Abstract
Reactive oxygen species (ROS) and mitochondrial defects in neurons are implicated in neurodegenerative disease. Here we find that a key consequence of ROS and neuronal mitochondrial dysfunction is the accumulation of lipid droplets (LD) in glia. In Drosophila, ROS triggers c-Jun-N-terminal Kinase (JNK) and Sterol Regulatory Element Binding Protein (SREBP) activity in neurons leading to LD accumulation in glia prior to or at the onset of neurodegeneration. The accumulated lipids are peroxidated in the presence of ROS. Reducing LD accumulation in glia and lipid peroxidation via targeted lipase overexpression and/or lowering ROS significantly delays the onset of neurodegeneration. Furthermore, a similar pathway leads to glial LD accumulation in Ndufs4 mutant mice with neuronal mitochondrial defects, suggesting that LD accumulation following mitochondrial dysfunction is an evolutionarily conserved phenomenon, and represents an early, transient indicator and promoter of neurodegenerative disease.
Keywords: ARSAL, CMT2A, Leigh Syndrome, glia, Aats-met, MARS2, Ndufs4, Marf, Mitofusin, sicily, C8Orf38, NDUFAF6, Drosophila melanogaster, Mus musculus
Introduction
Many neurodegenerative diseases are characterized by a gradual demise of neurons, coupled with a reduction in antioxidative capacity and/or an increase in oxidative stress (Turner et al., 2009; Gilgun-Sherki et al., 2001; Lin and Beal, 2006; Keller et al., 2005). Oxidative stress may result from impaired mitochondrial function and/or glutamate excitotoxicity, and causes DNA and protein damage as well as lipid peroxidation (Niki, 2009; Reed, 2011). These stresses promote damage to neurons and glia and contribute to the demise of neurons (Barnham et al., 2004; Listenberger et al., 2003). Importantly, signs of oxidative stress precede the neurological signs of neurodegeneration (ND) in disease models (Pratico et al., 2001; Keller et al., 2005; Xie et al., 2013). Complexes I and III of the mitochondrial electron transport chain are the major contributors of free radicals and ROS (Beal, 2007).
Lipid droplets (LDs) are dynamic organelles that emerge from the endoplasmic reticulum (ER) membrane and serve as a site of triglyceride and cholesterol storage. Under normal conditions, LDs are mostly found in liver and adipose tissues and actively respond to cellular signaling (Murphy, 2001; Farese, Jr. and Walther, 2009). One important regulator of LD biogenesis is sterol regulatory element binding protein (SREBP), a highly conserved, membrane bound, basic helix-loop-helix leucine zipper transcription factor that is crucial for lipid homeostasis (Horton et al., 2002). SREBP regulates lipogenesis and responds to levels of sterols in mammals and palmitate in Drosophila, respectively (Shao and Espenshade, 2012; Dobrosotskaya et al., 2002). Importantly, Drosophila SREBP mutants are auxotrophs, and SREBP levels are critical in larval stages (Kunte et al., 2006). Inactive SREBP is a transmembrane protein located in the ER membrane which relocates to the Golgi upon activation where it forms a complex with SREBP-cleavage activating protein (SCAP) (Horton et al., 2002). In the Golgi, SREBP is cleaved by site-1 and site-2 proteases to generate active SREBP. Active SREBP is then translocated into the nucleus and promotes transcription of genes involved in lipid metabolism (Shao and Espenshade, 2012). Although SREBP expressed in the gut responds to levels of circulating lipids and cholesterol, the blood-brain-barrier prevents lipids from entering the brain in mammals and de novo lipogenesis is critical for nervous system function (Camargo et al., 2009). Though the Drosophila nervous system can incorporate lipids from the gut, brain triacylglycerol levels are protected under stress and starvation (Palm et al., 2012; Cheng et al., 2011).
Cells other than adipocytes can form LDs as a response to cellular inflammation and stress (Santos and Schulze, 2012). In cancer cells, LDs are hypothesized to be an important source of energy for proliferation and may serve a protective role under conditions of hypoxia and cellular stress by gathering free fatty acids to protect cells against lipotoxicity (Bozza and Viola, 2010). However, ND has typically not been associated with LD formation in the nervous system, although few mouse mutants have been documented to have LDs in the brain (Mato et al., 1999; Hulshagen et al., 2008; Wang et al., 2002). Finally, lipid metabolism defects have been implicated in some forms of ALS (Ilieva et al., 2009; Turner et al., 2009; Pratico et al., 2001), but not other neurodegenerative diseases.
In this study, we document that ROS induced LD accumulation presages ND in several Drosophila mutants affecting mitochondrial function. We describe a ROS activated pathway in neurons, which induces LD formation in glia in a cell non-autonomous fashion. Reducing LD accumulation is sufficient to delay ND. Finally, we find that the LD accumulation also occurs in mice with mitochondrial dysfunction, suggesting that LD formation upon oxidative stress is an evolutionarily conserved phenomenon.
Results
Mitochondrial mutants exhibit lipid droplet accumulation in glia
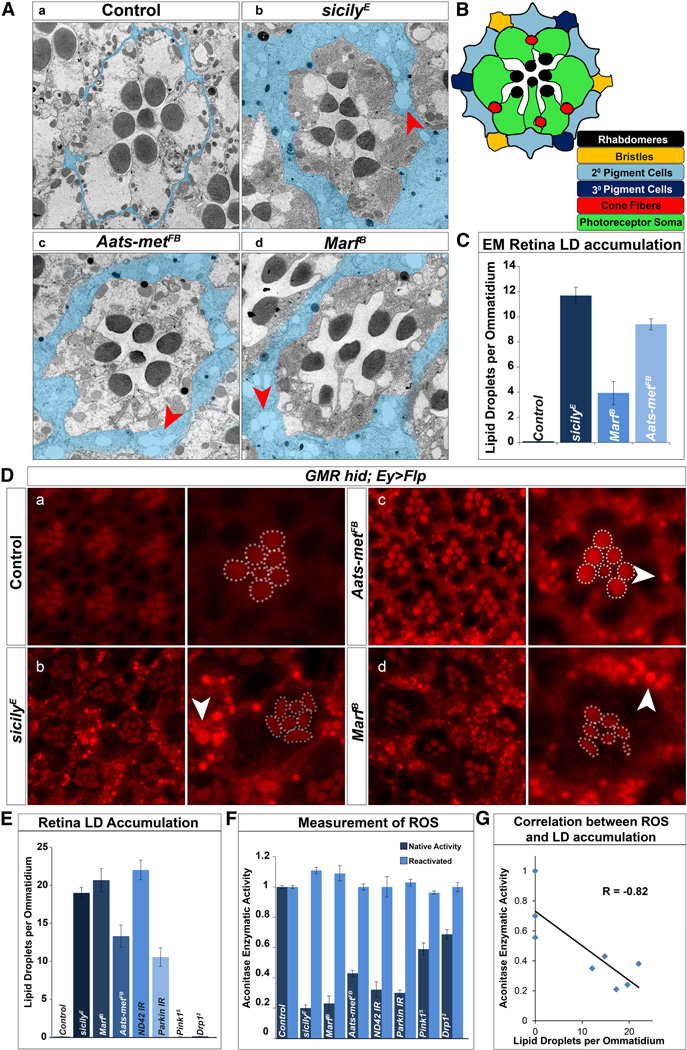
We previously performed forward genetic mosaic screens of essential genes in the Drosophila visual system to uncover genes that cause ND of photoreceptors (Yamamoto et al., 2014). While characterizing the phenotypes by transmission electron microscopy (TEM) in the retina and brain, we found structures reminiscent of LDs. In Drosophila, photoreceptors (neurons) project their axons directly into the brain and form synapses with post-synaptic neurons. Pigment cells (glia) ensheath the photoreceptor cell body in the retina and epithelial glia ensheath their axonal terminals (Edwards and Meinertzhagen, 2010). We found that three different mutants, sicily (Zhang et al., 2013), Aats-met (Bayat et al., 2012) and Marf (Sandoval et al., 2014) exhibit abundant LD accumulations in pigment (Figure 1A–C) and epithelial glia (Figure S1A–C).
Figure 1. LD accumulate in glia of mitochondrial mutants with elevated levels of reactive oxygen species (ROS).
