Abstract
Dysregulation of polycystin-1 (PC1) leads to autosomal dominant polycystic kidney disease (ADPKD), a disorder characterized by the formation of multiple bilateral renal cysts, the progressive accumulation of extracellular matrix (ECM), and the development of tubulointerstitial fibrosis. Correspondingly, cystic epithelia express higher levels of integrins (ECM receptors that control various cellular responses, such as cell proliferation, migration, and survival) that are characteristically altered in cystic cells. To determine whether the altered expression of ECM and integrins could establish a pathologic autostimulatory loop, we tested the role of integrin-β1 in vitro and on the cystic development of ADPKD in vivo. Compared with wild-type cells, PC1-depleted immortalized renal collecting duct cells had higher levels of integrin-β1 and fibronectin and displayed increased integrin-mediated signaling in the presence of Mn2+. In mice, conditional inactivation of integrin-β1 in collecting ducts resulted in a dramatic inhibition of Pkd1-dependent cystogenesis with a concomitant suppression of fibrosis and preservation of normal renal function. Our data provide genetic evidence that a functional integrin-β1 is required for the early events leading to renal cystogenesis in ADPKD and suggest that the integrin signaling pathway may be an effective therapeutic target for slowing disease progression.
Keywords: autosomal dominant polycystic kidney disease, polycystic kidney disease, cell–matrix interactions, fibrosis, genetic renal disease
Autosomal dominant polycystic kidney disease (ADPKD) is a common life-threatening genetic disorder, and the main clinical manifestation is the bilateral formation and progressive expansion of renal fluid-filled cysts, which eventually lead to kidney failure. The disease is caused by the mutation of the Pkd1 or Pkd2 genes encoding polycystin-1 (PC1) or polycystin-2 (PC2), respectively.1 Distinctive features of ADPKD are the alteration of epithelial extracellular matrix (ECM)2,3 and the progressive renal fibrosis, which parallels the course of the disease.4 The involvement of the ECM in the pathogenesis of ADPKD has long been suspected5,6 and is supported by the observations that the altered expression of the ECM components laminin-α5 and proteoglycans7,8 or the focal adhesion protein tensin lead to renal cystogenesis.9 ECM is essential in not only maintaining the normal tubule architecture, but also wound repair and developmental processes as well as the regulation of cell growth, polarity, migration, and differentiation.10,11 These functions are mediated by integrins, a superfamily of heterodimeric transmembrane receptors assembled by the noncovalent association of one α-subunit and one β-subunit. In mammals, 18 α-subunits and 8 β-subunits have been described that associate to form 24 heterodimers that specifically bind different ECM substrates. Ligand-engaged integrins organize specific focal adhesions that link ECM to cytoskeletal filaments and transform mechanical tension into chemical signals.12,13 At the same time, the binding to the ligand is regulated by the intracellular interaction of the integrins’ cytoplasmic domain with activating proteins in an inside-out manner.14,15 This dynamic bidirectional control of integrin receptor signaling allows the integration of extracellular cues and intracellular pathways. Importantly, integrin signaling extends to the activation of receptor tyrosine kinases, such as EGF and TGF-β receptors, further amplifying their regulatory functions. Many of the downstream pathways triggered by integrins, such as Ras/Raf/ERK, PI3K, JNK/AP1, or Ca2+ modulation, are also activated in ADPKD cells, suggesting the contribution of integrins to the pathogenesis of ADPKD.16–20
Indeed, PC1 and integrins may intersect at different levels. The extracellular region of PC1 contains multiple consensus motifs that are shared by cell adhesion molecules and possesses affinity for matrix components, thus suggesting that it may have adhesive properties.21,22 PC1 colocalizes within integrins at the focal adhesions, suggesting that it may affect the interaction of integrins with their ECM ligands.2,6,23,24 Growing evidence indicates that integrins expressed on cilia mediate the mechanical stimulation by ECM components through cues that may involve PC1.20,25–28
The upregulation of integrin in ADPKD alters the adhesion and motility properties of cystic cells, possibly triggering a stimulatory loop that can be reinforced by the aberrant deposition of ECM.29–31 These changes may contribute to the establishment of the cystic phenotype, which was suggested by the increased integrin-α2β1–mediated survival of PC1 knockdown cells during anoikis.32 However, despite the large body of evidence supporting a possible relationship between integrin and PC1, a functional role of integrin in the pathogenesis of ADPKD has never been directly determined.
We hypothesized that, as mediators of the ECM signaling, integrins may contribute to the cystogenic process. To test this hypothesis, we focused on integrin-β1 (Intβ1), because it is the most prevalent β-chain of the heterodimers expressed in kidney.33 Our findings unveil a novel cystogenic signal downstream of PC1 by showing that Intβ1 is essential for the development of ADPKD. The dramatic extent of the reversal also suggests that Intβ1 presides to early key events in the cystic signal induced by the depletion of PC1.
