Abstract
Background
The aim of our study was to evaluate the relevance of magnesium and FGF-23 in terms of cardiovascular disease in a population of type 2 diabetic patients with nephropathy.
Methods
In a cross-sectional study, we included 80 type 2 diabetic patients with chronic kidney disease (CKD) stages 2, 3 and 4. We analysed mineral metabolism, inflammation, oxidative stress and insulin resistance. Our population was divided into two groups according to their pulse pressure (PP) as follows: G-1 with PP < 50 mmHg (n = 34) and G-2 with PP ≥ 50 mmHg (n = 46).
Results
We found that G-2 patients showed lower calcium (P = 0.004), eGFR (P = 0.001), magnesium (P = 0.0001), osteocalcin (P = 0.0001) and 25(OH)D3 (P = 0.001), and higher iPTH (P = 0.001), FGF-23 (P = 0.0001), malonaldehyde (P = 0.0001), interleukin 6 (P = 0.001) and HOMA-IR (P = 0.033). No differences were found between the two groups regarding age, duration of disease, haemoglobin, HgA1c and phosphorus. In a multivariate analysis, we found that FGF-23 and magnesium independently influenced the PP [OR = 1.239 (1.001–2.082), P = 0.039 and OR = 0.550 (0.305–0.727), P = 0.016, respectively].
Conclusions
In our diabetic population with early stages of CKD, FGF-23 as well as lower magnesium levels were significantly and independently associated with higher PP levels, an established marker of cardiovascular morbidity and mortality.
Keywords: diabetes, FGF-23, magnesium, pre-dialysis, pulse pressure
Introduction
Cardiovascular disease (CVD) is the leading cause of death in chronic kidney disease (CKD) patients with a higher incidence than in the general population [1–7], even after adjusting for age and diabetic status [1, 8]. This increased cardiovascular morbidity and mortality progressively increases with the decline of the glomerular filtration rate (GFR) [8]. Nontraditional risk factors like disturbances of the mineral metabolism play a critical role in these adverse outcomes [3, 7, 8]. Large artery stiffness, secondary to medial calcification due to these disturbances in mineral metabolism, is the main determinant of pulse pressure (PP) [9–13].
Hypomagnesaemia is probably the most frequently undiagnosed electrolyte deficiency. Some reports indicate that there is a significant inverse correlation between magnesium levels, metabolic dysfunctions and CVD [12, 14–16] as well as mortality in haemodialysis patients [17].
Fibroblast growth factor-23 (FGF-23), secreted by osteocytes and osteoblasts, is also a major regulator of mineral metabolism in health and disease [2, 18, 19]. Emerging evidence from observational studies in the CKD population suggests a strong association between FGF-23 levels and cardiovascular events [8].
The aim of our study was to evaluate the relevance of magnesium and FGF-23 in terms of CVD in a population of type 2 diabetic patients with nephropathy.
Materials and methods
Study population
In a cross-sectional study, we included 80 type 2 diabetic patients, recruited between 2007 and 2011 with a diagnosis of diabetic nephropathy (stages 2–4) in a stable clinical condition attending our outpatient clinic. CKD stages were defined by the estimated glomerular filtration rate (eGFR) calculated with the modification of diet in renal disease (MDRD) formula at the time of the assessment. Exclusion criteria were previous CVD, uncontrolled hypertension, magnesium therapy, eGFR of <15 mL/min/1.73 m2 and neoplastic or infectious diseases.
The study was approved by the local Ethnics Committee, and written informed consent was obtained from each participant. The study was conducted according to the principles of the Declaration of Helsinki.
Blood measurements
Several laboratory parameters were determined using a standard methodology in routine blood samples drawn after an overnight fast. We analysed haemoglobin (Hg), glycosylate haemoglobin (HgA1c) and eGFR according to the MDRD formula. We also analysed markers of mineral metabolism including intact parathormone (iPTH), 25-hydroxyvitamin D3 [25(OH)D3], FGF-23, osteocalcin, phosphorus, calcium and magnesium. Inflammation (interleukin 6—IL6), oxidative stress (malonaldehyde) and insulin resistance (HOMA-IR) were also evaluated. FGF-23 serum levels were quantified using an enzyme-linked immunosorbent assay, Human FGF-23 (C-Term) ELISA kit, according to the manufacturer's instructions, adapted to the Triturus automatic microplate apparatus. Results were calculated using the apparatus curve-fitting software in 4 parameter logistics mode.
