Abstract
Exposure to adult smoking can have deleterious effects on children. Interventions that assist families with smoking cessation/reduction and environmental tobacco smoke (ETS) avoidance can improve child health outcomes and reduce the risk of smoking initiation. The purpose of this review was to describe the state of the science of interventions with families to promote smoke-free home environments for infants and young children, including parent smoking reduction and cessation interventions, ETS reduction, and anti-smoking socialisation interventions, using the socio-ecological framework as a guide. A systematic review of peer-reviewed articles identified from journal databases from 2000 to 2014 was undertaken. Of 921 articles identified, 28 were included in the review. Considerable heterogeneity characterised target populations, intervention types, complexity and intensity, precluding meta-analysis. Few studies used socio-ecological approaches, such as family theories or concepts. Studies in early parenthood (child age newborn to one year) tended to focus on parent smoking cessation, where studies of families with children aged 1–5 years were more likely to target household SHSe reduction. Results suggest that interventions for reduction in ETS may be more successful than for smoking cessation and relapse prevention in families of children aged less than 5 years. There is a need for a range of interventions to support families in creating a smoke free home environment that are both tailored and targeted to specific populations. Interventions that target the social and psychodynamics of the family should be considered further, particularly in reaching vulnerable populations. Consideration is also required for approaches to interventions that may further stigmatise families containing smokers. Further research is required to identify successful elements of interventions and the contexts in which they are most effective.
Keywords: child, family, smoking, smoking cessation, second hand smoke, antismoking socialisation
1. Introduction
Tobacco smoking in Western countries has declined in response to a range of policy, health promotion and education initiatives. While the prevalence of smoking in Western developed countries is now generally less than 20% in adults [1], people who continue to smoke include those in families with infants and children.
Exposure to adult smoking presents several risks to children. The World Health Organisation (WHO) estimates that one third of premature deaths attributable to environmental tobacco smoke (ETS) occur in children and that ETS contributes to the premature death of approximately 1100 children with asthma per annum [2]. Environmental tobacco smoke includes not only secondhand smoke exposure (SHSe) through passive exposure to tobacco smoke, but also thirdhand smoke exposure (THSe), via exposure to the toxic contaminants of tobacco smoke that remain in the environment particularly on clothing, hair and surfaces [3,4]. Where smoke-free legislation has been introduced, there has been a clear and corresponding decrease in preterm births and hospital admissions for asthma [5]. In addition to the physical risks from adult tobacco smoking, there are risks to children in the forms of behavioural effects of smoking in that children who have parents or siblings who smoke are more likely to smoke themselves [6,7,8,9] and to begin at an earlier age [10]. If both parents and siblings smoke, the risk of smoking is greater still [6,11].
Although smoking most commonly begins during adolescence, even young children recognise and respond to observed smoking behaviours. By the time children start school, they have begun to understand tobacco use. For example, at 5 years of age, children can recognise and identify cigarettes [12] and, in role play, demonstrate an awareness of how adults obtain and use tobacco [13,14]. By age 9, children can begin to identify reasons why someone may choose to smoke, including image, role modelling, stress relief and mood enhancement [15]. This suggests that parental role modelling of smoking is influential in children’s views and beliefs, even when children are aware of detrimental health effects and that interventions with parents and families in the early years of childhood may be important to children’s views and beliefs about smoking [15].
Concerns about the impact of smoking on young children have led to the development of interventions to assist families with harm minimisation including smoking cessation, ETS reduction, and antismoking socialisation. Antismoking socialisation has been defined as parenting behaviours and interactions that influence children’s cognitive and behavioural responses against smoking [16]. Parents’ behaviours and interactions may include communication about the risks of smoking, the setting of rules around smoking both for themselves and their children, monitoring of children’s behaviour and other methods of socialisation. Such interventions are important, as family is the first smoking socialisation context for children and young people. It is within the context of family that parents can positively or negatively influence children’s health behaviours [17].
There is evidence that smoking is associated with socioeconomic disadvantage and lower education and income [18,19]. As an example, single parent mothers are twice as likely to smoke as mothers living with a partner [20]. Almost half (47%) of Australian Indigenous people aged 15 years and older report being current smokers, compared with 17% of the broader Australian population [21]. Current smokers are more likely than non-smokers to be dealing with emotional and social difficulties, including psychological distress [22,23] and racial discrimination [23].
As such, a socio-ecological framework may provide a useful tool for organising and addressing these influencing agents from different environmental spheres [24]. Implicit in the model is an assumption that individual health behaviour is influenced by both individual beliefs and values as well as the beliefs and values of the individuals’ primary social groups, their social and community institutions and networks, and public policy [24]. These multiple levels of influence include intrapersonal (e.g., age, gender, knowledge, behaviour, self-efficacy, skills), interpersonal (personal networks, such as family, workplace and friends), institutional factors (e.g., neighbourhood, practices and policies of workplace, child care), community (community norms, relationships between organisations and institutions), and public policy (local and national laws and regulations).
Factors across the levels of the socio-ecological framework need consideration when developing interventions for smoking abstinence, cessation, and socialisation. However, they have been largely ignored by previous literature reviews [25,26,27]. One review assessed interventions designed to support families in their efforts to promote non-smoking in children [28], but excluded studies where the parent intervention was not tested separately to the other parts of the intervention. A more holistic approach is needed to understand what levels and components of interventions are most effective.
Objectives
The purpose of this review was to describe the state of the science of family-focussed interventions to promote smoke-free home environments for infants and children under 5 years, including parent smoking reduction and cessation interventions, SHSe reduction, and anti-smoking socialisation interventions, using the socio-ecological framework as a guide. All interventions that planned to intervene with families to support parent smoking cessation or reduction, or reduce ETS in the home or any other targeted program aimed at families of children aged 0–5 years were included. The outcome measures included any changes in the smoking behaviour of families, including smoking cessation or reduction, household restrictions on smoking, knowledge, attitudes and beliefs about smoking, child smoking behaviour (longitudinal), exposure to ETS (including biochemical measures and parent reported exposure), child health outcomes (illness events, respiratory symptoms, change in lung function, utilization of health care services). Studies published from 2000 to 2014 were included to ensure that the most contemporary research relevant to the current context of interventions in smoking cessation and harm reduction was captured.
2. Methods
2.1. Protocol
This review was guided by current methods for systematic searching and selecting evidence for a literature review [29,30].
2.2. Eligibility Criteria
Papers were included if they were: (1) empirical study reports of interventions aimed at smoking cessation, promoting a smoke free home environment or antismoking socialisation; and (2) focused on primary carers (parents, guardians, foster carers or grandparents) involved in the parenting of infants and young children and/or young children. Where child age range exceeded 0–5 years, a mean age within the 0–5 year range was used as a criterion. Included papers were published between 2000 and 2014 in peer reviewed journals to ensure a focus on the most recent research in the topic. Papers were excluded if they were not written in English.
2.3. Information Sources
Electronic databases searched included MEDLINE, Cochrane Database of Systematic Reviews, PubMed, and CINAHL. Search terms included cigarettes, smoking, tobacco, parent, and family, as well as terms aimed at identifying intervention studies (An example appears in Table 1). The reference lists of included studies were searched manually.
Table 1.
Medline search strategy.
| Term set 1: Child * |
| Term set 2: Parent * OR father * OR mother * OR caregivers OR famil * OR school * OR communit * |
| Term set 3: Cigar * OR tobacco * OR smok * OR smoking cessation OR tobacco cessation OR tobacco smoke pollution OR smoking abstinence |
| Term set 4: prevent * OR control * |
| Term set 5: intervention OR clinical trial OR pilot study OR outcomes OR randomised control trial |
| Term set 6: 1 and 2 and 3 and 4 and 5 |
2.4. Study Selection
All literature identified from the electronic searches were imported into the Endnote Reference Management System version 5. The title and abstract of each study were reviewed against the inclusion criteria, with full text being reviewed as required.
2.5. Data Collection Process and Data Items
Data were extracted using a standardised form. Data included country, intervention setting (e.g., community health, acute health care service, school, preschool), participants (demographic information), intervention details, and primary and secondary outcomes for the study. In accordance with Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA) guidelines [30] and critiques of the reporting of interventions for behaviour change [31], details were extracted for each intervention by one of the reviewers (NB), including content, delivery personnel, method of communication, intensity, complexity, environment and conceptual framework. Any concerns about the nature of the articles selected were discussed in conjunction with a second reviewer (TL).
2.6. Risk of Bias
The quality of the included studies was assessed by the first author using the United States Preventative Services Taskforce (USPST) procedures for critical appraisal of research [32]. USPST procedures include appraisal of the research design, internal and external validity, study population, location and provider (Table 2).
Table 2.
