Abstract
Bisphenol A (BPA) is a synthetic industrial reactant used in the production of polycarbonate plastics, and genistein is a natural phytoestrogen abundant in the soybean. Current studies investigating the endocrinedisrupting effects of concomitant exposures to BPA and genistein have warranted the development of an analytical method for the simultaneous measurement of BPA and genistein, as well as their primary metabolites, bisphenol A ß-d-glucuronide (BPA gluc) and genistein 4′-ß-d-glucuronide (genistein gluc), respectively. All four analytes were extracted from rat plasma via solid phase extraction (SPE). Three SPE cartridges and four elution schemes were tested. Plasma extraction using Bond Elut Plexa cartridges with sequential addition of ethyl acetate, methanol, and acetonitrile yielded optimal average recoveries of 98.1±1.8% BPA, 94.9±8.0% genistein, 91.4±6.1% BPA gluc, and 103±6.1% genistein gluc. Identification and quantification of the four analytes were performed by a validated HPLC-MS/MS method using electrospray ionization and selective reaction monitoring. This novel analytical method should be applicable to the measurement of BPA, genistein, BPA gluc, and genistein gluc in urine, cultures, and tissue following in vivo exposures. While reports of the determination of BPA and genistein independently exist, the simultaneous optimized extraction and detection of BPA, genistein, BPA gluc, and genistein gluc have not previously been reported.
Keywords: Bisphenol A (BPA), Genistein, Bisphenol A ß-d-glucuronide, Genistein 4′-ß-d-glucuronide, HPLC, Mass spectrometry, Electrospray ionization, Chromatographic techniques
Introduction
Endocrine-disrupting compounds (EDCs) are chemicals that perturb the endocrine system either by acting as agonists or antagonists to sex hormone receptors, thus mimicking or preventing the action of endogenous estrogens and androgens. Laboratories have reported the estrogenic activity for EDCs of various classes such as naturally occurring phytoestrogens (e.g., genistein, daidzein, biochanin A) [1, 2], man-made plasticizers (e.g., bisphenol A, phthalates) [3–5], pesticides (e.g., methoxychlor, atrazine) [6], and other synthetic industrial chemicals (e.g., polybrominated diphenyl alkanes) [7].
Bisphenol A [2,2-bis(4-hydroxyphenyl)propane (BPA)] (Fig. 1) is a monomer used in the synthesis of polycarbonate plastics and epoxy resins worldwide and is commonly found in baby bottles, food containers, and linings of food and beverage cans. BPA can migrate from containers into the contents, allowing for the direct exposure to humans via ingestion [8]. BPA is weakly estrogenic [9], and numerous laboratories have demonstrated the endocrine-disrupting properties of BPA, including in vitro proliferation of MCF-7 human breast cancer cells [7] and in vivo alteration of reproductive function and postnatal growth rate [10–12]. After much review of the risk of human exposure to BPA, the National Toxicology Program declared that there is some concern for adverse effects to human development and reproduction, specifically on the behavior, brain, and prostate gland of fetuses, infants, and children exposed to BPA [13].
Fig. 1. Chemical structures of BPA, BPA gluc, genistein, and genistein gluc.
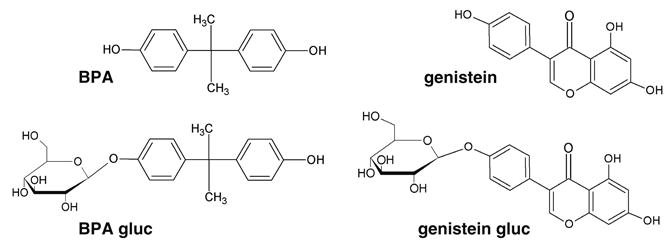
Genistein (4′,5,7-trihydroxyisoflavone) (Fig. 1) is a naturally occurring phytoestrogen found in many legumes and grains, with soybeans being a particularly rich source. The majority of commercially available laboratory rodent diets naturally contain variable concentrations of genistein, usually in the form of soybean meal or soy protein, thus making genistein a ubiquitous component of laboratory rodent chow [14]. Like BPA, genistein exhibits weak estrogenic activity both in vitro and in vivo [9, 15]. The interpretation of data on the effects of genistein is controversial to date, with some groups focusing on the beneficial effects of genistein such as the phytoestrogen's chemotherapeutic properties and its ability to prevent osteoporosis [16, 17], while other laboratories tout genistein's ability to cause adverse health effects in humans and laboratory animals, namely, endocrine disruption [18, 19]. Genistein has been shown to be detrimental to the differentiation and development of reproductive organs in embryos, fetuses, and neonates, suggesting that there is a critical window of exposure which may lead to latent effects [20, 21]. Analyses of BPA and genistein have increased in recent years due to their emergence as prevalent endocrine-disrupting compounds.
