Abstract
Liver kinase B1 (LKB1, also known as serine/threonine kinase 11, STK11) is a tumor suppressor mutated in Peutz-Jeghers syndrome and in a variety of sporadic cancers. Herein, we demonstrate that LKB1 controls the levels of intracellular reactive oxygen species (ROS) and protects the genome from oxidative damage. Cells lacking LKB1 exhibit markedly increased intracellular ROS levels, excessive oxidation of DNA, increased mutation rates, and accumulation of DNA damage, which are effectively prevented by ectopic expression of LKB1 and by incubation with antioxidant N-acetylcysteine (NAC). The role of LKB1 in suppressing ROS is independent of AMPK, a canonical substrate of LKB1. Instead, under the elevated ROS, LKB1 binds to and maintains the activity of cdc42-PAK1 (p21 activated kinase 1) complex, which triggers the activation of p38 and its downstream signaling targets, such as ATF-2, thereby enhancing the activity of SOD-2 and catalase, two antioxidant enzymes that protect the cells from ROS accumulation, DNA damage, and loss of viability. Our results provide a new paradigm for a non-canonical tumor suppressor function of LKB1 and highlight the importance of targeting ROS signaling as a potential therapeutic strategy for cancer cells lacking LKB1.
Keywords: LKB1, p38, ROS, DNA damage, mutation
INTRODUCTION
The liver kinase B1 (LKB1) gene is mutated in Peutz-Jeghers syndrome, an autosomal dominant inherited disorder characterized by mucosal gastrointestinal polyps and increased risk for malignant tumors.1–2 LKB1 mutation is also found in sporadic cancers, such as lung adenocarcinoma and cervical cancers.2–7 Physiologically, LKB1 is involved in multiple processes including embryonic development, cell polarity, cell-cycle arrest, apoptosis, metabolism, and hematopoietic stem cell (HSC) maintenance.2,8–11 LKB1 forms a heterotrimeric complex with mouse protein 25 and Ste20-related adaptor protein (STRAD), and phosphorylates at least 13 members of the AMP-activated protein kinase (AMPK) super family.12–17 It was established that LKB1 regulates cell growth and proliferation through AMPK by controlling tuberous sclerosis complex (TSC) and p53.18,19
LKB1 plays a role in the maintenance of HSC quiescence.9–11 Deletion of Lkb1 resulted in an initial expansion of HSCs and multipotent progenitor cells and eventually depletion of not only these cell populations but also mature blood cell types leading to pancytopenia.9–11 One of the explanations for the depletion of HSCs is that LKB1 deficiency leads to increased DNA damage in response to metabolic and genotoxic stresses. Gurumurthy et al. observed enhanced expression of phosphorylated histone H2AX (γ-H2AX), a marker of DNA damage, in hematopoietic tissues of LKB1-deficient mice, indicating an altered DNA damage response (DDR) in LKB1 deficient cells.10 Consistently, a recent study showed that LKB1-AMPK signaling regulates non-homologous end joining-associated DNA repair and contributes to genome stability.20 Thus, it seems plausible that LKB1 plays a role in DDR.
Reactive oxygen species (ROS) are a source of DNA damage, in particular DNA base damage, which may lead to the accumulation of point and/or deletion mutations and contribute to tumorigenesis.21–23 Some tumor suppressors and chemical compounds can directly regulate intracellular redox state to reduce ROS.24–26 A DNA microarray profile assay showed that LKB1 antagonizes Ras,27 activation of which may induce intracellular ROS accumulation and oncogenic transformation.28 This study also showed that LKB1 deficient cells possess low GADD45, NAD(P)H menadione oxidoreductase 1, and lysozyme P, which may participate in oxidative responses.27 To address the possible role of LKB in regulating cellular oxidative response, in this study, we analyze ROS levels in genetically manipulated LKB1 cells and provide evidence showing that LKB1 protects the genome from ROS-induced oxidation by regulating antioxidant gene products.
RESULTS
Increases of intracellular ROS in LKB1 deficient cells
To determine whether LKB1 regulates intracellular oxidative stress response, we measured ROS levels in LKB1 intact and compromised cells. Under unstressed conditions, LKB1-null mouse embryo fibroblasts (MEFs) possessed an approximately two-fold higher ROS than wild-type (WT) MEFs did (Figure 1a). Upon exposure to H2O2, both WT and LKB1-null cells exhibited a dosage–dependent increase in intracellular ROS (Figure 1a). However, LKB1-null cells consistently showed a higher level of ROS as compared with WT cells (Figure 1a). A kinetic analysis of the induction of ROS in cells treated with 50 μM H2O2 showed a consistently higher level in ROS in LKB1 null cells than in their WT counterparts (Figure 1b). We observed a similar increase in ROS in U2OS/shR-LKB1 cells, a cell line we established with a stable knockdown of LKB1 (Figure 1c). The antioxidant N-acetyl-L-cysteine (NAC) reduced ROS levels in both LKB1 intact and compromised cells and abrogated the difference between the two cell lines (Figure 1d). Together, our results suggest that LKB1 deficiency leads to elevated intracellular ROS levels.
Figure 1.
LKB1 status affects intracellular ROS accumulation. (a) Intracellular ROS levels in wild-type and LKB1 null MEFs treated with different dosages of H2O2 for 16 h. One hour prior to the termination of the treatment, 100 ng/ml dihydroethidium was added to the medium. The cells were harvested, washed, and analyzed by flow cytometry with the red laser channel (FL-3) using a FACscan analyzer. The results represent an average ± SEM of three independent experiments. *P < 0.01 compared with wild-type MEFs treated with the same dosage of H2O2. (b) The kinetic analysis of ROS in wild-type and LKB1 null MEFs treated with 50 μM H2O2. After each treatment, the cells were collected for intracellular ROS determination. The results represent an average ± SEM of three independent experiments. *P < 0.01 compared with wild-type cells exposed to H2O2 at the same time point. (c) Intracellular ROS levels in LKB1 knockdown cells. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were exposed to different dosages of H2O2 for 16 h. The cells were then treated and harvested as in Figure 1a for the determination of ROS. The results represent an average ± SEM of three independent experiments. *P < 0.01 and **P < 0.05 compared with U2OS/shR-Ctrl cells exposed to the same dosage of H2O2. (d) NAC reduces LKB1 deficiency-induced elevation of ROS. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were pre-treated with 5 mM NAC for 2 h. The cells were then treated with 200 μM H2O2 for 16 h. Intracellular ROS levels were detected with H2DCFDA staining. The average fluorescence intensity is expressed as mean ± SEM. *P < 0.01 compared with U2OS/shR-Ctrl cells with the same treatment. N.S., not significant (n = 3). (e and f) Ectopic expression of LKB1 reduces intracellular ROS levels. Proliferating HeLa/myc-LKB1 and HeLa/myc cells were cultured in the presence or absence of H2O2 for 16 hours. Cells were then treated as in a for the determination of ROS. (e) Endogenous O2− accumulation in HeLa/myc-LKB1 and HeLa/myc cells. Dotted lines indicate the mean fluorescence intensity. The results shown are representatives of three independent experiments. (f) Mean fluorescence intensity (O2−) in HeLa/myc-LKB1 and HeLa/myc cells treated with 0–50 μM H2O2. The results represent an average ± SEM of three independent experiments. *P < 0.01 compared with HeLa/myc cells exposed to the same dosage of H2O2.
