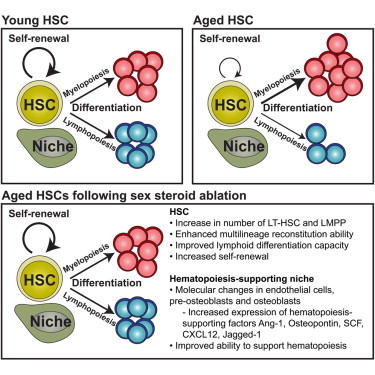
Summary
Mechanisms underlying age-related defects within lymphoid-lineages remain poorly understood. We previously reported that sex steroid ablation (SSA) induced lymphoid rejuvenation and enhanced recovery from hematopoietic stem cell (HSC) transplantation (HSCT). We herein show that, mechanistically, SSA induces hematopoietic and lymphoid recovery by functionally enhancing both HSC self-renewal and propensity for lymphoid differentiation through intrinsic molecular changes. Our transcriptome analysis revealed further hematopoietic support through rejuvenation of the bone marrow (BM) microenvironment, with upregulation of key hematopoietic factors and master regulatory factors associated with aging such as Foxo1. These studies provide important cellular and molecular insights into understanding how SSA-induced regeneration of the hematopoietic compartment can underpin recovery of the immune system following damaging cytoablative treatments. These findings support a short-term strategy for clinical use of SSA to enhance the production of lymphoid cells and HSC engraftment, leading to improved outcomes in adult patients undergoing HSCT and immune depletion in general.
Graphical Abstract
Highlights
-
•
Sex steroid ablation (SSA) increases number of hematopoietic stem cells (HSCs)
-
•
SSA enhances reconstitution potential and self-renewal of HSCs
-
•
SSA reverses the age-associated decline in Foxo1 expression by hematopoietic niche
-
•
There is an increase in niche expression of hematopoiesis-associated factors after SSA
Age-related declines in immune function underlie a wide range of diseases and impaired capacity to recover adaptive immunity following therapeutic immune depletion. Increasing evidence implicates a functional decline in hematopoietic stem cells (HSCs). Here, Chidgey, Dudakov, and colleagues demonstrate that reversal of HSC aging by blocking the immunosuppressive actions of sex steroids involves mechanisms both intrinsic and extrinsic to HSCs. These findings will enable progress in understanding bone marrow and HSC aging and in developing strategies for immune regeneration.
Introduction
One key etiological factor underlying a wide range of diseases is the progressive decline in immune function with age (Dorshkind et al., 2009). At its core is a reduction in lymphopoiesis within the bone marrow (BM) and thymus (Miller and Allman, 2003; Rodewald, 1998), attributed in part to a decrease in the number and function of lymphoid progenitors (Min et al., 2004, 2006). Increasing evidence suggests that intrinsic changes to the earliest hematopoietic stem cells (HSCs) also contribute toward age-related immune degeneration (Geiger et al., 2013). Deficiency in DNA repair, altered DNA methylation patterns, aberrant metabolism and reactive oxygen species, and skewed upregulation of myeloid- (at the expense of lymphoid-) associated genes all contribute to altered HSC function with age (expertly reviewed in Geiger et al., 2013). However, in addition to intrinsic functional changes, extrinsic alterations to the HSC niche also likely to contribute toward the degeneration of HSC function with age (Woolthuis et al., 2011).
Evidence suggests that sex steroids play at least some role in age-related degeneration of lymphopoiesis (Chinn et al., 2012), and we, and others, have previously shown that sex steroid ablation (SSA) is able to rejuvenate aged and immunodepleted BM and thymus, enhance peripheral T and B cell function, and promote immune recovery following hematopoietic stem cell transplantation (HSCT) (Dudakov et al., 2009a; Goldberg et al., 2009; Heng et al., 2005; Sutherland et al., 2005; Velardi et al., 2014). However, the mechanisms underlying SSA-mediated immune regeneration are still unresolved. In particular, the effects of SSA on hematopoietic stem and progenitor cells (HSPCs) are likely to be pertinent given that sex steroids regulate HSC function as well as lymphoid-primed multipotent progenitor (LMPP) cells (Medina et al., 2001; Nakada et al., 2014; Thurmond et al., 2000). In this study, we sought to examine the events upstream of SSA-mediated lymphoid regeneration, focusing on the earliest HSPCs.
Results
SSA Increases the Number of Hematopoietic Stem and Progenitor Cells
Although age-induced reduction in HSC function does not reach its nadir until at least 24 months of age in mice (Morrison et al., 1996), it is clear that significant defects in the capacity for T and B cell differentiation are already evident by middle age (9 months) (Dudakov et al., 2009a; Heng et al., 2005; Sutherland et al., 2005). To determine whether SSA initiates its impact early in hematopoiesis, we enumerated HSCs by flow cytometry (Figure S1A) at multiple time points after surgical castration of 9-month-old mice. Consistent with previous reports, there was a phenotypic increase in the absolute number of long-term HSCs (LT-HSCs) during aging with a 2-fold increase by middle age (Figure 1A). Following SSA, there was a further increase in the absolute number of LT-HSCs and short-term HSCs (ST-HSCs) from day 14 (d14SSA), which was maintained through to d56SSA compared to sham-SSA (shSSA) control mice (Figures 1A and 1B). While there was no observable impact of age on multipotent progenitors (MPPs), and SSA did not significantly alter their total number (Figure 1C), there was a selective decrease in LMPPs by 9 months, which was reversed following SSA (Figure 1D). This change in HSC number caused by SSA was extremely long-lived with increases in FLT3− (LT-HSC and ST-HSC) and FLT3hi (LMPPs) still observed 1 year later (Figure 1E).
Figure 1.
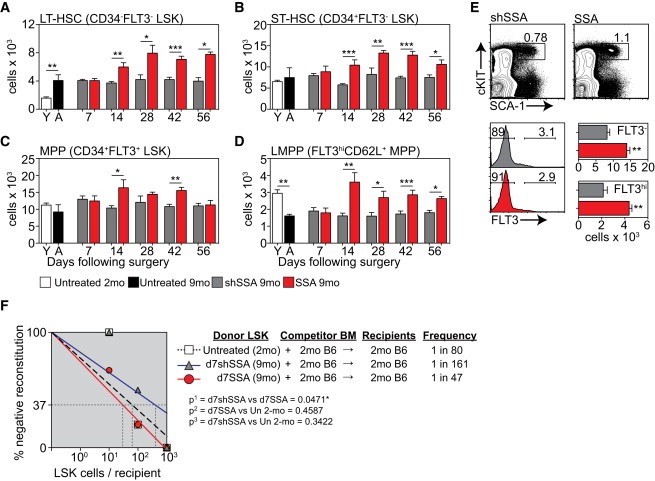
SSA Increases the Number of Multilineage HSCs in Middle-Aged Mice
(A–D) Lin−SCA1+cKIT+ (LSK) BM can be subdivided into populations of LT-HSCs (CD34−FLT3−), ST-HSCs (CD34+FLT3−), and MPPs (CD34+FLT3+). The MPP population can be further fractionated based on FLT3 and CD62L expression for analysis of LMPPs (FLT3hiCD62L+). Absolute number of LT-HSCs (A), ST-HSCs (B), MPPs (C), and LMPPs (D) (n = 5–12/group/time point).
(E) Concatenated flow cytometry plots, gated on Lineage− cells, and absolute number of FLT3− LSK cells, 1 year after surgical SSA of 9-month male mice (n = 5/group).
