Abstract
Objective
The role of receptors for endogenous metabolic danger signals-associated molecular patterns (DAMPs) has been characterized recently as bridging innate immune sensory systems for DAMPs to initiation of inflammation in bone marrow-derived cells such as macrophages. However, it remains unknown whether endothelial cells (ECs), the cell type with the largest numbers and the first vessel cell type exposed to circulating DAMPs in the blood, can sense hyperlipidemia. This report determined whether caspase-1 plays a role in ECs in sensing hyperlipidemia and promoting EC activation.
Approach and Results
Using biochemical, immunological, pathological and bone marrow transplantation methods together with the generation of new apoplipoprotein E (ApoE)−/−/caspase-1−/− double knock-out mice we made the following observations: 1) early hyperlipidemia induced caspase-1 activation in ApoE−/− mouse aorta; 2) caspase-1−/−/ApoE−/− mice attenuated early atherosclerosis; 3) caspase-1−/−/ApoE−/− mice had decreased aortic expression of pro-inflammatory cytokines and attenuated aortic monocyte recruitment; and 4) caspase-1−/−/ApoE−/− mice had decreased EC activation including reduced adhesion molecule expression and cytokine secretion. Mechanistically, oxidized lipids activated caspase-1 and promoted pyroptosis in ECs by a ROS mechanism. Caspase-1 inhibition resulted in accumulation of sirtuin 1 (Sirt1) in the ApoE−/− aorta, and Sirt1 inhibited caspase-1 upregulated genes via activator protein-1 (AP-1) pathway.
Conclusions
Our results demonstrate for the first time that early hyperlipidemia promotes EC activation before monocyte recruitment via a caspase-1-Sirt1-AP-1 pathway, which provides an important insight into the development of novel therapeutics for blocking caspase-1 activation as early intervention of metabolic cardiovascular diseases and inflammations.
Keywords: caspase-1, endothelial cell activation, monocyte recruitment, inflammation, atherosclerosis
Introduction
Hyperlipidemia, a risk factor for CVD, is defined as pathologically elevated plasma concentrations of cholesterol and other lipids, which are commonly found in patients with atherosclerosis1. We and others previously reported that hyperlipidemia, pro-inflammatory mediators, and other risk factors promote endothelial cell (EC) activation and atherosclerosis via several mechanisms, which include inducing endothelial activation and injury2, 3, increasing monocyte recruitment and differentiation4, 5, and decreasing regulatory T cell population6, 7.
Endothelial cells (ECs) that line the inner surface of vessel wall are the first cells exposed to metabolite-related endogenous danger signals in the circulatory system1. Endothelial activation is, therefore, defined as the initial event responsible for monocyte recruitment in atherogenesis8. However, questions such as how hyperlipidemia can be sensed by ECs, and how hyperlipidemia-induced vascular inflammation is initiated, remain largely unanswered.
The cellular “receptors”, which can recognize the risk factors for atherogenesis such as hyperlipidemia, have been under intensive search. The role of receptors for pathogen-associated molecular patterns (PAMPs) has been characterized recently as bridging innate immune sensory systems for exogenous infectious agents and endogenous metabolic danger signals-associated molecular patterns (DAMPs) to initiation of inflammation9. The Toll-like receptors (TLRs), mainly located in the plasma membrane, recognize a variety of conserved microbial PAMPs and metabolic DAMPs, and promote inflammatory gene transcription. As we described previously10, for inflammation privileged tissues in which inflammasome component genes are not constitutively expressed, TLRs also work in synergy with cytosolic sensing receptor families including NLRs [NOD (nucleotide binding and oligomerization domain)-like receptors] in recognizing endogenous DAMPs and in mediating upregulation and activation of a range of inflammatory genes11. Caspase-1, a member the cysteine protease family of caspases, is present in the cell cytosol as pro-caspase-1, an inactive zymogen, and requires the assembly of a NLR family member-containing protein complex called “inflammasome” for activation. Activated caspase-1 is required for cleaving/processing pro-interleukin-1β (IL-1β) and pro-IL-18 into mature pro-inflammatory cytokines IL-1β and IL-18, respectively, and activation of other inflammatory pathways. However, it remains unclear whether in early atherosclerosis, the caspase-1-inflammasome pathway in ECs can sense elevated lipids as a DAMP and promote endothelial activation.
Previous reports showed that cholesterol crystals activate NLRP3 inflammasome in macrophages12, 13, suggesting that NLRP3 inflammasome in macrophages can sense cholesterol crystals formed in advanced stage of atherosclerosis14. However, monocyte migration into the aorta after 3 weeks of high fat (HF) diet feeding is detected in atherosclerotic apolipoprotein E (ApoE)−/− mice15, suggesting that prior to cholesterol crystal formation in the vessels, ECs may respond to hyperlipidemia and activate caspase-1 precedent for monocyte recruitment. It has been reported that in response to various proinflammatory stimuli including lipopolysaccharide (LPS), human endothelial cells secrete IL-1β, resulted from the cleavage of pro-IL-1β by activated caspase-1. However, IL-1β secretion from human ECs, detected by ELISA, are 70.6 folds lower than that secreted from human monocytes16, suggesting that IL-1β role in endothelial cells as functional consequence of caspase-1 activation may not be as significant as that in monocytes. Thus, additional roles of caspase-1 in ECs need to be further explored. Although pro-atherogenic functions of caspase-117, NLRP312, IL-1β18, and IL-1819 have been reported, important knowledge gaps remain such as: 1) whether caspase-1 sensing system in ECs can sense early hyperlipidemia (non-cholesterol crystals lipid stimulus); and 2) whether caspase-1 activation in ECs can promote endothelial activation, monocyte recruitment, and atherogenesis.
Our previous report showed that caspase-1 can have more than 70 protein substrates20, the list of which is getting longer. A recent report showed that caspase-1 specifically cleaves sirtuin 1 (Sirt1), a nicotinamide adenine dinucleotide (NAD)-dependent protein/class III histone deacetylase, in adipose tissue during metabolic stress21. However, the question of whether caspase-1 cleaves Sirt1 in aortic ECs remains unanswered.
In this study, we examined a novel hypothesis that caspase-1 in ECs can sense hyperlipidemia in mice fed a HF diet for three weeks, and that caspase-1 activation in ECs, potentially via the caspase-1-Sirt1 pathway, can promote endothelial activation, monocyte recruitment, and atherogenesis. We generated double gene knockout (KO) mice that are deficient of caspase-1 and ApoE (ApoE−/−/caspase-1−/−) by crossing caspase-1−/− mice into ApoE−/− mouse background. Our results demonstrate that caspase-1 activation significantly contributes to endothelial activation, monocyte recruitment, and atherogenesis via the caspase-1-Sirt1-AP1 pathway. Therefore, our results indicate a role for caspase-1 activation in sensing hyperlipidemia as a DAMP and promoting endothelial activation.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
1. Hyperlipidemia induces the upregulation of caspase-1 expression and caspase-1 activation in ApoE−/− aorta
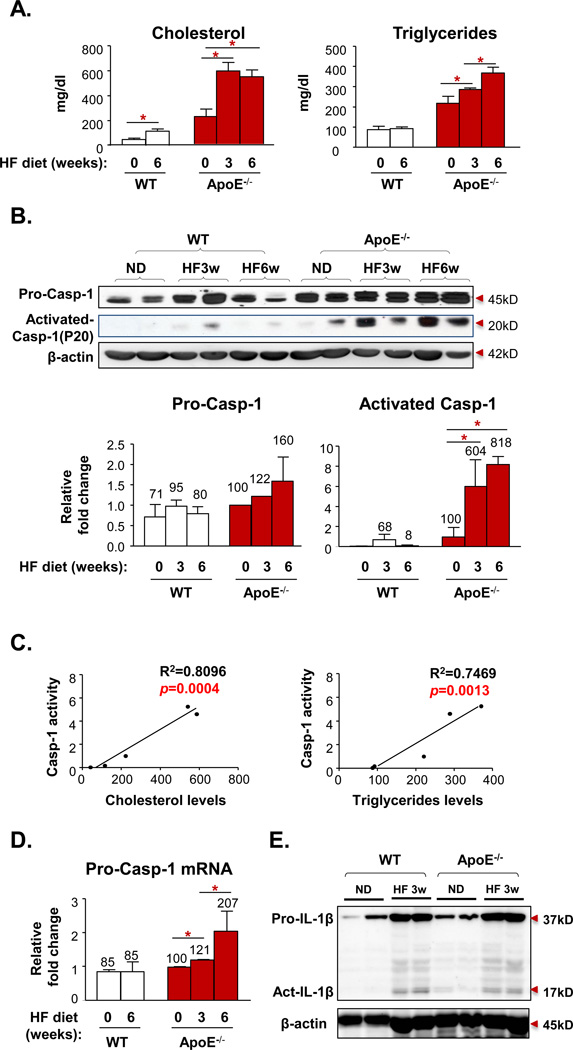
To examine our hypothesis that early hyperlipidemia activates the caspase-111 in the aortic tissue, we performed Western blot analysis with caspase-1 antibodies on mouse aortic protein lysates collected from wild-type (WT) mice and ApoE−/− mice fed a HF diet for 0, 3, and 6 weeks. Plasma lipid profiling data (Fig. 1A) showed that 3-week HF diet feeding significantly increased plasma cholesterol levels and triglyceride levels in ApoE−/− mice, reaching hyperlipidemic conditions (>200mg/dl). More importantly, pro-caspase-1 expression levels (Fig. 1B) were significantly upregulated in ApoE−/− mouse aorta after feeding a HF diet for 3 (122%) and 6 weeks (160%), respectively. Since catalytic activation of pro-caspase-1 (45 kD) into two smaller subunits, p20 and p10 in a protein complex termed inflammasome is required for its protease activity, we also examined the expression of activated caspase-1 p20 subunit. The results (Fig. 1B) showed that activated caspase-1 was increased in ApoE−/− mouse aorta fed with a HF diet for 3 weeks (604%) and 6 weeks (818%), respectively. Of note, upregulation of pro-caspase-1 induced by 6 weeks of HF diet feeding in ApoE−/− mouse aorta was about 2 folds higher than that of WT mouse aorta. In contrast, activated caspase-1 p20 expression in HF diet fed ApoE−/− mouse aorta was >8 folds higher than that of WT mouse aorta. With the lipid profiling data, we performed regression analysis of the lipid data against expression data of p20 activated caspase-1 detected by Western blot in Fig. 1B. We found that activated caspase-1 p20 expression in ApoE−/− mouse aorta was correlated well with increased plasma cholesterol levels (R2=0.8096; p=0.0004<0.01) and increased triglyceride levels (R2=0.7469; p=0.0013<0.01) (Fig. 1C), suggesting that caspase-1 activation is very tightly associated with elevated cholesterol and triglycerides levels, as early as 3 weeks of hyperlipidemia. Of note, the expression of pro-caspase-1 in non-HF diet fed ApoE−/− mouse aorta was not significantly higher than that of non-HF diet fed WT mouse aorta, suggesting that upregulation of pro-caspase-1 in HF diet fed ApoE−/− mouse aorta was not due to deficiency of the ApoE gene. The results showed that hyperlipidemia also upregulated the expression of caspase-1 mRNA about 2 folds (Fig. 1D), which was similar to the upregulation of pro-caspase-1 detected by Western blots. These results suggest that upregulation of pro-caspase-1 induced by hyperlipidemia in HF diet fed ApoE−/− mouse aorta results from the hyperlipidemia-induced transcriptional mechanism and the posttranslational mechanism. As the substrate of activated caspase-1, cleaved and activated IL-1β was induced after 3 weeks of high fat diet in the aortas of WT and ApoE−/− mice. In addition, the expression of pro-IL-1β was also induced (Fig. 1E). Taken together, the results demonstrated that early hyperlipidemia induces the upregulation of caspase-1/IL-1β expression and caspase-1/IL-1β activation in mouse aorta. Since Dr. Ross and his colleagues pointed out that significant monocyte recruitment into ApoE−/− mouse aorta does not happen until 6 weeks after HF diet feeding15, our results suggest that caspase-1 is activated in aortic residential cells at the early stage of atherosclerosis.