Ectopic expression of LKB1 reduces intracellular ROS
To further define the role of LKB1 in the regulation of ROS, we analyzed intracellular ROS levels in LKB1-deficient HeLa cells and stable HeLa/LKB1 cells that express ectopic LKB1. At the endogenous level, the average fluorescence intensity (O2−) of HeLa cells was approximately 25 +/− 3.2. HeLa/LKB1 cells showed a fluorescence intensity of 10 +/− 1.3, revealing a 60% lower in ROS levels (Figure 1e). Both cell lines displayed a five- to six-fold increase in the ROS levels following exposure to 5–50 μM H2O2, with ROS levels being consistently lower in HeLa/LKB1 cells (Figure 1f). The data provide further evidence that cancer cells expressing LKB1 display lower ROS levels.
LKB1 modulates ROS independent of AMPK
LKB1 activates AMPK under stress conditions, such as bioenergetics perturbation.17 It was reported that ROS are induced when cells are incubated with fatty acids.30 Activation of AMPK with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) is capable of reducing fatty acids-induced ROS, although AICAR had no affect on ROS levels under basal conditions.30 To determine whether the antioxidant function of LKB1 is mediated by AMPK, we detected ROS levels in WT and AMPKα-null MEFs. Surprisingly, AMPK status did not affect intracellular ROS accumulation under endogenous and stress conditions (Supplementary Figures 1a and b). Next, we knocked down LKB1 using siRNA in WT and AMPK-null MEFs. The cells were then treated with H2O2. LKB1 knockdown increased ROS accumulation in both WT and AMPK-null MEFs. Exposure to H2O2 further enhanced the accumulation of ROS in both cell lines (Supplementary Figures 1c and d). We noted that LKB1 deletion contributed to a 20% to 100% increase in intracellular ROS regardless the AMPK status (Supplementary Figure 1d). Thus, we conclude that LKB1-mediated ROS regulation is at least partially independent of AMPK.
Impaired p38 activation in response to ROS in LKB1 deficient cells
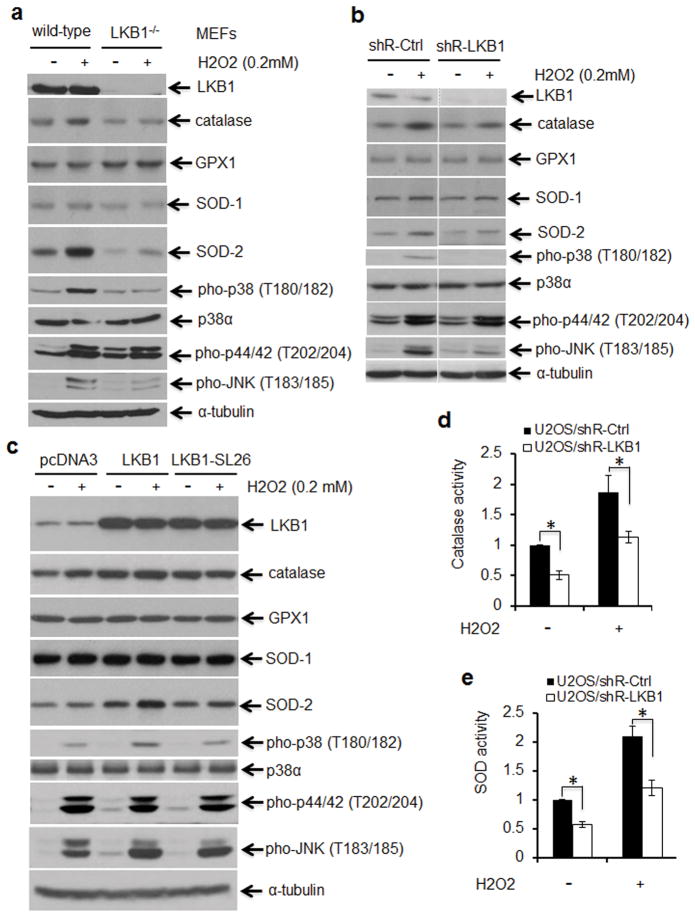
p38 and its mediated signaling are critical targets responding to ROS stress.31–33 To determine whether LKB1 regulates ROS accumulation through p38, we evaluated p38 signaling in response to H2O2 in WT and LKB1-null MEFs. Phosphorylation of p38 and JNK was markedly lower in LKB1-null MEFs as compared with that in WT MEFs under the similar treatment, whereas activation of ERK, another MAPK family member, was not affected by LKB1 status (Figure 2a). Similarly, phospho-p38 was substantially lower in LKB1-knocked-down cells treated with H2O2 as compared with that in LKB1-intact cells under the same condition (Figure 2b). Thus, our results indicate that p38 activation responding to H2O2 is impaired in the absence of LKB1.
Figure 2.
Impaired p38 activation in response to ROS in LKB1 deficient cells. (a and b) ROS responsive markers in LKB1-intact and compromised cells. Wild-type (WT) and LKB1-null MEFs (a) or LKB1 knockdown and control U2OS cells (b) were exposed to 200 μM H2O2 for 2 h. After the treatment, whole cell extracts (WCEs) were isolated for Western blot analysis. α-Tubulin served as a loading control. (c) ROS responsive markers in U2OS cells with enforced expression of LKB1. U2OS/pcDNA3, U2OS/pcDNA3-LKB1 and U2OS/pcDNA3-LKB1 SL26 cells were treated with or without 400 uM H2O2 for 15 min. The WCEs were isolated and subjected to Western blot analysis. α-Tubulin served as a loading control. (d, e) U2OS/shR-Ctrl and U2S/shR-LKB1 cells were exposed to 200 μM H2O2 for 2 h. After the treatment, the cells were washed and lysed for the measurement of catalase (d) and SOD (e) activities. The values of U2OS/shR-Ctrl cells without treatment were set as “1”. Fold changes were shown. *P < 0.01 (n = 4)
To further determine the role of p38 in mediating the effects of LKB1 on ROS, we tested the activation of p38 in H2O2-treated U2OS/LKB1 cells, a cell line with stable overexpression of LKB1. Phospho-p38 was higher in LKB1 overexpression cells than in control cells when treated with H2O2. A loss-of-function LKB1 mutant, SL26, which does not associate with STRAD,34 showed only a marginal effect. Phospho-JNK was minimally elevated, whereas phospho-ERK was not altered by the overexpression of LKB1 (Figure 2c). Pre-treatment with NAC substantially reduced p38 activation in both LKB1 intact and depleted cells (Supplementary Figure 2). Thus, our results suggest a role of LKB1 in the activation of p38 in response to ROS.
To explore whether LKB1 controls ROS through p38-regulated antioxidant enzymes,32 we detected the expression of glutathione peroxidase 1 (GPX1), superoxide dismutase (SOD) −1 and −2, and catalase in LKB1 intact and deficient cells. GPX1 and SOD-1 were not altered by LKB1 status and by ROS treatments in our settings (Figures 2a–c). In contrast, SOD-2 and catalase displayed an appreciable increase in LKB1 intact cells treated with H2O2, whereas elevations of these two enzymes were markedly restricted in LKB1 compromised cells with the similar treatments (Figures 2a–c). Consistently, enzyme activities of SOD and catalase were significantly lower in LKB1 deficient cells than those in LKB1 intact cells regardless of the presence or absence of H2O2 (Figures 2d and e), suggesting that LKB1 may physiologically regulate SOD and catalase antioxidant enzymes.
