Abstract
Background
Wound infections are traditionally thought to occur when microbial burden exceeds the innate clearance capacity of host immune system. Here we introduce the idea that the wound environment itself plays a significant contributory role to wound infection.
Methods
We developed a clinically relevant murine model of soft tissue infection to explore the role of activation of microbial virulence in response to tissue factors as a mechanism by which pathogenic bacteria cause wound infections. Mice underwent abdominal skin incision and light muscle injury with a crushing forceps versus skin incision alone followed by topical inoculation of P. aeruginosa. Mice were sacrificed on postoperative day 6 and abdominal tissues analyzed for clinical signs of wound infection. To determine if specific wound tissues components induce bacterial virulence, P. aeruginosa was exposed to skin, fascia, and muscle.
Results
Gross wound infection due to P. aeruginosa was observed to be significantly increased in injured tissues vs non-injured (80% vs 10%) tissues (n=20/group, p<0.0001). Exposure of P. aeruginosa to individual tissue components demonstrated that fascia significantly induced bacterial virulence as judged by the production of pyocyanin, a redox-active phenazine compound known to kill immune cells. Whole genome transcriptional profiling of P. aeruginosa exposed to fascia demonstrated activation of multiple genes responsible for the synthesis of the iron scavenging molecule pyochelin.
Conclusion
We conclude that wound elements, in particular fascia, may play a significant role in enhancing the virulence of P. aeruginosa and may contribute to the pathogenesis of clinical wound infection.
Keywords: wound infection, fascia, Pseudomonas aeruginosa, pyochelin, pyocyanin
Background
The development of a wound infection following elective surgery or traumatic injury remains a major cause of morbidity to patients and can incur enormous costs and disability (1). Current strategies to prevent wound infections focus on decreasing exposure of tissues to bacteria, improving tissue integrity and defenses, and administering antibiotics. Most often the pathogenesis of wound infection is framed as simple matter of the size of the bacterial inoculum pitted against host clearance mechanisms. Yet missing in this equation is the dynamic response of bacteria that can shift their phenotype in response to “cues” released by local conditions in the wound. While it is well established that host tissues dynamically respond to bacterial invasion via pathogen recognition molecules (2), it is now becoming increasingly clear that bacteria similarly respond to host tissues and the signals they release (3). Thus there is a complex molecular dialogue that develops in tissues exposed to microorganisms and is a context dependent. Here we posit that the outcome of a healing wound from bacterial exposure is not merely a matter of the number of the bacteria pitted against the resilience of the host tissues, but rather is a dynamic process by which ongoing information processing occurs between the microbe and host tissues during healing. This hypothesis could explain many of the risk factors that are associated with increased wound infection such as blood loss, hypoxia, operative time, tissue trauma, etc. (4). Although such risk factors certainly might decrease the clearance mechanisms of tissues, they also in turn, might release soluble host factors which stimulate bacteria to express a more virulent phenotype. Bacteria with more evolved information processing systems, such as the well described quorum sensing system of Pseudomonas aeruginosa (5), might be better positioned to express a more invasive phenotype during traumatic conditions.
Here we modeled the activation of bacterial virulence using a prototype P. aeruginosa strain that is well characterized for its ability to respond to local environmental cues. Our laboratory has previously reported that P. aeruginosa can express quorum sensing dependent virulence activation in response to several host compensatory cues such as opioids (morphine, dynorphin) (6, 7), ischemic end products (adenosine) (8), and inflammatory mediators (interferons) (9). Here we developed a wound infection model in which we demonstrated that despite equal inocula, P. aeruginosa can lead to a wound infection when tissues are traumatically injured. In particular we identify structural component of tissues (such as fascia) that appear to contain factors resulting in the production of virulent P. aeruginosa through scavenging of iron ions and affecting host immune system. The data herein provided shed new light on mechanisms of wound infection that can be further interrogated in molecular detail.
Methods
Bacterial Strains
Pseudomonas aeruginosa MPAO1(10) and luminescent strain XEN41 (11) were used in the experiments. Strain XEN41 (Calpier, Inc) is a constitutively luminescent derivative of PAO1.
