnaire. Principal component analysis was used to generate nutrient patterns (NP) from the full nutrient panel. Statistical parametric mapping and voxel based morphometry were used to assess the associations of the identified NPs with AD biomarkers.
Results
None of the participants were diabetics, smokers, or met criteria for obesity. Five NPs were identified: NP1 was characterized by most B-vitamins and several minerals [VitB&Minerals]; NP2 by monounsaturated and polyunsaturated fats, including ω-3 and ω-6 PUFA, and vitamin E [VitE&PUFA]; NP3 by vitamin A, vitamin C, carotenoids and dietary fibers [Anti-oxidants&Fibers]; NP4 by vitamin B12, vitamin D and zinc [VitB12&D]; NP5 by saturated, trans-saturated fats, cholesterol and sodium [Fats]. Voxel-based analysis showed that NP4 scores [VitB12&D] were positively associated with METglc and GMV, and negatively associated with PiB retention in AD-vulnerable regions (p<0.001). In addition, both METglc and GMV were positively associated with NP2 scores [VitE&PUFA], and negatively associated with NP5 scores [Fats] (p<0.001), and METglc was positively associated with higher NP3 scores [Anti-oxidants&Fibers] (p<0.001). Adjusting for age, gender, ethnicity, education, caloric intake, BMI, alcohol consumption, family history and Apolipoprotein E (APOE) status did not attenuate these relationships. The identified ‘AD-protective’ nutrient combination was associated with higher intake of fresh fruit and vegetables, whole grains, fish and low-fat dairies, and lower intake of sweets, fried potatoes, high-fat dairies, processed meat and butter.
Conclusion
Specific dietary NPs are associated with brain biomarkers of AD in NL individuals, suggesting that dietary interventions may play a role in the prevention of AD by modulating AD-risk through its effects on Aβ and associated neuronal impairment.
Keywords: Alzheimer’s disease, nutrition, aging, Positron Emission Tomography (PET), Magnetic Resonance Imaging (MRI)
Introduction
There is increasing evidence to suggest that diet, one of the most important modifiable lifestyle factors, may play a role in preventing or delaying cognitive decline and Alzheimer’s disease (AD), a major public health problem (1–7). AD is the most common cause of dementia and is associated with presence of amyloid-beta (Aβ) plaques, neurofibrillary tangles and neuronal loss. As pharmacological treatments for AD are limited, there is a growing interest in understanding how diet could mitigate AD risk and progression (8, 9). Despite studies showing protective effects of several nutrients against AD, the overall picture remains equivocal (10). These studies would greatly benefit from biomarkers for early AD pathology and associated neuronal injury, which are needed to assess the impact of diet on brain health and to monitor treatment efficacy (10), especially during the recently conceptualized preclinical period of AD (11). In vivo biomarkers are needed to clarify how nutrition promotes healthy brain aging (10), and can therefore be protective against AD, which is critical prior to implementing dietary recommendations for prevention and treatment.
There are very few studies that examined the relationships between dietary nutrients and brain biomarkers of AD in cognitively normal (NL) individuals. A few Magnetic Resonance Imaging (MRI) studies investigated the relationship between ω3 polyunsaturated fatty acids (PUFA) and brain volumes in non-demented elderly, and showed a correlation between higher baseline ω3-PUFA levels and lower atrophy rates over time (12–14). However, according to current hypothetical models of AD progression, structural MRI changes are secondary to Aβ deposition and neuronal hypometabolism (11), and previous studies included only individuals of age >65 y. To our knowledge, there are no published studies that examined the the associations of dietary nutrients with brain Abeta and metabolic activity in NL individuals.
The goal of this study was to examine the relationships between dietary nutrient patterns (NPs) and three major AD-biomarkers: brain Aβ load (i.e., a hallmark of AD pathology) assessed using 11C-Pittsburgh Compound-B (PiB) Positron Emission Tomography (PET), glucose metabolism (METglc, i.e. a proxy for neuronal activity) assessed using 18F-fluorodeoxyglucose (FDG) PET, and gray matter volumes (GMV, a marker of brain atrophy) on MRI in a cohort of young to late middle aged NL individuals. Using these imaging techniques, several studies have shown preclinical biomarker abnormalities in non-demented individuals several years, if not decades, prior to AD symptoms (11).
Given the interactive nature of nutrient action and metabolism, in this study we used principal component analysis (PCA) to generate NPs from a panel of 35 nutrients which have been related to AD or cognitive function or which are known to interact with those AD-related nutrients. NPs are advantageous as they capture the interactive effect of nutrients in combination (15–17). The present multi-modality brain imaging study uses voxel-based analysis techniques such as Statistical Parametric Mapping and Voxel-Based Morphometry to simultaneously examine Aβ deposition, METglc and GMV to define which NPs are protective against AD (as reflected in lower brain Aβ, higher metabolic activity and larger GMV among NL individuals, controlling for AD-risk factors such as age, gender, education, ethnicity, BMI, alcohol consumption, family history of AD, and Apolipoprotein E (APOE) genotype.
Methods
Participants
Among a larger pool of clinically and cognitively normal (NL) individuals participating in longitudinal brain imaging studies at New York University (NYU) Langone School of Medicine, this study focused a sub-set of 65 NL participants who were invited to participate in a lifestyle survey between 2013–2014. This study examined 52 participants who completed all clinical, MRI, PiB- and FDG-PET exams and dietary questionnaires within 6 months of each other. Of the remaining 13 subjects, 9 did not receive either the PiB or the FDG scan, and 4 returned only partially completed dietary questionnaires and were excluded from this examination. Subjects were derived from multiple community sources, including individuals interested in research participation, family members and caregivers of impaired patients. Informed consent was obtained from all subjects for participation in this NYU institutional review board-approved study.
Individuals with medical conditions or history of conditions that may affect brain structure or function, i.e. stroke, diabetes, head trauma, any neurodegenerative diseases, depression, hydrocephalus, intracranial mass, and infarcts on MRI, and those taking psychoactive medications were excluded. Subjects were 25–72 y of age, with education>12 y, Clinical Dementia Rating (CDR)=0, Global Deterioration Scale (GDS)<2, Mini Mental State Examination (MMSE)>28, Hamilton depression scale<16, Modified Hachinski Ischemia Scale<4 and normal cognitive test performance for age and education (18). A family history of late-onset AD that included at least one 1st degree relative whose AD onset was after age 60 was elicited using standardized questionnaires (18–20). APOE genotypes were determined using Polymerase Chain Reaction (PCR) using standardized protocols (21).