Definitions
Brachial blood pressure was measured by a trained technician using a validated oscillometric device at the clinic visit after the patient had rested for at least 5 min in a seated position. PP was calculated as the difference between the systolic blood pressure (SBP) and the diastolic blood pressure (DBP). Subjects were classified into two groups accordingly to the mean calculated PP: G-1 (PP < 50 mmHg, n = 46) and G-2 (PP ≥ 50 mmHg, n = 34).
Statistical analyses
Statistical analysis was performed with SPSS 17.0 for Windows. Descriptive statistics, Student's t-test and logistic regression were used. For comparison between CKD stages, we used ANOVA and a post hoc analysis with Scheffe test. The association between magnesium and other markers of mineral metabolism and HOMA-IR was evaluated with Pearson's correlation test. Continuous variables were presented as mean ± standard deviations. All tests were two-sided, and a P-value of <0.05 was considered significant.
Results
The clinical characteristics of the population (n = 80) are provided in Table 1. Age ranged from 40 to 85 years with a mean age of 65.8 years. CKD stage 2 represents 35% of the patients, stage 3 represents 45% and stage 4 the remaining. Estimated GFR ranged from 16 to 88 mL/min/1.73 m2.
Table 1.
Baseline patient characteristics (n = 80)
| Characteristics | Values |
|---|---|
| Age (years) | 65.8 ± 9.2 |
| Gender (male/female) | 51/29 |
| Duration of disease (years) | 13.6 ± 4.9 |
| eGFR (mL/min/1.73 m2) | 49.8 ± 19.5 |
| SBP (mmHg) | 129.1 ± 12.1 |
| DBP (mmHg) | 78.8 ± 10.3 |
| Hg, g/L (g/dL) | 129 ± 15 (12.9 ± 1.5) |
| HgA1c, % | 7.1 ± 1.3 |
| Calcium, mmol/L (mg/dL) | 2.35 ± 0.175 (9.4 ± 0.7) |
| Phosphorus, mmol/L (mg/dL) | 1.16 ± 0.190 (3.6 ± 0.6) |
| Magnesium,, mmol/L (mg/dL) | 1.1 ± 0.25 (2.2 ± 0.5) |
| 25(OH)D3, nmol/L (ng/mL) | 48.67 ± 24.96 (19.5 ± 10.0) |
| iPTH, ng/L (pg/mL) | 13.489 ± 8.78 (128.1 ± 83.4) |
| FGF-23, RU/mL | 137.2 ± 88.4 |
| Osteocalcin, nmol/L (µg/L) | 10.9 ± 8.5 (10.9 ± 8.5) |
| Malonaldehyde, µmol/L (nmol/mL) | 3.8 ± 1.2 (3.8 ± 1.2) |
| IL6, pg/mL | 6.2 ± 2.8 |
| HOMA-IR | 3.5 ± 2.5 |
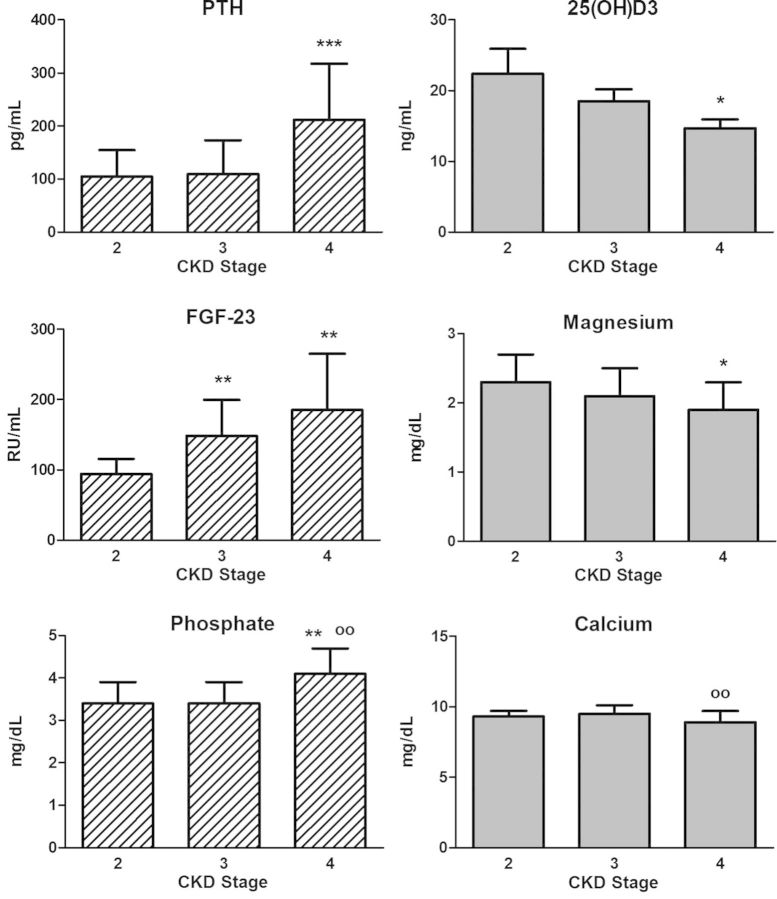
In Figure 1, mean values of osteomineral parameters according to the stage of renal disease are listed. iPTH, phosphorus and FGF-23 increase with inclining CKD stages, whereas magnesium and vitamin D decrease.
