Abstract
Ionizing radiation alone or in combination with chemotherapy is the main treatment modality for brain tumors including glioblastoma. Adult neurons and astrocytes demonstrate substantial radioresistance; in contrast, human neural stem cells (NSC) are highly sensitive to radiation via induction of apoptosis. Irradiation of tumor cells has the potential risk of affecting the viability and function of NSC. In this study, we have evaluated the effects of irradiated glioblastoma cells on viability, proliferation and differentiation potential of non-irradiated (bystander) NSC through radiation-induced signaling cascades. Using media transfer experiments, we demonstrated significant effects of the U87MG glioblastoma secretome after gamma-irradiation on apoptosis in non-irradiated NSC. Addition of anti-TRAIL antibody to the transferred media partially suppressed apoptosis in NSC. Furthermore, we observed a dramatic increase in the production and secretion of IL8, TGFβ1 and IL6 by irradiated glioblastoma cells, which could promote glioblastoma cell survival and modify the effects of death factors in bystander NSC. While differentiation of NSC into neurons and astrocytes occurred efficiently with the corresponding differentiation media, pretreatment of NSC for 8 h with medium from irradiated glioblastoma cells selectively suppressed the differentiation of NSC into neurons, but not into astrocytes. Exogenous IL8 and TGFβ1 increased NSC/NPC survival, but also suppressed neuronal differentiation. On the other hand, IL6 was known to positively affect survival and differentiation of astrocyte progenitors. We established a U87MG neurosphere culture that was substantially enriched by SOX2+ and CD133+ glioma stem-like cells (GSC). Gamma-irradiation up-regulated apoptotic death in GSC via the FasL/Fas pathway. Media transfer experiments from irradiated GSC to non-targeted NSC again demonstrated induction of apoptosis and suppression of neuronal differentiation of NSC. In summary, intercellular communication between glioblastoma cells and bystander NSC/NPC could be involved in the amplification of cancer pathology in the brain.
Keywords: Glioblastoma, Neural stem cells (NSC), Neural progenitor cells (NPC), Ionizing radiation, Bystander response, Signaling pathways, Apoptosis
Introduction
Glioblastoma is the most common primary malignant brain tumor affecting more than 10,000 new patients in the US each year. Despite advances in radiation therapy and chemotherapy, outcomes remain really poor with a median survival rate of 12–15 months [1]. Detailed investigations of the somatic genomic landscape of glioblastoma using numerous human glioblastoma samples and established cancer lines demonstrated a connection between gene alteration and signaling pathway modifications in glioblastoma cells, which include RTK pathways (EGFR1, FGFR2 or PDGFRA), PI3K/PTEN-AKT pathway, MAPK pathways (together with NF1 and BRAF), p53 pathway (together with MDM2, MDM4) and RB1 pathway (RB1, CDK4 and CDK6) [2, 3]. A role for the IKK-NF-κB pathway via activating mutations was additionally highlighted in some types of glioblastoma [4].
U87MG is a commonly studied grade IV glioblastoma cell line that has been analyzed in numerous studies over many years. The mutational landscape of the U87MG genome is extremely complicated. Protein coding sequences of genes were disrupted predominantly in this cancer cell line due to small insertions and deletions, large deletions, and translocations. In total, 512 genes were homozygously mutated, including 154 by single nucleotide variation, 178 by small insertions and deletions, 145 by large microdeletions, and 35 by interchromosomal translocations to reveal a highly mutated cell line genome [5]. Among these changes known homozygous mutations in PTEN and TP53, as well as EGFR amplification were identified.
Ionizing radiation alone or in combination with chemotherapy is the main treatment procedure for glioblastoma. Normal adult neurons and glial cells, which are terminally differentiated cells, exhibit a substantial radioresistance. In contrast, neural stem and progenitor cells (NSC/NPC) having significant proliferative capacities are highly sensitive to ionizing radiation. Numerous clinical observations and experiments with animals demonstrated that cranial irradiation used for treatment of brain tumors may cause substantial cognitive deficits such as impairing learning, attention and memory, due to inhibition of the proliferation and death of neural stem cells [2, 6–12]. Ionizing irradiation causes DNA damage via generation of reactive oxygen species (ROS) that further affect numerous cell signaling pathways and the corresponding gene expression followed by inhibition of cell proliferation, induction of the DNA repair mechanisms and, finally, either cell survival (that is achieved using multiple mechanisms, including protective autophagy) or cell death (via apoptosis, necrosis and destructive autophagy) [13, 14]. Directly irradiated cells, dramatically change the regulation of gene expression by induction of survival programs, including induction of gene expression of numerous cytokines, growth factors directed by activation of the transcription factors NF-κB, STAT3, AP1 and several others. This is a common feature of stress response and, furthermore, a basis for the induction of a bystander response (which might include apoptosis and genomic instability as endpoints) in non-targeted cells [15, 16]. The tumor microenvironment actively regulates cell signaling pathways and gene expression in cancer cells [17]. On the other hand, radiation-induced signals from treated tumors to non-irradiated bystander cells [18, 19], could be modulated by tumor microenvironment. Numerous investigations of the radiation-induced bystander response of non-targeted cells during the last two decades have dramatically changed the paradigm of radiobiology concerning general regulation of radiation response [18–20].
In spite of great importance of neural stem cells (NSC) in the development and maintenance of the nervous system, molecular mechanisms of the radiation-induced bystander effects in NSC remain mostly unknown. In the present study we investigate radiation-induced signaling in directly targeted human glioblastoma cells and NSC, as well as the subsequent induction of intercellular crosstalk between irradiated glioblastoma cells and non-targeted (bystander) NSC that could ultimately affect apoptosis, survival, proliferation and differentiation of non-targeted NSC.
Results
Cell signaling pathways in human neural stem cells (NSC) and U87MG glioblastoma cells before and after γ-irradiation
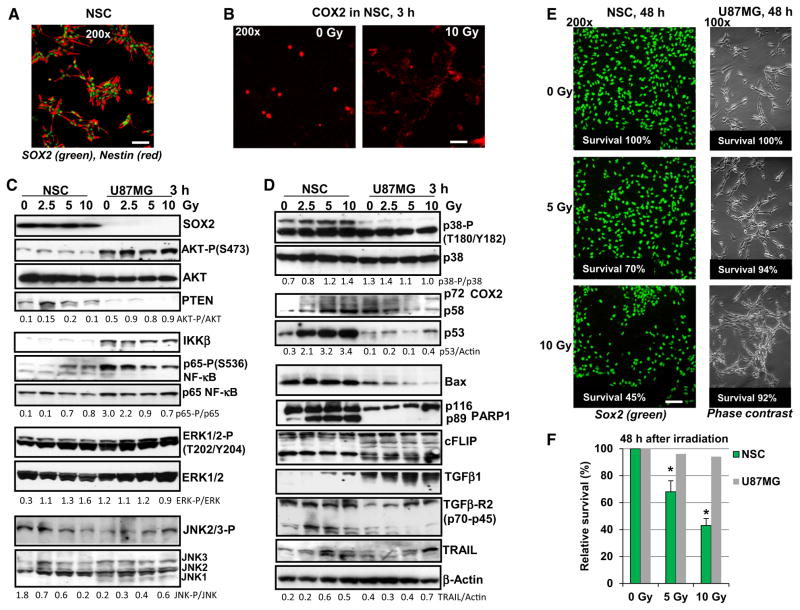
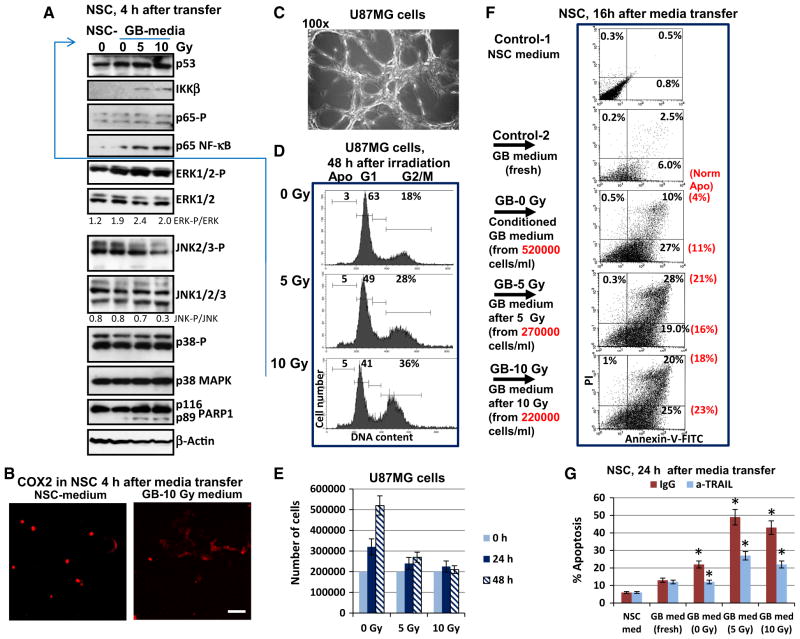
Human SOX2+, Nestin+ neural stem cells (NSC) (Fig. 1) and U87MG human glioblastoma cells (Fig. 1c–f) were either non-irradiated or exposed to graded doses of γ-irradiation (2.5–10 Gy). In a close correlation with previously published data [3, 4], constitutive activation of AKT (due to EGFR amplification and PTEN deficiency) and IKK-NF-κB, but a strong down-regulation of p53 levels were determined in non-treated U87MG glioblastoma cells. In contrast, substantially lower levels of the active (phosphorylated) AKT and NF-κB p65 were revealed in normal human NSC (Fig. 1c, d). However, active phosphorylated forms of ERK1/2 and MAPK p38 were permanently present in both NSC and U87MG cells. Irradiation markedly up-regulated the levels of active NF-κB p65-P(S536) in NSC, but gradually decreased its high basal levels in glioblastoma cells. γ-Irradiation further up-regulated phospho-ERK(T202/Y204) levels in NSC, while these levels were relatively stable in irradiated U87MG cells 3 h after treatment. Changes in the levels of phospho-JNK had the opposite trends in NSC and U87MG cells after irradiation (Fig. 1c). γ-Irradiation strongly increased p53 protein levels in dose-depended manner in NSC, while only the trace amount of p53 were detected in glioblastoma cells (Fig. 1d). BAX protein expression level (controlled by transcription factor p53) was also dramatically down-regulated in glioblastoma cells, compared to NSC, resulting in a general suppression of the p53-BAX-dependent apoptotic signaling in glioblastoma cells (Fig. 1d).