Dietary assessments
Dietary data regarding average food consumption over the prior year were obtained using the 116-item version of Harvard/Willett’s semi-quantitative food frequency questionnaire (SFFQ) (22, 23). Trained interviewers administered the SFFQ in English. The SFFQ has been validated for determination of nutrient intake in the elderly and young adults (22, 23) and against plasma measurements (24–26). The validity (using two 7-day food records) and reliability (using two 3-month frequency assessments) of various components of the SSFQ was replicated by several studies (3, 4, 27, 28). The food items were categorized into 30 food groups based on similarities in food and nutrient composition, and intake (g/day) of each food group was calculated by summing the intakes of member food items. The daily intake of nutrients from food sources was computed by multiplying the consumption frequency of each portion of every food by the nutrient content of the specified portion (22). The daily total caloric intake (kilocalories) was included as a confound.
A panel of 35 nutrients that have been associated with cognitive function and AD was examined, including fats: monounsaturated fatty acid (MUFA), ω-3 polyunsaturated fatty acid (PUFA), ω-6 PUFA, other PUFA, saturated fatty acid (SFA), trans-saturated fats and cholesterol[6, 29–34]; vitamins and precursors: α- and β-carotene, β-cryptoxanthin, β- γ- and δ-tocopherol, vitamin A, B vitamins including B1, B2, B3, B6, B9 (folate) and B12, vitamin C, vitamin D, vitamin E, lycopene, lutein and zeaxanthin (7, 27, 35–41); minerals: calcium, copper, iron, magnesium, phosphorus, potassium, selenium, and zinc (8, 42); and dietary fibers (43). As moderate alcohol drinking may be protective against dementia[28], alcohol intake (g/day) was also calculated.
Brain imaging
All subjects received volumetric 1.5 T MRI (124 slice T1-weighted Fast-Gradient-Echo, 1.2 mm sections, no interslice gaps), PiB- and FDG-PET scans following standardized procedures (18–20, 44, 45). For PET, subjects were positioned in the scanner 60 min after injection of 15 mCi of 11C-PiB, and scanned for 30 min in 3D-mode on an LS Discovery or BioGraph PET/CT scanner. The FDG scan was performed 30 min after completion of the PiB scan or on a separate day. After an overnight fast, subjects were injected with 5 mCi of 18F-FDG, positioned in the scanner 35 min after injection, and scanned for 20 min. All images were corrected for photon attenuation, scatter, and radioactive decay and smoothed for uniform resolution (46).
Image analysis was done blind to clinical data. For each subject, summed PET images corresponding to 40–60 min of FDG data and 60–90 min of PiB data were coregistered to MRI using the Normalized Mutual Information (NMI) routine of Statistical Parametric Mapping (SPM8) (47). Parametric standardized uptake value ratio (SUVR) images were generated by normalizing PiB uptake by cerebellar grey matter uptake (48) and FDG by pons activity (49). MRIs were segmented into grey (GM), white matter (WM) and cerebrospinal fluid (CSF) and normalized to Montreal Neurological Institute (MNI) space by high-dimensional warping (DARTEL) using VBM8 (47). MRI-coregistered PET scans were spatially normalized using subject-specific transformation matrixes obtained from MRI, and smoothed with a 10mm FWHM filter.
MRIs were examined using voxel-based morphometry (VBM) (47, 50). A custom template was created using MRI from all subjects by normalizing and segmenting the MRIs using the unified segmentation model with the MNI template and tissue probability maps (TPMs), and averaging the normalized subject TPMs. Individual scans were then processed using the custom TPMs. Jacobian modulation was applied to restore absolute GM volumes (GMV) in the GM images, which were smoothed with an 8-mm FWHM kernel. Total GM, WM, CSF and intracranial volumes were calculated.
Statistical Analysis
SPSS v.21 (SPPS Inc., 2013) and SPM8 were used for data analysis. Clinical and demographical measures were examined using descriptive statistics.
Nutrient pattern construction
Nutrient patterns (NPs) were derived from the pre-defined panel of 35 nutrients using multivariate analysis (principal component analysis, PCA). The PCA is used to derive factor scores (i.e. patterns), which are linear combinations of all of the variables (i.e., nutrients), and with each factor score being weighted towards groups of variables with the highest association with each other. This process generates principal components, whereby principal components can be characterized into patterns. There are no pre-defined hypotheses as to which nutrient variables will be included in the patterns as the classification of variables into patterns is based on statistical associations between variables. Furthermore, the factor scores are derived to be totally independent from each other (the correlation between them is 0), so that, by using factor scores, one not only reduces the number of variables but also reduces any potential problems with multicolinearity in the model. With this procedure, the factor scores do not interfere with each other, yielding more confidence in the accuracy of the p-values
Five distinct nutrient patterns (NPs) were extracted from the original set of nutrients via PCA (rotation method: varimax with kaiser normalization). An eigenvalue >1.0 was set a priori to determine the NPs to carry into hypothesis testing. Each participant receives a standardized NP score for each pattern that corresponds to a linear combination of the nutrients that load heavily within each pattern.
Primary models
Linear regressions were used to test for associations between demographical and neuropsychological measures (dependent variables) and each NP (independent variables) at p<0.05. The General Linear Model (GLM, i.e. multiple regressions) with post-hoc linear t-contrasts implemented in SPM8 was used to test for associations between FDG, PiB and MRI scans (dependent variables), each NP (independent variables), and confounds. Positive and negative associations between biomarkers and NPs were examined. Age and total caloric intake were included as covariates in all analysis. Gender, education, ethnicity, body mass index (BMI), alcohol consumption, APOE and family history were then examined as covariates in separate models to avoid over-fitting. Education and BMI were modeled as continuous variables. Gender (male vs. female), FH (positive vs. negative, FH+ vs. FH−), and APOE status (APOE4 carriers vs. non-carriers, APOE4+ vs. APOE4−) were examined as dichotomous variables. Ethnic group was based on self-report using the format of the 1990 census. Ethnicity was used as a dummy variable (White/non-Hispanic vs. other ethnic groups). Alcohol intake was used as a dichotomous variable (mild-moderate (0–30 g/day) vs. no (0 g/day) or more than moderate (>30 g/day) consumption) (16). No proportional scaling was performed as PET scans were normalized to reference regions. GMV images were corrected for total intracranial volume.
As we had specific a priori hypotheses on which brain regions would show possible biomarker effects, results were examined at p<0.001, uncorrected (cluster extent >20 voxels, i.e., >2 times the FWHM) (47), in the search volume defined by a masking image created from a set of predefined AD-vulnerable regions, which included: posterior cingulate cortex/precuneus, inferior and superior parietal lobule, lateral and medial temporal cortex (including hippocampus, amygdala and parahippocampal gyrus), medial and prefrontal cortex, striatum (18–20, 44, 45). Anatomical location of brain regions showing significant effects was described using Talairach and Tournoux coordinates, after conversion from MNI space.
