Abstract
Vitamin D is a promising, though under-explored, potential modifiable risk factor for acute respiratory infections (ARIs). We sought to investigate the association of vitamin D status with ARI in a large, nationally-representative sample of non-institutionalized individuals from the United States. We analyzed 14,108 individuals over 16 years of age in the National Health and Nutrition Survey (NHANES) 2001–2006 in this cross-sectional study. We used locally weighted scatterplot smoothing (LOWESS) to depict the relationship between increasing 25-hydroxyvitamin D (25OHD) levels and ARI. We then performed a multivariable regression analysis to investigate the association of 25OHD levels with ARI, while adjusting for known confounders. The median serum 25OHD level was 21 (IQR 15–27) ng/mL. Overall, 4.8% (95% CI: 4.5–5.2) of participants reported an ARI within 30 days before their participation in the national survey. LOWESS analysis revealed a near-linear relationship between vitamin D status and the cumulative frequency of ARI up to 25OHD levels around 30 ng/mL. After adjusting for season, demographic factors, and clinical data, 25OHD levels <30 ng/mL were associated with 58% higher odds of ARI (OR 1.58; 95% CI: 1.07–2.33) compared to levels ≥30 ng/mL. Among the 14,108 participants in NHANES 2001–2006, 25OHD levels were inversely associated with ARI. Carefully designed, randomized, controlled trials are warranted to determine the effect of optimizing vitamin D status on the risk of ARI.
Keywords: vitamin D, 25OHD, respiratory, infection
1. Introduction
Acute respiratory infections (ARIs) are typically classified into two distinct categories—upper respiratory infections (URIs) and lower respiratory infections (LRIs). URIs involve the ear, nose, paranasal sinuses, pharynx, larynx, trachea, or bronchi, and are the most frequent reason for ambulatory medical visits in the United States [1]. On average, adults experience 2–4 URIs every year [2] and although mortality is relatively low (~3000 deaths/year) [3], the direct and indirect costs of non-influenza-related URIs exceed $40 billion, annually [4]. The financial impact of each influenza epidemic is estimated at $12 million in additional direct and indirect costs [5]. On the other hand, LRIs involve the alveoli and most commonly manifest as pneumonia [6]. In the United States, there are over 4 million ambulatory care visits for community-acquired pneumonia (CAP) annually [7], which lead to over 1 million hospitalizations [8] and roughly 50,000 deaths [9]. The direct and indirect costs attributable to CAP, in the United States alone, is in excess of $17 billion every year [10].
Given the significant impact of ARIs on health and healthcare expenditures, considerable interest has focused on factors that may influence susceptibility to such respiratory illnesses. Research over the past few decades has led to the appreciation of independent risk factors for ARIs, such as fall/winter season, age, exposure to cigarette smoke, population density, and various comorbidities (e.g., asthma and emphysema) [11,12,13,14]. Public health policies based on these risk factors, have at best, been modestly successful in limiting the annual incidence and spread of ARIs [14,15,16,17,18]. Since ARIs occur when microbes overrun natural defenses within various segments of the respiratory system, more recent efforts are investigating interventions that optimize host immune responses in at-risk individuals [19,20,21,22]. Since vitamin D is a potent regulator of the innate and adaptive immune systems [23], vitamin D status has been proposed as a potentially modifiable risk factor for ARIs [24]. To date, only a few, large studies have investigated the association of 25-hydroxyvitamin D (25OHD) levels (widely recognized as the best indicator of total body vitamin D status [25]) with either URIs or LRIs in the general population [26,27,28,29,30]. Since the source data in these studies were collected several decades ago, our goal was to investigate the association of 25OHD level with ARI in a more recent, large, nationally-representative sample of non-institutionalized individuals from the United States.