Fig. 1.
Effects of γ-radiation on signaling pathways and survival of NSCs and human U87MG glioblastoma cells. a Immunostaining of NSC using polyclonal antibody to SOX2 (a pluripotency marker) and monoclonal antibody to Nestin (early neuroprogenitor marker). Bar = 50 μm. b Immunostaining COX2 expression in NSC before and 3 h after irradiation. c, d Western blot analysis of expression of the indicated proteins in human NSC and U87MG cells was performed 3 h after treatment by increasing doses of γ-irradiation. A ratio of active phosphorylated form to total level of signaling protein is indicated. For TRAIL and p53, normalization was based on beta-actin levels. e, f Effect of γ-irradiation on survival of human NSC and U87MG cells. Numbers of live cells attached to fibronectin matrix were determined before irradiation at time point 0 and 48 h after treatment. Immunostaining SOX2 in NSC was then performed. Bar = 50 μm. The number of U87MG cells was detected using a phase contrast microscopy. Relative cell survival (normalized to initial cell density at time point 0) is indicated. Pooled results of four independent experiments are shown in f. Error bars represent means ± SD (p < 0.05, Student’s t test); star indicates a significant difference. A typical result is shown in e
Early induction of radiation-induced apoptotic pathway reflected by caspase-3-mediated cleavage of PARP-1 was observed in human NSC [12], but not in irradiated glioblastoma cells (Fig. 1d). Both cell lines, NSC and U87MG, had quite similar levels of the anti-apoptotic survivin (data not shown) and cFLIP (Fig. 1d) suggesting that these proteins were not critically responsible for a sharp difference in the induction of apoptosis in two cell lines. Changes in the COX2 protein level (two bands, non-glycosylated p58 and glycosylated p72 protein subunit), a classical NF-κB target, showed opposite trends in NSC and U87MG cells after irradiation: a strong up-regulation in NSC and down-regulation of basal levels in U87MG cells 3 h after treatment (Fig. 1d). Interestingly, non-irradiated NSC contained low basal levels of COX2 protein with predominantly nuclear localization, while after irradiation cytoplasmic COX2 levels dramatically increased in NSC (Fig. 1b). In contrast, high basal levels of COX2 with cytoplasmic localization before and after treatment were observed in many human glioblastomas, including U87MG [21]. Furthermore, high protein levels of the endogenous TGFβ1 and modest levels of TGFβ-R2 were detected in glioblastoma cells after irradiation. Radiation-induced upregulation of TGFβ1 and TGFβR2 gene expression and a radioprotective role for TGFβ1-induced signaling pathways are known as characteristic features of many types of cancer, including glioblastoma [22]. The opposite trend of the permanent upregulation of TGFβ-R2 levels was characteristic for NSC (Fig. 1d) highlighting a higher sensitivity of irradiated NSC to TGFβ-R mediated signaling with the exogenous TGFβ. Endogenous production of death ligand TRAIL at moderate levels was detected in both cell lines (Fig. 1d). As early as 48 h after irradiation, a general survival of NSC was substantially decreased, while only modest changes in survival were observed among U87MG cells based on number of matrix-attached cells and Trypan blue exclusion test for floating cells (Fig. 1e, f).
Effects of small molecule inhibitors of cell signaling pathways on radiation-induced apoptosis and survival of U87MG cells
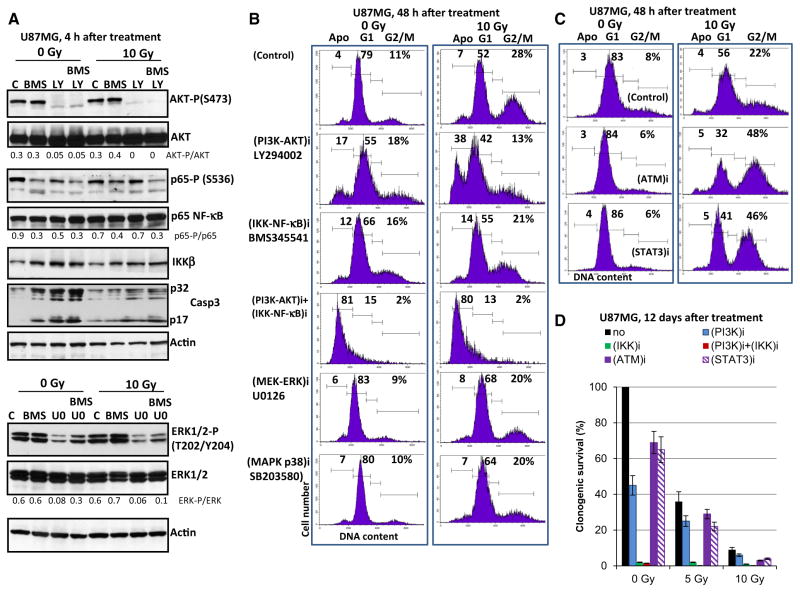
To further evaluate a role of cell signaling pathways for pro-apoptotic sensitization of glioblastoma cells, small molecule inhibitors specific for several pathways were used either alone or in combination with radiation (Fig. 2). Optimal doses for the various inhibitors (including inhibition of AKT-S473 and IKK-S176/180) employed in this study were chosen carefully on the basis of an earlier study in NSC [23]. Figure 2a demonstrates the data on the specificity of small molecule inhibitors in U87MG cells obtained with inhibitors alone or in combination with irradiation. LY294002 (50 μM) by inhibiting PI3K-AKT in non-irradiated and irradiated cells substantially up-regulated radiation-induced apoptosis of U87MG cells 48 h after treatment (Fig. 2b). BMS345541 significantly blocked IKK-NF-κB in non-irradiated cells, but was less effective in irradiated glioblastoma cells. Correspondingly, it had only a modest, but statistically significant effect on the increase of radiation-induced apoptosis (Fig. 2b and data nit shown). All other inhibitors used were not efficient for rapid up-regulation of apoptosis (Fig. 2b). For example, U0121, a MEK-ERK inhibitor (Fig. 2a, the bottom panel) increased the percentage cells at G1 phase, without up-regulating apoptosis (Fig. 2b). Inhibition of ATM-kinase by KU55933 (20 μM) or STAT3-dimerization by STAT-inhibitor-6 (50 μM) resulted in the enrichment of cells at G2/M phase without notable up-regulation of apoptosis (Fig. 2c). In contrast, a combination of LY294002 (50 μM) with BMS345541 (10 μM) was very effective for an extensive apoptosis induction involving a large fraction of cells. However, γ-irradiation did not further increase pro-apoptotic effects of this treatment (Fig. 2b). Cleavage and activation of caspase-3 (an additional proof of apoptotic commitment) were observed after treatment with BMS345541, an IKK-NF-κB inhibitor, LY294002, a PI3K-AKT inhibitor, alone or in combination, before and after irradiation (Fig. 2a).
Fig. 2.
Effects of small molecule inhibitors on cell cycle, apoptosis and survival of U87MG cells. a Specificity of inhibitors LY294002 (PI3K-AKT; 50 μM), BMS345541 (IKK-NF-κB; 10 μM) and U0121 (MEK-ERK, 10 μM) in U87MG cells. Western blot analysis of indicated proteins was performed in non-irradiated and irradiated glioblastoma cells in the absence or in the presence of small molecule inhibitors. A ratio of active phosphorylated form to total level of signaling protein is indicated. b, c U87MG cells were γ-irradiated (10 Gy) in the presence or in the absence of small molecule inhibitors: LY294002 (PI3K-AKT; 50 μM), U0126 (MEK-ERK; 10 μM), BMS345541 (IKK-NF-κB; 10 μM); KU55933 (ATM-kinase; 10 μM) and STAT3-inhibiror-6 (50 μM). Cell cycle-apoptosis analysis was performed 48 h after treatment. d Clonogenic survival assay was performed for control or irradiated cells in the absence or in the presence of indicated inhibitors (i). Pooled results of three independent experiments are shown. Error bars represent means ± SD (p <0.05)
High levels of total (relatively slow, non-apoptotic) death of G2/M-arrested glioblastoma cells were detected only 10–12 days after irradiation at 10 Gy, using clonogenic survival assay (Fig. 2d). Combined treatment by γ-radiation and LY294002 (50 μM), further decreased clonogenic survival of glioblastoma cells, as well as increased levels of cell death (Fig. 2b, c). Surprisingly, a strong effect of up-regulation of total cell death was achieved after treatment of U87MG cells with BMS345541 (10 μM) (Fig. 2d). Interestingly, BMS345541 alone or in a combination with irradiation induced only low to moderate levels of apoptosis, while it almost completely killed cancer cell population, probably, via non-apoptotic mechanisms 12 days after treatment (Fig. 2d). ATM-kinase- and STAT3- inhibitors also further decreased clonogenic survival after γ-irradiation (Fig. 2d). Based on the results of the induction of apoptosis and clonogenic survival data, combined treatment of glioblastoma cells by PI3K-AKT and IKK-NF-κB inhibitors might have a therapeutic significance. The main problem, however, is a certain sensitivity of human NSC to the above mentioned treatment [23].
Radiation-induced changes in glioblastoma secretome and in surface expression of death receptors and their ligands
Since IKK-NF-κB is one the critical pathways that regulate glioblastoma survival, the expression levels of some of the well-known downstream targets of IKK-NF-κB pathway, IL8, IL6 and TRAIL [24], as well as TGFβ1, which could serve as a upstream regulator for NF-κB activation [25], were examined in glioblastoma cells. Non-irradiated U87MG cells secreted high basal levels of IL8, IL6 and TGFβ1 into the culture media. BMS345541, an inhibitor of IKK-NF-κB, substantially reduced the basal secretion of IL6 and IL8 (Suppl. Figure 1A and B). In line with earlier studies [22, 26], our experiments demonstrated radiation-induced up-regulation of production and secretion of TGFβ1, IL8 and IL6 by U87MG cells. Interestingly, NF-κB suppression by BMS345541 was less pronounced in irradiated glioblastoma cells, compared to non-irradiated cells, in concert with highly stable ERK- and AKT activities (Fig. 2a), correlating with incomplete suppression of IL6 and IL8 secretion by inhibitor in irradiated cells (Suppl. Figure 1A and B). Levels of soluble TRAIL were very low in conditioned media but significantly increased after irradiation (10 Gy) of glioblastoma cells (Suppl. Figure 1D).