Fig. 1.
Parameters of mineral metabolism according to CKD stages. Most patients (65%) were in stages 3 and 4 of chronic kidney disease (stage 2, n = 28; stage 3, n = 36 and stage 4, n = 16). *: versus stage 2, P < 0.05; **: versus stage 2, P < 0.01; ***: versus stage 2, P < 0.001; oo: versus stage 3, P < 0.01
Subjects were classified into two groups according to the calculated PP: G-1 (PP < 50 mmHg, n = 46) and G-2 (PP ≥ 50 mmHg, n = 34). Table 2 illustrates the differences between these two groups. G-2 patients displayed lower values for calcium (P = 0.004), GFR (P = 0.001), magnesium (P = 0.0001), osteocalcin (P = 0.0001) and 25(OH)D3 (P = 0.001), and higher values for iPTH (P = 0.001), FGF23 (0.0001), malonaldehyde (P = 0.0001), interleukin 6 (P = 0.001) and HOMA-IR (P = 0.033). No differences were found between the two groups regarding age, duration of disease, haemoglobin, HgA1c and phosphorus.
Table 2.
Differences between groups
| G-1 (PP < 50 mmHg) n = 46 | G-2 (PP ≥ 50 mmHg) n = 34 | P | |
|---|---|---|---|
| Age (years) | 64.7 ± 9.8 | 67.1 ± 8.2 | ns |
| Gender (male) | 60.9% | 67.6% | ns |
| Disease time (years) | 13.3 ± 4.7 | 14.0 ± 5.3 | ns |
| Hg, g/L (g/dL) | 132 ± 14 (13.2 ± 1.4) | 125 ± 14 (12.5 ± 1.4) | 0.037 |
| HgA1c, % | 6.9 ± 0.6 | 7.4 ± 1.7 | ns |
| Calcium, mmol/L (mg/dL) | 2.4 ± 0.150 (9.6 ± 0.6) | 2.275 ± 0.175 (9.1 ± 0.7) | 0.004 |
| Phosphorus, mmol/L (mg/dL) | 1.131 ± 0.162 (3.5 ± 0.5) | 1.163 ± 0.258 (3.6 ± 0.8) | ns |
| iPTH, ng/L (pg/mL) | 10.825 ± 6.297 (102.8 ± 59.8) | 17.111 ± 10.351 (162.5 ± 98.3) | 0.001 |
| eGFR, mL/min/1.73 m2 | 55.8 ± 17.1 | 41.6 ± 19.7 | 0.001 |
| FGF-23, RU/mL | 80.3 ± 22.0 | 214.3 ± 85.9 | 0.0001 |
| Magnesium, mmol/L (mg/dL) | 1.250 ± 0.15 (2.5 ± 0.3) | 0.9 ± 0.15 (1.8 ± 0.3) | 0.0001 |
| Osteocalcin, nmol/L (µg/L) | 15.6 ± 6.8 (15.6 ± 6.8) | 4.5 ± 5.9 (4.5 ± 5.9) | 0.0001 |
| 25(OH)D3, nmol/L (ng/mL) | 61.651 ± 22.214 (24.7 ± 8.9) | 31.2 ± 16.476 (12.5 ± 6.6) | 0.0001 |
| IL-6, pg/mL | 5.3 ± 2.5 | 7.5 ± 2.8 | 0.001 |
| Malonaldehyde, µmol/L (nmol/mL) | 3.3 ± 0.9 (3.3 ± 0.9) | 4.5 ± 1.2 (4.5 ± 1.2) | 0.0001 |
| HOMA-IR | 3.0 ± 2.7 | 4.2 ± 2.1 | 0.033 |
| Total cholesterol, mmol/L (mg/dL) | 4.947 ± 1.0256 (191.0 ± 39.6) | 5.1101 ± 1.086 (197.3 ± 40.1) | ns |