Murine Model of Surgical Site Infection
All experiments were approved by the Animal Care and use Committee at the University of Chicago (IACUC Protocol 72090). Specific pathogen free C57B/L6 mice weighing 18 to 22 g and 7-8 weeks old were used for all experiments. Mice were routinely fed tap water and Harland Teklad feed under 12 hour light/dark cycles and were allowed to acclimate for at least 48 hours prior to surgery. Mice were anesthetized with a combination of 90 mg/kg of ketamine and 5 mg/kg of xylazine via intraperitoneal injection. Once anesthetized, mice were restrained supine, shaved in their lower abdomens, and draped in a sterile field. The abdominal wall was sterilized with betadine solution. A vertical lower abdominal incision was made approximately 1 cm in length and only through the depth of the skin and the abdominal wall muscles were exposed on both sides. To simulate tissue trauma, a small needle driver was used to crush three small areas (2-3 mm2) of muscle injury to the right rectus abdominus, leaving the other side intact; for controls, no tissue injury was performed. The bacterial inoculum of P. aeruginosa was applied to the surgical site and left for 15 minutes. Briefly, bacterial cells grown overnight on TSB agar were suspended into a 10% glycerol solution to an optical density (OD 600nm) of 0.05 (2.5 × 106 CFU/ml) for P. aeruginosa, and 10 μL of the bacterial suspension was mixed with 190 μL of a solution containing homogenized sterile mouse feces suspended in sterile normal saline (0.9% NaCl). The bacterial inoculum was used within two hours of preparation. After inoculation, the surgical site was irrigated using 4 mL of sterile saline solution to wash off the inoculum and the skin was closed using two interrupted 4-0 silk sutures. Mice were observed for 7 days for development of a surgical site infection, which was defined as the presence of pus within the healing wound.
Quantitative Cultures
In vivo growth of P. aeruginosa was examined by quantitative tissue cultures. Groups of mice in reiterative experiments were sacrificed on post-operative days 1, 3 and 7 and a 1 × 1 cm area of abdominal wall (including muscle and fascia) was dissected from the previously injured site. Tissue samples were homogenized in 1 mL of sterile normal saline solution, and serial 10-fold dilutions of the homogenates were plated onto Pseudomonas Isolation Agar for overnight growth in 37°C. Results were expressed as CFU per gram of mouse tissue.
Bioluminescent Imaging
Photon-camera imaging was used to determine the in vivo surface distribution patterns of P. aeruginosa. A bacterial inoculum of 107 CFU/ml of bioluminescent strain XEN41 was prepared and applied to our murine model of wound infection. Mice were sacrificed at 4, 24, and 48 hours, and skin was excised over the prior surgical site to expose the abdominal wall surface for imaging using the Xenogen IVIS-200 system.
Ex vivo experiments of P. aeruginosa exposed to tissue components
To determine which components of murine tissue were capable of activating bacterial virulence, ex vivo tissue homogenates were prepared. For this, 7-8 weeks old C57BL/6 mice weighing 18-22 grams were sacrificed and their skin, anterior rectus muscle, and fascia were collected. Samples were normalized by weight (20 mg) and homogenized into 1 mL of TY medium consisting of 10 g/L tryptone (EMD) and 5 g/L yeast extract (Sigma). P. aeruginosa MPAO1 culture grown overnight in TY medium was diluted (1:5,000) in fresh TY medium, and 10 μL of this inoculum was added to each 1 ml of tissues component homogenate solution. Samples were incubated at 37°C for 24 hours, after which pyocyanin was extracted into chloroform followed by re-extraction into 0.2 N HCl and measured as previously described (12).
Microarray Analysis
To investigate the transcriptional changes of P. aeruginosa in response to fascial tissue, total RNA was isolated using the Ribopure Bacteria Kit (Invitrogen) after 12 hours of incubation under conditions described above. Three biological replicates in each group were used. Prior to RNA isolation, samples were preserved in 2 mL of RNA Protect (Qiagen) to stabilize RNA. DNase I treatment was performed using DNA-free DNase kit (Ambion). RNA quality and quantity were assessed using the Agilent Bioanalyzer 2100 electrophoresis system (Agilent Technologies) and the Nanodrop ND-1000 spectrophotometer (Thermal Scientific), The cDNA synthesis, fragmentation, and labeling were performed according to the protocol for GeneChip®R Pseudomonas aeruginosa Genome Array (Affymetrix). Microarray slides were scanned using the 2500 GeneArray Scanner (Agilent Technologies). Gene expression values were calculated and normalized using the “gcrma” function (http://watson.nci.nih.gov/bioc_mirror/packages/2.13/bioc/manuals/gcrma/man/gcrma.pdf) in the R (v3.02) Bioconductor “affy” (v1.40.0) package (13). Robust multichip average (RMA) approach was used for background correction and quantile normalization of expressional data. To identify differentially expressed genes, we used Significance Analysis of Microarrays (SAM) (14) with two-class un-paired comparisons. The criteria for differentially expressed genes were set at false discover rate (FDR) <10% and fold change >1.4. The microarray data have been deposited in NCBI's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) under the GEO Series accession number GSE61925.
