Abstract
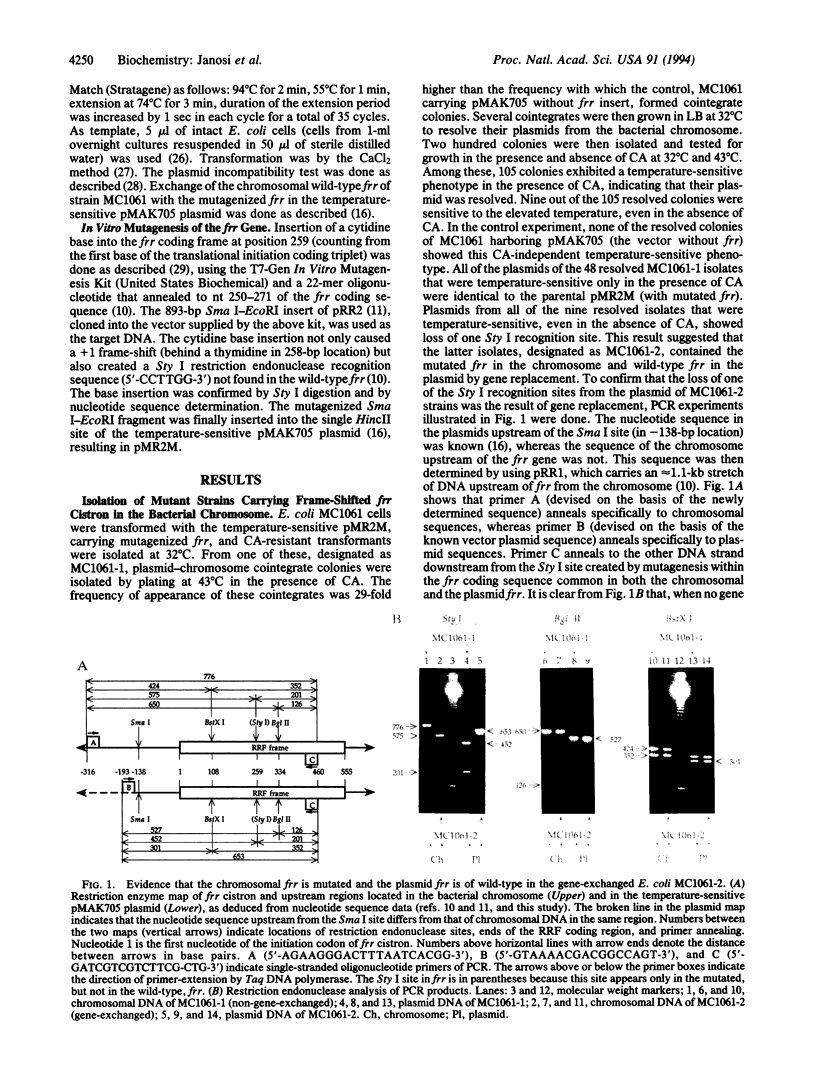
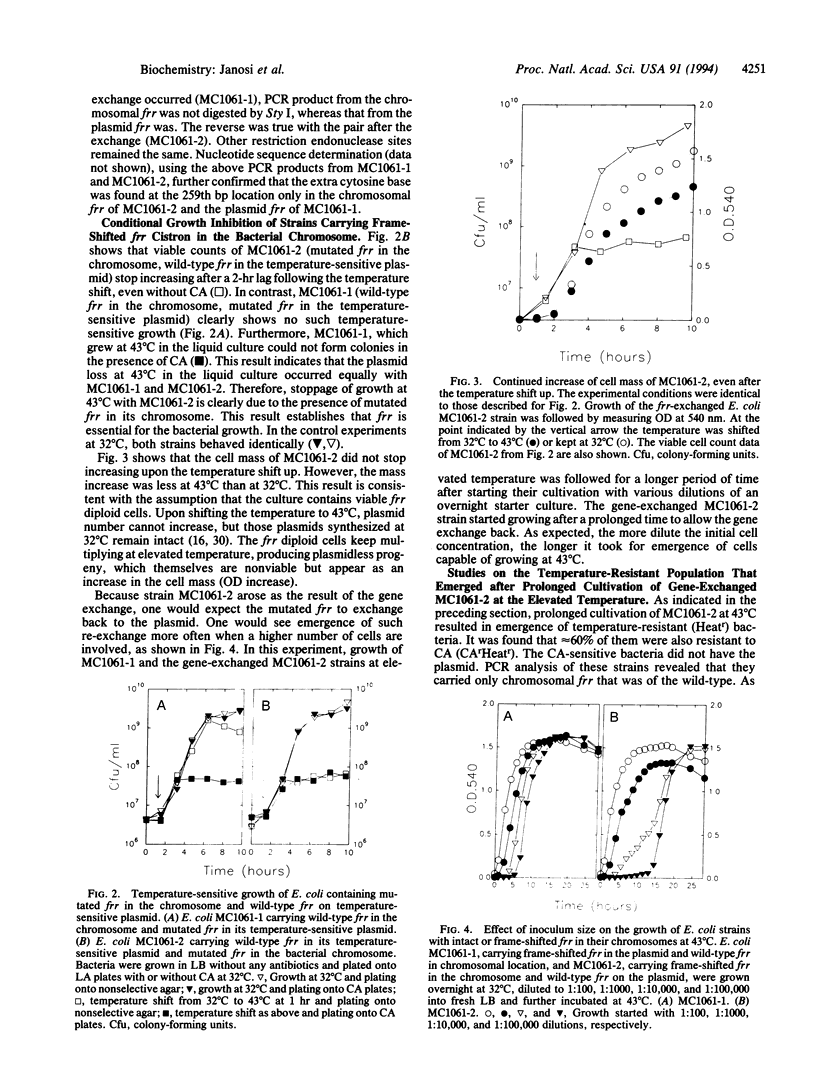
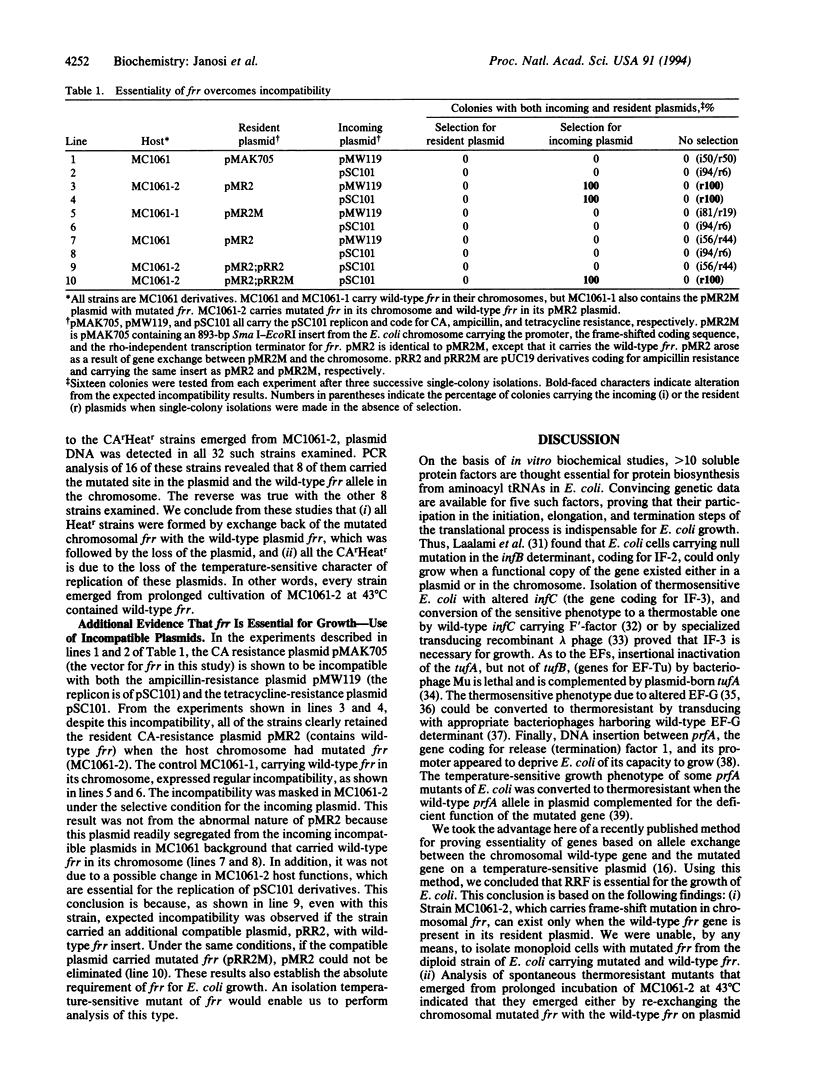
Ribosome releasing factor, product of the frr gene in Escherichia coli, is responsible for dissociation of ribosomes from mRNA after the termination of translation. It functions to "recycle" ribosomes and is renamed ribosome recycling factor in this paper. An E. coli strain was constructed (MC1061-2), which carried frame-shifted frr in the chromosome and wild-type frr on a temperature-sensitive plasmid. MC1061-2 is temperature-sensitive in its growth and does not segregate its frr-carrying plasmid under the plasmid incompatibility pressure. In contrast, isogenic E. coli carrying wild-type frr in the chromosome and mutated frr on the temperature-sensitive plasmid is not temperature-sensitive in its growth and segregates its plasmid from incompatible plasmids. All spontaneously formed thermoresistant colonies derived from MC1061-2 carried wild-type frr that resided either in the bacterial chromosome by re-exchange or in the plasmid, which became temperature-resistant. These observations establish that frr is an essential gene for cell growth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch L., Nilsson L., Vijgenboom E., Verbeek H. FIS-dependent trans-activation of tRNA and rRNA operons of Escherichia coli. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):293–301. doi: 10.1016/0167-4781(90)90184-4. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. doi: 10.1128/jb.132.2.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti L., Tocchini-Valentini G. P., Di Matteo G. F. The role of G factor in protein synthesis. Studies on a temperature-sensitive Escherichia coli mutant with an altered G factor. Biochemistry. 1969 Aug;8(8):3428–3432. doi: 10.1021/bi00836a044. [DOI] [PubMed] [Google Scholar]