LKB1 regulates cdc42-PAK1 complex formation and involves in MKK3/6-p38-ATF-2 signaling activation in response to ROS
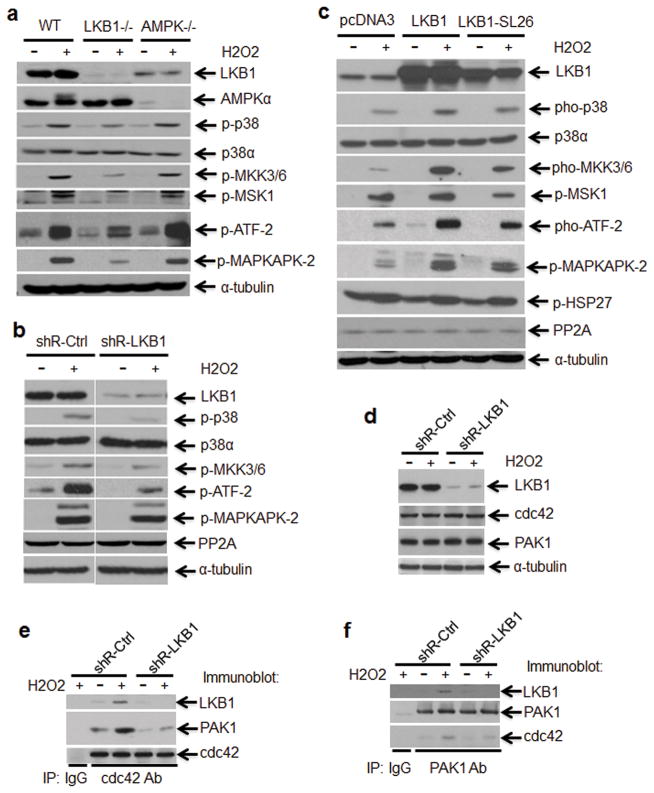
To determine the functional outcome of amplified p38 activation in LKB1-intact cells, we detected the downstream targets of p38 in WT and LKB1-null MEFs treated with H2O2. In WT MEFs, phosphorylation of ATF-2 and MAPKAPK-2, two p38 substrates,35 was strikingly induced by H2O2, consistent with the activation of p38 (Figure 3a). In contrast, both ATF-2 and MAPKAPK-2 phosphorylation in LKB1-null MEFs was much less induced than that in WT MEFs by H2O2 (Figure 3a). Data from genetically manipulated cells with depletion or overexpression of LKB1 recapitulated these findings (Figures 3b and c). We also investigated the activation of p38 in response to H2O2 treatment in WT and AMPK-null MEFs. There was no difference between WT and AMPK-null MEFs in terms of phospho-p38 as well as its downstream targets, ATF-2, MSK1, and MAPKAPK-2 (Figure 3a), which is consistent with the notion that LKB1-mediated ROS regulation is independent of AMPK.
Figure 3.
Cdc42/PAK1-MKK3/6-p38 signaling is involved in LKB1-mediated ROS response. (a and b) MKK3/6-p38-ATF-2 signaling is insensitive to ROS in LKB1 deficient cells. WT, LKB1-null, and AMPKα-null MEFs (a) or U2OS/shR-Ctrl and U2OS/shR-LKB1 cells (b) were treated with or without 400 μM H2O2 for 2 h. After the treatment, WCEs were isolated and subjected to Western blot analysis. α-Tubulin served as a loading control. (c) MKK3/6-p38-ATF-2 signaling is escalated in LKB1 overexpression cells in response to ROS. U2OS/pcDNA3, U2OS/pcDNA3-LKB1 and U2OS/pcDNA3-LKB1 SL26 cells were treated with 400 μM H2O2 for 15 min. The WCEs were isolated and subjected to Western blot analysis. α-Tubulin served as a loading control. (d–f) Proliferating U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were exposed to 200 μM H2O2 for 2 h. After the treatment, the WCEs were isolated. Expression of LKB1, cdc42, and PAK1 were determined by Western blot (d). cdc42-PAK1 complex formation in cells treated with or without H2O2 were analyzed with co-immunoprecipitation assay (co-IP) (e, f). Eight hundred micrograms of total protein were applied for each co-IP.
To define the link between LKB1 and p38 phosphorylation under ROS stress, we detected the expression of protein phosphatase 2A (PP2A), one of the phosphatases that mediate the termination of p38 kinase catalytic activity.35 The levels of PP2A were not affected by genetic manipulations of LKB1 (Figures 3b and c). We then evaluated the activation of p38 upstream kinases under ROS stress in the presence or absence of LKB1. Deficiency of LKB1 led to a marked decrease in the activation of MAP kinase kinase (MKK) 3/6, two essential kinases mediated stresses-induced p38 activation (Figures 3a and b).36 Moreover, ectopic expression of LKB1 increased the phosphorylation of MKK3/6 as compared with that in empty vector-transfected U2OS cells, whereas mutant LKB1 displayed only a marginal activity on MKK3/6-p38 signaling (Figure 3c). Together, our data suggest that LKB1 is required to fully activate the MKK3/6-p38 cascade under ROS stress.
Low molecular weight GTP-binding proteins in the Rho family such as Rac1 and Cdc42 have been reported to activate p38 upstream kinases.36–41 p21-activated kinases (PAKs) are another group of p38 activators by binding to Cdc42 and Rac.37,38 Interestingly, Zhang et al recently reported that LKB1 colocalizes with and is required for the function of cdc42 and PAK1 at the cellular leading edge.42 LKB1 interacts only with active form of cdc42 and PAK.42 To determine whether LKB1-mediated p38 activation involves in cdc42-PAK1, we analyzed the impact of LKB1 on cdc42-PAK1 complex formation, an essential for the activity of cdc42-PAK1 on MAP3Ks.36–41 Consistent with previous report,42 LKB1 could not affect the protein levels of cdc42 and PAK1 (Figure 3d). However, cdc42-PAK1 binding (complex) was substantially reduced due to lack of LKB1. LKB1 not only affected cdc42-PAK1 complex formation, but also located in the complex (Figures 3e and f). Thus, our data and previous report 42 clearly indicate that LKB1 facilitates the formation of the cdc42-PAK complex, and hence regulates the signaling events required to activate p38 under stress conditions.
Integrity of the p38 signaling is necessary for the anti-oxidant function of LKB1
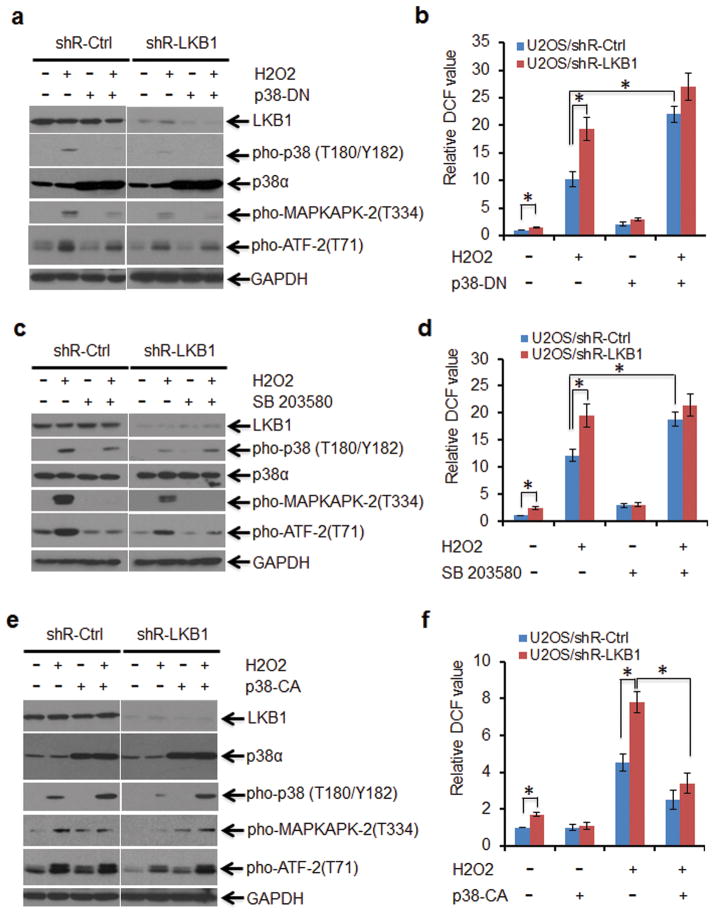
To elucidate the role of p38 activation in LKB1-mediated ROS regulation, transfection of a dominant negative p38 construct (p38-DN) 43 and a specific p38 kinase inhibitor were used to inhibit p38 signaling in U2OS/shR-Ctrl and U2OS/shR-LKB1 cells followed by treatment with H2O2. Phospho-p38 decreased dramatically in cells transfected with p38-DN irrespective of the status of LKB1 and the presence of H2O2 (Figure 4a). Phospho-ATF-2 and phospho-MAPKAPK-2 were also inhibited (Figure 4a). Intracellular ROS levels were significantly increased in p38-DN transfected U2OS/shR-Ctrl cells as compared to control vector transfected cells upon the treatment of H2O2 (Figure 4b). A marginal further increase in ROS was observed in LKB1-depleted cells under the similar treatment (Figure 4b), which is likely due to residual p38 activity in these cells (Figure 4a).