Irradiation additionally increased Fas expression on the cell surface; practically, the whole population of U87MG cells became Fas positive after treatment with 10 Gy of γ-rays (Suppl. Figure 2A). In contrast, only 50 % of U87MG cell population was positive for TRAIL-R2/DR5 surface expression in these conditions; no TRAIL-R1/DR4 surface expression was detected. A modest increase in surface expression of the membrane forms of FasL and TRAIL was detected after irradiation (Suppl. Figure 2A). Increased TRAIL production in irradiated U87MG cells was also confirmed by Western blotting (see Fig. 1d).
Our previous results demonstrated that NSC were very sensitive to treatment with the exogenous TRAIL, and radiation-induced apoptosis of NSC was mediated by the endogenous TRAIL [12]. In contrast, U87MG cells were resistant to endogenous or exogenous TRAIL alone. However, TRAIL in combination with CHX dramatically increased apoptosis and total death in U87MG cells (Suppl. Figure 2B and C). On the other hand, U87MG responded to treatment by the exogenous FasL (40 ng/ml) inducing apoptosis that was further increased in the presence of CHX (2 μg/ml) (Suppl. Figure 2B). Clonogenic survival assay confirmed an almost 100 % death rate of glioblastoma cells after combined treatment of FasL and CHX or after treatment with TRAIL and CHX (Suppl. Figure 2C). Furthermore, γ-irradiation up-regulated levels of both apoptotic and total death of FasL-pretreated U87MG cells (Suppl. Figure 2B and C).
Effects of the exogenous TGFβ, IL8, IL6 and TRAIL on viability and neuronal differentiation of NSC
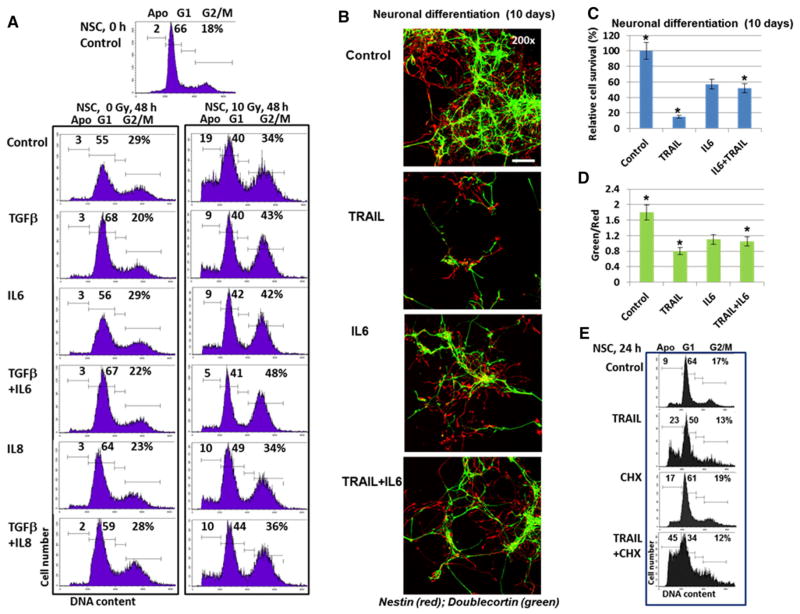
Abundant expression of cytokines and growth factors by glioblastoma cells, especially after irradiation, provoked an intriguing question regarding their potential role in intercellular communication between glioblastoma cells and NSC, which produced only the trace endogenous levels of these cytokines. Using the individual recombinant proteins, well-pronounced protective effects of TGFβ1 (20 ng/ ml), IL6 (50 ng/ml) and IL8 (50 ng/ml) either alone or in combination were observed against radiation-induced apoptosis of NSC (Fig. 3a). We used higher concentrations of exogenous cytokines compared to the corresponding endogenous levels in glioblastoma media after irradiation (see Suppl. Figure 1), because protein stability of recombinant non-glycosylated cytokines, which were also used without protective structures, such as exosomes or specific binding proteins, in the culture media was substantially lower than for the corresponding endogenously produced cytokines. Interestingly, TGFβ1 alone substantially increased % NSC at G1 phase, reflecting its inhibitory effects on NSC proliferation [27]. However, a combination of TGFβ1 (20 ng/ml) and irradiation (5–10 Gy) demonstrated a dramatic increase in survival of irradiated NSC (Fig. 3a). Of note, is that the lower levels of TGFβ1 (1–5 ng/ml) did not exhibit protective anti-apoptotic effects in irradiated NSC [12]. PGE2, the main signaling molecule downstream of COX2, also induced a modest pro-apoptotic effect for irradiated NSC in this range of concentrations (1–5 ng/ml) (data not shown).
Fig. 3.
Cell cycle and neuronal differentiation of NSC: effects of cytokines and TGFβ1. a Effects of growth factor/cytokines on the cell cycle-apoptosis of control and irradiated NSC. Recombinant TGFβ1 (20 ng/ml), IL6 (50 ng/ml) and IL8 (50 ng/ml) alone or in combination were added to serum-free NSC media. Cell cycle-apoptosis analysis was performed using PI staining and FACS analysis 48 h after culture initiation. Results of a typical experiment (one from four) are shown. b, c, d Neuronal differentiation of NSC was initiated by serum-free differentiation media in the presence or in the absence of TRAIL (50 mg/ml), IL6 (50 ng/ml) or TRAIL + IL6. After 10 days of growth cells were fixed and used for immunostaining. Confocal analysis of immunofluorescent images was performed using monoclonal Ab against an early neuroprogenitor marker, Nestin (red), and polyclonal Ab against a neuronal marker, Doublecortin (green). Bar = 50 μm. Typical images are shown in b. A ratio of the number of green cells to the number of red cells and relative cell survival 10 days after initiation of differentiation were determined. Pooled results of three independent experiments are shown in c, d. Error bars represent means ± SD (p <0.05, Student’s t test); star indicates a significant difference. e Effects of TRAIL (50 ng/ml), CHX (1 μg/ ml), and TRAIL + CHX on induction of apoptosis in NSC 24 h after treatment
The question arose regarding probable interactions of pro-apoptotic and pro-survival factors during differentiation of NSC/NPC. Neuronal differentiation of human NSC was induced by the corresponding serum-free differentiation medium without FGF2 and EGF (Fig. 3b–d). During this differentiation process high level of precursor cell death was observed. The gradual progression from cells positive for neural stem/progenitor cell specific marker Nestin (red) to cells positive for neuron specific marker Doublecortin (green) was evident in differentiating cells (Fig. 3b). Green/red ratio (a ratio of the number of Doublecortin-positive green cells to the number of Nestin-positive red cells) reflected the degree of neuronal differentiation (Fig. 3d). Relative cell survival of control or treated cells was determined by manual counting of cells that were attached to the laminin matrix in six microscopic areas 10 days after initiation of differentiation (Fig. 3c). As we previously observed, TRAIL (50 ng/ml) strongly decreased the final survival of NSC/NPC and the yield of differentiated neurons [12]. IL6 (50 ng/ml) also decreased the extent of differentiation and cell survival, even significantly less than TRAIL. The presence of IL6, however, partially suppressed negative effects of TRAIL on survival of NSC (Fig. 3b–d). A control experiment confirmed pro-apoptotic effects of TRAIL alone or in combination with CHX on the native NSC (Fig. 3e).
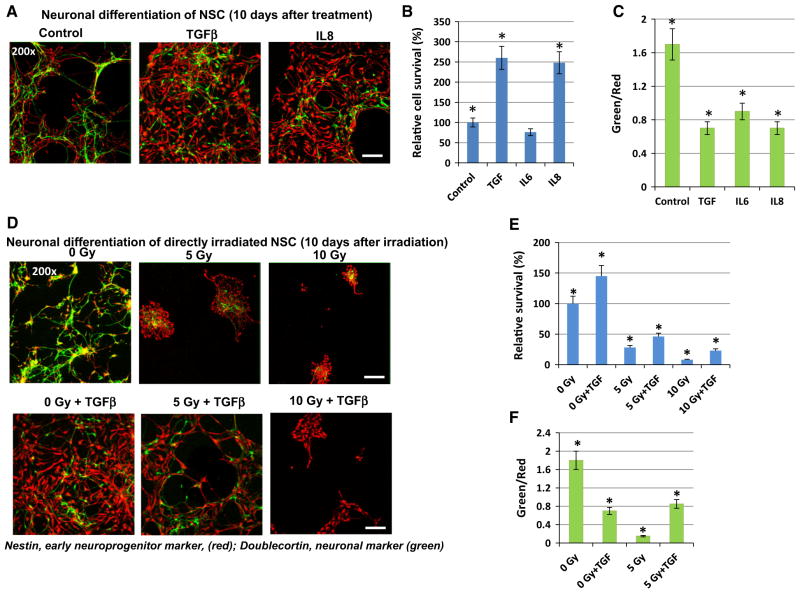
In contrast, TGFβ and IL8 demonstrated a strong pro-survival effect for differentiating NSC/NPC with a significant suppression of the degree of differentiation (Fig. 4a–c). Furthermore, we and others previously demonstrated [8, 12] that direct γ-irradiation before the induction of differentiation substantially reduced the efficiency of neuronal differentiation by down-regulating the survival of neural progenitor cells. The presence of TGFβ1 (20 ng/m) could partially maintain neuronal differentiation after irradiation at 5 Gy. Furthermore, TGFβ1 still protected a small subpopulation of neural precursors after irradiation with 10 Gy (Fig. 4d–f). Surprisingly, cell survival during astrocyte differentiation of NSC (10 days after initiation of differentiation) was not notably affected by irradiation (at 5 Gy), probably reflecting a deficiency of surface expression of death receptors in astrocyte precursors. The degree of astrocyte differentiation (based on the number of GFAP-positive cells) was still low in both cases of non-treated and irradiated cells 10 days after initiation of differentiation (data not shown).
Fig. 4.