- Hamilton C. M., Aldea M., Washburn B. K., Babitzke P., Kushner S. R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Sekiguchi M. Mutations of temperature sensitivity in R plasmid pSC101. J Bacteriol. 1977 Aug;131(2):405–412. doi: 10.1128/jb.131.2.405-412.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Kaji A. Factor dependent breakdown of polysomes. Biochem Biophys Res Commun. 1970 Nov 25;41(4):877–883. doi: 10.1016/0006-291x(70)90165-8. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Kaji A. Factor-dependent release of ribosomes from messenger RNA. Requirement for two heat-stable factors. J Mol Biol. 1972 Mar 14;65(1):43–58. doi: 10.1016/0022-2836(72)90490-1. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Kaji A. Purification and properties of ribosome-releasing factor. Biochemistry. 1972 Oct 24;11(22):4037–4044. doi: 10.1021/bi00772a005. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Kaji A. Role of elongation factor G and a protein factor on the release of ribosomes from messenger ribonucleic acid. J Biol Chem. 1973 Nov 10;248(21):7580–7587. [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Ichikawa S., Kaji A. Molecular cloning and expression of ribosome releasing factor. J Biol Chem. 1989 Nov 25;264(33):20054–20059. [PubMed] [Google Scholar]
- Ichikawa S., Ryoji M., Siegfried Z., Kaji A. Localization of the ribosome-releasing factor gene in the Escherichia coli chromosome. J Bacteriol. 1989 Jul;171(7):3689–3695. doi: 10.1128/jb.171.7.3689-3695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A. K., Baichwal V., Ames G. F. Rapid polymerase chain reaction amplification using intact bacterial cells. Biotechniques. 1991 Jan;10(1):42, 44-5. [PubMed] [Google Scholar]