Statistical Analysis
Z-tests were used to determine statically significant differences between rates of wound infections. A student's unpaired t-test was used to test for significant differences in quantitative cultures and pyocyanin production.
Results
Tissue injury significantly increases the rate of post-operative soft tissue Infection due to P. aeruginosa
The two-hit model of murine wound infection included wound trauma combined with contamination by P. aeruginosa (total ∼ 104 cells). In the majority of mice (i.e., 80%), wound infection developed as indicated by visible pus directly overlying the site of injury (Figure 1A, right panel). Wound trauma alone or P. aeruginosa contamination of non-traumatized wounds resulted in a negligible amount of pus development (Fig.1A, left panel). The percentage of post-operative soft-tissue infections is presented in Fig.1B (n=20/group), where a significant increase (p<0.001) in purulence was observed in mice subjected to tissue injury+ P. aeruginosa contamination.
Figure 1. Tissue injury significantly increases the rate of post-operative soft tissue Infection.
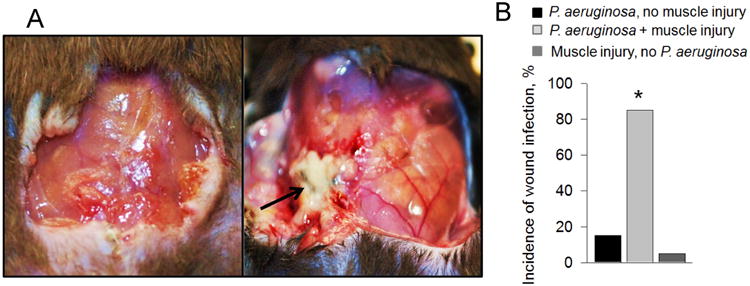
(A) Clinical soft tissue infection defined as pus localized to the wound cavity was observed by POD #7 in injured (right panel, shown by arrow) but not in non-injured (left panel), as demonstrated by the mouse that received tissue injury (right). (B) The injured group demonstrated a significantly higher (*p<0.001) rate of post-operative soft tissue infection for P. aeruginosa (n=20/group).
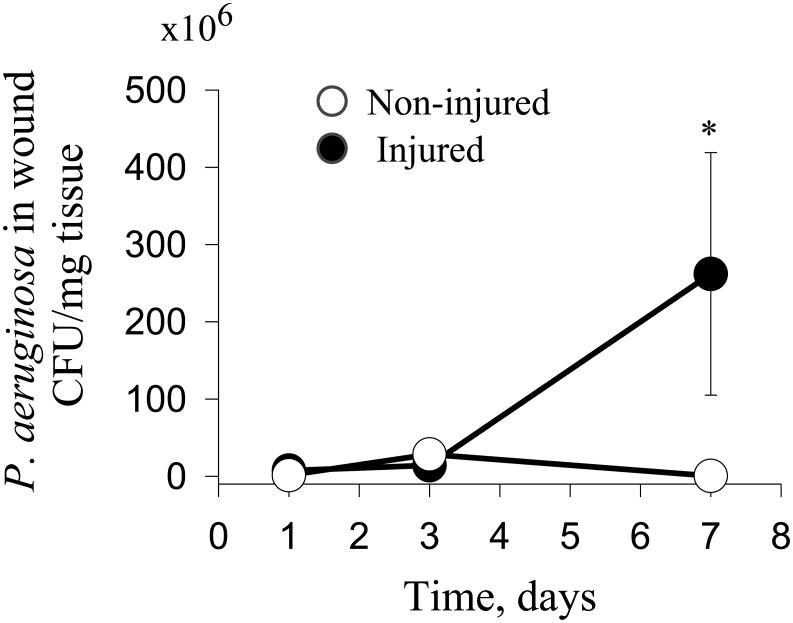
The number of P. aeruginosa cells is similar in injured vs non-injured groups up to day 3
In order to show in the early progression of the model that the bacterial inoculum remained the same in both muscle injured and non-injured mice, we performed reiterative experiments in mice sacrificed on POD1, POD3, and POD7. At sacrifice, abdominal tissues were homogenized and then cultured on Pseudomonas isolation agar plates. Numbers of colonies were normalized to the weight of isolated tissues. Results demonstrated negligible differences in bacterial counts between the groups at sacrifice on POD1 and POD3. However, by POD7, P. aeruginosa growth in wounds with injured muscle increased while growth in the non-injured muscles decreased (Fig.2).
