Summary
The generation of in vivo repopulating hematopoietic cells from in vitro differentiating embryonic stem cells has remained a long-standing challenge. To date, hematopoietic engraftment has mostly been achieved through the enforced expression of ectopic transcription factors. Here, we describe serum-free culture conditions that allow the generation of in vivo repopulating hematopoietic cells in the absence of ectopically expressed factors. We show that repopulating activity arises immediately upon the commitment of mesodermal precursors to the blood program, within the first wave of hematopoietic specification. We establish that the formation of these progenitors is extremely transient and exquisitely sensitive to the cytokine milieu. Our findings define the precise differentiating stage at which hematopoietic repopulating activity first appears in vitro, and suggest that during embryonic stem cell differentiation, all hematopoietic programs are unraveled simultaneously from the mesoderm in the absence of cues that restrict the coordinated emergence of each lineage as is normally observed during embryogenesis.
Graphical Abstract
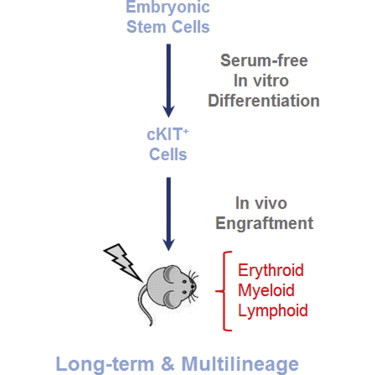
Highlights
-
•
In vivo repopulating hematopoietic cells from serum-free differentiated ESCs
-
•
Repopulating activity arises immediately upon commitment of mesoderm
-
•
In vivo repopulating progenitors are extremely transient
-
•
In vivo repopulating progenitors are exquisitely sensitive to the cytokine milieu
In this article, Kouskoff, Lacaud, and colleagues show that serum-free in vitro differentiated embryonic stem cells give rise to in vivo repopulating hematopoietic progenitors, that the repopulating activity arises immediately upon the commitment of mesodermal precursors to the blood program, and that the formation of these progenitors is extremely transient and exquisitely sensitive to the cytokine milieu.
Introduction
Recent advances in the generation, propagation, and differentiation of pluripotent stem cells (PSCs) offer great promise in the field of regenerative medicine. Both embryonic stem cells (ESCs) and induced PSCs (iPSCs) provide limitless sources of self-renewing cells endowed with the potential to generate tissue-specific cell populations that can be used in transplantation therapy (Grabel, 2012; Keller, 2005). However, one major hurdle in realizing this potential is the lack of specific and efficient protocols for differentiating these PSCs to specific populations that can be used for therapeutic applications. Although stem-cell-based regenerative medicine is still a distant goal, outstanding progress has been made in generating and engrafting ESC-derived lineages such as dopamine neurones (Kriks et al., 2011) and cardiomyocytes (Shiba et al., 2012; Yang et al., 2008). In contrast, since the first report of blood cell generation from ESCs 30 years ago (Doetschman et al., 1985), progress in deriving hematopoietic cells that are able to engraft in vivo has been rather modest. To date, the most successful in vitro derivation of hematopoietic cells capable of repopulating mouse models has relied on the ectopic expression of transcription factors such as HOXB4 (Kyba et al., 2002), CDX4 (Wang et al., 2005b), LHX2 (Kitajima et al., 2011), and RUNX1a (Ran et al., 2013). However, although HOXB4 overexpression has been shown to confer reproducible engraftment capability in differentiating mouse ESCs (Bonde et al., 2008; Kyba et al., 2002; Lesinski et al., 2012; Matsumoto et al., 2009), this approach has not been successfully translated to human ESCs (Wang et al., 2005a). An alternative approach to the use of HOXB4 in differentiated human ESCs was recently reported by Doulatov et al. (2013), who showed that the ectopic expression of transcription factors (HOXA9, ERG, RORA, SOX4, and MYB) in differentiating ESCs promotes short-term erythroid and myeloid engraftment. Few reports have documented the in vitro generation of hematopoietic repopulating potential from unmanipulated ESCs (Burt et al., 2004; Hole et al., 1996; Müller and Dzierzak, 1993; Potocnik et al., 1997). However, these approaches have not been reproduced or pursued, suggesting that they involve serum-dependent conditions that cannot be easily replicated. The use of high serum concentrations (Wang et al., 2005a) and/or stroma cell lines (Ledran et al., 2008) to support the formation of repopulating hematopoietic cells derived from human ESCs has also shown promising results, but to date, no follow-up studies have further validated or extended these differentiation protocols. It is likely that the reported successes in deriving repopulating hematopoietic cells relied on specific factors present in rare batches of serum—parameters that are impossible to control for and thus are extremely difficult to reproduce.
It is thought that a better understanding of the molecular and cellular mechanisms that regulate the emergence and maintenance of long-term repopulating hematopoietic stem cells (HSCs) during embryonic development would aid in the development of optimal protocols to generate such cells in vitro from PSCs. HSCs have been shown to emerge first from the aorta-gonad-mesonephros (AGM) region around embryonic day 10.5 (E10.5) in murine embryos (Medvinsky and Dzierzak, 1996). This occurs several days after the actual onset of hematopoietic activity, which is observed first in the yolk sac from E7.5 and next in the embryo proper from E9.0 (Palis et al., 1999). These early waves of hematopoiesis successively give rise to primitive erythroid, myeloid, definitive erythroid, and lymphoid progenitors (Costa et al., 2012; Lin et al., 2014). Several studies, including lineage tracing (Zovein et al., 2008) and in vivo imaging (Boisset et al., 2010) studies, have revealed the endothelial origin of HSCs emerging from a hemogenic endothelium (HE) population within the AGM region. Similarly, earlier waves of hematopoietic progenitors were also shown to derive from the HE (Ema et al., 2006; Lancrin et al., 2010; Nishikawa et al., 1998).
The in vitro differentiation of ESCs has been widely used as a model system to dissect and understand the early events of hematopoietic specification in terms of both molecular mechanisms and cellular steps. The careful dissection of this in vitro program has demonstrated that, similarly to in vivo development, blood cells are generated from mesodermal hemangioblast precursors through an HE intermediate (Choi et al., 1998, 2012; Eilken et al., 2009; Fehling et al., 2003; Huber et al., 2004; Kennedy et al., 2007; Lancrin et al., 2009; Wang et al., 2004) and that the same network of transcription factors orchestrates both in vivo and in vitro processes (Moignard et al., 2013). Detailed studies of the generation of primitive erythroid, myeloid, and lymphoid progenitors have suggested a temporal emergence of these blood lineages in vitro, reflecting their sequential emergence in vivo during embryonic development (Irion et al., 2010). This led to the concept that repopulating activity might emerge at late stages of the hematopoietic program during ESC differentiation (Kardel and Eaves, 2012; Lis et al., 2013; Sturgeon et al., 2013) and that the emergence of lymphoid potential marks the establishment of the definitive program (Kennedy et al., 2012; Slukvin, 2013). To date, however, attempts to derive in vivo repopulating hematopoietic cells from late stages of ESC differentiation have been largely unsuccessful. To revisit this long-standing challenge, we took an alternative approach and explored the very first step of hematopoietic specification from the mesoderm. We hypothesized that multilineage progenitors with in vivo repopulating ability might be specified very early upon commitment of mesoderm to the blood program, and might be difficult to maintain as such in the presence of serum or hematopoietic cytokines. We first evaluated the growth factor requirement for optimal specification of hemangioblast to HE. Next, defining the full hematopoietic potential of this emerging population, we observed the concomitant emergence of erythroid, myeloid, and lymphoid progenitors. Interestingly, this early population was also endowed with the capability to engraft immunocompromised mice and to confer multilineage, long-term engraftment. Further studies allowed us to define the temporal emergence of this repopulating ability and to determine the growth factor requirement and immunophenotypic characteristic of this population. Collectively, our findings demonstrate that in vitro repopulating cells emerge very rapidly from mesoderm precursors, are extremely transient, and are exquisitely sensitive to the growth factors present in the differentiating conditions.