Methods have been previously established for the extraction and detection of either BPA or genistein. Established methods allow for the extraction of BPA from environmental water samples, as well as from biological fluids including plasma, serum, amniotic fluid, breast milk, and urine [22, 23]. Genistein has been routinely extracted from food and beverage products, as well as urine, serum, and plasma [24–26]. Solid phase extraction (SPE) is the predominant isolation method for both BPA and genistein. Traditional SPE sorbent materials such as C18 and other reversed phase sorbents are commonly used to extract BPA [27], while more unique SPE sorbents such as bambooactivated charcoal are reportedly utilized as well [28]. The current leading methods for the extraction of genistein employ C18 or Oasis hydrophilic–lipophilic balance (HLB) SPE cartridges [24]. The methods for the separation and detection of BPA or genistein routinely include HPLC-ESI/MS/MS [28], HPLC-APCI/MS/MS [24], HPLC-UV [29], and UPLC-ESI/MS/MS [30].
Several studies have maximized the recovery and/or detection of either BPA or genistein, but no studies have focused on the optimized simultaneous measurement of the two compounds to date. BPA and genistein are rarely analyzed together, possibly due to the fact that analytes of interest and methodologies are often grouped based on chemical structure similarities or are members within the same compound class, such as pesticides or phytoestrogens. The prevalence of co-exposures to BPA and genistein warrant their simultaneous analysis. There are a number of scenarios in which individuals may be exposed to BPA and genistein simultaneously, such as in the situation of an infant drinking soy-based formula from a polycarbonate baby bottle or a woman taking a menopausal relief aid containing genistein who recently had dental work performed that exposed her to BPA [31, 32]. In this work, BPA and genistein are analyzed simultaneously because they are relevant in common binary exposure scenarios, in addition to the fact that they share numerous common biological endpoints, including precocious puberty, irregular cyclicity, and altered cellular differentiation [1, 4, 12].
The ability to simultaneously recover and detect BPA and genistein and their primary metabolites is immensely beneficial because it requires smaller sample sizes, reduced maintenance of sample integrity, decreased costs of materials and personnel, and reduced analysis time. The work presented here is significant because it provides a novel analytical method for the simultaneous measurement of BPA, genistein, BPA gluc, and genistein gluc.
Materials and methods
Chemicals and reagents
Bisphenol A, genistein, and ammonium acetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bisphenol A ß-d-glucuronide and genistein 4′- ß-d-glucuronide were purchased from TRC Canada (North York, Ontario). Ethyl acetate (pesticide grade), methanol (for LC/MS), and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Honeywell Burdick and Jackson (Muskegon, MI, USA) supplied high-purity solvents acetonitrile and water, both of HPLC grade.
SPE cartridges
The extraction of BPA, BPA gluc, genistein, and genistein gluc from rat plasma was performed via SPE. Three cartridges were tested for the efficiency of co-extraction: Bond Elut Plexa cartridges (Varian Inc., Palo Alto, CA, USA; 60 mg, 1 mL), Oasis HLB cartridges (Waters, Milford, MA, USA; 30 mg, 1 mL), and United Chemical Technologies (UCT) C18 cartridges (United Chemical Technologies, Bristol, PA, USA; 100 mg, 1 mL). Bond Elut Plexa cartridges feature hydrophilic surfaces and a gradient of polarity that allow for good transfer of small analytes into the polymer core, while excluding large proteins, and generally show efficient extraction of analytes across a broad range of polarities and acid/base properties. Oasis HLB cartridges are routinely used to extract parent compounds as well as their polar metabolites. UCT C18 cartridges are hydrophobic, featuring a sorbent that is composed of a silica backbone with hydrocarbon chains; these relatively non-selective cartridges are often used to extract non-polar or neutral analytes from complex matrices. Various combinations of ethyl acetate, methanol, and acetonitrile were used as elution solvents, with each combination tested per cartridge type.
