Abstract
Birt–Hogg–Dubé syndrome (BHD) is an inherited disorder associated with a germline mutation of the folliculin gene (FLCN). The affected families have a high risk for developing multiple renal cell carcinomas (RCC). Diagnostic markers that distinguish between FLCN-related RCC and sporadic RCC have not been investigated, and many patients with undiagnosed BHD fail to receive proper medical care. We investigated the histopathology of 27 RCCs obtained from 18 BHD patients who were diagnosed by genetic testing. Possible somatic mutations of RCC lesions were investigated by DNA sequencing. Western blotting and immunohistochemical staining were used to compare the expression levels of FLCN and glycoprotein non-metastatic B (GPNMB) between FLCN-related RCCs and sporadic renal tumors (n = 62). The expression of GPNMB was also evaluated by quantitative RT-PCR. Histopathological analysis revealed that the most frequent histological type was chromophobe RCC (n = 12), followed by hybrid oncocytic/chromophobe tumor (n = 6). Somatic mutation analysis revealed small intragenic mutations in six cases and loss of heterozygosity in two cases. Western blot and immunostaining analyses revealed that FLCN-related RCCs showed overexpression of GPNMB and underexpression of FLCN, whereas sporadic tumors showed inverted patterns. GPNMB mRNA in FLCN-related RCCs was 23-fold more abundant than in sporadic tumors. The distinctive expression patterns of GPNMB and FLCN might identify patients with RCCs who need further work-up for BHD.
Keywords: Birt–Hogg–Dubé syndrome (BHD), familial cancer, folliculin (FLCN), glycoprotein non-metastatic B (GPNMB), renal tumor
Birt–Hogg–Dubé syndrome, also called Hornstein–Knickenberg syndrome, is an inherited disorder characterized by skin fibrofolliculomas, pulmonary cysts, pneumothorax, and renal tumors.1,2 Renal cell carcinoma is the most serious and life-threatening manifestation. Since the discovery of the responsible gene, named FLCN,3 increasing numbers of BHD patients have been diagnosed by genetic testing.4–6 Bilateral and metachronous RCCs are often observed.5,6 Patients are at risk for developing independent tumors in the remaining renal tissues after surgical intervention. However, many physicians are still not aware of BHD-related symptoms, and undiagnosed patients are treated as sporadic RCC. Characterization of RCC associated with BHD is important to identify previously undiagnosed patients.
With regard to histological features, only a few studies have been published.5–7 Birt–Hogg–Dubé syndrome is morphologically characterized as a HOCT and composites of more than one histological type.5 Hybrid oncocytic/chromophobe tumor is an oncocytic neoplasm that has areas suggestive of chromophobe RCC and oncocytoma. Many large eosinophilic cells possess intracytoplasmic vacuoles.5,8 However, for a patient with an unknown genetic background, these renal tumors are virtually indistinguishable from sporadic ones solely by microscopic findings. Hybrid oncocytic/chromophobe tumor might also be found in individuals without an FLCN germline mutation.9–11 Detailed information about diagnostic markers for FLCN-related RCC is unavailable.
In the current study, we investigated the pathological features and expression of FLCN and another key molecule, GPNMB, in RCCs of BHD patients. Recently, GPNMB was identified as a downstream targeting molecule induced by FLCN inactivation.12 Here we show that RCCs with FLCN germline mutations overexpressed GPNMB and that they tended to lose the intact form of FLCN. Sporadic renal tumors showed inverted expression patterns of GPNMB and FLCN.
Materials and Methods
Samples
Eighteen patients (17 families) were enrolled in this study, providing 27 renal tumors (Tables2). Written informed consent was obtained from each patient. The study was approved by the Institutional Review Board of Yokohama City University (Yokohama, Japan). Resected tissues were fixed with 10% buffered formalin and embedded in paraffin. Hematoxylin and eosin staining was carried out for histological diagnosis. In seven tumors, RCCs and normal parts of the kidneys were snap-frozen and stored in liquid nitrogen until use. Sporadic renal tumors (n = 62) were used for comparison. The possibilities of von Hippel-Lindau disease, tuberous sclerosis complex, hereditary papillary RCC, and hereditary leiomyomatosis RCC were carefully examined and excluded in all patients by thorough medical examination and family history. The following points were checked in the studied cases in accordance with the diagnostic criteria proposed by the European BHD Consortium1: (i) pulmonary cysts; (ii) fibrofolliculomas; (iii) pneumothorax; (iv) familial history of pneumothorax, fibrofolliculoma, and renal tumor; and (v) multiple RCC or hybrid features in histology. All patients who were diagnosed with BHD by genetic testing had at least two manifestations. In the sporadic tumor groups, one patient with unilateral chromophobe RCC had pulmonary cysts and genetic testing was carried out. Other patients diagnosed with sporadic renal tumors did not have any of the manifestations listed above. Histological types were determined by two pathologists with expertise in renal tumors (N. Kuroda and Y. Nagashima).
Table 2.