(F) LSK cells were FACS purified from untreated CD45.2+ 2-month; CD45.2+ 9-month mice 7 days following surgical shSSA (d7shSSA); or CD45.2+ 9-month mice 7 days following surgical SSA (d7SSA) (n = 6 recipients/group/dose) and graded doses of cells were transferred into lethally irradiated congenic CD45.1 recipients along with 5 × 105 CD45.1+ supporting BM cells. Multilineage reconstitution (>1% B cell, T cell, macrophage, and granulocyte) was analyzed 12 weeks after transplant and the frequency of repopulating cells was calculated by Poisson statistics.
Bar graphs represent mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figures S1 and S2.
A defining characteristic of HSC function is the ability to differentiate into multiple lineages. The frequency of multilineage repopulating cells was therefore enumerated using a limiting-dilution competitive repopulation assay (Figure 1F). 10, 100, or 1,000 fluorescence-activated-cell-sorted (FACS) lineage-negative, Sca1+, c-Kit+ (LSK) cells from untreated 2-month mice; 9-month mice 7 days following sham surgery (9-month d7shSSA); or 9-month mice 7 days following surgical SSA (9-month d7SSA) were transferred along with 5 × 105 supporting BM cells into lethally irradiated congenic recipients. We chose the d7SSA time point to better delineate qualitative from quantitative changes in HSCs as this was prior to any SSA-induced increase in absolute cell number (Figure 1A). Given there was no quantitative or functional differences in HSCs between untreated 9-month and 9-month shSSA mice (Figures 1A and S1B), all of our functional studies compared SSA mice to age-matched shSSA and untreated 2-month mice. Mice were considered reconstituted by a single HSC if there was ≥1% contribution of donor cells within both lymphoid (T and B cells) and myeloid (macrophage and granulocyte) lineages 12 weeks after transplant (Figure 1F). While there was no decrease in the frequency of multilineage repopulating cells in 9-month d7shSSA compared to 2-month mice (Figure 1F), functional LSK cells from 9-month d7SSA were significantly increased compared to 9-month d7shSSA controls (Figure 1F). When individual lineages were analyzed, mice reconstituted with 9-month d7SSA LSKs had an increased frequency of B-lineage and macrophage reconstituting cells (Figure S1C). All recipients, regardless of the number of cells transplanted, had detectable levels of granulocyte engraftment (data not shown), and there were no significant differences detected in T cell engraftment (Figure S1C). Ki67 and Annexin V (AnnV) were used to determine whether the increase in functional HSC frequency was due to a change in their cycling status or apoptosis. At day 7, there was no change in Ki67 or AnnV expression on CD34−, CD34+, or CD34+CD62L+ LSK subsets following SSA (Figures S1D and S1E).
SSA Significantly Enhances HSC Repopulation Potential
To assess long-term functional capacity for multilineage reconstitution, BM (2.5 × 106 cells) from 9-month CD45.1+ d7shSSA or d7SSA was co-transplanted at a 1:1 ratio with congenic untreated young (2 month) competitor BM into lethally irradiated 2-month CD45.2+ recipients. Over the 17 weeks analyzed, total CD45.1+ donor-derived peripheral leukocyte reconstitution of mice transplanted with 9-month d7SSA BM was significantly higher than those reconstituted by 9-month d7shSSA mice (Figures 2A and 2B). Reflecting this, 9-month d7SSA-derived BM significantly improved B cell engraftment, while T cell engraftment transiently increased at 4 weeks after transplant compared to 9-month d7shSSA controls (Figure 2C). However, enhanced reconstitution by d7SSA was not restricted to the lymphoid compartment, with a significant improvement also observed in engraftment of both monocytes/macrophages and granulocytes (Figure 2C).
Figure 2.
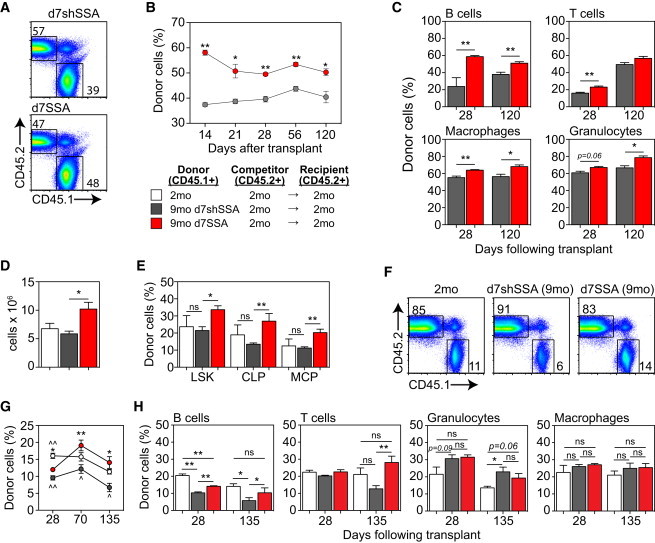
SSA Enhances the Functional Repopulation Potential of HSCs Derived from Middle-Aged Mice
(A–C) CD45.1 BM cells were harvested and pooled from 9-month d7shSSA, (n = 5 recipients/donor group) or 9-month d7SSA (n = 5 recipients/donor group) and 2.5 × 106 cells were transferred with an equal dose of untreated 2-month CD45.2 BM cells into lethally irradiated 2-month CD45.2 recipients. Reconstitution was analyzed at the designated time points. (A) Concatenated donor (CD45.1) versus competitor (CD45.2) flow cytometry profiles of spleen 17 weeks after transplant. (B) Total peripheral reconstitution measured by CD45.1 in spleen or peripheral blood over 120 days. (C) Lineage-specific reconstitution of B220+ B cells, TCRβ+ T cells, Gr1+CD11b+ granulocytes, and Gr1loCD11b+ monocyte/macrophages in the spleen at 28 and 120 days after transplant.
(D–G) 2,000 FACS purified CD45.1 LSK cells from untreated 2-month; 9-month d7shSSA; or 9-month d7SSA were transferred along with 2 × 106 BM cells from untreated 2-month CD45.2 competitors into lethally irradiated CD45.2 recipients (n = 5–7/donor group). (D) Total number of CD45.1 donor-derived cells in the BM of recipients 28 days after transplant. (E) Proportion of CD45.1+ whole BM, LSK, CLP, and MCP cells in the BM 28 days after transplant. (F) Concatenated donor (CD45.1) versus competitor (CD45.2) flow cytometry profiles of spleen 19 weeks after transplant. (G) Peripheral reconstitution in 28, 70, and 135 days after transplant. ∗Compared with 9-month shSS A mice. ˆCompared with untreated 2-month mice.
(H) Lineage repopulation was measured among B220+ B cells, TCRβ+ T cells, Gr1+CD11b+ granulocytes, and Gr1loCD11b+ monocyte/macrophages.
Results are expressed as mean ± SEM. ∗/ˆp < 0.05, ∗∗/ˆˆp < 0.01, ∗∗∗/ˆˆˆp < 0.001. See also Figure S2.
To determine the intrinsic impact on the stem and progenitor cell compartment, 2,000 purified LSK cells from untreated 2-month, 9-month d7shSSA, or 9-month d7SSA mice were co-transplanted with 2 × 106 BM cells from untreated congenic competitors into lethally irradiated recipients. Although there was no age-induced impact at 9-month on the ability of donor cells to engraft the BM 28 days after transplant, 9-month d7SSA-LSK significantly increased the overall number of CD45.1+ cells in the marrow (Figure 2D), comprising donor LSK cells as well as downstream Lin−SCA1−cKIT+ myeloid and erythroid progenitors and Lin−IL7Rα+cKIT+ CLP populations (Figure 2E). On the other hand, long-term peripheral repopulation was significantly impaired with 9-month d7shSSA compared to untreated 2-month LSK (Figures 2F and 2G). Over the same time frame, there was a significant increase in the repopulation potential of LSK cells isolated from 9-month d7SSA mice, restoring the reduced middle-aged (9 month) LSK repopulation potential to 2-month levels. Increased reconstitution consisted principally of improved B cells, but also improved long-term T cell repopulation at day 135 after transplant (Figure 2H). Although myeloid engraftment increased with age, primarily due to granulocyte engraftment (Figure 2H), we found that donor LSKs from 9-month d7SSA did not further increase this engraftment above that seen in recipients of 9-month shSSA LSK cells (Figure 2H).