Results
BMP4, Activin A, and VEGF Are Critically Required for the Generation of HE and CD41+ Progenitor Cells
We previously showed that a combination of BMP4, Activin A, FGF, and VEGF was sufficient to efficiently drive the formation of blood precursors from differentiating ESCs in serum-free culture conditions (Pearson et al., 2008). However, optimal specification to each differentiation stage is likely to require precise temporal exposure to cytokine stimuli. Therefore, we set out to define which cytokines were specifically required for the transition from hemangioblast to HE, and then from HE to hematopoietic progenitors, with a particular emphasis on HE, from which repopulating cells are known to emerge in vivo (Bertrand et al., 2010; Boisset et al., 2010; Kissa and Herbomel, 2010). As depicted in Figure 1A, ESCs were differentiated via embryoid body (EB) for 3 days in serum-free culture with the successive addition of BMP4 at day 0, and then Activin A and FGF at day 2.5. This sequential exposure to growth factors was previously shown to promote hemangioblast specification efficiently in developing mesoderm (Pearson et al., 2008). At day 3 of the EB culture, FLK1+ cells enriched for hemangioblast were isolated and then further cultured with no added factors, a combination of four factors (BMP4 [B], Activin A [A], FGF [F], and VEGF [V]), or various combinations of these factors. The successful differentiation of FLK1+ cells into HE was measured at day 2 of the culture by the coexpression of TIE2 and cKIT, as previously described (Lancrin et al., 2009). The efficient generation of hematopoietic progenitors was assessed at day 3 by CD41 expression, which is known to mark emerging progenitors (Ferkowicz et al., 2003; Mikkola et al., 2003). In the absence of added factors, few cells coexpressed TIE2 and cKIT (Figure 1B), and the generation of CD41+ cells was limited (Figure 1C). In contrast, the addition of all factors (BAFV) led to the detection of a substantial TIE2+cKIT+ population and the enhanced generation of CD41+ cells. Dissecting the role of each factor individually or in combination revealed that individual factors on their own and most combinations were not able to generate or maintain an HE population (Figure 1B) and/or to produce a substantial frequency of CD41+ cells (Figure 1C). Both Activin A and VEGF appeared to be critically required for the generation and maintenance of HE cells, since only culture conditions containing both factors led to the formation of a clear TIE2+cKIT+ population (AV, AFV, and BAV). As observed in V, BV, and BFV culture conditions, the absence of Activin A in the culture led to CD41 cell production associated with a decrease in TIE2+cKIT+ frequency that was already observed at day 2 (Figures 1B and 1C). In contrast, the absence of BMP4 in the culture led to a dramatic decrease in CD41+ cell production, as observed in A, AV, and AFV culture conditions, suggesting that while this factor is dispensable for the generation or maintenance of a TIE2+cKIT+ population, BMP4 is required for the emergence of CD41+ cells. To address this issue, we supplemented AV culture with BMP4 at day 2 and assayed for CD41 expression at day 3 (Figures S1A and S1B). However, the delayed addition of BMP4 did not enhance the generation of CD41+ cells, suggesting that although it does not impact the generation of a TIE2+cKIT+ immunophenotypic population from FLK1+ cells, BMP4 exposure is nonetheless critical for shaping the hematopoietic potential of this population at the onset of FLK1 differentiation. The expression of a panel of endothelial markers, such as ICAM2, FLK1, and CD144 (VE-cadherin), further revealed that the presence of both BMP4 and Activin A was critical to maintain the endothelial identity of the cKIT+ population at day 2 of the culture (Figure S1C). Only a fraction of cKIT+ cells maintained the expression of these endothelial markers when cultured in the presence of AFV or BFV. Altogether, these data revealed that the combination of BMP4, Activin A, and VEGF is critical for the generation of both HE and CD41+ cells. Interestingly, early exposure to BMP4 appears to confer hematopoietic potential to the TIE2+cKIT+ population.
Figure 1.
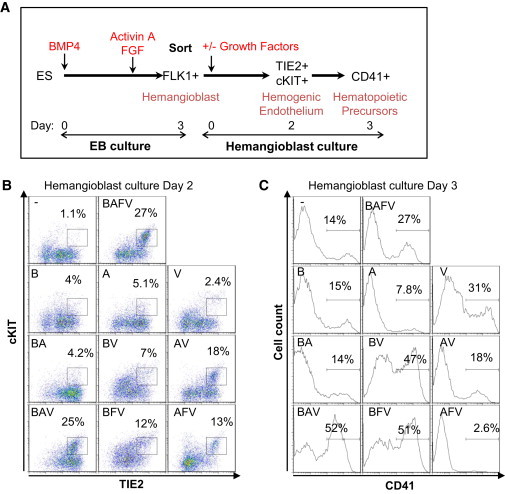
Optimal Cytokine Combinations for HE and Blood Cell Generation from FLK1+ Mesoderm Cells
(A) Schematic representation of the experimental strategy. ESCs were differentiated via embryoid body (EB) formation in serum-free culture supplemented with BMP4 at day 0 and with Activin A and FGF2 at day 2.5. FLK1+ cells sorted from day 3 EBs were seeded on gelatinized plates in serum-free media supplemented or not with cytokines (−, no cytokines; B, BMP4; A, ActivinA; V, VEGF; F, FGF2).
(B) Representative flow cytometry of cells analyzed at day 2 of the culture for the coexpression of TIE2 and cKIT marking HE.
(C) Representative flow cytometry of cells analyzed at day 3 of the culture for CD41 expression marking the emergence of blood cells. Data are representative of four independent experiments.
See also Figure S1.
Inhibitory Effect of FGF and Activin A on Further Hematopoietic Commitment
We next compared the emergence and frequency of HE when FLK1+ cells were cultured with BAV and BAFV, as these two conditions were the most effective for generating HE (Figure 1B). In both cases, a low frequency of TIE2+cKIT+ cells was observed at day 1 of the culture; the frequency of this population peaked at day 2 and decreased thereafter (Figure 2A). No noticeable differences were observed in the temporal emergence and frequency of this population regardless of whether FGF was added to the culture or not (Figures 2A and 3D). In contrast, the formation of CD41+ cells was negatively affected by the presence of FGF in the culture, with on average a 2-fold increase in the frequency of CD41+ cells produced in the absence of FGF from day 2 onward (Figures 2B and 3E). Both cultures gave rise to primitive erythroid and definitive colonies upon replating in clonogenic assays; however, FLK1+ cells cultured in the BAV condition resulted in the production of higher frequencies of hematopoietic precursors (Figure 2C), in agreement with the CD41 flow cytometry data. Altogether, these data suggest that although FGF does not affect the temporal emergence and frequency of HE, this growth factor negatively impacts the formation of hematopoietic progenitors.