Solid phase extraction
All samples had a total volume of 1.0 mL and consisted of 100 μL of rat plasma with citrate (Fisher Scientific, Pittsburgh, PA, USA), 100 μL of 250 mM ammonium acetate (pH 5), 80 μL of 1 M formic acid, water, and 0.1 μg/mL BPA, BPA gluc, genistein, and genistein gluc. Samples were sonicated for 5 min then centrifuged at 2,500 rpm for 10 min at 4 °C. Each SPE cartridge was conditioned with 3 mL of methanol followed by 3 mL of water before samples were loaded. Two milliliters of 9:1 water/methanol (v/v) wash solution was passed through each column prior to elution. Elution was performed at a flow rate of 1–2 mL/min with one of the following elution schemes, with elution solvents added sequentially in the order they are listed: 3 mL ethyl acetate and 3 mL methanol (EM), 3 mL ethyl acetate and 3 mL acetonitrile (EA), 3 mL methanol and 3 mL acetonitrile (MA), or 3 mL ethyl acetate, 2.5 mL methanol, and 2.5 mL acetonitrile (EMA). SPE extracts were evaporated to dryness under vacuum and a stream of nitrogen then reconstituted in 200 μL of 75% acetonitrile in water.
Standard solutions
Initial genistein stock solutions were prepared by dissolving genistein in methanol, whereas BPA, BPA gluc, and genistein gluc were initially dissolved in acetonitrile. Subsequent standards for all analytes were prepared via serial dilution in acetonitrile. Standard solutions ranged from 1 to 1,000 μg/mL and were stored at –20 °C.
Blank controls
Blank controls contained high-purity water in place of standard solutions and were processed alongside samples using all of the same supplies and reagents. While blank controls are always important, they are especially vital when analyzing ubiquitous compounds such as BPA, which is known to be prevalent in many common laboratory supplies and other materials required for SPE and HPLCMS/MS analysis due to its use as a plasticizer [33]. Efforts were made to minimize the contact of samples with plastics, and plasticware was replaced by glassware wherever possible. Despite significant efforts made to minimize the use of plastic materials by substitution with glass products, contamination with BPA is still a common challenge in the laboratory. Blank controls were performed for each cartridge type and elution scheme.
Chromatographic conditions
Of each reconstituted extract, 10 μL was directly injected via a Finnigan Surveyor Autosampler Plus (Thermo Fisher Scientific, Waltham, MA, USA). Chromatographic separation was carried out with either a Kinetex C18 column (Phenomenex, Torrance, CA, USA; 100×4.6-mm ID, 2.6 μm) or a Discovery C8 column (Supelco, St. Louis, MO, USA; 50×4.6-mm ID, 5 μm). A Krud Katcher Ultra In-Line Filter guard column (Phenomenex, 0.5 μm) was used with both HPLC columns. Mobile phase Awas 2 mM ammonium acetate (pH 9) and mobile phase B was acetonitrile. Replacing acetonitrile with methanol in mobile phase solution has been known to contribute to poor peak shape and variable baseline [27]. For this purpose, we chose to use acetonitrile rather than methanol in our mobile phase. Optimal chromatographic separation relative to discrete non-overlapping peaks with distinct baseline resolution between xenoestrogen analytes of interest and interfering substances was achieved (Fig. 2a, b) when the following gradient was employed—0–3 min 50% B, 3– 14 min 50% to 90% B, 14–18 min 90% B, 18–18.2 min 90% to 50% B, 18.2–20 min 50% B—using a Finnigan Surveyor MS Pump Plus (Thermo Fisher Scientific). Flow rate was maintained at 250 μL/min for the duration of each 20-min analysis.
Fig. 2.