| HDL, mmol/L (mg/dL) | 1.494 ± 0.658 (52.1 ± 25.4) | 1.354 ± 0.619 (52.3 ± 23.9) | ns |
| Triglycerides, mmol/L (mg/dL) | 1.603 ± 0.7526 (141.9 ± 66.6) | 1.651 ± 0.758 (146.1 ± 67.1) | ns |
A model of logistic regression analysis (Table 3) revealed that FGF-23 and magnesium independently influenced PP [OR = 1.239 (1.001–2.082), P = 0.039 and OR = 0.550 (0.305–0.727), P = 0.016, respectively].
Table 3.
Logistic regression model—predictors of PP ≥50 mmHg
| Adjusted OR (95% CI) | P-Value | |
|---|---|---|
| Age | 1.099 (1.000–1.112) | 0.62 |
| Sex | 0.344 (0.035–3.358) | 0.358 |
| eGFR | 1.009 (0.954–1.067) | 0.78 |
| iPTH | 1.005 (0.990–1.021) | 0.52 |
| Phosphorus | 0.595 (0.114–3.101) | 0.538 |
| FGF-23 | 1.239 (1.001–2.082) | 0.039 |
| Osteocalcin | 1.221 (0.954–1.562) | 0.112 |
| Magnesium | 0.550 (0.305–0.727) | 0.016 |
| 25(OH)D3 | 0.987 (0.861–1.130) | 0.846 |
| Malonaldehyde | 1.009 (0.999–3.099) | 0.852 |
| Il-6 | 1.010 (0.899–1.623) | 0.991 |
| HOMA-IR | 0.991 (0.897–1.843) | 0.385 |
Pearson analysis showed that magnesium was negatively correlated with PTH (R = −0.398, P = 0.0001), FGF-23 (R = −0.814, P = 0.0001), calcium (R = −0.248, P = 0.026) and HOMA-IR (R = −0.319, P = 0.003), but positively correlated with 25(OH)D3 (R = 0.690, P = 0.0001). No correlation was found with phosphorus.
Discussion
CVD is the leading cause of mortality in CKD patients [2–5]. Although traditional risk factors of CVD (hypertension, older age, hyperlipidaemia and diabetes) are highly prevalent in this population, they do not explain the severity and extent of this particular association. Other risk factors, related to abnormal mineral metabolism, endothelial dysfunction and inflammation, also play an important role in the pathogenesis of CVD [3, 7, 8]. Disturbances of the mineral metabolism are associated with increased arterial stiffness, left ventricular hypertrophy and vascular calcification, which are considered as independent risk factors for CVD in both pre-dialysis and dialysis patients [4, 5, 8, 20]. CKD patients develop extensive medial arterial calcification, which causes increased arterial stiffness of the large elastic arteries resulting in a widening of PP, which is ‘per se’—as pointed out before—an independent predictor of cardiovascular mortality.