Results
Increased Intβ1-Mediated Signaling in Pkd1-Ablated Cells
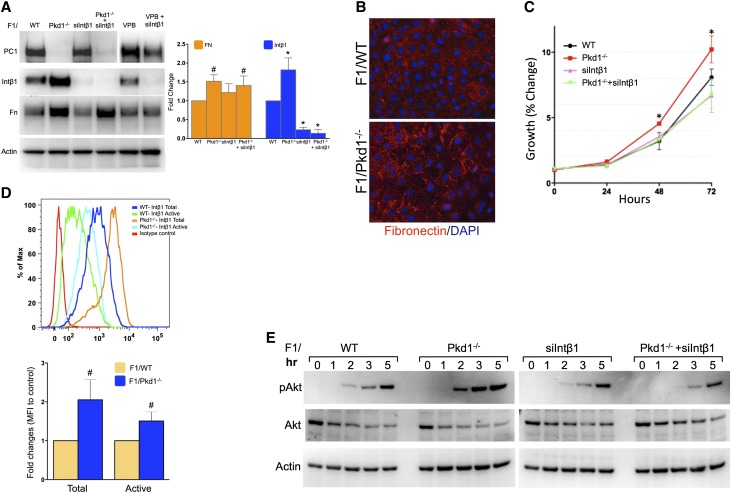
Our previous work indicated that Intβ1 was important for the hyperproliferation of PC1 knockdown IMCD3 cells (Supplemental Figure 1).34 To confirm these findings in cells depleted of PC1, we isolated epithelial cells from the kidney papillae of Pkd1fl/fl mice35 and established a collecting duct cell line (hereafter referred to as F1) by immortalization with the mouse telomerase reverse transcription (mTert), similarly to what was previously described.36 Generation and characterization of the renal collecting duct F1 cells are detailed in Supplemental Figure 2. On lentiviral-mediated Cre expression (VPB/Cre), PC1 was completely ablated in the resulting F1/Pkd1−/− cells, whereas the expression of total Intβ1 and fibronectin deposition were upregulated (Figure 1, A and B). Knockdown of Intβ1 did not affect the fibronectin expression in Pkd1-ablated cells, although it did suppress the hyperproliferative phenotype of the F1/Pkd1−/− cells (Figure 1C). No changes were detected in the F1/VPB cells transduced with the control empty VPB lentivector (Figure 1A).
Figure 1.
Ablation of Pkd1 increases activated Intβ1 and fibronectin expression. (A) Expression of PC1, Intβ1, and fibronectin in F1/WT cells before and after the ablation of Pkd1 (Pkd1−/−) and/or the knockdown of Intβ1 (siIntβ1). The bar graph in the right panel shows the quantification of the protein expression as fold change with respect to WT from five independent experiments. (B) Fibronectin immunostaining before and after Pkd1 ablation in F1 cells. (C) The knockdown of Intβ1 expression reverts the hyperproliferation of PC1-ablated cells. (D) The levels of total (MB1.2) and active (9EG7) Intβ1 were analyzed by FACS in the different cell populations. Isotype antibody and F1/siIntβ1 cells were used as controls. Upper panel shows a profile representative of three independent experiments. The bar graph in the lower panel shows the relative fold increase in mean fluorescence intensity (MFI) of Pkd1−/− versus WT. (E) Immunoblot detection of phosphorylated and total Akt expression in different cell populations stimulated for the indicated times (hours) with Mn2+ and collagen. Actin was used as gel loading control. Statistical comparison was performed by two-way ANOVA with (A and C) Bonferroni post-test and (D) two-tailed t test. FN, fibronectin. *P<0.001; #P<0.05.