Study design and level of quality (AHRQ 2008).
| Reference | Focus | Design | Internal Validity | External Validity |
|---|---|---|---|---|
| Phillips et al. 2012. USA [35] | Smoking relapse prevention | RCT | Good | Good |
| Hovell et al. 2009. USA [34] | Smoking cessation/SHS reduction | RCT | Good | Good |
| Kuiper et al. 2005. Schonberger et al. 2005. Netherlands [36,37] | Smoking cessation/SHS reduction | RCT | Fair | Good |
| Chan-Yeung et al. 2000; Becker et al. 2004, Chan-Yeung et al. 2005. Canada [55,56,57] | SHSe reduction | RCT | Fair | Good |
| Conway et al. 2004. USA [59] | SHSe reduction | RCT | Fair | Good |
| Joseph et al. 2014. USA [43] | Smoking cessation | Pilot Quasi-experimental | Fair | Fair |
| Jiminez-Muro et al. 2013. Spain [38] | Smoking cessation/relapse prevention | RCT | Fair | Fair |
| Storrø et al. 2010. Norway [42] | Smoking reduction | Cohort control trial with one year time difference | Fair | Fair |
| Winickoff et al. 2010. USA [40] | Smoking cessation/reduction | Quasi RCT | Fair | Fair |
| Hannover et al. 2009. Germany [39] | Smoking cessation/relapse prevention | Quasi RCT | Fair | Fair |
| Kallio et al. 2006. Finland [46] | Smoking cessation/reduction/SHS reduction | RCT (longitudinal) | Fair | Fair |
| Abdullah et al. 2005. Hong Kong [45] | Smoking cessation | RCT | Fair | Fair |
| Wiggins et al. 2005. UK [47] | Smoking cessation | RCT | Fair | Fair |
| Baheiraei et al. 2011. Iran [53] | SHSe reduction | RCT | Fair | Fair |
| Emmons et al. 2001. USA [52] | SHSe reduction | RCT | Fair | Fair |
| Kitzman et al. 2010. USA [61] | Smoking prevention | RCT (longitudinal) | Fair | Fair |
| Øien et al. 2008. Norway [44] | Smoking cessation | Control trial | Fair | Poor |
| Culp et al. 2007. USA [48] | Smoking cessation | Quasi-experimental | Fair | Poor |
| Wilson et al. 2013. Scotland [54] | SHSe reduction | Pilot RCT | Fair | Poor |
| Huang et al. 2013. Taiwan [60] | SHSe reduction | RCT | Poor | Fair |
| Harutyunyan et al. 2013. Armenia [50] | SHSe reduction | RCT | Poor | Fair |
| Fossum et al. 2004. Sweden [58] | SHSe reductions | CT | Poor | Fair |
| Zakarian et al. 2004. USA [51] | SHSe reduction | Quasi-experimental | Fair | Poor |
| Disantis et al. 2010. USA [41] | Smoking cessation/relapse prevention | Pilot 2 arm experimental | Poor | Poor |
| Yücel et al. 2014. Turkey [49] | SHSe reduction | RCT | Poor | Poor |
2.7. Synthesis of Results
The main aim of the literature review was to appraise and synthesize evidence across a broad range of interventions with families using the framework of the socio-ecological model. It was anticipated that there would be considerable heterogeneity of study aims, designs, methods and outcomes and that existing systematic reviews would be included, and thus narrative synthesis rather than meta-analysis was used to guide data synthesis. The synthesis followed a combination of methods recommended by Popay and colleagues [29], including tabulation and content analysis. These guidelines were developed to facilitate narrative synthesis in systematic reviews where the effectiveness of interventions and the factors influencing the implementation of interventions are central [33].
3. Results
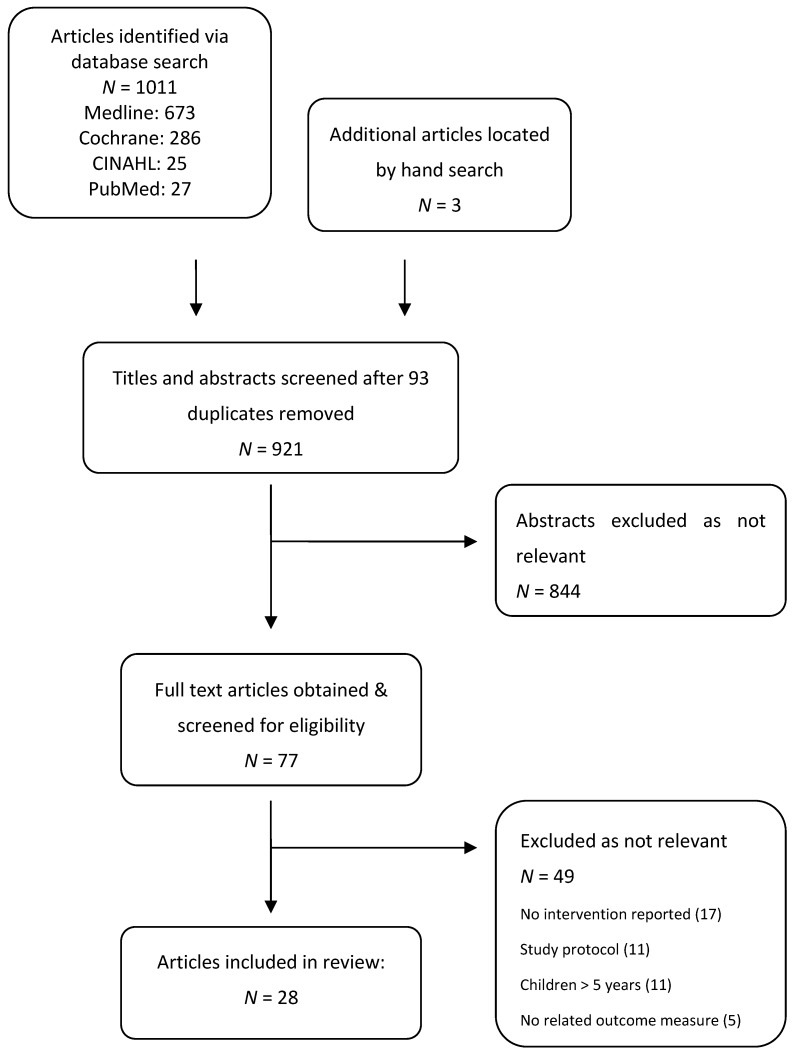
The initial search located 921 articles following removal of duplicates (Figure 1). After review against inclusion criteria, 28 articles were included including smoking cessation (n = 15), ETS reduction (n = 12) and anti-smoking socialisation interventions (n = 1).
Figure 1.
Search strategy.
The studies were assessed for quality against USPSTF methods, and were categorised as good, fair or poor (Table 3). The majority of studies were fair quality, with only two of the studies rated as good [34,35]. The main concerns with studies rated as fair or poor were related to limitations with randomisation or allocation concealment encountered in intervention design and delivery.
Table 3.
Study designs and outcomes.
| Reference | Focus | Participants | Design | Outcomes/Results |
|---|---|---|---|---|
| Joseph et al. 2014. USA [43] | To investigate feasibility of screening serum cotinine with lead screening to increase parental smoking cessation and implementation of home smoking restrictions. | 80 smoking parents of children at well child clinics for 12 and 24 month checks. | Pilot Quasi-experimental | Parent smoking cessation: 74% engaged in smoking counselling and 24% accessed NRT. 7 day point prevalence abstinence at 8 weeks: IG 11/40 (29%) vs. CG 1/40 (p = 0.001). |
| Home smoking restrictions: High levels of smoking restriction at baseline in both groups, change not significant (IG full ban: 67.5% at baseline vs. 86.8% at 8 weeks; CG full ban 77.5% at baseline vs. 80% at 8 weeks). | ||||
| Jiminez-Muro et al. 2013. Spain [38] | To analyse the efficacy of a motivational interview intervention in postpartum women to prevent relapse in recent quitters and encourage behaviour change in those still smoking. | 412/626 postpartum women smokers. 64% Spanish, 34% immigrants. | RCT | Continuous abstinence: Probability of remaining abstinent at 12 week was 74% (IG) & 37% (CG) (p < 0.001). |
| Urine Cotinine: Only 49% of participants attended 3 month visit and therefore biochemical validation was not statistically significant (int 31%, control 23%, n.s.). | ||||
| Phillips et al. 2012. USA [35] | To reduce smoking relapse and prolong breastfeeding in mothers during the first 8 weeks postpartum. | 54 mothers of an infant in NICU. Mothers had a history of tobacco use during or within one year of pregnancy, but currently not smoking. | RCT | Maternal smoking status at 8 weeks postpartum: Significant decrease in smoking relapse at 8 weeks postpartum in the int gp (IG: 81% vs. CG: 46%, p < 0.001). |
| Salivary cotinine: A 94% agreement was found between salivary cotinine level and mothers reported smoking status. | ||||
| Disantis et al. 2010. USA [41] | To pilot a postpartum smoking intervention that combined postpartum smoking cessation & relapse prevention with breastfeeding counselling. | 31 low income women who were either current smokers or recent ex-smokers. Hispanic (50%), African-American (25%). Primiparous (45.8%). 62.5% completed high school or higher education. Years of smoking M = 6.96 years (SD = 5.67). Daily cigarettes M = 12.5 (SD = 7.7) 51% quit smoking prior to pregnancy. | Pilot 2 arm experimental | 7-day point prevalence: S + B: 50%; RP: 75%, not significant. |
| Days to relapse: related to duration of breastfeeding (r = 0.92, p = 0.08). | ||||
| S + B: mothers who quit before or during pregnancy had higher rates of smoking abstinence than those who smoked through pregnancy (x2 = 4.00, p < 0.05). | ||||
| Storrø et al. 2010. Norway [42] | To evaluate the impact of a primary prevention intervention program on risk behaviour for allergic disease in primary health care settings (increase cod liver and oily fish intake, reduce parental smoking, reduce indoor dampness). | 2860 pregnant women or women with a child <2 years of age. | Cohort control trial with one year time difference | Maternal smoking frequencies: Significant and stable decline in smoking from pregnancy to 2 years postnatal, not attributable to intervention. In addition, there was a statistically significant annual trend in the control cohort. (Baseline: IG 17.3% vs. CG 23.6%. OR 0.70, 95% CI 0.60-0.82. 6 weeks: IG 5.3% vs. CG 10.8%. OR 0.55, 95% CI 0.42-0.70. 2 years: IG 9.9% vs. CG 19%. OR 0.50, 95% CI 0.41–0.61). |
| Winickoff et al. 2010. USA [40] | To test an intervention to address maternal and paternal smoking during postpartum hospitalization. | 101/173 parents. 71% current smokers, 29% recent quitters. 67% female. | Quasi RCT | 7 day point prevalence of cotinine verified tobacco abstinence for 3 months: Self-reported 7 day abstinence not significant (IG: Decreased 31% to 25%; CG: 38% to 28%. Effect 9.4%, n.s.). Cotinine confirmed 7 day abstinence rate at follow up IG: 9% vs. CG: 3% (n.s.). |