Summary of genetic and histopathological information in patients with Birt–Hogg–Dubé syndrome (BHD)
| Sample no. | Histology | Germline mutation of FLCN | Somatic mutation of FLCN | IHC | |
|---|---|---|---|---|---|
| GPNMB | FLCN | ||||
| BHD1 | Chromophobe | Exon 11c.1285dupC | ND | ND | ND |
| BHD2-T1 (proband) | Unclassified | Exon 5c.332_349del | Exon 13 c.1528_1530delGAG† | (++) | Cytoplasmic |
| BHD2-T2 (proband) | Unclassified | Exon 5c.332_349del | NA† | (++) | Cytoplasmic |
| BHD2-T3 (daughter) | HOCT | Exon 5c.332_349del | Exon 6 c.453delGT | (++) | Cytoplasmic |
| BHD3 | Papillary | Exon 13 c.1533_1536delGATG | ND | ND | ND |
| BHD4-T1 | Chromophobe | Exon 11c.1285dupC | NA† | ND | ND |
| BHD4-T2 | Chromophobe | Exon 11c.1285dupC | Undetectable† | ND | ND |
| BHD4-T3-Chr‡ | Chromophobe | Exon 11c.1285dupC | ND | (++) | Cytoplasmic |
| BHD4-T3-P§ | Papillary | Exon 11c.1285dupC | ND | (−) | (−) |
| BHD4-T3-Cle¶ | Clear cell | Exon 11c.1285dupC | ND | (−) | (−) |
| BHD5 | HOCT | Exon 13 c.1533_1536delGATG | Exon 5c.327_328delCC† | (++) | (−) |
| BHD6 | Unknown | Exon 11c.1285dupC | ND | ND | Not done |
| BHD7 | Papillary | Exon 11c.1285dupC | Exon 11 LOH | (++) | Cytoplasmic |
| BHD8 | Oncocytic tumor†† | Exon 13 c.1533_1536delGATG | NA† | ND | ND |
| BHD9-T1 | Chromophobe | Exon 11c.1285dupC | Undetectable | (++) | Nuclear |
| BHD9-T2 | HOCT | Exon 11c.1285dupC | Exon 10 c.1174delC | (++) | Cytoplasmic |
| BHD10 | HOCT | Exon 11c.1285dupC | Exon 10 c.1174delC† | (++) | Cytoplasmic |
| BHD11-T1 | Chromophobe | Exon 9c.906dupT | ND | (++) | Cytoplasmic |
| BHD11-T2 | Chromophobe | Exon 9c.906dupT | ND | (+) | Cytoplasmic |
| BHD12 | HOCT | Exon 11c.1285dupC | ND | (+) | Cytoplasmic |
| BHD13-T1 | Chromophobe | Exon 12c.1347_1353 dupCCACCCT | NA† | (++) | Cytoplasmic |
| BHD13-T2 | Clear cell | Exon 12c.1347_1353 dupCCACCCT | NA† | (−) | (−) |
| BHD14 | Chromophobe | Exon12c.1429 C>T | Exon 11c.1234 delGinsAGA | (+) | Cytoplasmic |
| BHD15 | Chromophobe | Exon 11c.1285dupC | Undetectable | (++) | Cytoplasmic |
| BHD16-T1 | HOCT | Exon 13 c.1533_1536delGATG | ND | (++) | Cytoplasmic |
| BHD16-T2 | Calcified nodule | Exon 13 c.1533_1536delGATG | ND | ND | ND |
| BHD16-T3 | Calcified nodule | Exon 13 c.1533_1536delGATG | ND | ND | ND |
| BHD17-T1 | Chromophobe | Exon 11c.1285dupC | Exon 11 LOH | (++) | Cytoplasmic |
| BHD17-T2 | Chromophobe | Exon 11c.1285dupC | Undetectable | (++) | Nuclear |
DNA was extracted from paraffin-embedded tumors.
Chromophobe lesion of BHD4-T3.
Papillary lesion of BHD4-T3. ¶Clear cell lesion of BHD4-T3.
Detailed histological analysis was not available due to postmortal autolysis. GPNMB immunostaining patterns were scored as: (−), no staining or <5% positive tumor cells; (+), occasional weak staining in ≥5% tumor cells; (++), moderate or intense staining in ≥5% tumor cells. FLCN, folliculin; GPNMB, glycoprotein non-metastic B; HOCT, hybrid oncocytic chromophobe tumor; IHC, immunohistochemistry; LOH, loss of heterozygosity; NA, not available; ND, not done; T, tumor.
Antibodies
For Western blotting of FLCN, rabbit mAb D14G9 (Cell Signaling Technology, Danvers, MA, USA) was used. For FLCN immunostaining, we used rabbit polyclonal antibody ab93196 (Abcam, Cambridge, UK) was used. For GPNMB immunostaining and Western blotting, we obtained goat polyclonal antibody AF-2550 (R&D Systems, Minneapolis, MN) was used. Mouse mAb against β-actin was purchased from Sigma (St. Louis, MO, USA).
Immunostaining
Four micrometer-thick paraffin sections were subjected to immunohistochemistry. For GPNMB immunostaining, sections were treated with proteinase K for 10 min at room temperature. For other antibodies, sections were autoclaved at 121°C for 15 min. The sections were treated with the diluted antibodies at 4°C overnight. Working dilutions were 1:200 for GPNMB and 1:50 for anti-FLCN antibody (ab93196). The FLCN immunostaining patterns were scored as follows. Tissues lacking staining or with <5% positive tumor cells regardless of cytoplasmic or nuclear pattern were termed “negative” (−). Weak to strong cytoplasmic staining without nuclear staining in ≥5% tumor cells was defined as “cytoplasmic”. Weak to strong nuclear staining regardless of cytoplasmic staining in ≥5% tumor cells was termed “nuclear”. The GPNMB immunostaining patterns were scored as follows: no staining or <5% positive tumor cells was categorized as “negative” (−); occasional weak staining in ≥5% tumor cells was categorized as (+); and moderate or intense staining in ≥5% tumor cells was categorized as (++).
DNA isolation
DNA from peripheral blood leukocytes was obtained using the LabPass Blood Mini kit (Cosmo Genetech, Seoul, Korea), and DNA from renal tissue was obtained using QIAamp DNA Mini kit (Qiagen, Hidden, Germany) according to the manufacturers’ instructions.
Direct sequencing
Exons 4–14 of the FLCN gene were amplified by PCR using the primers described previously.3 The PCR conditions were described in our previous study.13 After purification, DNA was labeled with the Big Dye Terminator version 1.1 Cycle Sequencing Kit (Applied Biosystems, Cleveland, OH, USA) and DNA sequencing was done using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems).
Western blot analysis
Lysates (12.5 μg protein) were electrophoresed on SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). Horseradish peroxidase-conjugated donkey anti-goat IgG and goat anti-rabbit IgG and anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used as the secondary antibodies. Bands were detected using an enhanced chemiluminescence system, according to the Hybond ECL protocol (GE Healthcare, Chalfont St. Giles, UK).
RNA isolation
Total RNAs from renal frozen tissues were obtained using RNeasy Mini kit (Qiagen) according to the manufacturer's instructions.
Quantitative RT-PCR
The QuantiTect SYBR Green PCR kit (Qiagen) was used for quantitative RT-PCR. Conditions for PCR were as follows: 50°C for 2 min, 95°C for 15 min, 40 cycles at 95°C for 30 s, and 60°C for 30 s. GAPDH was used as an internal control gene. The primers used for real-time PCR were: GPNMB, (F) 5′-TGCGAGATCACCCAGAACACA-3′ and (R) 5′-CGTCCCAGA- CCCATTGAAGGT-3′;12 GAPDH, (F) 5′-CCACCCATG GCAAATTCC-3′ and (R) 5′-TGATGGGATTTCCATTGATGAC-3′. The mRNA levels were expressed as the absolute number of copies normalized against GAPDH mRNA. Differences in amplification were determined using the standard curve method.