Figure 4.
The integrity of the p38 signaling is necessary for the anti-oxidant function of LKB1. (a and b) U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were transfected with pcDNA3 (Ctrl) or pcDNA3/p38-DN constructs and selected with 200 μg/ml hygromycin for 2 weeks for the establishment of stable expression cell lines. Cells were treated with 200 μM H2O2 for 2 h. (a) After the treatment, the WCEs were isolated for Western blot detection of p38 signaling targets. (b) Intracellular ROS levels were determined by H2DCFDA staining. The DCF value of U2OS/shR-Ctrl was set as 1. The relative DCF values are expressed as mean ± SEM. *P < 0.01 (n = 3). (c and d) U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were pre-treated with 10 μM SD203580 for 6 h. The cells were then exposed to 200 μM H2O2 for 2 h. (c) WCEs were isolated for Western blot detection of p38 signaling targets. (d) Intracellular ROS levels were determined by H2DCFDA staining. The DCF value of U2OS/shR-Ctrl was set as 1. The relative DCF values are expressed as mean ± SEM. *P < 0.01 (n = 3). (e and f) U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were transfected with pcDNA3 (Ctrl) or pcDNA3/p38-CA constructs and selected with 200 μg/ml hygromycin for 2 weeks for the establishment of stable expression cell lines. Cells were treated with 200 μM H2O2 for 2 h. (e) WCEs were isolated for Western blot detection of p38 signaling targets. (f) Intracellular ROS levels were determined by H2DCFDA staining. The DCF value of U2OS/shR-Ctrl was set as 1. The relative DCF values are expressed as mean ± SEM. *P < 0.01 (n = 3).
Next, we blocked p38 signaling with SB203580, a pyridinyl imidazole that inhibits the kinase activity of p38α and p38β without affecting p38 phosphorylation.35 Administration of SB203580 inhibited the activity of p38 toward its downstream targets and substantially enhanced the accumulation of ROS in both U2OS/shR-Ctrl and U2OS/shR-LKB1 cells (Figures 4c and d). Similarly, inhibition of p38 in MEFs led to the accumulation of ROS (Supplementary Figures 3a and b). In contrast, PD98059 (2′-amino-3′-methoxyflavone), a flavone derivative and selective p44/42 MAPK inhibitor, did not affect LKB1-associated ROS regulation (Supplementary Figure 3c). Together, our results suggest that the integrity of p38 signaling is necessary for LKB1-mediated suppression of ROS.
Ectopic expression of constitutively activated p38α prevents LKB1-deficient cells from the accumulation of ROS
To validate the role of p38 in mediating LKB1-dependent ROS regulation, we established U2OS/shR-Ctrl and U2OS/shR-LKB1 cells with stable expression of pcDNA3 (Ctrl), p38-WT, and a constitutively active form of p38 (p38-CA). Phospho-ATF-2 and Phospho-MAPKAPK-2 were up-regulated in p38-CA and to a lesser extent in p38-WT stable cells regardless of H2O2 stress (Figure 4e and data not shown). ROS levels were substantially reduced in U2OS/shR-Ctrl cells transfected with p38-WT and p38-CA as compared with control vector transfected cells when the cells were exposed to H2O2 (Figure 4f and Supplementary Figure 4). More importantly, the levels of ROS were also markedly reduced by the overexpression of p38-CA and p38-WT in U2OS/shR-LKB1 although ectopic expression of p38-WT showed a relatively lesser effect (Figure 4f and Supplementary Figures 4b and c). Taken together, our data suggest that p38 activation is sufficient to abrogate ROS accumulation resulting from LKB1 deficiency.
LKB1 deficiency promotes DNA oxidation and mutagenesis
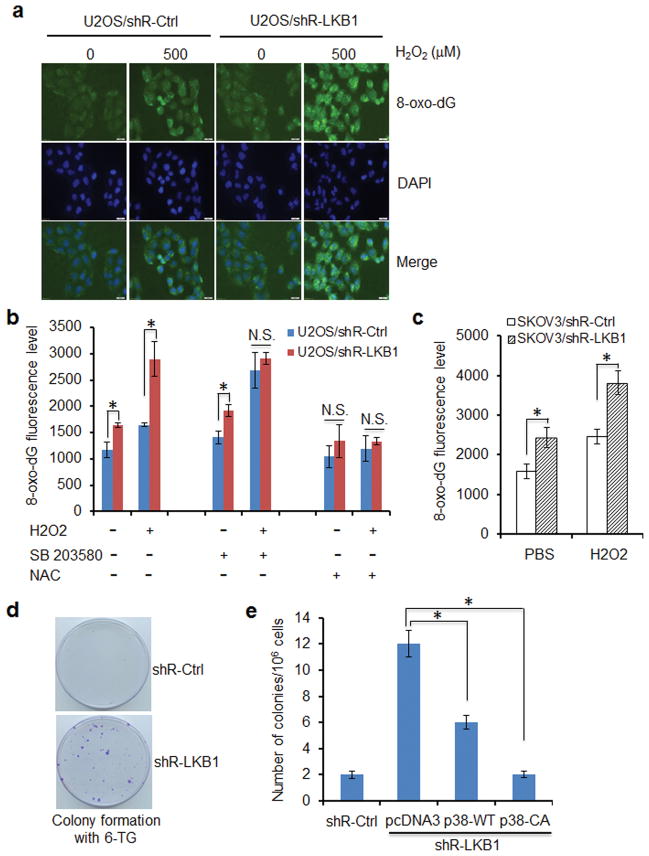
A direct consequence of elevated ROS is oxidative DNA damage and mutagenesis. To determine whether LKB1-mediated reduction in ROS could translate into a protective role against oxidative DNA damage, we monitored the formation of 8-oxoguanine (8-oxo-dG), a major product of DNA oxidation and a source of mutations.25 LKB1 RNAi in U2OS and SKOV3 cells led to a 60–80% increase in 8-oxo-dG as compared with that in control cells (Figures 5a–c). In contrast, transfection of LKB1 reduced cellular DNA oxidation (Supplementary Figure 5a), supporting an antioxidant function of LKB1. Consistently, inhibition of p38 with SB203580 increased levels of 8-oxo-dG in U2OS/shR-Ctrl cells, whereas overexpression of p38-CA and p38-WT or administration of NAC markedly reversed LKB1 deficiency-induced 8-oxo-dG increase (Figure 5b and Supplementary 5b). Taken together, our data suggest that LKB1 protects the cells from oxidative DNA damage through the activation of p38 signaling.
Figure 5.