Figure 1. Early Hyperlipidemia Induces Caspase-1 (casp-1) Expression and Activation in Mouse Aorta.
A. Plasma levels of cholesterol and triglycerides in wild type mice (WT) and apolipoprotein E gene deficient mice (ApoE−/−) after 0 week (ND), 3 weeks (HF3w), or 6 weeks (HF6w) of HF diet (n=5 for each group). B. The protein expression of pro-casp-1 and active casp-1 p20 subunit in mouse aorta lysate of WT and ApoE−/− mice after 0, 3, or 6 weeks of HF diet (n=2 for each group). C. Correlation of caps-1 activation and plasma lipid levels (of A and B). D. Casp-1 mRNA expression in aortas of WT and ApoE−/− after 0, 3, or 6 weeks of HF diet (n=3 for each group). E. The protein expression of pro-IL-1β and active IL-1β in mouse aorta lysate of WT and ApoE−/− mice with or without HF diet for 3 weeks. Data are expressed as mean ± SE. *, p<0.05, changes with the statistical significance.
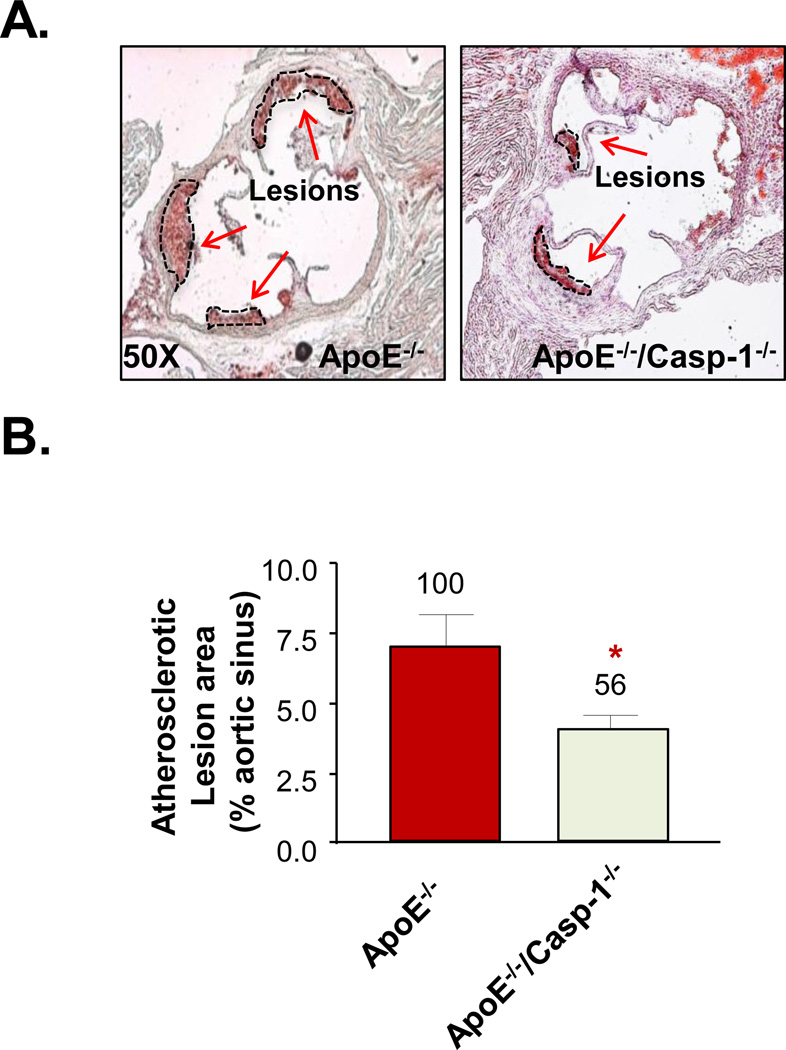
2. Deficiency of caspase-1 in ApoE−/− background results in decreased atherosclerotic lesion in the early stage of atherogenesis
To examine the hypothesis that caspase-1 plays an important role in early atherogenesis, ApoE−/−/caspase-1−/− double gene knock-out mice were generated. The protein expression of pro-caspase-1 in mouse aorta (Suppl. Fig. IA right panel) was absent in the double KO mice which were verified with the mouse tail genomic DNA analysis (Suppl. Fig. IA left panel). General health, body weight, heart and spleen weights (Suppl Fig. IB), and plasma cholesterol and triglyceride levels (Suppl Fig. IC) of ApoE−/−/caspase-1−/− mice were not significantly different from those of ApoE−/− mice. More importantly, after 3 weeks of HF diet, the atherosclerotic lesions in the aortic sinus area, the most sensitive atherogenic area in the aorta, of the double KO mice were significantly decreased by 44% (lesion area mean ± 2SD = 3.92% ± 1.42%) comparing to that of ApoE−/− mice (6.98% ± 2.67%) (p=0.0147) (Figs. 2A and B). The results demonstrated that caspase-1 plays a critical role in promoting early atherogenesis.
Figure 2. Caspase-1 Deficiency Attenuates Early Atherosclerotic Lesion Formation in Aortic Sinus of ApoE−/−/Casp-1−/− Fed with a 3-week HF Diet.
A. Representative images of atherosclerotic lesion staining of ApoE−/− (n=6) and ApoE−/−/Casp-1−/− mice (n=9) in mouse aortic sinus, as the arrows indicated. B. Atherosclerotic lesion quantification. Data are expressed as mean ± SE. *, p<0.05, changes with statistical significance.
3. Deficiency of caspase-1 in ApoE−/− background results in decreased expression of pro-inflammatory cytokines and chemokines in the aorta
Since pro-inflammatory cytokines and chemokines play essential roles in recruiting inflammatory cells into the aorta during atherogenesis22, to determine the molecular mechanism underlying the reduction in atherosclerotic lesion formation in ApoE−/−/caspase-1−/− mice, we examined the hypothesis that the decrease in atherosclerotic lesion may be a result of the decreased generation of pro-inflammatory cytokines and chemokines in mouse aorta. We utilized an antibody array to compare simultaneously the expressions of 40 cytokines and chemokines in ApoE−/−/caspase-1−/− mouse aorta and ApoE−/− mouse aorta (Suppl. Fig. II). The results showed that the expressions of 17 cytokines and chemokines out of 40 examined in ApoE−/− mouse aorta were higher than those in caspase-1−/−/ApoE−/− mouse aorta. These 17 upregulated cytokines and chemokines included soluble intercellular adhesion molecule-1 (sICAM-1), chemokine (C-C motif) ligand-17 (CCL17), granulocyte-macrophage colony stimulation factor (GM-CSF, CSF2), tissue inhibitor of metalloproteinases-1 (TIMP-1), interleukin-27 (IL-27), IL-2, CCL1, IL-23, IL-7, IL-10, IL-16, IL-1α, CCL11, CCL2, CCL4, IL-1 receptor antagonist (IL-1ra), and CCL12. Most of these cytokines and chemokines are pro-inflammatory except TIMP-1, IL-10, and IL-1ra, suggesting that early hyperlipidemia promotes the generation of pro-inflammatory cytokines and chemokines more than anti-inflammatory cytokines/chemokines while caspase-1 deficiency attenuates the generation of these pro-inflammatory cytokines and chemokines. It has been reported previously that besides being the converting enzyme for IL-1β and IL-18 maturation, caspase-1 also serves as a regulator for the expression of IL-1α, TNF-α, and IL-623 and for the secretion of unconventional proteins24. Of note, IL-1β was not detected in ApoE−/− mouse aorta after three weeks of HF feeding, suggesting that the cytokine array used here is not sensitive enough to detect the IL-1β differences between the groups. Several pro-inflammatory cytokines including IL-4, IL-5, IL-6, and IL-12 were reported to express in mouse plasma samples collected from 10 week-old HF diet fed ApoE−/− mice25, which was not evident in our results. This discrepancy may be due to the fact that our experiments were designed to examine early hyperlipidemia (3 weeks of HF diet feeding)-induced cytokine expression. Taken together, our results suggest that deficiency of caspase-1 results in decreased expression of pro-inflammatory cytokines and chemokines in the aorta.