Figure 2.
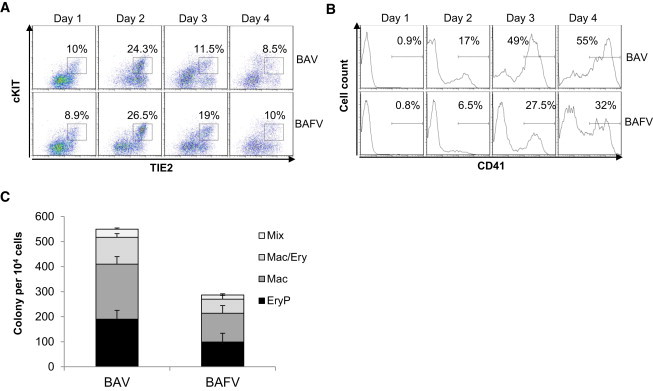
FGF2 Impairs the Generation of CD41+ Cells from HE
FLK1+ cells sorted from day 3 EBs were seeded on gelatinized plates in serum-free media supplemented with a BAV or BAFV cytokine combination (B, BMP4; A, Activin A; V, VEGF; F, FGF2).
(A and B) Cells were analyzed daily by flow cytometry for (A) coexpression of TIE2 and cKIT marking HE and (B) CD41 expression marking blood cells.
(C) At day 2 of the culture, cells were plated in clonogenic assay for hematopoietic precursors, and colonies were counted at day 5 for primitive erythroid (EryP) and day 8 for all other colonies (Mac, macrophages; Mac/Ery, macrophages and erythroid; Mix, multilineage myeloid and erythroid). Data shown are representative of at least three experiments. In (C), data are presented as the mean number of colonies from three dishes from one representative experiment; bars represent SEM.
Figure 3.
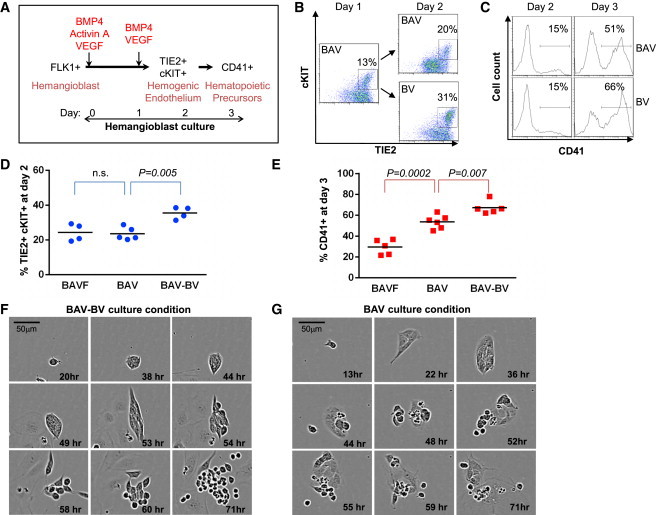
Activin A Impairs the Maintenance of HE
(A) Schematic representation of the experimental strategy. FLK1+ cells sorted from day 3 EBs were seeded on gelatinized plates in serum-free media supplemented with BAV for the first day and then with BV from day 1 onward (B, BMP4; A, Activin A; V, VEGF).
(B) Flow cytometry analysis of TIE2 and cKIT coexpression at day 1 and 2 of FLK1+ cell culture grown in a BAV or BAV-BV cytokine combination.
(C) Flow cytometry analysis of CD41 expression at days 2 and 3 of the same cultures.
(D and E) Graph of data obtained from BAFV, BAV, and BAV-BV cultures, showing the frequencies of TIE2+cKIT+ cells at day 2 (D) and CD41+ cells at day 3 (E). Each point represents an independent experiment.
(F and G) Representative time-lapse imaging of FLK1+ sorted cells cultured in serum-free media supplemented with a BAV-BV (F) or BAV (G) cytokine combination. Data shown are representative of at least three independent experiments (n.s., nonsignificant).
See also Figure S2.
We next assessed the influence of Activin A on the emergence of CD41+ progenitor cells because this factor was previously shown to negatively affect the generation of definitive hematopoiesis (Kennedy et al., 2012). For this purpose, sorted FLK1+ cells were cultured for 1 day in the presence of BMP4, Activin A, and VEGF (BAV), and then switched to media containing only BMP4 and VEGF (BV) as depicted in Figure 3A. Flow cytometric analysis revealed that removing Activin A after 1 day of culture significantly enhanced the frequency of TIE2+cKIT+ cells (Figures 3B and 3D), as well as the generation of CD41+ cells (Figures 3C and 3E). Time-lapse imaging of these cultures over a 3-day period illustrated the formation of adherent colonies of endothelial cells followed by the emergence of individual floating blood cells (Figures 3F and 3G), as previously shown in serum-supplemented cultures (Lancrin et al., 2009). The BAV-BV culture condition led to the emergence of large and healthy clusters of round floating cells (Figures 3F and S2A). In contrast, maintenance of Activin A in the culture (BAV) appeared to reduce the viability and size of the clusters of round floating cells (Figures 3G and S2B). This was further highlighted by the overall growth of the cultures in which a change of media from BAV to BV led to a marked increase in cell confluency (Figure S2C). Altogether, these data reveal that restricting the temporal exposure to Activin A is critically important for optimal specification of HE and hematopoietic precursors.
Multilineage Hematopoietic Potential of cKIT+ Cells
Having defined the optimal serum-free culture condition for the early steps of hematopoietic specification, we next evaluated the biological characteristics of cKIT-expressing cells generated in this condition. As shown by multiparameter flow cytometry analysis, cKIT+ cells coexpressed all endothelial markers tested (Figure 4A), including ICAM2 and CD40, which were previously shown to mark HE (Pearson et al., 2010). At this stage of the culture, a small fraction of cKIT+ cells also coexpressed low levels of CD41, but none expressed CD45 (Figure 4A; both of these markers are indicative of a further commitment to hematopoiesis). We next evaluated the biological potential of this cKIT+ population sorted at day 2 of BAV-BV culture. When cKIT+ cells were plated in clonogenic assays for hematopoietic progenitors, we observed the formation of both primitive erythroid colonies and definitive colonies (Figures 4B, S3A, and S3B). After 1 week, the culture of cKIT+ cells on OP9 or OP9-DL1 stroma in lymphoid-promoting conditions led to the formation of clusters of free-floating cells in the culture media and cobblestone-like areas underneath the stromal layer (Figure 4C). Analysis of cells derived from the OP9-DL1 cocultures after 3–4 weeks revealed the generation of T lymphocytes as defined by the expression of T cell-specific genes (Figure S3C); CD4, CD8, and CD3 expression; an immature CD4+CD8+ population; and low frequencies of more mature CD4+ and CD8+ cells (Figure 4D). Similarly, analysis of cells derived from OP9 cocultures demonstrated the generation of B lymphocytes as marked by the expression of B cell-specific genes (Figure S3D) and the coexpression of B220 and CD19, with a small fraction of these cells expressing immunoglobulin M (IgM; Figure 4E). Altogether, these data reveal that FLK1+ mesodermal progenitors that have grown for 2 days in the sequential presence of BMP4, Activin A, and VEGF (BAV) followed by BMP4 and VEGF (BV) give rise to a population of cKIT+ cells endowed with the capacity to generate erythroid, myeloid, and lymphoid cells.