| Self-reported 24 h quit attempts: IG: 64%; CG: 18%, p = 0.005. | ||||
| Hannover et al. 2009. Germany [39] | To test the efficacy of an intervention to aid cessation/relapse prevention for postpartum women. | 644 women from 6 hospitals with postpartum units. | Quasi RCT | Sustained abstinence (Still not smoking at 6 months or since birth): No statistically significant difference at follow up. |
| Repeated 4 week point prevalence (not smoking 4 weeks prior to follow up). | ||||
| No statistically significant difference in sustained abstinence at either follow up. Statistically significant 4 week point prevalence abstinence at 6 months only. | ||||
| Hovell et al. 2009. USA [34] | To test the effects of SHS and smoking counselling in high risk families. | 150/244 mothers of children aged less than 4 years exposed to minimum of 3 maternal cigarettes per day. | RCT | Reported SHS exposure: Decrease in both IG (80%) & CG (55%) in first 6 months. Group main effect 6–18 months significant for IG (p = 0.011). |
| Child urine cotinine: Decreased baseline to 6 months only (25% both gps). Only the group main effect significant for 6–18 months (p = 0.026). Controls higher throughout baseline & follow up. | ||||
| Maternal smoking (self-report): 6 months: IG decreased by 34%, CG decreased 5%. 6–18 months: IG decreased by 33% CG, 4.6%. | ||||
| Smoking cessations: 17% IG and 5.4% CG quit smoking for 7 days before one or more study measures. | ||||
| Øien et al. 2008. Norway [44] | Investigate parental smoking behaviour during pregnancy after introduction of a prenatal, structure, multidisciplinary smoking cessation intervention. | 3839 pregnant women attending primary health care settings. Estimated participation rate of 44% of eligible women in the location (Tondheim). Low smoking prevalence at inclusion (IG: 4.9%, CG: 7.1%). | Control trial | Self-reported smoking behaviour 6 weeks postnatal. No significant difference between IG and CG. |
| Culp et al. 2007. USA [48] | Evaluate health and safety intervention with first time mothers. | 355 pregnant women in rural south-western states (IG: n = 156, CG: n = 107). 61% smokers. | Quasi-experimental | Maternal smoking behaviour (no. of cigarettes/day): Baseline: n/s between IG and CG. Six months: IG smoking 2.4 fewer cigarettes per day (IG: M = 6.34; SD = 6.95 vs. CG: M = 8.72, SD 7.26, t (147) = 2.0, p = 0.023). Twelve months: IG smoking 2.1 fewer cigarettes per day (IG: M = 7.28, SD = 6.79 vs. CG: M = 9.41, SD = 7.09) t (147) = 1.82, p = 0.071.) |
| Knowledge of the effects of smoking on child development: e.g., Impaired brain development (IG 59.2% vs. CG 41.7%, p ≤ 0.01); lower mental health scores (IG 52.6% vs. CG 32.3%, p < 0.001). | ||||
| Kallio et al. 2006. Finland [46] | To determine whether repeated lifestyle counselling alters parental smoking and child exposure to tobacco smoke. | 1062/1105 parents of infants attending a well baby clinic. | RCT (longitudinal) | Parent smoking: Decreased across IG and CG over time. No significant difference between groups. |
| Serum cotinine of children: 46% of 8 year olds had been exposed to nicotine in last few days. None had high enough levels to confirm that they had smoked. Serum cotinine highest in children with both parent smokers. Serum cotinine higher in families where only father smoked than where only mother smoked. 24% of children from non-smoking families had cotinine higher than 1 ng/mL. | ||||
| Abdullah et al. 2005. Hong Kong [45] | To evaluate whether telephone counselling based on stages of change could help non-motivated smoking parents of young children to cease. | 952 smoking parents of Chinese children aged 5 years (85.3% fathers). | RCT | 7 point prevalence quite rate at 6 months: Higher in IG (15.3%: 68/444) than CG (7.4%: 34/459) p < 0.001. Absolute risk reduction 7.9% (95% CI: 3.78% to 12.01%). Number needed to treat 13 (95% CI: 8–26). |
| Kuiper et al. 2005. Schonberger et al. 2005. Netherlands [36,37] | To evaluate a multifaceted intervention strategy to reduce occurrence of severe asthma (smoking cessation, SHSE avoidance, dust mite avoidance, breastfeeding, timing of introduction of solid food). | Parents of 476 infants at high risk of severe asthma. | RCT | Self-report of SHSe at one year: No data reported. Authors state “No difference was found in the intervention compared with the control group concerning the exposure to tobacco smoke” (p. 329). |
| CO monitoring: No results reported. | ||||
| Wiggins et al. 2005. UK [47] | To evaluate the effect of two forms of postpartum social support (support health visitor (SVH) or community group support (CGS) on maternal and child health outcomes (maternal smoking). | 731 women with infants from culturally diverse and disadvantaged inner city areas of London. Approx 26%–30% smokers across groups. 14% non-English speakers. | RCT | Maternal smoking: not significantly reduced (SVH vs. CG: 95% CI 0.86 (0.62, 1.19); CGS vs. CG: 95% CI 0.97 (0.72, 1.33). |
| Yücel et al. 2014. Turkey [49] | To evaluate the effectiveness of an intensive intervention vs. a minimal intervention to reduce SHSe. | Parents of 182 children aged 1–5 years. | RCT | Urinary cotinine–pre and post intervention: Urine cotinine decreased across time in both groups. Decrease greater in intensive IG than minimal IG, but n.s. (p = 0.831). |
| Complete home smoking bans: Authors report that 30.6% of Intensive IG households who did not have a ban at baseline, did have a total ban at 3 months (p = 0.001). In the minimal IG, 10.5% more families had ban at 3 months, but n.s (p = 0.125). | ||||
| Wilson et al. 2013. Scotland [54] | To investigate feasibility of an intervention (REFRESH) to reduce SHSe for children in their homes. | 59/1693 smoking mothers with at least one child younger than 6 years. Maternal age M = 30 years; child age M = 3.5 years (range 1.2–5.7 years). | Pilot RCT | Difference in PM2.5 from visit 2 to visit 4: Greater reduction achieved for maximum PM. |
| Peak concentration of PM2.5: IG 67 vs. CG 148 (p = 0.006). | ||||
| The percentage of time when household PM2.5 concentrations exceeded 35 μ/m3: IG 0.49 vs. CG 3.6 (p = 0.017). | ||||
| Children’s salivary cotinine: No significant difference. | ||||
| Feasibility, acceptability and understanding of intervention: Qualitative data–intervention was acceptable and mothers were able to understand the data. | ||||
| Motivators and mechanisms of change: Personalised data made the concept of the dangers of SHSe more real to them and mothers reported a greater sense of motivation for change. | ||||
| Huang et al. 2013. Taiwan [60] | To evaluate the effectiveness of a transtheoretical model- based passive smoking prevention program for pregnant women and mothers of young children. | 294/335 women recruited from obstetrics and paediatric departments of four hospitals. IG: 48% pregnant. CG: 45% pregnant. Remainder mothers of children aged <3 years. | RCT | Stages of change: 73% were already in target stage at baseline. Less than 30% of the remaining changed stage. Distribution of stages of change statistically different after intervention between participant groups (mothers with children: F = 11.978, p = 0.003; pregnant women: F = 6.689, p = 0.035). |
| Knowledge: No significant difference between groups pre or post test. | ||||
| Frequency of avoiding passive smoking: Significant difference in intervention group (F = 5.115, p = 0.25) at post-test. | ||||
| Self-efficacy: No significant difference. | ||||
| Harutyunyan et al. 2013. Armenia [50] | To test an intense intervention to reduce child SHSe. | 250 households with children aged 2–6 years recruited via paediatrician primary health care clinics.Maternal age M = 30 years (SD 5.2 years). 53% employed, 36% had a university degree. Household smokers predominately fathers (80%). Child age M = 4 years (SD 1.2 years). Smoking was permitted in all households, some restrictions in approximately half of homes. | RCT | Child hair nicotine concentration: 17% lower in IG than CG although not significant (p = 0.239). Significantly decreased in IG from baseline to follow up (0.30 ng/mg to 0.23 ng/mg; p = 0.77). |