LKB1 deficiency promotes DNA oxidation and mutagenesis. (a) LKB1 depletion increases 8-oxo-dG. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells growing on fibronectin-coated coverslips were treated with 500 μM H2O2 in serum-free and phenol-red-free medium for 2 h at 37°C, fixed in absolute methanol (− 20°C, 20 minutes) and permeabilized with 0.1% Triton X-100 at room temperature for 15 min. After blocking, the cells were stained with FITC-conjugated avidin for 1 h at 37°C. Fluorescence is captured with an Olympus IX51 fluorescence microscope. (b) Levels of 8-oxo-dG in LKB1-depleted cells treated with p38 inhibitor and ROS scavenger. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells cultured in 96-well plate were pre-treated with 10 μM SB203580 or 5 mM NAC for 2 h before exposure to 500 μM H2O2 for additional 2 h. After the treatment, the cells were fixed in 4 % paraformadehyde and permeabilized with 0.1 % triton X-100 at room temperature for 15 min. The cells were washed with PBS for 3 times and stained with FITC-conjugated avidin for 1 h at 37°C. The supernatants were discarded and 100 ul PBS per well was added for analysis by fluorescence multi-well plate reader with excitation and emission wavelengths of 495 nm and 525 nm. Intensity of fluorescence is expressed as the mean ± SEM. *P < 0.01; N.S., not significant (n = 3). (c) Levels of 8-oxo-dG in SKOV3/shR-Ctrl and SKOV3/shR-LKB1 cells insulted by H2O2. Measurement of 8-oxo-dG was performed as in b. Intensity of fluorescence is expressed as the mean ± SEM. *P < 0.01 (n = 3). (d) Mutations within HPRT locus in U2OS/shR-Ctrl and U2OS/shR-LKB1 cells. One million cells were treated with 1.35 μM 6-TG. Three weeks later, the growth colonies were fixed with 4 % paraformaldehyde and stained with crystal violet. (e) p38 reduces LKB1-deficiency-induced mutation. HPRT assays were performed as in d in U2OS/shR-Ctrl and U2OS/shR-LKB1 cells stably transfected with pcDNA3 (vector), p38-WT, and p38-CA. Mutation frequencies within HPRT locus were calculated. The number of colonies is expressed as the mean ± SEM. *P < 0.01 (n = 3).
Oxidative DNA damage is known to be associated with an increased incidence of mutations. To test the contribution of LKB1-modulated antioxidant mechanism in the protection against mutations, we monitored the changes in mutation frequencies within the hypoxanthine phosphoribosyltransferase (HPRT) gene locus. Mammalian cells bear a single functional copy of the hprt gene that codes for HPRT, an enzyme catalyzes phosphoribosylation in the purine salvage pathway, as well as phosphoribosylation of purine analogues such as 6-thioguanine (6-TG) resulting in cytotoxicity. Only cells with an inactivating hprt gene mutation are able to proliferate in the presence of 6-TG. Thus, resistance to 6-TG indicates an hprt gene mutation.44 U2OS/shR-LKB1 cells formed 6-fold more 6-TG-resistant colonies than U2OS/shR-Ctrl cells did (Figures 5d and e). Over-expression of p38-WT in U2OS/shR-LKB1 cells reduced the number of 6-TG-resistant colonies to about 50% of those in U2OS/shR-LKB1 cells, whereas ectopic expression of p38-CA completely abolished the effect of LKB-depletion (Figure 5e). These results suggest that LKB1 suppresses the mutation phenotype in ROS-treated cells.
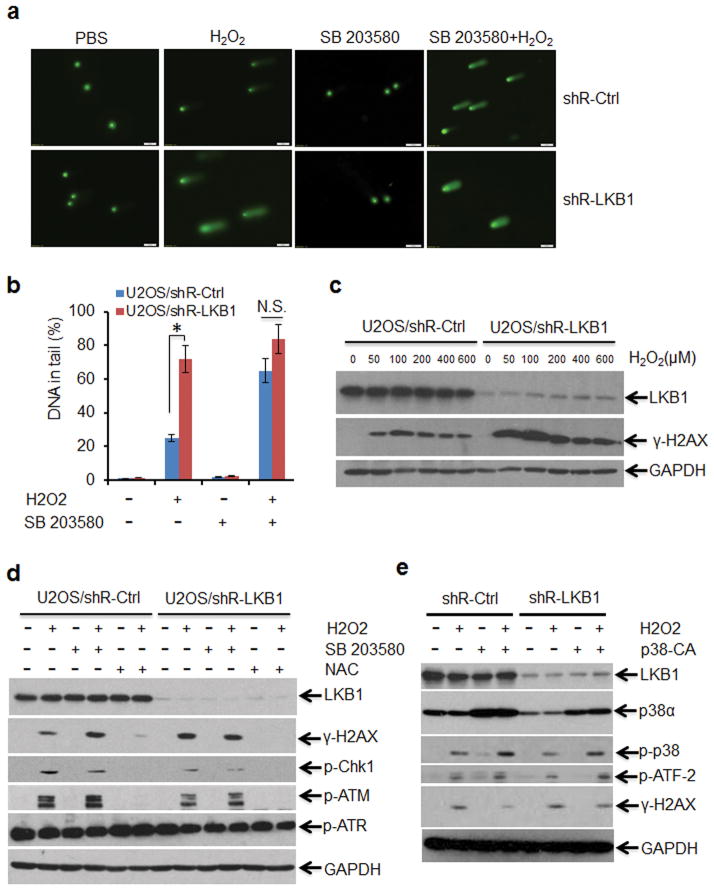
LKB1 deficiency enhances DNA damage induced by ROS
To identify the function of LKB1 in the protection against DNA damage, we exposed LKB1 compromised and intact cells to H2O2 and measured the formation and repair of DNA SSB and alkali-labile sites (mainly abasic sites) by the comet assay. The tail moment that reflects the frequency of breaks was used to quantify DNA damage. In U2OS/shR-LKB1, the level of damage induced by H2O2 was considerably higher than that in U2OS/shR-Ctrl cells (Figures 6a and b). When p38 inhibitor was applied, damaged DNA tail moment was markedly extended in U2OS/shR-ctrl but not U2OS/shR-LKB1 cells. γ-H2AX, another DNA damage marker, was also increased when the cells were treated with 50–600 μM H2O2. γ-H2AX levels were much higher in U2OS/shR-LKB1 cells than in U2OS/shR-Ctrl cells (Figure 6c). Consistent with our findings that p38 mediated the response of LKB1 to ROS, p38 inhibitor enhanced and p38-CA reduced γ-H2AX formation (Figures 6d and e). Additionally, the levels of CHK1 and ATM phosphorylation following ROS treatment were also reduced in LKB1-depleted cells (Figure 6d), suggesting incomplete or delayed DNA-damage repair in addition to the previously reported impaired DNA-damage sensing function in LKB1 deficient cells.20 Taken together, our results suggest that LKB1 protects cells from DNA damage induced by ROS and support the notion that p38 is the mediator in the process.
Figure 6.
LKB1 deficiency enhances ROS-induced DNA damage. (a and b) Comet assay demonstrating elevated DNA damage in LKB1 deficient cells treated with ROS. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were treated with 10 μM SB203580 for 2 h followed by the treatment of H2O2 (500 μM) for 30 min. The cells were then trypsinized and washed with PBS. Two thousand cells were mixed with 100 μl low melting agarose for alkaline comet assay. Cells in the gel were stained and visualized with epifluorescence microscopy (a). (b) Percentage of DNAs in the tail (damaged DNA) was calculated. *P < 0.01; N.S., not significant (n = 3). (c) DNA damage escalates in LKB1 deficient cells. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were treated with different dosages of H2O2 for 1 h. WCEs were isolated and analyzed with Western blot. GAPDH served as loading control. (d) NAC reduces LKB1-deficiency-induced DNA damage. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells were pretreated with 10 μM SB203580 or 5 mM NAC for 2 h, followed by treatment with H2O2 (200 μM) for 1 h. After the treatment, WCEs were isolated for Western blot analysis. GAPDH served as loading control. (e) p38-CA alleviates LKB1-deficiency-induced DNA damage. U2OS/shR-Ctrl and U2OS/shR-LKB1 cells with stable expression of p38-CA were treated with 200 μM H2O2 for 1 h. After the treatment, WCEs were isolated for Western blot analysis. GAPDH served as loading control.