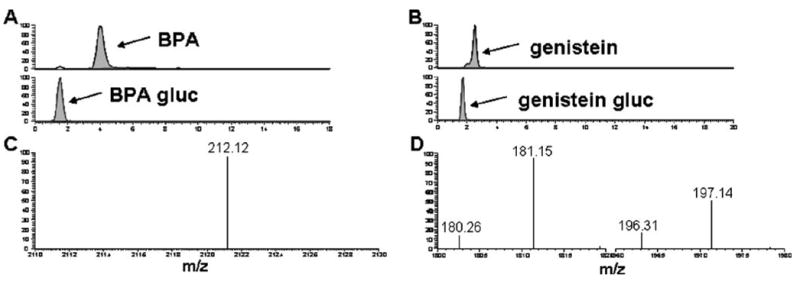
Chromatograms of BPA and BPA gluc (A) and genistein and genistein gluc (B) from Discovery C8. Elution times of BPA, BPA gluc, genistein, and genistein gluc are 4.0, 1.6, 2.5, and 1.7 min, respectively. Mass spectra of BPA (C) and genistein (D) acquired in SRM mode with isolation width of 2 m/z
Mass spectrometry
The HPLC eluate was directed into a Thermo LTQ mass spectrometer (Thermo Fisher Scientific) using electrospray ionization (ESI) in negative ion mode and a linear ion trap as an analyzer, all under the regulation of Xcalibur 2.0.7 software. Optimized ESI source conditions include a sheath gas flow rate of 40 (arbitrary units), spray voltage of 5.0 kV, heated capillary temperature of 275 °C, capillary voltage of –25 V, and spray current of 3.0 μA. To achieve optimal sensitivity, parameters including tube lens offset, multipole offset, gate and front lens voltages, and multipole RF amplitude were optimized prior to each analysis using a 1-μg/mL standard solution of BPA that was infused at a flow rate of 3 μL/min together with HPLC mobile phase at a flow rate of 250 μL/min. Data were acquired in the selective reaction monitoring (SRM) mode with an isolation width of 2 m/z for each analyte. Product ions were generated by applying a collision energy of 35 for BPA, BPA gluc, and genistein gluc, and a collision energy of 40 for genistein. Mass spectrometric parameters are summarized in Table 1. High-purity nitrogen was used as the sheath gas.
Table 1. Summary of mass spectrometric parameters.
| Analyte | Precursor ion (m/z) | Product ion (m/z) | Collision energy |
|---|---|---|---|
| BPA | 227 | 212 | 35 |
| BPA gluc | 403 | 227 | 35 |
| Genistein | 269 | 181 | 40 |
| Genistein gluc | 445 | 269 | 35 |
Data were acquired in SRM mode with an isolation width of 2 m/z for each analyte
Statistical analyses
Data are reported as average recovery ± relative standard deviation (RSD). Known concentrations of each analyte were spiked into the mobile phase and directly injected into the HPLC-MS/MS system multiple times. An average of 10 or 11 replicate injections of the standard was calculated, and the blank-subtracted quantity of each analyte measured in the corresponding unknown samples was then compared with the mean quantity of analyte detected in replicate injections of spiked standard to yield a “percent recovery.” This method of using a single-point calibration was used to verify that instrumental drift was not a major factor since analytical runs often exceed 40 h of continuous HPLC-MS/MS analysis. Reported values have been blank-subtracted from raw data prior to the calculation of percent recovery. Three separate extractions were performed per cartridge type and elution scheme, and each reconstituted extract was injected into the HPLC-MS/MS three times. Data acquisition was performed using Xcalibur 2.0.7, and data were analyzed by Qual Browser 2.0.7 (Thermo Fisher Scientific). Quantitation was performed with Excel 2003 based on manually integrated peak areas using genesis peak integration and 15-point smoothing with Xcalibur 2.0.7. Residual percent error was calculated using Stata 11.2 (Stata Corp., College Station TX, USA).
Results
Retention times, limits of detection, and calibration curves
Retention times with the Kinetex C18 column were approximately 6.7, 2.7, 3.5, and 2.7 min for BPA, BPA gluc, genistein, and genistein gluc, respectively. When separated with the Discovery C8 column, the retention times for BPA, BPA gluc, genistein, and genistein gluc were approximately 4.0, 1.6, 2.5, and 1.7 min, respectively (Fig. 2a, b). On-column limits of detection were 100, 250, 50, and 25 pg for BPA, BPA gluc, genistein, and genistein gluc, respectively. To quantitate the amount of each analyte, separate calibration curves were prepared over the ranges of 0.1 and 5 μg/mL for all four analytes. Standard solutions were injected into the HPLC-MS/MS system and calibration curves were obtained using integrated area. All analytes of interest showed good linearity between 0.1 and 5 μg/mL, with r2 values of 0.999, 0.999, 0.995, and 0.997 for BPA, BPA gluc, genistein, and genistein gluc, respectively. Residual percent error ranged from 0.08% for BPA gluc to 2.16% for genistein (Electronic supplementary material).