TEM of a single ommatidium of 1-day-old flies. A) a, Pigment cells that surround the photoreceptors are typically very thin (blue) in wild type eyes (y w FRT19A clones). They are vastly expanded in b, sicily, c, Aats-met, and d, Marf mutants and contain numerous LD (red arrowhead). B) Schematic of a single ommatidium in cross-section. C) Quantification of retina LD per ommatidium. D) a–d, Nile Red stained whole-mount retina show LD accumulation (white arrowhead) in sicily, Marf and Aats-met mutant clones of 1-day-old flies, but not in controls (y w FRT19A clones). Rhabdomeres outlined in white. Abnormal rhabdomere morphology can be seen in sicily and Marf mutant clones. E) Quantification of LD per ommatidium of panel D. A minimum of 16 eyes were stained and approximately 36 ommatidia were quantified. F) Aconitase activity is used to measure ROS - before and after enzymatic reactivation with reducing agents. RNAis were driven by the ubiquitously expressed daughterless[da]-GAL4 G) Correlation between aconitase enzymatic activity and LD per ommatidium. R = correlation coefficient. Data are represented as mean ± SEM. See also figure S1–3.
These three fly genes play important but distinct roles in mitochondrial biology and mutations in the human homologs cause neurological diseases. sicily is the homolog of the human nuclear encoded mitochondrial gene NDUFAF6 (C8orf38). Its loss leads to an early onset neurodegenerative disease known as Leigh Syndrome (Pagliarini et al., 2008). sicily functions as a chaperone for Complex I proteins and its loss leads to severe mitochondrial dysfunction (Zhang et al., 2013; McKenzie et al., 2011). Marf encodes the Drosophila homolog of the mitochondrial fusion GTPase, Mitofusin 1 and 2 (Debattisti and Scorrano, 2013). Mutations in MFN2 cause Charcot-Marie-Tooth disease type 2A2 (CMT2-A2), an autosomal dominant adult onset peripheral neuropathy (Kijima et al., 2005) as well as Hereditary motor and sensory neuropathy VI (HMSN6) (Del et al., 2008). Lastly, Aats-met is the Drosophila homolog of mitochondrial methionyl-tRNA synthase 2, or MARS2. Proteins encoded by these genes mediate the transfer of methionine to their cognate tRNA in the mitochondria matrix. Mutations in MARS2 lead to a disease known as Autosomal Recessive Spastic Ataxia with Leukoencephalopathy (ARSAL) (Thiffault et al., 2006; Bayat et al., 2012).
Transmission electron micrographs of 1-day-old wild-type retinal clones show the stereotypical organization of photoreceptors in each ommatidium (Figure 1Aa). Each ommatidium is separated by thin pigment cells (Figure 1Aa and B, pseudocolored in blue). Loss of sicily, Aats-met or Marf leads to an accumulation of LDs (arrow) in glia (Figures 1Aa–d and 1C), which is not observed in controls. Importantly, LDs are not observed in photoreceptors. In addition to the retina, LDs also accumulate in the epithelial glia of the lamina (Figure S1A a–d, S1B, and S1C). In summary, LD-like structures are present in cells that function as glia but not in neurons.
To determine whether the structures observed in Figure 1 are indeed LDs, we created large mutant clones in the visual system by GMR-hid (Stowers and Schwarz, 1999) and observed LD accumulation in the glia in sicily, Marf and Aats-met mutants using Nile Red (Figure 1Da–d) and BODIPY493/503 (Figure S1D), (Greenspan and Mayer, 1985; Pavlopoulos et al., 1990). The rhabdomeres (dashed white) are also labeled and serve as reference point for each ommatidium. sicily, Aats-met and Marf clones show LD accumulation in the area where the glia are located whereas control animals do not show staining (Figure 1E). In summary, mutations in genes involved in three separate mitochondrial processes, Complex I activity, fusion, and protein translation, exhibit LD accumulations the glia of newly eclosed adults prior to the onset of ND. However, not all mitochondrial mutations cause LD accumulation (see below).
The morphology of sicily, Aats-met and Marf photoreceptors is mostly normal or only mildly affected at day 1 based on TEM (Figure 1A). They begin to degenerate several days after eclosion and the process occurs faster in sicily and Marf mutants than in Aats-met mutant clones (Figure S1E) as revealed by fluorescently conjugated phalloidin labeling of actin rich rhabdomeres. Elongated, or diffuse rhabdomeres are characteristic for mutants that cause a progressive photoreceptor degeneration (Xiong and Bellen, 2013).
Lipid droplets accumulate transiently in glia
Next, we sought to characterize the temporal nature of the LD accumulation phenotype. One-day-old sicily, Aats-met and Marf clones exhibit approximately 10 LD per ommatidium. Upon aging, we noticed a significant decrease in the number of LD in the mutant clones (Figure S2A a–i, j–l and S2B), indicating that LD accumulation occurs prior to or concurrent with the onset of ND and is a transient phenomenon (Figure S2C).
Increased ROS correlates with increased LD accumulation
Since sicily, Aats-met and Marf affect mitochondrial function, we sought to investigate the potential link between LD accumulation and mitochondrial dysfunction for other nuclear encoded mitochondrial genes. We knocked down ND42, a CI subunit that is chaperoned by sicily (Zhang et al., 2013), and parkin, an E3 ubiquitin ligase involved in mitochondrial clearance using RNAi. Whole eye knockdown of both ND42 and parkin leads to LD accumulation (Figure S3A a–b and Figure 1E). However, escapers of Pink15 (which encodes a mitochondrial kinase (Clark et al., 2006) do not exhibit LD accumulation (Figure S3A c and Figure 1E). In addition, retinal clones of Drp12, which encodes a protein involved in mitochondria fission (Verstreken et al., 2005), also do not result in LD accumulation (Figure S3A d and Figure 1E). The presence of LD accumulations in a subset of mitochondrial mutants suggests that the loss of these genes share a common pathway.
Previously, we noticed elevated levels of reactive oxygen species (ROS) in sicily and Aats-met mutants (Zhang et al., 2013; Bayat et al., 2012). To determine whether increased ROS production and subsequent oxidative stress may promote LD formation, we measured aconitase enzymatic activity in third instar larvae (Yan et al., 1997). Consequently, enzymatic activity for aconitase is less than 50% of wild-type activity in all three mutants as well as ND42 and parkin RNAi knockdown, documenting the presence of high levels of ROS and possibly a threshold effect. Moreover, aconitase activity is less affected (more than 50% of wild-type activity) in Drp12 and Pink15 mutants (Figure 1F). As shown in Figure 1G, there is a strong correlation between decreased aconitase enzymatic activity and the number of LD per ommatidium (R = − 0.82). These data suggest that ROS may play a role in LD accumulation.
Reducing ROS ameliorates lipid droplet accumulation
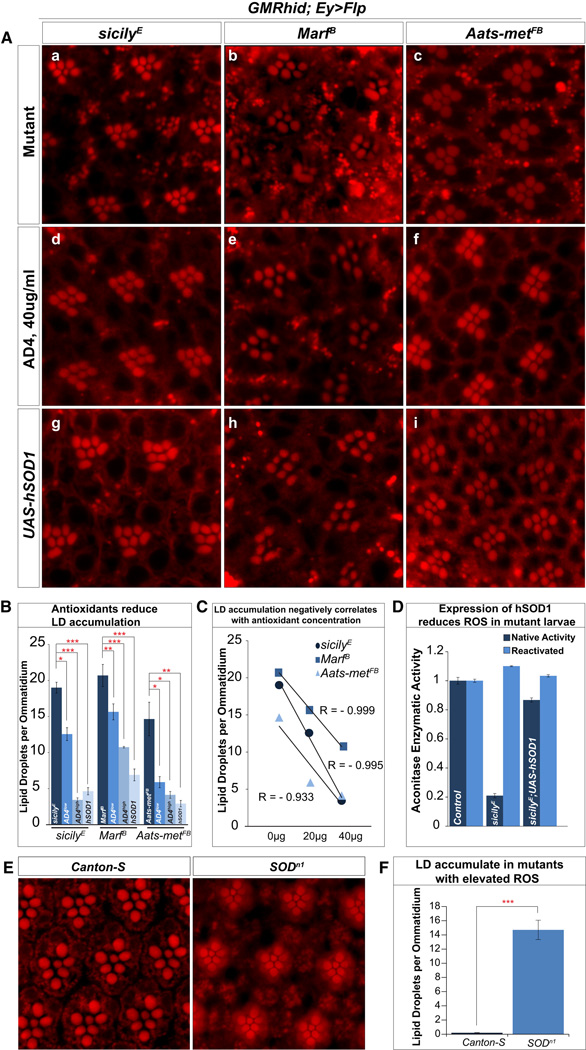
To establish a causative relationship between ROS and LD accumulation, we employed pharmacologic and genetic methods to reduce ROS and assessed LD. N-acetyl cysteine amide (AD4) is a blood-brain-barrier penetrating antioxidant that can decrease oxidative stress in vitro and in vivo (Schimel et al., 2011; Amer et al., 2008). We administered AD4 via fly food at concentrations of 20µg/ml and 40µg/ml (Bayat et al., 2012). Compared to mutants raised in standard fly food (Figure 2A a–c), animals raised on AD4 food (Figure 2A d–f) show a dose dependent reduction in LD accumulation (Figure 2B and C). In addition, we over-expressed human copper-zinc superoxide dismutase (hSOD1), a conserved antioxidative enzyme that has been shown to rescue or extend Drosophila lifespan (Parkes et al., 1998). Overexpression of hSOD1 in mutant larvae reduces ROS levels (Figure 2D) and considerably suppressed the LD accumulations in glia (Figure 2A g–i). To further determine whether the presence of ROS causes LD accumulation, we assessed LD accumulation in SOD mutant flies. SODn1 mutant (Sun et al., 2012) glia also exhibit LD accumulations, similar in levels to what we observe in Aats-met clones (Figure 2E–F and Figure 2B). In summary, these results provide compelling evidence that removing ROS reduces LD accumulation and increasing ROS induces LD accumulation.