Correspondingly, flow cytometry using antibodies that distinguish between total and active forms of Intβ1 (MB1.2 and 9EG7, respectively) also showed higher cell surface levels of total and active Intβ1 expression in F1/Pkd1−/−, suggesting an increased susceptibility of these cells to integrin stimulation (Figure 1D). To determine whether changes in Intβ1 levels were functionally relevant, we compared the response of the cells with the integrin activators Mn2+ and collagen (Figure 1E). When plated on collagen type I in the presence of Mn2+, F1/Pkd1−/− cells showed an enhanced Akt phosphorylation (Ser473) compared with F1/wild-type (WT) cells, thus confirming the higher integrin-mediated signaling of the PC1-depleted cells. Overall, these results confirmed the association between the loss of PC1 and the changes in ECM deposition4,6 and suggested that a positive feedback loop between Intβ1 and ECM may potentiate the cystic mechanisms in the absence of PC1.34,37
Reduced Cystogenesis and Restored Renal Function in Pkd1 Knockout Kidneys Lacking Intβ1
To assess the role of β1 integrin in ADPKD cystogenesis, we generated a conditional double knockout (DKO) mouse model in which the floxed Pkd135 and floxed Itgb138 genes are inactivated specifically in the collecting ducts by aquaporin-2 (Aqp2) promoter-driven Cre recombinase.39 The activation of the Aqp2 promoter at late developmental stages circumvents the embryonic lethality caused by the earlier ablation of either Pkd1 or Itgb1. Pkd1fl/fl, Itgb1fl/fl, and Aqp2-Cre mice were bred to generate single Pkd1 (Pkd1-KO) or Itgb1 (Itgb1-KO) KO, DKO of Pkd1 and Itgb1, and WT control genotypes within an experimental litter (Concise Methods). All four genotypes were obtained with the expected Mendelian distribution, and Itgb1-KO showed normal phenotype as previously reported.38 Efficient inactivation of the Pkd1 gene in single Pkd1-KO and DKO was confirmed by semiquantitative RT-PCR (Supplemental Figure 3).
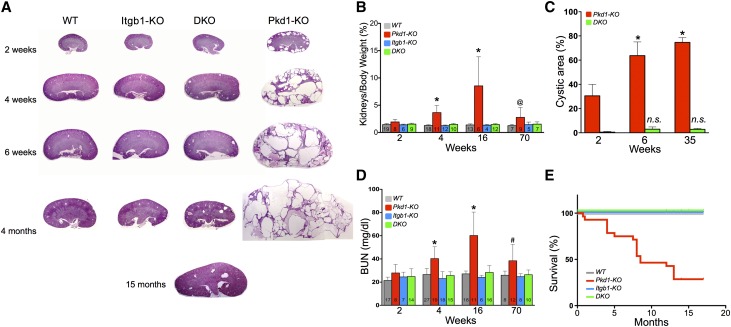
Because Aqp2 expression commences around E18.5, Pkd1-KO kidneys presented tubular dilation by postnatal day 7 (P7) and became overtly cystic by P14, with gross enlargement and progressive loss of renal architecture (Figure 2A). Positive staining with Dolichos bifluoros agglutinin (DBA) confirmed the cysts’ collecting duct origin (Supplemental Figure 3). In contrast, the kidneys of DKO littermates showed a dramatic reduction of the cystic phenotype and retained a mostly normal morphology akin to the controls WT or the Itgb1-KO, even at the oldest age (15 months) (Figure 2A). Although the kidneys-to-body weight ratio of Pkd1-KO mice increased over time, representing the significant renal cystic enlargement, the kidneys-to-body weight ratio of DKO mice remained indistinguishable from that of control WT and Itgb1-KO animals (Figure 2B). In fact, although few cysts appeared early postnatally (P14) in the DKO kidneys, their relative cystic/kidney area did not change significantly in time (Figure 2C). These results suggest a role of Intβ1 on not only cystic conversion but also, cystic progression.
Figure 2.
Pkd1-dependent cystic phenotype is reduced in Itgb1-KO mice. (A) Histologic periodic acid–Schiff-stained preparations from representative kidneys of littermates of indicated ages. The progression of the disease is blunted by the lack of Intβ1, which is shown in a representative 15-month specimen from a DKO mouse at the bottom. (B) Progressive enlargement of Pkd1-ablated kidneys determined as the percentage of body weight is prevented in the absence of Intβ1 expression. (C) The cystic area of DKO kidneys does not increase in time. (D) Ablation of Intβ1 in Pkd1 KO kidneys preserves the normal renal function, which was determined by BUN levels. (E) DKO mice have a normal survival rate. Numbers within each columns denote the number of animals (n) analyzed per group. Statistical comparison by two-way ANOVA with Bonferroni post-test. *P<0.001; #P<0.002; @P<0.04.
Importantly, the measurements of BUN indicated that the renal function of DKO animals remained normal for the whole duration of the experiments, whereas that of Pkd1-KO mice rapidly deteriorated (Figure 2D). The restoration of normal kidney function in the DKO mice was further reflected in the normalization of their survival rate, despite the lack of Pkd1. A normal survival pattern of the DKO mice was observed for the duration of the experiments, whereas the Pkd1-KO mice had an average life of approximately 7 months (Figure 2E). Regardless of the early blatant manifestation of the cystic phenotype, the lifespan of our animals is longer than the one originally reported, likely because of their mixed genetic background (our C57BL/6×129Sv/J versus original C57BL/6 background39). A small number of Pkd1-KO animals that survive longer do present a renal cystic phenotype and decreased BUN levels that remain life-compatible, emphasizing the variable penetrance of the disease and the role of gene modifiers in the development and progression of ADPKD. However, the large number of animals tested in each group (n>60) and the very high significance in the statistical differences within all of the measured parameters exclude the possibility that gene modifiers only and not the deletion of Intβ1 are responsible for the reversion of the cystic phenotype. On the contrary, the slower progression model may more accurately resemble the human course of the disease, and the dramatic absence of cystic phenotype of the DKO in the mixed background emphasizes the relevance of the Intβ1 signaling in ADPKD.