Neuronal differentiation of directly irradiated NSC: effects of TGFβ1. NSC were non-treated or directly γ-irradiated at 5–10 Gy in the presence or in the absence of TGFβ1 (20 ng/ml), IL6, IL8 (50 ng/ ml). a–c Neuronal differentiation of non-irradiated NSC in the absence or in the presence of indicated cytokines was initiated by differentiation medium. Confocal analysis of immunofluorescent images was performed using monoclonal Ab against an early neuroprogenitor marker, Nestin (red), and polyclonal Ab against a neuronal marker, Doublecortin (green). Bar = 50 μm. A relative cell survival and a degree of neuronal differentiation (green/red ratio) for control and irradiated cells 10 days after initiation of differentiation were determined. Pooled results of three independent experiments are shown in b, c. Error bars represent means ± SD (p < 0.05, Student’s t test); star indicates a significant difference. d–f Neuronal differentiation of non-irradiated and directly irradiated NSC was initiated by differentiation media in the absence or in the presence of TGFβ1 (20 ng/ml). Confocal analysis of immunefluorescent images was performed using monoclonal Ab against an early neuroprogenitor marker, Nestin (red), and polyclonal Ab against a neuronal marker, Doublecortin (green)
Intercellular crosstalk between glioblastoma cells and NSC before and after γ-irradiation
As we already observed (Fig. 2b), gamma-irradiation at 10 Gy induced a G2/M irreversible arrest of U87MG cell proliferation that was not accompanied by notable levels of cell death even 48–72 h after treatment (see also Fig. 5d).
Fig. 5.
Effects of U87MG conditioned and irradiated media transfer on cell signaling, cell cycle and death in the bystander NSC. a, b 4 h after media transfer (the NSC-control medium and GB-irradiated media), western blot analysis of NSC proteins was performed (a) and COX2 was detected by immunostaining and microscopy (b). Bar = 50 μm. c–e U87MG cells attached to the matrix (c) were non-treated or irradiated at 5–10 Gy in six-well plates at cell density 400,000 cells per a well or 200,000 cells per ml of culture medium. 48 h after irradiation, cell cycle—apoptosis analysis of U87MG was performed using PI staining and FACS analysis (d). Cell growth of control and irradiated U87MG cells is shown in e (f) 48 h after treatment, control-1 (NSC serum-free medium), control-2 (fresh glioblastoma medium), conditioned (non-irradiated) U87MG glioblastoma (GB) medium and media from irradiated U87MG cells (at 5 and 10 Gy) were transferred to naive NSC. 16 h after media transfer, Annexin-V-FITC/PI staining of NSC followed by FACS assay (f) was performed. Normalized levels of apoptosis (induced by secreted activity of 200,000 U87MG cells per ml) are shown in brackets. g The fresh NSC medium, fresh GB medium, conditioned GB medium and irradiated GB media were transferred to the native NSC in the presence of non-specific IgG or anti-TRAIL inhibitory antibody (5 μg/ml) for 24 h. Then cell-cycle apoptosis analysis of PI-stained nuclei of NSC was performed using FACS assay. Pooled results of four independent experiments are shown in g. Error bars represent means ± SD (p < 0.05, Student’s t test); star indicates a significant difference
To detect the non-targeted effects after irradiation of glioblastoma (GB) cells on naïve NSC, conditioned media and media from irradiated cultures of U87MG cells (48 h after irradiation) were transferred to the naïve NSC for short (4 h) and prolonged (16–24 h) exposure (Fig. 5). Levels of the main cytokines in conditioned and irradiated glioblastoma media used for treatment of NSC are shown on Suppl. Figure 1. Expression changes in IKK-NF-κB and, especially, ERK activation in NSC (due to high levels of IL6, IL8 and TGFβ1 in GB media) and down-regulation of JNK activity were revealed by Western blotting 4 h after treatment. Initial up-regulation of Caspase-3-mediated cleavage of PARP-1 in NSC, a characteristic feature of apoptotic commitment, was also detected after irradiated media transfer (Fig. 5a). Changes in the intracellular localization and up-regulation COX2 were revealed in experiments with short exposure (4 h) of NSC to irradiated glioblastoma media (Fig. 5b).
16 h after treatment with media transferred from GB cells, increased apoptotic levels were detected among exposed NSC using Annexin-V-FITC/PI staining of cells, reflecting a strong up-regulation in levels of the early and late phases of apoptosis (Fig. 5f). When apoptotic levels observed in NSC were normalized to the equal number of glioblastoma cells used in cultures, we revealed an increase in apoptotic levels after irradiated media transfer, compared to conditioned media (Fig. 5f; normalized apoptosis levels shown in the brackets). PI-staining of the cell nuclei and FACS analysis also demonstrated high apoptotic (pre-G1) levels in NSC after media transfer (Fig. 5g). Control fresh GB media had some toxic effect for NSC that was relatively modest for 8–16 h of treatment. There was a good correlation between two methods of detection of apoptotic cells (Fig. 5f, g). Dilution of transferred GB media by fresh NSC media (1:1) decreased the apoptotic level, which was found to be intermediate between NSC treated with medium without dilution and the fresh medium (data not shown).
Since moderate level of up-regulation of the endogenous TRAIL production was detected in U87MG after irradiation, we investigated effects of TRAIL suppression in transferred glioblastoma culture for NSC cells. Anti-TRAIL inhibitory antibody (5 μg/ml) in the media did indeed decrease the level of apoptosis among NSC after media transfer from glioblastoma cells (Fig. 5g). On the other hand, NSC efficiently responded to control treatment with the exogenous recombinant TRAIL (40 ng/ml), especially in combination with CHX (Fig. 3e). It is possible, however, that additional factor/s could be involved in the induction of NSC apoptosis, since only partial suppression of apoptosis by anti-TRAIL Ab (5 μg/ml) was observed (Fig. 5g).
In summary, results of these experiments demonstrated well pronounced effects of glioblastoma secreted factors on survival and regulation of apoptosis in bystander NSC/NPC before and, especially, after irradiation of cancer cells. Interestingly, non-targeted effects of irradiated glioblastoma cells on non-irradiated glioblastoma cells for the induction of death were not detected (data not shown). This is in a sharp contrast to induction of TRAIL-mediated death in the population of bystander NSC after media transfer from directly irradiated NSC [12], highlighting a relative non-efficiency of TRAIL/TRAIL-R apoptotic pathway in irradiated glioblastoma cells.
Bystander effects of irradiated media transfer on neuronal differentiation of NSC
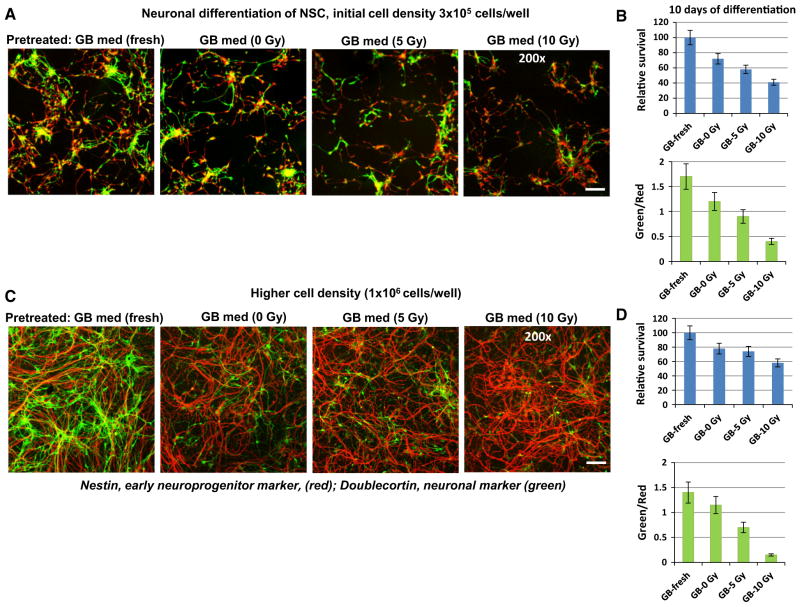
In our opinion, apoptosis as a biological indicator of radiation-induced bystander response in NSC induced by media transfer from irradiated glioblastoma cells was highly convincing. We next determined whether the signaling triggered by irradiated glioblastoma cells affect the differentiation potential of NSC. The experimental design of this investigation is shown in Suppl. Figure 3. We made a 8-h pretreatment of the naive NSC using media transferred from non-irradiated (control) and from directly irradiated U87MG glioblastoma cell (48 h after treatment) followed by neural differentiation of NSC that was induced by defined differentiation media. Media transfer from irradiated U87MG cells to naive bystander NSC affected both cell survival and the degree of differentiation determined as the ratio of green Doublecortin-positive young neurons among differentiating progenitor cells identified by Nestin expression (red) (Fig. 6a, b). We should highlight that 8-h pretreatment of NSC with the fresh GB-media (mock control) had only non-significant effects on neural differentiation of NSC, compared to NSC without pre-treatment. The decline of neuronal differentiation by media transfer from conditioned and, especially, irradiated glioblastoma cultures was convincing for optimal initial NSC density (Fig. 6a, b). NSC at higher initial density (106 cells/ well), which were pretreated with transferred media, exhibited stronger suppression of neuronal differentiation, but higher levels of relative survival (Fig. 6c, d). Increased survival of NSC/NPC, which were pretreated and initiated for neuronal differentiation at high initial cell density, was a quite expectable phenomenon, due to a possible maintenance of the endogenous secretion of the pro-survival factors, including FGF2 and EGF [28].
Fig. 6.