Several factors are causative for the calcified vasculature in dialysis patients, such as dialysis vintage, uraemic toxins, diabetes and inflammation, but abnormalities in bone mineral metabolism are considered to play a central role [6, 8, 13]. Recently, it was reported that magnesium and FGF-23 are also associated with increased vascular calcification in dialysis patients [1, 3, 20].
In this study, where only pre-dialysis diabetic CKD patients were assessed, lower serum magnesium levels and higher FGF-23 levels were significantly and independently associated with increased PP after adjusting for possible confounding factors. These findings suggest that these disturbances of mineral metabolism are linked to vascular stiffness and consequently can be considered as predictors of cardiovascular events [9–12, 14].
Other studies used central pulse wave velocity, the current gold standard [13], to measure vascular stiffness [9, 10, 14, 21–23] rather than PP, a simpler and less expensive technique [11]. As in the prospective study of Beigel et al., which analyses PP, endothelial function and CVD [22], the subjects were classified into two groups according to their mean calculated PP (threshold of 50 mmHg). Other epidemiologic studies showed that PP is associated with significant cardiovascular risk at values of >60 mmHg [24] or 70 mmHg [11]. Dividing the population by PP values, no differences with respect to age, haemoglobin, glycaemic control (HgA1c) and lipid profile were observed. However, G-2 showed a worse renal function, which is by itself considered as a cause of increased cardiovascular morbidity and mortality [8].
Magnesium could protect against vascular calcification via multiple molecular mechanisms, and decreased serum levels of magnesium are associated with vascular calcification, as revealed in both humans and in animal studies [1, 25–27].
Van Laecke and colleagues have reported that hypomagnesaemia is related to hypertension, endothelial dysfunction, dyslipidaemia and inflammation and is also associated with an increase in pulse wave velocity [22]. Experimental findings on magnesium-deficient, spontaneously hypertensive rats of the Munster strain revealed a high brachial PP and abnormal elastic material in the aortic wall [9]. It was also shown that magnesium deficiency is significantly and independently associated with greater intima-media thickness [1], suggesting that hypomagnesaemia alters vascular mechanical properties [1, 14, 28]. The Paris Prospective Study found that decreased serum magnesium was associated with cardiovascular and all-cause mortality [29], and the Atherosclerosis Risk in Communities (ARIC) study, a multicenter, prospective cohort study, showed an inverse association between serum magnesium and the risk for coronary heart disease [1, 29–31]. Overall, these observational data suggest that magnesium may play an important role in the development and/or acceleration of arterial atherosclerosis and vascular calcification both in patients with CKD and in the general population [1, 28].
In contrast, Khan and colleagues examined 3531 participants in the Framingham Heart Study offspring cohort and found no association between baseline serum magnesium levels and the development of hypertension, CVD or all-cause mortality [29]. In the National Health and Nutritional Examination Survey Epidemiologic Follow-up Study, there was no significant association between serum magnesium and incidence of CVD, although serum magnesium was inversely associated with all-cause mortality from ischemic heart disease [29].
There are other conflicting results [29] of studies evaluating the relationship between magnesium supplementation and endothelial function, arterial stiffness, carotid structure [14] and blood pressure [14, 32]. Some reports point towards a beneficial effect of magnesium supplementation with respect to improving endothelial function [14], vascular calcification [1], insulin sensitivity and lowering blood pressure [14]. Other authors failed to show a significant association between magnesium intake and blood pressure reduction [14, 32, 33] or CVD [33]. Magnesium supplementation is not indicated as a part of an antihypertensive treatment [14] as this supplementation has not produced consistent results in hypertensive patients [14, 32].
Our study evaluated also the association between magnesium and other markers of mineral metabolism, using a Pearson correlation test. A negative correlation with PTH was found, confirming previous findings that magnesium influences PTH secretion [34, 35].