Food sources
The food sources of the NPs were examined by testing for correlations between foods and NPs using Pearson’s coefficients of determination. Results were considered significant at p<0.05 (2-sided tests).
Results
Subjects’ characteristics are found in Table 1. None of the participants were diabetics, smokers, or met criteria for obesity as defined by a Body-Mass index (BMI)>30 kg/m2.
Table 1.
Demographic and clinical characteristics
| N | 52 |
| Age, y, mean (SD) | 54(11) |
| Gender, female/male (n, % female) | 37/15 (71%) |
| Education, y, mean (SD) | 16(2) |
| Family history of LOAD, % positive | 79% |
| APOE ε4 status, % positive | 47% |
| Ethnicity (%) | |
| White | 83% |
| Black | 8% |
| Hispanic | 6% |
| Other | 3% |
| Body Mass Index [unitless], mean (SD) | 25(4) |
| Hip to waist ratio [unitless], mean (SD) | 1.3(0.6) |
| Blood pressure (mm/Hg), mean (SD) | |
| Systolic | 119(14) |
| Diastolic | 72(8) |
| Glucose (mg/dl) | 78(12) |
| Cholesterol (mg/dl) | 197(37) |
| HDL (mg/dl) | 62(18) |
| LDL (mg/dl) | 117(32) |
| Triglycerides (mg/dl) | 89(39) |
| Homocysteine (micromol/l) | 10(3) |
| Neuropsychological tests, mean (SD) | |
| Mini Mental State Exam | 29(1) |
| Digit symbol substitution | 63(10) |
| Paired associates delayed recall | 7(2) |
| Paragraph delayed recall | 10(3) |
| Designs | 8(2) |
| Object naming | 55(12) |
| Digit span, forward | 7(13) |
| Digit span, backward | 5(1) |
| WAIS-vocabulary | 65(13) |
Nutrient patterns
Table 2 displays the composition of 5 extracted NPs (all loading coefficients >0.55), which accounted for 86% of the total variance in the nutrient panel. NP1 was characterized by B-vitamins (B1, B2, B3, B6 and B9) and several minerals (i.e., calcium, iron, magnesium, phosphorus, potassium and selenium) [VitB&Minerals]; NP2 by monounsaturated and polyunsaturated fats, including ω-3 and ω-6 PUFA, and vitamin E (mostly β- and γ-tocopherol) [VitE&PUFA]; NP3 by vitamin A, carotenoids (α- and β-carotene, β-cryptoxanthin, lutein and zeaxanthin), vitamin C, and dietary fibers [Anti-oxidants&Fibers]; NP4 by vitamin B12, vitamin D and zinc [VitB12&D]; NP5 by saturated, trans-saturated fats, cholesterol and sodium [Fats].
Table 2.
Nutrient pattern construction: Pattern structure and variance explained
| Nutrients | Nutrient patterns (NP) a | ||||
|---|---|---|---|---|---|
| NP1 | NP2 | NP3 | NP4 | NP5 | |
| Calcium | 0.77 b | 0.26 | |||
| Folic acid (B9) | 0.79 b | 0.33 | 0.44 | ||
| Iron | 0.77 b | 0.40 | 0.35 | ||
| Magnesium | 0.67 b | 0.54 | 0.33 | ||
| Niacin (B3) | 0.81 b | 0.39 | |||
| Phosphorus | 0.80 b | 0.47 | |||
| Potassium | 0.60 b | 0.46 | 0.49 | ||
| Pyridoxal phosphate (B6) | 0.72 b | 0.40 | 0.42 | ||
| Riboflavin (B2) | 0.84 b | ||||
| Selenium | 0.76 b | 0.45 | |||
| Thiamin (B1) | 0.88 b | 0.31 | |||
| Beta Tocopherol | 0.57 | 0.73 b | |||
| Copper | 0.38 | 0.63 b | 0.39 | 0.34 | −0.38 |
| Delta Tocopherol | 0.49 | 0.60 b | |||
| Gamma Tocopherol | 0.38 | 0.87 b | |||
| Monounsaturated fats | 0.38 | 0.82 b | |||
| Omega3 PUFA | 0.83 b | 0.36 | |||
| Omega6 PUFA | 0.33 | 0.92 b | |||
| Polyunsaturated fats | 0.32 | 0.92 b | |||
| Vitamin E | 0.36 | 0.84 b | 0.28 | ||
| Alpha carotene | 0.81 b | ||||
| Beta carotene | 0.85 b | ||||
| Beta Cryptoxanthin | 0.59 b | ||||
| Dietary Fibers | 0.55 | 0.44 | 0.60 b | ||
| Lutein and Zeaxanthin | 0.80b | ||||
| Vitamin A | 0.32 | 0.79 b | |||
| Vitamin C | 0.45 | 0.74 b | |||
| Calciferol (Vitamin D3) | 0.35 | 0.86 b | |||
| Cobalamin (B12) | 0.95 b | ||||
| Lycopene | −0.23 | 0.33 | 0.40 b | 0.30 | |
| Zinc | 0.45 | 0.69 b | −0.32 | ||
| Cholesterol | −0.24 | 0.31 | 0.75 b | ||
| Saturated fats | 0.49 | 0.71 b | |||
| Sodium | 0.55 | 0.72 b | |||
| Trans-saturated fats | 0.43 | 0.31 | 0.56 b | ||
| % variance explained by each component | 30 | 26 | 16 | 10 | 4 |
| Cumulative % of variance explained with each extraction | 30 | 56 | 72 | 82 | 86 |
Loading coefficients represent the actual correlations between each variable and the identified factor scores from PCA (i.e., patterns). Nutrient pattern interpretation is based on the strongest loading coefficients within each pattern. Each standardized summary score is a linear combination of the nutrients that mostly represent the respective pattern. Coefficients −0.20> and <0.2 were excluded to simplify the table and emphasize dominant nutrients within each pattern. As such, blank spaces reflect non-significant to weak associations;
Considered the dominant nutrients in the pattern. For example, a high pattern NP1 score is interpreted as high intake of B-vitamins (B1, B2, B3, B6, B9) and most minerals (i.e., calcium, iron, magnesium, phosphorus, potassium and selenium). Nutrients within in each pattern are listed in alphabetical order.
Nutrient patterns and clinical-demographic characteristics
As shown in eTable 1, age, gender, education, ethnicity, FH, APOE4 carrier status and BMI were not associated with any NP. Higher NP2 scores [VitE&PUFA] were associated with a more favorable HDL/LDL and hip-to-waist ratio (p<0.05). Systolic blood pressure was negatively associated with NP1 [VitB&Minerals] and positively associated with NP5 scores [Fats] (p<0.05). NP5 scores [Fats] were also positively associated with plasma triglycerides (p<0.05) and showed a trend for an association with BMI (p=0.08) and homocysteine levels (p=0.09). There were no associations between NPs and neuropsychological test scores.