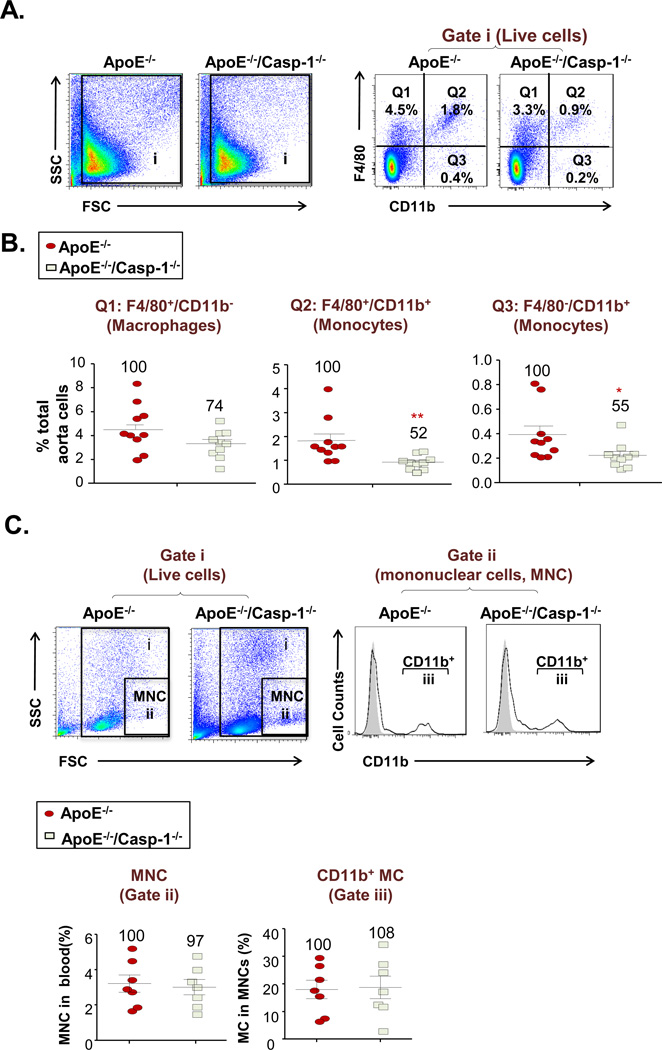
4. Deficiency of caspase-1 in ApoE−/− background results in decreased recruitment of monocytes into the aorta
Since recruitment of monocytes and other inflammatory cells into the mouse aorta and other arteries is essential for atherogenesis1, based on our above results of decreased expression of inflammatory cytokines and chemokines in double KO aorta, we hypothesized that caspase-1 deficiency may result in reduced monocyte recruitment into the mouse aorta. We performed single cell analysis of mouse aortic cells with fluorescence-conjugated antibody staining for F4/80 and CD11b followed by flow cytometric analysis as reported previously26, 27. The results (Figs. 3A and B) showed that caspase-1 deficiency in ApoE−/− background decreased F4/80+/CD11b− macrophage recruitment into the aorta but the reduction did not have statistical significance (p=0.0621). In contrast, the results also showed that caspase-1 deficiency significantly decreased F4/80+CD11b+ monocyte recruitment into the aorta (p=0.0045) and F4/80−CD11b+ monocyte recruitment into the aorta (p=0.0194), respectively. In addition, we further determined whether aortic monocyte composition changes resulted from the changes in the peripheral blood. The results in Fig. 3C showed that total mononuclear cells (MNCs) and CD11b+ monocytes in ApoE−/−/Caspase-1−/− mouse blood had no statistical differences to that of ApoE−/− mice. Moreover, we determined whether aortic monocyte composition changes as a result of alterations in the proliferation of recruited monocytes in mouse aorta. Since cell size of cell populations detected by the forward scatter with flow cytometry could be an estimate of cell proliferation status28, the results in Supple. Fig. III showed that the three cell size fractions (large, middle and small) in three cell subsets including F4/80+CD11b− macrophages, F4/80+CD11b+ monocytes and F4/80−CD11b+ monocytes in ApoE−/−/Caspase-1−/− mouse aortas had no statistical differences in comparison to that of ApoE−/− mouse aortas. Taken together, our results demonstrated that firstly, caspase-1 deficiency in ApoE−/− background decreased the recruitment of monocytes into the mouse aorta in early atherosclerosis; secondly, caspase-1 deficiency in ApoE−/− background did not significantly decrease F4/80+CD11b− macrophage recruitment into the aorta in early atherosclerosis, suggesting that caspase-1 deficiency did not result in a defect of monocyte-to-macrophage differentiation in the early atherosclerosis; and thirdly, the aortic data of caspase-1 deficiency in ApoE−/− background was a result of aortic recruitment of monocytes but not due to the percentage changes of mononuclear cell and CD11b+ monocyte populations in the peripheral blood in early atherosclerosis.
Figure 3. Caspase-1 Deficiency Attenuates Monocyte Infiltration into Mouse Aorta in ApoE−/−/Casp-1−/− Mice fed with a 3-week HF Diet.
A. Representative flow cytometric dot plots of live cells (Gate i) in mouse aortic single cell preparations. Monocytes were gated as CD11b+/F4/80+ and CD11b+/F4/80−. Macrophages were gated as CD11b−/F4/80+. B. Percentage of macrophages (CD11b−/F4/80+), monocytes(F4/80+/CD11b+ and F4/80−CD11b+) in total aortic cell population in ApoE−/− and ApoE−/−/Casp-1−/− mice after 3 weeks of a HF diet (n=10 for each group). C. Representative flow cytometric dot plots of live cells (Gate i) in mouse peripheral blood. Mononuclear cells (MNC, Gate ii) were first gated according the forward scatter (FSC) and side scatter (SSC). Monocytes (MC) were identified as CD11b+ mononuclear cells (Gate iii) (n=7 for each group). Data are expressed as mean ± SE. *, p<0.05, and **, p<0.01, indicate changes with the statistical significance.
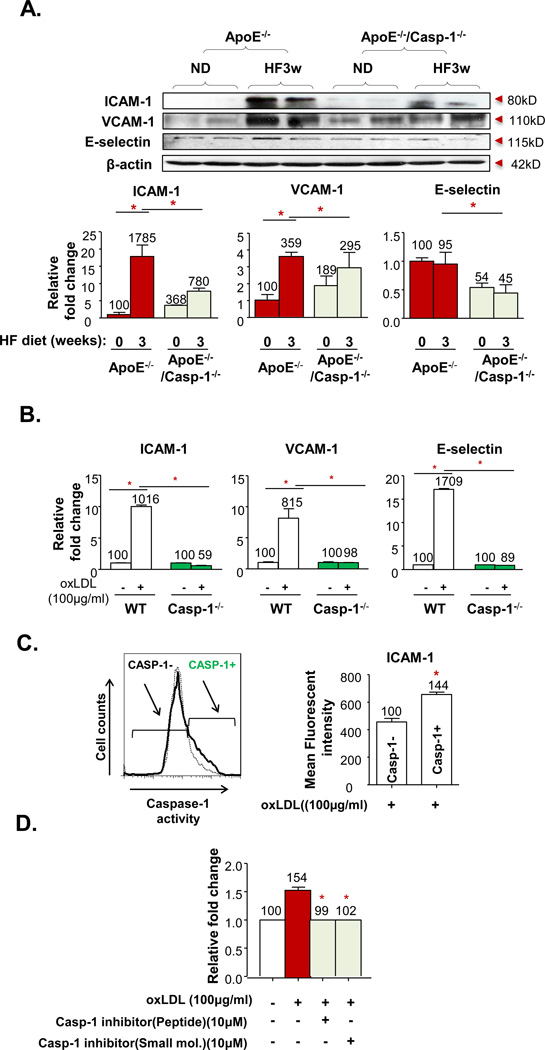
5. Deficiency of caspase-1 in ApoE−/− background results in decreased endothelial activation including reduced cell adhesion molecule expression and attenuated cytokine and chemokine secretion
A significant decrease in the recruitment of monocytes into the mouse aorta without changes in the peripheral blood monocyte compositions leads to our hypothesis that caspase-1 deficiency in early atherosclerosis decreases endothelial activation rather than reducing the potency of monocyte infiltration into the mouse aorta. Endothelial activation can be examined from two prospectives. First, we reasoned that decreased endothelial activation would result in decreased secretion of cytokines and chemokines. To examine this possibility, mouse aortic endothelial cells (MAECs) from WT mice and caspase-1−/− mice were cultured and primed with 50ng/mL LPS and treated with 200µg/mL of oxLDL (first signals for the inflammasome activation)10 for 24 hr followed with adenosine-5'-triphosphate (ATP) (5mM) spike (second signal for the inflammasome activation)11 for 20 min. The antibody array results (Suppl. Fig. IV) showed that caspase-1 deficiency significantly attenuated the secretion of C-X-C motif chemokine 10 (CXCL10), CCL3, CXCL2 (MIP-2) and GM-CSF levels from MAECs.