Figure 2. Antioxidants reduce LD accumulation in mutant retinas.
A) a–c, Nile Red stains of 1-day-old sicily, Marf and Aats-met mutant retinas reveal LD accumulation. d–f, Animals fed with AD4 at 40µg/ml (AD4high in quantification) reduces the number of LD. Notice the partial restoration of rhabdomere integrity in Marf mutants. g–i, Suppressing ROS by overexpression of a copy of hSOD1 (eyeless[ey]-Gal4, UAS-Flp) also reduces LD accumulations. B) Quantification of the data presented in A and animals with mutant clones raised on food supplemented with 20µg/ml (AD4low) of AD4 the show a reduction of LD accumulation. C) Linear regressions show a negative correlation between the numbers of LD accumulated and the amount of supplemented AD4. D) Expression of hSOD1 with da-Gal4 in sicily mutants significantly suppresses the loss of aconitase activity and hence ROS E) Nile Red staining of wild type (Canton-S) flies show no LD accumulation whereas staining of SODn1 mutant escapers reveal high levels of LD accumulation. F) Quantification of SODn1 LD accumulation. Data are represented as mean ± SEM. Significance was calculated compared to controls using t-tests (*P<0.05, **P<0.005, ***P<0.0005).
Elevated ROS promotes JNK/SREBP activation and reducing JNK/SREBP activity suppresses LD accumulation
Prolonged increase of ROS triggers a stress response that is mediated by c-Jun-N-terminal Kinase (JNK) signaling in both Drosophila and mammals. JNK activates the transcription of downstream genes, including its own inhibitor, JNK phosphatase. In Drosophila, JNK phosphatase is encoded by puckered (puc), whose expression is used as readout for JNK activity (Martin-Blanco et al., 1998). ROS can suppress JNK phosphatase activity and therefore allows sustained JNK activation (Kamata et al., 2005).
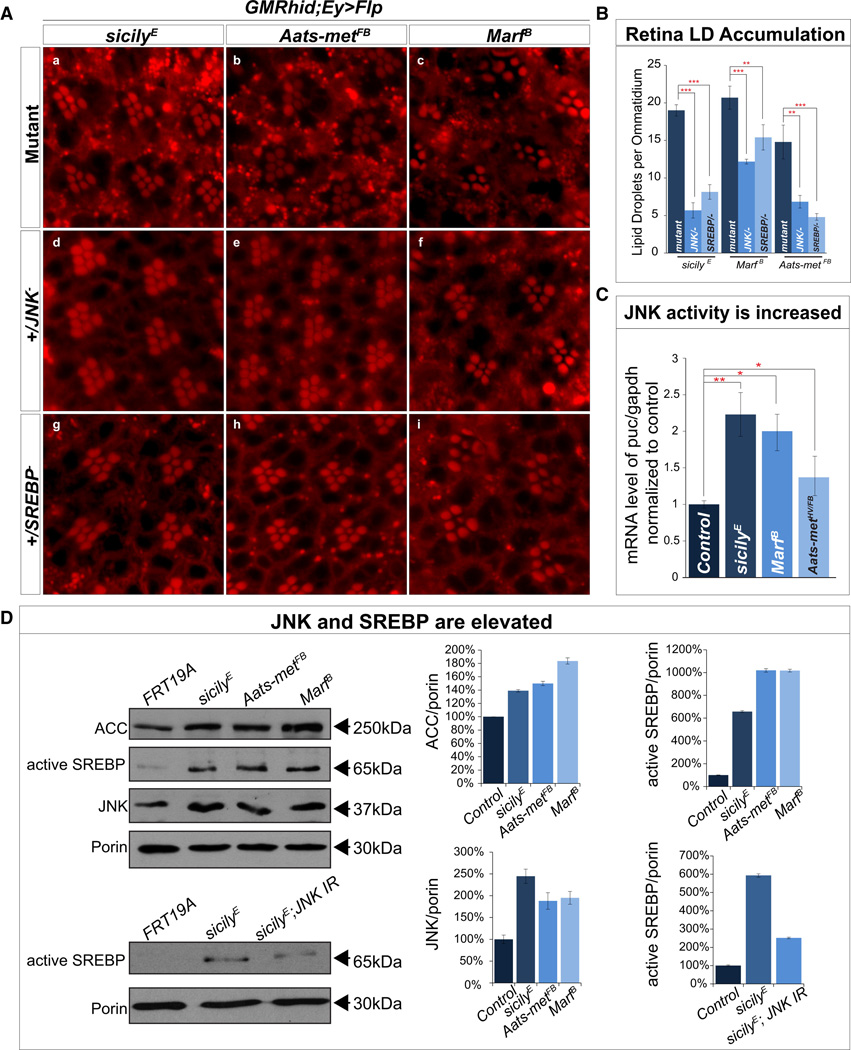
Given the presence of LD, we assessed SREBP activity. To determine whether JNK and SREBP are involved in the LD accumulation observed in sicily, Aats-met and Marf clones, we removed a copy of either JNK or SREBP in each mutant background. Removal of a single copy of either JNK or SREBP is sufficient to ameliorate the LD accumulation in all three mutants (Figure 3A, 3B). In addition, visual system (ey-GAL4) knockdown of JNK in the sicily and Marf mutant background also significantly reduces LD accumulation (Figure S4A and B). Furthermore, JNK may be upstream of SREBP as the LD accumulations caused by overexpressing JNK with a neuronal driver in SREBP hemizygous (SREBP189/+) flies is strongly suppressed (Figure S6C and quantified in Figure S6D)(Kunte et al., 2006).
Figure 3. ROS leads to aberrant activation of JNK and SREBP and reducing JNK or SREBP in mutants reduces LD accumulation.
A) a–c, sicily, Aats-met and Marf mutant clones exhibit high levels of LD accumulation but when one copy of JNK (d–f) or SREBP (g–i) is removed, LD accumulation is significantly reduced. B) Quantification of retinas in A. C) qRT-PCR quantification of puc shows a significant upregulation of puc mRNA in all three mutant larvae compared to control (y w FRT19A). D) Immunoblot of sicily, Aats-met and Marf heads with mutant eye clones show an increase in ACC, total JNK and active SREBP when compared to control (y w FRT19A). Knockdown of JNK in the sicily mutant background (heads), reduces active SREBP levels compared to heads with mutant visual systems. Data are represented as mean ± SEM. Significance was calculated compared to controls and immunoblots were normalized to loading control (porin). (*P<0.05, **P<0.005, ***P<0.0005). See also Figure S4.
To determine the extent of JNK upregulation in these mutants, we first performed quantitative RT-PCR in all three mutant larvae and observed an upregulation of puc (Figure 3C), suggesting JNK activation. To determine whether JNK is elevated, we used brain lysates from mutant larvae and immunoblotted against total JNK and phosphorylated JNK (pJNK) and found both to be elevated (Figure S4C). To further document a persistent elevation of JNK, we performed immunoblots for total JNK in adult heads with mutant eye clones. Although most of the brain is wild type, much of the visual system is mutant. sicily, Aats-met and Marf mutants exhibit elevated levels of total JNK (Figure 3D), yet only an estimated 40% of the loaded tissue is homozygous mutant. These data indicate that JNK activation correlates with ROS levels.
To test whether SREBP is upregulated in these mutants, we immunoblotted protein extracted from mutant larvae and observed a ~fivefold increase in level of active/cleaved SREBP (Figure S4C). Mutants that do not exhibit LD accumulation do not exhibit elevated levels (Pink15) or modest increases of SREBP (Drp12) (Figure S4D). Furthermore, we observe an upregulation of active SREBP in adult heads with mutant clones (Figure 3D), and Acetyl CoA carboxylase (ACC), whose transcription levels are directly regulated by SREBP (Seegmiller et al., 2002) are upregulated in mutant clones (Figure 3D). Moreover, reducing ROS by overexpressing hSOD1 in sicily mutant larvae decreases the levels of JNK and active SREBP (Figure S4E). Finally, reducing ROS with AD4 reduces levels of active SREBP in mutant larvae (Figure S4C). Our results therefore strongly indicate that ROS stimulates the activation of JNK and SREBP and this axis directly contributes to the LD accumulation phenotype in the nervous system.