Nutrient patterns and AD-biomarkers
FDG-PET
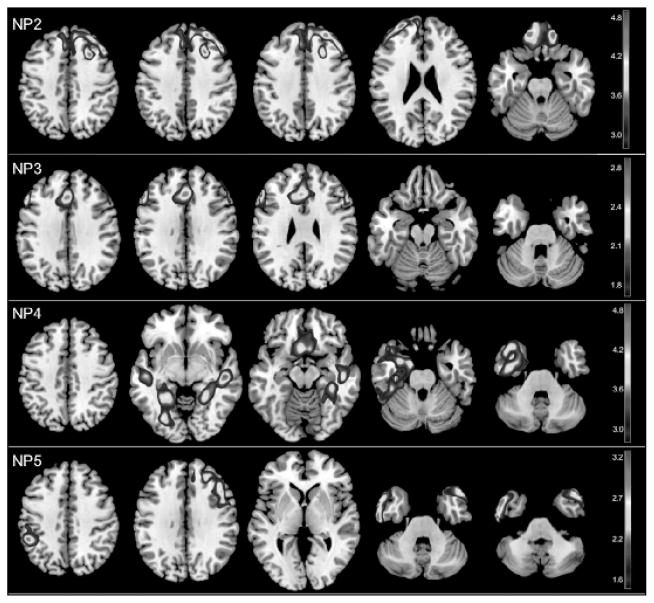
Results from voxel-based analysis are reported in terms of statistical parametric maps (SPMs), e.g. brain areas showing significant associations between NPs and brain biomarkers. As shown in Table 3, SPMs are defined in terms of cluster extent (i.e., number of voxels in the cluster), cluster coordinates in the Talairach space (x, y, z), Z and P values for each set of coordinates, anatomical description of the brain regions included in each cluster, and corresponding Brodmann areas. Correcting for age and total caloric intake, NP2 scores [VitE&PUFA] were positively associated with METglc in medial, inferior and lateral frontal cortex, bilaterally (p<0.001, Table 3). NP3 scores [Anti-oxidants&Fibers] were positively associated with METglc in middle frontal and cingulate cortex of the left hemisphere (p<0.001, Table 3). NP4 scores [VitB12&D] were positively associated with METglc in several temporal regions, including superior and medial temporal areas, bilaterally (p<0.001, Table 3). NP5 scores [Fats] were negatively associated with METglc in middle and inferior temporal cortex, bilaterally, right frontal cortex, and left parietal cortex (p<0.001, Table 3). Adjustment for gender, education, ethnical group, BMI, APOE, family history, and alcohol consumption did not attenuate these relationships (Figure 1). There were no other brain regions showing positive or negative correlations with the remaining NPs.
Table 3.
Brain regions showing significant relationships between NPs and brain glucose metabolism on FDG-PET
| Cluster extent | x* | y | z | Z † | Anatomical region | Brodmann area |
|---|---|---|---|---|---|---|
| Positive associations between FDG uptake and NP2 | ||||||
| 71 | 14 | 38 | −19 | 3.61 | Right Cerebrum, Frontal Lobe, Inferior Frontal Gyrus | 11 |
| 54 | −14 | 40 | −19 | 3.51 | Left Cerebrum, Frontal Lobe, Middle Frontal Gyrus | 11 |
| 40 | −25 | 48 | 30 | 3.48 | Left Cerebrum, Frontal Lobe, Superior Frontal Gyrus | 9 |
| 31 | 24 | 37 | 41 | 3.35 | Right Cerebrum, Frontal Lobe, Middle Frontal Gyrus | 8 |
| 39 | 24 | 14 | 45 | 3.32 | Right Cerebrum, Frontal Lobe, Middle Frontal Gyrus | 8 |
| Positive associations between FDG uptake and NP3 | ||||||
| 40 | −54 | 27 | 28 | 2.71 | Left Cerebrum, Frontal Lobe, Middle Frontal Gyrus | 46 |
| 47 | −2 | 26 | 31 | 2.57 | Left Cerebrum, Limbic Lobe, Cingulate Gyrus | 32 |
| Positive associations between FDG uptake and NP4 | ||||||
| 277 | 44 | −21 | −4 | 4.08 | Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 22 |
| 259 | −15 | −3 | −17 | 3.64 | Left Cerebrum, Limbic Lobe, Parahippocampal Gyrus | 34 |
| −28 | −1 | −15 | 3.32 | Left Cerebrum, Limbic Lobe, Parahippocampal Gyrus, Amygdala | ||
| 278 | −36 | 14 | −22 | 3.55 | Left Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 38 |
| 116 | −39 | −5 | −32 | 3.42 | Left Cerebrum, Temporal Lobe, Middle Temporal Gyrus | 21 |
| −34 | −8 | −29 | 3.27 | Left Cerebrum, Limbic Lobe, Uncus | 20 | |
| 141 | −6 | 13 | −10 | 3.41 | Left Cerebrum, Frontal Lobe, Medial frontal Gyrus | 25 |
| 59 | 23 | −43 | −5 | 3.39 | Right Cerebrum, Limbic Lobe, Parahippocampal Gyrus | 36 |
| 68 | −29 | −44 | −8 | 3.32 | Left Cerebrum, Limbic Lobe, Parahippocampal Gyrus | 37 |
| Negative associations between FDG uptake and NP5 | ||||||
| 333 | −47 | 8 | −35 | 3.52 | Left Cerebrum, Temporal Lobe, Middle Temporal Gyrus | 21 |
| −56 | −10 | −30 | 3.21 | Left Cerebrum, Temporal Lobe, Inferior Temporal Gyrus | 20 | |
| 235 | 36 | 20 | 37 | 3.33 | Right Cerebrum, Frontal Lobe, Middle frontal Gyrus | 9 |
| 43 | −48 | −44 | 39 | 2.87 | Left Cerebrum, Parietal Lobe, Inferior Lobule | 40 |
| 44 | 42 | 13 | −33 | 2.72 | Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 38 |
Coordinates (x, y, z) from Talairach and Tournoux.
Z values at the peak of maximum significance at p<0.001, correcting for age and total caloric intake. Only contrasts yielding significant results are reported.
Abbreviations: NP1 = Vit B & minerals, NP2 = PUFA & Vit E, NP3 = Anti-oxidants & Fibers, NP4 = Vit B12 & D and zinc, NP5 = Fats and sodium
Figure 1. Statistical parametric maps (SPMs) showing associations between nutrient patterns (NPs) and brain glucose metabolism (METglc) on FDG-PET.