| Maternal knowledge of SHSe and smoking hazards: IG: From 9.5 at baseline to 11.3 at follow up. CG: From 9.8 to 10.5. 10% higher in IG than CG after controlling for baseline score (p = 0.006). | ||||
| Baheiraei et al. 2011. Iran [53] | To assess whether counselling both mother and father reduces infant SHSe. | 130 parents of health infants (<12 months) with at least one parent smoker. Families from predominately lower SES. | RCT | Urine cotinine: Decreased for both groups but significantly decreased in IG (Baseline: IG 48.72 vs. CG 40.83; 3 months IG: 28.68 vs. CG 3.32). p = 0.029). |
| Total daily cigarette consumption: Greater decrease in presence of child in IG (median = 0, interquartile range: 0, 2.71) than CG (median = 1, interquartile range: 0, 3.21) at the 3 month follow up (one tailed p, 0.3). No significant correlation between cigarettes consumed and reported level of SHSe. | ||||
| Home and car smoking bans: Increase in both IG & CG, but not significant in CG. Statistically significant between groups (p = 0.49). | ||||
| Fossum et al. 2004. Sweden [58] | To evaluate the effects of a counselling intervention (Smoke Free children). | 41 mothers of newborn infants attending child health clinics. | CT | Self reported smoking: More IG mothers reported smoking at baseline (M = 13.1, SD 6.5 than CG (M = 10.8, SD 5.7) and after intervention (M = 12.8, SD 5.9) than CG (M = 8.2, SD 4.3). |
| Maternal saliva cotinine: Cotinine levels increased by 40% in CG and decreased by 10% in IG (F = 5.501, df = 1, p = 0.027). | ||||
| Zakarian et al. 2004. USA [51] | To evaluate the effectiveness of a behavioural counselling program for reducing child SHSe. | 150 mothers of children aged less than 4 years attending a well-child community clinic. Most mothers were White, not employed, low education. Approximately 40% were single parents. | Quasi-experimental | Maternal report of child SHSe (number of maternal cigarettes child exposed to per week: Declined for baseline to 6 months post-test for both groups (IG: 18.89 at baseline to 5.41 at 12 months. CG: 13.25 at baseline to 5.23 at 12 months) (p < 0.001). Data presented in graph difficult to report exact results. Priest et al. (2008) reported data. Total exposure to cigarettes/week (IG 53.2 at baseline to 21.99 at 12 months. CG: l 54.48 atbaseline to 18.22 at 12 months) (p < 0.001). |
| No significant group x time differences. Number of counselling sessions completed was not a significant covariate. | ||||
| Children’s urinary cotinine concentration: No significant change over time in either group. No significant group x time or group differences. | ||||
| Maternal smoking rates: Similar to SHSe above, a sharp decline from baseline to post-test across both groups. | ||||
| Maternal smoking cessation: Self-reported 7-day quit status did not vary by experimental group at any time point. | ||||
| Chan-Yeung et al. 2000; Becker et al. 2004, Chan-Yeung et al. 2005. Canada [55,56,57] | Prevention of asthma in high-risk infants via multifaceted intervention program (house dust mite control, pet avoidance, avoidance of ETS, promotion of breastfeeding). | 545 infants at high risk for asthma and their families. 7% of mothers smoking at baseline (36/493). | RCT | Parental smoking cessation: No significant difference in proportion of mothers, fathers or others who gave up or acquired smoking at 12 months. |
| Conway et al. 2004. USA [59] | To evaluate the effectiveness of a lay delivered intervention to reduce ETS exposure in Latino children. | 143 Latino parent-child pairs. Child age 1–9 years (M = 4 years). | RCT | Child hair nicotine (log ng/mg): Baseline (IG: 0.25 vs. CG 0.23), post intervention (IG: 0.17 vs. CG: 0.19, 3 months (IG: 0.28 vs. CG 0.32), 12 months: (IG: 0.23 vs. CG: 0.23). No significant differences between groups over time. |
| Child hair cotinine (log ng/mg): Baseline (IG 0.05 vs. CG 0.05), post intervention (IG 0.03 vs. CG 0.03), 3 months (IG 0.04 vs. CG 0.04), 12 month (IG 0.02 vs. CF 0.04). No significant differences between groups, but time effect detected (p < 0.001). | ||||
| Parent report of number of cigarettes child exposed to in household over one month: Baseline (IG 1.75 vs. CG 1.85), post intervention (IG 1.42 vs. CG 1.62), 3 months (IG: 1.27 vs. CG 1.44), 12 months (IG: 1.06 vs. CG 1.27). No significant difference between groups, trending toward significance over time (p = 0.048). | ||||
| Confirmed reduction (dichotomous variable based on parent report and child hair biomarkers: Not significant. | ||||
| Emmons et al. 2001. USA [52] | Outcome evaluation of project KISS (Keep Infants Safe From Smoke). | 291 smoking low-income parent/caregivers. Children younger than 3 years. | RCT | Nicotine levels in household: significant time-by-treatment effect (F (2406) = 4.80, p < 0.01). IG: Levels at 3 & 6 months significantly lower than baseline (F (2200) = 4.36; p < 0.5). |
| Smoking cessation: Overall cessation 7.5% CG vs. 10.1% IG. No significant difference between groups. | ||||
| Kitzman et al. 2010. USA [61] | To test the effect of prenatal and infancy home visits by nurses on 12 year old first born children’s use of substances (cigarettes, alcohol, marijuana). | 1139 low SES African-American women pregnant with first child. | RCT (longitudinal) | Substance use by children: IG less likely to have used substances (CG: 5.1 vs. IG 1.7, OR 0.31, p = 0.04), to have used fewer of these substances (incidence ratio = 0.22, p = 0.02) and to have used these substances for fewer days (incidence ratio, 0.15, p = 0.02). |
CG: Control group, IG: Intervention group, NRT: Nicotine replacement therapy, PM2.5: Airborne particulate matter < 2.5 μm in size, RR: Response rate, SC: Standard care; SES: Socioeconomic status, SHSe: Second-hand smoke exposure, ETS: Environmental tobacco smoke, UK: United Kingdom, USA: United States of America.
3.1. Smoking Cessation Interventions
Fifteen articles on smoking cessation were reviewed and, of these, two articles were drawn from the same study [36,37]. The majority were from the United States and Europe and used a prospective single centre randomised controlled trial design (Table 3).
3.1.1. Target Populations
Most studies targeted families in the postpartum period. Of these, five studies were designed to prevent relapse in parents who had stopped smoking in response to pregnancy, or to encourage smoking behaviour change or cessation in parents who were still smoking [35,38,39,40,41]. One study specifically targeted parents of infants at high risk for severe asthma [36,37]. Only two studies reported on family based interventions of children aged 1–5 years [42,43]. Studies varied considerably in sample size–from 31 to 3889 (Table 3).
3.1.2. Interventions
The content and focus of interventions ranged considerably (Table 4). Four studies reflected existing smoking cessation intervention practice guidelines or programs [40,42,44] or smoking cessation information tailored to stages of change [45]. Two studies used education relating to healthy behaviours and risk of smoking [38,46]. Two studies had no direct intervention that focussed on smoking or associated risk at all. Instead, the focus was on the promotion of bonding and attachment between the parents and newborn infant as a way to promote smoking cessation [35] or through different models of social support during the early postpartum period [47]. A further three studies included smoking cessation interventions within the context of a universal health promotion program [46,48] or as one part of a multifaceted intervention to reduce the risk of severe asthma in at risk infants [36,37].
Table 4.
Characteristics of interventions.
