Highlight
Arabidopsis RNase J plays an important role in maintaining chloroplast function, and the impairment of chloroplast development in rnj mutants may lead to aberrant embryos with disrupted pattern formation.
Key words: Arabidopsis, chloroplast, embryo, pattern formation, RNase J.
Abstract
Chloroplasts perform many essential metabolic functions and their proper development is critically important in embryogenesis. However, little is known about how chloroplasts function in embryogenesis and more relevant components need to be characterized. In this study, we show that Arabidopsis Ribonuclease J (RNase J) is required for chloroplast and embryo development. Mutation of AtRNJ led to albino ovules containing aborted embryos; the morphological development of rnj embryos was disturbed after the globular stage. Observation of ultrastructures indicated that these aborted embryos may result from impaired chloroplast development. Furthermore, by analyzing the molecular markers of cell fate decisions (STM, FIL, ML1, SCR, and WOX5) in rnj embryos, we found that this impairment of chloroplast development may lead to aberrant embryo patterning along the apical-basal axis, indicating that AtRNJ is important in initiating and maintaining the organization of shoot apical meristems (SAMs), cotyledons, and hypocotyls. Moreover, the transport and response of auxin in rnj embryos was found to be disrupted, suggesting that AtRNJ may be involved in auxin-mediated pathways during embryogenesis. Therefore, we speculate that RNJ plays a vital role in embryo morphogenesis and apical-basal pattern formation by regulating chloroplast development.
Introduction
In higher plants, embryogenesis starts from a single fertilized egg cell (zygote). Arabidopsis zygotes undergo a series of programmed cell divisions and differentiation processes that result in the formation of mature embryos. Although some of the molecular factors that regulate embryo patterning have been identified (Capron et al., 2009; Jeong et al., 2011; Wendrich and Weijers, 2013; Costa et al., 2014; Huang et al., 2014), the overall process and particular mechanisms involved remain poorly understood. Since the release of the Arabidopsis genome sequence in 2000 and the isolation and analysis of the embryo-defective (emb) mutants, a large number of genes involved in embryogenesis have been characterized as essential. Many such genes were predicted through enrichment analysis to function in basic cellular processes such as DNA, RNA, and protein synthesis, and these genes are likely to have counterparts in yeast and Caenorhabditis elegans. Fewer regulatory genes, such as transcription factors and signalling components, were identified in this analysis (Tzafrir et al., 2004). It has been suggested that embryogenesis is precisely controlled by a complicated network of gene expression, and that about 1000 genes play crucial roles during Arabidopsis embryo development (Meinke et al., 2008). Only a portion of these genes have been studied experimentally in detail. The characterization and functional dissection of ever more unknown yet essential genes will deepen our understanding of the molecular mechanisms and regulatory networks underlying embryogenesis.
Many of the essential genes have been shown to encode proteins that are localized in chloroplasts (Bryant et al., 2011). During Arabidopsis embryogenesis, chloroplasts are derived from the proplastids in meristematic cells when the embryo develops up to the late globular stage (Mansfield and Briarty, 1991). The proplastid is a kind of undifferentiated plastid with very few internal membrane vesicles; it is small and colourless. In response to light, the invaginated inner envelope of proplastids enfolds to form more vesicles that are linked and merged together, finally resulting in the development of thylakoid membranes (López-Juez, 2007). Accompanied by the accumulation of proteins and lipids required for photosynthesis, chlorophyll starts to be synthesized in heart stage embryos; subsequent photosynthesis in chloroplasts supplies energy for later stages of embryo development. The synthesis of compounds that are critical for embryogenesis, including amino acids, lipids, and phytohormones, also occurs in chloroplasts (Ruuska et al., 2004).