Brain regions showing positive associations between METglc and (NP2) intake of vitamin E, monounsaturated and polyunsaturated fats (ω-3 and ω-6 PUFA); (NP3) intake vitamin A, vitamin C, carotenoids and dietary fibers; (NP4) intake of vitamin B12, vitamin D and zinc; brain regions showing negative associations between METglc and (NP5) intake of saturated, trans-saturated fats and sodium. SPMs are represented on a color-coded scale at p<0.001, and displayed onto a standardized MRI. Results are adjusted for age, gender, education, BMI, APOE, family history and total caloric intake.
MRI
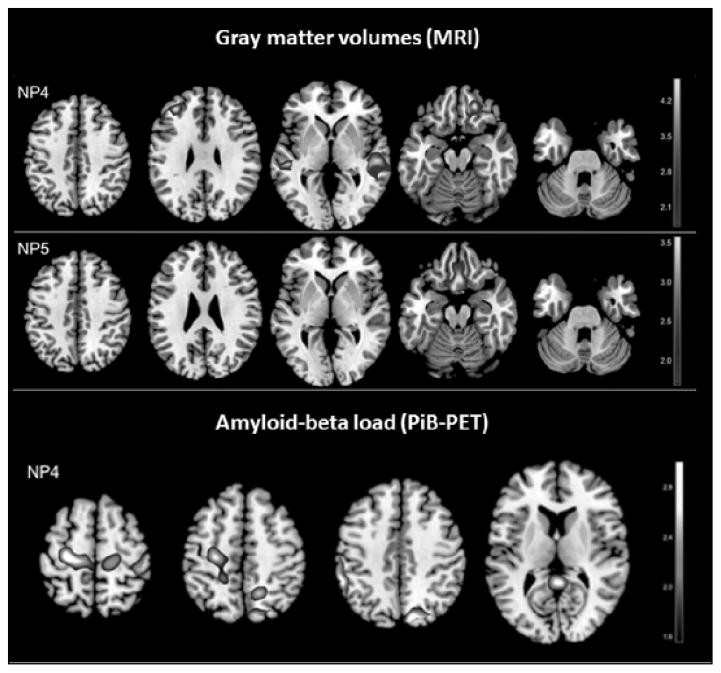
NP4 scores [VitB12&D] were positively associated with GMV in temporal and frontal cortex, mostly of the right hemisphere (p<0.001, Table 4). NP5 scores [Fats] were negatively associated with GMV in frontal cortex (p<0.001, Table 4). Adjustment for gender, education, ethnical group, BMI, APOE, family history, and alcohol consumption did not attenuate these relationships (Figure 1). There were no other brain regions showing positive or negative correlations with the remaining NPs. Overall, with regard to the number of regions affected and magnitude of impairment in those regions, the association between NPs and biomarkers were less robust with MRI than with FDG measures (Figure 1).
Table 4.
Brain regions showing significant relationships between NPs and brain gray matter volumes (GMV) on MRI
| Cluster extent | x* | y | z | Z † | Anatomical region | Brodmann area |
|---|---|---|---|---|---|---|
| Positive associations between GMV and NP1 | ||||||
| 62 | −28 | 45 | 17 | 4.16 | Left Cerebrum, Frontal Lobe, Superior Frontal Gyrus | 10 |
| Positive associations between GMV and NP4 | ||||||
| 629 | 50 | −20 | −6 | 4.48 | Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 22 |
| 52 | −25 | 6 | 3.95 | Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 41 | |
| 44 | −25 | 5 | 3.75 | Right Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 22 | |
| 51 | −48 | −25 | 1 | 4.17 | Left Cerebrum, Temporal Lobe, Superior Temporal Gyrus | 21 |
| 81 | 19 | 40 | −17 | 4.15 | Right Cerebrum, Frontal Lobe, Middle Frontal Gyrus | 11 |
| 79 | −34 | 32 | 32 | 4.09 | Left Cerebrum, Frontal Lobe, Middle Frontal Gyrus | 9 |
| Negative associations between GMV and NP5 | ||||||
| 123 | −5 | 10 | −16 | 3.64 | Left Cerebrum, Frontal Lobe, Medial Frontal Gyrus | 25 |
| 0 | 22 | −12 | 3.59 | Left Cerebrum, Limbic Lobe, Anterior Cingulate, Gray Matter | 32 | |
Coordinates (x, y, z) from Talairach and Tournoux.
Z values at the peak of maximum significance at p<0.001, correcting for age and total caloric intake. Only contrasts yielding significant results are reported.
Abbreviations: see legend to Table 3
PiB-PET
Only NP4 scores [VitB12&D] were negatively associated with PiB retention in parietal, frontal and PCC regions (p<0.001, Table 5). Adjustment for gender, education, ethnical group, BMI, APOE, family history, and alcohol consumption did not attenuate these relationships (Figure 2). There were no other brain regions showing positive or negative correlations with the remaining NPs.
Table 5.
Brain regions showing significant relationships between NPs and brain Aβ load on PiB-PET
| Cluster extent | x* | y | z | Z † | Anatomical region | Brodmann area |
|---|---|---|---|---|---|---|
| Negative associations between PiB retention and NP4 | ||||||
| 307 | −14 | −12 | −42 | 2.90 | Left Cerebrum, Limbic Lobe, Cingulate Gyrus | 24 |
| 768 | −29 | 25 | 50 | 2.79 | Left Cerebrum, Frontal Lobe, Superior Frontal Gyrus | 8 |
| 279 | 0 | −43 | 8 | 2.78 | Left Cerebrum, Limbic Lobe, Posterior Cingulate Gyrus | 29 |
| −23 | −10 | 50 | 2.72 | Left Cerebrum, Frontal Lobe, Superior Frontal Gyrus | 8 | |
| 134 | 7 | −76 | 43 | 2.72 | Right Cerebrum, Parietal Lobe, Precuneus | 7 |
| 30 | −53 | −45 | 40 | 2.66 | Left Cerebrum, Parietal Lobe, Inferior Parietal Lobule | 40 |
| 77 | 12 | −58 | 46 | 2.64 | Right Cerebrum, Parietal Lobe, Precuneus | 7 |
| 85 | 37 | −37 | 54 | 2.63 | Right Cerebrum, Parietal Lobe, Inferior Parietal Lobule | 40 |
Coordinates (x, y, z) from Talairach and Tournoux.
Z values at the peak of maximum significance at p<0.001, correcting for age and total caloric intake. Only contrasts yielding significant results are reported.
Abbreviations: see legend to Table 3
Figure 2. Statistical parametric maps (SPMs) showing significant regional associations between nutrient patterns (NPs), gray matter volumes (GMV) on MRI, and reduced brain amyloid load on PiB-PET.