2. Materials and Methods
2.1. Source Data
The National Health and Nutrition Examination Survey (NHANES) data are nationally representative, cross-sectional samples of the non-institutionalized, civilian population of the United States [31]. They have been used extensively to report on the association of various biomarkers with major diseases. Conducted by the National Center for Health Statistics (Atlanta, GA, USA), and after three large phases between 1971 and 1994, the survey was converted into an annual process between 1999 and 2010. The most recent surveys with 25OHD assessments were 2001–2002, 2003–2004, and 2005–2006. During this period, 31,509 individuals were interviewed and 30,070 individuals completed physical examinations and laboratory testing. In addition, to allow for better population estimates, there was oversampling of individuals from the following categories: Age 12–19; age ≥70 years; non-Hispanic black; Mexican American; and low-income white. We conducted a secondary analysis of this large dataset (NHANES 2001–2006), after the Partners Human Research Committee (local Institutional Review Board) granted an “exempt” status for this retrospective study.
2.2. Data Collection
Detailed survey methods, including sampling, interview, examination, laboratory measurements, ethics approval, and informed consent have previously been reported [31]. In summary, the survey used a complex, stratified, multistage probability sample design to recruit nationally representative samples. Approximately 12,000 individuals were invited to participate in each 2-year NHANES cycle. The surveys were performed during scheduled in-home interviews to obtain demographic information as well as data on health and nutrition. Physical examinations and laboratory testing were performed in either a mobile examination center or during a home visit. Blood samples collected during the examination were centrifuged, aliquoted, and stored at −70 °C on-site. They were then shipped on dry ice to central laboratories, where they were stored at −70 °C until analysis. Serum 25OHD levels were measured using a radioimmunoassay kit after extraction with acetonitrile (DiaSorin, Stillwater, MN, USA) by the National Center for Environmental Health (Atlanta, GA, USA). Standards for 25OHD assays were released in July 2009 using isotope dilution tandem mass spectrometry (LC-MS/MS) candidate reference measurement procedures to assign certified values by the National Institute of Standards and Technology (NIST). The Centers for Disease Control and Prevention will release a revised analytic note in the future to improve 25OHD data interpretation including across NHANES 2001–2006 and NHANES III by generating regression equations to adjust 25OHD data using the NIST standards from LC-MS/MS. This will allow more accurate assessment of possible correlations with 25OHD and health conditions. NHANES also modified the 2003–2006 values for 25OHD in November 2010 to address observed drifts in assay performance following reagent and calibration lot fluctuation [32]. Quality control pool data from the drift period was used for a statistical adjustment model since the sample subject values would be independent of empirical trends [32].
2.3. Data Abstraction
We limited our analysis to the 14,108 individuals, 17 years and older, with reported 25OHD levels (primary exposure). To most accurately adjust for the effect of season on 25OHD levels, we recorded the 6-month block within which the laboratory samples were collected. We also abstracted information on all participants in the NHANES 2001–2006 datasets related to age, sex, race, body mass index (BMI), and poverty-to-income ratio (measure of socioeconomic status). We also abstracted smoking status, exposure to second-hand smoke, alcohol consumption, pneumococcal vaccination status, and several self-reported current diseases: Asthma, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), diabetes mellitus (DM), and stroke. A diagnosis of COPD was based on responses to questions on emphysema and/or chronic bronchitis. Furthermore, we used laboratory data to determine the estimated glomerular filtration rate (eGFR) to assess for chronic kidney disease (CKD), and to document cases of neutropenia. The primary outcome, ARI, was based on the response to the question spanning both upper and lower ARI: “Did you have flu, pneumonia, or ear infections that started during [the past] 30 days?”
2.4. Statistical Analysis
All statistical analyses were performed using Stata 12.0 (StataCorp LP, College Station, TX, USA). Using survey commands, we applied the recommended subsample weights for the interview plus examination data to account for unequal probabilities of selection and to accurately represent estimates for the population of the United States. All of the results are presented as weighted values. We calculated variance based on NHANES-provided masked variance units using the Taylor series linearization method. All reported p values are 2-tailed, with p < 0.05 considered statistically significant. We calculated proportions with 95% confidence intervals (CIs) for demographic features and other factors thought to be related to ARI, overall and in the subset of participants with self-reported ARI, within 30 days of the interview.