Figure 4.
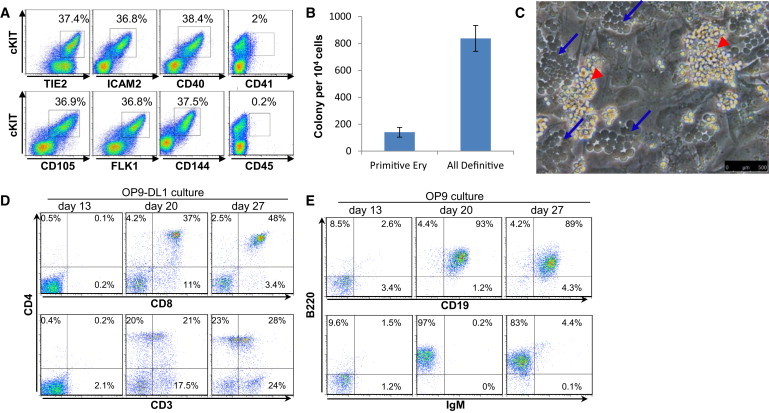
The cKIT+ Cell Population Derived from FLK1+ Hemangioblast Contains Erythroid, Myeloid, and Lymphoid Potential
(A and B) FLK1+ cells sorted from day 3 EBs were seeded on gelatinized plates in serum-free media supplemented with BAV for the first day and then with BV from day 1 onward (B, BMP4; A, Activin A; V, VEGF). At day 2 of culture, cells were analyzed for the coexpression of cKIT with a panel of endothelium and hematopoietic cell-surface markers.
(B) Day 2 sorted cKIT cells were plated in clonogenic assay for hematopoietic precursors. Primitive Ery: primitive erythrocytes; all definitive colonies: macrophages, macrophages/erythrocytes, GM, and GEMM colonies. Data are presented as the mean number of colonies from three dishes; bars represent SEM.
(C) Bright-field picture taken at 1 week of culture. Blue arrows mark cobblestone areas; red arrowheads mark free-floating hematopoietic clusters.
(D) Cells derived from OP9-DL1 culture were stained at the indicated time for the coexpression of CD4, CD8, and CD3 marking T lymphocytes.
(E) Cells derived from OP9 culture were stained at the indicated time for the coexpression of B220, CD19, and IgM marking B lymphocytes. Data shown are representative of at least three experiments.
See also Figure S3.
Long-Term and Multilineage Engraftment Potential of the cKIT+ Population
Given the in vitro multilineage capacity of the cKIT+ population isolated from FLK1+ cells that had differentiated for 2 days, we next investigated whether this population contained cells that were able to engraft in vivo. For this purpose, cKIT+ cells were isolated from BAV-BV culture at day 2 and injected into sublethally irradiated recipient mice as depicted in Figure 5A. Blood samples were taken every 4 weeks over a 16-week period and analyzed by flow cytometry for the expression of CD45.1 (marking recipient cells) and CD45.2 (marking in vitro-derived donor cells). In the initial experiments, the persistence of a small CD45.2+ population was observed in four out of ten mice over the 16-week period (Figures 5B and 5C). The in vitro origin of the CD45.2+ cells was further confirmed by detection of the Brachyury-GFP knockin allele, which was present in the starting ESC line but absent from the recipient mice (Figure 5D). Similar in vivo engraftment results were also obtained with cKIT+ cells derived from the in vitro differentiation of two other ESC lines (Figures S5D and S5G–S5J). Analysis of the lineage contribution in bone marrow and spleen at 22 weeks after engraftment revealed the presence of CD45.2+ cells expressing cell-surface markers characteristic of erythroid (TER119 and CD71), myeloid (Gr1 and CD11b), and B lymphoid (IgM and B220) lineages (Figures 5E and 5F). The B lymphocytes generated upon engraftment coexpressed IgM, CD19, and B220 (Figure S4A), and were able to secrete immunoglobulins (Figure S4B). It has been shown that embryonic hematopoietic precursors preferentially give rise to B-1 B lymphoid cells involved in innate immunity (Montecino-Rodriguez and Dorshkind, 2012). In addition to IgM expression, these B lymphocytes are characterized by the expression of CD11b and CD5 for the B-1a subset and CD11b for the B-1b subset. Given the embryonic origin of the cKIT+ donor cells, we further assessed the immunophenotype of the B lymphoid population generated upon engraftment. None of the IgM+ cells expressed CD11b or CD5, suggesting that these cells are B-2 type B lymphocytes (Figure S4C). Altogether, these data demonstrate that cKIT+ cells isolated from ESCs that have differentiated in serum-free culture with restricted temporal exposure to specific growth factors are able to confer long-term and multilineage engraftment in vivo.
Figure 5.
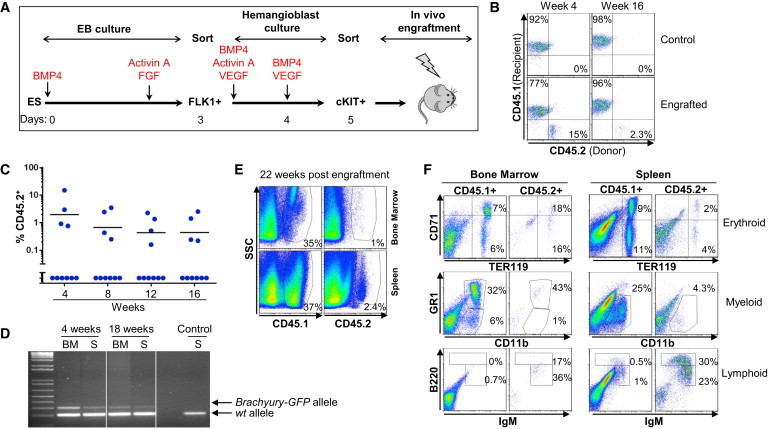
The cKIT+ Population Contains In Vivo Repopulating Activity
(A) Schematic representation of the experimental strategy.
(B) Blood cells from control and engrafted mice were stained for CD45.1 expression (marking recipient cells) and CD45.2 expression (marking ESC-derived cells).
(C) Frequency of CD45.2+ cells (blue circles) in the blood at the indicated week. Each point represents a mouse (n = 2, with 5 mice per experiment).
(D) PCR detection of the Brachyury endogenous and Brachyury-GFP knockin alleles carried by ESC-derived blood cells (BM, bone marrow; S, spleen).
(E) Bone marrow and spleen cells harvested 22 weeks after engraftment were stained for CD45.1 and CD45.2.
(F) Staining for lineage analysis is shown for CD45.1+ recipient and CD45.2+ ESC-derived cells from bone marrow and spleen at 22 weeks after engraftment. CD71 and TER119 mark erythroid cells, CD11b and GR1 mark myeloid cells, and IgM and B220 mark B lymphocytes.
See also Figure S4.