| Author | Content | Delivery Personnel | Method of Communication | Intensity/Complexity | Environment | Conceptual Framework | Socio-Ecological Model | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking cessation/relapse prevention | |||||||||||||||||||
| Joseph et al. 2014 [43] | Serum cotinine feedback, SHSe education, optional counselling, optional NRT | Trained tobacco advisor | Mail and phone | Weekly for 8 weeks | Home | MI, CBT | Intrapersonal | ||||||||||||
| Jiminez-Muro et al. 2013 [38] | Risks of smoking, health behaviours | Research student | Phone | 5 × 15 minute calls over 3 months | Home (phone) | MI | Intrapersonal | ||||||||||||
| Phillips et al. 2012 [35] | Newborn cues | Not stated. Partially self-administered | DVD Brochure | Not described | Hospital and home | Attachment theory | Intrapersonal | ||||||||||||
| Disantis et al. 2010 [41] | Smoking and breastfeeding counselling OR relapse prevention | Counsellor | Face to face Written materials | 15 minutes + written materials | Clinic | Not stated | Intrapersonal | ||||||||||||
| Storro et al. 2010 [42] | Brief 5As | GP or midwife | Face to face | At least 5 occasions | Clinic | Brief 5As | Intrapersonal Interpersonal | ||||||||||||
| Winickoff et al. 2010 [40] | Brief 5 As | Trained study staff | Face to face | 15 minutes + offer to enroll in Quitline | Hospital | Brief 5As | Intrapersonal Interpersonal | ||||||||||||
| Hannover et al. 2009 [39] | Relapse prevention/smoking cessation counselling | Trained study staff | Face to face + phone | Single interview + phone follow up × 2 | Home | MI | Intrapersonal | ||||||||||||
| Hovell et al. 2009 [34] | SHSe reduction and tailored smoking cessation including option of NRT | Study counsellor | Face to face + phone | 14 sessions over 7 weeks. Mean time/session: 23 minutes | Home | Learning theory | Intrapersonal Interpersonal | ||||||||||||
| Oien et al. 2008 [44] | Brief office intervention (Fiore et al. 2000) | Midwives, GP, nurses | Face to face | Not clear | Primary health care | Not stated | Intrapersonal | ||||||||||||
| Culp et al. 2007 [48] | Universal program, including smoking and effect of SHSe on infant growth and development | Visitors with child development degree level qualifications | Face to face | Average 10.9 visits before birth + 20.7 visits after birth (approx 1 h per visit) | Home | Not reported | Intrapersonal | ||||||||||||
| Kallio et al. 2006 [46] | Universal program including smoking | Paediatrician and dietician | Face to face | Paediatrician: every 1–3 months until 2 years Dietician: every 4–6 months until 2 years. Dietician and paediatrician every 6 months until 7 years | Clinic | Not reported | Intrapersonal | ||||||||||||
| Abdullah et al. 2005 [45] | Smoking cessation and SHSe reduction tailored to stage of change. No NRT information | Nurse | Phone + written materials | Three phone calls × 20–30 min | Home via phone | Transtheoretical model (stages of change) | Intrapersonal | ||||||||||||
| Kuiper et al. 2005. Schonberger et al. 2005 [36-37] | Smoking cessation and home bans on smoking | Research nurse | Face to face | Once | Not explained | Not explained | Intrapersonal Interpersonal | ||||||||||||
| Wiggens et al. 2005 [47] | Social support | Health visitor OR non-professional | Face to face | 1.5–10 h | Home OR community centre | Not explained. ? social support | Intrapersonal Interpersonal | ||||||||||||
| SHSe reduction interventions | |||||||||||||||||||
| Yucel et al. 2014 [49] | SHSe information, goal setting, use of resources, urine cotinine feedback | Researcher | Face to face Phone Written materials | Intensive group: Home visits at baseline, 1 & 3 months. Phone calls at 6 & 8 weeks. Minimal intensity group: Home visit at baseline and 3 months. Mail out urine cotinine result | Home | Not stated | Intrapersonal | ||||||||||||
| Wilson et al. 2013 [54] | 24 h measure on home air quality PM2.5 (particulate matter) & motivational interview | Research staff | Face to face | Four visits over a one month period | Home | MI | Intrapersonal | ||||||||||||
| Huang et al. 2013 [60] | Impact of passive smoking, avoiding passive smoke in public and at home. Sections tailored to stages of change. | Research staff | Face to face, audiovisual, written materials, phone | Time not stated. Included DVD, booklet, stickers, phone follow up at 2 weeks and 3 weeks post intervention | Home | Transtheoretical model (stages of change) | Intrapersonal | ||||||||||||
| Harutyunyan et al [50]. | Importance of healthy environment, dangers of smoking and SHSe, smoking cessation, smoke-free home, PM25 feedback, written materials. CG: written materials only | Research staff | Face to face Written materials Phone | 40 minute MI + 2 follow up phone calls (timeframe not specified) | Home | MI | Intrapersonal Interpersonal | ||||||||||||
| Baheiraei et al. 2011 [53] | Smoke free children (Fossum et al. 2004 [58]) | Research student | Face to face Phone Written materials | One face to face interview + two phone interviews (max. 20 min each) | Home | MI | Intrapersonal | ||||||||||||
| Chan-Yeung et al. 2000, Becker et al. 2004, Chan-Yeung et al. 2005 [55,56,57] | Counselled on smoking cessation and instructed to keep house smoke free | Research nurse | Face to face | Single prenatal visit | Home | Risk factors for asthma | Intrapersonal Interpersonal | ||||||||||||
| Conway et al. 2004 [59] | Problem solving aimed at lowering child ETS in the household | Lay bicultural and bilingual Latina community health advisors. All received 20 h training over 4 weeks | Face to face Phone | Six sessions over four months | Home | Not stated, but problem solving, positive reinforcement & social support described. | Intrapersonal Interpersonal | ||||||||||||
| Fossum et al. 2004 [58] | Counselling for effects of SHSe, monitoring SHSe, changing smoking habits, supporting non-smoking | Child health nurses | Face to face | Not explained | Child health clinic | Self-efficacy | Interpersonal | ||||||||||||
| Zakarian et al. 2004 [51] | Behavioural counselling including contracting to reduce SHSe, problem solving, goal setting and self-monitoring | Health educators Nurses Medical assistants | Face to face | Seven counselling sessions over 6 months | Clinic (× 3) Home via phone (× 4) | SLT (Bandura 1977) and behavioural ecological model (Hovell, Wahlgreen & Gehrman, 2002 [ref]) | Interpersonal | ||||||||||||
| Emmons et al. 2001 [52] | Choice, personal responsibility for change, sel-efficacy, feedback on CO level. Tailored to interest in quitting smoking or reducing SHSe | Health educator | Face to face Phone | One 30–45 motivational interview + four follow up phone calls | Home | MI | Interpersonal | ||||||||||||
| Anti-smoking socialisation | |||||||||||||||||||
| Kitzman et al. 2010 [61] | Nurse Family Partnership. Home visiting program during first two years of child’s life (health promotion, parenting support, developmental screening, planning for pregnancies, education and employment) | Nurse | Face to face | Mean visits during pregnancy = 7 (range 0–118). Mean visits during first two years = 26 visits (range 0–71) | Home | Family partnership model | Intrapersonal Interpersonal | ||||||||||||
In most instances, the intervention was delivered either by research personnel who had received additional training in smoking cessation [36,37,38,39,40] or health care professionals [42,44,45,47]. Most interventions took place in an individual face to face counselling session. Some studies augmented these sessions with phone counselling [39] or with written or audio-visual materials [35,38].
There was considerable variation in the intensity and duration of interventions. They ranged from brief, single interventions [40] to a repeated intervention over a seven year period [46]. Interventions took place either in the home or a clinical environment.
Limited detail of the conceptual frameworks underpinning interventions was provided in the retrieved studies. Those that did provide details had utilised the principles of motivational interviewing [38,39,43], the 5A model for smoking cessation [40,42] or the transtheoretical model of behavioural change [45]. In the two studies where the intervention did not focus on smoking as a risk, the intervention designs suggested that attachment theory [35] or social support [47] were used.
3.1.3. Outcome Measures
All studies used primary outcome measures that were based on self-report of smoking abstinence status such as 7-day point prevalence [40,41,43], self-report of smoking status at a time point [35,44,46,47,48], or self-report of continuous smoking abstinence [38,39] (Table 3). Four studies used biochemical measures as a secondary outcome to verify the self-report measures including maternal urine cotinine [38,40], maternal salivary cotinine [35], or cotinine measures from the parent’s children [46]. Carbon monoxide monitoring [36,37] was used, but results were unreported. Additional secondary outcomes included home smoking restrictions or bans [43] and maternal knowledge of second hand smoke effects [48].
3.1.4. Effectiveness
Of the 13 studies reviewed, only four reported statistically significant positive effects [35,38,43,45].
3.2. Environmental Tobacco Smoke (ETS) Interventions
Twelve articles reporting on ten studies of family based interventions to reduce ETS were located (Table 3). The majority of studies focused on SHSe reduction, and used an RCT design. Participant retention ranged from 76% to 88%.
3.2.1. Target Populations
The studies targeted families of young children (1–5 years) or those pregnant or caring for infants [49,50,51,52]. Four studies targeted populations with lower socioeconomic status [51,52,53,54] and one study targeted parents of infants at high risk for asthma [55,56,57]. The numbers of participants ranged from 41 to 545.
3.2.2. Interventions
Specific details of the intervention content were not always well described (Table 4). One program used a previously validated SHSe intervention program [53]. The remaining studies developed new interventions or materials using a range of strategies to engage with families such as motivational interviewing [50,52,53,54] or counselling [49,51,55,56,57,58,59]. Four studies used some form of biochemical monitoring and feedback as part of the intervention including home air quality [50,52,54] and child urine cotinine [49].
The studies provided limited information regarding personnel responsible for implementation of the intervention. Most studies reported use of research staff for the intervention, but few provided additional details of professional background. Methods of communication included a mixture of face to face counselling or education, supplemented with telephone support and written materials.
There was considerable variation in intensity of interventions ranging from a single prenatal visit [55,56] to seven counselling sessions over a 6 month period [51]. Little information on session length was provided. The majority of interventions took place, either partially or wholly, in participants’ homes.
The conceptual framework underpinning interventions was not consistently described. Motivational interviewing, the transtheoretical model of behaviour change, social learning theory and the behavioural ecological model were named.
3.2.3. Outcomes
Eight studies used biochemical measures either as a primary outcome for the study, or as a secondary outcome to validate parental self-report of smoking behaviour, including household and child measures (Table 3). Biochemical measures based in the household included air particulate matter (PM2.5) [54] and household nicotine levels [52], while child biochemical measures included urine cotinine [49,59], hair nicotine concentration [50,59] and salivary cotinine [54]. One study used maternal salivary cotinine as a secondary outcome measure to verify maternal self-report outcomes [58].