Second, we further reasoned that decreased endothelial activation in caspase-1 deficient mice would result in decreased upregulation of endothelial adhesion molecules including ICAM-1, VCAM-1 and E-selectin. To examine this possibility, we first examined the adhesion molecule expression in aortas from ApoE−/− mice and ApoE−/−/caspase-1−/− mice. The results (Fig. 4A) showed that 3 weeks of HF feeding induced upregulation of ICAM-1 (17.8 folds) and VCAM-1 (3.5 fold) protein expressions in ApoE−/− mouse aorta, respectively. On the contrary, HF diet feeding upregulated ICAM-1 and VCAM-1 expressions only by 2 folds and 1.5 folds, respectively, in the ApoE−/−/caspase-1−/− aorta. Of note, we did not find a difference in E-selectin expression between ApoE−/− and ApoE−/−/caspase−/− aortas. We then used RT-PCR to further examine the mRNA transcripts of ICAM-1, VCAM-1, and E-selectin in MAECs from WT mice and caspase-1−/− mice stimulated with oxLDL (100µg/mL). The results (Fig. 4B) showed that oxLDL stimulation induced mRNA upregulation of ICAM-1, VCAM-1, and E-selectin in WT MAECs by 10 folds, 8 folds, and 17 folds, respectively. In contrast, oxLDL stimulation induced no mRNA upregulation of ICAM-1, VCAM-1, and E-selectin in caspase-1−/− MAECs. The differences between the protein expression of adhesion molecules in mouse aortas and their mRNA expressions in MAECs may be due to the fact that in addition to ECs some adhesion molecules are also expressed in other vascular cells including smooth muscle cells in mouse aorta29. Regardless of the differences between the two experimental systems, caspase-1 deficiency resulted in decreased induction of EC adhesion molecules ICAM-1 and VCAM-1 in mouse aorta and MAECs in response to hyperlipidemic stimulations. Since attenuation of hyperlipidemia-induced ICAM-1 upregulation by caspase-1 deficiency was most dramatic among adhesion molecules examined, we looked into the possibility that caspase-1 activity positive ECs may have higher ICAM-1 expression than caspase-1 inactive ECs. The results (Fig. 4C) showed that ECs with active caspase-1 have higher ICAM-1 expression than caspase-1 inactive ECs, suggesting that caspase-1 activation promotes ICAM-1 upregulation and endothelial activation. Furthermore, we wanted to determine whether caspase-1 activation functionally promotes human aortic ECs (HAECs) to be more adhesive to unstimulated monocytes. Indeed, we found that oxLDL increased adhesiveness of ECs to monocytes (Fig. 4D), which were inhibited by caspase-1 inhibitors, suggesting that caspase-1 activation increases upregulation of adhesion molecules, promotes endothelial activation, and makes ECs more adhesive to monocytes.
Figure 4. Caspase-1 Activation Regulates Hyperlipidemia-Induced EC Activation in vivo and in vitro.
A. Protein expression of ICAM-1, VCAM-1, and E-selectin in aortic tissues from ApoE−/− and ApoE−/−/Casp-1−/− mice after a HF diet for 3 weeks. Representative western blots (top). Quantification of protein expression normalized to the levels of β-actin (bottom). B. mRNA expressions of ICAM-1, VCAM-1, and E-selectin in mouse aortic endothelial cells (MAECs) from WT and Casp−/− mice, cultured and treated with oxLDL (100µg/mL) for 24 hours. C. Expression level of ICAM-1 in Casp-1 active human aortic endothelial cells (HAECs) after oxLDL treated for 6 hours. D. Effect of Casp-1 inhibition on oxLDL-induced monocytic THP-1 cell static adhesion to HAECs. HAECs were cultured and treated with oxLDL (100µg/mL) for 24 hours. Caspase-1 peptide inhibitor (10µM, z-YVAD-FMK) and caspase-1 small molecular inhibitor (10mM) were added 1 hr before the treatment. Data are expressed as mean ± SE. *, p<0.05.
6. Deficiency of caspase-1 in the aorta of ApoE−/− mice results in decreased recruitment of transplanted caspase-1+/+ bone marrow-derived inflammatory Ly6Cmiddle/high monocytes into the aorta
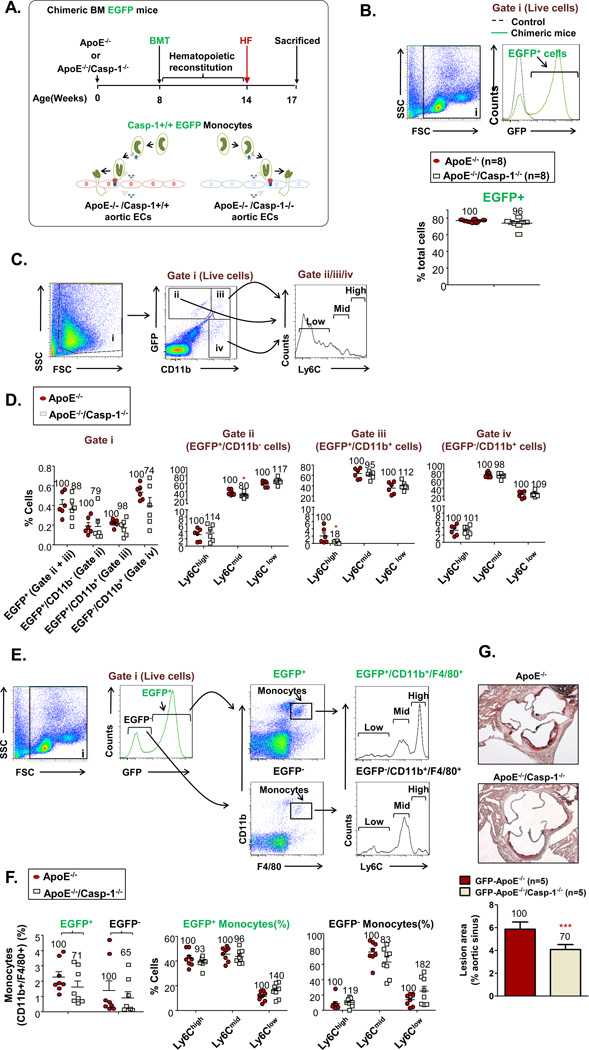
To further consolidate our finding on the role of caspase-1 in promoting aortic endothelial activation and monocyte recruitment into the aorta, we performed chimeric bone marrow (BM) transplantation with enhanced green fluorescence protein (EGFP) transgenic mouse BM as the donor group and ApoE−/− mice and ApoE−/−/caspase-1−/− mice as the two recipient groups (Figs. 5A and B). We reasoned that if caspase-1 activation promotes endothelial activation and monocyte recruitment, then more caspase-1 activity+ EGFP+ BM-derived Ly6Cmiddle/high inflammatory monocytes should migrate into the ApoE−/− aorta than the ApoE−/−/caspase-1−/− aorta. Indeed, we found that significantly more GFP+CD11b−Ly6Cmiddle cells and GFP+CD11b+Ly6Chigh BM-derived monocytes migrated into the ApoE−/− aorta than the ApoE−/−/caspase-1−/− aorta (Figs. 5C and D) (p<0.05). As control experiments, we examined the peripheral blood monocyte subsets in the two recipient mouse groups. In contrast, we did not find any significant difference in peripheral blood monocyte subsets between the two recipient groups (Figs. 5E and F). In addition, after caspase-1+/+(wild-type) GFP transgenic bone marrow cell transplantation into either ApoE−/− recipient mice or caspase-1−/−/ApoE−/− double gene KO recipient mice, caspase-1−/−/ApoE−/− double gene KO recipient mice had significantly less atherosclerotic lesions than ApoE−/− recipient mice (Fig. 5G). Although that ECs are not the only vascular residential cells that have caspase-1 activation in response to inflammatory stimuli30 and that EC-specific role of caspase-1 may ultimately require the model of EC-specific deficient mice of caspase-1, the results correlated well with our previous findings and suggested that caspase-1 activation in aortic ECs promotes monocyte recruitment into the aorta.
Figure 5. Caspase-1 Deficient Aortas are Less Efficient in Recruiting Inflammatory Monocytes during Early Atherogenesis.