Enhanced Repopulation Ability following SSA Is Not due to Improved Homing of HSCs
To assess whether improved homing of transplanted cells to the BM niche could explain our observed SSA-mediated improvements to HSC repopulation, we examined LSK cells for expression of the homing and adhesion molecules VLA-4, VLA-5, and CXCR4. Although we found a decrease in the proportion of LSK cells expressing VLA-4, VLA-5, and CXCR4 with age, there was no change in their expression following SSA (Figures S2A and S2B), suggesting that the impacts of SSA on HSCs were intrinsic to the cells’ function. To directly examine the homing ability of LT-HSCs in vivo, we harvested BM from 9-month d7shSSA or d7SSA mice and transplanted into unablated congenic 2- or 9-month recipients at a saturating dose of 20 × 106 BM cell and homing and engraftment of donor LT-HSCs analyzed 40 hr later (Figure S2C). The same proportion of LT-HSCs homed to BM irrespective of the age of the recipient, the age of the donor, or whether donors had undergone SSA. This result also held true for downstream ST-HSCs and MPPs (data not shown). Consistent with previous studies (Dykstra et al., 2011; Liang et al., 2005), when the number of transfused LT-HSCs was taken into account, there was a significant decrease in HSC homing in recipients of 9-month shSSA and SSA BM, as well as in aged recipients in all groups (Figure S2D).
HSC Self-Renewal Is Improved following SSA
To assess the impact of SSA on HSC self-renewal, lethally irradiated 2-month CD45.2 mice were transplanted with 2,000 purified LSK cells from untreated 2-month, 9-month d7shSSA, or 9-month d7SSA mice together with 2.5 × 106 untreated congenic 2-month CD45.2 BM as support cells. 12 weeks after transplantation, 2.5 × 106 BM cells from primary recipients were transferred into secondary recipients along with 2.5 × 106 BM cells from untreated 2-month competitors. In both primary and secondary recipients, 9-month d7shSSA and untreated 2-month cells gave a similar level of donor engraftment, although with age there was a significant decline in the proportion of donor-derived cells upon secondary transplant (Figure 3A). However, 9-month d7SSA had significantly higher levels of engraftment in both primary and secondary recipients compared to cells from 9-month d7shSSA and untreated 2-month mice. Consistent with previous reports (Dykstra et al., 2011), there was a reduction in donor contribution between primary and secondary recipients when cells derived from 9-month d7shSSA mice were transplanted. In contrast, secondary recipients receiving 9-month d7SSA cells or 2-month cells showed no reduction in the proportion of total donor cells engrafted compared to the primary grafts (Figure 3A).
Figure 3.
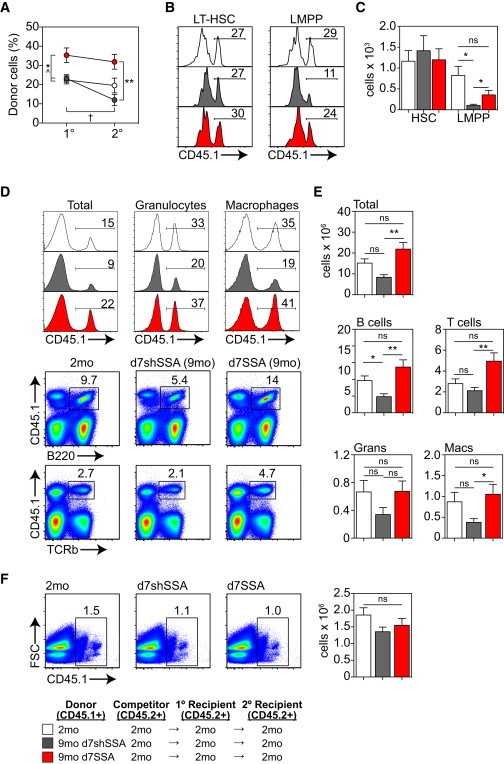
SSA Enhances the Self-Renewal Capacity of HSCs Derived from Middle-Aged Mice
2,000 CD45.1 purified LSK cells from CD45.1 2-month untreated, 9-month d7shSSA, or 9-month d7SSA were transferred along with 2.5 × 106 untreated 2-month CD45.2 BM cells into lethally irradiated 2-month CD45.2 primary recipients (n = 6 recipients/donor group, one individual recipient per individual donor). 12 weeks after transplant, primary recipient BM was harvested, and 2.5 × 106 BM cells were transplanted along with an equal dose of untreated 2-month CD45.2 BM cells into lethally irradiated 2-month CD45.2 secondary recipients (n = 12 recipient/donor groups, two recipients per individual donor). Reconstitution of secondary recipients was measured 12 weeks after transplant.
(A) Proportion of CD45.1+ donor cells in BM 12 weeks following transplant in primary and secondary recipients (∗Compared with 9-month d7shSSA mice within primary or secondary recipients. ˆCompared to untreated 2-month mice within either primary or secondary recipients. †Compared to 9-month d7shSSA between primary and secondary recipients).
(B) Proportion of donor-derived CD150+CD34−FLT3− LT-HSCs and CD34+FLT3hiCD62L+ LMPPs in the BM of secondary recipients 12 weeks after transplant.
(C) Absolute number of donor-derived LT-HSCs and LMPPs in the BM of secondary recipients 12 weeks after transplant.
(D) Concatenated flow cytometric profiles of total donor reconstitution, as well as donor B220+ B cells, TCRβ+ T cells, Gr-1+CD11b+ granulocytes, and Gr1loCD11b+ in the spleen of secondary recipients 12 weeks after transplant.
(E) Absolute number of CD45.1+ donor cells in the spleen of secondary recipients 12 weeks after transplant.
(F) 12 weeks after secondary transplant, 2.5 × 106 BM cells from secondary recipients was transplanted into tertiary recipients at a 1:1 ratio with competitor CD45.2+ BM, and peripheral reconstitution was assessed at 12 weeks. Concatenated FACS plots of the proportion, and the absolute number of CD45.1+ donor cells, in tertiary recipients (n = 6/group).
Results are expressed as mean ± SEM. ∗/ˆp < 0.05, ∗∗/ˆˆp < 0.01.
This defect in the reconstitution of secondary recipients was not attributable to a numerical reduction in stem cell engraftment, as there was no change in the proportion or number of donor-derived LT-HSCs in the BM of secondary recipients as a consequence of age or SSA (Figures 3B and 3C). However, consistent with the functional decline in lymphopoiesis with age, there was a significant decrease in donor-derived LMPPs from 9-month d7shSSA, which was reversed in secondary recipients of 9-month d7SSA cells (Figures 3B and 3C). Within specific hematopoietic lineages, although there was no loss in the proportion of donor-derived T cells or CD11b+ myeloid cells with age, there was a decline in B cell reconstitution, which contributed toward a global decline in CD45+ cells (Figures 3D and 3E). Importantly, secondary reconstitution from 9-month d7SSA BM significantly increased the donor contribution of all leukocyte subsets (Figures 3D and 3E), but this effect of SSA on HSC self-renewal was finite as there was no difference in donor contribution between 9-month d7shSSA and 9-month d7SSA in tertiary recipients (Figure 3F).