A comprehensive data set of 119 nuclear genes encoding Arabidopsis chloroplast proteins associated with embryo development was established by querying the literature and the SeedGenes database (Tzafrir et al., 2004; Bryant et al., 2011). These genes/proteins were then divided into three major groups according to their probable functions: (i) enzymes required for the biosynthesis of amino acids, vitamins, nucleotides, and fatty acids; (ii) essential proteins required for the import, modification, and localization of chloroplast proteins; and (iii) proteins required for expression of the chloroplast genes. It is known that there are many diverse chloroplast proteins that are required for embryo development in Arabidopsis. The elimination of biosynthetic functions within the chloroplast and interferance with the expression of chloroplast genes often results in embryo death. Several chloroplast proteins that are known to perform crucial functions in embryogenesis have recently been studied; examples include Hsp90C (Inoue et al., 2013), PGDH1 (Benstein et al., 2013), and NAD–MDH (Selinski et al., 2014). An essential role for Arabidopsis chloroplast heat shock protein 90 (Hsp90C) in protein import into chloroplasts had been demonstrated; the siliques from heterozygous hsp90c plants contained ~25% albino and aborted seeds that failed to develop properly. Arabidopsis phosphoglycerate dehydrogenase1 (PGDH1) is localized in plastids and required for ammonium assimilation and tryptophan biosynthesis. Analysis of pgdh1 mutants revealed an embryo-lethal phenotype. The plastid-localized NAD-dependent malate dehydrogenase (NAD–MDH) enzyme is crucial for energy homeostasis in developing Arabidopsis seeds. Embryos in homozygous knockout seeds only grew to reach the globular stage and the seeds developed into tiny wrinkled shapes. These results demonstrate that chloroplast proteins play essential roles in embryo development.
Chloroplasts originated when a photosynthetic prokaryote was engulfed and ‘enslaved’ by the primitive eukaryotic ancestor of land plants. They are semiautonomous organelles and contain their own genomes. Most of the endosymbiotic prokaryote genes were transferred to the nuclear genome or lost, though modern chloroplasts retain some metabolic activities, genetic mechanisms, and protein transport complexes that clearly reflect their prokaryotic origins (Reyes-Prieto et al., 2007; Keeling, 2010). RNase J is a well studied nuclease that plays an important role in RNA metabolism in bacteria (Mathy et al., 2007). In Arabidopsis, this protein has exoribonuclease activity and compensates for inefficient transcription termination by removal of antisense RNA in chloroplasts. Therefore, it plays an important role in the regulation of chloroplast gene expression (Sharwood et al., 2011). However, the biological function of AtRNJ in embryogenesis is still unknown.
In this study, we found that plants are the only eukaryotes that have genes encoding RNJs. Further, we observed that RNJ family members from both monocots and eudicots all display a high degree of sequence homology. AtRNJ is highly expressed in green tissues and reproductive organs, and its expression level is greatly dependent on light. Three null rnj mutants all displayed the same phenotype: about 25% aborted seeds distributed in siliques of heterozygous plants, and no homozygous plants could be identified. Furthermore, we show that chloroplast development in rnj embryo cells is impaired. Mutation of the RNJ gene caused impediments in cell differentiation, apical-basal patterning, and auxin metabolism during early embryogenesis. Therefore, AtRNJ plays a vital role in chloroplast development and in embryo cell fate determination.
Materials and methods
RNJ sequence analysis
We obtained the protein sequences of the Arabidopsis RNJ, CPSF73-I, CPSF73-II, and TRZ genes from the Arabidopsis Information Resource (http://www.arabidopsis.org), and the sequences of their homologues in various species from the National Center for Biotechnology Information by data bank searching using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple sequence alignment was performed on ClustalX (version 1.83), and MEGA4 software was used to display the phylogenetic tree based on the Neighbour-Joining method (Tamura et al., 2007).
Plant materials and growth conditions
The three Arabidopsis thaliana L. rnj alleles, rnj-1 (CS16191), rnj-2 (CS815990), and rnj-3 (CS24091), were obtained from the Arabidopsis Biological Resource Center (http://abrc.osu.edu/). The T-DNA insertion sites in the three mutants were confirmed by PCR and sequencing. Plant lines carrying pDR5rev::3XVENUS-N7, pPIN1::PIN1-GFP, pSTM::STM-VENUS, and pFIL::dsRED-N7 (Heisler et al., 2005) were obtained from Elliot Meyerowitz (Division of Biology, California Institute of Technology, CA, USA); the pSCR::H2B-YFP and pWOX5::GFP lines (Heidstra et al., 2004; Blilou et al., 2005) were obtained from Ben Scheres (Department of Biology, University of Utrecht, The Netherlands); and the pAtML1::NLS-3xEGFP line (Takada and Jürgens, 2007) was obtained from Gerd Jürgens (Developmental Genetics, Centre for Molecular Biology of Plants, University of Tübingen, D-72076 Tübingen, Germany). The Arabidopsis plants were cultivated in a greenhouse at Wuhan University at 22±2°C with a 16-h light/8-h dark cycle.