Somatic mutation analysis of FLCN in renal tumors
DNA was extracted from renal tumor tissues. If only the germline mutational signal was amplified and the wild-type sequence was unreadable, it was determined that LOH occurred as the second hit. Exons other than genetically mutated sites were also amplified, and if somatic intragenic mutation was suggested, the PCR product was subcloned and sequenced to clarify the second hit.
Results
Clinical histories and FLCN mutation patterns
Patient characteristics are summarized in Table1. All the patients in this study were Japanese, and the patient group included nine women and nine men. Two patients had lung metastases. One of them (BHD7) had a 10-cm-sized solitary RCC and multiple lung metastases at the time of diagnosis. The patient died 78 months after nephrectomy and successive chemotherapy and targeting therapies. All of the patients had pulmonary cysts and four patients had skin tumors.
Table 1.
Summary of clinical information of patients with Birt–Hogg–Dubé syndrome (BHD)
| Family no. | Gender | Age, years | No. of RCC | Treatments | Other findings | Prognosis (follow-up period, months) |
|---|---|---|---|---|---|---|
| BHD1 | Female | 46 | 1 | Nx | PTX, PC | NED (30) |
| BHD2 (proband) | Female | 69 | 2 | Rt. Nx | Skin tumors, PC | NED (54) |
| BHD2 (daughter) | Female | 46 | 1 | Lt. NSS | PC | NED (42) |
| BHD3 | Male | 61 | 1 | Nx, VATS | PTX, PC | Lung metastasis NED (42) |
| BHD4 | Male | 67 | 3 | Lt. Nx | Skin tumors, PC, Adrenal adenoma | NED (30) |
| BHD5 | Male | 54 | >5 | Lt. Nx, Rt. NSS | PC | NED (27) |
| BHD6 | Male | 65 | >5 | Bilateral Nx | PTX, PC | Dyalisis NED (25) |
| BHD7 | Male | 56 | 1 | Lt. Nx | PTX, PC | Lung metastasis DOD (78) |
| BHD8 | Male | 79 | 3 | Unoperated | PTX, PC, Prostatic Ca. | DEAD (23) |
| BHD9 | Female | 68 | 3 | Lt. NSS | PTX, PC Skin tumors | NED (18) |
| BHD10 | Female | 45 | 5 | Lt. Nx, Rt. Unoperated | PTX, PC Thyroid Ca. | AWD (390) |
| BHD11 | Male | 48 | 2 | Lt. Nx, Rt. NSS | PTX, PC | NED (174) |
| BHD12 | Female | 29 | 1 | Lt. NSS | PTX, PC | NED (19) |
| BHD13 | Male | 63 | 2 | Rt. Nx | PTX, PC Skin tumors | NED (18) |
| BHD14 | Female | 46 | 1 | Rt. NSS | PC | NED (9) |
| BHD15 | Female | 65 | 1 | Lt. NSS | PTX, PC | NED (9) |
| BHD16 | Male | 45 | 3 | Lt. Nx | PTX, PC | NED (9) |
| BHD17 | Female | 49 | 2 | Lt. and Rt. NSS | PC | NED (9) |
AWD, alive with disease; Ca, carcinoma; DEAD, died of prostatic cancer; DOD, died of disease; Lt., left; NED, no evidence of disease; NSS, nephron-sparing surgery; Nx, nephrectomy; PC, pulmonary cysts; PTX, pneumothorax; RCC, renal cell carcinoma; Rt., right; VATS, video-associated thoracic surgery.
The C8 tract in exon 11 (c.1285dupC) was the most frequent germline mutation pattern. It was detected in 9 of 18 patients. Deletion of GATG in exon 13 (c.1533_1536delGATG) was detected in four patients. Other affected families showed different mutation patterns (Table2).
In the sporadic RCC group, one patient with unilateral chromophobe RCC had a few small air cystic spaces in the lung. Although the patient and family members had neither fibrofolliculoma nor pneumothorax, genetic testing was carried out. No mutation was detected, and the possibility of BHD was excluded. Other patients diagnosed with sporadic tumors did not have any manifestation related to BHD, thus genetic testing was not carried out.
Histology of FLCN-related renal tumors
Two patients were excluded from histological study. Patient BHD8 died of a prostatic tumor and had not undergone surgery; at autopsy, renal tumors showed autolysis. Patient BHD6 experienced chronic renal failure. Twelve tumor lesions were pathologically diagnosed as chromophobe RCCs (44.4%), six tumors were HOCTs (22.2%), three tumor lesions were papillary RCCs (11.1%), and two tumor lesions were clear cell RCCs (7.4%). In BHD4, one of three tumors (BHD4-T3) was composed of more than one histological type. In BHD16, two tumors were mostly replaced by calcified material. In BHD2-proband, two independent tumors were diagnosed as unclassified.14
Chromophobe RCCs and HOCTs grew in an expansive manner. A few cystic tubules were composed of low columnar benign-looking cells that were intermingled in the peripheries or the centers of the tumors. That is, intratumoral peripheral small papillary tufts15 were associated with fibrotic stroma or thick-walled blood vessels (Fig.1a,b, arrows). In some areas, tumor cells looked clear due to intracytoplasmic vacuoles (Fig.1c,d).
Figure 1.

Histological and gross features of FLCN-related renal tumors. (a, b) H&E staining of chromophobe renal cell carcinoma (RCC) in Birt–Hogg–Dubé (BHD) syndrome patient BHD13 (a) and hybrid oncocytic/chromophobe tumor (HOCT) in BHD5 (b). A few benign-looking cystic tubules migrated in tumor nodules (arrows). (c, d) High magnification of chromophobe RCC in BHD17 (c) and HOCT in BHD10 (d). HOCT is composed of oncocytoma-like granular cells with round nuclei and chromophobe RCC-like cell borders and perinuclear halo. (e) Sectioned surface of BHD7 shows a brownish solid tumor. (f, g) H&E staining of papillary RCC in BHD7 at low magnification (f) and high magnification (g). (h) Tumor cells were diffusely immunostained for α-methylacyl-CoA racemase, supporting the histology of papillary RCC.