Neuronal differentiation of NSC after pretreatment (8 h) with conditioned media or media from irradiated U87MG cells. a, b NSC at the initial density (3 × 105 cells/well) were used for pretreatment with transferred media and induction of differentiation. U87MG cells were non-treated or directly γ-irradiated at 5–10 Gy. Naïve NSC was primed by 8-h incubation with transferred media from non-irradiated glioblastoma cells (GB-0 Gy) or from irradiated glioblastoma cells (GB-5 Gy; GB-10 Gy). Fresh GB media was used as a control. Eight hours after GB media transfer, neuronal differentiation was initiated by replacement GB media with serum-free differentiation medium. Confocal analysis of immunofluorescent images was performed using monoclonal Ab against an early neuroprogenitor marker, Nestin (red), and polyclonal Ab against a neuronal marker, Doublecortin (green). Bar = 50 μm. A relative cell survival and a ratio of the number of green cells to the number of red cells (reflecting neuronal differentiation) 10 days after initiation of differentiation are indicated in b. c, d NSC at the higher initial cell density (1 × 106 cells/well) were used for pretreatment with transferred media and induction of differentiation. Pooled results of four independent experiments are shown in b, d. Error bars represent means ± SD (p < 0.05, Student’s t test); star indicates a significant difference
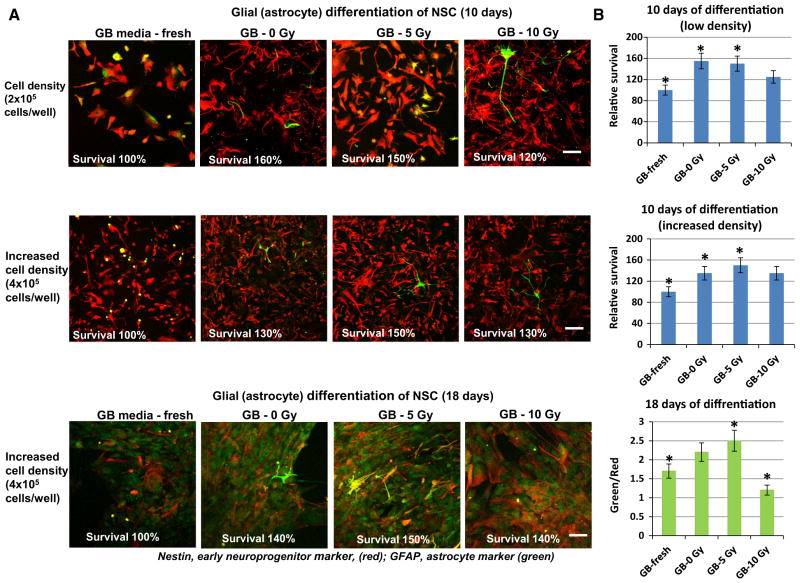
In contrast, media transfer experiments demonstrated the absence of negative regulation of progenitor survival during astrocyte differentiation at 10- and 18-days after initiation of differentiation (Fig. 7). Furthermore, priming with irradiated GB media (at 5 Gy, but not at 10 Gy) actually promoted astrocyte differentiation (Fig. 7, the bottom panel). Taken together, the results demonstrated the early and delayed radiation-induced bystander effects of U87MG glioblastoma cells on NSC/NPS, which were mediated by soluble factors from cancer cells secreted into culture media.
Fig. 7.
Glial differentiation of NSC (10–18 days) after pretreatment (8 h) with conditioned media or media from irradiated U87MG cells. Pretreatment of NSC with media from U87MG was performed as described on Fig. 6. Glial differentiation was induced by astrocyte differentiation medium. Confocal analysis of immunofluorescent images was performed using monoclonal Ab against an early neuroprogenitor marker, Nestin (red), and polyclonal Ab against an astrocyte marker, GFAP (green). Pooled results of three independent experiments on cell survival and a degree of astrocyte differentiation are shown in b. Error bars represent means ± SD (p < 0.05, Student’s t test); star indicates a significant difference
Effects of glioblastoma stem-like cells on viability, apoptosis and differentiation of neural stem cells
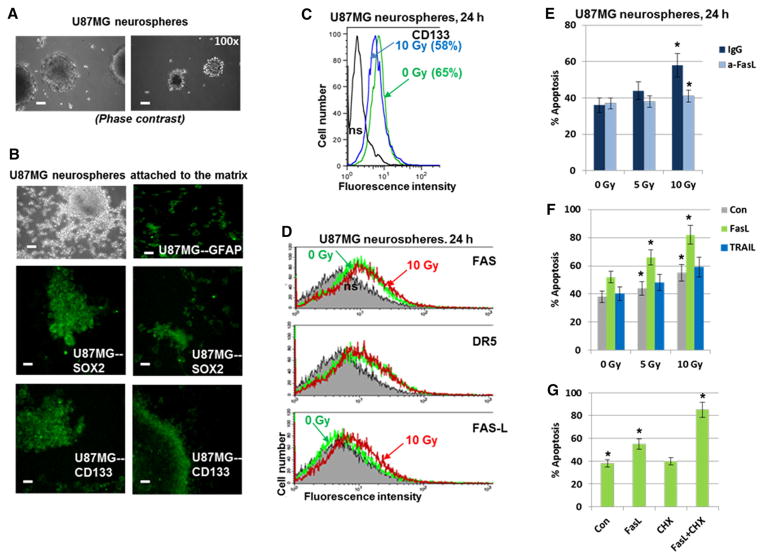
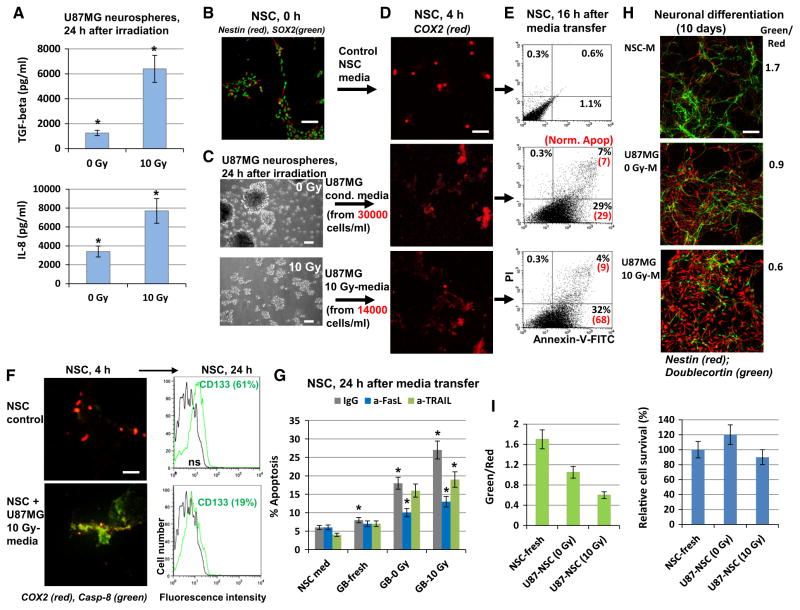
Although the precise origin of glioblastoma (GB) cells is not known, the current hypothesis is that glioma-initiating cells, which have characteristics of cancer stem-like cells, might give rise to GB [29]. To directly assess the intercellular communication between glioblastoma stem-like cells (GSC) and NSC after ionizing irradiation of GSC, experiments were performed with direct irradiation of GB subpopulation enriched with GSC and evaluation of the induction of a bystander response in non-targeted NSC. A U87MG neurosphere population enriched by GSC was established after 15–20 passages of U87MG cells in the serum-free NSC medium with EGF and FGF2 (Fig. 8a–d), as previously described [30]. Immunostaining demonstrated that the vast majority of neurospheres after 20 passages were GFAP+, SOX2+ and CD133+ (Fig. 8b). FACS assay further confirmed that approximately 65 % of cells in neurospheres were CD133+. The percentage of CD133+ cells to some extent dropped 24 h after irradiation, reflecting, probably, a change in CD133 surface expression on GSC and death of a fraction of CD133+ cells (Fig. 8c). Furthermore, notable fractions of the U87MG neurosphere population were FAS+ or DR5+. Radiation induced moderate expression of the endogenous membrane FasL, but did not really affect TRAIL (Fig. 8d). Interestingly, gamma-irradiation of U87MG neurospheres induced apoptosis that was partially blocked by anti-FasL antibody (Fig. 8e). On the other hand, addition of the exogenous recombinant FasL 4 h after irradiation significantly upregulated (+30 %) apoptosis in GSC, while exogenous TRAIL exhibited only marginal effects (Fig. 8f). Relatively high basal percentages (35–38 %) of the sub-G1 cells reflected the permanent presence of contaminating dead cells in the core of non-adherent spheroids during cell culturing. Taken together, these results highlighted a notable role of FasL/Fas and a marginal role of TRAIL/ DR5 for radiation-induced apoptosis of U87MG neurospheres. CHX further increased FasL-mediated apoptosis of neurospheres (Fig. 8g).
Fig. 8.
Establishing of enriched population of glioma stem-like cells among U87MG neurospheres. a, b U87MG neurosphere culture was established after 15 passages in serum-free media with EGF and FGF2. Immunostaining of neurospheres attached to the fibronectin matrix revealed numerous CD133+ and SOX2+ U87MG cells. Furthermore, all cells were GFAP+. Bar = 50 μm. c, d Immunostaining and FACS analysis of U87MG neurosphere culture. Surface expression levels of CD133, FAS, DR5 and FAS-L were determined before and 24 h after irradiation (10 Gy). A fraction of CD133+ dropped from 65 to 58 % after irradiation at 10 Gy. e Effects of anti-FasL Ab on apoptotic levels of U87MG neurospheres before and after irradiation. Anti-FasL Ab (5 μg/ml) and normal IgG were added to the media before irradiation. Apoptotic levels were determined 24 h after irradiation using PI staining and FACS analysis. f Additional effects of the exogenous FasL and TRAIL (40 ng/ml) on radiation-induced apoptosis of U87MG neurospheres. Death ligands were added 4 h after irradiation. g FasL+CHX induced apoptosis of neurospheres
Using ELISA, we observed dramatic up-regulation of TGFβ1 and IL8 production 24 h after irradiation of glioblastoma neurospheres (Fig. 9a). GSC produced relatively similar levels of IL8, but substantially higher levels of TGFβ1, especially after irradiation, compared to non-stem GB cells (see Suppl. Figure 1). A critical role TGFβ in self-renewal of GSC is well established [22, 31]. Media transfer experiments from non-treated and irradiated neurospheres to the naïve NSC were performed for evaluation of non-targeted bystander effects. Of note, for culture of both neurospheres and NSC the same NSC-media (serum-free, with EGF and FGF2) was used. Media transfer experiments from neurospheres to the naïve NSC further demonstrated COX2 induction in NSC (Fig. 9b–d) and notable up-regulation of NSC apoptosis, especially after irradiated media transfer. Levels of apoptosis were detected using Annexin-V-FITC + PI staining of NSC and FACS analysis (Fig. 9e). Normalization of apoptotic levels in NSC was performed to the equal number of glioblastoma cells used for secretion into the culture media (Fig. 9c, e). Immunostaining with antibody against cleaved (active) fragment of caspase-8 further confirmed caspase-8-mediated apoptosis in NSC induced by media from glioblastoma neurospheres. Apoptosis of NSC was accompanied by a strong decrease in the fraction of CD133+ NSC (Fig. 9f). Furthermore, apoptosis that was induced by media transfer to NSC could be significantly blocked by anti-FasL Ab, and relatively slightly by anti-TRAIL Ab (after irradiation at 10 Gy) indicating a notable role for FasL and somewhat a lesser role for TRAIL from GB-transferred media in the regulation of NSC apoptosis (Fig. 9g).