LKB1 deficiency sensitizes cells to ROS treatments
In keeping with our data showing that LKB1 deficiency dramatically enhanced ROS accumulation (Figures 1a–c), we set out to determine whether cancer cells lacking LKB1 might be more vulnerable to ROS-mediated stress response, in particular, to drugs that further enhance intracellular ROS. To test our hypothesis, we exposed control and LKB1 knocked-down U2OS and SKOV3 cells to different dosages of H2O2 and analyzed the cytotoxicity of H2O2. Indeed, depletion of LKB1 markedly sensitized the cells to H2O2, with an IC50 being 12.5 – 25 μM for LKB1 knocked down cells and 50 – 75 μM for LKB1-intact cells (Supplementary Figures 6a and b). To validate the impact of LKB1 status on cellular viability to oxidative stress, we induced LKB1 expression in A549 cells, a cell line lacking endogenous LKB1, and exposed the cells to H2O2. Induction of LKB1 substantially desensitized the cells to H2O2 treatment (Supplementary Figure 6c). Consistent with our previous notion that LKB1 regulated ROS through activation of p38, application of p38 inhibitor, SB203580 enhanced the sensitivity of LKB1-intact cells to H2O2 and eliminated the difference in susceptibility to H2O2 between LKB1 intact and compromised cells (Supplementary Figure 7). In contrast, NAC blocked the cytotoxicity of H2O2 to both cell lines (Supplementary Figure 8). Thus, our data demonstrate that LKB1 deficiency renders the cells more susceptible to ROS stress.
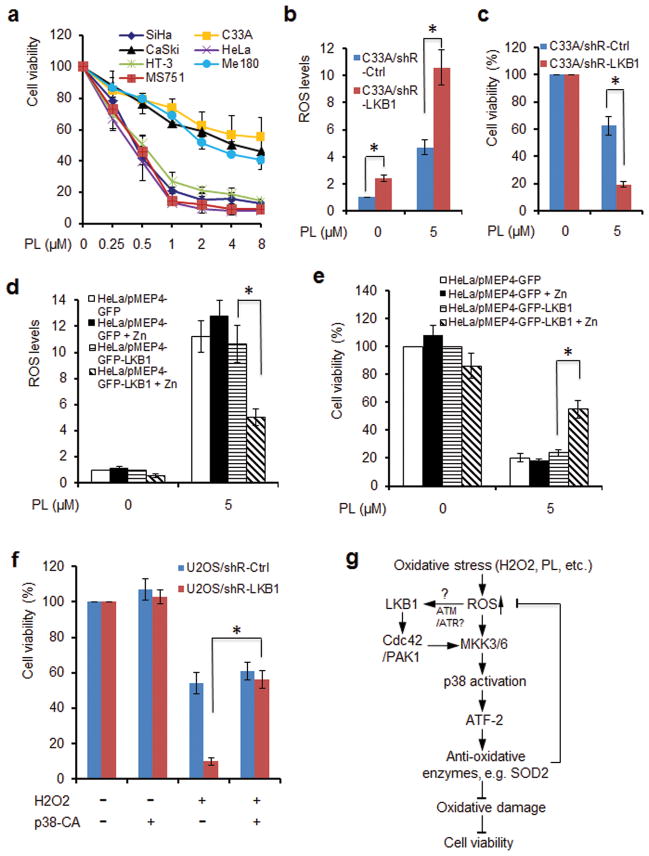
Piperlongumine (PL) is an alkaloid isolated from Piper longum L., which possesses selective anti-cancer properties.45,46 We and several other groups demonstrated that PL treatment dramatically increases intracellular ROS levels, a likely mechanism by which PL exerts its selective antitumor effect.45–47 Thus, we interrogated whether LKB1 deficient cells are more susceptible to PL. We exposed cervical cancer cell lines with or without LKB1 to various dosages of PL. LKB1 deficient HeLa, SiHa, MS751, and HT-3 cells were generally sensitive to PL treatments with IC50s around 0.5 μM following a 3-day exposure. In contrast, LKB1 intact C33-A, CaSki, and ME-180 cells exhibited a relative resistance to the same treatment with IC50s of 4 μM or higher (Figure 7a, Supplementary Figure 9). Thus, our results indicate that LKB1 status may be a predictor for cellular outcomes in response to ROS-related treatments.
Figure 7.
LKB1 deficiency sensitizes cells to ROS treatments. (a) LKB1 deficient cells are highly sensitive to ROS inducer, piperlongumine (PL). LKB1 proficient human cervical cancer cell lines C33A, CaSki, and Me180, and LKB1 deficient cervical cancer cell lines HeLa, SiHa, MS751, and HT-3 were exposed to 0–8 μM PL for 72 h. Cell viability was determined by MTT assay. The value of OD530/620nm in cells without treatment was set as 100%. Data represent average of three independent experiments ± SEM. (b and c) LKB1 depletion elevates intracellular ROS induced by PL and sensitizes the cells to PL. C33A/shR-Ctrl and C33A/shR-LKB1 cells were exposed to 5 μM PL for 72 h. After the treatment, the cells were collected for ROS determination by H2DCFDA staining (b) or cell viability measurement by MTT assay (c). The values for shR-Ctrl cells without treatment were set as “1” or 100%. The values are expressed as the mean ± SEM. *P < 0.01 (n = 3). (d and e) Inducible expression of LKB1 reduces intracellular ROS levels induced by PL and desensitizes the cells to PL. Expression of GFP-LKB1 (GFP) in HeLa/pMEP4-GFP-LKB1 (HeLa/pMEP4-GFP) cells was induced with 100 μM Zn. The cells were then exposed to 5 μM PL for 72 h. After the treatment, the cells were collected for ROS determination by H2DCFDA staining (d) or cell viability measurement by MTT assay (e). The values for control cells without induction and PL treatment were set as “1” or 100%. The values are expressed as the mean ± SEM. *P < 0.01 (n = 3). (f) Establishment of p38-CA and control cell lines was described in Figures 4e and f. The cells were treated with 100 μM H2O2 for 72 h. Cell viability was determined by MTT assay. The value of OD530/620nm in cells without treatment was set as 100%. Data represent average of three independent experiments ± SEM. *P < 0.01. (g) A schematic model for LKB1-mediated cellular response to ROS.
To further validate the specific role of LKB1 in determining the sensitivity to PL, we treated C33A/shR-Ctrl and C33A/shR-LKB1 cells with 5 μM PL. We found that treatment of PL markedly increased intracellular ROS, which were further escalated by the knock-down of LKB1 (Figure 7b). More importantly, depletion of LKB1 strikingly enhanced the cytotoxicity of PL (Figure 7c). To corroborate the findings, we established inducible LKB1 expression in HeLa cells. Consistently, reconstitution of LKB1 reduced intracellular ROS accumulation and conferred a resistance on the cells to PL treatment (Figures 7d and e). Thus, our results demonstrate a crucial role of LKB1 in determining cellular sensitivity to ROS-based treatments.