Overall recoveries
Recoveries of BPA (Fig. 3a) and genistein (Fig. 3b) varied among cartridge types as well as elution schemes. The overall background-subtracted recoveries ranged from 62.1% to 108% for BPA and 4.28% to 108% for genistein.
Fig. 3.
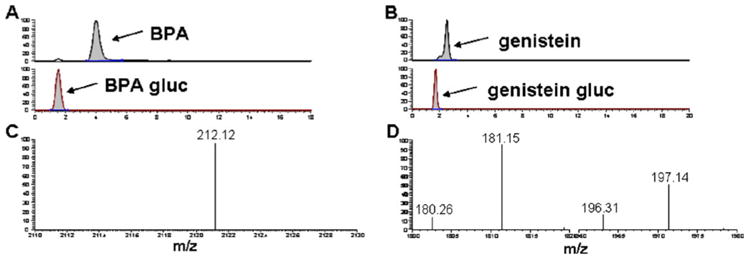
Average recovery of BPA (A) and genistein (B) via SPE performed with different elution paradigms (EM ethyl acetate, methanol; EA ethyl acetate, acetonitrile; MA methanol, acetonitrile; EMA ethyl acetate, methanol, acetonitrile) on three different cartridge types (Bond Elut Plexa, Oasis HLB, and UCT C18). The optimal recovery of BPA and genistein is achieved with Bond Elut Plexa cartridges and EMA (98.1±1.8% and 94.9±8.0%, respectively)
Bond Elut Plexa SPE cartridges
All four elution schemes resulted in adequate recoveries for both BPA and genistein. EMA recovered the highest amount of BPA, followed by EM, EA, and MA (98.1±1.8%, 96.4± 18%, 93.9±11%, and 78.6±16%, respectively). Elution with EMA also yielded substantially more reproducible results than any other elution scheme, as expressed by RSD. The recoveries of genistein by the different elution solvents, in order from highest recovery to least were: 108±20% using EM, 108±11% using EA, 94.9±8.0% using EMA, and 92.9± 8.2% using MA.
Oasis HLB SPE cartridges
When analyzed across the elution scheme, EMA yielded the highest recovery of BPA on Oasis HLB cartridges, followed by EA, EM, and MA, with recoveries of 108±16%, 85.5±12%, 67.2±14%, and 62.1±9.9%, respectively. Genistein recoveries from SPE with Oasis HLB cartridges were also highest when eluted with EMA, followed by MA, EM, and EA: 90.4±9.7%, 83.9±7.2%, 83.7±14%, and 4.28±21%, respectively.
UCT C18 SPE cartridges
As observed with both of the aforementioned cartridges, BPA recovery was maximized when eluted with EMA from UCT C18 cartridges. The average recovery was 107±10% with EMA, followed by 87.0±10% with EM, 77.1±11% with EA, and 70.6±12% with MA. Genistein recoveries from SPE with UCT C18 cartridges were 96.7±15% using MA, 92.1±4.1% using EMA, 90.7±14% using EA, and 82.3±7.1% using EM.
Recovery of glucuronides
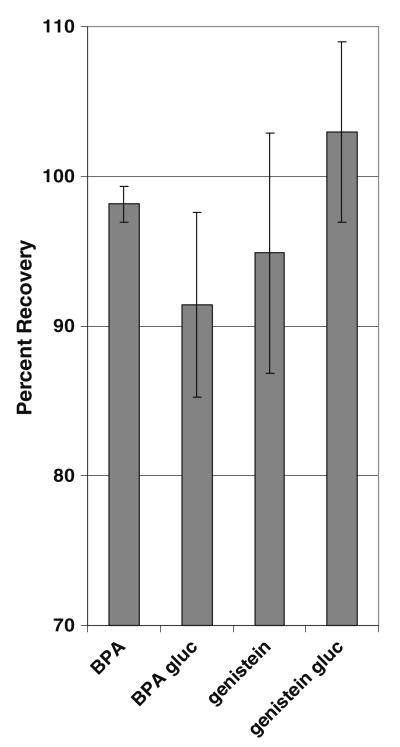
The analytical method presented here focuses on the optimization of the simultaneous measurement of BPA and genistein. Upon optimization, the method was tested for its ability to recover and detect the primary glucuronides of BPA and genistein, which are each more hydrophilic than their parent compound (Fig. 1). Despite these differences in water solubility, the glucuronides were successfully eluted with the optimized method using Bond Elut Plexa cartridges and elution with EMA, yielding background-subtracted average recoveries of 91.4±6.1% for BPA gluc and 103±6.1% for genistein gluc (Fig. 4), demonstrating that the novel method presented here is effective at recovering and detecting not only BPA and genistein but also their primary metabolites. The overall background-subtracted recoveries for BPA gluc and genistein gluc ranged from 85.9% to 101% and from 91.4% to 110%, respectively.