It should be noted that, in previously described models, the late inactivation of Pkd1 (>6-week-old mice) significantly retarded cystic events but did not prevent the onset of the disease.39–42 However, despite the embryonic inactivation of Pkd1, DKO kidneys showed no change in the cystic index in time and maintained a very mild phenotype, even at 15 months (Figure 2A). In fact, we observe a much larger variable degree of cystic phenotype in the Pkd1-KO kidneys than in the DKO kidneys (Supplemental Figure 4), presumably because of inhibition of cyst initiation, further strengthening the notion that a functional Intβ1 is necessary for the cystic transformation and the continuous expansion of the cystic epithelia.
Lack of Intβ1 Function Abrogates Fibrosis in Pkd1 KO Kidneys
To further characterize the reversal of cystic disease, we determined whether the depletion of Intβ1 affected the persistent cell proliferation, the accumulation of ECM proteins, and interstitial fibrosis that accompany the cystic progression in ADPKD.
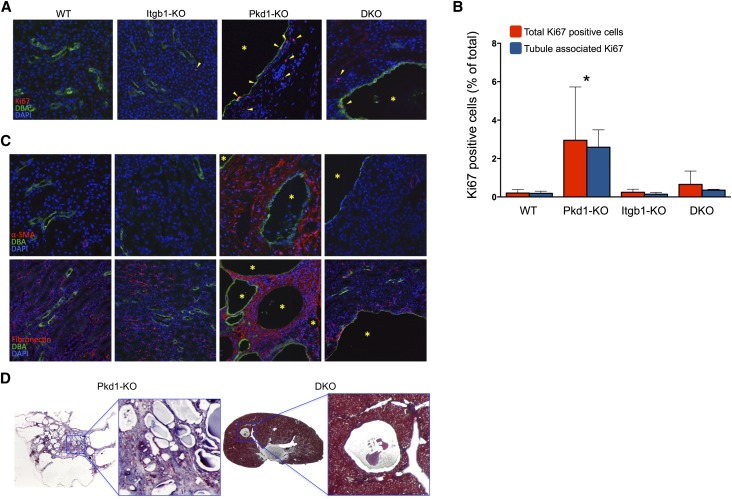
Staining for the nuclear cell proliferation marker Ki67 was easily detectable in the specimens from 6-week-old Pkd1-KO kidneys and only sporadically observed in age-matched DKO and control WT or Itgb1-KO kidneys (Figure 3, A and B). Interestingly, a significant number of the DBA-negative cells outside the cyst lining epithelia were also Ki67-positive, suggesting a crosstalk between cystic and noncystic neighboring cells (Figure 3B).
Figure 3.
Proliferation and fibrosis are abrogated in the DKO kidneys. (A) Representative immunofluorescence staining for Ki67 (red), DBA (green), and DAPI (blue). Arrowheads indicate Ki67-positive cells. Asterisks indicate cysts. (B) Quantification of total Ki67-positive cells and Ki67-positive cells of confirmed tubular origin from sections of the indicated genotypes (n=3, five ×200 fields of approximately 2000 DAPI-positive nuclei/field per animal). *P<0.001. (C) Immunostaining for α-SMA (upper panel) and fibronectin (lower panel; both in red). All renal specimens from 4-week-old mice counterstained with DBA (green) and DAPI (blue). Asterisks indicate cysts. (D) Collagen staining with Masson’s trichrome (blue) of representative 6-week-old Pkd1-KO and DKO renal specimens. Original magnification, ×200.
As expected, the cystic phenotype of Pkd1-KO kidneys was associated with intense α-smooth muscle actin (α-SMA) immunoreactivity, particularly in areas proximal to the cysts, indicating an extensive level of fibrosis (Figure 3C). Similarly pronounced and diffuse was the staining for fibronectin and collagen (Figure 3, C and D). In contrast, even in areas surrounding the few cysts, only traces of α-SMA, fibronectin, or collagen staining could be detected in DKO kidneys (Figure 3C), indicating that the absence of Intβ1 was also sufficient to blunt the progression of fibrosis.