Somatic mutations of FLCN
Possible somatic mutations of FLCN were investigated in 16 tumors (7 freshly frozen tumors and 9 paraffin-embedded tumors). Second hit intragenic mutations were detected in 6 tumors. In these tumors, the second hits occurred in a different exon from the genetically affected exon (Table2). Among them, in BHD9-T2, monoallelic mutations between exon 10 and exon 11 were identified by the cloning (Figs2a,S1). It was unknown in the other five tumors which allele was affected due to large-sized intervening introns or the quality of DNA from paraffin-embedded tumors. Apart from six tumors with second hits, two tumors showed LOH (Fig.2b) in which the heterozygous mutation pattern was completely replaced by a hemizygous mutation pattern. The germline mutation was retained and the wild-type allele was lost, thus both copies were affected.
Figure 2.
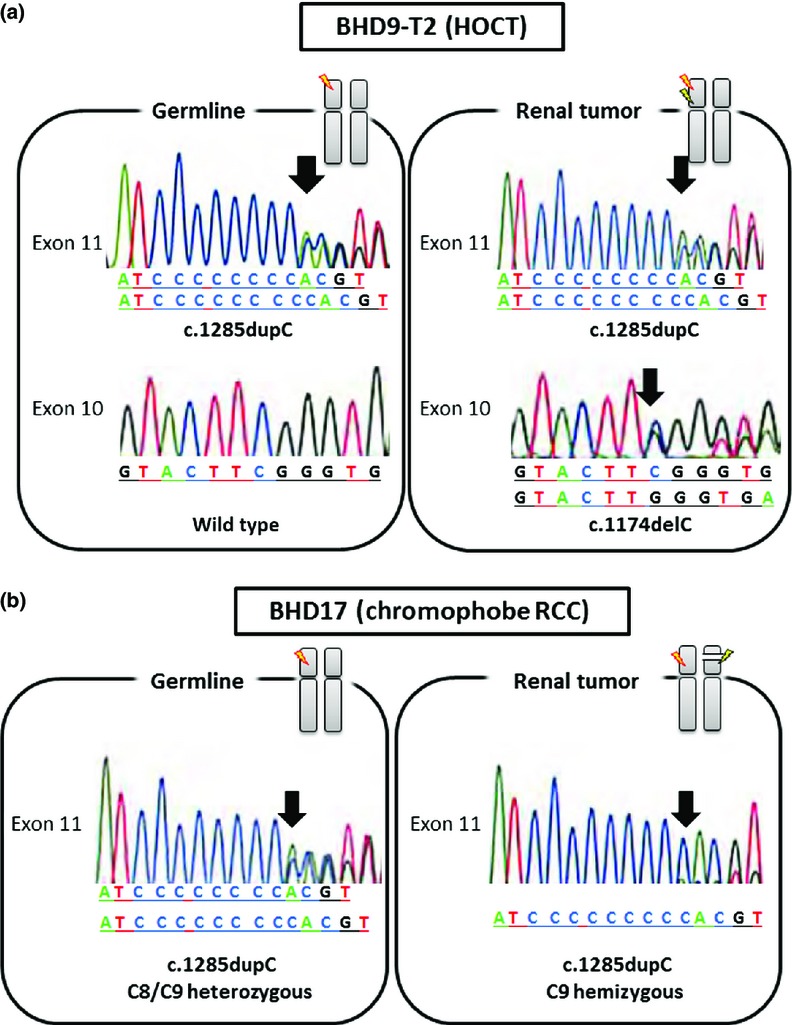
Germline and somatic mutation patterns of FLCN. (a) Birt–Hogg–Dubé (BHD) syndrome patient BHD9 carried FLCN germline mutation in exon 11 (upper left). The exon 10 sequence was confirmed to be wild-type (lower left). The patient's tumor BHD9-T2 showed somatic mutation in exon 10 (lower right) in addition to germline mutation (upper right). (b) BHD17 carried FLCN germline mutation in exon 11 (left). The mutation pattern was c.1285dupC. In the tumor lesion, heterozygous mutation in the C8 tract was completely replaced by a C9 tract, indicating loss of heterozygosity (right). No additional mutation was found in the other exons. Arrows indicate starting point of mutation.
Expression of FLCN protein in BHD and sporadic renal tumors
Next, we investigated FLCN protein expression. Using the antibody D14G9 for Western blotting, the FLCN bands (70 kDa) stained strongly in non-tumor regions of BHD kidneys, whereas FLCN bands were either undetectable or much weaker in RCCs of BHD patients, compared with the corresponding non-tumor parts (Fig.3a). In sporadic tumors, the FLCN bands were clearly detected (Fig.3b). We also obtained similar results using antibodies sc-25168 and ab93196 (data not shown).
Figure 3.
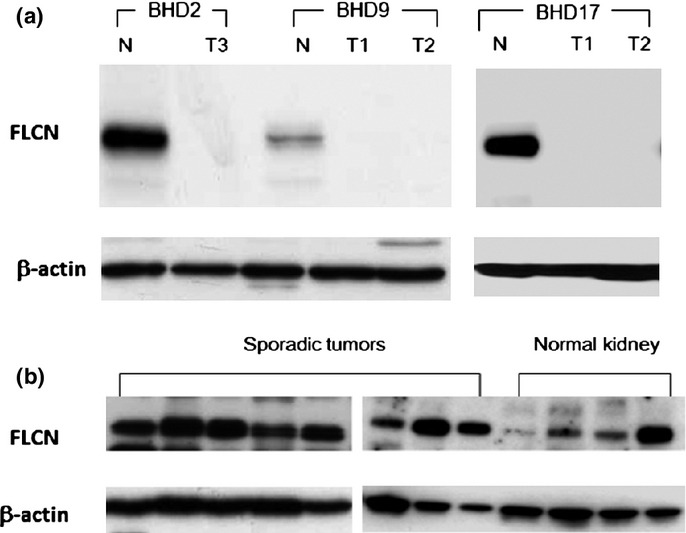
Folliculin (FLCN) expression in Birt–Hogg–Dubé (BHD) and sporadic tumors. (a) Representative results of Western blot analysis of BHD kidneys. Patients BHD9 and BHD17 had two independent tumors (T1, T2). The FLCN bands (70 kDa) were clearly detected in non-tumor lanes but barely seen in tumor lanes. N, normal-looking region; T, tumor region. (b) Representative results of Western blotting analysis of sporadic renal tumors and normal kidneys without the background of BHD. Sporadic renal tumors include clear cell renal cell carcinomas (RCCs) (n = 3), oncocytoma (n = 1), papillary RCC (n = 1), and chromophobe RCCs (n = 3).