Top panel: brain regions showing positive associations between GMV and (NP4) intake of vitamin B12, vitamin D and zinc; brain regions showing negative associations between GMV and (NP5) intake of saturated, trans-saturated fats and sodium. Bottom panel: brain regions showing negative associations between PiB retention and (NP4) higher intake of vitamin B12, vitamin D and zinc. SPMs are represented on different color-coded scales at p<0.001, and displayed onto a standardized MRI. Results are adjusted for age, gender, education, BMI, APOE, family history and total caloric intake.
Food sources
Correlations between NPs and food groups showed that NP1 [VitB&Minerals] was mainly from low fat dairies, whole grains and cereals, fresh fruit, nuts, green leafy and cruciferous vegetables with correlation coefficients (r) of 0.47, 0.40, 0.37, 0.33, 0.29 and 0.27 respectively (p’s<0.05); NP2 [VitE&PUFA] was from vegetable oil, nuts, fish, green leafy and other vegetables, and fresh fruit (r = 0.61, 0.57, 0.36, 0.32 and 0.32; p’s<0.05); NP3 [Anti-oxidants&Fibers] was from fresh fruit, green leafy cruciferous and dark leafy vegetables, legumes, and fruit juice (r = 0.63, 0.63, 0.62, 0.58, 0.47 and 0.34, p’s<0.02); NP4 [VitB12&D] was from fish, eggs, tomato, and low-fat dairies (r = 0.63, 0.44, 0.30 and 0.28, p<0.05); and NP5 [Fats] was from sweets, fried potatoes, high-fat dairies, processed meat and butter (r = 0.45, 0.32, 0.30, 0.28 and 0.27, p’s<0.05).
Discussion
Present results show an association between nutrient patterns, as derived from principal component analysis, and three major brain AD-biomarkers in NL individuals. Specifically, the pattern characterized by vitamin B12, vitamin D and zinc (NP4) was significantly associated with all biomarkers, so that the higher intake of these nutrients, the lower Aβ load, and the higher METglc and GMV in AD-vulnerable regions. Additionally, metabolic activity and GMV were negatively associated with intake of saturated, trans-saturated fats, cholesterol and sodium (NP5). Finally, METglc was positively associated with intake of vitamin E, mono- and polyunsaturated fats such as ω3 and ω6-PUFA (NP2), and with carotenoids, vitamin A, vitamin C and dietary fibers (NP3). Results were independent of AD-risk factors such as age, gender, education, ethnicity, FH and APOE status, as well as BMI and alcohol consumption. The identified ‘AD-protective’ patterns were linked to higher intake of vegetables, fruit, whole grains, fish, low fat dairies and nuts, and lower intake of sweets, friend potatoes, processed meat, high-fat dairies and butter, indicating that such dietary pattern might be particularly useful to support brain aging.
Most studies on nutrition and risk for AD investigated nutrients in their isolated forms, although the human diet is strongly influenced by synergy or antagonism among components (17). In this study, we used PCA to generate NPs from a panel of nutrients that have been related to AD or cognitive function, and their combinations. The nutrient pattern associated with all biomarkers (NP4) included neuro-protective vitamin B12 (39, 51), vitamin D (40, 41) and zinc, one of the most important transitional metals for human metabolism that is involved in Aβ adhesiveness and amyloid precursor protein synthesis (52). A recent randomized controlled trial showed that treatment with a combination of B vitamins, but especially B12, slowed gray matter volume loss in AD-regions of amnestic MCI patients (53). Our findings suggest that vitamin B12 may have beneficial effects on AD-biomarkers also during the normal stages of cognition.
Higher intake of fats (NP5) was associated with reduced METglc and GMV, consistent with the notion that “bad fats” may have negative effects on cognitive function (31, 32). Additionally, METglc was positively associated with higher intake of vitamin E, a strong anti-oxidant (35, 38), and fatty acids such as ω-3 and ω-6PUFA which are known for their neuroprotective properties through anti-inflammatory, antioxidant, and energy metabolism pathways (29). These results are consistent with previous MRI studies of NL elderly that showed a correlation between higher ω3-PUFA levels and lower atrophy rates of the medial temporal lobes over time (12–14). Together with previous studies, our data from a substantially younger population (mean age 54 y vs. 72–78 y (12–14)) free of possible confounding comorbidities such as depression or diabetes (12–14), indicate that AD biomarkers are affected by dietary food intake already at middle age, further supporting an early role for nutrition in the prevention of AD. METglc was also positively associated with intake of vitamin A, C, several carotenes, and dietary fibers. These nutrients are known to have beneficial effects via their strong antioxidant and Aβ anti-oligomerization effects (7, 35, 37, 38, 54), and dietary fibers help regulate glucose levels (43).
A community-based study of NL elderly reported an association between a diet rich in ω-3PUFA and lower plasma Aβ levels (30). Our results showed an association between higher PUFA/vitamin E scores (NP3), higher metabolic activity and GMV, as well as with a more favorable HDL/LDL ratio, though there were no significant associations with PiB retention. Differences may depend on measurement of central nervous system vs peripheral (plasma) Aβ measures, as well as on age effects, as our NL cohort was substantially younger than in the previous report (mean age 75 y (30)) and fibrillar Aβ accumulation increases in an age-dependent fashion (55). Nonetheless, it is possible that FDG and MRI effects reflect toxic effects of Aβ oligomers, which are known to occur early in AD (55) and which are not currently visible on PET imaging. It is also possible that the combination of PUFA with other nutrients into a single pattern might overshadow the specific impact of PUFA on PiB retention. To our knowledge there are no previous studies that examined the correlation between ω-3PUFA and PiB-PET in NL individuals. While the present study aimed at identifying patterns of nutrients rather than individual nutrients, other studies are warranted to test for associations between brain Aβ and specific nutrients such as ω-3PUFA.
NP1 [VitB&Minerals] was the only pattern that did not show significant associations with AD-biomarkers. This pattern included several B vitamins and minerals which have been related to better cognitive functioning or lower AD risk in the elderly (27, 36, 39, 42, 51). While all these nutrients loaded into the same PCA-derived NPs, in keeping with their interactive biochemical qualities, their data reduction into a single pattern might hinder detection of each nutrient’s specific effect on biomarkers. To our knowledge, there are no previous studies that investigated the associations of individual B vitamins and minerals with brain AD biomarkers in NL individuals. More studies with larger samples and longitudinal examinations are needed to replicate these preliminary results and investigate whether isolated nutrients show effects.
The food sources associated with our biomarker-identified NPs are consistent with current definitions of an AD-preventative diet, in terms of recommended food choices (for review see (56)). Present data are consistent with epidemiological findings showing that high adherence to dietary patterns characterized by higher intakes of fruits, vegetables, fish, nuts and legumes, and lower intake of meat, high-fat dairies and sweets, is consistently associated with reduced risk for AD (3, 4, 16, 17, 57–59). Our biomarker data supports previous epidemiological studies by offering a possible pathophysiological substrate to the observed reduced risk for AD. The NPs identified using brain imaging in the present study provide biological evidence that a healthy diet is associated with a more favorable “AD-brain profile”, characterized by lower Aβ load, higher metabolic activity and GMV in NL individuals, several years prior to possible symptoms onset.