Temporal Emergence of Hematopoietic Repopulating Activity
The repopulating activity observed upon engraftment of cKIT+ cells isolated from day 2 culture was reproducible but remained low in terms of both the chimerism level and frequency of mouse repopulation (Table 1). Therefore, we explored whether we could achieve a higher repopulation activity by changing the timing of cKIT+ cell isolation during the course of FLK1 differentiation to hematopoiesis. The cKIT+ population isolated from day 3 culture was unable to engraft in vivo, whereas day 1 cKIT+ cells gave rise to reproducible engraftment capability (Figure 6A). The overall level of chimerism observed with cKIT+ cells derived from day 1 culture was higher than that observed with cells derived from day 2 culture. Given this very rapid onset of repopulating activity upon culture of FLK1+ mesodermal precursors, we also evaluated the potential of FLK1+ cells to engraft directly without further culture. However, FLK1+ cells isolated from EBs and directly injected in vivo did not give rise to any repopulation activity (Figure 6A). Altogether, these findings suggest that the repopulating ability of ESC-derived hematopoietic precursors emerges rapidly upon mesoderm specification and is very transient.
Table 1.
Summary of the In Vivo Engraftment Experiments
| Population Tested | Cytokines in FLK1 Culture | n | Engrafted Mice | Chimerism Frequency at 4 Weeks (Percentage CD45.2+) |
|---|---|---|---|---|
| FLK1+ | no further culture | 2 | 0/8 | 0 |
| cKIT+ day 1 | BAFV | 6 | 14/26 | 4, 5.9, 6.5, 0.37, 4.5, 2.32, 0.77, 21, 3.36, 2.17, 10.04, 15.66, 5.04, 16.36 |
| BAV | 2 | 3/8 | 0.4, 19.9, 15.65 | |
| AV | 1 | 1/4 | 0.43 | |
| BNVF | 1 | 0/4 | 0 | |
| BNV | 1 | 1/4 | 0.09 | |
| BFV | 1 | 0/4 | 0 | |
| cKIT+ day 2 | BAV(day 1)-BV(day 2) | 7 | 9/28 | 2.96, 15.09, 0.76, 0.91, 1.44, 0.18, 0.58, 0.44, 0.26 |
| BAV(day 1)-A(day 2) | 1 | 2/4 | 0.12, 2.9 | |
| BAV(day 1)-SB(day 2) | 1 | 2/4 | 0.19, 2.5 | |
| BAV(day 1)-B(day 2) | 1 | 1/4 | 1.5 | |
| BAV(day 1)-none(day 2) | 1 | 1/3 | 0.24 | |
| BAFV | 2 | 5/12 | 0.78, 1.04, 1.3, 2.9, 6.1 | |
| BAV | 1 | 1/8 | 1.42 | |
| BNVF | 1 | 0/4 | 0 | |
| BNV | 1 | 0/4 | 0 | |
| cKIT+ day 3 | BAV(day 1)-BV(day 2) | 2 | 0/12 | 0 |
B, BMP4; A, Activin A; V, VEGF; F, FGF2; N, Nodal; SB, SB-431542. See also Figure S5.
Figure 6.
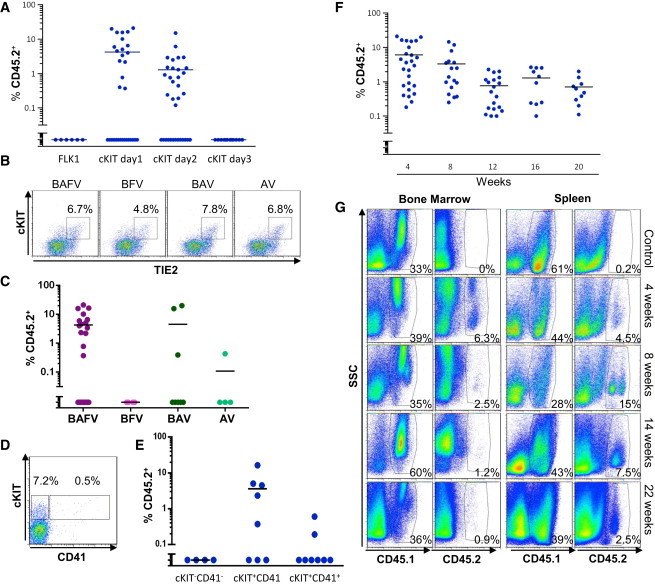
Successful Engraftment Is Highly Dependent on Cytokine Exposure
(A) Frequency of CD45.2+ cells (blue circles) in the blood of recipient mice 4 weeks after engraftment with the indicated population. The data presented in this graph for cKIT+ cells at days 1 and 2 were obtained under BAFV, BAV, and BAV-BV culture conditions. Each point represents one mouse; the number of experiments conducted for each population is shown in Table 1 (B, BMP4; A, ActivinA; V, VEGF; F, FGF2).
(B) Representative flow cytometric analysis of cKIT and TIE2 expression for cells obtained from day 1 FLK1 culture grown in the indicated cytokines.
(C) Frequency of CD45.2+ cells in the blood of recipient mice 4 weeks after engraftment with cKIT+ cells isolated from day 1 FLK1 culture grown in the indicated cytokine mix (BAFV, purple circles; BFV, pink circles; BAV, dark green circles; AV, light green circles). Each point represents one mouse; the numbers of mice and experiments are detailed in Table 1.
(D) Representative flow cytometric analysis of cKIT and CD41 expression for cells obtained from day 1 FLK1 culture grown in the presence of BAFV cytokines.
(E) Frequency of CD45.2+ cells (blue circles) in the blood of recipient mice 4 weeks after engraftment with the indicated cells isolated from day 1 FLK1 culture grown in the presence of the BAFV cytokine mix. Each point represents one mouse (n = 2, with 4 mice per experiment, except for group CD41−cKIT− with 2 mice per experiment).
(F) Frequency of CD45.2+ cells (blue circles) in the blood of recipient mice at the indicated number of weeks after engraftment. Data in this graph represent a summary of all recipients that successfully engrafted. Each point represents one mouse, n = 18 with 4 mice per experiment as detailed in Table 1.
(G) Flow cytometric analysis of CD45.1 and CD45.2 expression relative to side scatter (SSC) for bone marrow and spleen cells at the indicated weeks after engraftment.
See also Figure S6.