Parent self-report of smoking behaviour was frequently included as an outcome measure, but the assessment varied considerably. One study asked parents to estimate the number of maternal cigarettes that the child was exposed to in one week [51], while another study sought parent reports of the number of household cigarettes that a child was exposed to in one month [60]. Other approaches included parent estimate of the frequency of SHSe avoidance [61], the introduction of household smoking bans [49] or child SHSe exposure before and after birth [55,56,57]. Four studies included current parent current smoking or cessation status [51,52,55,56,57,58]. Two studies included an assessment of maternal knowledge of SHSe and smoking risk [50,60],
3.2.4. Effectiveness
Most studies reported positive results following interventions. These included increased self-reported household restrictions on smoking, decreased cigarette consumption, or avoidance of SHSe [49,51,53,60]. Some confirmation was validated through decreased cotinine levels [52,58,59] or improved air quality [54]. There were no significant changes in parent report of smoking cessation in these studies.
3.3. Anti-Smoking Socialisation Interventions
One study analysed the impact of a family-based intervention on children’s smoking behaviour later in life [61] (Table 3 and Table 4). This longitudinal RCT investigated the effect of a two year home visiting model (Nurse Family Partnership) during pregnancy and infancy (through age 2) on the use of substances by children at age 12 years. The Nurse Family Partnership model uses an individualised family approach to improving the outcomes of pregnancy through health promotion of maternal health behaviours, promoting effective parental care and enhancing parent outcomes in pregnancy planning, education and finding employment. While no specific data on tobacco use was described, outcome measures included first born child self-report of substances use at 12 years of age. Children of mothers participating in Nurse Family Partnership were less likely to have used substances, to have used fewer of these substances and to have used these substances for fewer days.
4. Discussion
Family based interventions for smoking cessation, relapse prevention and ETS reduction have taken place in a wide range of contexts, targeting families at different stages of family life. Heterogeneity among approaches to interventions, target populations, contexts and efficacy makes it difficult to draw firm conclusions about the best approach. However, interventions for parent smoking cessation and relapse prevention seem to have been less successful than interventions to reduce SHSe. No studies were found that considered third hand smoke contamination.
Whilst it is tempting to argue that SHSe reduction interventions should be considered as an element of any family based intervention, there is some evidence that interventions that try to address more than one element of a smoke free home or are based on universal precautions for substance abuse may be less effective than those that focus on a single target [28]. In previous reviews, both Patnode et al. [25] and Rosen et al. [62] observed that smoking cessation interventions were more likely to be effective when the focus was on smoking cessation only. At the same time, it is important to recognise that smoking cessation is difficult to achieve and commonly requires multiple quit attempts [63]. In the meantime, ETS reduction remains an important harm reduction strategy.
For studies that targeted parents in pregnancy and early parenthood, the focus was more likely to be on maternal smoking, due to the higher risks from prenatal and postnatal exposure. Early pregnancy and transition to parenting are often perceived to be a powerful motivator for change in health behaviour, but this may be counter-balanced by demographic factors in the smoking trajectory of women during their childbearing and childrearing years related to maternal age, education, ethnicity and socioeconomic status [64,65]. Smoking is often generational and embedded in social network [66]. The smoking of fathers and other family members should not be overlooked. For example, fathers are increasingly taking on primary care roles, and the transition to becoming a parent may also be a motivator to change smoking behaviour [67].
There is some indication that parents of infants or very young children may not be as responsive to intervention as parents of children in the pre-school to school age range [68]. Parents of infants are making their first transition to parenting or coping with the new infant in the context of an already busy family life. Nonetheless, they should not be excluded from interventions as they indicate that they are receptive to the message, and can increase knowledge, even though they may not be ready to implement change [40]. More programs that compare interventions with families at different stages of development (e.g., pregnancy/first year and children over 1 year) are required.
Surprisingly few studies seem to have explicitly considered any of the parenting or family based theories in the development and delivery of their interventions. The positive results reported by Phillips et al. [35] suggest that including such theoretical frameworks may be useful in increasing parent motivation for change when used in conjunction with other smoking behaviour interventions in the pre and postnatal period. Furthermore, the interventions used individual techniques, such as motivational interviewing or counselling. This is unsurprising, as few studies truly considered the wider family as part of their target group, yet intrapersonal factors such as knowledge, attitudes, beliefs and values are affected by relationships with others [69].
Interventions that are “family based” should incorporate or offer both intra- and interpersonal level interventions and need further consideration in the context of family based interventions. Given that social cohesion and support is an important factor in continuing abstinence, [70], the importance of interventions that are truly inclusive of the family, not just the smoking parent, are required. Reviews of older children and families have reported studies that included a wider community component in their intervention, and there is some evidence that multi-sector programs that encompass individual, family and community contexts may be more likely to succeed [26]. However, the number of studies are limited and conducted mainly in Western developed countries and have yet to assess efficacy in families with younger children. Consideration of extended family and community level interventions may be critical in the development and delivery of interventions in developing countries as these levels of intervention may be more cost-effective and culturally appropriate [71].
Given the decrease in adult smoking in Western developed countries, it would seem appropriate to target families where smoking is more likely, particularly those of lower socioeconomic status. Yet, little is understood about the best ways in which to reach such families [72]. Depending on their circumstances, families with vulnerabilities may need more support that is offered in brief or individual programs [73]. For example, few studies considered increased availability, access to, or financial support for nicotine replacement therapy.
The use of biochemical markers and environmental air monitoring as either an intervention or outcome measure may be contentious. There is considerable cost associated with these methods and some evidence that parent self-report is a reasonably successful alternative when cost limitations prohibit the use them. Furthermore, such methods may not detect small changes in exposure level over time and monitoring of the control group participants may have an intervention effect [62]. In this review, some studies using biochemical markers or environmental monitoring reported higher refusal rates [50] and of parents who did participate, some would not consent or did not complete biochemical monitoring [38,57] or did not complete. While not conclusive, it is possible that some families may not be comfortable with the level of intrusion that biochemical or environmental monitoring might entail. The use of such devices may exacerbate the sense of stigma associated with being a smoker and thus affect participation in research [73]. Studies that explore parental perceptions of biochemical and environmental monitoring as either intervention or outcome are absent from the literature.
Limitations
Limitations of this review include the English language-only literature inclusion and search terminology that did not encompass substance use or drug references. The majority of studies included in this review were from Western developed countries. More studies are needed from developing countries, particularly as this is a “growth” area for tobacco use. Some studies were excluded because child age data was not provided.
5. Conclusions
Smoking cessation interventions are critically important and there is a need for a range of interventions that are both tailored and targeted to specific populations and also opportunistic models of interventions that can be activated during clinical encounters. As in many non-pharmacological interventions, quality of reporting challenges identification of intervention elements. Based on this review, interventions that target the social and psychodynamics of the family should be considered further, particularly with regard to vulnerable populations.
