Abstract
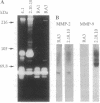
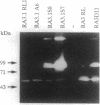
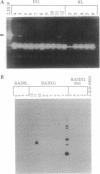
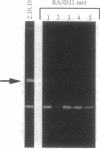
Members of the matrix metalloproteinase (MMP) family have been implicated in the metastasis of tumor cells, but no direct evidence linking any given member of the MMP family to metastatic behavior has been presented. Rat embryo cells transformed by the Ha-ras and v-myc oncogenes or by Ha-ras alone are metastatic in nude mice and release the 92-kDa gelatinase/collagenase (MMP-9), whereas those transformed by Ha-ras plus the adenovirus E1A gene are not metastatic and do not release MMP-9. Here we demonstrate that MMP-9 expression can be induced in these tumorigenic but nonmetastatic rat cells by transfection with an MMP-9 expression vector. Transfection of a MMP-9 expression vector, but not control DNAs, conferred metastatic capacity on the nonmetastatic cells. The majority of colonies isolated after continued passage either in vivo or in vitro had lost the MMP-9 expression vector. However, occasional cells were isolated from metastases which retained MMP-9 expression after passage. These cells retained metastatic capacity. In contrast, cells isolated after losing MMP-9 expression also lost the ability to metastasize. These results provide direct evidence that MMP-9 has a role in tumor metastasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O. A., Carmichael D. F., DeClerck Y. A. Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. J Natl Cancer Inst. 1990 Apr 4;82(7):589–595. doi: 10.1093/jnci/82.7.589. [DOI] [PubMed] [Google Scholar]
- Ballin M., Gomez D. E., Sinha C. C., Thorgeirsson U. P. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988 Aug 15;154(3):832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Behrendtsen O., Alexander C. M., Werb Z. Metalloproteinases mediate extracellular matrix degradation by cells from mouse blastocyst outgrowths. Development. 1992 Feb;114(2):447–456. doi: 10.1242/dev.114.2.447. [DOI] [PubMed] [Google Scholar]
- Bernhard E. J., Muschel R. J., Hughes E. N. Mr 92,000 gelatinase release correlates with the metastatic phenotype in transformed rat embryo cells. Cancer Res. 1990 Jul 1;50(13):3872–3877. [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Campbell E. J., Cury J. D., Shapiro S. D., Goldberg G. I., Welgus H. G. Neutral proteinases of human mononuclear phagocytes. Cellular differentiation markedly alters cell phenotype for serine proteinases, metalloproteinases, and tissue inhibitor of metalloproteinases. J Immunol. 1991 Feb 15;146(4):1286–1293. [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Garbisa S., Pozzatti R., Muschel R. J., Saffiotti U., Ballin M., Goldfarb R. H., Khoury G., Liotta L. A. Secretion of type IV collagenolytic protease and metastatic phenotype: induction by transfection with c-Ha-ras but not c-Ha-ras plus Ad2-E1a. Cancer Res. 1987 Mar 15;47(6):1523–1528. [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hoidal J. R., Kang A. H. Expression of a metalloproteinase that degrades native type V collagen and denatured collagens by cultured human alveolar macrophages. J Clin Invest. 1987 Dec;80(6):1644–1650. doi: 10.1172/JCI113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Khokha R., Waterhouse P., Yagel S., Lala P. K., Overall C. M., Norton G., Denhardt D. T. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989 Feb 17;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- Kubota S., Mitsudomi T., Yamada Y. Invasive human fibrosarcoma DNA mediated induction of a 92 kDa gelatinase/type IV collagenase leads to an invasive phenotype. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1539–1547. doi: 10.1016/0006-291x(91)92114-y. [DOI] [PubMed] [Google Scholar]