Neuronal but not glial mitochondrial dysfunction induces cell non-autonomous LD accumulation in glia
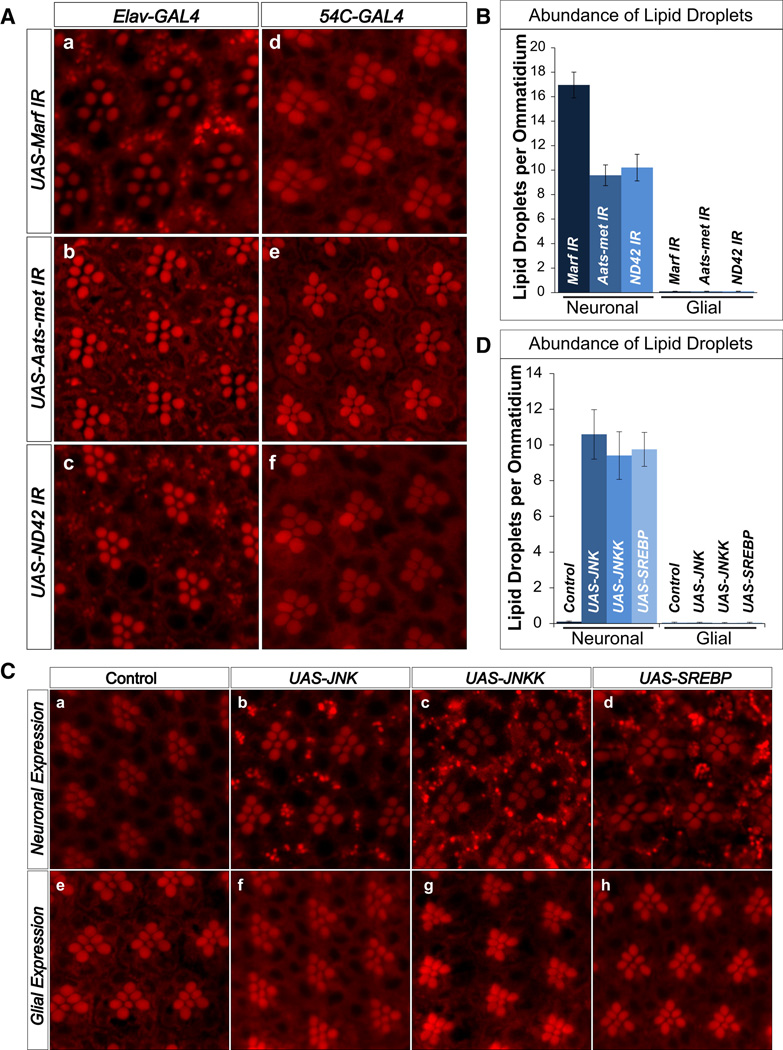
To determine the cell specific defects that lead to LD accumulation, we knocked down Marf, Aats-met, and ND42 expression with RNAi lines driven by a neuronal driver or pigment glia driver (Bao et al., 2010). To demonstrate that the glial driver is able to reduce the expression of genes in pigment cells we crossed it to an RNAi against the white gene. As shown in Figure S5, the 54C-GAL4 driver efficiently reduces pigment levels.
Neuronal expression of RNAi against Marf, Aats-met and ND42 causes significant LD accumulation in glia but not neurons (Figure 4A a–c and Figure 4B). However, RNAi in glia for these genes does not result in LD accumulation (Figure 4A d–f and Figure 4B). Hence, neuronal knockdown of Marf, Aats-met and ND42 is sufficient to lead to LD accumulation glia.
Figure 4. Photoreceptor neurons, but not glia, induce LD accumulation cell non-autonomously.
A) a–c, Nile Red stains show that neuronal knockdown (elav-GAL4) of Marf, Aats-met, and ND42 result in LD accumulation in the glia. d–f, Glial (54C-GAL4) knockdown of Marf, Aats-met, and ND42 does not result in LD accumulation. B) Quantification of A. C) a,e, Control (white) do not exhibit LD accumulation. b–d, Overexpression of JNK, JNKK and SREBP in the neurons (elav-GAL4) leads to LD accumulation in the glia. e–h, However, overexpression of JNK, JNKK and SREBP in the glia (54C-GAL4) does not result in LD accumulation. D) Quantification of LD abundance. Data are represented as mean ± SEM. See also Figure S5.
To assess if overexpression of JNK or SREBP is sufficient to induce LD accumulation, we overexpressed JNK, Hemipterous (the Drosophila JNK Kinase), or full-length SREBP using neuronal or glia specific drivers. As shown in Figure 4C, neuronal expression of JNK or SREBP leads to glial cell specific LD accumulation, whereas glial cell specific expression did not. Activation of JNK is required for the LD accumulation phenotype as overexpression of JNKK also leads to LD accumulation. In summary, activation of JNK or SREBP in neurons is sufficient to cause LD in glia.
It is interesting to note that neuronal overexpression of JNK or SREBP alone does not cause ND upon aging (Figure S5B) and LDs are retained in aged animals (data not shown). These data show that glial LD accumulation is not sufficient to cause ND, and additional insults are likely to be required.
Neuronal knockdown of Marf, Aats-met and ND42 leads to LD accumulation in glia. To assess whether a reduction in ROS or JNK in neurons alone is sufficient to reduce LD accumulation in glia, we expressed Marf RNAi under the control of the photoreceptor specific Rhodopsin (Rh1) promoter (Rh-Marf RNAi). Rh-Marf RNAi leads to LD accumulation in glia, but expression of hSOD1 or reduction of JNK (RNAi) using a neuronal driver, significantly reduce LD accumulation. However, when we reduce JNK in glia, LDs are retained (Figure S7A and B). Hence, elevated ROS and JNK activation in neurons is sufficient and necessary to cause LD accumulation in glia, providing compelling evidence that this neuronal pathway plays a critical role in LD accumulation.
Reduction of LDs delays neurodegeneration
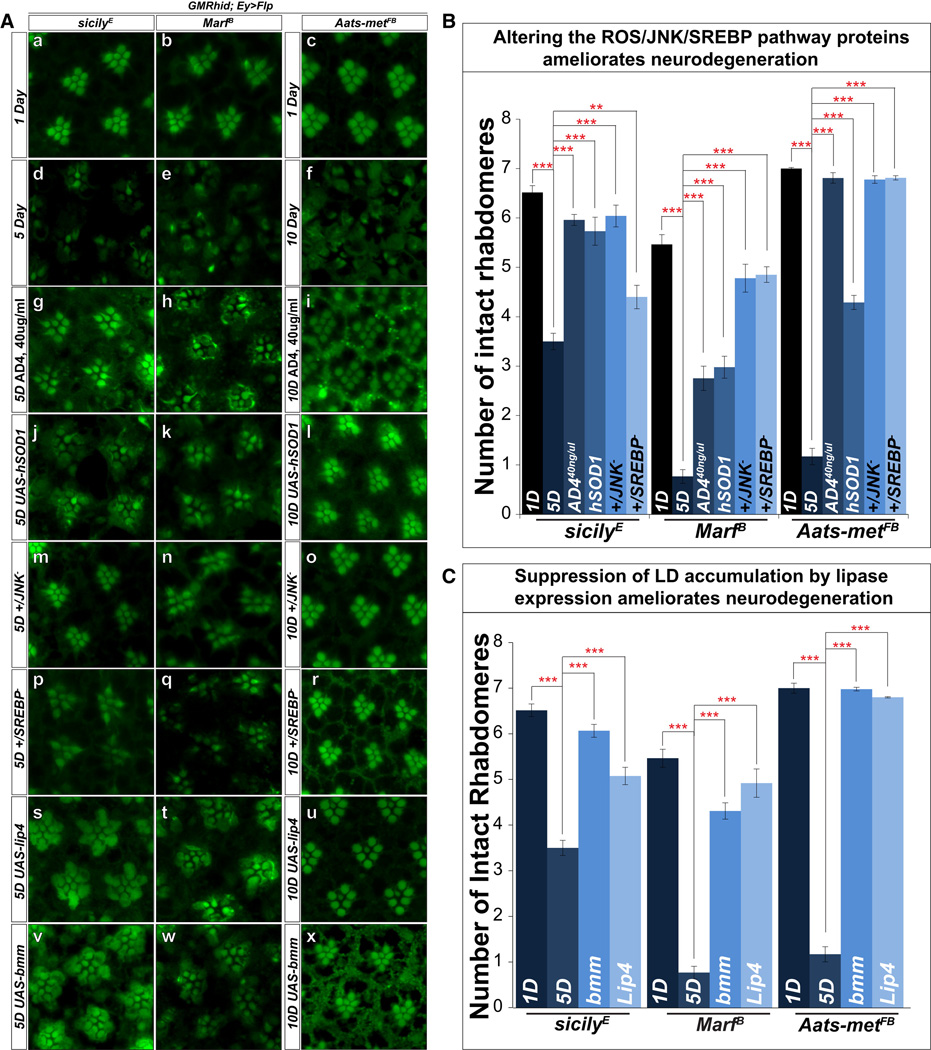
To assay ND in various genetic backgrounds, we assessed degeneration as previously mentioned. Upon aging, mutant flies exhibit a significant loss of f-actin staining in rhabdomeres when compared to day 1 (Figure 5A a–f). To assay whether reduction of the levels of ROS, JNK or SREBP suppress LD as well as ND, we analyzed the rhabdomere phenotypes of flies fed with 40µg/ml of AD4, overexpressing hSOD1, or lacking one copy of JNK or SREBP, by phalloidin staining (Figure 5A g–r). These animals contain significantly more intact rhabdomeres when aged compared to mutants alone (Figure 5B). These data show that suppression of ROS/JNK/SREBP suppress LD formation and delays ND.