Plant lines carrying different markers were crossed with rnj-2 heterozygotes, and the progenies were identified by PCR and observed under a fluorescence microscope. The rnj-2/+ mutants carrying a homozygous fluorescence marker were used for subsequent experiments.
For dark treatment, Arabidopsis seeds were sterilized and plated on solid 1/2 MS medium. After stratification for 2 days at 4°C, the seeds grew under the normal light conditions for 6 days at 22°C. At 6 days after germination (DAG), seedlings were transferred to dark conditions for 1 day, while the control-tested seedlings grew under the normal light cycle.
RT-PCR and quantitative RT-PCR
Total RNA from different kinds of Arabidopsis tissue were isolated using TRIZOL reagent (Sigma, http://www.sigmaaldrich.com/), and transcribed into cDNA with a Reverse Transcription System (TOYOBO, http://www.toyobo.co.jp/e/) after digestion with DNase I (Fermentas). Next, the cDNA was used as the template for PCR analysis with gene-specific primers (Supplementary Table S1). Real-time PCR was performed using TransStart Top Green qPCR SuperMix (TransGen, China) with a Rotor-Gene 6000 machine (Corbett Research, http://www.corbettlifescience.com/). At least two independent biological replicates were made for quantitative PCR analysis, and three technical replicates were taken in each biological replicate. The GAPC was applied as a reference gene for quantitative PCR analysis. The relative expression levels were analysed as described by Ren et al. (2012).
RNJ genomic DNA cloning and rnj mutant complementation
An RNJ genomic DNA fragment including 2100bp of the promoter and 5′-UTR region was cloned from wild-type genomic DNA using a pair of specific primers (Supplementary Table S1). The amplified DNA fragment was verified by sequencing, cloned into pCambia1300 vector (Cambia, http://www.cambia.org/), and transferred into three rnj/+ mutants by the floral-dip method (Clough and Bent, 1998). After screening the transgenic seeds on hygromycin plates, positive transformants were identified by PCR, and then used for subsequent analysis.
Ovule clearing and embryo observation
Fresh ovules were dissected from siliques using forceps and mounted in Hoyer’s solution [chloral hydrate:glycerol:water, 8:1:2 (w/v/v)] for minutes to hours depending on the embryo developmental stage (Berleth and Jurgens, 1993). Next, the cleared ovules with embryos were examined by differential interference contrast microscopy under an inverted microscope (Olympus TH4-200; http://www.olympus-global.com/) equipped with a CCD of SPOT Digital Microscope Camera (Diagnostic Instruments, http://www.spotimaging.com/).
RNJ promoter and GUS/GFP fusion
The promoter fragment of RNJ was amplified with genome-specific primers (Supplementary Table S1). After verification by sequencing, the amplified DNA fragment was cloned into pCAMBIA1381Xb and pCBIm–eGFPm binary vector (Cambia, http://www.cambia.org/), and then transformed into Arabidopsis plants using the method described above.
GUS staining analysis
The homozygous T4 generation pRNJ::GUS transformants were used for GUS staining analysis. The GUS staining was carried out as described by Yuan et al. (2008). The samples were observed under an Olympus SZX12 stereomicroscope (http://www.olympus-global.com/en/) and photographed using a digital camera (Cool SNAP, RS Photometric; http://www.photometrics.com/products/ccdcams).
Confocal laser scanning microscopyConfocal laser scanning microscopy (CLSM) was used to detect the fluorescent signal of molecular markers and transgenic lines. Fresh embryos were isolated from ovules, mounted in 10% glycerol, and then observed under an Olympus FV1000 confocal microscope.
Transmission electron microscopy
The 5 DAP ovules of wild-type and rnj mutants were fixed, embedded, and sectioned as described by Qin and Zhao (2006), and then the prepared samples were observed and photographed using transmission electron microscopy (TEM; TEHitachi H-7000 FA).