Discussion
Our findings show that a functional Intβ1 is a necessary component in the cystogenic program triggered by the deletion of Pkd1. The course of ADPKD depends on both the frequency of cystic events (that is, the formation of new cysts) and their progressive expansion.43 The significant reduction in the cyst number that follows the simultaneous ablation of Pkd1 and Intβ1 indicates that Intβ1 signaling is involved in the early events that trigger the cystic transformation. However, the sustained suppression of the cystic load in the DKO strongly suggests that Intβ1 may be important in controlling the disease progression as well possibly by supporting cell survival and proliferation.32
Integrins are transmembrane, bidirectional signaling molecules that provide mechanical links between ECM and actin cytoskeleton and control the deposition of ECM molecules.44 The dramatic reduction of both fibronectin and collagen in the DKO kidneys suggests that more than one integrin heterodimer may be involved in and respond to the increased expression of these ECM components. We confirmed the increase of Intα2 expression in F1/Pkd1−/− cells, which we previously observed in PC1 knockdown MDCK cells32 (data not shown), but at least four Intβ1 heterodimers (α1/β1, α2/β1, α5/β1, and αV/β1) with collagen or fibronectin binding specificity can be expressed in the renal tubule. Their identification may be important to develop specific targeting approaches, but it is beyond the scope of this study. The overall evidence suggests that the concomitant increase of integrin receptors and their ligands may trigger a self-stimulatory activation loop that supports the survival and proliferation of the PC1-deficient cells. This effect may be further exacerbated by the positive role of Intβ1 in signaling by TGF-β receptor.45 Recently, Intα2/β1 was shown to exert a deleterious effect in glomerular injury by upregulating collagen production.46 Our findings in a model of ADPKD expand these observations to the collecting ducts, thus strengthening the role of Intβ1 in the renal fibrotic process.47
The reciprocal control of integrins on matrix deposition48 and ECM on integrin activation makes it difficult to determine whether the upregulation of integrin or ECM comes first.
Although the mechanisms controlling the increased expression of Intβ1 and fibronectin remain to be determined, it will be important to assess whether the fibrosis associated with CKD and possibly, diseases of other organs is similarly dependent on Intβ1 function.
Recent genetic studies have uncovered an important relationship between PCs and cilium by showing that cystogenesis caused by the ablation of either Pkd1 or Pkd2 progresses more rapidly in the presence of the cilium than in its absence.49 These findings imply a suppressive role of PCs on the cilium-dependent cystic signal. Our data suggest that a loss of PC1 leads to the sustained activation of Intβ1 and that, under normal conditions, PC1 may act as a repressor of Intβ1, possibly by regulating the ECM–integrin crosstalk.6 This role may be facilitated by the physical proximity of PC1 and Intβ1, because they colocalize at multiple subcellular compartments, including focal adhesions, cell–cell adhesions, and cilium.2,50,51 Although no direct association has been found between PC1 and Intβ1, PC1 interacts with vinculin and paxillin, adaptor proteins essential for integrin signaling and the control of adhesion complexes, suggesting that PC1 might affect the interaction of integrins with their ECM ligands.6,23 An intriguing possibility is that PC1 participates in the dynamic crosstalk between integrins and E-cadherin that regulates the cytoskeletal rearrangements required during tissue morphogenesis and repair, phases that are particularly susceptible to the cystogenic switch.40,52 Presently, however, it cannot be distinguished whether the lack of either PC1 or Intβ1 in a specific cellular compartment is responsible for the control of cystogenesis. Future experiments in cilia mutants will be necessary to establish whether Intβ1 also functions downstream of cilium-dependent cystogenesis. Equally important will be to address whether Intβ1 participates in the cystogenic process triggered by other cystic proteins, particularly PC2, with a mutant phenotype that largely overlaps with that of PC1.
Integrin expression is often altered in cancer, where it plays a role in the susceptibility and progression of the disease.53 The remarkable effects of the inhibition of Intβ1 on the ADKPD phenotype establish Intβ1 as a genetic modifier of the disease and lead to the possibility that the aberrant regulation of integrins may contribute to the variable penetrance of ADPKD.54
Overall, our data provide the first genetic evidence that Intβ1 is functionally linked to ADPKD cystogenesis. The dramatic inhibition of the cystic disease suggests that Intβ1 is involved in the early cystogenic events triggered by the lack of PC1. An important implication of this work is the identification of a previously unrecognized signaling pathway that may be targeted to interfere with ADPKD progression and fibrosis.