Figure 2. Quantitation of P. aeruginosa in traumatized and non-traumatized wounds.
Quantitative cultures of P. aeruginosa demonstrated no significant differences in the early post-operative period (POD3) (n=5, p=0.14). However, significant differences in bacterial counts were observed on POD7 (*n=5, p<0.05).
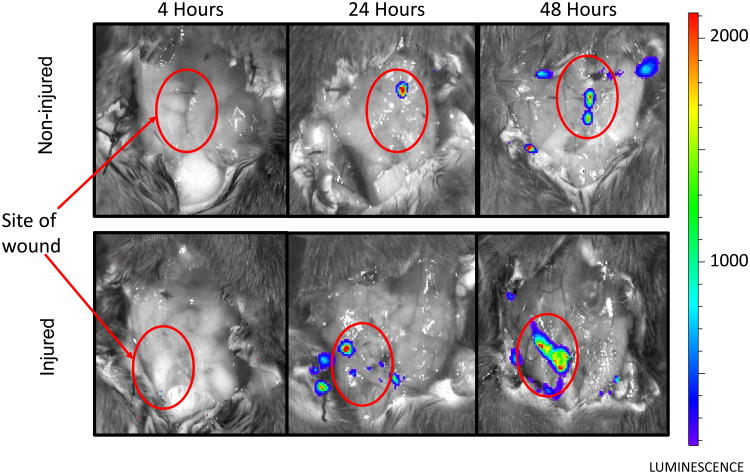
Localization of P. aeruginosa following in vivo inoculation
In vivo bioluminescent imaging was used to track the surface colonization patterns of P. aeruginosa in murine model of wound infection. In non-injured mice, the growth of P. aeruginosa was observed.in the center of the wound cavity, immediately under the incision site. In group of mice with injured tissues, bacteria mostly localized to the site of trauma. This led us to hypothesize that the exposure of P. aeruginosa to some tissue components may play a role in the development of clinical infection.
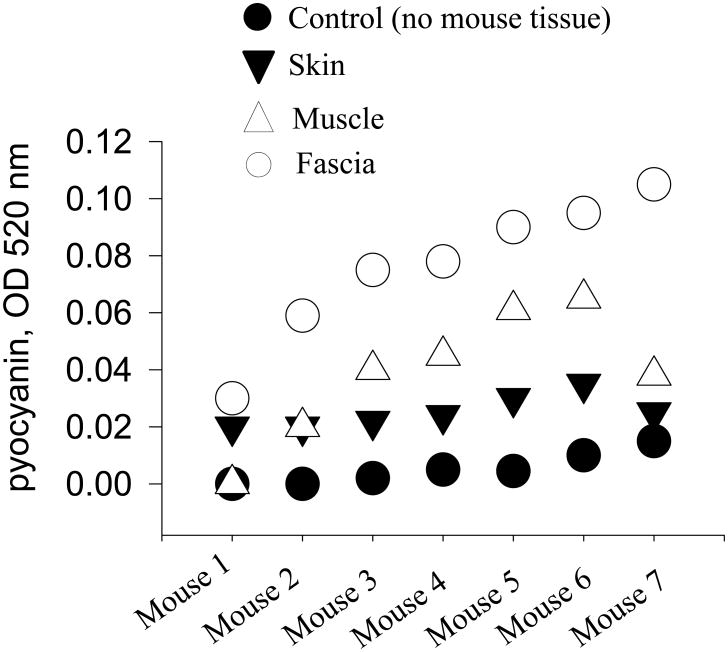
Fascial tissues causre the greatest activation of the virulence of P. aeruginosa Ex Vivo
To determine the independent effect of wound components on P. aeruginosa virulence, wound tissue components (i.e., skin, muscle and fascia) were isolated and homogenized. The homogenized components were co-incubated with P. aeruginosa. Bacterial virulence activation was assayed by pyocyanin production, an important virulence factor capable of inducing apoptosis in neutrophils (15). After 24 hours of incubation, while all tissue homogenates appeared to induce the production of pyocyanin, only fascia was observed to achieve statistically significant effect (Fig. 4, p<0.001).