SSA Increased the Lymphoid Differentiation Capacity of LT-HSCs
The dominant downstream effects of SSA in steady-state aged mice are on lymphopoiesis, with little if any impact observed on myeloid cells. To test the lymphoid differentiation capacity of the BM as a whole, we transferred 5 × 106 BM cells derived from 2-month, 9-month d7shSSA, or 9-month d7SSA mice into unirradiated Il7ra−/− recipients, which bypasses the confounding effects of total body irradiation (TBI) (Gossens et al., 2009). We found a significant defect by 9 months in the ability of BM to engraft IL7Rα−/− recipients. As expected, this was predominantly composed of declines in donor-derived TCRβ+ T cells and B220+ B cells (Figures 4A and 4B). To test the relative functional changes between HSCs and their downstream progenitors, we transferred 2,000 LT-HSCs or LMPPs derived from untreated 2-month, 9-month d7shSSA, or 9-month d7SSA mice into unirradiated Il7ra−/− recipients. LT-HSCs isolated from 9-month d7shSSA mice exhibited reduced engraftment at day 70 compared to untreated 2-month LT-HSCs in both B and T cell lineages. However, Il7ra−/− recipients transplanted with 9-month d7SSA cells restored the differentiation capacity of aged LT-HSCs to that of untreated 2-month (Figures 4C and 4D). Interestingly, although we found a significant decline in their differentiation capacity with age, there was little effect of SSA on lymphoid differentiation of LMPPs on a per-cell basis (Figures 4E and 4F). Thus, increased lymphopoiesis seems to be predicated wholly on the intrinsic increase in HSC function. Given that Il7ra−/− mice have a selective deficiency in lymphopoiesis, we would only expect limited myeloid engraftment, and indeed that was the case when we transplanted whole bone marrow (WBM) (Figures S3A and S3B). However, interestingly, when LT-HSCs were transplanted, although there was no change in engraftment of granulocytes either as a consequence of age or SSA (Figure 4G), there was a significant decrease in monocyte/macrophage reconstitution with age, which was reversed after SSA (Figure 4H).
Figure 4.
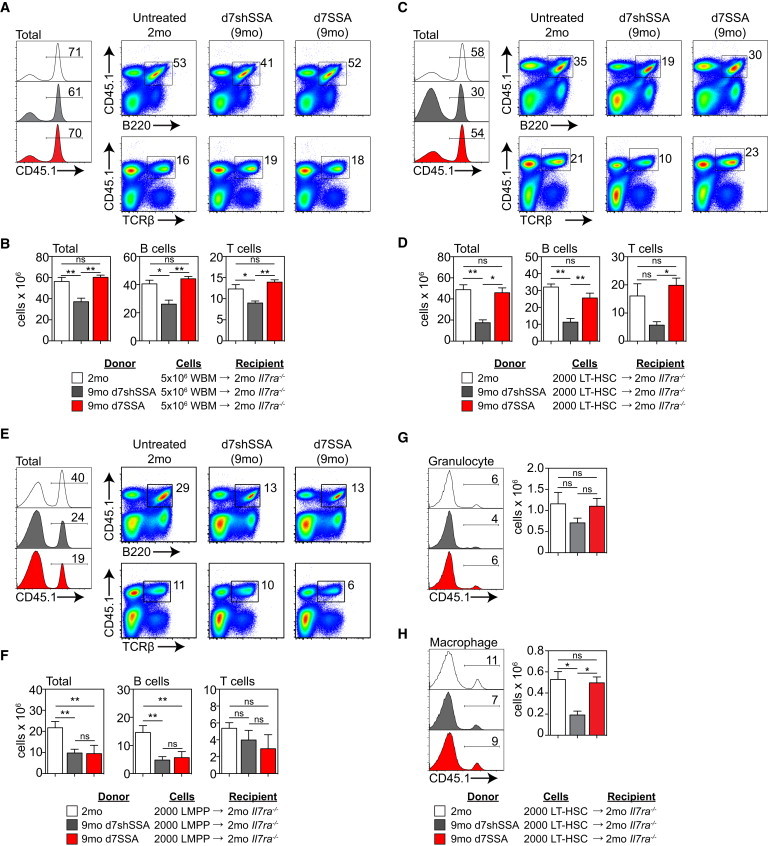
SSA Promotes Lymphoid Differentiation Potential of HSCs Derived from Middle-Aged Mice
(A and B) 5 × 106 CD45.1 BM cells from untreated 2-month; 9-month d7shSSA; or 9-month d7SSA were transferred into unablated 2-month Il7ra−/− recipients (n = 6 recipient/donor groups, one recipient per donor). (A) Concatenated flow cytometry plots displaying total donor reconstitution as well as donor Gr1+CD11b+ granulocyte, Gr1loCD11b+ monocyte/macrophage, B220+ B cells, and TCRβ+ T cells reconstitution at day 42. (B) Absolute number of donor cells as well as the number of donor B cells and T cells at day 42.
(C and D, and G and H) 2,000 CD45.1 LT-HSCs from untreated 2-month; 9-month d7shSSA; or 9-month d7SSA were transferred into unablated 2-month Il7ra−/− recipients (n = 6 recipient/donor groups, one recipient per donor) and donor-derived hematopoiesis measured in the spleen 70 days after transfer. (C) Concatenated flow cytometry profiles showing total donor reconstitution as well as donor Gr1+CD11b+ granulocyte, Gr1loCD11b+ monocyte/macrophage, B220+ B cells, and TCRβ+ T cells reconstitution. (D) Absolute number of donor-derived cells in the periphery of Il7ra−/− recipients.
(E and F) 2,000 LMPPs from untreated CD45.1+ 2-month; CD45.1+ 9-month d7shSSA); or CD45.1+ 9-month d7SSA were transferred into unablated 2-month Il7ra−/− recipients (n = 6 recipient/donor groups) and donor-derived hematopoiesis measured in the spleen 70 days after transfer. (E) Concatenated flow cytometry profiles showing total donor reconstitution as well as donor B220+ B cells, and TCRβ+ T cells reconstitution. (F) Absolute number of donor-derived cells in the periphery of Il7ra−/− recipients.
(G) Concatenated flow cytometry plots displaying donor reconstitution and absolute numbers of donor Gr1+CD11b+ granulocytes.
(H) Concatenated flow cytometry plots displaying donor reconstitution and absolute numbers of donor Gr1loCD11b+ monocyte/macrophages.
Results are expressed as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S3.