Several data reduction techniques have been proposed for analysis of nutrients, including PCA (17). Bowman et al. (15) applied PCA to analysis of plasma nutrients and showed that a nutrient pattern characterized by higher intake of anti-oxidants, vitamin B12, vitamin D and ω-3PUFA was associated with more favorable cognitive and MRI white matter hyper-intensity profiles in NL elderly (15). More studies are needed to assess the relationships between brain imaging of AD pathology and NPs, from both SFFQs and plasma measures. While SFFQs are fairly comprehensive and SFFQ-derived dietary patterns remain quite stable over time (16, 23, 60), this method may be subject to faulty recall of dietary intake and portion size. In support of the accuracy of SFFQ, we detected the expected associations between reported diet and objective measures, e.g. higher NP2 [VitE&PUFA] scores were associated with a more favorable HDL/LDL ratio; higher NP5 [Fats] scores were positively associated with higher systolic blood pressure, plasma triglycerides, homocysteine and BMI. Moreover, the SFFQ used in our study was validated against plasma nutrient measures by several investigators, showing good correlations (24–26).
In our data set, none of the NPs were associated with neuropsychological measures, possibly because our subjects were relatively young to late-middle aged adults and all high-school graduates, which resulted in a “ceiling-effect”. Longitudinal studies with larger samples are warranted to assess whether the relationship between nutrients, AD-biomarkers and cognitive performance varies with age and disease. There is evidence that dietary interventions can alleviate AD pathology in MCI patients (61), consistent with our observations that diet might modulate AD-risk through its effects on Aβ and associated neuronal dysfunction.
This study has several limitations. The NL population selected in our study consists of a group with a high a priori risk of preclinical AD-changes, and results were found in small numbers of carefully screened subjects under controlled clinical conditions. As we did not request participants to modify their diet in any way, some were taking supplements at the time of the exam. Descriptively, 31/52 (60%) participants reported taking no supplements for >1 year prior to brain imaging, and the remaining 21/52 (40%) subjects reported taking a multivitamin regularly, as well as additional fish oil supplements (15%), vitamin D (300–1000 IU) and/or vitamin E (>600 IU) (10%), or vitamin B12 (4%), for at least 1 year prior to brain imaging. This study examined nutrient intake from food sources only, showing significant associations between NPs and brain AD biomarkers in NL individuals. Other studies with a supplement-free control group, as well as other groups of individuals stratified by supplementation type are warranted to replicate these preliminary findings. Second, participation in our studies is completely voluntary, and we do not screen individuals based on ethnicity. At present, the proportion of underrepresented groups (including African American, Hispanic, Asian, and individuals of mixed race) in our center cohort in midtown Manhattan is approx. 16%. Other studies including individuals with a more diversified ethnical background are needed to assess the impact of ethnicity on the observed associations between nutrients and AD biomarkers. Third, the age range of our study cohort was relatively wide (25–72 years). The small sample led us to examine the associations between NPs and biomarkers across all subjects rather than by age group so as to avoid further reducing statistical power to detect significant effects. Although our results were corrected for age by setting this variable as a covariate in SPM analysis, the associations between NPs and biomarker may differ in younger vs. older subjects. As our study cohort is not large enough to reliably test for age differences in NP/biomarkers associations, other studies with larger samples are warranted to determine whether the observed associations change with age and disease. Finally, most participants belonged to middle-class, none were smokers, diabetics, or met criteria for obesity. Replication of these preliminary findings in community-based populations with more diversified demographical, socio-economic, ethnical and medical status, as well as with other biomarkers of AD, is warranted and clinical application is not justified. There is evidence that, among newly diagnosed AD patients, those at risk of malnutrition have more severe impairments of basic and complex daily functioning than well-nourished AD patients (62). These data indicate that assessment of nutritional status is valuable in the diagnostic work up of AD, and that aging individuals might benefit from nutritional interventions implemented prior to developing disease-related malnutrition. Nutritional recommendations that are focused on supporting brain and cognitive function would be helpful not only to the patients but also to the caregivers, as managing the diet of older adults with AD can be particularly challenging (63).
Conclusions
This multi-modality brain imaging study identified nutrient patterns associated with healthy brain aging, which provides support for further exploration of nutritional quality and associated dietary choices for the prevention of AD.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIA grants AG035137, AG13616, P30AG008051.
Footnotes
Contributions: Dr. Berti – study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content; Mr. Murray –acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content; Ms. Davies – acquisition of data, critical revision of the manuscript for important intellectual content; Ms. Spector; Dr. Tsui – acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content; Dr. Li – acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content; Ms. Williams – acquisition of data, analysis and interpretation, study supervision; Dr. Pirraglia – analysis and interpretation, critical revision of the manuscript for important intellectual content; Dr. Vallabhajosula -acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content; Dr. McHugh – study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content; Dr. Pupi - analysis and interpretation, critical revision of the manuscript for important intellectual content; Dr. de Leon – study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content; Dr. Mosconi - study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Statistical Analyses were done by Valentina Berti, Lisa Mosconi and Elizabeth Pirraglia
Competing Interest: Disclosures: Dr. Berti reports no disclosures; Mr. Murray reports no disclosures; Ms. Davies reports no disclosures; Ms. Spector; Dr. Tsui has a patent on a technology that was licensed to Abiant Inc. by NYU and, as such, has a financial interest in this license agreement and hold stock and stock options on the company; Dr. Li has received compensation for consulting services from Abiant Inc; Ms. Williams reports no disclosures; Dr. Pirraglia reports no disclosures; Dr. Vallabhajosula reports no disclosures; Dr. McHugh was PI on an investigator initiated clinical trial supported by Bayer Healthcare Pharmaceuticals; Dr. Pupi reports no disclosures; Dr. de Leon has a patent on a technology that was licensed to Abiant Inc. by NYU and, as such, has a financial interest in this license agreement and hold stock and stock options on the company. Dr. de Leon has received compensation for consulting services from Abiant Inc., has received honoraria from the French Alzheimer Foundation, and was PI on an investigator initiated clinical trial supported by Neuroptix; Dr. Mosconi has a patent on a technology that was licensed to Abiant Inc. by NYU and, as such, has a financial interest in this license agreement and hold stock and stock options on the company. Dr. Mosconi has received compensation for consulting services from Abiant Inc;