Author Contributions
Nicola Brown, Tim Luckett, Patricia M. Davidson and Michelle Di Giacomo contributed to the development of the study objectives and the design of the review protocol. Nicola Brown obtained and reviewed literature, and had responsibility for manuscript drafts. All authors discussed findings of the review, read, reviewed and contributed to the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization WHO Report on the Global Tobacco Epidemic, 2013. [(accessed on 2 May 2014)]. Available online: http://www.who.int/tobacco/global_report/2013/en/
- 2.Öberg M., Woodward A., Jaakkola M.S., Peruga A., Prüss-Ustün A. Global Estimate of the Burden of Disease from Second-Hand Smoke. World Health Organisation; Geneva, Switzerland: 2010. [Google Scholar]
- 3.Matt G.E., Quintana P.J.E., Destaillats H., Gundel L.A., Sleiman M., Singer B.C., Jacob P., III, Benowitz N., Winickoff J.P., Rehan V., Talbot P., Schick S., Samet J., Yinsheng W., Bo H., Martins-Green M., Pankow J.F., Hovell M. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ. Health Perspect. 2011;119:1218–1226. doi: 10.1289/ehp.1103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Protano C., Vitali M. The new danger of thirdhand smoke: Why passive smoking does not stop at secondhand smoke. Environ. Health Perspect. 2011;119:A422–A422. doi: 10.1289/ehp.1103956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Been J.V., Nurmatov U.B., Cox B., Nawrot T.S., van Schayck C.P., Sheikh A. Effect of smoke-free legislation on perinatal and child health: A systematic review and meta-analysis. Lancet. 2014;383:1549–1560. doi: 10.1016/S0140-6736(14)60082-9. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi-Bee J., Jere M.L., Britton J. Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: A systematic review and meta-analysis. Thorax. 2011;66:847–855. doi: 10.1136/thx.2010.153379. [DOI] [PubMed] [Google Scholar]
- 7.Loke A.Y., Wong Y.P.I. Smoking among young children in Hong Kong: Influence of parental smoking. J. Adv. Nurs. 2010;66:2659–2670. doi: 10.1111/j.1365-2648.2010.05419.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson A.V., Shete S., Prokhorov A.V., Wilkinson A.V., Shete S., Prokhorov A.V. The moderating role of parental smoking on their children’s attitudes toward smoking among a predominantly minority sample: A cross-sectional analysis. Subst. Abuse Treat. Prev. Policy. 2008;3 doi: 10.1186/1747-597X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bricker J.B., Peterson A.V., Jr., Leroux B.G., Andersen M.R., Rajan K.B., Sarason I.G. Prospective prediction of children’s smoking transitions: Role of parents’ and older siblings’ smoking. Addiction. 2006;101:128–136. doi: 10.1111/j.1360-0443.2005.01297.x. [DOI] [PubMed] [Google Scholar]
- 10.Chang H.-Y., Wu W.-C., Wu C.-C., Cheng J.Y., Hurng B.-S., Yen L.-L. The incidence of experimental smoking in school children: An 8-year follow-up of the child and adolescent behaviors in long-term evolution (CABLE) study. BMC Public Health. 2011;11 doi: 10.1186/1471-2458-11-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuolo M., Staff J. Parent and child cigarette use: A longitudinal, multigenerational study. Pediatrics. 2013;132:568–577. doi: 10.1542/peds.2013-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn E.J., Hall L.A., Rayens M.K., Burt A.V., Corley D., Sheffel K.L. Kindergarten children’s knowledge and perceptions of alcohol, tobacco, and other drugs. J. School Health. 2000;70:51–55. doi: 10.1111/j.1746-1561.2000.tb07241.x. [DOI] [PubMed] [Google Scholar]
- 13.Dalton M.A., Bernhardt A.M., Gibson J.J., Sargent J.D., Beach M.L., Adachi-Mejia A.M., Titus-Ernstoff L.T., Heatherton T.F. Use of cigarettes and alcohol by preschoolers while role-playing as adults: “Honey, have some smokes”. Arch. Pediatr. Adolesc. Med. 2005;159:854–859. doi: 10.1001/archpedi.159.9.854. [DOI] [PubMed] [Google Scholar]
- 14.De Leeuw R.N., Engels R.C., Scholte R.H. Parental smoking and pretend smoking in young children. Tob. Control. 2010;19:201–205. doi: 10.1136/tc.2009.033407. [DOI] [PubMed] [Google Scholar]
- 15.Hruba D., Zaloudikova I. Why to smoke? Why not to smoke? Major reasons for children’s decisions on whether or not to smoke. Cent. Eur. J. Public Health. 2010;18:202–208. doi: 10.21101/cejph.a3626. [DOI] [PubMed] [Google Scholar]
- 16.Jackson C., Dickinson D. Enabling parents who smoke to prevent their children from initiating smoking: Results from a 3-year intervention evaluation. Arch. Pediatr. Adolesc. Med. 2006;160:56–62. doi: 10.1001/archpedi.160.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Kumpfer K.L., Alvarado R. Family-strengthening approaches for the prevention of youth problem behaviours. Amer. Psychol. 2003;58:457–465. doi: 10.1037/0003-066X.58.6-7.457. [DOI] [PubMed] [Google Scholar]
- 18.Huisman M., Van Lenthe F.J., Giskes K., Kamphuis C.B., Brug J., Mackenbach J.P. Explaining socio-economic inequalities in daily smoking: A social-ecological approach. Eur. J. Public Health. 2012;22:238–243. doi: 10.1093/eurpub/ckr039. [DOI] [PubMed] [Google Scholar]
- 19.Scollo M., Winstanley M. Melbourne: Cancer Council Victoria. 3rd ed. Cancer Council Victoria; Melbourne, Australia: 2008. [(accessed on 20 August 2014)]. Tobacco in Australia: Facts and issues. Available online: http://www.tobaccoinaustralia.org.au. [Google Scholar]
- 20.Siahpush M. Why is lone-motherhood so strongly associated with smoking? Aust. N. Zeal. J. Public Health. 2004;28:37–42. doi: 10.1111/j.1467-842X.2004.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 21.Australian Bureau of Statistics National Aboriginal and Torres Strait Islander Social Survey, 2008. [(accessed on 11 October 2014)]; Available online: http://www.abs.gov.au/AUSSTATS/abs@.nsf/mf/4714.0/
- 22.Lyvers M., Hall T., Bahr M. Smoking and psychological health in relation to country of origin. Int. J. Psychol. 2009;44:387–392. doi: 10.1080/00207590802511759. [DOI] [PubMed] [Google Scholar]
- 23.Purnell J.Q., Peppone L.J., Alcaraz K., McQueen A., Guido J.J., Carroll J.K., Shacham E., Morrow G.R. Perceived discrimination, psychological distress, and current smoking status: Results from the behavioral risk factor surveillance system reactions to race module, 2004–2008. Amer. J. Public Health. 2012;102:844–851. doi: 10.2105/AJPH.2012.300694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLeroy K.R., Bibeau D., Steckler A., Glanz K. An ecological perspective on health promotion programs. Health Educ. Behav. 1988;15:351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 25.Patnode C.D., O’Connor E., Whitlock E.P., Perdue L.A., Soh C., Hollis J. Primary care–relevant interventions for tobacco use prevention and cessation in children and adolescents: A systematic evidence review for the U.S. preventive services task force. Ann. Intern. Med. 2013;158:253–260. doi: 10.7326/0003-4819-158-4-201302190-00580. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Riemenschneider F., Bockelbrink A., Reinhold T., Rasch A., Greiner W., Willich S.N. Long-term effectiveness of behavioural interventions to prevent smoking among children and youth. Tob. Control. 2008;17:301–302. doi: 10.1136/tc.2007.024281. [DOI] [PubMed] [Google Scholar]
- 27.Thomas R., Perera R. School-based programmes for preventing smoking. Cochrane Database Syst. Rev. 2013;8:1616–2040. [Google Scholar]
- 28.Thomas R.E., Baker P., Lorenzetti D. Family-based programmes for preventing smoking by children and adolescents. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD004493.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions. [(accessed on 20 October 2013)]. Available online: http://www.cochrane-handbook.org/
- 30.Moher D., Liberati A., Tetziaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham C., Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 32.Agency for Healthcare Research and Quality U.S. Preventative Services Taskforce Procedures Manual. [(accessed on 6 February2014)]. Available online: http://www.uspreventiveservicestaskforce.org/uspstf08/methods/procmanual.pdf.
- 33.Popay J., Roberts H., Sowden A., Petticrew M., Arai L., Rodgers M., Britten N., Roen K., Duffy S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Lancaster University; New York, NY, USA: 2006. [Google Scholar]
- 34.Hovell M.F., Zakarian J.M., Matt G.E., Liles S., Jones J.A., Hofstetter C.R., Larson S.N., Benowitz N.L. Counseling to reduce children’s secondhand smoke exposure and help parents quit smoking: A controlled trial. Nicotine Tob. Res. 2009;11:1383–1394. doi: 10.1093/ntr/ntp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips R.M., Merritt T.A., Goldstein M.R., Deming D.D., Slater L.E., Angeles D.M. Prevention of postpartum smoking relapse in mothers of infants in the neonatal intensive care unit. J. Perinatol. 2012;32:374–380. doi: 10.1038/jp.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schonberger H.J.A.M., Dompeling E., Knottnerus J.A., Maas T., Muris J.W.M., van Weel C., van Schayck C.P. The PREVASC study: The clinical effect of a multifaceted educational intervention to prevent childhood asthma. Eur. Respir. J. 2005;25:660–670. doi: 10.1183/09031936.05.00067704. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper S., Maas T., van Schayck C.P., Muris J.W.M., Schonberger H.J.A.M., Dompeling E., Gijsbers B., van Weel C., Knottnerus J.A., the PREVASC group The primary prevention of asthma in children study: Design of a multifaceted prevention program. Pediatr. Allergy Immunol. 2005;16:321–331. doi: 10.1111/j.1399-3038.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 38.Jimenez-Muro A., Nerin I., Samper P., Marqueta A., Beamonte A., Gargallo P., Oros D., Rodriguez G. A proactive smoking cessation intervention in postpartum women. Midwifery. 2013;29:240–245. doi: 10.1016/j.midw.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Hannover W., Thyrian J.R., Roske K., Grempler J., Rumpf H.-J., John U., Hapke U. Smoking cessation and relapse prevention for postpartum women: Results from a randomized controlled trial at 6, 12, 18 and 24 months. Addict. Behav. 2009;34:1–8. doi: 10.1016/j.addbeh.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 40.Winickoff J.P., Healey E.A., Regan S., Park E.R., Cole C., Friebely J., Rigotti N.A. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: The NEWS study. Pediatrics. 2010;125:518–525. doi: 10.1542/peds.2009-0356. [DOI] [PubMed] [Google Scholar]