We carried out FLCN immunostaining using the antibody ab93196 (Fig.4). The staining sensitivity and intensity of this product were the best among the three examined antibodies. In normal renal cortices, FLCN was strongly stained in the nuclei and cytoplasms of distal tubules and collecting ducts (Fig.4a,b). The majority of FLCN-related tumors showed weak cytoplasmic staining (Table2, Fig.4c,e,g). Nuclear staining was observed in only two FLCN-related tumors. In normal areas of BHD kidneys, strong nuclear staining was detected (Fig.4c,e,g, insets). In sporadic cases, most tumors (except chromophobe RCCs) showed nuclear staining. In chromophobe RCCs, 11 of 18 cases showed nuclear staining (61.1%) (Table3, Fig.4d,f,h).
Figure 4.
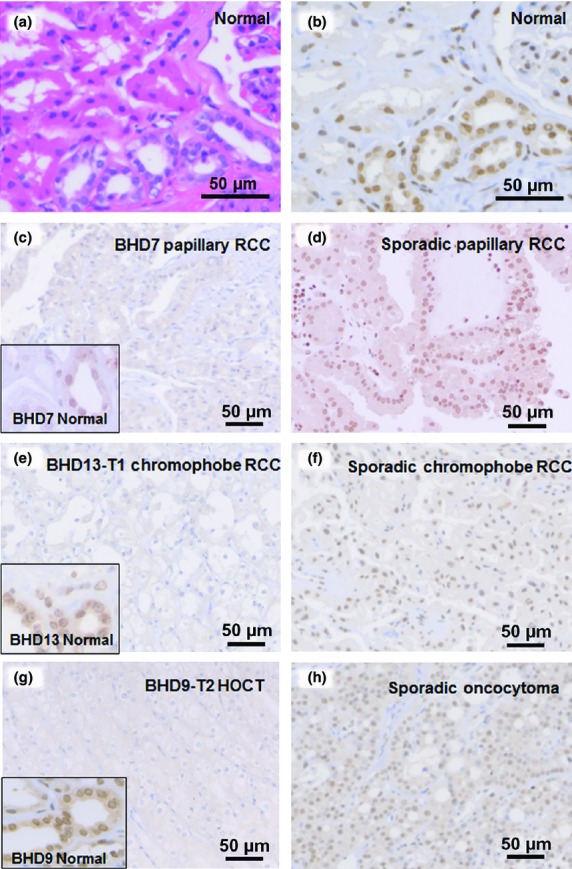
Folliculin (FLCN) immunostaining in normal kidney, and Birt–Hogg–Dubé (BHD) and sporadic tumors. (a) H&E staining of a normal kidney. (b) Serial section of sample A immunostained for FLCN. Nuclei of distal tubules were strongly positive for FLCN. (c–h) Immunostaining for FLCN in BHD and sporadic tumors. Weak cytoplasmic staining was evident in tumors of BHD patients (c, e, h). Insets highlight distal tubules of non-tumor areas, showing immunoreactivity to FLCN. Sporadic renal tumors (d, f, h) showed strong nuclear staining.
Table 3.
Summary of folliculin (FLCN) and glycoprotein non-metastic B (GPNMB) immunostaining in Birt–Hogg–Dubé (BHD) and sporadic renal tumors
| Immunostaining | HOCT/oncocytoma | Chromophobe | Papillary | Clear cell | ||||
|---|---|---|---|---|---|---|---|---|
| BHD, n = 6 (%) | Sporadic, n = 8 (%) | BHD, n = 9 (%) | Sporadic, n = 18 (%) | BHD, n = 2 (%) | Sporadic, n = 11 (%) | BHD, n = 2 (%) | Sporadic, n = 20 (%) | |
| FLCN | ||||||||
| Nuclear | 0 (0) | 6 (75) | 2 (22) | 11 (61) | 0 (0) | 9 (82) | 0 (0) | 16 (80) |
| Cytoplasmic | 5 (83) | 2 (25) | 7 (78) | 6 (33) | 1 (50) | 0 (0) | 0 (0) | 1 (5) |
| Negative | 1 (17) | 0 (0) | 0 (0) | 1 (6) | 1 (50) | 2 (18) | 2 (100) | 3 (15) |
| GPNMB | ||||||||
| (−) | 0 (0) | 8 (100) | 0 (0) | 9 (50) | 1 (50) | 10 (91) | 2 (100) | 20 (100) |
| (+) | 1 (17) | 0 (0) | 2 (22) | 8 (44) | 0 (0) | 1 (9) | 0 (0) | 0 (0) |
| (++) | 5 (83) | 0 (0) | 7 (78) | 1 (6) | 1 (50) | 0 (0) | 0 (0) | 0 (0) |
(−), No staining or <5% of the tumor cells positive; (+), occasional weak staining; (++), moderate or intense staining. HOCT, hybrid oncocytic chromophobe tumor.
Overexpression of GPNMB protein in FLCN-related tumors
Western blotting clearly indicated the loss of FLCN, but immunostaining for FLCN was not sufficiently specific to predict RCC associated with BHD. Some auxiliary markers were desirable to give an indication for genetic analysis. In the current study, we compared GPNMB expression between BHD and sporadic tumors. In quantitative RT-PCR, RCCs from BHD patients expressed GPNMB mRNA at levels that averaged 23-fold higher compared to sporadic RCCs (P < 0.01, Student's t-test) (Fig.5a). GPNMB mRNA levels of non-neoplastic renal tissues of BHD patients were as low as those of sporadic RCCs (data not shown).
Figure 5.