Figure 5. Reduction of LD accumulation restores rhabdomere integrity and partially suppresses neurodegeneration.
A) a–c, Phalloidin staining of whole mount retina of 1-day-old sicily, Marf and Aats-met mutant clones exhibit mostly intact rhabdomeres at this early stage. d–f, Upon aging, sicily (5d), Marf (5d), and Aatsmet (10d) mutant clones show obvious signs of degeneration. g–I, animals fed with 40µg/ml of AD4 exhibit more intact rhabdomeres after aging for all three mutants. j–l, Reducing LD accumulation by overexpression of hSOD1, or m–r, one copy of JNK or SREBP all partially restore rhabdomere integrity after aging based on phalloidin staining. s–x, The expression of lipases (brummer and Lip4) in the mutant background also delays degeneration compared to mutant clones alone. B) Quantification of rhabdomere numbers after aging show that expression of hSOD1, feeding AD4 and removing one copy of JNK or SREBP restores rhabdomere integrity. C) Quantification of rhabdomere numbers after aging shows that expression of both brummer and Lip4 significantly restore the number of photoreceptors in all three mutant backgrounds. Data are represented as mean ± SEM. Significance was calculated compared to controls (*P<0.05, **P<0.005, ***P<0.0005). See also Figure S6.
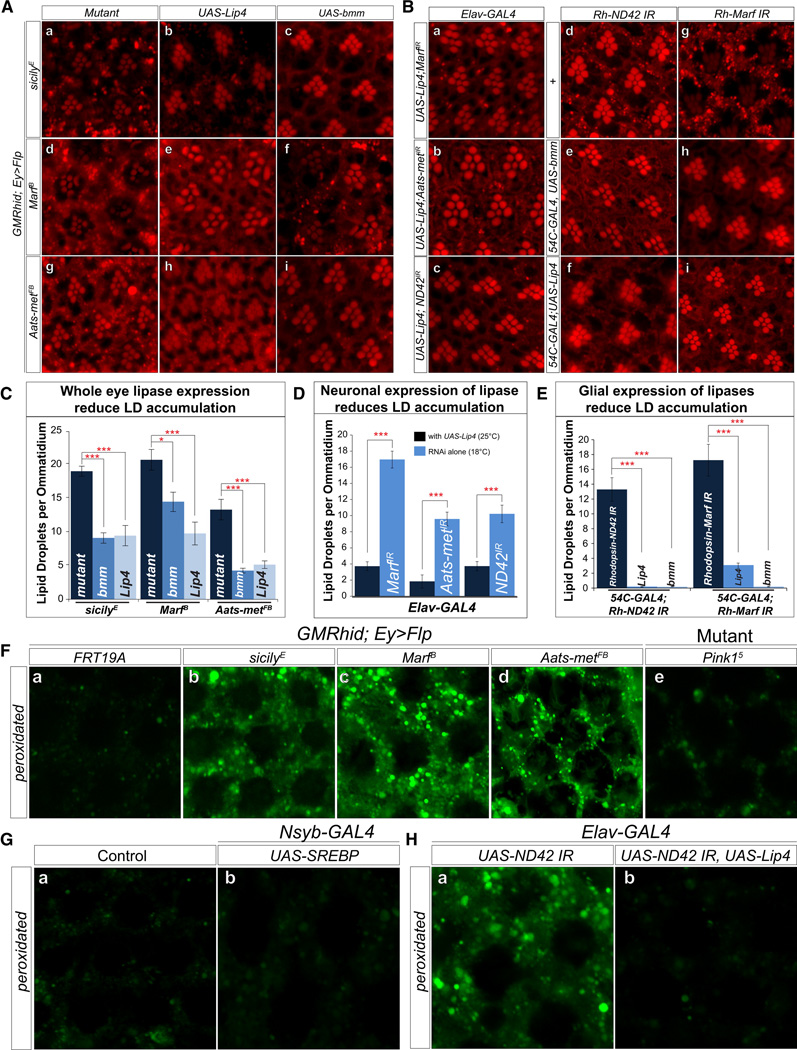
To determine whether reducing LD by ectopic expression of lipases are protective in ND, we expressed two different lipases in mutant clones and assessed ND: Lipase 4 (Lip4), homolog of human acid lipases, functions in starvation mediated lipolysis, and brummer, homolog of adipocyte triglyceride lipase (ATGL), localizes to the LD surface, and acts in lipolysis (Gronke et al., 2005). When we expressed the lipases in mutant clones, the expression of either lipase partially restores the shape, density, and number of identifiable rhabdomeres (Figure 5A s–x, and C), indicating that reducing LD accumulation partially alleviates ND. This was confirmed by TEM (Figure S5C).
Lipid peroxidation in glial cells affects the demise of neurons
LD accumulation occurs early and disappears by the time the features of ND become more apparent (Figure S2C). To demonstrate that the lipases reduce the formation of LD, we assessed the loss of LD upon overexpression of lipases in the eye at day 1. Overexpression of the lipases significantly reduces LD formation (Figure 6 Aa–i and Figure 6C). To assess the specific cellular requirements, we determined whether LD accumulation in glia induced by knockdown of Marf, Aatsmet or ND42 (Figure 4) is suppressed by co-expression with Lip4 in neurons. Neuronal expression of Lip4 suppresses LD accumulation in glia (Figure 6Ba–c and quantified in Figure 6D) and ND (Figure S6E and F).
Figure 6. Lipid peroxidation in glial cells affects the demise of neurons.
A)a–i, Nile Red staining of whole mount retina show that overexpression of brummer and Lip4 in the visual system reduces LD accumulations in sicily, Marf and Aats-met mutant clones. B) a–c, Neuronal expression of lipases and knockdown of Marf, Aats-met and sicily also reduces LD accumulation in the glia. Flies with elav-GAL4 mediated knockdown of the RNAis alone were raised at 18°C (efficient RNAi leads to high percentage of death) and flies expressing Lip4 and RNAi were raised at 25°C. d–i, Rh-ND42 and Marf RNAi in the neurons results in glial LD accumulation. Expression of brummer and Lip4 in the glia (54C-GAL4) reduces the formation of LD accumulation. C) Quantification of A. D) Quantification of B a–c, Significance was calculated compared to Figure 4Aa–c. E) Quantification of B d–i. F) a, e. Measurement of peroxidated lipids shows basal level of peroxidated lipids in control (FRT19A) clones and pink15 mutant retinas. b–d, 1-day-old mutant clones exhibit elevated levels of peroxidated lipids. G) Neuronal overexpression of SREBP does not result in elevated levels of peroxidated lipids compared to control (UAS-SREBP). H) Neuronal RNAi knockdown of ND42 results in elevated levels of peroxidated lipids, which is rescued with neuronal expression of lipase. Data are represented as mean ± SEM. Significance was calculated compared to controls. (*P<0.05, **P<0.005, ***P<0.0005). See also Figure S6.
Is expressing lipases in glia sufficient to reduce LD and delay ND? A knockdown of Marf and ND42 (Rh-ND42 RNAi and Rh-Marf RNAi) in neurons and overexpression of Lip4 or brummer in glia lowers LD accumulation and delays ND (Figure 6B d–I and Figure S6G and H). In summary, reducing lipid load in neurons or glia reduces LD accumulation (Figure 6D and E) and delays ND. Note that with elav-GAL4 or Rh promoter driven knockdowns of ND42 or Marf, the onset of ND is significantly delayed compared to the ND observed in mutant clones. Finally, reducing lipid load in both cell types seems more potent in delaying ND than reducing lipid load in each cell type separately.