Fig. 4.
Optimized SPE conditions (Bond Elut Plexa cartridge and EMA) yield average recoveries for BPA, BPA gluc, genistein, and genistein gluc of 98.1±1.8%, 91.4±6.1%, 94.9±8.0%, and 103± 6.1%, respectively
Comparison of Kinetex C18 and Discovery C8 HPLC columns
For both columns, discrete separation of BPA and genistein with distinct baseline resolution was obtained within 7 min. On both columns, BPA gluc was the first analyte to elute, followed by genistein gluc, genistein, and finally BPA. On average, all four analytes eluted within a range of 4 min on the Kinetex C18 column and within 2.5 min on the Discovery C8 column (Fig. 2). Reproducibility of replicate injections performed with the Discovery C8 column was far superior to that of the Kinetex C18 column (RSDs 3.6 and 13 for six replicate BPA injections on Discovery C8 and Kinetex C18, respectively). The Kinetex C18 column is stable over a pH range of 1.5–10, while the Discovery C8 column is stable between pH 2 and 7.5. With the solvent gradient used in this method, the measured pH ranged from 5 to 7, which fell within the stability range for both columns used.
Discussion
Elution with EMA resulted in the highest recovery of BPA for all three cartridge types (Fig. 3a). SPE with Bond Elut Plexa cartridges yielded the highest recovery of BPA for all elution schemes, except for elution with EMA (98.1±1.8%) which was not appreciably less than that observed with either Oasis HLB (108±16%) or UCT C18 (107±10%). Additionally, the variability of BPA recovery among SPE replicates eluted from Bond Elut Plexa cartridges with EMA is substantially less than that observed with Oasis HLB or UCT C18 cartridges (RSDs 1.8, 16, and 10, respectively). Collectively, these data indicate that elution with EMA from Bond Elut Plexa cartridges provides optimal recovery of BPA.
Elution of genistein via SPE with Bond Elut Plexa cartridges yielded highest genistein recovery for all four elution schemes compared with the other cartridges tested. The exception was MA, where the average recovery of genistein from Bond Elut Plexa was very near the recoveries from both other cartridge types (Fig. 3b). While EMA did not yield the overall highest recovery of genistein from Bond Elut Plexa cartridges, its recovery was not substantially less than those observed with the other elution schemes. While some mean recoveries exceed 100%, none fall outside the acceptable range for measurement error. When extracted with Bond Elut Plexa cartridges, elution with EMA resulted in markedly less variability among replicate samples as compared with the other elution schemes. Reproducibility is an important factor when determining ideal extraction conditions. When considering reproducibility in conjunction with the recovery efficiency for both BPA and genistein, it is clear that optimal simultaneous recoveries for BPA and genistein are achieved by SPE performed on the Bond Elut Plexa cartridge, with sequential additions of 3 mL ethyl acetate, 2.5 mL methanol, and 2.5 mL acetonitrile. This optimized method also demonstrated superb recovery, efficiency, and reproducibility when used to extract the primary metabolites of BPA and genistein from rat plasma, achieving average recoveries of 91.4±6.1% and 103±6.1% for BPA gluc and genistein gluc, respectively (Fig. 4).
The on-column detection limits reported here are not as low as those achieved using a triple quadrupole mass spectrometer as an analyzer [24]. Additionally, analytical methodologies aimed to measure individual analytes are often capable of achieving lower limits of detection than those presented here; however, in the present method, it was acceptable to compromise higher detection limits for a comprehensive method allowing for the simultaneous measurement of BPA, BPA gluc, genistein, and genistein gluc.