Locally weighted scatter plot smoothing (LOWESS) was used to graphically represent the association between 25OHD level and the cumulative frequency of ARI. LOWESS is a type of nonparametric regression, which summarizes the relationship between two variables in a fashion that initially relies on limited assumptions about the form or strength of the relationship [33]. The rationale and methods underlying the use of LOWESS for depicting the local relationship between measurements of interest across parts of their ranges are available elsewhere [34].
For our primary analysis, we first considered serum 25OHD level as a continuous variable using LOWESS analysis, and then as a dichotomous variable based on the LOWESS results. To improve interpretability of the analysis, we converted some variables into commonly used groupings: Age (17–39, 40–59, and ≥60) and BMI in kg/m2 (<20, 20–24.9, 25–29.9, ≥30). In addition, we dichotomized other variables as follows: Season (1 May–31 October as high ambient ultraviolet B radiation versus 1 November–30 April as low ambient ultraviolet B radiation), race (non-white versus white), poverty-to-income ratio (≤federal poverty level versus >federal poverty level), alcohol consumption (≤30 drinks/month versus >30 drinks/month), CKD (eGFR <60 mL/min/1.73m2 versus ≥60 mL/min/1.73m2), and neutropenia (white blood cell count <3.5 × 109/L versus ≥3.5 × 109/L). We also dichotomized self-reported histories of: Active smoking, exposure to second-hand smoke in the household, pneumococcal vaccination, asthma, COPD, CHF, DM, and stroke. We determined unadjusted associations between risk factors and the outcome of ARI using the Pearson chi-squared test for categorical variables and simple ordinal logistic regression for ordinal variables. To evaluate the independent association between serum 25OHD level and ARI, we created multivariable models by progressively adding covariates that might confound or alter the association of 25OHD with ARI. All adjusted odds ratios (ORs) for the variables in the models are reported with 95% confidence intervals (CIs).
3. Results
Characteristics of the analytic sample are given in Table 1. The median age of the participants was 45 (IRQ 28–63) years; 51% were female and 51% were white. Overall, the median serum 25OHD level was 21 (IRQ 15–27) ng/mL. Overall, 4.8% (95% CI: 4.5–5.2) of the sample reported an ARI within 30 days before their NHANES interview. The proportion of participants with recent ARI, stratified by individual characteristics, is also presented in Table 1.
Table 1.
Overall sample characteristics and sub-groups with acute respiratory infections.