Early Intrinsic Functional Changes in LT-HSCs
To identify whether any functional effects could be observed in HSCs earlier than day 7, 8,000–10,000 CD34-FLT3− LT-HSCs from 9-month d2SSA mice, 9-month d7SSA mice, or their shSSA controls were co-cultured with OP9-DL1 cells for 12 days. Consistent with our in vivo data, LT-HSCs derived from 9-month SSA mice progressed more rapidly to the DN2 and DN3 stages of T cell development when compared to LT-HSCs isolated from 9-month shSSA mice (Figure 5A). To explore the intrinsic mechanistic changes to the stem cell compartment after SSA, we performed transcriptome analysis on LT-HSCs from 9-month shSSA and 9-month SSA mice, 2 days after surgery. 251 genes were significantly altered (p < 0.05; >1.5-fold change), 55 upregulated and 196 downregulated (Figure 5B). Core pathway analysis revealed 24 genes upregulated and 13 downregulated in d7SSA LT-HSCs under the “Hematological System Development and Function” pathway (Table S1), indicating that even using an unbiased approach revealed hematopoietic-related gene changes. Upon further dissection, many of the 251 altered genes could be broadly grouped into those that impacted on cell cycle and cell differentiation (Figures 5C and 5D), including downregulation of cell-cycle inhibitors such as ARF proteins Nkx3-1, Dkk1, and Cebpa and pro-apoptotic factors including Cebpe, Prg3, and Nlrp10. There was also an upregulation of genes promoting proliferation such as Foxq1, Hoxb7, Zfhx1a, and Fgfbp3. Consistent with the marked shift in lineage differentiation potential of HSCs with age, previous studies have found considerable gene usage skewing in HSCs from old mice away from lymphoid-associated and toward myeloid-associated genes (Rossi et al., 2005). We found that as early as day 2 after SSA, there was a significant decrease in genes associated with myeloid differentiation (Prg2, Ela2, Mpo, and Ear-2, -4) as well as upregulation of genes previously associated with lymphopoiesis (Zbtb7a, Rag1ap1, Tbx1, and Tcta) (Figure 5D).
Figure 5.
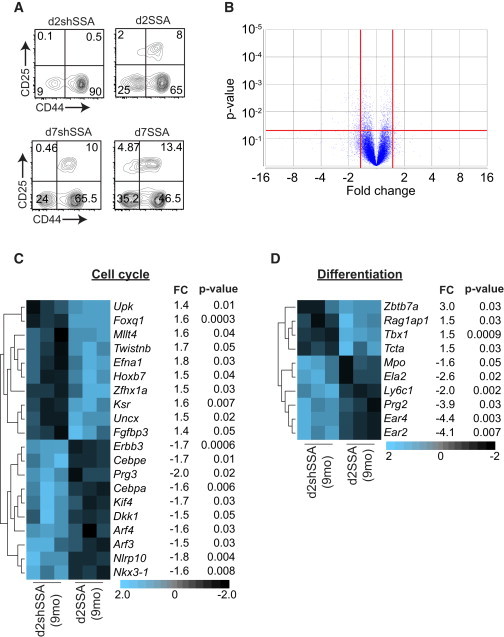
Intrinsic Molecular Changes within HSCs after SSA
(A) 10,000 LT-HSCs from 9-month shSSA or 9-month SSA mice 2 or 7 days after surgery were plated onto OP9-DL1 cells and T cell differentiation assessed at day 12. Concatenated FACS plots gated on CD4−CD8−CD3−.
(B–D) LT-HSCs from 9-month d2shSSA or 9-month d2SSA mice were FACS purified, and transcriptome analysis was performed (n = 3 biological replicates, where each replicate included cells pooled from 10–20 animals). (B) Volcano plot outlining gene expression between 9-month d2shSSA and 9-month d2SSA LT-HSCs. 251 genes were significantly altered (p < 0.05; >1.5-fold change), 55 upregulated and 196 downregulated. (C and D) Significant changes between 9-month d2shSSA and 9-month d2SSA LT-HSCs in genes involved with cell-cycle regulation (C) or differentiation (D).
SSA Alters Intrinsic Gene Expression by Hematopoietic Niche Cells
Given the intimate relationship between HSCs and their microenvironment, we performed transcriptome analysis on non-hematopoietic cells from the sinusoidal niche of 2- and 9-month untreated mice, 9-month shSSA, and 9-month SSA mice at days 2, 4, 7, and 10 post-SSA. 160 genes were downregulated and 72 upregulated (>2-fold) between untreated 2- and 9-month mice (Figure S4A). Of the 160 downregulated genes, pattern discovery (correlation >0.7) revealed 116 that became progressively more similar after SSA to untreated 2-month mice (Figure 6A; Table S2). Genes of note following this pattern included Spib, which can be induced by RANKL, a factor critical for osteoblast (OBL) differentiation (de Lau et al., 2012; Wilson, 2011); Lgr5, which marks stem cells in the intestine, liver, and ovary (Koo and Clevers, 2014); and Foxo1, which has been implicated in protection from age-associated pathologies in other tissues (van der Horst and Burgering, 2007). qPCR of highly purified niche subsets confirmed an increase in Foxo1 expression by pre-OBLs as early as day 2 after SSA (Figure 6B). Gene ontology analysis of the 116-gene cluster revealed 50 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (Figure S4B), including (1) gap junction molecules, which have been found to regulate hematopoiesis and prevent HSC senescence (Schajnovitz et al., 2011; Taniguchi Ishikawa et al., 2012), (2) cell adhesion molecule-associated pathways, which modulate the interaction of HSCs with their stromal niche (Mendelson and Frenette, 2014), and (3) the MAPK signaling pathway, which regulates the balance between HSC expansion, survival, and differentiation and has also been implicated in promoting thymopoiesis (Geest and Coffer, 2009).
Figure 6.
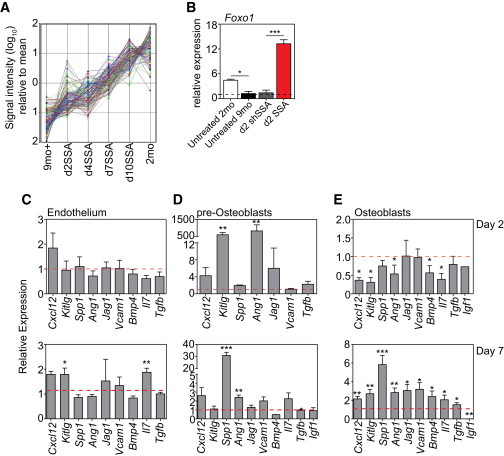
SSA Induces Molecular Changes to the Niche and Its Ability to Support Hematopoiesis
Transcriptome analysis was performed on purified sinusoidal CD45−TER119− cells from 2- and 9-month untreated mice, 9-month shSSA control, and 9-month SSA mice at days 2, 4, 7, and 10 after surgery (n = 1, ten mice pooled/sample).
(A) Pattern discovery (correlation >0.7) identified 132 probes that demonstrated an increasing gene expression trend toward young profiles following castration of middle-aged mice.
(B) qPCR of Foxo1 expression in pre-OBLs purified from untreated 2- or 9-month mice, and 9-month d2shSSA or 9-month d2SSA mice.
(C–E) Expression of Cxcl12, Kitlg, Spp1, Ang1, Jag1, Vcam1, Bmp4, Il7, Tgfb, and Igf1 in endothelial cells (C), pre-OBLs (D), and OBLs (E) from 9-month d2SSA and 9-month d7SSA. Expression is represented as 9-month d7SSA relative to 9-month d7shSSA control mice, n = 3–11 independent experiments.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S4.
To further probe the basis underlying SSA-mediated changes to HSC function, molecular changes to genes previously implicated with maintenance of hematopoietic function were examined within specific isolated niche populations. Endothelial cells (CD45−TER119−CD31+) and pre-OBLs (CD45−TER119−CD31−CD51+) were isolated from the sinusoidal compartment after flushing bones while mature endosteal niche OBLs (CD45−TER119−CD31−CD51+) were obtained by digesting bone fragments with collagenase. Precursor status of sinusoidal-resident OBLs was confirmed by their expression of Runx2 and their reduced expression of Gpnb (Osteoactivin), Bglap (Osteocalcin), and Spp1 (Osteopontin), compared with endosteal OBLs (Figure S4C); consistent with data suggesting that CD51+PDGFRα+ cells in the sinusoidal niche correspond with HSC niche-forming Nestin+ mesenchymal stromal cells (Pinho et al., 2013). Analysis revealed no significant changes in niche population size with age or SSA (data not shown). Although there was no notable difference in endothelial gene expression at d2SSA (9 month), there was a significant increase in the expression of Kitlg and Il7 by day 7 (Figure 6C), both of which could aid in lymphoid differentiation. Within the pre-OBL population, there was a large early transient increase in Scf expression and Angiopoietin-1 (Ang-1) at day 2, which subsided by day 7 (Figure 6D). Despite sinusoidal niche-residing pre-OBLs expressing a fraction of the Opn compared to endosteal OBLs on a per-cell basis (Figure S4C), 7 days following SSA sinusoidal pre-OBLs showed a 30-fold increase in Opn production compared to shSSA control mice. Within the mature endosteal OBL population, there was an initial decline in the expression of Cxcl12, Scf, Angpt1, Bmp4, and Il7 at day 2. However, by day 7 there was a significant increase in many of these factors including Spp1, Ang1, Cxcl12, Kitlg, Jag1, Vcam1, Bmp4, and Tgfb as well as decreased Igf1 (Figure 6E).