Figure 4. Pyocyanin production by P. aeruginosa upon the contact with components of wound tissues.
Ex-vivo tissue experiments performed to test for virulence activation of P. aeruginosa (measured as pyocyanin production) demonstrated significant increases by fascia compared to control (growth in TY medium without addition of mouse tissues) (p<0.001). Each symbol represents a mouse wound component incubated with P. aeruginosa in TY medium. Pyocyanin reading were performed after 24 hours of growth at 37°C, 200 rpm.
Fascia induces a specific transcriptional response in P. aeruginosa characterized by the activation of pyochelin biosynthesis
Given the potential of fascia to induce P. aeruginosa virulence, we performed whole genome expressional profiling of P. aeruginosa MPAO1 exposed to homogenates of mouse fascia. Microarray analysis was performed after incubation of P. aeruginosa for 12 hours with fascia homogenates vs. controls (grown in TY media only). Differentially expressed genes were selected as described in Methods; in total out of 5,900 genes, presented on the array, 136 were identified as differentially expressed under given conditions. Clustering of these genes revealed that they contain two major groups- those, from which 46 were up-regulated upon incubation with fascia homogenates and 90 were down-regulated (Fig. 5A, and supplemental Table S1). Analysis of up-regulated genes revealed that cluster of pyochelin biosynthesis (PA4221 fptA, PA4223 pchH, PA4224 pchG, PA4226 pchE, PA4228-PA4230 pchD, pchB, pchC) (16) was up-regulated due to exposure to fascia. (Fig.5B). While activation of the siderophore pyochelin could be hypothesized to be a result of the recognition of iron limitation by P. aeruginosa, the overall gene expression pattern did not confirm this. Except for pyochelin, none of the other iron limitation-induced genes, however the iron-sulphur [Fe-S] cluster assembly genes (PA3812-PA3814, iscA, iscU, iscS) (17) was up-regulated in our study (Fig.5C, supplemental table S1) possibly to reduce the total intracellular iron content increased due to pyochelin action. Genes encoding multidrug efflux pump MexGHI-OpmD (PA4207 mexI, PA4208 opmD) were also activated. It was shown that the MexGHI-OpmD controls growth, antibiotic resistance and virulence including the production of pyocyanin (18). Analysis of down-regulated genes revealed the suppression of the important phosphate limitation-induced PA5369 pstS gene whose expression is induced by phosphate limitation (19). Downregulation of pstS in response to fascia may have been due to the release of phosphate from damaged tissues. Interestingly, we also observed downregulation of 29 bacteriophage related genes in the fascia exposed bacteria (Fig.5D, supplemental Table S1). That may suggest that P. aeruginosa becomes less predisposed to bacteriophage-mediated lysis (20) when contacts fascia. An another important cluster of down-regulated genes relates to the Mg2+ transport system regulated by PhoP that is known to maintain magnesium homeostasis in low Mg2+ environment (21) (Fig.5E, supplemental Table S1). These data suggest an increase of magnesium concentration in damaged tissues. In general, these data demonstrated coordinated transcriptional response of P. aeruginosa to fascia extracts with specific involvement of genes related to pyochelin biosynthesis despite nutrient rich environment.
Figure 5. Transcriptional response in P. aeruginosa to fascia.