Results
RNase J is a metallo-beta-lactamase that is conserved in plants
RNase J (RNJ) is a member of the metallo-beta-lactamase protein family that has been studied extensively in bacteria (Bebrone, 2007). It plays an important role in rRNA maturation and in the 5′ stability of mRNA (Mathy et al., 2007). Cloning of the Arabidopsis RNJ gene (At5g63420) from genomic DNA and cDNA confirmed that it contains 17 exons and 16 introns (Fig. 1B). Bioinformatic analysis showed that there are three other types of RNJ-like metallo-beta-lactamases involved in RNA metabolism in eukaryotes: TRZ (tRNase Z), CPSF73-I (cleavage and polyadenylation specificity factor 73-I), and Int11 (Integrator 11). The TRZ and CPSF73-I proteins can be found in plants, humans, and yeast, while Int11 can be found in plants and humans but not in yeast; this protein was first studied and designated as CPSF73-II in Arabidopsis (Supplementary Figure 1A). However, the RNJs are only encoded in plants among eukaryotes, and AtRNJ displays a high degree of homology with other RNJs in both monocots and eudicots (Supplementary Figure 1A). The fact that no proteins homologous to RNJ are found in animal or yeast genomes suggests that this protein may play a special function in plants. Protein comparison showed the five signature motifs as described by Dominski (2007), indicating that the four tRNase Z proteins are metallo-beta-lactamase (Supplementary Figure 1B). The most characteristic feature of the RNJ, CPSF73-I, and CPSF73-II proteins is the lack of a readily identifiable motif 5 and instead the presence of three conserved motifs, A–C (Supplementary Figure 1B).
Fig. 1.
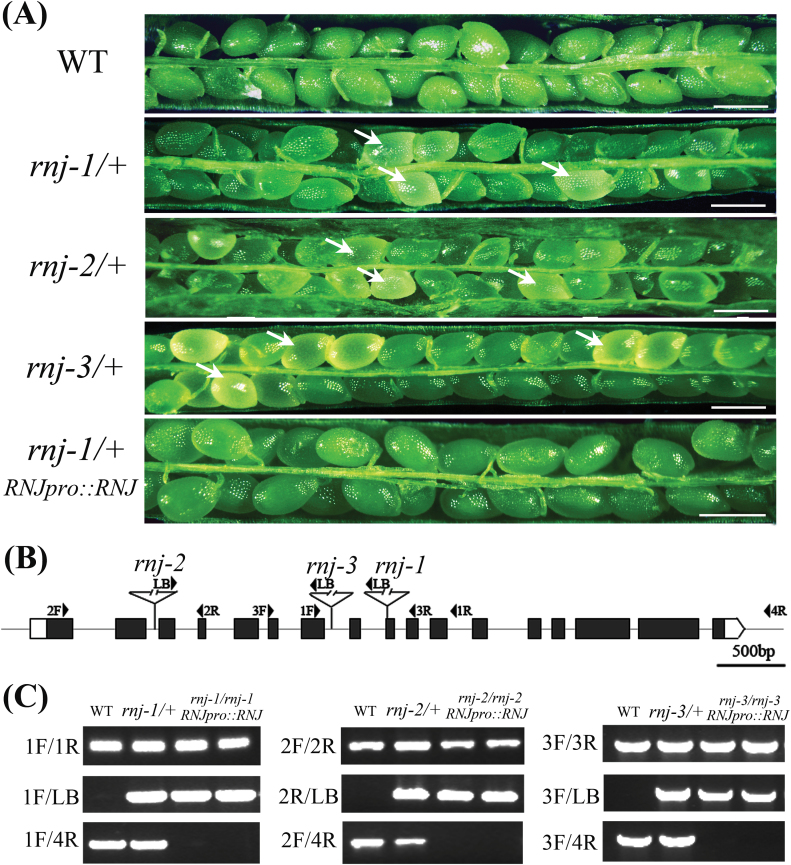
T-DNA insertion mutants of RNJ and functional complementation. (A) Seed development in wild-type, rnj-1/+, rnj-2/+, rnj-3/+, and functionally complemented rnj-1/+ transgenic plants. The arrows show the aborted white ovules. Scale bars = 0.5mm. (B) Schematic diagram of three T-DNA insertions in the Arabidopsis RNJ gene. The three mutants rnj-1 (CS16191), rnj-2 (CS815990), and rnj-3 (CS24091) have T-DNA insertions in exon 9, intron 2, and intron 7, respectively. Black boxes indicate exons, grey lines indicate introns, and arrowheads indicate the positions of primers used for genotyping. (C) PCR analysis of three rnj mutants and functionally complemented transgenic plants. This figure is available in colour at JXB online.