We then sought to determine whether p38α-CA was able to rescue ROS-induced cell death in LKB1 compromised cells. Indeed, while constitutive expression of p38α-CA significantly increased the viability of LKB1 knocked-down cells treated with H2O2, ectopic expression of p38α-CA had little effect on LKB1-intact cell viability (Figure 7f). These data suggest that the antioxidative role of LKB1 is mediated by p38.
DISCUSSION
We have provided new data showing that LKB1 plays an important role in reducing intracellular ROS in response to oxidant challenge. LKB1 deficiency enhanced oxidative DNA damage and mutations induced by the accumulation of ROS. Ectopic expression of LKB1 reduced ROS and ROS-induced DNA damage and mutation. We further characterized that the protective effect of LKB1 against oxidative stress was mediated by the activation of p38 MAPK and its downstream targets, such as ATF2, SOD2, and catalase. In summary, we have proposed a working model for LKB1-induced activation of p38 and its functional outcome in response to ROS stress (Figure 7g).
ROS are the major sources of DNA damage and are substantial factors contributing to the accumulation of mutations and deletions and chromosome instability, which may lead to cancer. Thus, reduction of ROS may represent a plausible mechanism underlying the tumor suppression function of LKB1. Interestingly, however, our data suggest that the antioxidant effect of LKB1 is independent of AMPK, which was thought to carry out much of the tumor suppressor function of LKB1.8,19,48 Alexander et al reported that in response to elevated ROS, ATM activates TSC2 via the LKB1/AMPK metabolic pathway in the cytoplasm to repress mTORC1 and to induce autophagy.49 This ROS-mediated activation of ATM in the cytoplasm is distinct from the classical pathway for ATM activation via DNA double-strand breaks in the nucleus. Based on previous reports and our studies that stresses, such as H2O2 and CDDP treatment (data not shown), enhance the accumulation of LKB1 in the nucleus and induce the colocalization of LKB1 with ATM, ATR, and other DDR proteins, we suspect that activation of LKB1 and ATM in the nucleus may still be an important pathway in response to ROS in addition to the non-classical cytoplasmic ATM-TSC2 signaling reported.49
Our data suggest that activation of p38 through its upstream kinases was indispensable for the action of LKB1. We characterized that LKB1 was necessary for maintaining the integrity of the cdc42-PAK1 complex, which is an important activator for p38 upstream kinases (MAP3Ks).36–41 A recent report demonstrated that LKB1 colocalizes with cdc42 and PAK1 at the cellular leading edge.42 LKB1 functionality is required for the accurate localization of cdc42, maintaining active cdc42 levels, and downstream PAK phosphorylation.42 Thus, our results and previous report42 clearly indicate that LKB1 is a critical facilitator for the cdc42-PAK signaling and the following response to activate p38 under stress conditions, such as ROS, UV treatment, and inflammation.
It was reported that activated p38 promotes the phosphorylation of ATF-2 and hence enhances the transcription of SOD-2.32 In addition, p38 activation may also stabilize the mRNA of SOD2 and catalase.32, 50 Consistent with these reports, we showed that not only was phospho-p38 reduced, but also the expression of SOD-2 and the activity of catalase and SOD were substantially decreased in LKB1 deficient cells under ROS stress. Thus, current evidence leads us to speculate that LKB1 possesses the antioxidant function most probably through p38 signaling-activated antioxidant enzymes, such as SOD2 and catalase.
Normal cells maintain redox homeostasis with a low level of basal ROS by controlling the balance between ROS generation and elimination. In cancer cells, the increase in ROS generation from metabolic abnormalities and oncogenic signaling may render cancer cells more dependent on the antioxidant system and more vulnerable to further oxidative stress.21 Therefore, increased ROS levels in LKB1 deficient cells may create an “Achilles’ heel” that can be exploited to develop improved, individualized therapies against LKB1 compromised cancers by ROS inducers. Indeed, Shackelford et al 51 recently reported that LKB1-mutant tumors were sensitive to phenformin, a biguanide compound, which is used to treat diabetes. The preferential cytotoxicity of phenformin to Lkb1-deleted tumors was associated with a greater decrease in intracellular ATP, decreased mitochondrial function, and increased intracellular ROS levels, suggesting that ROS can be targeted for antitumor in the absence of LKB1. Consistent with the concept, we showed that LKB1 deficient cells were more sensitive to H2O2 and PL, the latter being a nature compound that produces ROS. Thus, we anticipate that oxidative stress inducers can be an effective means of selectively eradicating LKB1 deficient cells, which comprise more than 20% cervical cancers, 30% non-small cell lung cancer, and many other cancer types.2,3,5,18
MATERIALS AND MATHODS
Cell cultures, gene transfection, and viability assays
The U2OS, SKOV3, HeLa and MEF cells were cultured in DMEM containing 10% fetal bovine serum. LKB1 knock-down with shRNA and control cells were reported previously.52 WT and AMPK-null MEFs were obtained from Dr. Laderoute.16 Gene transfection was performed with Fugene 6 (Roche Diagnostics Corp., Indianapolis, IN.) in accordance with the manufacturer’s instructions. Cell viability was determined by 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay.52
Plasmids and establishment of over-expression stable cell lines
The GFP-LKB1, pMEP4/LKB1, and shR-LKB1 plasmids were constructed as described previously.52 TetR-null and TetR-LKB1 lentiviral expression system was purchased from GenTarget (San Diego, CA). The pcDNA3.1/Hygro(+)-p38α WT, CA, and DN constructs originally from Dr. Mousseau 43 were transfected to U2OS/shR-Ctrl and U2OS/shR-LKB1 cells and selected with 200 μg/ml hygromycin for 2 weeks. Hygromycin-resistant stable clones were pooled and passaged.
Antibodies and chemicals
Antibodies against phospho- and/or total ATM, ATR, CHK1, Histone H2AX, HSP27, JNK/SAPK, MKK3/MKK6, MAPKAPK-2, MSK1, p38α, p44/42, and PAK1 were purchased from Cell Signaling Technology, Inc. (Boston, MA). Anti-cdc42 antibody was purchased from Cytoskeleton, Inc. (Denver, CO). Anti-LKB1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-α-tubulin monoclonal antibody, SB203580, PD98059, and 6-TG were purchased from Sigma Aldrich (St. Louis, MO).
RNAi and Western blot
Four pairs of small interfering RNAs (siRNAs) targeting human or mouse LKB1 and a non-targeting siRNA pool were purchased from Dharmacon Inc. (Lafayette, CO). 50 nM of siRNAs were used per well in a six-well plate. The method for Western blot was reported previously.43
Detection of intracellular ROS
Cells were treated with H2O2 for various durations. One hour prior to the termination of the treatment, 100 ng/ml dihydroethidium was added to the medium. The cells were harvested, washed, and analyzed by flow cytometry with the red laser channel (FL-3) using a FACscan analyzer.29,47 ROS were also determined using a 96-well plate-based intracellular ROS assay as we previously reported.53
Measurement of antioxidant enzymes
Catalase activities were determined by spectrophotometric method as described by Slaughter and O’Brien.54 SOD activities were estimated as per method described by Madesh and Balasubramanian.55
Detection of 8-oxo-dG
Cells were treated with 500 μM H2O2 in serum-free and phenol-red-free medium for 2 h at 37°C, fixed in absolute methanol (− 20°C, 20 minutes) and permeabilized with 0.1% Triton X-100 at room temperature for 15 min. The cells were washed and stained with FITC-conjugated avidin for 1 h. Fluorescence was measured using a microplate reader or analyzed with Olympus IX51 fluorescence microscope.25
HPRT assay
The mutation frequency within the HPRT locus was determined by counting 6-TG–resistant colonies.44 Cells were pre-selected in medium containing hypoxanthine, aminopterine and thymidine (HAT; Sigma) for 72 h, and grown exponentially for 1–2 weeks to allow mutations to accumulate. After the treatment, 1×106 cells were seeded onto a 100 mm2-plate with medium containing 1.35 μM of 6-TG. The number of colonies was counted after 3 weeks.