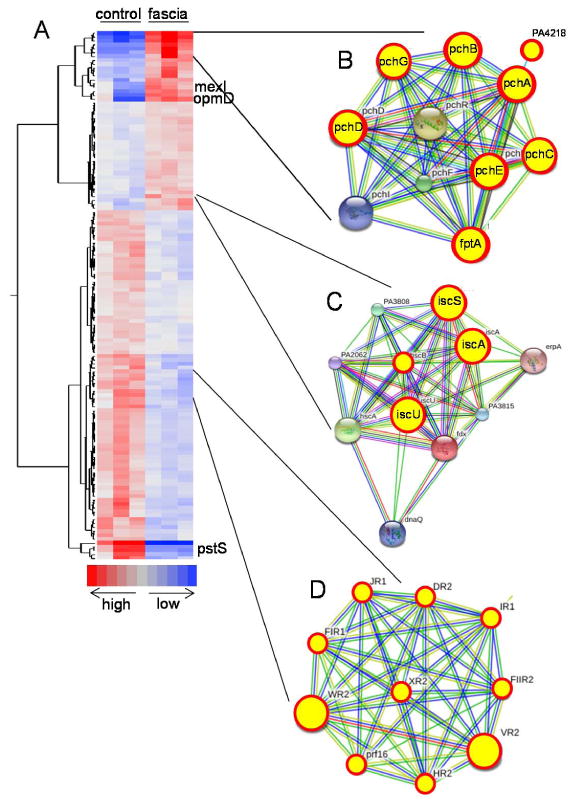
(A) Heat map generated from microarray data depicts specific pattern of gene clusters involved in the activation of pyochelin biosynthesis (B), and iron-sulfur cluster biogenesis (C), while suppression of bacteriophage genes (D). B, C, and D represent gene clusters with predicted functional links retrieved from a Search Tool for the Retrieval of Interacting Genes/Proteins (STRING 9.1). Genes in circular red yellow circles represent genes with alter expression in the response to fascia.
Discussion
Wound infection models in mice are highly problematic due to the lack of subcutaneous tissue and the overall resilience of mice to infection. Others have described models introducing different stressors such as foreign bodies (22, 23), ischemia (24), burn injury (25). These models often require highly virulent strains of bacteria to produce a wound infection such as P. aeruginosa or Staphylococcus aureus (26). We focused on traumatic soft-tissue infections that can be classified as crushed contaminated wounds (26, 27), which are more relevant to surgical site infections (28) that develop after elective surgery or trauma. Here we introduce the concept that dynamic virulence activation of the contaminating pathogen in the response to the wound environment itself may be an unrecognized yet highly contributory element in the pathogenesis of wound infection. We have previously reported that P. aeruginosa is an organism with a highly evolved information processing system termed quorum sensing. In the intestinal tract, P. aeruginosa can “sense” soluble elements released during systemic injury and “respond” with enhanced virulence. Elucidating the precise host elements that trigger this response and uncovering the mechanisms by which P. aeruginosa and other pathogens activate their virulence could lead to novel anti-virulence strategies to prevent infection. The density of P. aeruginosa detected in the wound of injured versus non- injured abdominal muscle was the same at day 3 but drastically increased at day 7 perhaps due to activation of siderophores and thereby increased capacities of host iron acquisition.
The main finding of this study was the induction of P. aeruginosa virulence by fascia. Fascia consists primarily of collagen fibers that enclose muscles and confer stability. Injury of fascia leads to the accumulation of macrophages and fibroblasts that are involved in the healing process (29). Fascia has attracted increasing interest in the process of wound healing and infection (30, 31). Necrotizing fasciitis (32) is the prototype soft tissue infection that can be severe and life threatening. The specific changes in the transcriptional pattern of P. aeruginosa when exposed to fascia demonstrates that much remains to be elucidated that may shed light on surgical wound infections
Data from the present study show for the first time, that wound tissues can induce bacteria virulence ex vivo and thus provide a system in which the provocative host factors may be able to be identified. One may consider a wound infection as a dynamic process that develops as a result of a complex bidirectional molecular dialogue between the host and the pathogen. Host tissue cues can activate bacteria to express highly potent virulence factors that impair neutrophil clearance (i.e., pyocyanin), and iron scavenging systems (i.e., pyochelin) allowing them to proliferate and cause tissue inflammation and gross clinical infection. Further work along this line of inquiry is needed to form a more complete understanding of why certain patients develop wound infections.
Supplementary Material
Supplemental table S1. List of genes of P. aeruginosa differentially responding to fascia.
Figure 3. P. aeruginosa localizes to the skin incision site.
Bioluminescent images demonstrating localization of P. aeruginosa XEN41 to the sites of skin incision at early post-operative period.