Concise Methods
Reagents
The following antibodies were used for Western blotting, immunofluorescence, and flow cytometry: Akt antibodies (9916; Cell Signaling Technology), Aqp2 (H-40; Santa Cruz Biotechnology), fibronectin (F3628; Sigma-Aldrich), Intβ1 (EP1041Y; Abcam, Inc.; clones 9EG7 and 18; BD Biosciences; MB1.2; EMD Millipore), Ki67 (SP6; Vector Laboratories; B56; BD Biosciences), PC1 (7E12; Santa Cruz Biotechnology), α-SMA (ab5694; Abcam, Inc.), and THP (H-135; Santa Cruz Biotechnology). FITC-conjugated lectins were purchased from Vector Laboratories. Rat-tail collagen type I was purchased from BD Biosciences.
Cell Culture
Mouse-F1 cells were established after the isolation of renal epithelial cells from kidney papillae of Pkd1fl/fl mice (B6.129S4-Pkd1tm2Ggg/J; The Jackson Laboratory, Bar Harbor, ME) by immortalization with the lentiviral vector VVPW/mTert expressing the mTert (provided by Ronald DePinho), similar to what was described by Steele et al.36 (Supplemental Figure 2), and maintained in DMEM/F12 supplemented with 5% FBS and 2 mM L-glutamine. F1 cells were transduced with the VPB/Cre and VIRHD/HY/siIntβ1/2363 lentivectors to generate the F1/Pkd1−/− and F1/siIntβ1 cell populations after selection with blasticidin (5 μg/ml) and hygromycin (400 μg/ml), respectively.55 Double transduction followed by double selection was used to obtain the F1/Pkd1−/−+siIntβ1 cells. Cell proliferation assays were performed using crystal violet dye. Briefly, 5000 cells were seeded in 96-well plates in sextuplets and allowed to adhere overnight. Unattached cells were then washed out with PBS, and the remaining adherent cells were fixed in 10% neutral-buffered formalin and collected as time 0 hours. Each plate was similarly collected every 24 hours as indicated. Fixed cells were then stained with 0.5% crystal violet, and the relative growth was calculated on the basis of the 570-nm absorbance of the solubilized dye in 1% SDS. To assess the collagen-dependent signaling, serum-starved cells were dissociated, plated on collagen-coated plates in the presence of 0.5 mM MnCl2, and allowed to adhere for the indicated time points.
Flow Cytometry and Immunocytochemistry
Flow cytometry was carried out as described previously32 with the following modifications. To detect total and active membrane pools, Intβ1-dissociated cells were incubated with integrin antibodies (MB1.2 or 9EG7) or isotype control at 4°C in the presence of 5% BSA and 0.05% NaN3 in 1× PBS. For binding of 9EG7, 1 mM MnCl2 was included in the primary antibody incubation. Mean channel fluorescence was obtained using Beckman FACScan. For immunocytochemistry, cells cultured on glass coverslips were fixed with 4% paraformaldehyde, permeablized, and blocked with 5% normal donkey serum and 5% BSA in 0.02% Triton X-100 in PBS (PBST). Primary and secondary antibody incubations were carried out in 5% BSA in PBST, and coverslips were mounted on glass slides using Fluoromount G mounting media with 4′,6-diamidino-2-phenylindole (DAPI; Southern Biotech). Images were acquired on Leica CTR 6000 inverted microscope using the Leica AF software (Leica Microsystems).
Generation of Pkd1fl/fl, Itgb1fl/fl, and Aqp2-cre Mice
Animal studies were approved by and conducted in accordance with the regulations of the Institutional Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai. The following mice were obtained from Jackson Laboratory and crossed to generate collecting duct-specific single KO of Pkd1 or Itgb1 or DKO of Pkd1 and Itgb1:Pkd1fl/fl (B6.129S4-Pkd1tm2Ggg/J), Itgb1fl/fl (B6;129-Itgb1tm1Efu/J), and Aqp2-cre [B6.Cg-Tg(Aqp2-cre)1Dek/J]. PCR genotyping was performed according to the protocols specified by The Jackson Laboratory. Because Aqp2 is expressed in both the collecting duct and sperm and a paternal transmission of cre transgene may result in gene ablation in multiple/all tissues in the offspring, we used a breeding strategy that would ensure a maternal transmission of cre. The experimental litters contained a single KO of Pkd1 (Pkd1fl/fl,Itg1fl/+;cre) or Itgb1 (Pkd1fl/+,Itg1fl/fl;cre) or a DKO of Pkd1 and Itgb1 (Pkd1fl/fl,Itgb1fl/fl;cre). Littermates without cre expression and compound heterozygotes of Pkd1 and Itgb1 (Pkd1fl/+,Itg1fl/+;cre) were indistinguishable in phenotype and served interchangeably as WT controls.