Since LD formation alone is not sufficient to induce ND (Figure 4C and Figure S5B), and since levels of ROS are elevated in mutants, we explored whether accumulated lipids are peroxidated. Lipids can be peroxidated in presence of ROS and mediate cellular stress (Niki, 2009; Reed, 2011). To visualize peroxidated lipids, staining with BODIPY® 581/591 C11 revealed an abundance of peroxidated lipids when compared to controls that do not exhibit LD accumulations (Figure 6F). Furthermore, as neuronal overexpression of SREBP leads to LD accumulation but not ND, we assessed lipid peroxidation in animals overexpressing SREBP and observed no difference compared to controls (Figure 6G). Finally, neuronal knockdown of ND42 results in LD accumulation and peroxidated lipids. However, this peroxidation is reduced when lipases are expressed neuronally (Figure 6H). Hence, these data show that LD accumulation alone is insufficient to cause ND, and ROS is required in conjunction with LD accumulation to promote ND.
Ndufs4−/− mice exhibit glial LD accumulation and neurodegeneration that is significantly ameliorated with presymptomatic antioxidant treatment
We hypothesized that mitochondrial defects in vertebrates may also lead to LD accumulation in glia, as mitochondrial genes as well as JNK and SREBP are conserved. A mouse model has been generated which permits modeling of Leigh Syndrome by removing a mitochondrial Complex I subunit, Ndufs4 (Quintana et al., 2012; Quintana et al., 2010; Kruse et al., 2008). Mutant Ndufs4 mice develop normally but exhibit signs of encephalomyopathy at postnatal day 30 (P30) and eventually die from breathing defects around P50. These mice develop brain lesions similar to those in Leigh Syndrome patients. The KO animals also exhibit reduced activity in Complex I and oxidative stress (Quintana et al., 2012; Assouline et al., 2012).
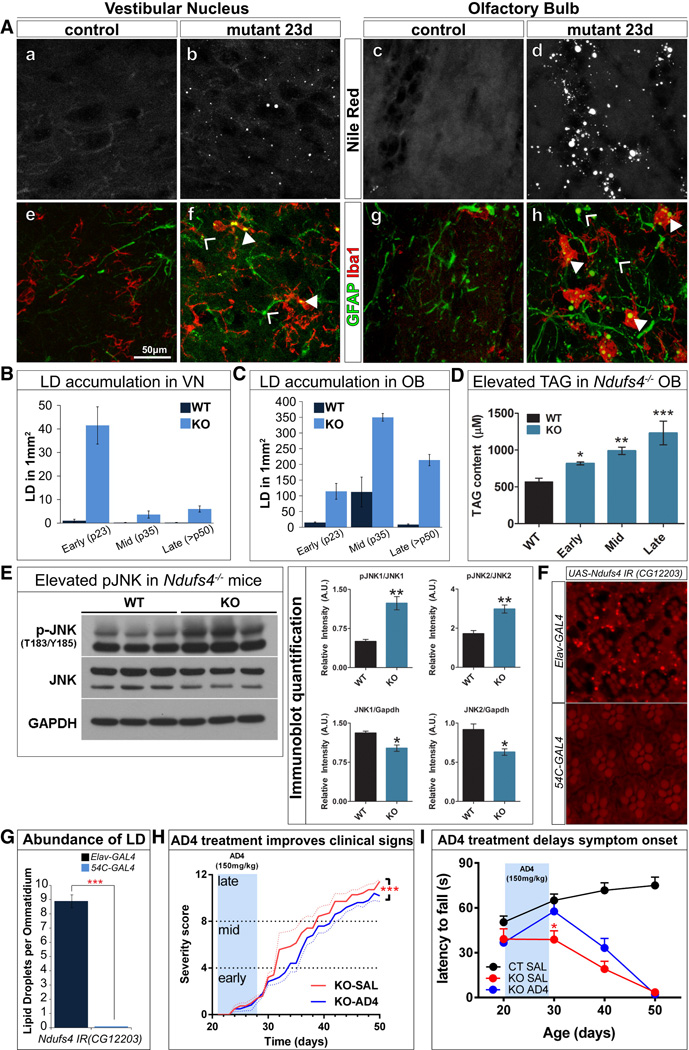
To determine whether LD accumulate in Ndufs4 KO mice (Ndufs4−/−), we systematically profiled the brain with neutral lipid stains (20 µm coronal sections from the olfactory bulb to the brain stem) of presymptomatic (P23), mid (P35) and late-stage (>P50) mice along with gender and age-matched controls. We observed an abundance of LD in the olfactory bulb (OB) and vestibular nucleus (VN) of P23 brain slices (Figure 7A a–d and S8C). We also observed mild LD accumulation in the periaqueductal gray, cerebellum, dorsal motor nucleus, vagus and abducens nuclei of mutant brains that are not observed in controls (data not shown). As quantified in Figure 7B–C, there is an abundance of LDs in the VN of presymptomatic brains but LDs progressively disappear in P34 and P50 mice. We also observed lesions at P34 and >P50 in the areas where LDs accumulated at P23 (Figure S7A and B). Aside from the VN, LDs also accumulate in the OB of presymptomatic animals. However, unlike in the VN, accumulations in the OB persist throughout the lifespan of the these mice, perhaps of newborn neurons in this region (Ming and Song, 2011). Interestingly, the areas of the brain with LD accumulation at P23 (VN and the OB) do not exhibit other obvious pathological phenotypes at this time. However, at P34, and especially at P50, the VN and the OB are the main affected regions by lesions and gliosis previously described in Quintana et al. (2012). These data show that LD accumulation precede physical and histological evidence of ND in mice. In addition, triglyceride (TAG) levels in OB of early-, mid- and late-stage Ndufs4−/− mice progressively increase (Figure 7D). No differences in TAG levels were observed in the brainstem, a small portion of which corresponds to the VN (data not shown).
Figure 7. LD accumulation is present in the astrocytes and microglia of Ndufs4−/− mice prior to neurodegeneration.
A) a–d 20µm coronal cryosections reveal LD accumulation in the VN and OB of Ndufs4−/− mice compared to control (C57Bl/6). e–h LD accumulates in the astrocytes and microglia of the VN and OB shown by colocalization with the astrocyte marker, GFAP and the microglia marker, Iba1. (B) LD accumulation in the VN is high in the P23 mutants but decreases in number in the P34 and >P50 mutants. (C) In the OB, LD accumulation occurs in the P23 mutants and increases in number in the P34 and >P50 animals. (D) Ndufs4−/− exhibit elevated triglyceride (TAG) levels in the OB in an age-dependent manner. E) pJNK is increased in the mutants. Right panels show the quantification of the immunoblots. F) Neuronal knockdown of dNdufs4 (CG12203) leads to LD accumulation in the glia, while glial knockdown results in no LD accumulation. (*P<0.05, **P<0.005, ***P<0.0005). G) Quantification of F. H) 7-day administration of AD4 in P21 animals via IP injection at 150mg/kg shows treated mutant animals with improved clinical signs (body weight loss, hypotonia, ataxia, piloerection, clasping, gasping, paralysis, tremor) upon aging and I) performing at a comparable level as control while mutants treated with saline show progressive decline in motor performance. (*p<0.05 KO SAL vs KO AD4, two-way ANOVA). See also Figure S7.
To determine the cellular specificity of this LD accumulation, we stained for neutral lipids and glial markers. In the OB and VN, LD co-localize with the astrocyte and microglia markers (Figure 7Aeh). However, LD are rarely seen at P34 and >P50 mice, when there are obvious pathological signs, such as reactive astrocytes and activated microglia. Hence, LDs accumulate in glia prior to the onset of the physical signs of ND in the mutants.
We also assessed JNK activation in the OB of WT and P34 Ndufs4−/− mice (Figure 7E); a stage at which the highest level of LD is observed in this area (Figure 7C). Levels of pJNK are increased in the OB of Ndufs4−/− mice (Figure 7E), suggesting activation of JNK. Finally, neuronal knockdown of fly Ndufs4 (CG12203) via RNAi in neurons also causes LD accumulations in glia, consistent with our observations in mice (Figure 7F–G).
As the levels of ROS, JNK and LD are elevated, we tested whether AD4 can delay ND in Ndufs4−/− mice. KOs were injected intraperitoneally with 150mg/kg/day from P21 to P28. This short treatment leads to a delay in onset and reduction in severity of clinical signs throughout the lifespan of treated mice (Figure 7H). By P30, the treated KO mice have a significantly delayed latency to fall in the rotarod assay compared to saline treated KO mice (Figure 7I). Thus, a brief administration of AD4 is sufficient to delay the onset of ND and motor defects in Ndufs4−/− mice, arguing that increased ROS plays a role in the pathogenesis.