Acknowledgments
This study was funded by NIH RO1 2R01GM062344-13A1 (JCA).
This work was supported by NIH RO1 2R01GM062344-13A1 (JCA) and DDRCC grant P30 DK42086 (JCA)
Footnotes
Author contributions: MK performed study design and run experiments; SC, NNK, and YH analyzed and interpreted the data; IF collected the data, performed a literature search, and wrote the draft of the manuscript; EC analyzed the data and critically revised the manuscript; OZ and JA designed and analyzed the experiments and wrote the manuscript.
No conflicts are declared
The data were partially presented at 32d Annual Meeting of the Surgical Infection Society, Dallas, Texas, April 18-21, 2012
References
- 1.Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The Preventive Surgical Site Infection Bundle in Colorectal Surgery: An Effective Approach to Surgical Site Infection Reduction and Health Care Cost Savings. JAMA surgery. 2014 doi: 10.1001/jamasurg.2014.346. Epub 2014/08/28. [DOI] [PubMed] [Google Scholar]
- 2.Roux A, Payne SM, Gilmore MS. Microbial telesensing: probing the environment for friends, foes, and food. Cell host & microbe. 2009;6(2):115–24. doi: 10.1016/j.chom.2009.07.004. Epub 2009/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flack CE, Zurek OW, Meishery DD, Pallister KB, Malone CL, Horswill AR, Voyich JM. Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(19):E2037–45. doi: 10.1073/pnas.1322125111. Epub 2014/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraday N, Rock P, Lin EE, Perl TM, Carroll K, Stierer T, Robarts P, McFillin A, Ross T, Shah AS, et al. Past history of skin infection and risk of surgical site infection after elective surgery. Annals of surgery. 2013;257(1):150–4. doi: 10.1097/SLA.0b013e3182588abf. Epub 2012/05/29. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor perspectives in medicine. 2012;2(11) doi: 10.1101/cshperspect.a012427. Epub 2012/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Annals of surgery. 2012;255(2):386–93. doi: 10.1097/SLA.0b013e3182331870. Epub 2011/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, Ciancio M, Zaborin A, Petrof EO, Turner JR, et al. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS pathogens. 2007;3(3):e35. doi: 10.1371/journal.ppat.0030035. Epub 2007/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel NJ, Zaborina O, Wu L, Wang Y, Wolfgeher DJ, Valuckaite V, Ciancio MJ, Kohler JE, Shevchenko O, Colgan SP, et al. Recognition of intestinal epithelial HIF-1alpha activation by Pseudomonas aeruginosa. American journal of physiology Gastrointestinal and liver physiology. 2007;292(1):G134–42. doi: 10.1152/ajpgi.00276.2006. Epub 2006/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, Patel N, Musch MW, Chang EB, Fu YX, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–7. doi: 10.1126/science.1112422. Epub 2005/07/30. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14339–44. doi: 10.1073/pnas.2036282100. Epub 2003/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink D, Romanowski K, Valuckaite V, Babrowski T, Kim M, Matthews JB, Liu D, Zaborina O, Alverdy JC. Pseudomonas aeruginosa potentiates the lethal effect of intestinal ischemia-reperfusion injury: the role of in vivo virulence activation. The Journal of trauma. 2011;71(6):1575–82. doi: 10.1097/TA.0b013e31821cb7e5. Epub 2011/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172(2):884–900. doi: 10.1128/jb.172.2.884-900.1990. Epub 1990/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–15. doi: 10.1093/bioinformatics/btg405. Epub 2004/02/13. [DOI] [PubMed] [Google Scholar]
- 14.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98(9):5116–21. doi: 10.1073/pnas.091062498. Epub 2001/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, Whyte MK. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J Immunol. 2005;174(6):3643–9. doi: 10.4049/jimmunol.174.6.3643. Epub 2005/03/08. [DOI] [PubMed] [Google Scholar]