The Kinetex C18 column utilizes a fused-core silica particle technology that is credited with increasing resolution, throughput, and sensitivity for ultra-high-performance liquid chromatography analyses; unfortunately, it was proven disadvantageous in the analytical method presented here. When compared with the Discovery C8 column for use in the present method, it became apparent that the Kinetex C18 column is inferior. The Discovery C8 HPLC column displayed dramatically increased reproducibility among replicate injections compared with the Kinetex C18 HPLC column. Additionally, variability of results when using the Kinetex C18 column increased substantially after only 200 total injections, whereas variability remained minimal after over more than 1,000 injections on the Discovery C8 column, making the longevity of Discovery C8 dramatically better than Kinetex C18. Due to superior column lifetime and reproducibility, Discovery C8 was the HPLC column of choice for the detection of BPA, genistein, BPA gluc, and genistein gluc.
Conclusion
In this paper, a highly sensitive SPE isolation method was coupled with LC-ESI-MS/MS quantitation for the simultaneous measurement of endocrine-disrupting compounds BPA, genistein, BPA gluc, and genistein gluc. Bond Elut Plexa SPE cartridges with ethyl acetate, methanol, and acetonitrile elution produced optimal recoveries of 98.1± 1.8% for BPA, 91.4±6.1% for BPA gluc, 94.9±8.0% for genistein, and 103±6.1% for genistein gluc and were more reproducible than either the Oasis or UCT SPE cartridges tested. The development of this analytical method is beneficial because it allows for BPA, BPA gluc, genistein, and genistein gluc to all be efficiently recovered from plasma in a single comprehensive method, allowing for the analysis of in vitro and in vivo toxicology exposure studies where BPA, genistein, and their primary metabolites are present. This novel analytical method may be adapted for the measurement of BPA, genistein, and their glucuronides from other biological matrices including urine, cultures, and possibly tissue, following exposures.
Supplementary Material
Acknowledgments
The authors would like to thank the National Institutes of Health (grant no. T32ES007148), National Institute of Environmental Health Sciences (grant no. ES05022), and Environmental and Occupational Health Sciences Institute for financial support. A special thank you to Stuart Shalat for performing the statistical analysis for residual percent error.
Abbreviations
- ESI
Electrospray ionization
- HPLC
High-performance liquid chromatography
- MS
Mass spectrometry
- MS/MS
Tandem MS
- RSD
Relative standard deviation
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00216-011-5151-8) contains supplementary material, which is available to authorized users.
Contributor Information
Janis L. Coughlin, Environmental and Occupational Health Sciences Institute, A Joint Institute of Rutgers University and the University of Medicine and Dentistry of New Jersey (UMDNJ), Piscataway, NJ 08854, USA; Joint Graduate Program of Toxicology, Rutgers University, Piscataway, NJ 008854-8075, USA; University of Medicine and Dentistry of New Jersey, Piscataway, NJ 08854-5635, USA
Bozena Winnik, Environmental and Occupational Health Sciences Institute, A Joint Institute of Rutgers University and the University of Medicine and Dentistry of New Jersey (UMDNJ), Piscataway, NJ 08854, USA.
Brian Buckley, Email: bbuckley@eohsi.rutgers.edu, Environmental and Occupational Health Sciences Institute, A Joint Institute of Rutgers University and the University of Medicine and Dentistry of New Jersey (UMDNJ), Piscataway, NJ 08854, USA; Joint Graduate Program of Toxicology, Rutgers University, Piscataway, NJ 008854-8075, USA; University of Medicine and Dentistry of New Jersey, Piscataway, NJ 08854-5635, USA.