Alkaline comet assay
We followed the method for alkaline comet assay that we reported previously.56 The cells were treated and run in alkaline buffer for 20 min, fixed, stained, and examined under the fluorescence microscope.
Statistical analysis
The Student t-test was used for the statistical analyses between two groups unless where it is specified. P < 0.05 was considered as statistically significant.
Supplementary Material
Figure S1. LKB1 modulates ROS independent of AMPK.
Figure S2. Antioxidant NAC alleviates LKB1-mediated activation of p38.
Figure S3. Inhibition of p38, but not p44/42, increases ROS in MEFs.
Figure S4. Overexpression of p38 prevents LKB1-deficiency-induced ROS.
Figure S5. Ectopic expression of LKB1 or p38 reduces ROS-induced oxidative DNA damage.
Figure S6. LKB1 deficiency sensitizes cells to ROS treatments.
Figure S7. Suppression of p38 sensitizes LKB1-intact cells to H2O2 and eliminates the difference in the susceptibility to H2O2 between LKB1 intact and compromised cells.
Figure S8. Antioxidant N-acetylcysteine (NAC) blocks the cytotoxicity of H2O2 to LKB1-compromised cells.
Figure S9. LKB1 deficient cells are sensitive to ROS inducer, piperlongumine (PL).
Acknowledgments
We thank Dr. Mousseau at University of Saskatchewan for providing p38-WT, CA, and DN constructs; Dr. Laderoute at SRI International for providing WT and AMPK-null MEFs, Dr. Frazier at University of Texas M.D. Cancer Center for providing the LKB1-null cells; Dr. Lizhong Wang from University of Alabama at Birmingham for providing WT and LKB1fl/fl MEFs. This work was supported by grants from National Cancer Institute R01CA133053 (Z.X.X) and the National 863 Program #2004AA205020 and the National Natural Science Foundation of China #30700872 (YLL).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)”
References
- 1.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 4.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 5.Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Y, Lin S, Li JL, Nakagawa H, Chen Z, Jin B, et al. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene. 2012;31:469–79. doi: 10.1038/onc.2011.247. [DOI] [PubMed] [Google Scholar]
- 7.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 8.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurumurthy S, Xie SZ, Alagesan B, Kim J, Yusuf RZ, Saez B, et al. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468:659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 18.Zhao RX, Xu ZX. Targeting the LKB1 tumor suppressor. Curr Drug Targets. 2014;15:32–52. doi: 10.2174/1389450114666140106095811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Ui A, Ogiwara H, Nakajima S, Kanno S, Watanabe R, Harata M, et al. Possible involvement of LKB1-AMPK signaling in non-homologous end joining. Oncogene. 2014;33:1640–1648. doi: 10.1038/onc.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 22.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 24.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero J, Dutta C, van Bodegom D, Weinstock D, Letai A. p53 regulates a non-apoptotic death induced by ROS. Cell Death Differ. 2013;20:1465–1474. doi: 10.1038/cdd.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez AI, Fernandez P, Dominguez O, Dopazo A, Sanchez-Cespedes M. Growth and molecular profile of lung cancer cells expressing ectopic LKB1: down-regulation of the phosphatidylinositol 3′-phosphate kinase/PTEN pathway. Cancer Res. 2003;63:1382–1388. [PubMed] [Google Scholar]
- 28.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 30.Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2010;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez-Uzquiza Á, Arechederra M, Bragado P, Aguirre-Ghiso JA, Porras A. p38 alpha mediates cell survival in response to oxidative stress via induction of antioxidant genes: effect on the p70S6K pathway. J Biol Chem. 2012;287:2632–2642. doi: 10.1074/jbc.M111.323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-Signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY) 2010;2:597–611. doi: 10.18632/aging.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signaling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 36.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Research. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, et al. Rho family GTPases regulate p38 MAP kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 38.Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 39.Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR, et al. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 40.Martin GA, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Schafer-Hales K, Khuri FR, Zhou W, Vertino PM, Marcus AI. The Tumor Suppressor LKB1 Regulates Lung Cancer Cell Polarity by Mediating cdc42 Recruitment and Activity. Cancer Res. 2008;68:740–748. doi: 10.1158/0008-5472.CAN-07-2989. [DOI] [PubMed] [Google Scholar]
- 43.Cao X, Rui L, Pennington PR, Chlan-Fourney J, Jiang Z, Wei Z, et al. Serine 209 resides within a putative p38(MAPK) consensus motif and regulates monoamine oxidase-A activity. J Neurochem. 2009;111:101–110. doi: 10.1111/j.1471-4159.2009.06300.x. [DOI] [PubMed] [Google Scholar]
- 44.Archer H, Bar-Sagi D. Ras and Rac as activators of reactive oxygen species (ROS) Methods Mol Biol. 2002;189:67–73. doi: 10.1385/1-59259-281-3:067. [DOI] [PubMed] [Google Scholar]
- 45.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Adams DJ, Dai M, Pellegrino G, Wagner BK, Stern AM, Shamji AF, et al. Synthesis, cellular evaluation, and mechanism of action of piperlongumine analogs. Proc Natl Acad Sci USA. 2012;109:15115–15120. doi: 10.1073/pnas.1212802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Wang JW, Xiao X, Shan Y, Xue B, Jiang G, et al. Piperlongumine induces autophagy by targeting p38 signaling. Cell Death Dis. 2013;4:e824. doi: 10.1038/cddis.2013.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci USA. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen P, Chakraborty PK, Raha S. p38 mitogen-activated protein kinase (p38MAPK) upregulates catalase levels in response to low dose H2O2 treatment through enhancement of mRNA stability. FEBS Lett. 2005;579:4402–4406. doi: 10.1016/j.febslet.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 51.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, et al. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol. 2012;127:249–255. doi: 10.1016/j.ygyno.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan J, She M, Xu ZX, Sun L, Yeung SC. Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005;65:3671–3681. doi: 10.1158/0008-5472.CAN-04-2744. [DOI] [PubMed] [Google Scholar]
- 54.Slaughter MR, O’Brien PJ. Fully-automated spectrophotometric method for measurement of antioxidant activity of catalase. Clin Biochem. 2000;33:525–534. doi: 10.1016/s0009-9120(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 55.Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- 56.Jian W, Xu HG, Chen J, Xu ZX, Levitt JM, Stanley JA, et al. Activity of CEP-9722, a poly (ADP-ribose) polymerase inhibitor, in urothelial carcinoma correlates inversely with homologous recombination repair response to DNA damage. Anticancer Drugs. 2014 doi: 10.1097/CAD.0000000000000114. e-pub ahead of print 7 April 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. LKB1 modulates ROS independent of AMPK.
Figure S2. Antioxidant NAC alleviates LKB1-mediated activation of p38.
Figure S3. Inhibition of p38, but not p44/42, increases ROS in MEFs.
Figure S4. Overexpression of p38 prevents LKB1-deficiency-induced ROS.
Figure S5. Ectopic expression of LKB1 or p38 reduces ROS-induced oxidative DNA damage.
Figure S6. LKB1 deficiency sensitizes cells to ROS treatments.
Figure S7. Suppression of p38 sensitizes LKB1-intact cells to H2O2 and eliminates the difference in the susceptibility to H2O2 between LKB1 intact and compromised cells.
Figure S8. Antioxidant N-acetylcysteine (NAC) blocks the cytotoxicity of H2O2 to LKB1-compromised cells.
Figure S9. LKB1 deficient cells are sensitive to ROS inducer, piperlongumine (PL).