- 16.Serino L, Reimmann C, Visca P, Beyeler M, Chiesa VD, Haas D. Biosynthesis of pyochelin and dihydroaeruginoic acid requires the iron-regulated pchDCBA operon in Pseudomonas aeruginosa. Journal of bacteriology. 1997;179(1):248–57. doi: 10.1128/jb.179.1.248-257.1997. Epub 1997/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romsang A, Duang-Nkern J, Leesukon P, Saninjuk K, Vattanaviboon P, Mongkolsuk S. The iron-sulphur cluster biosynthesis regulator IscR contributes to iron homeostasis and resistance to oxidants in Pseudomonas aeruginosa. PloS one. 2014;9(1):e86763. doi: 10.1371/journal.pone.0086763. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aendekerk S, Diggle SP, Song Z, Hoiby N, Cornelis P, Williams P, Camara M. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology. 2005;151(Pt 4):1113–25. doi: 10.1099/mic.0.27631-0. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 19.Zaborin A, Romanowski K, Gerdes S, Holbrook C, Lepine F, Long J, Poroyko V, Diggle SP, Wilke A, Righetti K, et al. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6327–32. doi: 10.1073/pnas.0813199106. Epub 2009/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrova OE, Schurr JR, Schurr MJ, Sauer K. The novel Pseudomonas aeruginosa two-component regulator BfmR controls bacteriophage-mediated lysis and DNA release during biofilm development through PhdA. Molecular microbiology. 2011;81(3):767–83. doi: 10.1111/j.1365-2958.2011.07733.x. Epub 2011/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou D, Han Y, Qin L, Chen Z, Qiu J, Song Y, Li B, Wang J, Guo Z, Du Z, et al. Transcriptome analysis of the Mg2+-responsive PhoP regulator in Yersinia pestis. FEMS microbiology letters. 2005;250(1):85–95. doi: 10.1016/j.femsle.2005.06.053. Epub 2005/08/03. [DOI] [PubMed] [Google Scholar]
- 22.McRipley RJ, Whitney RR. Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrobial agents and chemotherapy. 1976;10(1):38–44. doi: 10.1128/aac.10.1.38. Epub 1976/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espersen F, Frimodt-Moller N, Corneliussen L, Riber U, Rosdahl VT, Skinhoj P. Effect of treatment with methicillin and gentamicin in a new experimental mouse model of foreign body infection. Antimicrobial agents and chemotherapy. 1994;38(9):2047–53. doi: 10.1128/aac.38.9.2047. Epub 1994/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagami G, Morohoshi T, Ikeda T, Ohta Y, Sagara H, Huang L, Nagase T, Sugama J, Sanada H. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in pressure ulcer infection in rats. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19(2):214–22. doi: 10.1111/j.1524-475X.2010.00653.x. Epub 2011/03/03. [DOI] [PubMed] [Google Scholar]
- 25.Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infection and immunity. 1999;67(11):5854–62. doi: 10.1128/iai.67.11.5854-5862.1999. Epub 1999/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011;2(4):296–315. doi: 10.4161/viru.2.4.16840. Epub 2011/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammers R, Henry C, Howell J. Bacterial counts in experimental, contaminated crush wounds irrigated with various concentrations of cefazolin and penicillin. The American journal of emergency medicine. 2001;19(1):1–5. doi: 10.1053/ajem.2001.18115. Epub 2001/01/09. [DOI] [PubMed] [Google Scholar]
- 28.Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale / AMMI Canada. 2008;19(2):173–84. doi: 10.1155/2008/846453. Epub 2009/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau FH, Pomahac B. Wound healing in acutely injured fascia. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22(Suppl 1):14–7. doi: 10.1111/wrr.12165. Epub 2014/05/13. [DOI] [PubMed] [Google Scholar]
- 30.Findley T, Chaudhry H, Stecco A, Roman M. Fascia research--a narrative review. Journal of bodywork and movement therapies. 2012;16(1):67–75. doi: 10.1016/j.jbmt.2011.09.004. Epub 2011/12/27. [DOI] [PubMed] [Google Scholar]
- 31.Bove GM. Weaving a mat of fascia research. Journal of bodywork and movement therapies. 2012;16(2):132–3. doi: 10.1016/j.jbmt.2012.01.004. Epub 2012/04/03. [DOI] [PubMed] [Google Scholar]
- 32.Kessenich CR, Bahl A. Necrotizing fasciitis: understanding the deadly results of the uncommon ‘flesh-eating bacteria’. The American journal of nursing. 2004;104(9):51–5. doi: 10.1097/00000446-200409000-00024. Epub 2004/09/15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table S1. List of genes of P. aeruginosa differentially responding to fascia.