References
- 1.Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HD. Developmental effects of dietary phytoestrogens in Sprague–Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci. 1999;51:236–244. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 2.Peterson G, Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22:335–345. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- 3.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposures to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannas BR, Furr J, Lambright CS, Wilson VS, Foster PMD, Gray LE., Jr Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the Sprague–Dawley rat with greater relative potency than other phthalates. Toxicol Sci. 2011;120:184–193. doi: 10.1093/toxsci/kfq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez P, Pulgar R, Olea-Serrano F, Villaloboa M, Rivas A, Metzler M, Pedraza V, Olea N. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect. 1998;106:167–174. doi: 10.1289/ehp.98106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim DS, Kwack SJ, Kim KB, Kim HS, Lee BM. Potential risk of bisphenol A migration from polycarbonate containers after heating, boiling, and microwaving. J Toxicol Environ Health A. 2009;72:1285–1291. doi: 10.1080/15287390903212329. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper GG, Lemmen JG, Carlsson BB, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 10.Xing L, Xu Y, Xiao Y, Shang L, Liu R, Wei X, Jiang J, Hao W. Embryotoxic and teratogenic effects of the combination of bisphenol A and genistein on in vitro cultured postimplantation rat embryos. Toxicol Sci. 2010;115:577–588. doi: 10.1093/toxsci/kfq081. [DOI] [PubMed] [Google Scholar]
- 11.vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 12.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 13.NIH. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. National Toxicology Program. 2008 Sep; NIH Publication no. 08-5994. 2008. [PubMed] [Google Scholar]
- 14.Thigpen JE, Setchell KDR, Saunders JE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR J. 2004;45:401–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 15.Shelnutt SR, Cimino CO, Wiggins PA, Badger TM. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev. 2000;9:413–419. [PubMed] [Google Scholar]
- 16.Albertazzi P. Purified phytoestrogens in postmenopausal bone health: is there a role for genistein? Climacteric. 2002;5:190–196. [PubMed] [Google Scholar]
- 17.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 18.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 19.Ball ER, Caniglia MK, Wilcox JL, Overton KA, Burr MJ, Wolfe BD, Sanders BJ, Wisniewski AB, Wrenn CC. Effects of genistein in the maternal diet on reproductive development and spatial learning in male rats. Horm Behav. 2010;57:313–322. doi: 10.1016/j.yhbeh.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Res Rev. 2008;57:352–362. doi: 10.1016/j.brainresrev.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta -mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 22.Yi B, Kim C, Yang M. Biological monitoring of bisphenol A with HLPC/FLD and LC/MS/MS assays. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2606–2610. doi: 10.1016/j.jchromb.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Rezaee M, Yamini Y, Shariati S, Esrafili A, Shamsipur M. Dispersive liquid–liquid microextraction combined with highperformance liquid chromatography-UV detection as a very simple, rapid and sensitive method for the determination of bisphenol A in water samples. J Chromatogr A. 2009;1216:1511–1514. doi: 10.1016/j.chroma.2008.12.091. [DOI] [PubMed] [Google Scholar]
- 24.Valentin-Blasini L, Blount BC, Rogers HS, Needham LL. HPLC-MS/MS method for the measurement of seven phytoestrogens in human serum and urine. J Expo Anal Environ Epidemiol. 2000;10:799–807. doi: 10.1038/sj.jea.7500122. [DOI] [PubMed] [Google Scholar]
- 25.Twaddle NC, Churchwell MI, Doerge DR. High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:139–145. doi: 10.1016/s1570-0232(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson AP, Wahala K, Williamson G. Identification and quantification of polyphenol phytoestrogens in foods and human biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:93–109. doi: 10.1016/s1570-0232(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Q, Li Y, Ouyang H, Xu P, Wu D. High-performance liquid chromatographic analysis of bisphenol A and 4-nonylphenol in serum, liver and testis tissues after oral administration to rats and its application to toxicokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:322–329. doi: 10.1016/j.jchromb.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Zhao RS, Wang X, Yuan JP. Highly sensitive determination of tetrabromobisphenol A and bisphenol A in environmental water samples by solid-phase extraction and liquid chromatography– tandem mass spectrometry. J Sep Sci. 2010;33:1652–1657. doi: 10.1002/jssc.201000010. [DOI] [PubMed] [Google Scholar]
- 29.Hosoda K, Furuta T, Yokokawa A, Ogura K, Hiratsuka A, Ishii K. Plasma profiling of intact isoflavone metabolites by highperformance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab Dispos. 2008;36:1485–1495. doi: 10.1124/dmd.108.021006. [DOI] [PubMed] [Google Scholar]
- 30.Churchwell MI, Twaddle NC, Meeker LR, Doerge DR. Improving LC-MS sensitivity through increases in chromatographic performance: comparisons of UPLC-ES/MS/MS to HPLC-ES/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:134–143. doi: 10.1016/j.jchromb.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Howes LG, Howes JB, Knight DC. Isoflavone therapy for menopausal flushes: a systematic review and meta-analysis. Maturitas. 2006;55:203–211. doi: 10.1016/j.maturitas.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiles R, Yang I, Lippincott RL, Murphy E, Buckley B. Potential sources of background contaminants in solid phase extraction and microextraction. J Sep Sci. 2007;30:1029–1036. doi: 10.1002/jssc.200600358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.