A. Schematic representation of chimeric bone marrow (BM) EGFP mice generation. Casp-1+/+ BM cells collected from EGFP+ mice were injected into irradiated ApoE−/− mice or ApoE−/−/Casp-1−/− mice to determine the effect of caspase-1 deficiency in vascular cells on monocyte migration into the aorta. After a 6-week reconstitution period, the chimeric mice were fed with a HF diet for 3 weeks. B. The reconstitution rates of EGFP+ nuclear cells in the peripheral blood 6 weeks after BM transplantation. C. Monocyte population in mouse aorta after reconstitution with EGFP+ BM. Representative dot plots of CD11b−/EGFP+ cells (Gate ii), CD11b+/EGFP+ monocytes (Gate iii), and CD11b+/EGFP− (Gate iv) monocyte in mouse aorta. Monocytes in each of the three gates were further divided into 3 subsets: Ly-6Chigh, Ly-6Cmid, and Ly-6Clow. D. Quantification of EGFP+ and CD11b−/EGFP+ cells, and CD11b+/EGFP+ and CD11b+/EGFP− monocytes within live cells, and Ly-6Chigh, Ly-6Cmiddle, and Ly-6Clow monocytes within indicated gates in ApoE−/− and ApoE−/−/Casp-1−/− mouse aortas after BM reconstitution. Number within each graph represents cells in the ApoE−/−/Casp-1−/− mouse group as a percentage of the ApoE−/− mouse group (n=6 for each group). E. Monocyte population in mouse peripheral blood after reconstitution with EGFP+ BM. Representative dot plots of CD11b+/F4/80+ monocytes in both EGFP+ and EGFP− peripheral blood cells. Monocytes were further divided into three subsets: Ly-6Chigh, Ly-6Cmid, and Ly-6Clow. F. Quantification of CD11b+/F4/80+ monocytes in both EGFP+ and EGFP− peripheral blood cells and Ly-6Chigh, Ly-6Cmiddle, and Ly-6Clow cells in EGFP+ and EGFP− monocytes. Number within each graph represents cells in the ApoE−/−/Casp-1−/− mouse group as a percentage of the ApoE−/− mouse group (n=8 for each group). G. Quantification of atherosclerotic lesion area in ApoE−/− and ApoE−/−/Casp-1−/− mouse aortas after BM reconstitution. Data are expressed as mean ± SE. *, p<0.05.
7. Atherogenic lipid products induce caspase-1 activation and endothelial inflammation via a reactive oxygen species dependent pathway
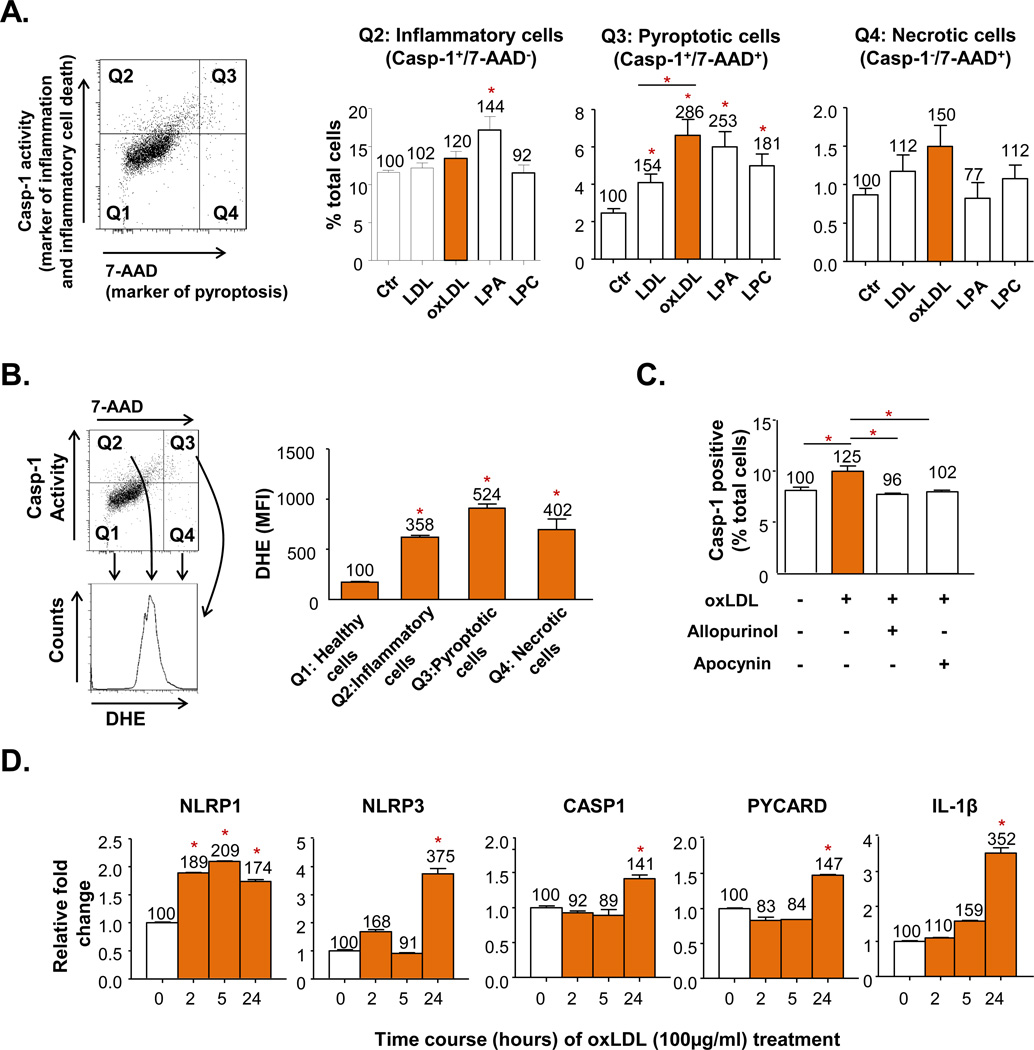
Our data demonstrated that caspase-1 plays a critical role in promoting EC activation and monocyte recruitment into the mouse aorta exposed to hyperlipidemia. To further determine whether atherogenic lipid products induce caspase-1 activation in ECs, and whether reactive oxygen species (ROS) play any role in caspase-1 activation in ECs, we used oxLDL and two oxLDL derivatives, lysophosphatidic acid (lysoPA) and lysophosphatidylcholine (lysoPC)31 to stimulate HAECs. Since plasma membrane rupture and caspase-1 activation are two key features of the newly characterized inflammatory cell death (pyroptosis)32, in addition to using a flow cytometry-based fluorescence-labeled caspase-1 enzymatic activity assay to detect caspase-1 activation, we also used fluorescence dye 7-AAD to measure plasma membrane integrity. We classified caspase-1 enzymatically active (capase-1+) and 7AAD− (caspase-1+/7-AAD−) cells as inflammatory ECs, caspase-1+/7-AAD+ cells as pyroptotic cells, and caspase-1−/7-AAD+ cells as necrotic cells. We found that oxLDL, lysoPA, and lysoPC induced inflammation, inflammatory cell death (pyroptosis), and necrosis after 6-hr stimulation in HAECs (Fig. 6A). We then examined whether ROS plays any role in oxidized lipids-induced caspase-1 activation by co-staining inflammatory, pyroptotic, and necrotic ECs with ROS probe dihydroethidium (DHE). Our results showed that the mean fluorescence intensities (MFI) of DHE stain in ruptured cells (either pyroptotic cells or necrotic cells) were higher than that of the inflammatory cells (Fig. 6B), suggesting that oxidized lipids increased ROS-mediated caspase-1 activation, and that cell death requires higher ROS levels to trigger than inflammation. We further verified the results with ROS inhibitors allopurinol (xanthine oxidase inhibitor) and apocynin (NADPH oxidase inhibitor) for inhibition of caspase-1 activation (Fig. 6C). Finally, we examined whether oxLDL induces upregulated caspase-1 and inflammasome components transcripts in ECs. The RT-PCR results (Fig. 6D) showed that treatment of oxLDL for 24 hr upregulated significantly NLRP1, NLRP3, caspase-1, PYCARD, and IL-1β transcripts. Since inflammasome assembly for caspase-1 activation requires NLRP, PYCARD, and pro-caspase-1, and effective upregulation of transcription of inflammasome and caspase-1 occurs approximately 24 hr after stimulation, these results suggest that post-translational caspase-1 activation is much earlier than upregulation of caspase-1 and inflammasome transcription in ECs.
Figure 6. Oxidized Low-Density Lipoprotein (oxLDL) and its Components Induce Caspase-1 Activation in HAECs via a Reactive Oxygen Species (ROS)-Mediated Pathway.
A. Pyroptotic cell death in HAECs caused by activation of casp1 induced by oxLDL and its components. HAECs were cultured and treated with LDL (100µg/mL), oxLDL(100µg/mL), oxLDL-derivatives lysophosphatidic acid (LPA, 100µM) or lysophosphatidylcholine (LPC, 15µM) as indicated for 6 hr. Casp-1 activity was determined by a commercial kit, and 7-Aminoactinomycin D (7-AAD) fluorescence dye was used to determine the cell membrane integrity. casp-1+/7-AAD+ cells were gated as pyrototic cells (Q3), casp-1+ single positive cells (Q2) were gated as inflammatory cells, and 7-AAD+ single positive cells (Q4) were gated as necrotic cells. B. ROS levels in pyroptotic cells. ROS levels were determined by dihydroethidium (DHE) fluorescence dye staining and the mean fluorescence intensity (MFI) of DHE+ cell fraction was determined. C. Attenuation of oxLDL-induced caspase-1 activation in HAECs with ROS inhibitors Allopurinol (xanthine oxidase inhibitor) and Apocynin (NADPH oxidase inhibitor). Allopurinol (1mM) and Apocynin (100µM) were added 1 hour before oxLDL treatment. HAECs were then treated with oxLDL (100µg/mL) for 6 hr and stained for caspase-1 activity. D. mRNA upregulation of inflammasome components including NLRP1 (Nod-like receptor protein 1), NLRP3 (Nod-like receptor 3), PYCARD (or ASC, inflammasome adaptor Apoptosis-associated speck-like protein containing a CARD), caspase-1, and IL-1β (interleukin-1β) in HAECs treated with oxLDL. Data are expressed mean ± SE. *; p<0.05, changes with statistical significance.