To the best of our knowledge, the present study is the first to assess only diabetic subjects with mild-to-moderate CKD. Several studies reported significant association between hypomagnesaemia and diabetes [15, 28, 30]. The ARIC study revealed a significant higher prevalence of diabetes in patients with lower serum magnesium levels [28]. Although many authors suggest that diabetes ‘per se’ may induce hypomagnesaemia, others suggest that decreased levels of magnesium may affect glycaemic control. It is believed that an impairment of magnesium homeostasis may favour the onset and progression of diabetic complications [15], and some studies reported that higher magnesium intake may improve diabetic control [30]. In fact, using a Pearson correlation analysis, we found that magnesium was negatively correlated with HOMA-IR (R = −0.319, P = 0.003).
Applying a multivariate logistic regression model adjusted to age, sex, eGFR, iPTH, phosphorus, FGF-23, osteocalcin, 25(OH)D3, malonaldehyde, IL-6 and HOMA-IR (Table 3), the present study demonstrates that decreased levels of magnesium independently predict PP of ≥50 mmHg. Clinical and experimental investigations have revealed that hypomagnesaemia accelerates atherosclerosis by promoting the elevation of inflammatory cytokines, lipid oxidation and the inhibition of endothelial cell growth [22, 28]. In line with this, patients grouped into G-2 in the present study indeed had lower levels of serum magnesium and higher levels of inflammatory and oxidative stress parameters.
FGF-23 is secreted by osteocytes and osteoblasts in response to dietary phosphate intake and 1,25(OH)2D3 [8, 36, 37], and their levels are progressively increased with the deterioration of the renal function [5, 8]. The main known physiological role of FGF-23 is to regulate urinary phosphate excretion by suppressing the expression of type IIa sodium phosphate co-transporter, thus regulating phosphate reabsorption in the renal proximal tubule. FGF-23 also appears to impair the synthesis and to accelerate the degradation of 1,25(OH)2D3. This rise of FGF-23 levels is detected during the early stages of CKD (2–3) with even modest decreases in GFR, as noted in the population assessed in this study. As a result, serum phosphate levels are maintained within the normal range up until late stages of CKD when estimated GFR falls below 25–30 mL/min [3, 8, 20, 36, 37]. Recent data suggest that FGF-23 directly inhibits the expression and secretion of PTH but by accentuating 1,25(OH)2D3, deficiency may also contribute to the development of secondary hyperparathyroidism [8, 20, 37].
Several studies documented that high levels of FGF-23 are an independent marker of cardiovascular and all-cause mortality [3–5, 8, 18, 37]. Moreover, FGF-23 levels were better predictors than serum phosphate levels in some studies [4] although, in other studies, FGF-23 levels did not demonstrate additional prognostic information when phosphate levels were introduced in the analysis [5]. In fact, in the present population with mild-to-moderate CKD, G-2 showed phosphorus levels within the normal range and elevated FGF-23 levels. Seiler et al. [4] were the first to report increased cardiovascular events in pre-dialysis CKD patients with elevated FGF-23 [4, 5]. Although the rise of FGF-23 levels in early stages of CKD is an appropriate mechanism to maintain normal phosphorus levels, it is associated with worse outcome [4]. Several studies involving dialysis and pre-dialysis CKD patients also suggest an association between increased FGF-23 levels and vascular calcification [3, 8, 19, 20, 37]. Larsson found that higher FGF-23 was related to arterial stiffness measured by pulse wave velocity as well as to endothelial dysfunction measured by an invasive forearm technique [36]. These findings were confirmed in another cohort study with early stage CKD patients, where a subset of these patients displayed a greater atherosclerotic burden measured by whole-body magnetic resonance angiography [5].
The literature supports that FGF-23 is linked to early changes in vascular function predisposing to an increased cardiovascular risk [36]. Although FGF-23 has been associated with vascular calcification, few studies reported this relationship [2, 19, 36], possibly due to disparity in diagnostic techniques and difficulties in standardizing the quantification of vascular calcification [5, 36].