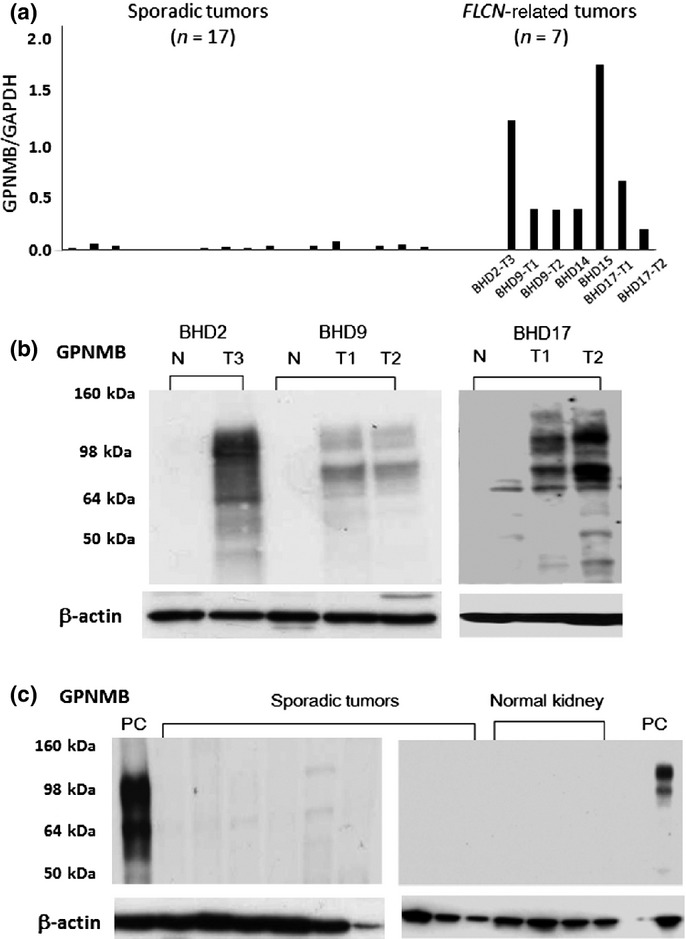
Glycoprotein non-metastatic B (GPNMB) expression in Birt–Hogg–Dubé (BHD) and sporadic tumors. (a) Expression levels of GPNMB mRNA were analyzed by quantitative RT-PCR as shown. Sporadic renal tumors include clear cell renal cell carcinomas (RCCs) (n = 9), oncocytoma (n = 1), papillary RCC (n = 1), and chromophobe RCCs (n = 6). (b) Representative results of Western blot analysis of BHD kidneys. Patients BHD9 and BHD17 had two independent tumors (T1, T2), respectively. Two isoforms (115 kDa and 80 kDa) of GPNMB bands were seen in tumor lanes, but not in normal-looking lanes. N, normal-looking region; T, tumor region. (c) Representative results of Western blot analysis of sporadic renal tumors and normal kidneys without the background of BHD. GPNMB bands were barely seen in sporadic tumor and normal kidney lanes. Sporadic renal tumors included clear cell RCCs (n = 3), oncocytoma (n = 1), papillary RCC (n = 1), and chromophobe RCCs (n = 4). PC, positive control using BHD9-T2.
In Western blot analysis, strong expression of GPNMB protein was detected only in FLCN-related tumors, and the bands were undetectable in non-tumor renal tissues of BHD patients. The GPNMB bands were barely detectable in sporadic RCCs and normal kidneys (Fig.5b,c).
Immunostaining revealed that GPNMB was undetectable in normal control kidneys (Fig.6). All FLCN-related RCCs (except for three non-chromophobe RCCs) were positively stained for GPNMB (Table2, Fig.6b,d). Immunostaining intensities of GPNMB were correlated with the relative expression of GPNMB mRNA, such that BHD15 with intense staining showed the highest expression whereas BHD2 with moderate staining showed lower expression compared with BHD15. Small nodules of renal oncocytosis in non-tumor regions, which is one of the diagnostic characteristics of BHD kidneys,5 also showed moderate immunostaining (Fig.6f). In sporadic cases, half of the chromophobe RCCs and most other types were negatively stained for GPNMB (Table3). Importantly, all sporadic oncocytomas were negative for GPNMB, whereas all HOCTs in BHD were positively stained. With regard to GPNMB-positive RCCs, all but one showed nuclear staining for FLCN. Only one GPNMB-positive RCC showed cytoplasmic staining for FLCN. The patient with this tumor also had a few pulmonary cysts. Genetic testing was carried out and the possibility of BHD was eliminated.
Figure 6.
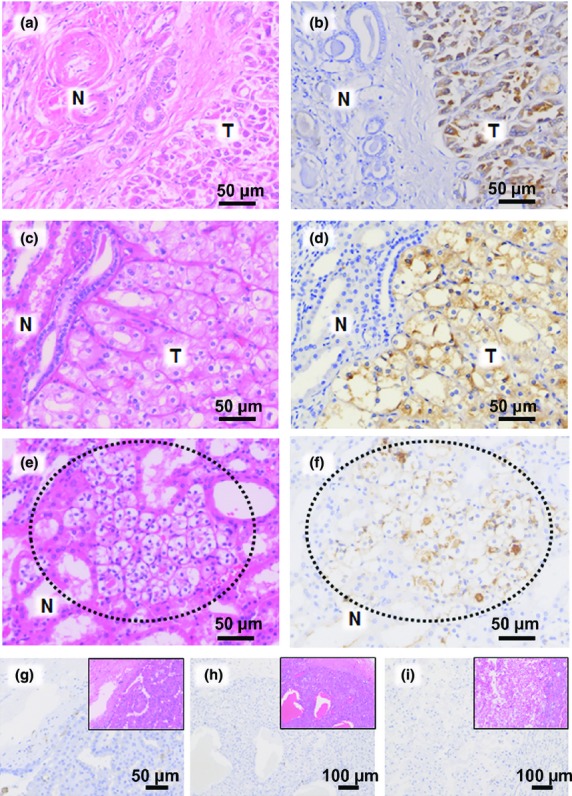
Glycoprotein non-metastatic B (GPNMB) immunostaining of Birt–Hogg–Dubé (BHD) and sporadic tumors. (a, c, e) H&E staining of BHD tumors (T) of patients BHD2-T1 (a), BHD13-T1 (c), and a nodule of oncocytosis in the normal-looking region (N) of BHD13 (e, dotted circle). (b, d, f) Serial sections of (a, c, e) immunostained for GPNMB. Tumor cells (b, d) were intensely stained for GPNMB. Tubules of normal-looking regions were negative for GPNMB. GPNMB was immunostained in the region of focal oncocytosis (f, dotted circle). (g–i) Immunostaining for GPNMB in sporadic renal tumors: papillary renal cell carcinoma (g), oncocytoma (h), and chromophobe renal cell carcinoma (i). Insets are H&E stains of the serial sections.