Homozygous rnj mutation causes seed abortion
To investigate the biological functions of the Arabidopsis RNJ gene, we obtained three independent T-DNA insertion mutants from the Arabidopsis Biological Resource Center (http://abrc.osu.edu/). The positions of the T-DNA insertions in rnj-1 (CS16191), rnj-2 (CS815990), and rnj-3 (CS24091) mutants were verified by genomic PCR and sequencing; these insertions where found to be located in exon9, intron2, and intron7, respectively (Fig. 1B). The functional domains of RNJ with respect to the T-DNA insertions are also indicated in Supplementary Figure 2. Genotypic analysis of the rnj/+ progeny (n > 100 per line) showed no homozygotes for any of the three mutants. None of the heterozygous plants exhibited any vegetative developmental defects, but a portion of the ovules in mature siliques were white (Fig. 1A), with frequencies of 24.9% in rnj-1/+ (n = 1653, P = 0.99), 25.7% in rnj-2/+ (n = 2422, P = 0.48), and 25.1% in rnj-3/+ (n = 1372, P = 0.96). The white ovules in rnj/+ siliques then turned brown and wrinkled, occurring at a frequency close to the expected value of 25% (Tzafrir et al., 2004). This finding suggests that the homozygous rnj mutation causes seed abortion.
To clarify whether a null mutation in RNJ could result in gametophyte sterility, we performed further genetic analysis on the rnj-2/+ lines. The T-DNA insertion in rnj-2/+ harbours a genetic tag of Basta (Bas) resistance for the mutant plants; this feature facilitates segregation analysis of mutant alleles. The progeny seedlings of self-pollinated rnj-2/+ plants segregated close to a 2:1 ratio of Bas resistant (BasR) to Bas sensitive (BasS) (Table 1). Because no homozygous seedlings were obtained, this segregation ratio was taken as being indicative of the expected theoretical ratio of 2:1 for heterozygous to wild-type plants. To determine whether T-DNA could be transmitted through the male and female gametophytes, we performed reciprocal crosses with rnj-2/+ and wild-type plants (Meinke et al., 2008). When rnj-2/+ plants were used as recipients in crosses with wild-type pollen, 48.3% of progeny were resistant to Basta and the transmission efficiency of female gametophytes was 93.5% (Table 1). When wild-type pistils were crossed with the rnj-2/+ pollen grains, 52.3% of progeny were resistant to Basta and the transmission efficiency of male gametophytes was 109.5% (Table 1). These results indicate that the transmission efficiency of the rnj-2/+ mutation was normal through both male and female gametophytes.
Table 1.
Transmission analysis of reciprocal crosses between Arabidopsis wild type and rnj-2/+
| Female × Male | BASTAR | BASTAS | BASTAR:Total | TE (%) | P value |
|---|---|---|---|---|---|
| rnj-2/+ × rnj-2/+ | 1249 | 628 | 0.67:1 | NA | NA |
| rnj-2/+ × +/+ | 288 | 308 | 0.48:1 | 93.5 | 0.24 |
| +/+ × rnj-2/+ | 243 | 222 | 0.52:1 | 109.5 | 0.18 |
BASTAR, BASTA resistant; BASTAS, BASTA sensitive; NA, not applicable; TE, transmission efficiency [(BASTAR/BASTAS) × 100]. The P value is based on an expected 100% TE; P > 0.05 indicates no significant differences.
To confirm that the observed seed lethality was caused by interruption of the RNJ gene, we employed a complementation strategy to test whether or not the full-length genomic sequence of RNJ could rescue the seed-lethal phenotype. The genomic fragment of RNJ, including the 5772bp gene sequence and 2100bp upstream of the ATG codon, was introduced into each of the three mutants (rnj-1/+, rnj-2/+, rnj-3/+). PCR screening and phenotypic analysis in the T2 progeny of the complementation lines allowed us to identify homozygous rnj mutants that showed no aborted seeds in siliques (Fig. 1A, C). These results indicated that RNJ was indeed the gene responsible for the seed lethality and that it is an essential gene in seed development.