| Covariate | Total Number of Observations | Number Reporting Acute Respiratory Infection (Row %) | p-value |
|---|---|---|---|
| 25-hydroxyvitamin D | |||
| <10 ng/mL | 1168 | 90 (7.7%) | <0.001 |
| 10–19.9 ng/mL | 4958 | 262 (5.3%) | |
| 20–29.9 ng/mL | 5416 | 245 (4.5%) | |
| ≥30 ng/mL | 2566 | 82 (3.2%) | |
| Season | |||
| High ambient ultraviolet B radiation | 7468 | 236 (3.2%) | <0.001 |
| Low ambient ultraviolet B radiation | 6640 | 443 (6.7%) | |
| Age | |||
| 17–39 years | 5934 | 285 (4.8%) | 0.06 |
| 40–59 years | 3836 | 208 (5.4%) | |
| ≥60 years | 4338 | 186 (4.3%) | |
| Sex | |||
| Female | 7252 | 386 (5.3%) | 0.004 |
| Male | 6856 | 293 (4.3%) | |
| Race | |||
| Non-white | 7178 | 303 (4.2%) | 0.001 |
| White | 6930 | 376 (5.4%) | |
| Poverty-to-income ratio | |||
| ≤Federal poverty limit | 2408 | 148 (6.2%) | <0.001 |
| >Federal poverty limit | 10,361 | 454 (4.4%) | |
| Body mass index | |||
| <20 kg/m2 | 744 | 34 (4.6%) | 0.06 |
| 20–24.9 kg/m2 | 3787 | 160 (4.2%) | |
| 25–29.9 kg/m2 | 4668 | 220 (4.7%) | |
| ≥30 kg/m2 | 4390 | 241 (5.5%) | |
| Active smoker | |||
| Yes | 2801 | 173 (6.2%) | <0.001 |
| No | 3416 | 127 (3.7%) | |
| Second-hand smoke | |||
| Yes | 2767 | 176 (6.4%) | <0.001 |
| No | 11,227 | 494 (4.4%) | |
| Alcohol consumption | |||
| ≤30 drinks/month | 952 | 37 (3.9%) | 0.53 |
| >30 drinks/month | 7167 | 310 (4.3%) | |
| Pneumococcal vaccination | |||
| Yes | 867 | 35 (4.0%) | 0.33 |
| No | 3278 | 158 (4.8%) | |
| Asthma | |||
| Yes | 1716 | 129 (7.5%) | <0.001 |
| No | 12,374 | 550 (4.4%) | |
| Chronic obstructive pulmonary disease | |||
| Yes | 929 | 87 (9.4%) | <0.001 |
| No | 11,694 | 504 (4.3%) | |
| Congestive heart failure | |||
| Yes | 421 | 36 (8.6%) | <0.001 |
| No | 12,916 | 556 (4.6%) | |
| Diabetes mellitus | |||
| Yes | 1290 | 72 (5.6%) | 0.15 |
| No | 12,612 | 591 (4.7%) | |
| Chronic kidney disease | |||
| eGFR < 60 mL/min/1.73m2 | 1697 | 82 (4.8%) | 0.97 |
| eGFR ≥ 60 mL/min/1.73m2 | 12,108 | 588 (4.9%) | |
| Stroke | |||
| Yes | 459 | 37 (8.1%) | <0.001 |
| No | 12,191 | 556 (4.6%) | |
| Neutropenia | |||
| WBC ≥ 3.5 × 109/L | 13,958 | 672 (4.8%) | 0.84 |
| WBC < 3.5 × 109/L | 135 | 6 (4.4%) |
A two-tailed p < 0.05 was considered statistically significant, p-values are based on simple ordinal logistic regression (for ordinal variables) and chi-square test (for categorical variables).
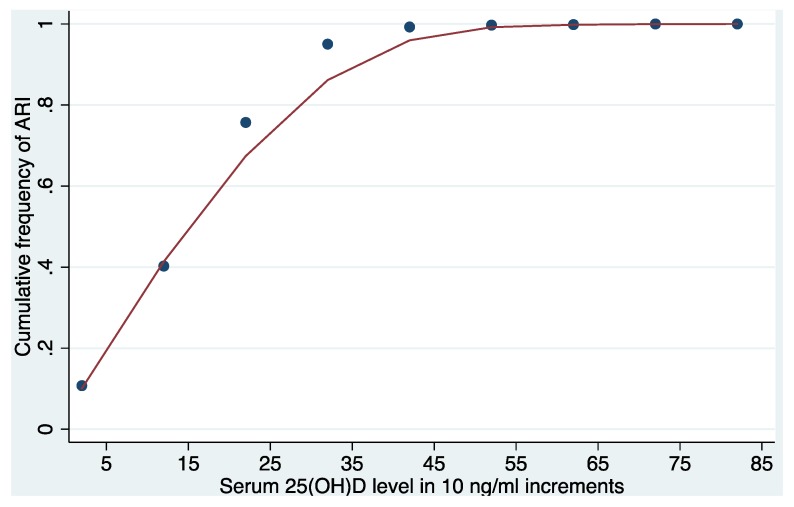
LOWESS analysis showed a near linear relationship between 25OHD level and the cumulative frequency of ARI up to 25OHD levels around 30 ng/mL (Figure 1). Between 25OHD levels of 30 ng/mL and 50 ng/mL there was an increasing flattening of the curve.
Figure 1.