Discussion
Several important signaling pathways are affected by FLCN deficiency, including mTOR and the MiTF family.12,16–18 We confirmed overexpression of phospho-S6, a downstream molecule of mTOR complex 1, in FLCN-related RCCs (data not shown). However, mTOR pathway molecules are also overexpressed in sporadic RCCs.19,20 These ubiquitously upregulated molecules cannot be used for differential diagnosis.
A previous analysis of FLCN-related RCCs revealed frequent somatic frameshift mutations or LOH.21 In situ hybridization analysis indicated that FLCN mRNA was undetectable in the RCCs of BHD patients.22 The results of the current study supported the notion that the intact form of FLCN protein is lost in RCCs of BHD patients. However, immunohistochemical studies of a substantial number of FLCN-related RCCs have not been carried out due to the specificity and sensitivity of antibodies.23 Investigators using a non-commercial polyclonal antibody reported strong immunostaining for FLCN in a BHD renal tumor with a second hit in the FLCN gene.24 We recognize that the antibody ab93196 showed strong nuclear staining in normal renal tubules. HeLa cells transiently transfected with FLCN showed nuclear staining in vitro.16 Nuclear accumulation of FLCN in normal cells has also been described in published reports.23 Therefore, a weak cytoplasmic staining in vivo might alert us to the possibility of BHD. The pathophysiological significance of nuclear localization of FLCN is poorly understood, and further study is needed.
In the present study, the expression of GPNMB mRNA was significantly higher in FLCN-related RCCs than in sporadic tumors, data that were supported by Western blotting. However, half of the sporadic chromophobe RCCs showed weak immunostaining for GPNMB. It should be noted that all of these RCCs showed a nuclear staining for FLCN. Although immunoreactivity for GPNMB alone cannot be sufficiently specific to predict RCC associated with BHD, a combined immunohistochemical panel for FLCN and GPNMB might predict appropriate cases for genetic testing. One sporadic RCC case with pulmonary cysts showed the immunostaining patterns suggestive of FLCN-related tumors. Although genetic testing excluded the possibility of BHD, careful follow-up is needed to better understand the unusual clinicopathological features of this case. Sporadic chromophobe RCCs rarely have somatic FLCN mutations but potentially have promoter methylation and LOH.25,26 Therefore, further studies are needed to understand the role of FLCN in sporadic RCCs.
Little is known about the roles of GPNMB in the human kidney. Glycoprotein non-metastatic B is upregulated in monocytes and macrophages in dialysis patients, suggesting that it has a role as an inflammatory mediator in a uremic milieu.27 Glycoprotein non-metastatic B plays an essential role in osteoblast maturation and osteoclast differentiation28 and is also involved in melanosome formation.29 In previous work on GPNMB in FLCN signaling, FLCN inactivation induced nuclear accumulation of TFE3, allowing the production of GPNMB.12 We also examined immunostaining of TFE3 in the current cases, but TFE3-positive cells were sparse (data not shown), suggesting that the overexpression of GPNMB might not be an event explained solely by the FLCN–TFE3 axis. Not only MiTF family members30 but also growth factors31 were reported to be involved in the cascade. For example, β-fibroblast growth factor and platelet-derived growth factor were shown to induce GPNMB overexpression in skeletal muscle cells.31 Although the detailed role of GPNMB in RCC needs to be analyzed in greater detail, the present study suggests that GPNMB is expressed more abundantly in FLCN-related RCC than sporadic RCC.
We suggest that urologists and pathologists should carefully analyze the background of unusual renal tumors with multifocal growth. It is hoped that our study of FLCN and GPNMB expression might help distinguish FLCN-related RCCs from sporadic tumors. It is important to understand the detailed role of GPNMB in renal carcinogenesis in BHD patients. Further studies will also be needed to find additional useful markers in the diagnosis of FLCN-related RCCs.
Acknowledgments
The authors thank Drs. Hiroko Gotoda, Yasushi Yatabe, Akira Okada, Suzuko Moritani, Fumi Kawakami, Toshiharu Sakuma, Shin Sasaki, Tomohiro Hasaka, Yoshiaki Imamura, Muneyuki Hiraishi, Yoshinori Matsui, and Keisuke Sugimoto for providing clinical information and renal tissues, and members of pathology laboratories in Yokohama City University Graduate School of Medicine and affiliated hospitals for excellent assistance, and Ms. Y Imanishi for sequence analysis. This work was supported by Japan Society for the Promotion of Science Kakenhi (Grant Nos. 23590406 [to M.F.] and 24590408 [to Y.N.]), the Ministry of Health, Labor and Welfare (Y.N.), Yokohama Foundation for Advancement of Medical Science (M.F.), Suzuki Hinyoki Igaku Shinko Zaidan (M.F.) and the Mitsubishi Foundation (M.F.).
Glossary
Abbreviations
- BHD
Birt–Hogg–Dubé syndrome
- FLCN
folliculin
- GPNMB
glycoprotein non-metastatic B
- HOCT
hybrid oncocytic/chromophobe tumor
- LOH
loss of heterozygosity
- MiTF
microphthalmia-associated transcription factor
- mTOR
mammalian target of rapamycin
- RCC
renal cell carcinoma
- TFE3
transcription factor E3
Disclosure Statment
The authors have no conflict of interest.
Supporting Information
Fig. S1. Cloning from exon 10 to exon 11 of folliculin (FLCN) in Birt–Hogg–Dubé (BHD) syndrome patient BHD9-T2. Exon 10 (114 bp) and exon 11 (124 bp) are intervened by intron 10 (565 bp). The sequence results of four clones (Nos. 1C–4C) are shown. Ex10 and Ex11 indicate wild-type sequences. Clone-2 (No. 2C) simultaneously shows somatic mutation in exon 10 (second line from the top) and germline mutation in exon 11 (bottom line).