Morphological development of the rnj mutant embryo is disturbed
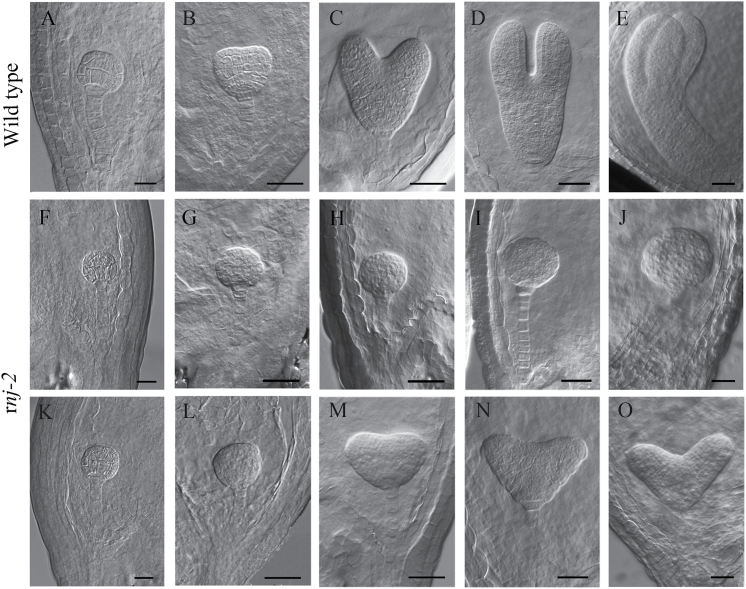
To investigate how the seed abortion phenotype occurred in the siliques of rnj heterozygotes, we examined the seed developmental processes in wild-type and rnj-2/+ plants with a whole mount clearing technique and differential interference contrast microscopy. The results showed that there were no obvious differences between wild-type and rnj-2 mutant embryos from the zygote up to the early globular stage (Fig. 2A, F, and K). Subsequently, disturbed embryos were observed in the three mutants; aberrant phenotypes became more obvious as embryo development progressed. By the time that the wild-type embryos had developed to the heart stage, two kinds of aborted embryos were distinguishable: irregular globular embryos and abnormal cotyledon embryos. The irregular globular embryos showed shape alterations and abnormal cell division and were unable to produce cotyledons (Fig. 2G–J). The abnormal cotyledon embryos had two cotyledons with unequal and asymmetric growth, accompanied by a much larger angle between the two cotyledons, as compared to wild-type embryos (Fig. 2L–O). Of 244 mutant embryos at 5 DAP, 185 (75.8%) embryos were irregularly globular, while 59 (24.2%) had abnormal cotyledons. The same embryonic defects could also be observed in rnj-1 and rnj-3 mutants (Supplementary Figure 3), suggesting the RNJ gene function is totally lost in each of the three alleles. These results showed that the homozygous rnj mutation caused embryo abortion at the late globular stage and that many rnj mutant embryos could not develop beyond this stage, indicating that morphological development of the embryos was disturbed in the rnj mutants.
Fig. 2.
Embryogenesis in wild-type and rnj-2/+ plants examined by differential interference contrast microscopy. (A–E) Embryos from the globular stage to the bent cotyledon stage in wild-type ovules: (A) globular stage; (B) transition stage; (C) heart stage; (D) torpedo stage; (E) bent cotyledon stage. (F–J) The irregular globular rnj embryos from the same siliques at the different development stages as in (A–E). (K–O) The rnj embryos with abnormal cotyledons from the same siliques at the different development stages as in (A–E). Scale bars = 20 μm.