- Librach C. L., Werb Z., Fitzgerald M. L., Chiu K., Corwin N. M., Esteves R. A., Grobelny D., Galardy R., Damsky C. H., Fisher S. J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991 Apr;113(2):437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990 Apr;1(2):99–106. [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T. Stromelysin/transin and tumor progression. Semin Cancer Biol. 1990 Apr;1(2):107–115. [PubMed] [Google Scholar]
- Matsubara M., Zieske J. D., Fini M. E. Mechanism of basement membrane dissolution preceding corneal ulceration. Invest Ophthalmol Vis Sci. 1991 Dec;32(13):3221–3237. [PubMed] [Google Scholar]
- McKenna W. G., Nakahara K., Muschel R. J. Site-specific integration of H-ras in transformed rat embryo cells. Science. 1988 Sep 9;241(4871):1325–1328. doi: 10.1126/science.3045971. [DOI] [PubMed] [Google Scholar]
- Murphy G., Docherty A. J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992 Aug;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- Nagase H., Ogata Y., Suzuki K., Enghild J. J., Salvesen G. Substrate specificities and activation mechanisms of matrix metalloproteinases. Biochem Soc Trans. 1991 Aug;19(3):715–718. doi: 10.1042/bst0190715. [DOI] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya H., Shimizu H., Tomita K., Nakanishi I., Sato H., Seiki M., Yamashita K., Hayakawa T. Induction and stimulation of 92-kDa gelatinase/type IV collagenase production in osteosarcoma and fibrosarcoma cell lines by tumor necrosis factor alpha. Biochem Biophys Res Commun. 1990 Sep 14;171(2):610–617. doi: 10.1016/0006-291x(90)91190-4. [DOI] [PubMed] [Google Scholar]
- Pozzatti R., McCormick M., Thompson M. A., Khoury G. The E1a gene of adenovirus type 2 reduces the metastatic potential of ras-transformed rat embryo cells. Mol Cell Biol. 1988 Jul;8(7):2984–2988. doi: 10.1128/mcb.8.7.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzatti R., Muschel R., Williams J., Padmanabhan R., Howard B., Liotta L., Khoury G. Primary rat embryo cells transformed by one or two oncogenes show different metastatic potentials. Science. 1986 Apr 11;232(4747):223–227. doi: 10.1126/science.3456644. [DOI] [PubMed] [Google Scholar]
- Rao J. S., Steck P. A., Mohanam S., Stetler-Stevenson W. G., Liotta L. A., Sawaya R. Elevated levels of M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res. 1993 May 15;53(10 Suppl):2208–2211. [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Sato H., Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993 Feb;8(2):395–405. [PubMed] [Google Scholar]
- Schultz R. M., Silberman S., Persky B., Bajkowski A. S., Carmichael D. F. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988 Oct 1;48(19):5539–5545. [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Sreenath T., Matrisian L. M., Stetler-Stevenson W., Gattoni-Celli S., Pozzatti R. O. Expression of matrix metalloproteinase genes in transformed rat cell lines of high and low metastatic potential. Cancer Res. 1992 Sep 15;52(18):4942–4947. [PubMed] [Google Scholar]
- Tsuchiya Y., Sato H., Endo Y., Okada Y., Mai M., Sasaki T., Seiki M. Tissue inhibitor of metalloproteinase 1 is a negative regulator of the metastatic ability of a human gastric cancer cell line, KKLS, in the chick embryo. Cancer Res. 1993 Mar 15;53(6):1397–1402. [PubMed] [Google Scholar]
- Wang M., Stearns M. E. Blocking of collagenase secretion by estramustine during in vitro tumor cell invasion. Cancer Res. 1988 Nov 15;48(22):6262–6271. [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Yamagata S., Ito Y., Tanaka R., Shimizu S. Gelatinases of metastatic cell lines of murine colonic carcinoma as detected by substrate-gel electrophoresis. Biochem Biophys Res Commun. 1988 Feb 29;151(1):158–162. doi: 10.1016/0006-291x(88)90573-6. [DOI] [PubMed] [Google Scholar]