8. Caspase-1 activation in the mouse aorta and human aortic endothelial cells decreases the expression of anti-inflammatory protein/histone deacetylase Sirtuin 1 (Sirt1) by cleaving Sirt1
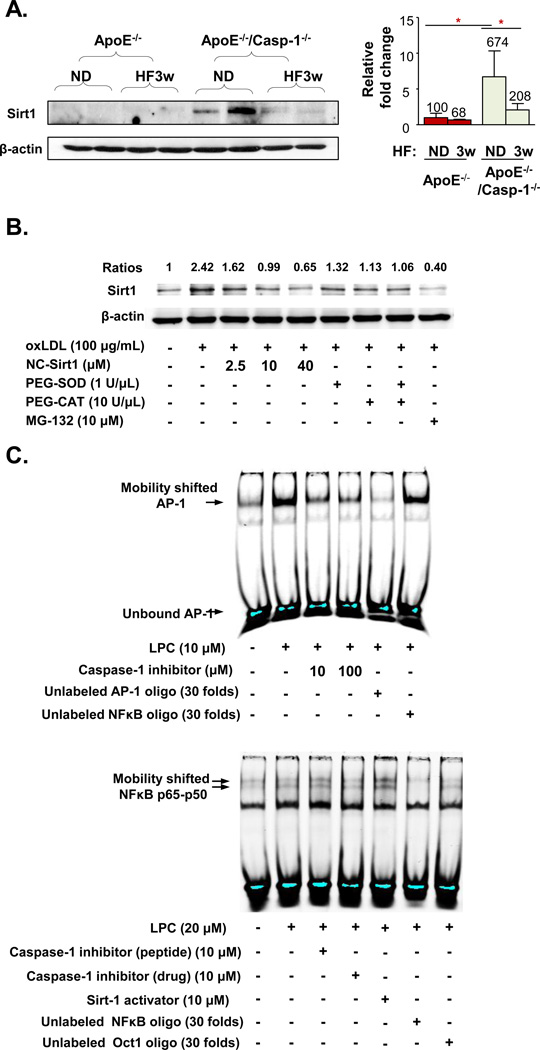
As we detected only weak active IL-1β expression in the mouse aorta (Fig. 1E) in ApoE−/− mice after 3 weeks of HF diet, we hypothesized that the effect of caspase-1 activation on endothelial activation is probably contributed more by other pathways rather than the IL-1β pathway. Thus, we attempted to search for novel substrate of caspase-1 that could modulate inflammation and endothelial activation. Among 24 experimentally verified caspase-1 substrates that we found in a literature search20, sirtuin 1 (Sirt1) has recently been suggested to be cleaved by caspase-121. Since Sirt1 has previously been reported to regulate endothelial activation, and has anti-atherogenic function33, we then hypothesized that caspase-1 deficient mouse aorta has accumulation of noncleaved Sirt1. To test this hypothesis, we examined Sirt1 expression by Western blot with Sirt1 antibody in the following four groups of mice (2 mice/group): 1) ApoE−/− mice fed a normal chow diet; 2) ApoE−/− mice fed a HF diet; 3) ApoE−/−/caspase-1−/− mice fed a normal chow diet; and 4) ApoE−/−/caspase-1−/− mice fed a HF diet. Our results showed that compared to ApoE−/− aorta fed a normal chow diet, ApoE−/−/caspase-1−/− aorta expressed significantly higher amount of Sirt1 (Fig. 7A). HF fed ApoE−/−/caspase-1−/− aorta had decreased Sirt1 accumulation to 1/3 of the level of ApoE−/−/caspase-1−/− mice fed a normal chow diet. These results suggest that a HF diet induces other proteinase(s) activities, which participate in Sirt1 cleavage in the absence of caspase-1. Of note, plasma cholesterol levels in ApoE−/− mice and ApoE−/−/caspase-1−/− mice (Suppl. Fig. 1C) were in the range of 220–320 mg/dL, which were a few folds higher than those in WT mice (average 109 mg/dL) (Fig. 1A). Our results suggest that caspase-1 activation induced by moderate hyperlipidemia is responsible for cleaving Sirt1, and hyperlipidemia induced by HF feeding further triggers additional uncharacterized proteinase(s) to cleave/degrade Sirt1. Then, we examined whether oxLDL decreases Sirt1 expression in HAECs by caspase-1 cleavage mechanism. The results (Fig. 7B) showed that oxLDL induced the expression of cleaved-Sirt1 by 2.4-folds in HAECs. To examine whether the induced cleavage form of Sirt1 was the result from the specific enzyme activity of Casp1, we designed a new cell permeable non-cleavable Sirt1 (NC-Sirt1) by replacing the aspartate (D) in the amino acid position 150 of human Sirt1 with alanine (A), the specific cleavage site of Sirt1 recognized by Casp121 (Suppl. Fig. V). Our results showed that NC-Sirt1 dose-dependently decreases the cleavage of Sirt1 induced by oxLDL. In addition, two different ROS scavengers (PEG-SOD and PEG-catalase) independently and synergistically inhibit oxLDL-induced, Casp1-mediated Sirt1 cleavage. Furthermore, the proteasome inhibitor MG-132 inhibited oxLDL-induced Sirt1 cleavage, suggesting that the cleaved Sirt1 may be further subjected to a putative proteolysis by an uncharacterized proteasome-controlled proteinase. Thus, when MG-132 inhibits proteasome, the expression of this uncharacterized proteasome-controlled proteinase is increased, which leads to decreased expression of caspase-1 cleaved Sirt1. These results suggest that oxLDL first increases ROS, which promotes caspase-1 activation for cleaving Sirt1. We then used the PeptideCutter database of the Swiss Institute of Bioinformatics to analyze the potential enzymes that can cleave the human Sirt1 protein sequence. The results (Suppl. Table I) showed that caspase-1 and caspase-3 are among the enzymes that can cleave Sirt1 and are regulated by ROS although the predicted cleavage site on Sirt1 for caspase-1 is not the same one as experimentally determined21. Taken together, our results suggest that caspase-1 in the mouse aorta and HAECs cleaves Sirt1 protein in response to hyperlipidemic stimuli.
Figure 7. Hyperlipidemia/dyslipidemia Decreases Sirtuin 1 (Sirt1) Expression in ApoE−/− Mouse Aorta and Induces Sirt1 Cleavage in Human Aortic ECs through Caspase-1 Activation.
A. Caspase-1 deficiency results in Sirt1 accumulation in mouse aorta in ApoE−/−/Casp-1−/− mice. ApoE−/− mice and ApoE−/−/Casp-1−/− mice were fed with a HF diet for 3 weeks. Mouse aortic tissues were collected for uncleaved Sirt1 protein expression analysis by Western blot with the specific antibody (left panel). The quantification of Sirt1 expression in the Western blot was presented after normalized with the expression of β-actin in the same sample (right panel). B. OxLDL induces Sirt1 cleavage in HAECs. HAECs were cultured and treated with oxLDL (100µg/mL) for 24 hr. Different doses of non-caspase-1 cleavable sirt1 peptide (NC-SIRT1), superoxide scavengers PEG-SOD and PEG-CAT, and proteasome inhibitor MG-132 were added 1 hr before oxLDL treatment. The protein lysates were collected, and cleaved-Sirt1 expression was determined by Western blot with anti-Sirt1 antibody. The relative changes of Sirt1 expression normalized by β-actin (ratios) were calculated based on the ratios of Sirt1 expression levels in treated samples over that in non-treated samples. C. Inhibition of caspase-1 attenuates LPC induced AP-1 binding to the AP-1 site, revealed by AP-1 electrophoretic gel mobility shift assay (upper panel), whereas inhibition of caspase-1 does not decrease NF-kB binding to the NF-kB site (lower panel). Data are expressed mean ± SE. *; p<0.05, changes with statistical significance.
9. Caspase-1 activation induces expression of cytokines, chemokines, and adhesion molecules via an Sirt1-AP-1-mediated pathway
Our data showed that caspase-1 activation induces the upregulation of several EC activation-associated cytokines, chemokines, and endothelial adhesion molecules. To further explore the mechanism underlying this caspase-1 function, we hypothesized that caspase-1 activation leads to a Sirt1-controlled transcription factor pathway to regulate these genes. Among the transcription factors that are modulated by Sirt1 are AP-134 and NF-κB35. We examined whether lysoPC-activated AP-1 activity and NF-κB activity can be inhibited by caspase-1 inhibitors by performing electrophoretic mobility shift assay (EMSA). The results (Fig. 7C) showed that lysoPC induced AP-1 binding to AP-1 consensus nucleotides were inhibited by caspase-1 inhibitor whereas lysoPC-activated NF-κB binding to NF-κB consensus probe was not significantly affected by caspase-1 inhibitors. We then searched for published experimental evidence that caspase-1-induced cytokines, chemokines, and adhesion molecules (Suppl. Fig. II, IV and Fig. 4)) are AP-1 targeted genes. The results (Suppl. Table IIA) showed that 11 out of 14 caspase-1 induced genes are experimentally verified AP-1 pathway-induced genes.
In addition, analysis from the microarray experimental results of Sirt1 gene deficient mice in comparison to WT mice showed that the expression of these AP-1 targeted genes are increased in Sirt1 deficient mice (Suppl. Table IIA), suggesting that Sirt1 inhibits the expression of AP-1 targets. Moreover, the data-mining results (Suppl. Table IIB) showed that the expressions of AP-1 genes themselves including Jun and Fos are increased in Sirt1 deficient mice. Taken together, the results suggest that caspase-1-cleavable Sirt1 inhibits the expression of caspase-1-induced cytokines, chemokines, and adhesion molecules by suppressing AP-1 gene transcription and AP-1 targeted gene transcription, which further suggest that caspase-1 induces the expression of cytokines, chemokines, and adhesion molecules in ECs by cleavage and inhibition of Sirt1.