Near linear relationship of acute respiratory infection and 25-hydroxyvitamin D up to 30 ng/mL in LOWESS analysis. Locally weighted scatterplot smoothing analysis = LOWESS; 25OHD = 25-hydroxyvitamin D in 10 ng/mL increments; ARI = acute respiratory infection.
Based on the analysis, we selected a cut-point of 30 ng/mL in the model where 25OHD was considered as a dichotomous variable. Compared with individuals with 25OHD levels ≥30 ng/mL, those with levels <30 ng/mL had a 58% higher adjusted odds of ARI (OR 1.58; 95% CI: 1.07–2.33) within 30 days of the interview. Other independent risk factors associated with increased odds of ARI in this model were low ambient ultraviolet B radiation, active smoking, higher poverty-to-income ratio, history of COPD, and CHF (Table 2). Based on existing clinical guidelines [35], we further sub-categorized 25OHD levels <30 ng/mL as <10 ng/mL, 10 to 19.9 ng/mL, and 20 to 29.9 ng/mL. Looking within this large group of participants with 25OHD levels <30 ng/ml, we found higher adjusted odds of ARI for those with levels <10 ng/mL (OR 2.75; 95% CI: 1.67–4.54), 10–19.9 ng/mL (OR 1.36; 95% CI: 0.88–2.08), and 20–29.9 ng/mL (OR 1.55; 95% CI: 1.02–2.36), compared to individuals with levels of 30 ng/mL or higher.
Table 2.
Multivariable model with odds ratios for acute respiratory infection risk factors.
| Risk Factor | Odds Ratio (95% Confidence Interval), p-value |
|---|---|
| 25OHD (<30 ng/mL versus ≥30 ng/mL) | 1.58 (1.07–2.33), p = 0.023 |
| Low ambient UVB radiation (1 November–30 April) | 2.19 (1.69–2.85), p < 0.001 |
| Poverty-to-income ratio (≤FPL versus >FPL) | 1.47 (1.10–1.96), p = 0.009 |
| Active smoking | 1.39 (1.01–1.91), p = 0.046 |
| Chronic obstructive pulmonary disease | 1.94 (1.39–2.72), p < 0.001 |
| Congestive heart failure | 1.85 (1.15–2.97), p = 0.012 |
25OHD = 25-hydroxyvitamin D; UVB = ultraviolet B radiation; FPL = Federal poverty limit.
4. Discussion
In this large, nationally-representative sample of non-institutionalized individuals in the United States, we investigated whether 25OHD level was associated with ARI. We demonstrated that 25OHD levels were inversely associated with the odds of ARI. This relationship was most pronounced when comparing individuals with 25OHD levels <10 ng/mL to those with levels ≥30 ng/mL. While previous studies have shown that vitamin D status is associated with the risk of specific respiratory diseases [26,27,28,29,30], our work demonstrates a near linear relationship between vitamin D status and the cumulative frequency of ARIs over a commonly encountered range of 25OHD levels in the general population. Such a growing body of evidence may thus support later randomized controlled trials to test the hypothesis that a therapeutic window of vitamin D supplementation may help reduce the risk of ARI. However, given the cross-sectional design of the present study, a causal inference about the effect of low 25OHD levels and higher risk of ARI is not possible. Nonetheless, the biological plausibility remains undeniable.
Cells of the innate and adaptive immune system express the vitamin D receptor [36], and in low vitamin D states, dysfunctional macrophage activity becomes evident [37]. Moreover, vitamin D is necessary for interferon-γ dependent T cell responses to infection [38] and it is also an important link between Toll Like Receptor (TLR) activation and antibacterial responses [39]. Macrophages stimulated by TLR-ligands induce further vitamin D receptor expression [40] and conversion of 25OHD to its most biologically active form (1,25-dihydroxyvitamin D) [41]. This, in turn, leads to increased production of cathelicidin, an endogenous antimicrobial peptide with potent activity against bacteria, viruses, fungi, and mycobacteria [42,43,44]. Cathelicidin is also highly expressed by epithelial cells at natural barrier sites (e.g., skin, gut, lungs) and may represent an important first-line of defense for the innate immune system [45] against pathogens that lead to ARI.