Fig. 9.
Cytokine secretion, apoptosis and bystander effects in bystander NSC after media transfer from non-treated and γ-irradiated U87MG neurospheres. a TGFβ1 and IL8 secretion before and after irradiation was determined by ELISA and normalized for 106 U87MG cells. b–i Media transfer experiments. Control (non-treated) NSC were stained for Nestin (red) and SOX2 (green) (b). U87MG neurospheres 24 h after irradiation are shown in c. Up-regulation of COX2 protein levels in NSC (d) was induced by exposure to media (4 h) that were transfered from non-irradiated and irradiated U87MG neurospheres. Apoptosis of NSC (e) was induced by exposure to media (16 h) that were transfer from non-irradiated and irradiated (10 Gy) U87MG neurospheres. Apoptosis levels were detected using Annexin-V-FITC + PI staining of and FACS analysis. Normalized levels of apoptosis (induced by secreted activity of 30,000 U87MG cells) are shown in brackets (e). The left side of f: induction of Caspase-8 activity in NSC treated by media from irradiated neurospheres. Immunostaining with anti-COX2 (red) and anti-cleaved fragment of Caspase-8 Abs (green). The right side of the f: immunostaining and FACS analysis of CD133+ NSC. Percentage of positive cells before and 24 h after media transfer are indicated. g Apoptosis levels in NSC were detected using PI staining of the NSC nuclei and the flow cytometry. Media transfer experiments were performed in the presence of antibodies in transferred media, anti-TRAIL, anti-FasL (5 μg/ml), or normal IgG as a control. Pooled results of three independent experiments are shown. Error bars represent means ± SD (p < 0.05, Student’s t test); star indicates a significant difference. h, i U87MG neurospheres were cultured in the NSC serum-free media. Effects of media transfer from non-treated (U87MG 0 Gy-M) and irradiated (U87MG 10 Gy-M) neurospheres with 8-h pretreatment of NSC on the subsequent neuronal differentiation of NSC (10 days). Differentiation of the native NSC in NSC-media (NSC-M) was used as a reference. Immunostaining for Nestin and Doublecortin was performed 10 days after initiation of differentiation. Bar = 50 μm. A ratio of green/red cells reflected a degree of neuronal differentiation
Finally, media generated after irradiation of neurospheres at 10 Gy substantially suppressed neuronal differentiation of NCS (Fig. 9h, i), reminiscent of the effects of media transfer experiments using non-stem U87MG glioblastoma cells with high radiation-induced secretion of IL8 and TGFβ. It should be highlighted the absence of negative effects for the control NSC by pretreatment with fresh serum-free culture medium, compared to small negative effects of the fresh GB media pretreatment described above (see Fig. 5f). It makes the media transfer experiments from irradiated neurospheres to non-targeted bystander NSC very convincing.
Discussion
One of the primary goals in radiation therapy is to enhance the efficiency of tumor cell killing without seriously hampering the functional integrity of normal differentiated cells and multipotent adult stem cells in the tissue micro-environment. However, demonstration of bystander effects in non-targeted cells in diverse human cell systems raises a serious health concern [18, 32]. Understanding the intrinsic cellular communication between cancer and non-cancer cells, especially after radiation exposure, is highly desirable for development of strategies to either minimize or prevent the harmful radiation effects on normal stem cells, which are critical for tissue homeostasis. In this study, we explored the concept that the cellular signaling trigged by radiation exposure in cancer cells can produce adverse effects on the integrity and function of adult stem cells.
The results of our study demonstrated the existence of intercellular crosstalk of glioblasoma (GB) cells, as well as a subpopulation of glioma stem-like cells (GSC), with NSC before and after ionizing irradiation. Although, we observed a qualitative similarity in the bystander effects of non-stem GB cells and GSC on the naive NSC via soluble factors, further investigation might reveal quantitative differences in the extent of bystander effects. For example, the well pronounced pro-apoptotic features of irradiation for GSC in our study (that was mediated, at least partially, via endogenous expression of both FasL and Fas) were in contrast to non-apoptotic mechanism of radiation-induced death for non-stem U87MG glioblastoma cells. Distinct mechanisms for radiation-induced non-apoptotic death of non-stem glioblastoma cells and for Fas-L/Fas-mediated apoptosis of these cells were previously described [33]. Interestingly, there are contradictory observations regarding the radioresistance of GSC. McCord et al. described establishing several GSC lines that were substantially more radiosensitive than the corresponding parent GB lines [34]. In contrast, other investigators observed a pronounced increase in the subpopulation of CD133+ glioblastoma stem-like cells after irradiation due to preferential activation of the DNA damage response [35]. It is still unclear whether this is due to selective expansion of CD133+ cells, or it is simply due to dedifferentiation of differentiated cancer cells. Mechanisms of dedifferentiation of cancer cells, including glioblastoma cells, are under active investigation.
An exciting recent publication identified a core set of neurodevelopmental transcription factors (SOX2, POU3F2, SALL2 and OLIG2) that were sufficient to fully reprogram differentiated GB cells to glioblastoma stem-like cells with recapitulating phenotype of native GSC. OLIG2-dependent LSD1 (Lysine-specific demethylase-1, one of the components of a chromatin-modifying complex) was necessary for GSC survival; treatment with a specific LSD1 inhibitor reduced cells survival [36]. It was supposed in this that inhibition of LSD1 had potential therapeutic significance. However, the well-known role of LSD1 in the positive control of human NSC proliferation [37] challenges this suggestion.
The ideal mechanism for killing cancer cells via death receptor-mediated apoptosis appeared to be rarely functional if ionizing irradiation was used alone. The most probable scenarios for cancer cells exposed to low linear energy transfer (LET) irradiation are: (i) undergo cell senescence (a permanent proliferative arrest) with the subsequent slow death due to changing balance between of autophagy and necrosis; (ii) activate the intrinsic mitochondria-mediated death pathways using ROS-induced DNA-damage response when these pathways were maintained in cancer cells [38, 39]. As a rule, the second scenario might require an additional suppression of cell survival pathways that are over-activated in cancer cells, for example, PI3K-AKT, as we observed in the current study, or TGFβ [22] and STAT3 [30, 40]. GSC also contained constitutive NF-κB and STAT3 activities [41] and could be radio-synthesized by the corresponding small molecule inhibitors. An additional opportunity for inhibition of glioblastoma proliferation is linked with high levels of expression of PKC-iota in these cancer cells. ICA-1, a specific inhibitor of PKC-iota, alone or especially in combination with TRAIL suppressed proliferation and induced apoptosis in glioblastoma lines [42, 43].
At present, due to significant technological advances, ionizing radiation can very selectively target the tumors with limited side effects for normal tissues. The existence of intercellular communication between targeted cancer cells and bystander (non-irradiated) normal cells, however, could dramatically affect the fate of the bystander normal cells [18]. For cranial irradiation of brain tumor, NSC could be in many cases a critical bystander target [6, 10]. Our previous study demonstrated that radiation-induced apoptosis of NSC in cell culture is mediated by TRAIL/ TRAIL-R2 interaction through autocrine or paracrine stimulation. It created mechanisms for killing of both directly irradiated NSC and bystander NSC. This finding was in contrast to the popular belief that TRAIL-dependent signaling mechanism preferentially killed cancer cells. Furthermore, ionizing irradiation of cancer cells, such as neuroblastoma [12] or glioblastoma (the current study), was accompanied by increased production and secretion of TRAIL and FasL resulting in upregulation of apoptosis of bystander NSC/NPC, while malignant cancer cells exhibited a substantial anti-apoptotic protective mechanisms. Indeed, massive productions of pro-inflammatory cytokines after irradiation of glioblastoma plays pronounced anti-apoptotic role for cancer cells. Furthermore, in certain circumstances, this cytokine secretion could increase the survival of bystander NSC/NPC, but with simultaneous inhibition of the neuronal pathway of differentiation. Interestingly, there was a strong asymmetry in bystander response of differentiating astrocyte precursors compared to differentiating neuronal precursors, since the media from irradiated glioblastoma cultures had favorable effects for astrocyte differentiation. To our best knowledge, inhibition of the neuronal pathway of NSC differentiation by factors that were produced by directly irradiated glioblastoma cells is a new finding, while positive effects of γ-irradiation on the astrocyte differentiation via up-regulation of IL6-STAT3 have been recently described [44].
The high basal levels of IL6, IL8 and COX2-driven production of PGE2 in glioblastoma cells are direct downstream targets of the constitutive IKK-NF-κB activity. On the other hand, COX2-dependent product PGE2 with numerous signaling functions actually could suppress the late NF-κB activation to establish the feedback mechanism allowing for restriction of COX2 induction and PGE2 secretion [45]. This may be a reason for a dose-dependent decrease of the high basal NF-κB phospo-p65 and COX2 activity 3–6 h after irradiation of glioblastoma cells (see Figs. 1c, d).
A strong dependence of glioblastoma cell survival on activation of two major signaling pathways IKK-NF-κB and PI3K-AKT, was further confirmed in our study by the synergistic induction of apoptosis of U87MG cells using combined treatment with small molecule inhibitors of both pathways (see Fig. 2b). Paradoxically, BMS345541, an IKK-NF-κB inhibitor, alone or in combination with γ-irradiation mainly induced non-apoptotic death of glioblastoma cells. An additional example of induction of death of glioblastoma cells via senescence and the subsequent necrosis was a combination of ATM-kinase inhibitor KU55933 and ionizing irradiation (see Fig. 2c). Since STAT3 activation and translocation is one of the critical downstream targets of ATM-kinase [46], suppression of STAT3 translocations by a specific small molecule inhibitor STX-0119 in concert with irradiation also resulted in G2/M arrest, senescence and slow cell death (see Fig. 2d). In a recent publication, the usage of Vacquinol-1, a MKK4-JNK inhibitor, had dramatic effects for cell physiology of GB in culture conditions and in vivo due to the induction of rapid endocytic-like activity followed by disruption of the cell membrane and cell death [47]. On the other hand, interferon-β via massive alteration in expression of its target genes could overcome therapy resistance (including irradiation) in GSC [48].