Growth Factor Requirement for the Emergence of Hematopoietic Repopulating Activity
We next evaluated how altering the cytokine combination during the differentiation of FLK1+ mesoderm might impact the generation of in vivo repopulating cells. In a first set of experiments, we assessed the growth factor requirement for the generation of this repopulating activity. We tested combinations of cytokines in which FLK1+ cells were cultured for the first day with BAV cytokines and then either maintained with BAV or changed to Activin A, BMP4, TGFβ inhibitor (SB-431542), or no cytokines for the second day of culture. In addition, cKIT+ cells isolated after 2 days of culture with BAFV were also tested in engraftment experiments. In all conditions tested, no improvement was observed in either the chimerism level or frequency of engrafted mice when compared with the BAV-BV cytokine combination (Table 1). Altogether, these data suggest that a variation in cytokine exposure during the second day of FLK1 culture does not affect the repopulating activity of the cKIT+ population isolated at day 2. Additionally, cKIT+ cells isolated from day 1 culture gave higher engraftment levels, suggesting that the first day of FLK1 culture is critical for determining in vivo repopulating competency. We therefore focused on the cytokine requirement for the first day of culture. Data presented in Figure 1A show that the cytokine combinations BAV, BAFV, and AV were the best conditions for generating a TIE2+cKIT+ HE population at day 2. When analyzed at day 1 of culture, the TIE2+cKIT+ population was detected at equivalent frequencies in those three cytokine mixes (Figure 6B). However, when tested in an in vivo repopulation assay, the absence of BMP4 was detrimental to engraftment, as shown by a comparison of engraftment for cKIT+ cells derived from AV and BAV (Figure 6C). Interestingly, cKIT+ cells derived from BFV culture did not give rise to any repopulating activity, even though this cytokine combination gave rise to a high frequency of blood progenitors (Figure S5A). Furthermore, although the chimerism levels observed in mice repopulated with cKIT+ cells derived from BAV and BAFV were similar, the number of mice engrafted was substantially higher in the presence of FGF (14/26; 53.8%) than in its absence (3/8; 37.5%; Figure 6C). It was recently shown that in vitro differentiated, Nodal-derived endoderm contributed in vivo to embryonic endoderm much more efficiently than Activin A-derived endoderm (Chen et al., 2013). Therefore, we assessed the in vivo repopulating ability of cKIT+ cells obtained from cultures in which Activin A was replaced by Nodal. Although Nodal was able to induce the formation of a TIE2+cKIT+ population at days 1 and 2 of FLK1+ cell culture (Figure S5B), we only observed a very low level of engraftment (0.09%) in one mouse out of 12 for all conditions tested (Figure S5C). Finally, to ensure the reproducibility and specificity of the differentiation process that is promoted by the BAFV cytokines, we performed the differentiation and engraftment protocol using BAFV cytokines from another commercial supplier. We observed similar levels of engraftment and contributions to erythroid, myeloid, and lymphoid lineages with these cytokines (Figures S5D–S5F). Altogether, these data demonstrate that very specific growth factor exposure during FLK1+ differentiation to cKIT+ is critically important to generate in vivo repopulating cells. Changes in a single cytokine can dramatically alter the potential of these in vitro-generated cells to engraft in vivo.
Immunophenotype of In Vitro-Derived Cells Endowed with Repopulation Activity
In order to refine our analysis of the cell population that harbored in vivo engraftment potential, we aimed to further define the immunophenotypic characteristics of this population. All endothelial cell-surface markers tested were coexpressed by cKIT+ cells and therefore could not be used to subfractionate the cKIT+ population. In contrast, CD41, which is known to be expressed on early embryonic HSCs (McKinney-Freeman et al., 2009; Robin et al., 2011), showed a distinct expression in a small subset of cKIT+ cells (Figure 6D). Surprisingly, however, when tested in in vivo engraftment, the cKIT+CD41− fraction was more enriched in repopulating activity than the cKIT+CD41+ fraction, whereas, as expected, the cKIT−CD41− subset was devoid of this activity (Figure 6E). These results establish that as soon as TIE2+cKIT+ HE cells acquire CD41 expression, their in vivo engrafting ability is dramatically decreased, further reinforcing the hypothesis that the potential for in vitro-derived hematopoietic cells to repopulate recipient mice is extremely transient.
Long-Term and Multilineage Engraftment
The two most fundamental characteristics of HSCs are their abilities to self-renew and to give rise to all lineages of the blood system. As shown above in Figure 5F, blood progenitor contribution to the myeloid, erythroid, and lymphoid lineages was observed at 22 weeks after engraftment, but also at all stages analyzed (Figure S6). To address the self-renewing property of these in vitro-derived repopulating cells, we followed the frequency of CD45.2+ cells in the peripheral blood of recipients for up to 20 weeks (Figure 6F), considering that engraftment past 16 weeks is a readout of long-term repopulation. From 4 to 12 weeks after engraftment, we observed a progressive decline in the contribution of donor cells, with the frequency of these cells plateauing from 12 weeks onward. Analysis of donor cell contributions in the bone marrow and spleen revealed that initially the frequency of CD45.2+ cells was high in the bone marrow and low in the spleen (Figure 6G), a ratio that was reversed over time. By 22 weeks, the contribution to the bone marrow was low but still clearly detectable. Altogether, these data suggest that in vitro-derived repopulating cells are able to provide long-term multilineage engraftment, but their self-renewing ability is not as robust as that of in vivo-derived repopulating cells. In the future, it will be important to determine whether this is an intrinsic property of in vitro-derived repopulating cells or whether self-renewal can be modulated by the culture conditions.
Discussion
The present study defines the temporal emergence of repopulating activity in vitro, identifying the precise step at which this population is generated. Altogether, our data establish that the presence of this repopulating activity is remarkably transient and the emergence/maintenance of this cell population is critically dependent on a precise set of growth factors.
To investigate the presence of long-term in vivo repopulating hematopoietic progenitor cells during ESC differentiation, we made the assumption that this activity might emerge rapidly upon specification of the hematopoietic program in mesoderm precursors, and we implemented several experimental strategies to test this hypothesis. First, the differentiating conditions were designed to avoid exposure to serum and cytokines such as SCF, IL6, and IL3 to prevent further differentiation of newly emerging hematopoietic progenitors as much as possible. Second, we only considered cKIT expression as a potential marker of repopulating activity, since to date it is the only cell-surface marker that has been shown to be expressed on all HSCs throughout embryonic development and adulthood (all other markers are either expressed transiently during embryonic development or are only expressed on adult HSCs). Finally, to test for engraftment potential, we used sublethally conditioned recipients, reasoning that, in contrast to lethal irradiation, the remaining endogenous hematopoietic system would allow recipient mice to survive even with very low levels of contribution from the donor cells. A possible drawback to this approach is that the full engraftment potential might be underestimated due to competition between recipient and donor cells. However, the combination of these various experimental settings allowed us to reproducibly monitor the serum- and stroma-free generation of ESC-derived repopulating hematopoietic cells.
Our data demonstrate that both the emergence and maintenance of in vivo repopulating cells are extremely sensitive to the cytokine milieu: a change in a single factor will completely abolish the detection of engrafting cells. When we compared the outcome of FLK1 cells cultured in the presence of BAFV versus BFV, the absence of Activin A dramatically affected the detection of in vivo engrafting cells, as they were most likely rapidly pushed toward differentiation when exposed only to BFV. Taken together, our results strongly suggest that the signaling pathway activated by Activin A, but not Nodal, is critical, but not sufficient, for maintaining the repopulating ability of the TIE2+cKIT+ population. The addition of FGF seems to reinforce the role of Activin A; however, on its own, FGF is not able to maintain the population of engrafting hematopoietic progenitors. Finally, BMP4 signaling appears to be critical for conferring hematopoietic competency to the HE, an observation that is consistent with the known role of BMP4 in the regulation of Runx1 (Burns et al., 2005; Pimanda et al., 2007). Although our study identifies differentiating conditions that allow the detection of engrafting hematopoietic cells, further work will be required to improve and optimize the culture conditions for the maintenance and expansion of these repopulating cells.