Although observational studies have suggested a link between vitamin D status and ARI [26,27,28], evidence from randomized controlled trials (RCTs) are conflicting. While one meta-analysis of existing RCTs concluded that there was no benefit to vitamin D supplementation for the prevention of ARIs [46], two separate meta-analyses, performed by independent research teams, found a lower risk of ARIs in vitamin D-treated groups [47,48]. Such discrepancies may be, at least partially, explained by the heterogeneity of the data included in the meta-analyses—e.g., the age of subjects (adults versus children), health status of participants (general population versus inpatients), frequency of vitamin D dosing (daily versus intermittent), dose of vitamin D supplementation, and baseline vitamin D status of the subjects. Nonetheless, these studies underscore the need for adequately powered, well-designed RCTs to determine whether vitamin D may be beneficial for the prevention of ARIs in the general population.
While our results provide further compelling evidence to suggest that vitamin D status may be a modifiable risk factor for ARIs in the general population, it is important to discuss the potential limitations of the present study. As with all observational studies, and any cross-sectional research design, there is potential for confounding due to the lack of a randomly distributed exposure. Selection bias may be present, since 25OHD was not recorded for all participants in the NHANES 2001–2006 survey. And despite adjusting for multiple potential confounders, there may still be residual confounding, which could account for the observed differences in outcomes. In particular, low 25OHD levels may be a reflection of poor general health or suboptimal nutritional state, for which we are unable to fully adjust. We are also unable to fully adjust for lack of sun exposure, use of sunscreens, physical activity, and influenza vaccination status. Given the confines of the NHANES survey, a further limitation is that we were unable to control for the exact amount of time between the self-reported ARI and the timing of blood draws (i.e., the ARI may have occurred at any point up to 30 days before the survey). As such, we cannot rule out the possibility of reverse causation (i.e., low 25OHD levels lead to ARIs versus ARIs lead to low 25OHD levels). However, in non-hospitalized individuals, 25OHD levels tend to be relatively consistent over time (intra-person Pearson correlation coefficient of 0.70 at three years between blood draws following adjustments for age, race, and season) [49]. Nonetheless, vitamin D status may be influenced by acute illness [25], and therefore 25OHD levels may have been different at the time that participants developed ARIs. Also, the NHANES dataset relies on a self-reported history of ARI, which may be prone to recall bias and is less valid as a measurement of true ARI status compared to evaluation by a medical professional. And finally, the ARI definition can include upper and lower respiratory tract infections that may be caused by different pathogens. These and other potential issues will need to be addressed in future studies in order to replicate and extend our findings.
5. Conclusions
In summary, these data demonstrate that low 25OHD levels are strongly associated with ARIs in a large, nationally-representative sample of non-institutionalized individuals from the United States. Longitudinal studies are required to confirm our findings and establish the mechanisms underlying these observations. Moreover, high-quality, randomized, controlled trials are warranted to determine whether vitamin D supplementation in individuals with low vitamin D status may affect the incidence and severity of ARIs in the general population.
Acknowledgments
C.A.C. received support from the US National Institutes of Health R01 AI093723 and U01 AI087881. S.A.Q. received support from the US National Institutes of Health 5T32GM007592 and UL1 RR025758.