Radiation-induced up-regulation of cytokine and death ligand secretion by glioblastoma cells established the conditions for radiation-induced bystander response of NSC that was mediated mostly soluble factors released in the media by cancer cells. The characteristic feature of apoptosis in the bystander NSC was its suppression by anti-TRAIL antibody. Human NSC were also sensitive to Fas-Ligand- and to TNFα-induced cell death, due to the presence of the corresponding receptors. TNFα could be produced in vivo by microglial cells of the brain [49], targeting via TNFR1 both apoptotic and necrotic pathways in NSC. On the other hand, beside induction of cell death, TNFα may serve as a classical inducer of cell survival pathways via NF-κB activation. Dual functions of TNFα highlight its central role in balancing between life and death during different types of stress responses [50–52].
In general, the irradiation of cells targets numerous cell signaling pathways and induces changes in expression of several hundred genes [53]. However, radiation-induced gene activation shares some common features with other types of cell stress, including modulation of the pro-inflammatory and anti-inflammatory gene expression of specific sets of cytokines and their receptors, COX2 expression and COX2-directed production of PGE2, expression of death ligands and death receptors [18, 54]. Paradoxically, non-active COX2 was located in the nuclei, while it was translocated to the cytoplasm upon activation [55]; (see also Fig. 1b). Production and secretion of cytokines and PGE2 after irradiation may serve as a basis for the induction of propagating the bystander response of non-targeted cells. Bystander suppression of neuronal differentiation was especially strong in the case of radiation-induced intercellular crosstalk between irradiated cancer (neuroblastoma or glioblastoma) cells and NSC.
Taken together, results of our study indicated that intercellular communication between glioblastoma cells and bystander NSC/NPC could be involved in the amplification of cancer pathology in the brain. We expect that further investigations of the mechanisms of radiation-induced neurotoxicity at the level of NSCs may open new opportunities to protect and maintain neurogenesis after anti-cancer therapy, especially for patients with brain tumors.
Materials and methods
Reagents
Fibronectin, laminin and polyornithine were obtained from Sigma-Aldrich (St. Louis, MO, USA). PI3 K inhibitor LY294002, IKK inhibitor BMS345541, STAT3 inhibitor-6 S3I-201 (also known as NSC 74859), MEK inhibitor U0126, MAPK p38 inhibitor SB203580 and JNK inhibitor SP600125 were purchased from Calbiochem (La Jolla, CA, USA). Human soluble Killer-TRAIL (recombinant), Fas-Ligand (recombinant), anti-human TRAIL and anti-human Fas-Ligand antibodies were purchased from Alexis (San Diego, CA, USA); human TNFα, TGFβ1, IL6 and IL8 were obtained from R&D Systems (Minneapolis, MN, USA).
Human embryonic neural stem cells (NSC) in culture
Cryopreserved human embryonic neural stem cells (NSC) were obtained from Gibco/Life Technologies (Carlsbad, CA, USA) as a commercially available product (N7800-200). The cells were derived from NIH approved H9 (WA09) human embryonic stem cells. The cells were plated in 6-well culture plates coated with fibronectin and incubated at 37 °C in complete growth medium NSC/SFM, which contained serum-free DMEM/F12 supplemented with 2 mM GlutaMAX, bFGF (20 ng/ml), EGF (20 ng/ml) and StemPRO neural supplement (2 %). All reagents were obtained from from Gibco/Life Technologies (Carlsbad, CA, USA).
Neuronal differentiation of NSCs in culture
Neural stem cells were plated on polyornithine- and laminin-coated 6-well plates, which contained similarly coated cover slips, in complete NSC/SFM. After 2 days, neuronal differentiation was initiated by neuronal differentiation media, which contains Neurobasal medium, B-27 Serum-free supplement (2 %) and 2 mM GlutaMAX (Gibco/Life Technologies). Medium was changed every 2 days. A neuronal phenotype was confirmed using immunofluorescence detection 10 days after initiation of differentiation.
Glial (astrocyte) differentiation of NSCs in culture
Neural stem cells were plated on polyornithine- and laminin-coated 6-well plates, which contained similarly coated cover slips, in complete NSC/SFM. After 2 days, astrocytes differentiation was initiated by astrocyte differentiation media, which contains DMEM, N-2 supplement (1 %), 1 % FBS and 2 mM GlutaMAX (Gibco/Life Technologies). Medium was changed every 2 days. An astrocyte phenotype was confirmed using immunofluorescence detection of GFAP 10–18 days after initiation of differentiation.
U87MG glioblastoma cells
Human glioblastoma (U87MG or HTB-14, ATCC) cell line was obtained from ATCC (Manassas, VA, USA). Cells were cultured in DMEM supplemented with 10 % FBS and 1 % pyruvate. For neurosphere formation, U87MG glioblastoma cells were cultured in the serum-free media DMEM/F12 supplemented with 2 mM GlutaMAX, bFGF (20 ng/ml), EGF (20 ng/ml) and B27 supplement (2 %). All reagents were obtained from from Gibco/Life Technologies (Carlsbad, CA, USA). After 15–20 passages, spheroid U87MG culture was significantly enriched by CD133+ cells. For radiation treatment and immunocytochemical analysis U87MG spheroids were attached to fibronectin matrix.
Immunocytochemistry analysis
Cells were fixed with 4 % paraformaldehyde in PBS for 60 min. Immunochemical staining was performed using standard protocols. Cells were stained for the undifferentiated NSC marker, Nestin (using mAb from Millipore, Temecula, CA, USA) and for the neuronal marker, Doublecortin using Ab from Cell Signaling, (Danvers, MA, USA). Additional markers include SOX2, GFAP, CD133 and COX2 (using Abs from Cell Signaling). The secondary Abs were Alexa Fluor 594 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG from Molecular Probes/Life Technologies (Carlsbad, CA, USA). A laser scanning confocal microscope (Nikon TE 2000 with EZ-C1 software, Tokyo, Japan) was used for immunofluorescence image analysis.
Irradiation procedures
To determine sensitivity to γ-rays, plated NSC, astrocytes and glioblastoma cells were exposed to radiation from a Gammacell 40 137Cs irradiator (dose rate, 0.82 Gy/min) at Columbia University. Six to 48 h after irradiation, cells were stained with PI and analyzed by flow cytometry for cell cycle-apoptosis studies.
FACS analysis of TRAIL, TRAIL-R2/DR5, TRAIL-R1/DR4, FAS, FAS-L and CD133 levels
Surface levels of TRAIL, TRAIL-R2/DR5, TRAIL-R1/ DR4, FAS, FAS-L and CD133 on human cell lines were determined by staining with a PE-conjugated Abs to the corresponding human proteins (R&D System, Minneapolis, MN, USA and eBioscience, San Diego, CA, USA) and subsequent flow cytometry. For detection of total levels of antigen proteins, cells were fixed and permeabilized using 0.5 % Triton X-100 in PBS. PE-conjugated nonspecific mouse IgG1 was used as an immunoglobulin isotype control. A FACS Calibur flow cytometer (Becton–Dickinson, Mountain View, CA, USA) and the CellQuest program were used to perform flow cytometric analysis. All experiments were independently repeated 3–5 times.
Cell death studies
For induction of apoptosis, cells were exposed to γ-irradiation (2–10 Gy) alone or in the presence of small molecule inhibitors of cell signaling pathways. Furthermore, apoptosis was induced TRAIL, TNFα, FasL, TGFβ and CHX alone or in combination. Media transfer experiments from conditioned and irradiated U87MG cells to naïve NSC were also used for induction of NSC apoptosis. Apoptosis levels were then assessed by propidium iodide (PI) staining and quantifying the percentage of hypodiploid nuclei (pre-G1) using FACS analysis or by quantifying the percentage of Annexin-V-FITC-positive cells (BD Pharmingen, San Diego, CA) that was performed on a FACS Calibur flow cytometer (Becton–Dickinson) using the CellQuest program. Trypan blue exclusion test was used for determination of cell viability and total death levels. Clonogenic survival assay of U87MG cells before and after treatment with increased doses of γ-radiation in the presence or absence of a PI3K-AKT inhibitor LY294002 (40 μM), an IKK-NF-κB inhibitor BMS345541 (10 μM) or several other small molecule inhibitors was also performed using a standard method.
Western blot analysis
Total cell lysates (50 μg protein) were resolved on SDS-PAGE, and processed according to standard protocols. The monoclonal antibodies used for Western blotting included: anti-β-Actin (Sigma, St. Louis, MO, USA); anti-caspase-3 (Cell Signaling, Danvers, MA, USA); The polyclonal antibodies used included anti-phospho-p44/p42 MAP kinase (T202/Y204) and anti-p44/p42 MAP kinase; anti-phospho-JNK and anti-JNK1-3; anti-phospho-AKT (S473) and anti-AKT; anti-phospho-p65 (S536) NF-κB and anti-p65 NF-κB, anti-phospho-STAT3 (Y705) and anti-STAT3; anti-p53, anti-BAX, anti-SOX2, anti-TGF-β1, anti-TGF-β-Receptor-2 and anti-PARP-1 (Cell Signaling, Danvers, MA, USA); anti-FAS, anti-FAS-Ligand, anti-DR5/TRAIL-R2, anti-DR4/TRAIL-R1 and anti-TRAIL (Alexis, San Diego, CA, USA); anti-SURVIVIN (R&D, USA) The secondary antibodies were conjugated to horseradish peroxidase; signals were detected using the ECL system (Thermo Scientific, Rockford, IL, USA).
ELISA for IL6, IL8, TGFβ1 and TRAIL detection in the media
The ELISA kits for detection of human cytokines were from R&D System, Minneapolis, MN, USA and eBioscience, San Diego, CA, USA.
Statistical analysis
Data from four to five independent experiments were calculated as means and standard deviations. Comparisons of results between treated and control groups were made by the Students’ t tests. A p value of 0.05 or less between groups was considered significant.