Tissue Preparation, Histology, and Immunofluorescence
Mice were anesthetized with ketamine/xylazine and perfused transcardially with 4% paraformaldehyde in PBS. One kidney was frozen in OCT compound (Tissue-Tek) after a sucrose (4%) immersion at 4°C overnight, and the other kidney was processed and embedded in paraffin. For histology, dewaxed paraffin sections of kidneys (4 μm) were hydrated and stained with periodic acid–Schiff or Masson’s trichrome reagent. For immunostaining of paraffin sections, antigen retrieval was performed on dewaxed hydrated sections by heating in 20 mM Tris-HCl (pH 9.0)/1 mM EDTA or 10 mM sodium citrate buffer (pH 6.5) at 95°C for 15 minutes. Cooled sections were blocked in 5% normal donkey serum in PBST, incubated with primary antibodies in blocking solution followed by Cy-3–conjugated secondary antibodies and FITC-labeled lectins, and coverslipped with DAPI-containing Fluoromount-G mountant (Southern Biotech).
For quantification of Ki67-positive tubules, paraffin sections from kidneys of each genotype (n=3) were colabeled with DBA and LTA lectins, anti-THP, and anti-Ki67 antibodies simultaneously. Five nonoverlapping fields were imaged at ×200 and quantified by counting the total number of Ki67-positive nuclei relative to the total number of DAPI-stained nuclei. Ki67-positive nuclei that were not part of the tubules stained by DBA, LTA, or THP and without the morphology of nuclei typical of tubules were excluded from the total count.
BUN Measurement and Cystic Index
Blood from control, single KO, and DKO mice were collected from the submandibular vein at specified time points, and serum samples were isolated by centrifugation. BUN (milligrams per deciliter) was calculated using the QuantiChrom Urea Assay Kit (BioAssay System, Hayward, CA). Cystic index was obtained from periodic acid–Schiff-stained transverse kidney section images of 2-, 6-, and 35-week-old animals at ×10 magnification and calculating the relative area of cyst lumen from the total kidney area using the ImageJ software.
Statistical Analyses
All data are presented as mean±SD. Two-tailed t test or two-way ANOVA with Bonferroni post-test were used for statistical analyses using The GraphPad Prism software. A P value<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Cijiang He for critically reviewing the manuscript.
This work was supported by National Institutes of Health Grant 5R01-DK78231 and Department of Defense Grant W81XWH-09-1-0269 (to G.L.G.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111179/-/DCSupplemental.
References
- 1.Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PD, Geng L, Li X, Burrow CR: The PKD1 gene product, “polycystin-1,” is a tyrosine-phosphorylated protein that colocalizes with α2β1-integrin in focal clusters in adherent renal epithelia. Lab Invest 79: 1311–1323, 1999 [PubMed] [Google Scholar]
- 3.Candiano G, Gusmano R, Altieri P, Bertelli R, Ginevri F, Coviello DA, Sessa A, Caridi G, Ghiggeri GM: Extracellular matrix formation by epithelial cells from human polycystic kidney cysts in culture. Virchows Arch B Cell Pathol Incl Mol Pathol 63: 1–9, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Norman J: Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD). Biochim Biophys Acta 1812: 1327–1336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvet JP: Polycystic kidney disease: Primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int 43: 101–108, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Drummond IA: Polycystins, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta 1812: 1322–1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shannon MB, Patton BL, Harvey SJ, Miner JH: A hypomorphic mutation in the mouse laminin α5 gene causes polycystic kidney disease. J Am Soc Nephrol 17: 1913–1922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condac E, Silasi-Mansat R, Kosanke S, Schoeb T, Towner R, Lupu F, Cummings RD, Hinsdale ME: Polycystic disease caused by deficiency in xylosyltransferase 2, an initiating enzyme of glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A 104: 9416–9421, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E: Progressive kidney degeneration in mice lacking tensin. J Cell Biol 136: 1349–1361, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daley WP, Peters SB, Larsen M: Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121: 255–264, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO, Naba A: Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4: a004903, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE: Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res 301: 23–30, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Dalagiorgou G, Basdra EK, Papavassiliou AG: Polycystin-1: Function as a mechanosensor. Int J Biochem Cell Biol 42: 1610–1613, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO: Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Wickström SA, Radovanac K, Fässler R: Genetic analyses of integrin signaling. Cold Spring Harb Perspect Biol 3: 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnould T, Kim E, Tsiokas L, Jochimsen F, Grüning W, Chang JD, Walz G: The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem 273: 6013–6018, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Parnell SC, Magenheimer BS, Maser RL, Zien CA, Frischauf A-M, Calvet JP: Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J Biol Chem 277: 19566–19572, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O’Hare MJ, Ong ACM: Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72: 157–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Praetorius HA, Praetorius J, Nielsen S, Frokiaer J, Spring KR: Beta1-integrins in the primary cilium of MDCK cells potentiate fibronectin-induced Ca2+ signaling. Am J Physiol Renal Physiol 287: F969–F978, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millán