Author Contributions
D.J.M. and S.A.Q. jointly conceived the study as well as designed and implemented the analysis with assistance from E.A.B., K.B.C., and C.A.C. E.A.B. and D.J.M. assembled input data, wrote code, ran the model and analyzed output data. D.J.M., C.A.C. and S.A.Q. wrote the manuscript. D.J.M., E.A.B., K.B.C., C.A.C. and S.A.Q. edited the manuscript and provided conceptual advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Cherry D.K., Hing E., Woodwell D.A., Rechtsteiner E.A. National ambulatory medical care survey: 2006 summary. Natl. Health Stat. Rep. 2008;3:1–39. [PubMed] [Google Scholar]
- 2.Allan G.M., Arroll B. Prevention and treatment of the common cold: Making sense of the evidence. CMAJ. 2014;186:190–199. doi: 10.1503/cmaj.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2014;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J., Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 6.Bariffi F., Sanduzzi A., Ponticiello A. Epidemiology of lower respiratory tract infections. J. Chemother. 1995;7:263–276. doi: 10.1179/joc.1995.7.4.263. [DOI] [PubMed] [Google Scholar]
- 7.File T.M., Jr., Marrie T.J. Burden of community-acquired pneumonia in North American adults. Postgrad. Med. 2010;122:130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 8.Watkins R.R., Lemonovich T.L. Diagnosis and management of community-acquired pneumonia in adults. Am. Fam. Physician. 2011;83:1299–1306. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Fast Stats: Pneumonia Deaths and Mortality. [(accessed on 15 August 2014)]. Available online: http://www.cdc.gov/nchs/fastats/pneumonia.htm.
- 10.Feikin D.R., Schuchat A., Kolczak M., Barrett N.L., Harrison L.H., Lefkowitz L., McGeer A., Farley M.M., Vugia D.J., Lexau C., et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am. J. Public Health. 2000;90:223–229. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.File T.M. The epidemiology of respiratory tract infections. Semin. Respir. Infect. 2000;15:184–194. doi: 10.1053/srin.2000.18059. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Allergy and Infectious Diseases Common Cold: Protect Yourself and Others. [(accessed on 15 August 2014)]. Available online: http://www.niaid.nih.gov/topics/commoncold/Pages/default.aspx.
- 13.World Lung Foundation: The Acute Respiratory Infections Overcrowding. [(accessed on 15 August 2014)]. Available online: http://www.ariatlas.org/drivers_of_aris/overcrowding.
- 14.Torres A., Peetermans W.E., Viegi G., Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: A literature review. Thorax. 2013;68:1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong V.W., Cowling B.J., Aiello A.E. Hand hygiene and risk of influenza virus infections in the community: A systematic review and meta-analysis. Epidemiol. Infect. 2014;142:922–932. doi: 10.1017/S095026881400003X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passioti M., Maggina P., Megremis S., Papadopoulos N.G. The common cold: Potential for future prevention or cure. Curr. Allergy Asthma Rep. 2014;14:413. doi: 10.1007/s11882-013-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrelet C., Bourrier M., Burton-Jeangros C., Schinder M. Unresolved issues in risk communication research: The case of the H1N1 pandemic (2009–2011) Influenza Other Respir. Vir. 2013;7(Suppl. S2):114–119. doi: 10.1111/irv.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Limiting Spread: Limiting the Spread of Pandemic, Zoonotic, and Seasonal Epidemic Influenza. [(accessed on 15 August 2014)]. Available online: http://www.who.int/influenza/resources/research/research_agenda_influenza_stream_2_limiting_spread.pdf.