- 41.DiSantis K.I., Collins B.N., McCoy A.C.S. Associations among breastfeeding, smoking relapse, and prenatal factors in a brief postpartum smoking intervention. Acta Obstet. Gynecol. Scand. 2010;89:582–586. doi: 10.3109/00016341003678435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storro O., Oien T., Dotterud C.K., Jenssen J.A., Johnsen R. A primary health-care intervention on pre- and postnatal risk factor behavior to prevent childhood allergy. The Prevention of Allergy among Children in Trondheim (PACT) study. BMC Public Health. 2010;10 doi: 10.1186/1471-2458-10-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph A., Murphy S., Thomas J., Okuyemi K.S., Hatsukami D., Wang Q., Briggs A., Doyle B., Winickoff J.P. A pilot study of concurrent lead and cotinine screening for childhood tobacco smoke exposure: Effect on parental smoking. Amer. J. Health Promot. 2014;28:316–320. doi: 10.4278/ajhp.120912-ARB-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oien T., Storro O., Jenssen J.A., Johnsen R. The impact of a minimal smoking cessation intervention for pregnant women and their partners on perinatal smoking behaviour in primary health care: A real-life controlled study. BMC Public Health. 2008;8 doi: 10.1186/1471-2458-8-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdullah A.S.M., Mak Y.W., Loke A.Y., Lam T.-H. Smoking cessation intervention in parents of young children: A randomised controlled trial. Addiction. 2005;100:1731–1740. doi: 10.1111/j.1360-0443.2005.01231.x. [DOI] [PubMed] [Google Scholar]
- 46.Kallio K., Jokinen E., Hamalainen M., Kaitosaari T., Volanen I., Viikari J., Ronnemaa T., Simell O. Impact of repeated lifestyle counselling in an atherosclerosis prevention trial on parental smoking and children’s exposure to tobacco smoke. Acta Paediatr. 2006;95:283–290. doi: 10.1080/08035250500375145. [DOI] [PubMed] [Google Scholar]
- 47.Wiggins M., Oakley A., Roberts I., Turner H., Rajan L., Austerberry H., Mujica R., Mugford M. The social support and family health study: A randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas. Health Technol. Assess. 2004;8:1–120. doi: 10.3310/hta8320. [DOI] [PubMed] [Google Scholar]
- 48.Culp A.M., Culp R.E., Anderson J.W., Carter S. Health and safety intervention with first-time mothers. Health Educ. Res. 2007;22:285–294. doi: 10.1093/her/cyl079. [DOI] [PubMed] [Google Scholar]
- 49.Yucel U., Ocek Z.A., Ciceklioglu M. Evaluation of an intensive intervention programme to protect children aged 1–5 years from environmental tobacco smoke exposure at home in Turkey. Health Educ. Res. 2014;29:442–455. doi: 10.1093/her/cyu005. [DOI] [PubMed] [Google Scholar]
- 50.Harutyunyan A., Movsisyan N., Petrosyan V., Petrosyan D., Stillman F. Reducing children’s exposure to secondhand smoke at home: A randomized trial. Pediatrics. 2013;132:1071–1080. doi: 10.1542/peds.2012-2351. [DOI] [PubMed] [Google Scholar]
- 51.Zakarian J.M., Hovell M.F., Sandweiss R.D., Hofstetter C.R., Matt G.E., Bernert J.T., Pirkle J., Hammond S.K. Behavioral counseling for reducing children’s ETS exposure: Implementation in community clinics. Nicotine Tob. Res. 2004;6:1061–1074. doi: 10.1080/1462220412331324820. [DOI] [PubMed] [Google Scholar]
- 52.Emmons K.M., Hammond S.K., Fava J.L., Velicer W.F., Evans J.L., Monroe A.D. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108:18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 53.Baheiraei A., Kharaghani R., Mohsenifar A., Kazemnejad A., Alikhani S., Milani H.S., Mota A., Hovell M.F. Reduction of secondhand smoke exposure among healthy infants in Iran: Randomized controlled trial. Nicotine Tob. Res. 2011;13:840–847. doi: 10.1093/ntr/ntr085. [DOI] [PubMed] [Google Scholar]
- 54.Wilson I., Semple S., Mills L.M., Ritchie D., Shaw A., O’Donnell R., Bonella P., Turner S., Amos A. REFRESH—Reducing families’ exposure to secondhand smoke in the home: A feasibility study. Tob. Control. 2013 doi: 10.1136/tobaccocontrol-2011-050212. [DOI] [PubMed] [Google Scholar]
- 55.Becker A., Watson W., Ferguson A., Dimich-Ward H., Chan-Yeung M. The Canadian asthma primary prevention study: Outcomes at 2 years of age. J. Allergy Clin. Immunol. 2004;113:650–656. doi: 10.1016/j.jaci.2004.01.754. [DOI] [PubMed] [Google Scholar]
- 56.Chan-Yeung M., Ferguson A., Watson W., Dimich-Ward H., Rousseau R., Lilley M., Dybuncio A., Becker A. The Canadian childhood asthma primary prevention study: Outcomes at 7 years of age. J. Allergy Clin. Immunol. 2005;116:49–55. doi: 10.1016/j.jaci.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Chan-Yeung M., Manfreda J., Dimich-Ward H., Ferguson A., Watson W., Becker A. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high-risk infants. Arch. Pediatr. Adolesc. Med. 2000;154:657–663. doi: 10.1001/archpedi.154.7.657. [DOI] [PubMed] [Google Scholar]
- 58.Fossum B., Arborelius E., Bremberg S. Evaluation of a counseling method for the prevention of child exposure to tobacco smoke: An example of client-centered communication. Prev. Med. 2004;38:295–301. doi: 10.1016/j.ypmed.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Conway T.L., Woodruff S.I., Edwards C.C., Hovell M.F., Klein J. Intervention to reduce environmental tobacco smoke exposure in Latino children: Null effects on hair biomarkers and parent reports. Tob. Control. 2004;13:90–92. doi: 10.1136/tc.2003.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C.-M., Wu H.-L., Huang S.-H., Chien L.-Y., Guo J.-L. Transtheoretical model-based passive smoking prevention programme among pregnant women and mothers of young children. Eur. J. Public Health. 2013;23:777–782. doi: 10.1093/eurpub/cks177. [DOI] [PubMed] [Google Scholar]
- 61.Kitzman H.J., Olds D.L., Cole R.E., Hanks C.A., Anson E.A., Arcoleo K.J., Luckey D.W., Knudtson M.D., Henderson C.R., Jr., Holmberg J.R. Enduring effects of prenatal and infancy home visiting by nurses on children: Follow-up of a randomized trial among children at age 12 years. Arch. Pediatr. Adolesc. Med. 2010;164:412–418. doi: 10.1001/archpediatrics.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosen L.J., Myers V., Hovell M., Zucker D., Ben Noach M. Meta-analysis of parental protection of children from tobacco smoke exposure. Pediatrics. 2014;133:698–714. doi: 10.1542/peds.2013-0958. [DOI] [PubMed] [Google Scholar]
- 63.Borland R., Partos T.R., Yong H.-H., Cummings K.M., Hyland A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction. 2012;107:673–682. doi: 10.1111/j.1360-0443.2011.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mumford E.A., Hair E.C., Yu T.-C., Liu W. Women’s longitudinal smoking patterns from preconception through child’s kindergarten entry: Profiles of biological mothers of a 2001 U.S. birth cohort. Mater. Child Health J. 2014;18:810–820. doi: 10.1007/s10995-013-1305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greaves L., Hemsing N. Women and tobacco control policies: Social-structural and psychosocial contributions to vulnerability to tobacco use and exposure. Drug Alcohol Depend. 2009;104:S121–S130. doi: 10.1016/j.drugalcdep.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 66.Edwards R. Spousal smoking as an indicator of total secondhand smoke exposure. Nicotine Tob. Res. 2009;11:606–613. doi: 10.1093/ntr/ntp024. [DOI] [PubMed] [Google Scholar]
- 67.Kayser J.W., Semenic S. Smoking motives, quitting motives, and opinions about smoking cessation support among expectant or new fathers. J. Addict. Nurs. 2013;24:149–157. doi: 10.1097/JAN.0b013e3182a4caf1. [DOI] [PubMed] [Google Scholar]
- 68.Rosen L.J., Noach M.B., Winickoff J.P., Hovell M.F. Parental smoking cessation to protect young children: A systematic review and meta-analysis. Pediatrics. 2012;129:141–152. doi: 10.1542/peds.2010-3209. [DOI] [PubMed] [Google Scholar]
- 69.Simons-Morton B.G., McLeroy K.R., Wendel M.L. Behavior Theory in Health Promotion Practice and Research. Jones & Bartlett Learning; Burlington, MA, USA: 2012. [Google Scholar]
- 70.Reitzel L., Kendzor D., Castro Y., Cao Y., Businelle M., Mazas C., Cofta-Woerpel L., Li Y., Cinciripini P., Ahluwalia J., et al. The relation between social cohesion and smoking cessation among black smokers, and the potential role of psychosocial mediators. Ann. Behav. Med. 2013;45:249–257. doi: 10.1007/s12160-012-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Letona P., Ramirez-Zea M., Caballero B., Gittelsohn J. Formative research to develop a community-based intervention for chronic disease prevention in Guatemalan school-age children. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carson K.V., Brinn M.P., Peters M., Veale A., Esterman A.J., Smith B.J. Interventions for smoking cessation in Indigenous populations. Cochrane Database of Syst. Rev. 2012;1 doi: 10.1002/14651858.CD009046.pub2. [DOI] [PubMed] [Google Scholar]
- 73.Burgess D.J., Fu S.S., van Ryn M. Potential unintended consequences of tobacco-control policies on mothers who smoke: A review of the literature. Am. J. Prev. Med. 2009;37:S151–S158. doi: 10.1016/j.amepre.2009.05.006. [DOI] [PubMed] [Google Scholar]