SSA Mediates Regeneration of the Hematopoietic Niche to Promote Lymphopoiesis
Consistent with these molecular changes in niche function, 9-month SSA-treated recipients of untreated 2-month HSCT had significantly greater engraftment of LSK cells compared with 9-month shSSA control recipients, even with ten times fewer cells transplanted (Figures 7A and 7B). This engraftment was reflected in the periphery at 4 and 12 weeks after transplant where there was an increased total number of donor cells (Figures 7C and 7D). In addition, the number of donor T cells, B cells, and macrophages was also significantly increased in recipients that had been given SSA regardless of which dose of cells they were given (Figure 7E). However, interestingly, within the granulocyte population we could detect no difference in engraftment in mice regardless of their SSA status or cell dose infused (Figure 7E). This suggests that, in addition to enhancing HSC function, SSA also enhances the function of the niche and its ability to support hematopoiesis.
Figure 7.
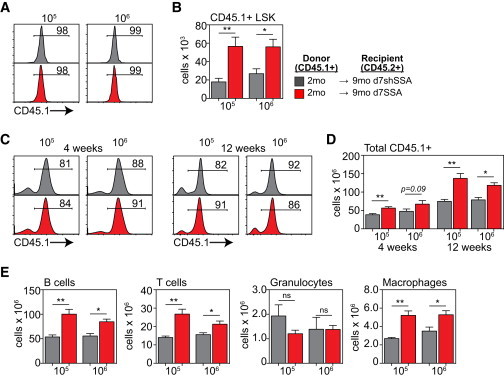
SSA Promotes the Ability of the Niche to Support Hematopoiesis
Lethally irradiated 9-month d7shSSA or 9-month d7SSA were reconstituted with either 5 × 105 or 5 × 106 BM cells from untreated 2-month CD45.1 donors (n = 5–6/group).
(A) Concatenated flow cytometry plots showing the proportion of donor LSK cells at 28 days after transplant.
(B) Absolute number of donor-derived LSK cells in the BM 28 days after transplant.
(C) Concatenated flow cytometry plots showing total donor reconstitution in the spleen at days 28 and 84 after transplant.
(D) Absolute number of donor-derived cells in the spleen at days 28 and 84 after transplant.
(E) Absolute number of donor-derived Gr1+CD11b+ granulocyte, Gr1loCD11b+ monocyte/macrophage, B220+ B cells, and TCRβ+ T cells in the spleen 84 days after transplant.
Results are expressed as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01.
Discussion
One of the hallmarks of aging is reduced generation of new lymphocytes. Although there is a profound decline in the number of lymphoid progenitors with age (Min et al., 2004, 2006) that can be reversed by SSA, consistent with their sensitivity to sex steroids (Medina et al., 2001), we have demonstrated here and in previous work that the function of lymphoid progenitors does not change on a per-cell basis after SSA (Heng et al., 2005). Rather, upstream HSCs exhibit improved overall function early after the cessation of testosterone production. One hypothesis that may explain this early functional shift would be an immediate and direct intrinsic impact on stem and progenitor cells due to the removal of the negative influences of sex steroids. This was supported by the early molecular and functional changes within a highly purified population of LT-HSCs 2 days after SSA. Noteworthy among the altered genes in LT-HSCs post-SSA were downregulation of Dkk1, a wnt inhibitor that can be upregulated by androgens and mediate aging degeneration (Kwack et al., 2008; Seib et al., 2013); decreased Erbb3, elevated levels of which correlate with androgen-responsive prostate cancers (Koumakpayi et al., 2006); increased expression of Efna1, which has been implicated in enhanced proliferation and is downregulated upon exposure to androgens (Nantermet et al., 2004); decreased Arf3 and Arf4, which have been implicated in regulating CDC42 (Erickson et al., 1996), a potential mediator of HSC aging and regeneration (Florian et al., 2012); upregulation of genes directly involved with lymphoid differentiation, such as Zbtb7a, Tbx1, and Rag1ap1 (Gennery, 2012; Maeda et al., 2007); and downregulation of Ear2, which has recently been described as a negative regulator of T cell development (Ichim et al., 2014).
In our previous studies, we demonstrated that after SSA there is a significant increase in the number of all developing B and T cell subsets (Dudakov et al., 2009a, 2009b; Goldberg et al., 2010; Heng et al., 2005; Sutherland et al., 2005). However, in this study there was a clear increase in B lymphopoiesis, while thymopoiesis was only sometimes increased. Both T and B lymphopoiesis occur in very close contact with their respective stromal microenvironments; however, T cell development is entirely dependent on these interactions, while some B lymphopoiesis can be achieved independently of the BM stromal microenvironment (Carsetti, 2000; Nagasawa, 2006; Takahama, 2006). Therefore, one hypothesis could be that altering the intrinsic function of HSCs—although enough to profoundly impact on B lymphopoiesis—is not sufficient to promote thymopoiesis, which may require additional stimulation of the stromal microenvironment. Consistent with this hypothesis, increases after SSA in T-lineage progenitors such as LMPP and CLP were not by themselves enough to promote increased thymopoiesis, without additional impact on the thymic stromal microenvironment.
In addition to intrinsic changes in HSCs, we also found a qualitative change to the BM niche, which likely contributes to the improved function of HSCs after SSA. Consistent with this, we have previously found that SSA can significantly improve hematopoietic reconstitution after HSCT (Goldberg et al., 2005, 2007, 2009), and here we could demonstrate that fewer cells were needed for effective engraftment. There is some rationale for these findings, as (1) exposure of aged satellite stem cells to a young microenvironment reverses their age-related changes (Conboy et al., 2005), and (2) subsequently several studies have reported that circulating factors in young mice can reverse age-related defects in a number of tissues including heart, brain, and skeletal muscle (Loffredo et al., 2013; Sinha et al., 2014; Villeda et al., 2014). Pattern analysis of the changed genes within the stromal microenvironment revealed a distinct shift in gene expression after SSA from a profile characteristic of an aged mouse to that of the young by d10 after SSA. Of particular interest within this group was the gene Foxo1, which has been described in the development and differentiation of OBLs, regulating their expression of Runx2 and Bglap (Teixeira et al., 2010; Yang et al., 2011), and preventing differentiation of mesenchymal progenitor cells into fat or muscle (Nakae et al., 2003). Foxo1 has also been implicated with protection during aging (van der Horst and Burgering, 2007), with Foxo1-deficient mice displaying aberrant hematopoietic phenotypes resembling those seen with age; however, it is unclear whether these are intrinsic to HSCs or mediated through the BM microenvironment (Kim et al., 2008; Tothova et al., 2007). Consistent with these changes after SSA, the androgen receptor can directly bind the Foxo1 promoter and regulate its action, and FOXO1 can inhibit androgen receptor transcription (Liu et al., 2008; Ma et al., 2009). In addition, IGF-1, expression of which is almost completely abrogated after SSA, triggers the inactivation of FOXO1 by nuclear exclusion (Yang et al., 2011). Analysis of known hematopoietic niche interactions (Mercier et al., 2012) revealed upregulation following SSA of Spp1, Ang1, Cxcl12, Kitlg (Scf), Tgfb, Jag1, and Vcam1—many of which can be directly regulated by FOXO1 (Ferdous et al., 2011; Martinez et al., 2008; Potente et al., 2005).