Discussion
Here, we show that neuronal mitochondrial defects that lead to elevated levels of ROS, induce activation of JNK and SREBP, which in turn elevate lipid synthesis in neurons and formation of LD in glial cells. These LDs contribute to and promote ND through elevated levels of lipid peroxidation. LDs form in glia prior to or at the onset of the appearance of obvious degenerative histological features in Drosophila and mice. Reducing the number and size of LD pharmacologically or genetically delays ND in the fly. To our knowledge, this is the first indication that SREBP, lipid droplet biogenesis and lipid metabolism play a role in the pathogenesis of several neurodegenerative diseases.
A growing body of evidence points to the importance of glial health and function in nervous system energy metabolism and homeostasis (Belanger et al., 2011). Nevertheless, given the number and prevalence of different types of neurodegenerative diseases, very few reports have documented the presence of LDs in either neuron or glia in patients and in animal models. LD accumulation in the brain has been reported in cells that line the ventricles in the globus pallidus and substantia nigra in mutant mice lacking both subunits of the liver X receptor (Wang et al., 2002), apolipoprotein E (Mato et al., 1999), or a peroxisomal biogenesis factor (Pex5) (Hulshagen et al., 2008). In addition, in vitro studies using immortalized cell lines and explants show that LD may form and accumulate in glia under conditions of nutrient deprivation or lipopolysaccharide induced stress (Cabodevilla et al., 2013; Bozza and Viola, 2010). However, LDs have not been shown to play an active role in neurodegenerative processes. Furthermore, LD accumulation has not been reported in patients with or animal models of Leigh Syndrome (NDUFS4/Ndufs4, NDUFAF6/sicily), CMT-2A2 or HMSN6 (MFN2/Marf), and ARSAL (MARS2/Aats-met) (Assouline et al., 2012; McKenzie et al., 2011; Pagliarini et al., 2008; Boaretto et al., 2010; Bayat et al., 2012; Thiffault et al., 2006). The lack of neuropathological reports of LDs in animal models or in patients with ND may be attributed to the fact that LD accumulation is transient and mostly occur during presymptomatic stages of the disease.
Although these genes/mutants are implicated in very different mitochondrial processes, they exhibit a common phenotype of elevated levels of ROS, leading to LD accumulation. Similar morphological changes of glia have been reported under stress conditions (Boche et al., 2013; Pekny and Nilsson, 2005). Interestingly, mid- and late-stage Ndufs4−/− mice exhibit CNS lesions in the same brain regions where the LD accumulate in early stage animals, showing a strong correlative relationship. Similarly, LD accumulation in Drosophila mutants occurs prior to or at the onset of physical signs of ND. Importantly, the delivery of AD4 is able to significantly ameliorate LD accumulation in Drosophila and delay the onset of ND in flies and mice. Hence, the molecular mechanisms underlying these phenotypes are likely to be conserved between these species and potentially also in higher organisms.
In the clinical setting, the prescription of antioxidants towards treatment of neurodegenerative diseases has been tested repeatedly on patients with neurodegenerative disorders (Galasko et al., 2012; Gilgun-Sherki et al., 2001; Gandhi and Abramov, 2012), without compelling results. The LD accumulation phenotype in our mutants occurs prior to histopathological and physical signs of ND. A brief period of AD4 delivery prior to the onset of symptoms in mutant mice is effective in delaying onset of clinical signs. Thus, therapy with an effective antioxidant that penetrates the blood-brain barrier should be started early and be sustained over long periods. In addition, pharmacological manipulation of JNK or lipid levels in the brain may serve as a potential therapy to delay the onset of ND. However, similar to antioxidant treatment, this may need to be administered at an early stage. Hence, early identification of potential ROS related neurological disease based on genetic/genomic diagnosis or by biomarkers may be critical. Since LD accumulation is one of the earliest presymptomatic changes that occurs in the nervous system, detection of LD itself or changes in neurometabolism may be a promising biomarker.
In summary, we provide evidence for the role of altered lipid metabolism and a neuron-glia interplay that promotes ND. In some mitochondrial mutants, we observe an upregulation of SREBP as well as lipid biogenesis and glial LD formation. The accumulation of LD is not sufficient to promote the ND process itself. However, in the presence of ROS the accumulated lipids are peroxidated and promote ND, possibly by promoting the release of lipids from LD, elevating the cytoplasmic load, and causing a progressive loss of LD. The released lipids likely become peroxidated and affect glial function. Hence, the synergistic effects of increased lipid synthesis and/or LD accumulation in combination with elevated ROS and lipid peroxidation promote ND. Finally, we show that LD accumulation occurs at the onset or precedes ND in flies and mice, suggesting that LD and changes in lipid metabolism in the nervous system may be a promising biomarker to identify brain regions susceptible to but not yet exhibiting symptoms of ND.
Materials and Methods
Lipid Droplet staining
For whole mount staining of fly retinas, heads were dissected in cold PBS and fixed in 3.7% formaldehyde for 1 hour. Subsequently, the retinas were dissected and fixed for an additional 30 minutes. Retinas were rinsed several times with 1× PBS and incubated for 10 minutes at 1:1000 dilution of PBS with 1 mg/ml Nile Red (Sigma) or BODIPY493/503 (Life Technologies). Subsequently, tissues were rinsed with PBS and immediately mounted with Vectashield (Vector Labs) for same-day imaging. Images were obtained with a Zeiss LSM 510 microscope.
For LD staining in the CNS, mice were perfused transcardially with 4% paraformaldehyde. Brains were extracted and post-fixed in the same fixative overnight at 4°C and then dehydrated in sucrose. They were then embedded in OCT (Tissue-Tek) and cryosectioned at 20µm per slice. Slides were submerged in 1× PBS for 10 min and then incubated for 10 min in Nile Red/BODIPY as above. Subsequently, the slides were washed twice with 1× PBS and immediately covered with Vectashield mounting medium with DAPI (Vector Labs) for same-day imaging.
Lipid peroxidation
Procedures with C11-BODIPY581/591 were adapted for tissue staining instead of cell culture as described in (Drummen et al., 2002). BODIPY was solubilized in 50µl DMSO and was added to a final concentration of 20µM in BSA. For live tissue staining of mutant retinas, whole flies were pinned to Sylgard ® silicone elastomer plates and submerged with 1% BSA and BODIPY. Tungsten needles were used to slice the cuticle around the eye for optimal dye penetration. Plates were incubated for 30 minutes at 37°C and subsequently rinsed with 1× PBS before the retinas were sliced out, mounted with Vectashield and imaged immediately.
Tissue triglyceride quantification
Triglyceride levels in OB samples (n=6 for WT mice, n=3 for each Ndufs4 mice group) were determined using the PicoProbe Triglyceride Quantification Assay Kit (Abcam) according to manufacturer’s directions. Wild-type mice included age-matched controls.
AD4 Administration
Control and KO mice were administered i.p. daily for 7 days with either saline (CT SAL, n=11; KO SAL, n=7) or 150 mg/kg AD4 (KO AD4, n=6) from day P21 unil P28.
Supplementary Material
Acknowledgements
We thank Karen Schulze, Richard Palmiter, Bertrand Mollereau and Kartik Venkatachalam for comments; Robert Rawson (SREBP antibody), Ronald Kühnlein (UAS-bmm), the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi center and the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing stocks and reagents, Ching-On Wong for help with westerns and Lita Duraine for TEM. Confocal microscopy was supported by the BCM Intellectual and Developmental Disabilities Research Center (NIH/NICHHD P30 HD024064). We acknowledge support of the NIH(1RC4GM096355), the Robert A. and Renee E. Belfer Family Foundation, the Huffington aging center and Target ALS to H.J.B. S.Y. is supported by the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital. H.J.B. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contribution
LL, KZ and HJB conceived and designed the project. LL conducted the lipid stainings, genetic interaction studies and analyzed the data. LL, KZ and HS prepared samples for TEM. LL, KZ, ZL, and BHG performed the mitochondria assays. ES, JH, AQ designed or performed, the mouse biochemical, behavioral, and drug studies. KZ, HS, SY and MJ performed the screen that isolated the mutants. LL and HJB wrote the manuscript.