Discussion
Although the role of caspase-1 in atherogenesis remains controversial36, the prevailing concept is that caspase-1 plays a proatherogenic role, which is supported by results collected from ApoE−/−/caspase-1−/− mice17, 37, inflammasome sensor NLRP3 KO bone marrow cells in low density lipoprotein receptor (LDLR)−/− mice12, ApoE−/−/IL-1β−/− mice38, and ApoE−/−/IL-18−/−mice39. Of note, Gage et al.17 and Usui et al.37 studied the role of caspase-1 deficiency in full-blown atherosclerosis in ApoE−/− mice after HF feeding for 8 weeks17 and 12 weeks37. In addition, it has been reported that NLRP3 medidates hemodynamic-induced endothelial cell activation40; and that the IL-1β mRNA/protein as well as NLRP3 mRNA are upregulated in 30 week HF feeding-induced atherosclerotic lesion and endothelium of diabetic pigs41. Along the line, we further asked whether in the early atherogenesis associated with early hyperlipidemia induced by only 3 week HF feeding, caspase-1 activation, as metabolic stress-related danger signal associated molecular pattern-sensing pathway11, could be involved in endothelial activation. Using biochemical, immunological, and pathological approaches, and our newly generated ApoE−/−/caspase-1−/− mice, we addressed this question and have the following results: 1) early hyperlipidemia induces the upregulation of caspase-1 expression and caspase-1 activation in ApoE−/− aorta, which supports our previously proposed “three-tier/inflammation privilege model” for determining tissue readiness to caspase-1 activation and inflammation initiation10; 2) caspase-1 deficiency in ApoE−/− background results in decreased early atherosclerotic lesion formation, suggesting that caspase-1 activation in ECs promotes early atherogenesis; 3) caspase-1 deficiency in ApoE−/− background results in decreased expression of pro-inflammatory cytokines and chemokines in the aorta. Of note, the expression of two anti-inflammatory cytokines IL-10 and IL-1ra were also decreased in caspase-1 deficient aorta. However, the decreased expressions of as many as 15 pro-inflammatory cytokines in caspase-1 deficient aorta outweigh concomitant reduction of two anti-inflammatory cytokines, suggesting that caspase-1 activation promotes an inflammatory environment and a chemokine gradient more than anti-inflammatory environment for the recruitment of monocytes and other inflammatory cells into the aorta; 4) caspase-1 deficiency in ApoE−/− background results in decreased recruitment of monocytes into the aorta but has no significant role in monocyte composition in the peripheral blood in the early stage of atherosclerosis, suggesting that caspase-1 activation promotes monocyte recruitment into the aorta presumably via promoting endothelial activation and not via increasing monocyte compositions in the peripheral blood; 5) caspase-1 deficiency in ApoE−/− background results in decreased endothelial activation including reduced cell adhesion molecule expression and attenuated cytokine and chemokine secretion, suggesting that increased caspase-1 activities promote endothelial activation; 6) caspase-1 deficiency in ApoE−/− mice results in decreased recruitment of transplanted caspase-1+ bone marrow-derived inflammatory Ly6Cmiddle/high monocytes into the caspase-1−/− aorta, suggesting that caspase-1 activation can lead to endothelial activation which subsequently recruits more monocyte into the aorta. Decreased recruitment of caspase-1+ bone marrow-derived inflammatory Ly6Cmiddle/high monocytes into the caspase-1−/− aorta results in less atherosclerosis than caspase-1+/+ aorta. In order to further determine the underlying molecular signaling mechanisms, we found: 7) atherogenic lipid products induce caspase-1 activation and endothelial inflammation via a ROS-dependent pathway; 8) caspase-1 deficiency in ApoE−/−/caspase-1−/− aorta and inhibition of caspase-1 in ECs result in accumulation of anti-inflammatory protein/histone deacetylase sirtuin 1 which is a substrate of caspase-1, suggesting that caspase-1 activation in early atherogenesis promotes endothelial activation via a Sirt1 pathway; and 9) caspase-1 activation induces the upregulation of cytokines, chemokines, and adhesion molecules in ECs via a Sirt1-AP-1 mediated pathway.
Although our previous report showed that caspase-1 can cleave numerous protein substrates20, it is generally considered that caspase-1 fulfills its pro-inflammatory functions predominately by cleaving pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18, respectively. Although the role of pro-inflammatory cytokines IL-1β38 and IL-1839, as the “classical substrates” of caspase-1, in the promotion of atherosclerosis has been reported, the role of caspase-1, IL-1β, and IL-18 in promoting EC activation in the early stage of atherogenesis remained unknown. As defined by Ross’ laboratory in ApoE−/− mice15, early atherosclerosis is the initiative stage precedent the occurrence of a large number of monocyte recruitment before 6 weeks of HF feeding in ApoE−/− mice. Our results were well correlated with a previous report that IL-1β secretion from human endothelial cells are 70.6 folds lower than that secreted from human monocytes16, suggesting that IL-1β role in endothelial cells may not be as significant as that in monocytes. These results indicate that caspase-1 may not only act through an IL-1β- or IL-18- dependent pathway to promote endothelial activation. Instead, we found that caspase-1 activation in mouse aorta in early atherogenesis and in human aortic ECs stimulated by oxLDL promotes endothelial activation via a Sirt1-inhibitable pathway. It was reported that Sirt1 reduces endothelial activation42, and overexpression of Sirt1 in ECs inhibits atherosclerosis33. Mechanistically, adenovirus-mediated over-expression of Sirt1 significantly inhibits PMA/Ionomycin-induced ICAM-1 expression in human umbilical vein ECs, whereas knockdown of Sirt1 by RNA interference (RNAi) results in increased expression of ICAM-1 and increases NF-κB p65 binding ability to the ICAM-1 promoter by ChIP assays in HUVECs43. However, the issue of whether caspase-1 in aortic ECs senses hyperlipidemia to initiate vascular inflammation via inhibiting Sirt1 was not examined until this report. Taken together, our results demonstrate a novel mechanism in early atherosclerosis: caspase-1 promotes EC activation and monocyte recruitment via decreasing Sirt1 expression and activating AP-1 pathway. The novel caspase-1-Sirt1-AP-1 pathway and the classical caspase-1-IL-1β-IL-18 are not mutually exclusive. HF diet feeding for more than 6 weeks promoted monocyte recruitment into the aorta15, thus, the classical caspase-1-IL-1β and Il-18 pathway in recruited monocytes and macrophages may interplay with caspase-1-Sirt1-AP-1 pathway in ECs during later stages of atherosclerosis.
Endothelial activation is the first and essential step for atherogenesis, which includes two molecular events - upregulation of cell surface adhesion molecules to make ECs more adhesive and increased secretion of pro-inflammatory cytokines and chemokines to attract monocytes and other inflammatory cells for transendothelial recruitment9. Monocytes and macrophages play an essential role in promoting atherogenesis; however, we reason that if ECs are not activated during the initiation of atherogenesis, then no monocytes in the peripheral blood can be recruited into the aorta. Our results showed that caspase-1 deficiency did not alter the composition of peripheral blood monocytes and macrophages in early hyperlipidemia but instead significantly decreased aortic monocyte recruitment, suggesting that caspase-1 deficient ECs are less activated for recruitment of monocytes into the aorta. These findings were further supported by our bone marrow transplantation results as well as the decreased ICAM-1 and VCAM-1, and pro-inflammatory cytokine and chemokine expressions/secretion in HAECs and in caspase-1 deficient mouse aorta. A recent report showed that suppression of monocyte recruitment results in removal of macrophage from atherosclerotic plaques of ApoE−/− mice44, which echoes the importance of our finding. It has been reported that chemokine CXCL16 and its receptor CXCR6 play a critical role in mediating T cell migration into aorta during atherogenesis45. To determine whether CXCL16 and CXCR6 expressions are regulated by caspase-1 pathway, we searched very extensively the NIH-GeoProfile microarray database and found that both CXCL16 and its receptor CXCR6 expressions are downregulated in caspase-1 KO mice microarray in comparison to that in wild-type control microarray (Geo-dataset GDS3925), suggesting that casapse-1 activation promotes CXCL16 and CXCR6 expression and presumably T cell migration into aorta during atherogenesis. Thus, T cell migration into caspase-1−/−/ApoE−/− mouse aortas may be decreased.