The present study also confirms the relationship between FGF-23 and arterial stiffness using PP, a simple but clinical relevant marker of vascular stiffness. The inverse relationship between 1,25(OH)2D3 and FGF-23 is well known, and low vitamin D status is associated with cardiovascular disorders [18], reflected by the lower vitamin D levels in the G-2 patients with higher PP.
Notwithstanding, the present study has several limitations. First, this is a cross-sectional study with a relatively small population. Second, blood pressure and laboratory parameters were measured at baseline, not accounting for natural variation that may occur over time [29]. Third, dietary information was not available at the point of examination, and consequently, it was not possible to correlate dietary intake with serum magnesium levels. Last, pharmacological medication was not included in the analysis and some common antihypertensive drugs interfere with magnesium homeostasis.
In conclusion, in a population of diabetic pre-dialysis patients, magnesium and FGF-23 levels are independently associated with PP. Further studies with more patients are warranted to confirm whether an increase in magnesium and a decrease in FGF-23 would reduce the PP and consequently the cardiovascular risk of our patients.
Conflict of interest statement
None declared.
Acknowledgement
We are indebted to Prof. Volker Schoder for his statistical advise.
References
- 1.Massy ZA, Drueke TB. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J. 2012;5(Suppl 1)):i52–i61. doi: 10.1093/ndtplus/sfr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkpantur A, Balci M, Gurbuz OA, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. 2011;26:1346–1354. doi: 10.1093/ndt/gfq539. [DOI] [PubMed] [Google Scholar]
- 3.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiler S, Reichart B, Roth D, et al. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Tranasplant. 2010;25:3983–3989. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 5.Damasiewicz MJ, Toussaint ND, Polkinghorne KR. Fibroblast growth factor 23 in chronic Kidney disease: new insights and clinical implications. Nephrology. 2011;16:261–268. doi: 10.1111/j.1440-1797.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- 6.Nitta K. Relationship between fibroblast growth factor-23 and mineral metabolism in chronic kidney disease. Int J Nephrol. 2010;2010:1–7. doi: 10.4061/2010/167984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigrist M, Bungay P, Taal MW, et al. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707–714. doi: 10.1093/ndt/gfi236. [DOI] [PubMed] [Google Scholar]
- 8.Toussaint ND, Pedagogos E, Tan SJ, et al. Phosphate in early chronic kidney disease: associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology. 2012;17:433–434. doi: 10.1111/j.1440-1797.2012.01618.x. [DOI] [PubMed] [Google Scholar]
- 9.Kisters K, Gremmler B, Hausberg M. Magnesium and arterial stiffness. Hypertension. 2006;47:e3. doi: 10.1161/01.hyp.0000197263.07085.e8. [DOI] [PubMed] [Google Scholar]
- 10.Ketteler M, Kruger T. Vascular complications in renal failure. EJHP Pract. 2006;12:57–59. [Google Scholar]
- 11.Ventura HO, Mehra MR. The interaction of vascular stiffness and cardiovascular events in women: insights from the heart and estrogen/progestin replacement study. Chest. 2005;127:1477–1480. doi: 10.1378/chest.127.5.1477. [DOI] [PubMed] [Google Scholar]
- 12.Jensky NE, Criqui MH, Wright MC, et al. Blood pressure and vascular calcification. Hypertension. 2010;55:990–997. doi: 10.1161/HYPERTENSIONAHA.109.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEniery CM, McDonnell BJ, So A, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. doi: 10.1161/HYPERTENSIONAHA.108.126615. [DOI] [PubMed] [Google Scholar]
- 14.Cunha A, Umbelino B, Correia ML, et al. Magnesium and vascular changes in hypertension. Int J Hypertension. 2012 doi: 10.1155/2012/754250. Article ID 754250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corica F, Corsonello A, Ientile R, et al. Serum ionized magnesium levels in relation to metabolic syndrome in type 2 diabetic patient. J Am Coll Nutr. 2006;25:210–215. doi: 10.1080/07315724.2006.10719534. [DOI] [PubMed] [Google Scholar]