Reference List
- Amer J, Atlas D, Fibach E. N-acetylcysteine amide (AD4) attenuates oxidative stress in betathalassemia blood cells. Biochim. Biophys. Acta. 2008;1780:249–255. doi: 10.1016/j.bbagen.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Assouline Z, Jambou M, Rio M, Bole-Feysot C, de LP, Barnerias C, Desguerre I, Bonnemains C, Guillermet C, Steffann J, Munnich A, Bonnefont JP, Rotig A, Lebre AS. A constant and similar assembly defect of mitochondrial respiratory chain complex I allows rapid identification of NDUFS4 mutations in patients with Leigh syndrome. Biochim. Biophys. Acta. 2012;1822:1062–1069. doi: 10.1016/j.bbadis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Bao S, Fischbach KF, Corbin V, Cagan RL. Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev. Biol. 2010;344:948–956. doi: 10.1016/j.ydbio.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Bayat V, Thiffault I, Jaiswal M, Tetreault M, Donti T, Sasarman F, Bernard G, Demers-Lamarche J, Dicaire MJ, Mathieu J, Vanasse M, Bouchard JP, Rioux MF, Lourenco CM, Li Z, Haueter C, Shoubridge EA, Graham BH, Brais B, Bellen HJ. Mutations in the mitochondrial methionyl-tRNA synthetase cause a neurodegenerative phenotype in flies and a recessive ataxia (ARSAL) in humans. PLoS. Biol. 2012;10:e1001288. doi: 10.1371/journal.pbio.1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Mitochondria and neurodegeneration. Novartis. Found. Symp. 2007;287:183–192. doi: 10.1002/9780470725207.ch13. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti P. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell metabolism. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Boaretto F, Vettori A, Casarin A, Vazza G, Muglia M, Rossetto MG, Cavallaro T, Rizzuto N, Carelli V, Salviati L, Mostacciuolo ML, Martinuzzi A. Severe CMT type 2 with fatal encephalopathy associated with a novel MFN2 splicing mutation. Neurology. 2010;74:1919–1921. doi: 10.1212/WNL.0b013e3181e240f9. [DOI] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:243–250. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Cabodevilla AG, Sanchez-Caballero L, Nintou E, Boiadjieva VG, Picatoste F, Gubern A, Claro E. Cell survival during complete nutrient deprivation depends on lipid droplet-fueled beta-oxidation of fatty acids. J. Biol. Chem. 2013;288:27777–27788. doi: 10.1074/jbc.M113.466656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo N, Smit AB, Verheijen MH. SREBPs: SREBP function in glia-neuron interactions. FEBS J. 2009;276:628–636. doi: 10.1111/j.1742-4658.2008.06808.x. [DOI] [PubMed] [Google Scholar]
- Cheng L, Bailey A, Leevers S, Ragan T, Driscoll P, Gould A. Anaplastic lymphoma kinase spares organ growth during nutrient restriction in Drosophila. Cell. 2011;146:435–447. doi: 10.1016/j.cell.2011.06.040. [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Debattisti V, Scorrano L. D. melanogaster, mitochondria and neurodegeneration: small model organism, big discoveries. Mol. Cell Neurosci. 2013;55:77–86. doi: 10.1016/j.mcn.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Del BR, Moggio M, Rango M, Bonato S, D'Angelo MG, Ghezzi S, Airoldi G, Bassi MT, Guglieri M, Napoli L, Lamperti C, Corti S, Federico A, Bresolin N, Comi GP. Mutated mitofusin 2 presents with intrafamilial variability and brain mitochondrial dysfunction. Neurology. 2008;71:1959–1966. doi: 10.1212/01.wnl.0000327095.32005.a4. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002;33:473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, Aisen P. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012;69:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell Longev. 2012;2012:428010. doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Greenspan P, Mayer E. Nile red: a selective fluorescent stain for intracellular lipid droplets. The Journal of cell biology. 1985 doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein R. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell metabolism. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshagen L, Krysko O, Bottelbergs A, Huyghe S, Klein R, Van Veldhoven PP, De Deyn PP, D'Hooge R, Hartmann D, Baes M. Absence of functional peroxisomes from mouse CNS causes dysmyelination and axon degeneration. J. Neurosci. 2008;28:4015–4027. doi: 10.1523/JNEUROSCI.4968-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kijima K, Numakura C, Izumino H, Umetsu K, Nezu A, Shiiki T, Ogawa M, Ishizaki Y, Kitamura T, Shozawa Y, Hayasaka K. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum. Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte AS, Matthews KA, Rawson RB. Fatty acid auxotrophy in Drosophila larvae lacking SREBP. Cell Metab. 2006;3:439–448. doi: 10.1016/j.cmet.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato M, Ookawara S, Mashiko T, Sakamoto A, Mato TK, Maeda N, Kodama T. Regional difference of lipid distribution in brain of apolipoprotein E deficient mice. Anat. Rec. 1999;256:165–176. doi: 10.1002/(SICI)1097-0185(19991001)256:2<165::AID-AR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McKenzie M, Tucker EJ, Compton AG, Lazarou M, George C, Thorburn DR, Ryan MT. Mutations in the gene encoding C8orf38 block complex I assembly by inhibiting production of the mitochondria-encoded subunit ND1. J. Mol. Biol. 2011;414:413–426. doi: 10.1016/j.jmb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, Sampaio J, Brankatschk M, Carvalho M, Mahmoud A, Shevchenko A, Eaton S. Lipoproteins in Drosophila melanogaster-Assembly, Function, and Influence on Tissue Lipid Composition. PLoS genetics. 2012;8 doi: 10.1371/journal.pgen.1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos TG, Boyer JH, Shah M, Thangaraj K, Soong ML. Laser action from 2,6,8-position trisubstituted 1,3,5,7-tetramethylpyrromethene-BF(2) complexes: part 1. Appl. Opt. 1990;29:3885–3886. doi: 10.1364/AO.29.003885. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J. Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10996–11001. doi: 10.1073/pnas.1006214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD. Fatal breathing dysfunction in a mouse model of Leigh syndrome. J. Clin. Invest. 2012;122:2359–2368. doi: 10.1172/JCI62923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic. Biol. Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Sandoval H, Yao CK, Chen K, Jaiswal M, Donti T, Lin YQ, Bayat V, Xiong B, Zhang K, David G, Charng WL, Yamamoto S, Duraine L, Graham BH, Bellen HJ. Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. Elife. 2014;3 doi: 10.7554/eLife.03558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- Schimel AM, Abraham L, Cox D, Sene A, Kraus C, Dace DS, Ercal N, Apte RS. Nacetylcysteine amide (NACA) prevents retinal degeneration by up-regulating reduced glutathione production and reversing lipid peroxidation. Am. J. Pathol. 2011;178:2032–2043. doi: 10.1016/j.ajpath.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller AC, Dobrosotskaya I, Goldstein JL, Ho YK, Brown MS, Rawson RB. The SREBP pathway in Drosophila: regulation by palmitate, not sterols. Dev. Cell. 2002;2:229–238. doi: 10.1016/s1534-5807(01)00119-8. [DOI] [PubMed] [Google Scholar]
- Shao W, Espenshade P. Expanding Roles for SREBP in Metabolism. Cell metabolism. 2012;16:414–419. doi: 10.1016/j.cmet.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Komatsu T, Lim J, Laslo M, Yolitz J, Wang C, Poirier L, Alberico T, Zou S. Nutrientdependent requirement for SOD1 in lifespan extension by protein restriction in Drosophila melanogaster. Aging cell. 2012;11:783–793. doi: 10.1111/j.1474-9726.2012.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiffault I, Rioux MF, Tetreault M, Jarry J, Loiselle L, Poirier J, Gros-Louis F, Mathieu J, Vanasse M, Rouleau GA, Bouchard JP, Lesage J, Brais B. A new autosomal recessive spastic ataxia associated with frequent white matter changes maps to 2q33-34. Brain. 2006;129:2332–2340. doi: 10.1093/brain/awl110. [DOI] [PubMed] [Google Scholar]
- Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wang L, Schuster GU, Hultenby K, Zhang Q, Andersson S, Gustafsson JA. Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13878–13883. doi: 10.1073/pnas.172510899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7904–7909. doi: 10.1073/pnas.1217938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong B, Bellen HJ. Rhodopsin homeostasis and retinal degeneration: lessons from the fly. Trends Neurosci. 2013;36:652–660. doi: 10.1016/j.tins.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Jaiswal M, Charng WL, Gambin T, Karaca E, Mirzaa G, Wiszniewski W, Sandoval H, Haelterman NA, Xiong B, Zhang K, Bayat V, David G, Li T, Chen K, Gala U, Harel T, Pehlivan D, Penney S, Vissers LE, de LJ, Jhangiani SN, Xie Y, Tsang SH, Parman Y, Sivaci M, Battaloglu E, Muzny D, Wan YW, Liu Z, Lin-Moore AT, Clark RD, Curry CJ, Link N, Schulze KL, Boerwinkle E, Dobyns WB, Allikmets R, Gibbs RA, Chen R, Lupski JR, Wangler MF, Bellen HJ. A drosophila genetic resource of mutants to study mechanisms underlying human genetic diseases. Cell. 2014;159:200–214. doi: 10.1016/j.cell.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li Z, Jaiswal M, Bayat V, Xiong B, Sandoval H, Charng WL, David G, Haueter C, Yamamoto S, Graham BH, Bellen HJ. The C8ORF38 homologue Sicily is a cytosolic chaperone for a mitochondrial complex I subunit. J. Cell Biol. 2013;200:807–820. doi: 10.1083/jcb.201208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.