An important conceptual aspect of our findings relates to the relationship between ESC-derived repopulating cells and their in vivo counterparts. Based on their immunophenotypic characteristics, the ESC-derived cells more closely resemble the VE-cad+CD45−CD41low pre-HSC type I population identified in the AGM region at E11.5 (Rybtsov et al., 2011). However, these type I pre-HSCs express a low level of CD41 and do not engraft recipients unless they are cocultured for 4 days with OP9, in contrast to ESC-derived repopulating cells, which do not express CD41 and are able to engraft directly, albeit when injected intrafemorally. Based on their limited self-renewal characteristics, the ESC-derived engrafting cells might correspond to lineage-committed progenitors with a repopulating ability in which the multilineage potential is dissociated from the self-renewal capacity, as recently described for adult mouse bone marrow progenitors (Yamamoto et al., 2013). An interesting finding in our study is the concomitant emergence of primitive erythroid, myeloid, definitive erythroid, and lymphoid potential very early upon mesoderm specification. This is in contrast to previous studies that reported the sequential generation of these progenitors during in vitro differentiation of ESCs (Keller et al., 1993; Kennedy et al., 2012; Rafii et al., 2013). In those studies, serum-supplemented factors may have conditioned or altered the timing of differentiation and delayed the emergence of specific progenitor subsets. During embryonic development, the emergence of hematopoietic progenitors occurs in successive waves, with primitive erythroid progenitors emerging first around E7.25, followed by erythro-myeloid progenitors from E8.25 and lymphoid progenitors from E9, whereas definitive HSCs are only detected from E10.5 onward (Costa et al., 2012; Lin et al., 2014). One possible explanation to account for our findings is that during serum-free ESC differentiation, all hematopoietic programs unravel simultaneously because there are no extrinsic factors restricting or altering the developmental timing for the emergence of each lineage. During embryonic development, these cues are provided by the microenvironment in which these precursors reside.
The present study establishes a first critical step toward the generation of in vitro-derived, repopulating hematopoietic cells that might be suitable for therapeutic applications. Further work will need to be carried out to translate this protocol to the differentiation of human ESCs and iPSCs.
Experimental Procedures
ESC Growth and Differentiation
Unless specified otherwise, the ESC line used in this study is an E14.1 (129/ola) carrying a GFP reporter cassette knocked in the Brachyury locus (Fehling et al., 2003). The F1 (129/B6) and RI (129/sv) ESC lines were also tested for engraftment potential. ESCs were maintained on irradiated mouse embryonic fibroblasts in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 50 μg/ml penicillin-streptomycin (GIBCO), 15% fetal calf serum (FCS; PAA Laboratories), 1% leukemia inhibitory factor (conditioned medium from Chinese hamster ovary cells), and 1.5 × 10−4 M monothioglycerol (Sigma). Prior to differentiation, ESCs were passaged twice on gelatinized tissue-culture-grade plates to remove the mouse embryonic fibroblasts. For the first passage, ESCs were grown in DMEM supplemented as above, and the second passage was performed in Iscove’s modified Dulbecco’s medium supplemented as above. For gelatin treatment, dishes were coated for 20 min with 0.1% w/v gelatin in ddH20. For EB generation, ESCs were trypsinized and plated at 50,000 cells/ml in petri-grade dishes in StemPro-34 SFM (GIBCO) supplemented with 2 mM L-glutamine (GIBCO), transferrin (Roche), 0.5 mM ascorbic acid (Sigma), and 4.5 × 10−4 M monothioglycerol (Sigma). BMP4, bFGF, Activin A, Nodal, and VEGFa (R&D Systems or PeproTech) were used at 5 ng/ml unless otherwise stated. For hemangioblast culture, FLK1+ sorted cells were seeded on gelatinized plates in StemPro-34 SFM supplemented as above with the addition of cytokines as stated for each experiment.
Mice Engraftments
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice were purchased from The Jackson Laboratory and bred in-house or at Harlan. Male mice were irradiated with a 125 cGy sublethal dose and injected intrafemorally with sorted cells. Depending on the experiment, between 105 and 5 × 105 sorted cells were injected per mouse. All animal work was performed in accordance with regulations established by Home Office Legislation under the 1986 Animal Scientific Procedures Act.
Author Contributions
S.P., S.C., and M.F. performed the research and analyzed the data. V.K. and G.L. designed and supervised the research, analyzed the data, and wrote the manuscript.
Acknowledgments
The authors thank members of V.K. and G.L.’s laboratories for critical readings of the manuscript, and the Cancer Research UK Manchester Institute facilities for technical support. Our work is funded by Cancer Research UK and the BBSRC.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Contributor Information
Georges Lacaud, Email: georges.lacaud@cruk.manchester.ac.uk.
Valerie Kouskoff, Email: valerie.kouskoff@cruk.manchester.ac.uk.
Supplemental Information
References
- Bertrand J.Y., Chi N.C., Santoso B., Teng S., Stainier D.Y., Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisset J.C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- Bonde S., Dowden A.M., Chan K.M., Tabayoyong W.B., Zavazava N. HOXB4 but not BMP4 confers self-renewal properties to ES-derived hematopoietic progenitor cells. Transplantation. 2008;86:1803–1809. doi: 10.1097/TP.0b013e31818fe741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C.E., Traver D., Mayhall E., Shepard J.L., Zon L.I. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R.K., Verda L., Kim D.A., Oyama Y., Luo K., Link C. Embryonic stem cells as an alternate marrow donor source: engraftment without graft-versus-host disease. J. Exp. Med. 2004;199:895–904. doi: 10.1084/jem.20031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.E., Borowiak M., Sherwood R.I., Kweudjeu A., Melton D.A. Functional evaluation of ES cell-derived endodermal populations reveals differences between Nodal and Activin A-guided differentiation. Development. 2013;140:675–686. doi: 10.1242/dev.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K., Kennedy M., Kazarov A., Papadimitriou J.C., Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Choi K.D., Vodyanik M.A., Togarrati P.P., Suknuntha K., Kumar A., Samarjeet F., Probasco M.D., Tian S., Stewart R., Thomson J.A., Slukvin I.I. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G., Kouskoff V., Lacaud G. Origin of blood cells and HSC production in the embryo. Trends Immunol. 2012;33:215–223. doi: 10.1016/j.it.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Doetschman T.C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Doulatov S., Vo L.T., Chou S.S., Kim P.G., Arora N., Li H., Hadland B.K., Bernstein I.D., Collins J.J., Zon L.I., Daley G.Q. Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13:459–470. doi: 10.1016/j.stem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken H.M., Nishikawa S., Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Ema M., Yokomizo T., Wakamatsu A., Terunuma T., Yamamoto M., Takahashi S. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood. 2006;108:4018–4024. doi: 10.1182/blood-2006-03-012872. [DOI] [PubMed] [Google Scholar]
- Fehling H.J., Lacaud G., Kubo A., Kennedy M., Robertson S., Keller G., Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- Ferkowicz M.J., Starr M., Xie X., Li W., Johnson S.A., Shelley W.C., Morrison P.R., Yoder M.C. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- Grabel L. Prospects for pluripotent stem cell therapies: into the clinic and back to the bench. J. Cell. Biochem. 2012;113:381–387. doi: 10.1002/jcb.23364. [DOI] [PubMed] [Google Scholar]