- 16.Van Laecke S, Van Biesen W, Vanholder R. Hypomagnesaemia, the kidney and the vessels. Nephrol Dial Transplant. 2012;0:1–8. doi: 10.1093/ndt/gfs126. [DOI] [PubMed] [Google Scholar]
- 17.Ishimura E, Okuno S, Yamakawa T, et al. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res. 2007;20:237–244. [PubMed] [Google Scholar]
- 18.Unsal A, Budak SK, Koc Y, et al. Relationship of fibroblast growth factor 23 with left ventricular mass index and coronary calcification in chronic renal disease. Kidney Blood Press Res. 2012;36:55–64. doi: 10.1159/000339026. [DOI] [PubMed] [Google Scholar]
- 19.Nasrallah MM, El-Shehaby AR, Salem MM, et al. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–2685. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 20.Bernheim J, Benchetrit S. The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrol Dial Transplant. 2011;26:2433–2438. doi: 10.1093/ndt/gfr208. [DOI] [PubMed] [Google Scholar]
- 21.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 22.Van Laecke S, Maréchal C, Verbeke F, et al. The relation between hypomagnesaemia and vascular stiffness in renal transplant recipients. Nephrol Dial Transplant. 2011;26:2362–2369. doi: 10.1093/ndt/gfq728. [DOI] [PubMed] [Google Scholar]
- 23.Salem S, Bruck H, Bahlmann FH, et al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol. 2012;35:31–39. doi: 10.1159/000334742. [DOI] [PubMed] [Google Scholar]
- 24.Safar ME, Vaisse B, Blacher J, et al. Pulse pressure monitoring of open antihypertensive therapy. Am J Hypertens. 2004;17:1088–1094. doi: 10.1016/j.amjhyper.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Ishimura E, Okuno S, Kitatani K, et al. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68:222–227. doi: 10.5414/cnp68222. [DOI] [PubMed] [Google Scholar]
- 26.Turgut F, Kanbay M, Metin MR, et al. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40:1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 27.Gorgels TG, Waarsing JH, de Wolf A, et al. Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med. 2010;88:467–475. doi: 10.1007/s00109-010-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto T, Hara A, Ohkubo T, et al. Serum magnesium, ambulatory blood pressure and carotid artery alteration: the Ohsama study. Am J Hypertens. 2010;23:1292–1298. doi: 10.1038/ajh.2010.168. [DOI] [PubMed] [Google Scholar]
- 29.Khan AM, Sullivan L, McCabe E, et al. Lack of association between serum magnesium and the risk of hypertension and cardiovascular disease. Am Heart J. 2010;160:715–720. doi: 10.1016/j.ahj.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham PCT, Pam PMT, Pham SVm Miller JM, et al. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2:366–373. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 31.Agus ZS. Hypomagnesemia. J Am Soc Nephrol. 1999;10:1616–1622. doi: 10.1681/ASN.V1071616. [DOI] [PubMed] [Google Scholar]
- 32.Gupta VK. Does hypomagnesemia have an adaptive role in hypertension. Hypertension. 2004;43:e29. doi: 10.1161/01.HYP.0000121463.32551.8f. [DOI] [PubMed] [Google Scholar]
- 33.Sontia B, Touyz RM. Role of magnesium in hypertension. Arch Biochem Biophys. 2007;458:33–39. doi: 10.1016/j.abb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Thumfart J, Jung S, Amasheh S, et al. Magnesium stimulates renal phosphate reabsorption. Am J Physiol Renal Physiol. 2008;295:F1126–F1133. doi: 10.1152/ajprenal.00353.2007. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Ortiz ME, Canalejo A, Herencia C. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expressions at moderately low calcium concentration. Nephrol Dial Transplant. 2013;0:1–8. doi: 10.1093/ndt/gft400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson TE. The role of FGF-23 in CKD-MBD and cardiovascular disease: friend or foe. Nephrol Dial Transplant. 2010;25:1376–1381. doi: 10.1093/ndt/gfp784. [DOI] [PubMed] [Google Scholar]
- 37.Juppner H, Wolf M, Salusky IB. FGF-23: more than a regulator of renal phosphate handling. J Bone Miner Res. 2010;25:2091–2097. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]