Taken together, these data indicate that the widespread downstream impacts of SSA on lymphopoiesis are at least partially attributable to the considerable effects on primitive HSC function, thereby demonstrating mechanisms by which lymphopoiesis can be rejuvenated following SSA. Given that SSA can be achieved clinically, and reversibly, using currently approved agonists and antagonists of the sex steroid pathway (e.g., LHRH/GnRH), and that these improve immune function (Goldberg et al., 2009; Velardi et al., 2014), it has important implications for replenishing the diminished repertoire of lymphoid cells following damaging cytoablative treatments associated with bone marrow transplantation and cancer therapies. While the capacity for long-term BM regeneration following SSA has not yet been established, this work provides the basis for more detailed investigations into hematopoietic niche aging and SSA-induced rejuvenation, to develop more targeted strategies for HSC recovery.
Experimental Procedures
Animals
Young (2 months) or middle-aged (9 months) male C57Bl/6 or Ly5.1 mice were obtained from the Animal Resources Centre or Monash Animal Services. Il7ra−/− mice were kindly provided by A. Strasser (Walter and Eliza Hall Institute, Melbourne). All animal experimentation was carried out at Monash University in accordance with animal experiment ethics committee guidelines. Surgical removal of the testes was performed on male mice as previously described (Heng et al., 2005). Recipient mice were lethally irradiated with 1,100-cGy total body irradiation (137Cs source) as a split dose (2 × 550 cGy) separated by 3 hr and provided with antibiotics (Baytril) in water for 2 weeks after transplantation.
Cell Isolation and Analysis
Cell suspensions of the sinusoidal compartment were obtained by flushing tibias and femurs. Absolute numbers were calculated based on the total numbers of viable nucleated cells harvested from two tibias and two femurs. Osteoblasts from the endosteal niche were isolated by crushing flushed bones and digesting with 0.3% (w/v) Collagenase/Dispase (Roche) and 0.1% (w/v) DNase I (Roche) in RPMI-1640. Flow cytometric analysis and sorting was performed on FACSCalibur, FACSCanto II, FACSVantage, FACSAria, or Influx (BD Biosciences) cytometers and cell sorters. Gates were typically set using appropriate isotype controls.
Microarray and qPCR Analysis
Microarrays and bioinformatics were performed at the Australian Genome Research Facility (AGRF) using Partek v.6.5. Pathway. Analysis on LT-HSCs was conducted using Ingenuity Pathway Analysis (IPA) software. qPCR was performed using Platinum SYBR Green Supermix-UDG (Invitrogen) on a Corbett Rotor-Gene 3000 (Corbett Research). Relative levels of target mRNA was compared to GAPDH using the 2−ΔΔCt method, comparing 9-month d7SSA mice to 9-month d7shSSA control mice.
Statistical Analysis
Statistical analysis was performed using nonparametric, unpaired Mann-Whitney U test. Limiting dilution CRU frequencies were calculated by L-calc software (STEMCELL Technologies), using Poisson statistics (two-tailed test). Comparative analysis of relative gene expression in SSA mice was performed using Student’s t test.
Author Contributions
A.P.C., R.L.B., and J.A.D. conceived the studies. A.P.C., R.L.B., J.A.D., and D.M.K. designed experiments and analyzed data, with intellectual input from G.L.G. and M.R.M.v.d.B. All experiments were performed at Monash University by D.M.K., J.A.D., and M.V.H., with assistance from M.I.J., S.M.L.K., T.U., L.S., and L.F.Y. The manuscript was written and prepared by J.A.D., D.M.K., and A.P.C. with assistance from R.L.B.
Acknowledgments
We acknowledge Jade Homann, Luciana Thompson, and Jade Barbuto for surgery and animal handling; Monash and AMREP core facilities for expert cell sorting; the Australian Genome Research Facility (AGRF) for bioinformatics analyses; and Mark Malin for helpful discussions. This study was funded by grants from the Australian Stem Cell Centre and the Australian National Health and Medical Research Council. J.A.D. was supported by a CJ Martin overseas biomedical training fellowship from the Australian National Health and Medical Research Council; a Scholar Award from the American Society of Hematology; and a K99/R00 Pathway to Independence Award from the NIH (K99-CA176376).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contributor Information
Jarrod A. Dudakov, Email: dudakovj@mskcc.org.
Ann P. Chidgey, Email: ann.chidgey@monash.edu.
Accession Numbers
The NCBI GEO accession number for gene expression data reported in this paper is GSE64841.
Supplemental Information
References
- Carsetti R. The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment. J. Exp. Med. 2000;191:5–8. doi: 10.1084/jem.191.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn I.K., Blackburn C.C., Manley N.R., Sempowski G.D. Changes in primary lymphoid organs with aging. Semin. Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- de Lau W., Kujala P., Schneeberger K., Middendorp S., Li V.S., Barker N., Martens A., Hofhuis F., DeKoter R.P., Peters P.J. Peyer’s patch M cells derived from Lgr5(+) stem cells require SpiB and are induced by RankL in cultured “miniguts”. Mol. Cell. Biol. 2012;32:3639–3647. doi: 10.1128/MCB.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Montecino-Rodriguez E., Signer R.A.J. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- Dudakov J.A., Goldberg G.L., Reiseger J.J., Chidgey A.P., Boyd R.L. Withdrawal of sex steroids reverses age- and chemotherapy-related defects in bone marrow lymphopoiesis. J. Immunol. 2009;182:6247–6260. doi: 10.4049/jimmunol.0802446. [DOI] [PubMed] [Google Scholar]
- Dudakov J.A., Goldberg G.L., Reiseger J.J., Vlahos K., Chidgey A.P., Boyd R.L. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J. Immunol. 2009;183:7084–7094. doi: 10.4049/jimmunol.0900196. [DOI] [PubMed] [Google Scholar]
- Dykstra B., Olthof S., Schreuder J., Ritsema M., de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J. Exp. Med. 2011;208:2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.W., Zhang Cj., Kahn R.A., Evans T., Cerione R.A. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J. Biol. Chem. 1996;271:26850–26854. doi: 10.1074/jbc.271.43.26850. [DOI] [PubMed] [Google Scholar]
- Ferdous A., Morris J., Abedin M.J., Collins S., Richardson J.A., Hill J.A. Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc. Natl. Acad. Sci. USA. 2011;108:16307–16312. doi: 10.1073/pnas.1107341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian M.C., Dörr K., Niebel A., Daria D., Schrezenmeier H., Rojewski M., Filippi M.-D., Hasenberg A., Gunzer M., Scharffetter-Kochanek K. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. 2012;10:520–530. doi: 10.1016/j.stem.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geest C.R., Coffer P.J. MAPK signaling pathways in the regulation of hematopoiesis. J. Leukoc. Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- Geiger H., de Haan G., Florian M.C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- Gennery A.R. Immunological aspects of 22q11.2 deletion syndrome. Cell. Mol. Life Sci. 2012;69:17–27. doi: 10.1007/s00018-011-0842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G.L., Sutherland J.S., Hammet M.V., Milton M.K., Heng T.S., Chidgey A.P., Boyd R.L. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- Goldberg G.L., Alpdogan O., Muriglan S.J., Hammett M.V., Milton M.K., Eng J.M., Hubbard V.M., Kochman A., Willis L.M., Greenberg A.S. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J. Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- Goldberg G.L., King C.G., Nejat R.A., Suh D.Y., Smith O.M., Bretz J.C., Samstein R.M., Dudakov J.A., Chidgey A.P., Chen-Kiang S. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J. Immunol. 2009;182:5846–5854. doi: 10.4049/jimmunol.0801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G.L., Dudakov J.A., Reiseger J.J., Seach N., Ueno T., Vlahos K., Hammett M.V., Young L.F., Heng T.S.P., Boyd R.L., Chidgey A.P. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J. Immunol. 2010;184:6014–6024. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- Gossens K., Naus S., Corbel S.Y., Lin S., Rossi F.M.V., Kast J., Ziltener H.J. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J. Exp. Med. 2009;206:761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng T.S., Goldberg G.L., Gray D.H., Sutherland J.S., Chidgey A.P., Boyd R.L. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- Ichim C.V., Dervović D.D., Zúñiga-Pflücker J.C., Wells R.A. The orphan nuclear receptor Ear-2 (Nr2f6) is a novel negative regulator of T cell development. Exp. Hematol. 2014;42:46–58. doi: 10.1016/j.exphem.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Kim J.Y., Yu B.P., Chung H.Y. The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology. 2008;9:33–47. doi: 10.1007/s10522-007-9114-6. [DOI] [PubMed] [Google Scholar]
- Koo B.K., Clevers H. Stem cells marked by the R-spondin receptor LGR5. Gastroenterology. 2014;147:289–302. doi: 10.1053/j.gastro.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Koumakpayi I.H., Diallo J.S., Le Page C., Lessard L., Gleave M., Bégin L.R., Mes-Masson A.M., Saad F. Expression and nuclear localization of ErbB3 in prostate cancer. Clin. Cancer Res. 2006;12:2730–2737. doi: 10.1158/1078-0432.CCR-05-2242. [DOI] [PubMed] [Google Scholar]
- Kwack M.H., Sung Y.K., Chung E.J., Im S.U., Ahn J.S., Kim M.K., Kim J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Invest. Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- Liang Y., Van Zant G., Szilvassy S.J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Li S., Gan L., Kao T.P., Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res. 2008;68:10290–10299. doi: 10.1158/0008-5472.CAN-08-2038. [DOI] [PubMed] [Google Scholar]
- Loffredo F.S., Steinhauser M.L., Jay S.M., Gannon J., Pancoast J.R., Yalamanchi P., Sinha M., Dall’Osso C., Khong D., Shadrach J.L. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Fu W., Li P., Nicosia S.V., Jenster G., Zhang X., Bai W. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol. Endocrinol. 2009;23:213–225. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Merghoub T., Hobbs R.M., Dong L., Maeda M., Zakrzewski J., van den Brink M.R.M., Zelent A., Shigematsu H., Akashi K. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S.C., Tanabe K., Cras-Méneur C., Abumrad N.A., Bernal-Mizrachi E., Permutt M.A. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–859. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- Medina K.L., Garrett K.P., Thompson L.F., Rossi M.I., Payne K.J., Kincade P.W. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat. Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- Mendelson A., Frenette P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F.E., Ragu C., Scadden D.T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.P., Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J. Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- Min H., Montecino-Rodriguez E., Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- Min H., Montecino-Rodriguez E., Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J. Immunol. 2006;176:1007–1012. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Wandycz A.M., Akashi K., Globerson A., Weissman I.L. The aging of hematopoietic stem cells. Nat. Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat. Rev. Immunol. 2006;6:107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- Nakada D., Oguro H., Levi B.P., Ryan N., Kitano A., Saitoh Y., Takeichi M., Wendt G.R., Morrison S.J. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505:555–558. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J., Kitamura T., Kitamura Y., Biggs W.H., 3rd, Arden K.C., Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Nantermet P.V., Xu J., Yu Y., Hodor P., Holder D., Adamski S., Gentile M.A., Kimmel D.B., Harada S., Gerhold D. Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J. Biol. Chem. 2004;279:1310–1322. doi: 10.1074/jbc.M310206200. [DOI] [PubMed] [Google Scholar]
- Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y., Frenette P.S. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M., Urbich C., Sasaki K., Hofmann W.K., Heeschen C., Aicher A., Kollipara R., DePinho R.A., Zeiher A.M., Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald H.R. The thymus in the age of retirement. Nature. 1998;396:630–631. doi: 10.1038/25251. [DOI] [PubMed] [Google Scholar]
- Rossi D.J., Bryder D., Zahn J.M., Ahlenius H., Sonu R., Wagers A.J., Weissman I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schajnovitz A., Itkin T., D’Uva G., Kalinkovich A., Golan K., Ludin A., Cohen D., Shulman Z., Avigdor A., Nagler A. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011;12:391–398. doi: 10.1038/ni.2017. [DOI] [PubMed] [Google Scholar]
- Seib D.R., Corsini N.S., Ellwanger K., Plaas C., Mateos A., Pitzer C., Niehrs C., Celikel T., Martin-Villalba A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Sinha M., Jang Y.C., Oh J., Khong D., Wu E.Y., Manohar R., Miller C., Regalado S.G., Loffredo F.S., Pancoast J.R. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J.S., Goldberg G.L., Hammett M.V., Uldrich A.P., Berzins S.P., Heng T.S., Blazar B.R., Millar J.L., Malin M.A., Chidgey A.P., Boyd R.L. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- Taniguchi Ishikawa E., Gonzalez-Nieto D., Ghiaur G., Dunn S.K., Ficker A.M., Murali B., Madhu M., Gutstein D.E., Fishman G.I., Barrio L.C., Cancelas J.A. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc. Natl. Acad. Sci. USA. 2012;109:9071–9076. doi: 10.1073/pnas.1120358109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C.C., Liu Y., Thant L.M., Pang J., Palmer G., Alikhani M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J. Biol. Chem. 2010;285:31055–31065. doi: 10.1074/jbc.M109.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond T.S., Murante F.G., Staples J.E., Silverstone A.E., Korach K.S., Gasiewicz T.A. Role of estrogen receptor alpha in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology. 2000;141:2309–2318. doi: 10.1210/endo.141.7.7560. [DOI] [PubMed] [Google Scholar]
- Tothova Z., Kollipara R., Huntly B.J., Lee B.H., Castrillon D.H., Cullen D.E., McDowell E.P., Lazo-Kallanian S., Williams I.R., Sears C. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van der Horst A., Burgering B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Velardi E., Tsai J.J., Holland A.M., Wertheimer T., Yu V.W.C., Zakrzewski J.L., Tuckett A.Z., Singer N.V., West M.L., Smith O.M. Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J. Exp. Med. 2014;211:2341–2349. doi: 10.1084/jem.20131289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. Osteocytes, RANKL and bone loss. Nat. Rev. Endocrinol. 2011;7:693. doi: 10.1038/nrendo.2011.176. [DOI] [PubMed] [Google Scholar]
- Woolthuis C.M., de Haan G., Huls G. Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects? Curr. Opin. Immunol. 2011;23:512–517. doi: 10.1016/j.coi.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Yang S., Xu H., Yu S., Cao H., Fan J., Ge C., Fransceschi R.T., Dong H.H., Xiao G. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J. Biol. Chem. 2011;286:19149–19158. doi: 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