Supplementary Material
Acknowledgments
We would like to thank Drs. Adayabalam Balajee and Peter Grabham for advice, critical reading of the manuscript and discussion. This work was supported by NIH Grant P01 CA049062 and Pilot Grant of the Department of Dermatology, Columbia University (P30AR044531-11, project GG006336).
Abbreviations
- CHX
Cycloheximide
- DR5
Death recepotor-5 (synonym for TRAIL-R2), FACS, Fluorescence-activated cell sorter
- FasL
Fas ligand
- FGF2
Fibroblast growth factor-2 (basic)
- GB
Glioblastoma
- GSC
Glioma stem-like cells
- IκB
Inhibitor of NF-κB
- IKK
Inhibitor nuclear factor kappa B kinase
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MEF
Median fluorescence intensity
- NF-κB
Nuclear factor kappa B
- NPC
Neural progenitor cells
- NSC
Neural stem cells
- PARP1
Poly (ADP-ribose) polymerase-1
- PI
Propidium iodide
- STAT
Signal transducers and activators of transcription
- TGFβ
Transforming growth factor beta
- TGFβ-R
TGFβ-receptor
- TNFα
Tumor necrosis factor alpha
- TRAIL
TNF-related apoptosis ligand
- TRAIL-R2
TRAIL-receptor-2
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10495-014-1040-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that there are no conflicts of interest.
Contributor Information
Vladimir N. Ivanov, Email: vni3@columbia.edu, Center for Radiological Research, Department of Radiation Oncology, College of Physicians and Surgeons, Columbia University, 630 West 168th Street, New York, NY 10032, USA
Tom K. Hei, Center for Radiological Research, Department of Radiation Oncology, College of Physicians and Surgeons, Columbia University, 630 West 168th Street, New York, NY 10032, USA
References
- 1.Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neurooncology. 2010;12:520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark MJ, Homer N, O’Connor BD, Chen Z, Eskin A, Lee H, Merriman B, Nelson SF. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS Genet. 2010;6:e1000832. doi: 10.1371/journal.pgen.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 7.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 8.Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, Limoli CL. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med. 2010;49:1846–1855. doi: 10.1016/j.freeradbiomed.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, Limoli CL. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71:4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellstrom NA, Bjork-Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27:634–641. doi: 10.1634/stemcells.2008-0732. [DOI] [PubMed] [Google Scholar]
- 11.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov VN, Hei TK. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis. 2014;19:399–413. doi: 10.1007/s10495-013-0925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 14.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov VN, Ghandhi SA, Zhou H, Huang SX, Chai Y, Amundson SA, Hei TK. Radiation response and regulation of apoptosis induced by a combination of TRAIL and CHX in cells lacking mitochondrial DNA: a role for NF-kappaB-STAT3-directed gene expression. Exp Cell Res. 2011;317:1548–1566. doi: 10.1016/j.yexcr.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov VN, Zhou H, Ghandhi SA, Karasic TB, Yaghoubian B, Amundson SA, Hei TK. Radiation-induced bystander signaling pathways in human fibroblasts: a role for interleukin-33 in the signal transmission. Cell Signal. 2010;22:1076–1087. doi: 10.1016/j.cellsig.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persano L, Rampazzo E, Basso G, Viola G. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol. 2013;85:612–622. doi: 10.1016/j.bcp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, Seyfried NT, Abe T, Chen LB, Carroll RS, Black PM. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60:4926–4931. [PubMed] [Google Scholar]
- 22.Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, Barcellos-Hoff MH. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res. 2012;72:4119–4129. doi: 10.1158/0008-5472.CAN-12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanov VN, Hei TK. Induction of apoptotic death and retardation of neuronal differentiation of human neural stem cells by sodium arsenite treatment. Exp Cell Res. 2013;319:875–887. doi: 10.1016/j.yexcr.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C, Wu J, Hu B, Cheng SY, Li M, Li J. TGF-beta induces miR-182 to sustain NF-kappaB activation in glioma subsets. J Clin Invest. 2012;122:3563–3578. doi: 10.1172/JCI62339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasi F, Facoetti A, Nano R. IL-8 and IL-6 bystander signalling in human glioblastoma cells exposed to gamma radiation. Anticancer Res. 2010;30:2769–2772. [PubMed] [Google Scholar]
- 27.Aigner L, Bogdahn U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell Tissue Res. 2008;331:225–241. doi: 10.1007/s00441-007-0466-7. [DOI] [PubMed] [Google Scholar]
- 28.Nieto-Estevez V, Pignatelli J, Arauzo-Bravo MJ, Hurtado-Chong A, Vicario-Abejon C. A global transcriptome analysis reveals molecular hallmarks of neural stem cell death, survival, and differentiation in response to partial FGF-2 and EGF derivation. PLoS ONE. 2013;8:e53594. doi: 10.1371/journal.pone.0053594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 30.Ashizawa T, Miyata H, Iizuka A, Komiyama M, Oshita C, Kume A, Nogami M, Yagoto M, Ito I, Oishi T, Watanabe R, Mitsuya K, Matsuno K, Furuya T, Okawara T, Otsuka M, Ogo N, Asai A, Nakasu Y, Yamaguchi K, Akiyama Y. Effect of the STAT3 inhibitor STX-0119 on the proliferation of cancer stem-like cells derived from recurrent glioblastoma. Int J Oncol. 2013;43:219–227. doi: 10.3892/ijo.2013.1916. [DOI] [PubMed] [Google Scholar]
- 31.Penuelas S, Anido J, Prieto-Sanchez RM, Folch G, Barba I, Cuartas I, Garcia-Dorado D, Poca MA, Sahuquillo J, Baselga J, Seoane J. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–327. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Brady D, O’Sullivan JM, Prise KM. What is the role of the bystander response in radionuclide therapies? Front Oncol. 2013;3:215. doi: 10.3389/fonc.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streffer JR, Schuster M, Pohl U, Belka C, Dichgans J, Bamberg M, Weller M. Irradiation induced clonogenic cell death of human malignant glioma cells does not require CD95/CD95L interactions. Anticancer Res. 1999;19:5265–5269. [PubMed] [Google Scholar]
- 34.McCord AM, Jamal M, Williams ES, Camphausen K, Tofilon PJ. CD133 + glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin Cancer Res. 2009;15:5145–5153. doi: 10.1158/1078-0432.CCR-09-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 36.Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, Curry WT, Martuza RL, Rivera MN, Rossetti N, Kasif S, Beik S, Kadri S, Tirosh I, Wortman I, Shalek AK, Rozenblatt-Rosen O, Regev A, Louis DN, Bernstein BE. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun G, Alzayady K, Stewart R, Ye P, Yang S, Li W, Shi Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol Cell Biol. 2010;30:1997–2005. doi: 10.1128/MCB.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology. 2013;2:e26536. doi: 10.4161/onci.26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borges HL, Linden R, Wang JY. DNA damage-induced cell death: lessons from the central nervous system. Cell Res. 2008;18:17–26. doi: 10.1038/cr.2007.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garner JM, Fan M, Yang CH, Du Z, Sims M, Davidoff AM, Pfeffer LM. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappaB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J Biol Chem. 2013;288:26167–26176. doi: 10.1074/jbc.M113.477950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel R, Win H, Desai S, Patel K, Matthews JA, Acevedo-Duncan M. Involvement of PKC-iota in glioma proliferation. Cell Prolif. 2008;41:122–135. doi: 10.1111/j.1365-2184.2007.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCray AN, Desai S, Acevedo-Duncan M. The interruption of PKC-iota signaling and TRAIL combination therapy against glioblastoma cells. Neurochem Res. 2014 doi: 10.1007/s11064-014-1361-8. [DOI] [PubMed] [Google Scholar]
- 44.Schneider L, Pellegatta S, Favaro R, Pisati F, Roncaglia P, Testa G, Nicolis SK, Finocchiaro G, d’Adda di Fagagna F. DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem cell reports. 2013;1(2):123–138. doi: 10.1016/j.stemcr.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov VN, Zhou H, Partridge MA, Hei TK. Inhibition of ataxia telangiectasia mutated kinase activity enhances TRAIL-mediated apoptosis in human melanoma cells. Cancer Res. 2009;69:3510–3519. doi: 10.1158/0008-5472.CAN-08-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitambi SS, Toledo EM, Usoskin D, Wee S, Harisankar A, Svensson R, Sigmundsson K, Kalderen C, Niklasson M, Kundu S, Aranda S, Westermark B, Uhrbom L, Andang M, Damberg P, Nelander S, Arenas E, Artursson P, Walfridsson J, Forsberg Nilsson K, Hammarstrom LG, Ernfors P. Vulnerability of glioblastoma cells to catastrophic vacuolization and death induced by a small molecule. Cell. 2014;157:313–328. doi: 10.1016/j.cell.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Happold C, Roth P, Silginer M, Florea AM, Lamszus K, Frei K, Deenen R, Reifenberger G, Weller M. Interferon-beta induces loss of spherogenicity and overcomes therapy resistance of glioblastoma stem cells. Mol Cancer Ther. 2014;13:948–961. doi: 10.1158/1535-7163.MCT-13-0772. [DOI] [PubMed] [Google Scholar]
- 49.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 50.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 51.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walczak H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol. 2013;5:a008698. doi: 10.1101/cshperspect.a008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghandhi SA, Ming L, Ivanov VN, Hei TK, Amundson SA. Regulation of early signaling and gene expression in the alpha-particle and bystander response of IMR-90 human fibroblasts. BMC Med Genomics. 2010;3:31. doi: 10.1186/1755-8794-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov VN, Ghandhi SA, Zhou H, Huang SX, Chai Y, Amundson SA, Hei TK. Radiation response and regulation of apoptosis induced by a combination of TRAIL and CHX in cells lacking mitochondrial DNA: a role for NF-[kappa]B-STAT3-directed gene expression. Exp Cell Res. 2011;317:1548–1566. doi: 10.1016/j.yexcr.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parfenova H, Parfenov VN, Shlopov BV, Levine V, Falkos S, Pourcyrous M, Leffler CW. Dynamics of nuclear localization sites for COX-2 in vascular endothelial cells. Am J Physiol Cell Physiol. 2001;281:C166–C178. doi: 10.1152/ajpcell.2001.281.1.C166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.