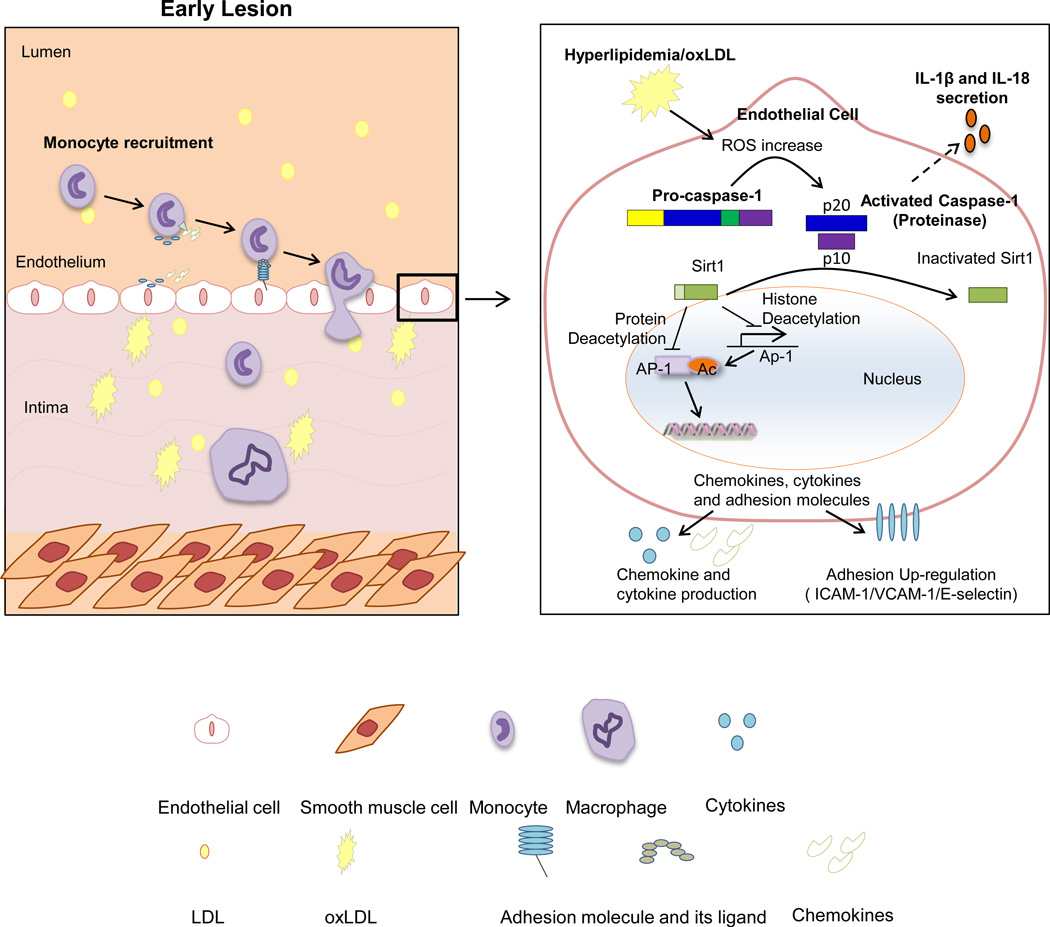
In our newly proposed working model, we summarize our findings and highlight current understanding (Fig. 8): 1) hyperlipidemia induces elevation of ROS via NADPH oxidase-dependent pathway; 2) increased ROS levels induce caspase-1 activation, EC inflammation, and endothelial pyroptosis (inflammatory cell death); 3) activated caspase-1 decreases anti-inflammatory protein/histone deacetylase Sirt1 expression by cleaving Sirt1; 4) Sirt1 is a high hierarchy gene that can deacetylate and inhibit pro-inflammatory transcription factors including AP-1; and 5) the caspase-1-Sirt1-AP-1 pathway can promote endothelial activation, inflammation and atherogenesis. Our results have demonstrated for the first time how hyperlipidemia, one of the most important metabolic risk factors, induces endothelial activation, which provides an important insight for future development of novel therapeutics for early intervention of cardiovascular diseases and other inflammatory diseases.
Figure 8. Schematic representation of a new model for the role of caspase-1 activation in ECs during early atherogenesis.
Supplementary Material
Significance.
The role of receptors for endogenous metabolic danger signals-associated molecular patterns (DAMPs) has been characterized recently as bridging innate immune sensory systems for DAMPs to initiation of inflammation in bone marrow-derived cells such as macrophages. However, an important question remained unknown whether endothelial cells (ECs), the cell type with the largest numbers and are first vessel cells exposed to circulating DAMPs, can sense hyperlipidemia. Using biochemical, immunological, pathological and bone marrow transplantation methods together with the generation of new apoplipoprotein E (ApoE)−/−/caspase-1−/− double knock-out mice, we found that caspase-1−/−/ApoE−/− mice attenuated early atherosclerosis and aortic EC activation, and monocyte recruitment into mouse aorta. Our results demonstrate for the first time that early hyperlipidemia promotes EC activation before monocyte recruitment via a caspase-1-Sirt1-AP-1 pathway, which provides an important insight into the development of novel therapeutics for blocking caspase-1 activation as early intervention of metabolic cardiovascular diseases and inflammations.
Acknowledgements
We are very grateful to Dr. Richard Flavell in Yale University School of Medicine for generously providing us caspase-1 gene knock-out mice and Dr. Wenchao Song in University of Pennsylvania, Drs. Michael Autieri, Muniswamy Madesh, Salim Merali, and Barrie Ashby in Temple University for the insightful suggestions.
Funding Sources
This work was partially supported by the National Institutes of Health Grants to XFY and HW.
Non-standard Abbreviations and Acronyms
- ECs
Endothelial cells
- HF
High fat
- HAECs
Human aortic endothelial cells
- MAECs
Mouse aorta endothelial cells
- WT
wild-type
Footnotes
Disclosures: None
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends in cardiovascular medicine. 2008;18:228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr, Randhawa JK, Rumbaut RE, Durante W, Schafer AI, Yang X, Wang H. Hyperhomocystinemia impairs endothelial function and enos activity via pkc activation. Arterioscler Thromb Vasc Biol. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Z, Yan Y, Song J, Fang P, Yin Y, Yang Y, Cowan A, Wang H, Yang XF. Expression of tctp antisense in cd25(high) regulatory t cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 8.Chowienczyk PJ, Watts GF, Cockcroft JR, Ritter JM. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992;340:1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- 9.Yang XF, Yin Y, Wang H. Vascular inflammation and atherogenesis are activated via receptors for pamps and suppressed by regulatory t cells. Drug discovery today. Therapeutic strategies. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front Biosci. 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the nlrp3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PloS one. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim RS, Suhalim JL, Miyazaki-Anzai S, Miyazaki M, Levi M, Potma EO, Tromberg BJ. Identification of cholesterol crystals in plaques of atherosclerotic mice using hyperspectral cars imaging. Journal of lipid research. 2011;52:2177–2186. doi: 10.1194/jlr.M018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. Apoe-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 16.Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, Surprenant A, Crossman DC. P2x receptor characterization and il-1/il-1ra release from human endothelial cells. British journal of pharmacology. 2007;151:115–127. doi: 10.1038/sj.bjp.0707213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gage J, Hasu M, Thabet M, Whitman SC. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein e-null mice. The Canadian journal of cardiology. 2012;28:222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein e-knockout mice. Cardiovasc Res. 2005;66:583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein e(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Yin Y, Mai J, Xiong X, Pansuria M, Liu J, Maley E, Saqib NU, Wang H, Yang XF. Caspase-1 recognizes extended cleavage sites in its natural substrates. Atherosclerosis. 2010;210:422–429. doi: 10.1016/j.atherosclerosis.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of sirt1 in adipose tissue to promote metabolic dysfunction. Cell metabolism. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 23.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 24.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Tabibiazar R, Wagner RA, Deng A, Tsao PS, Quertermous T. Proteomic profiles of serum inflammatory markers accurately predict atherosclerosis in mice. Physiological genomics. 2006;25:194–202. doi: 10.1152/physiolgenomics.00240.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The nod2 sensor promotes intestinal pathogen eradication via the chemokine ccl2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves R, Zhang X, Cohen H, Debrabant A, Mosser DM. Platelet activation attracts a subpopulation of effector monocytes to sites of leishmania major infection. The Journal of experimental medicine. 2011;208:1253–1265. doi: 10.1084/jem.20101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohmer RM, Bandala-Sanchez E, Harrison LC. Forward light scatter is a simple measure of t-cell activation and proliferation but is not universally suited for doublet discrimination. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2011;79:646–652. doi: 10.1002/cyto.a.21096. [DOI] [PubMed] [Google Scholar]
- 29.Braun M, Pietsch P, Schror K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/s0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- 30.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med. 2000;191:1535–1544. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z, Subramanian P, Sevilmis G, Globke B, Soehnlein O, Karshovska E, Megens R, Heyll K, Chun J, Saulnier-Blache JS, Reinholz M, van Zandvoort M, Weber C, Schober A. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing cxcl1 from the endothelium. Cell metabolism. 2011;13:592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class iii deacetylase sirt1 decreases atherosclerosis in apolipoprotein e-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, Yang RF, Zhang Y, Xu J, Wei YS, Liu DP, Liang CC. Sirt1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J Biol Chem. 2010;285:7097–7110. doi: 10.1074/jbc.M109.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, Leung S, Zhong Z, Zhao H, Sweitzer S, Considine T, Riera T, Suri V, White B, Ellis JL, Vlasuk GP, Loh C. Sirt1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of nf-kappab activity. PLoS ONE. 2012;7:e46364. doi: 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L, Tschopp J. Atherosclerosis in apoe-deficient mice progresses independently of the nlrp3 inflammasome. Cell death & disease. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S, Takahashi M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in western diet-fed apolipoprotein e-deficient mice. Biochem Biophys Res Commun. 2012;425:162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 38.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 39.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F, Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein e-knockout mice. Cardiovasc Res. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 40.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JY. Sterol regulatory element binding protein 2 activation of nlrp3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128:632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Xu S, Jiang B, Cohen RA, Zang M. Activation of sterol regulatory element binding protein and nlrp3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS ONE. 2013;8:e67532. doi: 10.1371/journal.pone.0067532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. Sirt1 reduces endothelial activation without affecting vascular function in apoe−/− mice. Aging. 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia Y, Gao P, Chen H, Wan Y, Zhang R, Zhang Z, Yang R, Wang X, Xu J, Liu D. Sirt1 suppresses pma and ionomycin-induced icam-1 expression in endothelial cells. Science China. Life sciences. 2013;56:19–25. doi: 10.1007/s11427-012-4407-7. [DOI] [PubMed] [Google Scholar]
- 44.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheikine Y, Sirsjo A. Cxcl16/sr-psox--a friend or a foe in atherosclerosis? Atherosclerosis. 2008;197:487–495. doi: 10.1016/j.atherosclerosis.2007.11.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.