JL, Gamble V, Harris PC: The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Malhas AN, Abuknesha RA, Price RG: Interaction of the leucine-rich repeats of polycystin-1 with extracellular matrix proteins: Possible role in cell proliferation. J Am Soc Nephrol 13: 19–26, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Zaidel-Bar R, Milo R, Kam Z, Geiger B: A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci 120: 137–148, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Humphries JD, Byron A, Humphries MJ: Integrin ligands at a glance. J Cell Sci 119: 3901–3903, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGlashan SR, Jensen CG, Poole CA: Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem 54: 1005–1014, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Muhammad H, Rais Y, Miosge N, Ornan EM: The primary cilium as a dual sensor of mechanochemical signals in chondrocytes. Cell Mol Life Sci 69: 2101–2107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wann AKT, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, Knight MM: Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J 26: 1663–1671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeger-Nukpezah T, Golemis EA: The extracellular matrix and ciliary signaling. Curr Opin Cell Biol 24: 652–661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Adelsberg J: Murine polycystic kidney epithelial cell lines have increased integrin-mediated adhesion to collagen. Am J Physiol 267: F1082–F1093, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Joly D, Morel V, Hummel A, Ruello A, Nusbaum P, Patey N, Noël L-H, Rousselle P, Knebelmann B: β4 integrin and laminin 5 are aberrantly expressed in polycystic kidney disease: Role in increased cell adhesion and migration. Am J Pathol 163: 1791–1800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daïkha-Dahmane F, Narcy F, Dommergues M, Lacoste M, Beziau A, Gubler MC: Distribution of α-integrin subunits in fetal polycystic kidney diseases. Pediatr Nephrol 11: 267–273, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Battini L, Fedorova E, Macip S, Li X, Wilson PD, Gusella GL: Stable knockdown of polycystin-1 confers integrin-alpha2beta1-mediated anoikis resistance. J Am Soc Nephrol 17: 3049–3058, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Kreidberg JA, Symons JM: Integrins in kidney development, function, and disease. Am J Physiol Renal Physiol 279: F233–F242, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Lee K, Battini L, Martinez-Romero C, Geng L, Gusella GL: Role of integrinß1 in cystogenesis and hyperproliferation of ADPKD cells. Presented at American Society of Nephrology Kidney Week, November 16–21, 2010 [Google Scholar]
- 35.Piontek KB, Huso DL, Grinberg A, Liu L, Bedja D, Zhao H, Gabrielson K, Qian F, Mei C, Westphal H, Germino GG: A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J Am Soc Nephrol 15: 3035–3043, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Steele SL, Wu Y, Kolb RJ, Gooz M, Haycraft CJ, Keyser KT, Guay-Woodford L, Yao H, Bell PD: Telomerase immortalization of principal cells from mouse collecting duct. Am J Physiol Renal Physiol 299: F1507–F1514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanian B, Ko W-C, Yadav V, DesRochers TM, Perrone RD, Zhou J, Kaplan DL: The regulation of cystogenesis in a tissue engineered kidney disease system by abnormal matrix interactions. Biomaterials 33: 8383–8394, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Mernaugh G, Yang D-H, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, Fässler R, Yurchenco P, Pozzi A, Zent R: β1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136: 3357–3366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raphael KL, Strait KA, Stricklett PK, Miller RL, Nelson RD, Piontek KB, Germino GG, Kohan DE: Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int 75: 626–633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG: A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takakura A, Contrino L, Beck AW, Zhou J: Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol 19: 2351–2363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJM: Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Pankov R, Clark K: Integrins in extracellular matrix assembly. In: Integrins and Development, edited by: Danen E, Georgetown, Landes Bioscience, 2005, 1–10 [Google Scholar]
- 45.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL: Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem 276: 46707–46713, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, Bennett JS, Degrado WF, Eckes B, Zent R, Pozzi A: Inhibition of integrin α2β1 ameliorates glomerular injury. J Am Soc Nephrol 23: 1027–1038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pozzi A, Zent R: Integrins in kidney disease. J Am Soc Nephrol 24: 1034–1039, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh P, Carraher C, Schwarzbauer JE: Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26: 397–419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huan Y, van Adelsberg J: Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest 104: 1459–1468, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoder BK, Hou X, Guay-Woodford LM: The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J: Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desgrosellier JS, Cheresh DA: Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer 10: 9–22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris PC, Rossetti S: Determinants of renal disease variability in ADPKD. Adv Chronic Kidney Dis 17: 131–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battini L, Macip S, Fedorova E, Dikman S, Somlo S, Montagna C, Gusella GL: Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum Mol Genet 17: 2819–2833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.