- Hole N., Graham G.J., Menzel U., Ansell J.D. A limited temporal window for the derivation of multilineage repopulating hematopoietic progenitors during embryonal stem cell differentiation in vitro. Blood. 1996;88:1266–1276. [PubMed] [Google Scholar]
- Huber T.L., Kouskoff V., Fehling H.J., Palis J., Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Irion S., Clarke R.L., Luche H., Kim I., Morrison S.J., Fehling H.J., Keller G.M. Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development. 2010;137:2829–2839. doi: 10.1242/dev.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardel M.D., Eaves C.J. Modeling human hematopoietic cell development from pluripotent stem cells. Exp. Hematol. 2012;40:601–611. doi: 10.1016/j.exphem.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Keller G., Kennedy M., Papayannopoulou T., Wiles M.V. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., D’Souza S.L., Lynch-Kattman M., Schwantz S., Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., Awong G., Sturgeon C.M., Ditadi A., LaMotte-Mohs R., Zúñiga-Pflücker J.C., Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Kissa K., Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Kitajima K., Minehata K., Sakimura K., Nakano T., Hara T. In vitro generation of HSC-like cells from murine ESCs/iPSCs by enforced expression of LIM-homeobox transcription factor Lhx2. Blood. 2011;117:3748–3758. doi: 10.1182/blood-2010-07-298596. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M., Perlingeiro R.C., Daley G.Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lancrin C., Sroczynska P., Stephenson C., Allen T., Kouskoff V., Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancrin C., Sroczynska P., Serrano A.G., Gandillet A., Ferreras C., Kouskoff V., Lacaud G. Blood cell generation from the hemangioblast. J. Mol. Med. 2010;88:167–172. doi: 10.1007/s00109-009-0554-0. [DOI] [PubMed] [Google Scholar]
- Ledran M.H., Krassowska A., Armstrong L., Dimmick I., Renström J., Lang R., Yung S., Santibanez-Coref M., Dzierzak E., Stojkovic M. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Lesinski D.A., Heinz N., Pilat-Carotta S., Rudolph C., Jacobs R., Schlegelberger B., Klump H., Schiedlmeier B. Serum- and stromal cell-free hypoxic generation of embryonic stem cell-derived hematopoietic cells in vitro, capable of multilineage repopulation of immunocompetent mice. Stem Cells Transl. Med. 2012;1:581–591. doi: 10.5966/sctm.2012-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Yoder M.C., Yoshimoto M. Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev. 2014;23:1168–1177. doi: 10.1089/scd.2013.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis R., Rafii S., James D. Wading through the waves of human embryonic hemogenesis. Cell Cycle. 2013;12:859–860. doi: 10.4161/cc.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Isagawa T., Nishimura T., Ogaeri T., Eto K., Miyazaki S., Miyazaki J., Aburatani H., Nakauchi H., Ema H. Stepwise development of hematopoietic stem cells from embryonic stem cells. PLoS ONE. 2009;4:e4820. doi: 10.1371/journal.pone.0004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney-Freeman S.L., Naveiras O., Yates F., Loewer S., Philitas M., Curran M., Park P.J., Daley G.Q. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009;114:268–278. doi: 10.1182/blood-2008-12-193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A., Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Mikkola H.K., Fujiwara Y., Schlaeger T.M., Traver D., Orkin S.H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- Moignard V., Woodhouse S., Fisher J., Göttgens B. Transcriptional hierarchies regulating early blood cell development. Blood Cells Mol. Dis. 2013;51:239–247. doi: 10.1016/j.bcmd.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A.M., Dzierzak E.A. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993;118:1343–1351. doi: 10.1242/dev.118.4.1343. [DOI] [PubMed] [Google Scholar]
- Nishikawa S.I., Nishikawa S., Hirashima M., Matsuyoshi N., Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- Palis J., Robertson S., Kennedy M., Wall C., Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- Pearson S., Sroczynska P., Lacaud G., Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- Pearson S., Lancrin C., Lacaud G., Kouskoff V. The sequential expression of CD40 and Icam2 defines progressive steps in the formation of blood precursors from the mesoderm germ layer. Stem Cells. 2010;28:1089–1098. doi: 10.1002/stem.434. [DOI] [PubMed] [Google Scholar]
- Pimanda J.E., Donaldson I.J., de Bruijn M.F., Kinston S., Knezevic K., Huckle L., Piltz S., Landry J.R., Green A.R., Tannahill D., Göttgens B. The SCL transcriptional network and BMP signaling pathway interact to regulate RUNX1 activity. Proc. Natl. Acad. Sci. USA. 2007;104:840–845. doi: 10.1073/pnas.0607196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocnik A.J., Kohler H., Eichmann K. Hemato-lymphoid in vivo reconstitution potential of subpopulations derived from in vitro differentiated embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1997;94:10295–10300. doi: 10.1073/pnas.94.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S., Kloss C.C., Butler J.M., Ginsberg M., Gars E., Lis R., Zhan Q., Josipovic P., Ding B.S., Xiang J. Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood. 2013;121:770–780. doi: 10.1182/blood-2012-07-444208. [DOI] [PubMed] [Google Scholar]
- Ran D., Shia W.J., Lo M.C., Fan J.B., Knorr D.A., Ferrell P.I., Ye Z., Yan M., Cheng L., Kaufman D.S., Zhang D.E. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121:2882–2890. doi: 10.1182/blood-2012-08-451641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin C., Ottersbach K., Boisset J.C., Oziemlak A., Dzierzak E. CD41 is developmentally regulated and differentially expressed on mouse hematopoietic stem cells. Blood. 2011;117:5088–5091. doi: 10.1182/blood-2011-01-329516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Sobiesiak M., Taoudi S., Souilhol C., Senserrich J., Liakhovitskaia A., Ivanovs A., Frampton J., Zhao S., Medvinsky A. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med. 2011;208:1305–1315. doi: 10.1084/jem.20102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y., Fernandes S., Zhu W.Z., Filice D., Muskheli V., Kim J., Palpant N.J., Gantz J., Moyes K.W., Reinecke H. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slukvin I.I. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013;122:4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon C.M., Ditadi A., Clarke R.L., Keller G. Defining the path to hematopoietic stem cells. Nat. Biotechnol. 2013;31:416–418. doi: 10.1038/nbt.2571. [DOI] [PubMed] [Google Scholar]
- Wang L., Li L., Shojaei F., Levac K., Cerdan C., Menendez P., Martin T., Rouleau A., Bhatia M. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang L., Menendez P., Shojaei F., Li L., Mazurier F., Dick J.E., Cerdan C., Levac K., Bhatia M. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J. Exp. Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yates F., Naveiras O., Ernst P., Daley G.Q. Embryonic stem cell-derived hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R., Morita Y., Ooehara J., Hamanaka S., Onodera M., Rudolph K.L., Ema H., Nakauchi H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126. doi: 10.1016/j.cell.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Yang L., Soonpaa M.H., Adler E.D., Roepke T.K., Kattman S.J., Kennedy M., Henckaerts E., Bonham K., Abbott G.W., Linden R.M. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Zovein A.C., Hofmann J.J., Lynch M., French W.J., Turlo K.A., Yang Y., Becker M.S., Zanetta L., Dejana E., Gasson J.C. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