References
- 1.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso BR, Cominetti C, Cozzolino SM. Importance and management of micronutrient deficiencies in patients with Alzheimer’s disease. Clin Interv Aging. 2013;8:531–542. doi: 10.2147/CIA.S27983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. 2010;22:483–492. doi: 10.3233/JAD-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–1349. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI Study. Neurology. 2014;82:435–442. doi: 10.1212/WNL.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, Amieva H, Allard M, Dartigues JF, Cunnane SC, Mazoyer BM, Barberger-Gateau P. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology. 2012;79:642–650. doi: 10.1212/WNL.0b013e318264e394. [DOI] [PubMed] [Google Scholar]
- 14.Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–664. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei B, Kaye JA, Shannon J, Quinn JF. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78:241–249. doi: 10.1212/WNL.0b013e3182436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y, Scarmeas N. Dietary patterns in Alzheimer’s disease and cognitive aging. Curr Alzheimer Res. 2011;8:510–519. doi: 10.2174/156720511796391836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, Murray J, Scheinin N, Nagren K, Williams S, Glodzik L, De Santi S, Vallabhajosula S, de Leon MJ. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci U S A. 2010;107:5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 23.Smith W, Mitchell P, Reay EM, Webb K, Harvey PW. Validity and reproducibility of a self-administered food frequency questionnaire in older people. Aust N Z J Public Health. 1998;22:456–463. doi: 10.1111/j.1467-842x.1998.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. 2010;92:1214–1222. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- 25.Pirouzpanah S, Taleban FA, Mehdipour P, Atri M, Hooshyareh-Rad A, Sabour S. The biomarker-based validity of a food frequency questionnaire to assess the intake status of folate, pyridoxine and cobalamin among Iranian primary breast cancer patients. Eur J Clin Nutr. 2014;68:316–323. doi: 10.1038/ejcn.2013.209. [DOI] [PubMed] [Google Scholar]
- 26.Feunekes GI, Van Staveren WA, De Vries JH, Burema J, Hautvast JG. Relative and biomarker-based validity of a food-frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr. 1993;58:489–496. doi: 10.1093/ajcn/58.4.489. [DOI] [PubMed] [Google Scholar]
- 27.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol. 2007;64:86–92. doi: 10.1001/archneur.64.1.86. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 29.Cunnane SC, Plourde M, Pifferi F, Begin M, Feart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res. 2009;48:239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Schupf N, Cosentino SA, Luchsinger JA, Scarmeas N. Nutrient intake and plasma beta-amyloid. Neurology. 2012;78:1832–1840. doi: 10.1212/WNL.0b013e318258f7c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 32.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 33.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 34.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira BF, Veloso CA, Nogueira-Machado JA, de Moraes EN, Santos RR, Cintra MT, Chaves MM. Ascorbic acid, alpha-tocopherol, and beta-carotene reduce oxidative stress and proinflammatory cytokines in mononuclear cells of Alzheimer’s disease patients. Nutr Neurosci. 2012 doi: 10.1179/1476830512Y.0000000019. [DOI] [PubMed] [Google Scholar]
- 36.Ford AH, Flicker L, Alfonso H, Thomas J, Clarnette R, Martins R, Almeida OP. Vitamins B(12), B(6), and folic acid for cognition in older men. Neurology. 2010;75:1540–1547. doi: 10.1212/WNL.0b013e3181f962c4. [DOI] [PubMed] [Google Scholar]
- 37.Kesse-Guyot E, Andreeva VA, Ducros V, Jeandel C, Julia C, Hercberg S, Galan P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br J Nutr. 2013:1–9. doi: 10.1017/S0007114513003188. [DOI] [PubMed] [Google Scholar]
- 38.Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 39.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, Schneider JA. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62:641–645. doi: 10.1001/archneur.62.4.641. [DOI] [PubMed] [Google Scholar]
- 40.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, Qui WQ, Bergethon P, Rosenberg IH, Folstein MF, Patz S, Bhadelia RA, Tucker KL. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74:18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doraiswamy PM, Finefrock AE. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 2004;3:431–434. doi: 10.1016/S1474-4422(04)00809-9. [DOI] [PubMed] [Google Scholar]
- 43.Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61:1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berti V, Mosconi L, Glodzik L, Li Y, Murray J, De Santi S, Pupi A, Tsui W, De Leon MJ. Structural brain changes in normal individuals with a maternal history of Alzheimer’s. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosconi L, Rinne JO, Tsui W, Murray J, Li Y, Glodzik L, McHugh P, Williams S, Cummings M, Pirraglia E, Goldsmith SJ, Vallabhajosula S, Scheinin N, Viljanen T, Nagren K, de Leon MJ. Amyloid and metabolic PET imaging of cognitively normal adults with Alzheimer’s parents. Neurobiol Aging. 2012;20 doi: 10.1016/j.neurobiolaging.2012.03.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi A, Koeppe RA, Fessler JA. Reducing between scanner differences in multi-center PET studies. Neuroimage. 2009;46:154–159. doi: 10.1016/j.neuroimage.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 48.Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 49.Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr. 1995;19:541–547. doi: 10.1097/00004728-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Morris MC, Schneider JA, Tangney CC. Thoughts on B-vitamins and dementia. J Alzheimers Dis. 2006;9:429–433. doi: 10.3233/jad-2006-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 53.Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A. 2013;110:9523–9528. doi: 10.1073/pnas.1301816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takasaki J, Ono K, Yoshiike Y, Hirohata M, Ikeda T, Morinaga A, Takashima A, Yamada M. Vitamin A has anti-oligomerization effects on amyloid-beta in vitro. J Alzheimers Dis. 2011;27:271–280. doi: 10.3233/JAD-2011-110455. [DOI] [PubMed] [Google Scholar]
- 55.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 56.Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, Barberger Gateau P, Berr C, Bonnefoy M, Dartigues JF, de Groot L, Ferry M, Galan P, Hercberg S, Jeandel C, Morris MC, Nourhashemi F, Payette H, Poulain JP, Portet F, Roussel AM, Ritz P, Rolland Y, Vellas B. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–152. [PubMed] [Google Scholar]
- 57.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 59.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158:1213–1217. doi: 10.1093/aje/kwg290. [DOI] [PubMed] [Google Scholar]
- 61.Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743–752. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Droogsma E, van Asselt DZ, Scholzel-Dorenbos CJ, van Steijn JH, van Walderveen PE, van der Hooft CS. Nutritional status of community-dwelling elderly with newly diagnosed Alzheimer’s disease: prevalence of malnutrition and the relation of various factors to nutritional status. J Nutr Health Aging. 2013;17:606–610. doi: 10.1007/s12603-013-0032-9. [DOI] [PubMed] [Google Scholar]
- 63.Silva P, Kergoat MJ, Shatenstein B. Challenges in managing the diet of older adults with early-stage Alzheimer dementia: a caregiver perspective. J Nutr Health Aging. 2013;17:142–147. doi: 10.1007/s12603-012-0385-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.