RNJ is expressed in green tissues and reproductive organs
To analyse the expression pattern of the RNJ gene, we used quantitative PCR to evaluate its mRNA levels in different tissues and organs, with GAPC used as a reference gene. The results showed that RNJ was expressed at different levels in nearly all organs, and the relative expression levels were most abundant in inflorescences and seedlings (Fig. 3J). To investigate the spatial expression pattern, we fused the RNJ promoter (2100bp) with a β-glucuronidase (GUS) reporter gene to monitor its expression in transgenic plants (pRNJ::GUS). In 7-day-old seedlings, strong GUS signals were detected in shoot meristems, hypocotyls, and in the vascular bundles of cotyledons, as well as in the veins of mature leaves (Fig. 3A, D). In reproductive organs, GUS expression was detected in inflorescences, especially in sepals, filaments, and stigmas (Fig. 3B, C), as well as in mature siliques and seeds (Fig. 3E, F). We also fused the RNJ promoter with green fluorescent protein (GFP) to evaluate the expression of RNJ in more detail during embryo development. During the early stages of embryo development, no GFP signal could be detected until the transition stage (Fig. 3G). In the heart and torpedo stages, GFP fluorescence was predominantly distributed in the upper part of embryos (Fig. 3H, I). These results showed that RNJ is expressed widely in green tissues and reproductive organs, and that it is expressed at particularly high levels in the heart and torpedo embryos as compared to the transition stages.
Fig. 3.
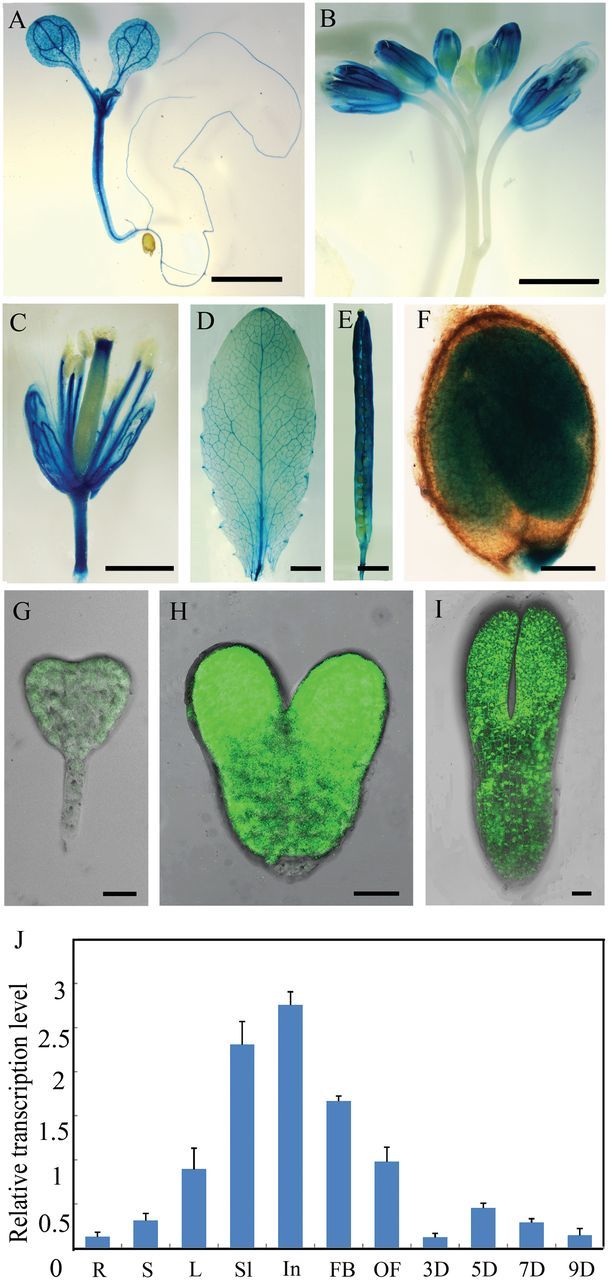
Expression pattern analysis of RNJ in Arabidopsis. (A–F) GUS staining signals in pRNJ::GUS transgenic plants: (A) 7 DAG seedling; (B) inflorescence; (C) flower; (D) rosette leaf; (E) adult silique; (F) mature seed. (G–I) GFP images of embryos from pRNJ::GFP transgenic plants: (G) transition stage; (H) heart stage; (I) torpedo stage. (J) Quantitative PCR analysis of RNJ in various tissues. R, root; S, stem; L, leaf; Sl, seedling; In, inflorescence; FB, flower bud; F, flower; 3Si, 3 DAP silique; 5Si, 5 DAP silique; 7Si, 7 DAP silique; 9Si, 9 DAP silique. Scale bars (A–E) = 2mm; scale bars (F–I) = 20 μm.