- Kung H. F., Treadwell B. V., Spears C., Tai P. C., Weissbach H. DNA-directed synthesis in vitro of beta-galactosidase: requirement for a ribosome release factor. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3217–3221. doi: 10.1073/pnas.74.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. G factor mutants of Escherichia coli: map location and properties. Biochem Biophys Res Commun. 1971 Feb 5;42(3):441–444. doi: 10.1016/0006-291x(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Laalami S., Putzer H., Plumbridge J. A., Grunberg-Manago M. A severely truncated form of translational initiation factor 2 supports growth of Escherichia coli. J Mol Biol. 1991 Jul 20;220(2):335–349. doi: 10.1016/0022-2836(91)90017-z. [DOI] [PubMed] [Google Scholar]
- Noll M., Noll H. Structural dynamics of bacterial ribosomes. V. Magnesium-dependent dissociation of tight couples into subunits: measurements of dissociation constants and exchange rates. J Mol Biol. 1976 Jul 25;105(1):111–130. doi: 10.1016/0022-2836(76)90197-2. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Kaji A. Requirement for ribosome-releasing factor for the release of ribosomes at the termination codon. Eur J Biochem. 1975 Oct 15;58(2):411–419. doi: 10.1111/j.1432-1033.1975.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Kaji A. Ribosome run through of the termination codon in the absence of the ribosome releasing factor. Biochim Biophys Acta. 1975 Sep 1;402(3):288–296. doi: 10.1016/0005-2787(75)90266-x. [DOI] [PubMed] [Google Scholar]
- Ryden M., Murphy J., Martin R., Isaksson L., Gallant J. Mapping and complementation studies of the gene for release factor 1. J Bacteriol. 1986 Dec;168(3):1066–1069. doi: 10.1128/jb.168.3.1066-1069.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Berland R., Kaji A. Reinitiation of translation from the triplet next to the amber termination codon in the absence of ribosome-releasing factor. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5973–5977. doi: 10.1073/pnas.78.10.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoji M., Karpen J. W., Kaji A. Further characterization of ribosome releasing factor and evidence that it prevents ribosomes from reading through a termination codon. J Biol Chem. 1981 Jun 10;256(11):5798–5801. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shimizu I., Kaji A. Identification of the promoter region of the ribosome-releasing factor cistron (frr). J Bacteriol. 1991 Aug;173(16):5181–5187. doi: 10.1128/jb.173.16.5181-5187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Graffe M., Grunberg-Manago M. Characterization of an E. coli mutant with a thermolabile initiation factor IF3 activity. Mol Gen Genet. 1977 Feb 28;151(1):17–26. doi: 10.1007/BF00446908. [DOI] [PubMed] [Google Scholar]
- Springer M., Graffe M., Hennecke H. Specialized transducing phage for the initiation factor 3 gene in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3970–3974. doi: 10.1073/pnas.74.9.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D. Release of 70 S ribosomes from polysomes in Escherichia coli. J Mol Biol. 1973 Feb 15;74(1):45–56. doi: 10.1016/0022-2836(73)90353-7. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Mattoccia E. A mutant of E. coli with an altered supernatant factor. Proc Natl Acad Sci U S A. 1968 Sep;61(1):146–151. doi: 10.1073/pnas.61.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Weiner M. P., Hutton C. J., Batt C. A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988 May 15;65(1):129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]
- Verkamp E., Chelm B. K. Isolation, nucleotide sequence, and preliminary characterization of the Escherichia coli K-12 hemA gene. J Bacteriol. 1989 Sep;171(9):4728–4735. doi: 10.1128/jb.171.9.4728-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