References
- Menko FH, van Steensel MA, Giraud S, et al. Birt–Hogg–Dube syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt–Hogg–Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet. 2008;45:321. doi: 10.1136/jmg.2007.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt–Hogg–Dube syndrome. Cancer Cell. 2002;2:157. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- Zbar B, Alvord WG, Glenn G, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt–Hogg–Dube syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393. [PubMed] [Google Scholar]
- Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt–Hogg–Dube syndrome. Am J Surg Pathol. 2002;26:1542. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Houweling AC, Gijezen LM, Jonker MA, et al. Renal cancer and pneumothorax risk in Birt–Hogg–Dube syndrome; an analysis of 115 FLCN mutation carriers from 35 BHD families. Br J Cancer. 2011;105:1912. doi: 10.1038/bjc.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hes O, Petersson F, Kuroda N, et al. Renal hybrid oncocytic/chromophobe tumors – a review. Histol Histopathol. 2013;28:1257–64. doi: 10.14670/HH-28.1257. [DOI] [PubMed] [Google Scholar]
- Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37:1469. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- Petersson F, Gatalica Z, Grossmann P, et al. Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch. 2010;456:355. doi: 10.1007/s00428-010-0898-4. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Tanaka A, Ohe C, et al. Review of renal oncocytosis (multiple oncocytic lesions) with focus on clinical and pathobiological aspects. Histol Histopathol. 2012;27:1407. doi: 10.14670/HH-27.1407. [DOI] [PubMed] [Google Scholar]
- Delongchamps NB, Galmiche L, Eiss D, et al. Hybrid tumour ‘oncocytoma-chromophobe renal cell carcinoma’ of the kidney: a report of seven sporadic cases. BJU Int. 2009;103:1381. doi: 10.1111/j.1464-410X.2008.08263.x. [DOI] [PubMed] [Google Scholar]
- Hong SB, Oh H, Valera VA, et al. Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLoS One. 2010;5:e15793. doi: 10.1371/journal.pone.0015793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M, Tanaka R, Koga S, et al. Pulmonary cysts of Birt–Hogg–Dube syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am J Surg Pathol. 2012;36:589. doi: 10.1097/PAS.0b013e3182475240. [DOI] [PubMed] [Google Scholar]
- Nagashima Y, Furuya M, Gotohda H, et al. FLCN gene-mutated renal cell neoplasms: mother and daughter cases with a novel germline mutation. Int J Urol. 2012;19:468. doi: 10.1111/j.1442-2042.2011.02945.x. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Furuya M, Nagashima Y, et al. Intratumoral peripheral small papillary tufts: a diagnostic clue of renal tumors associated with Birt–Hogg–Dube syndrome. Ann Diagn Pathol. 2014;18:171. doi: 10.1016/j.anndiagpath.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci USA. 2006;103:15552. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasumi Y, Baba M, Ajima R, et al. Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2. Proc Natl Acad Sci USA. 2009;106:18722. doi: 10.1073/pnas.0908853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TP, Gruber JJ, Hartman TR, et al. Loss of the Birt–Hogg–Dube tumor suppressor results in apoptotic resistance due to aberrant TGFbeta-mediated transcription. Oncogene. 2011;30:2534. doi: 10.1038/onc.2010.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz L, Chaux A, Albadine R, et al. Immunoexpression status and prognostic value of mTOR and hypoxia-induced pathway members in primary and metastatic clear cell renal cell carcinomas. Am J Surg Pathol. 2011;35:1549. doi: 10.1097/PAS.0b013e31822895e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaux A, Schultz L, Albadine R, et al. Immunoexpression status and prognostic value of mammalian target of rapamycin and hypoxia-induced pathway members in papillary cell renal cell carcinomas. Hum Pathol. 2012;43:2129. doi: 10.1016/j.humpath.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocke CD, Yang Y, Pavlovich CP, et al. High frequency of somatic frameshift BHD gene mutations in Birt–Hogg–Dube-associated renal tumors. J Natl Cancer Inst. 2005;97:931. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- Warren MB, Torres-Cabala CA, Turner ML, et al. Expression of Birt–Hogg–Dube gene mRNA in normal and neoplastic human tissues. Mod Pathol. 2004;17:998. doi: 10.1038/modpathol.3800152. [DOI] [PubMed] [Google Scholar]
- Adley BP, Smith ND, Nayar R, et al. Birt–Hogg–Dube syndrome: clinicopathologic findings and genetic alterations. Arch Pathol Lab Med. 2006;130:1865. doi: 10.5858/2006-130-1865-BSCFAG. [DOI] [PubMed] [Google Scholar]
- Menko FH, Johannesma PC, van Moorselaar RJ, et al. A de novo FLCN mutation in a patient with spontaneous pneumothorax and renal cancer; a clinical and molecular evaluation. Fam Cancer. 2012;12:373–9. doi: 10.1007/s10689-012-9593-8. [DOI] [PubMed] [Google Scholar]
- Khoo SK, Kahnoski K, Sugimura J, et al. Inactivation of BHD in sporadic renal tumors. Cancer Res. 2003;63:4583. [PubMed] [Google Scholar]
- Gad S, Lefevre SH, Khoo SK, et al. Mutations in BHD and TP53 genes, but not in HNF1beta gene, in a large series of sporadic chromophobe renal cell carcinoma. Br J Cancer. 2007;96:336. doi: 10.1038/sj.bjc.6603492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl MV, Vaziri ND, Yuan J, et al. Upregulation of monocyte/macrophage HGFIN (Gpnmb/Osteoactivin) expression in end-stage renal disease. Clin J Am Soc Nephrol. 2010;5:56. doi: 10.2215/CJN.03390509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, et al. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J Cell Biochem. 2001;84:12. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- Tomihari M, Hwang SH, Chung JS, et al. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp Dermatol. 2009;18:586. doi: 10.1111/j.1600-0625.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Liu W, Zhu C, et al. Silencing of GPNMB by siRNA inhibits the formation of melanosomes in melanocytes in a MITF-independent fashion. PLoS One. 2012;7:e42955. doi: 10.1371/journal.pone.0042955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Nikawa T, Furochi H, et al. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am J Physiol Cell Physiol. 2005;289:C697. doi: 10.1152/ajpcell.00565.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cloning from exon 10 to exon 11 of folliculin (FLCN) in Birt–Hogg–Dubé (BHD) syndrome patient BHD9-T2. Exon 10 (114 bp) and exon 11 (124 bp) are intervened by intron 10 (565 bp). The sequence results of four clones (Nos. 1C–4C) are shown. Ex10 and Ex11 indicate wild-type sequences. Clone-2 (No. 2C) simultaneously shows somatic mutation in exon 10 (second line from the top) and germline mutation in exon 11 (bottom line).