- 19.Evans S.E., Tuvim M.J., Fox C.J., Sachdev N., Gibiansky L., Dickey B.F. Inhaled innate immune ligands to prevent pneumonia. Br. J. Pharmacol. 2011;163:195–206. doi: 10.1111/j.1476-5381.2011.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui D.S., Lee N. Adjunctive therapies and immunomodulating agents for severe influenza. Influenza Other Respir. Vir. 2013;7(Suppl. S3):52–59. doi: 10.1111/irv.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darwish I., Mubareka S., Liles W.C. Immunomodulatory therapy for severe influenza. Expert Rev. Anti Infect. Ther. 2011;9:807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 22.Sallenave J.M. Secretory leukocyte protease inhibitor and elafin/trappin-2: Versatile mucosal antimicrobials and regulators of immunity. Am. J. Respir. Cell Mol. Biol. 2010;42:635–643. doi: 10.1165/rcmb.2010-0095RT. [DOI] [PubMed] [Google Scholar]
- 23.Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Taylor C.E., Camargo C.A., Jr. Impact of micronutrients on respiratory infections. Nutr. Rev. 2010;69:259–269. doi: 10.1111/j.1753-4887.2011.00386.x. [DOI] [PubMed] [Google Scholar]
- 25.Quraishi S.A., Camargo C.A., Jr. Vitamin D in acute stress and critical illness. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quraishi S.A., Bittner E.A., Christopher K.B., Camargo C.A., Jr. Vitamin D status and community-acquired pneumonia: Results from the third National Health and Nutrition Examination Survey. PLoS One. 2013;8:e81120. doi: 10.1371/journal.pone.0081120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginde A.A., Mansbach J.M., Camargo C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirani V. Associations between vitamin D and self-reported respiratory disease in older people from a nationally representative population survey. J. Am. Geriatr. Soc. 2013;61:969–973. doi: 10.1111/jgs.12254. [DOI] [PubMed] [Google Scholar]
- 29.Zhao G., Ford E.S., Tsai J., Li C., Croft J.B. Low concentrations of serum 25-hydroxyvitamin D associated with increased risk for chronic bronchitis among US adults. Br. J. Nutr. 2013;107:1386–1392. doi: 10.1017/S0007114511004417. [DOI] [PubMed] [Google Scholar]
- 30.Berry D.J., Hesketh K., Power C., Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 2011;106:1433–1440. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 31.Zipf G., Chiappa M., Porter K.S., Ostchega Y., Lewis B.G., Dostal J. National Health and Nutrition Examination Survey: Plan and operations, 1999–2010. Vital Health Stat. 2013;56:1–37. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention Revised Analytical Note for NHANES 2001–2006 and NHANES III (1988–1994) 25-hydroxyvitamin D analysis. [(accessed on 15 August 2014)]. Available online: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/VID_D.htm#Analytic_Notes.
- 33.Cleveland W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979;74:829–836. [Google Scholar]
- 34.Cleveland W.S., Devlin S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988;83:596–610. [Google Scholar]
- 35.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 36.Adams J.S., Hewison M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kankova M., Luini W., Pedrazzoni M., Riganti F., Sironi M., Bottazzi B., Mantovani A., Vecchi A. Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology. 1991;73:466–471. [PMC free article] [PubMed] [Google Scholar]
- 38.Fabri M., Stenger S., Shin D.M., Yuk J.M., Liu P.T., Realegeno S., Lee H.M., Krutzik S.R., Schenk M., Sieling P.A., et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu P.T., Stenger S., Tang D.H., Modlin R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 40.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., Ochoa M.T., Schauber J., Wu K., Meinken C., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;31:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 41.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 42.Bhalla A.K., Amento E.P., Krane S.M. Differential effects of 1,25-dihydroxyvitamin D3 on human lymphocytes and monocyte/macrophages: Inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell Immunol. 1986;98:311–322. doi: 10.1016/0008-8749(86)90291-1. [DOI] [PubMed] [Google Scholar]
- 43.Pinheiro da Silva F., Machado M.C. Antimicrobial peptides: Clinical relevance and therapeutic implications. Peptides. 2012;36:308–314. doi: 10.1016/j.peptides.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Dürr U.H., Sudheendra U.S., Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 45.Tollin M., Bergman P., Svenberg T., Jörnvall H., Gudmundsson G.H., Agerberth B. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides. 2003;24:523–530. doi: 10.1016/s0196-9781(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 46.Mao S., Huang S. Vitamin D supplementation and risk of respiratory tract infections: A meta-analysis of randomized controlled trials. Scand. J. Infect. Dis. 2013;45:696–702. doi: 10.3109/00365548.2013.803293. [DOI] [PubMed] [Google Scholar]
- 47.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. J. Pharmacol. Pharmacother. 2012;3:300–303. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergman P., Lindh A.U., Björkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Platz E.A., Leitzmann M.F., Hollis B.W., Willett W.C., Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]