A previous study confirmed that the Arabidopsis RNJ protein is localized in the chloroplast (Sharwood et al., 2011). In this study, we found 18 light-response elements in the RNJ promoter sequence (http://arabidopsis.med.ohio-state.edu/AtcisDB/), including the GATA factors that were reported by Teakle et al. (2002). We therefore wondered whether or not RNJ expression was influenced by light. Quantitative PCR was performed to detect the mRNA levels in the 7 DAG seedlings. After 1 day of dark treatment, the expression level of the RNJ gene decreased by 67.3% compared to the controls (Supplementary Figure 4A). GUS staining also indicated obviously lower expression levels in dark-treated pRNJ::GUS transgenic seedlings than in the controls (Supplementary Figure 4B, C). These results verified our supposition that light is an important signal in the regulation of RNJ expression. Since the GATA factors are type-IV zinc-finger proteins with DNA-binding and transcriptional activation activities, and several GATA factor genes had been verified to be in response to light (Manfield et al., 2007), we suggest that RNJ expression may be under the indirect control of light, perhaps via the products of photosynthesis.
Chloroplast development is impaired in homozygous rnj embryos
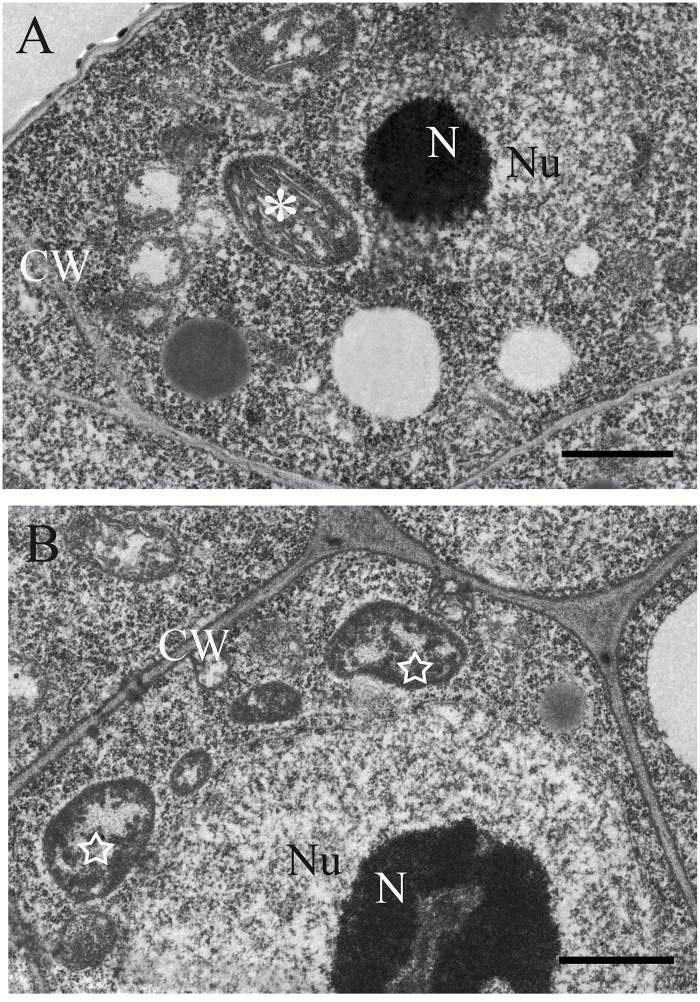
Since the rnj heterozygous mutants produced about 25% albino seeds in siliques, we wondered whether the homozygous embryo lethality was due to impaired chloroplast development in the mutant embryo cells. We prepared wild-type and rnj albino ovules from 5 DAP siliques as samples for ultrastructural observation with TEM. In the embryo wild-type samples, chloroplasts had organized thylakoid membranes stacked into grana that were well developed (Fig. 4A). However, in the rnj embryo samples, we observed many immature plastids that lacked internal thylakoid membranes but contained darkly stained aggregations (Fig. 4B). The lack of normal chloroplasts in the mutant embryo cells indicated that the rnj mutation may disturb the formation of internal thylakoid membranes during embryo development and lead to impaired chloroplasts, strongly suggesting that the RNJ gene is required for chloroplast development.
Fig. 4.
TEM of chloroplast development in wild-type and rnj-2 embryo cells. (A) Cells in wild-type embryos at 5 DAP. (B) Cells in rnj-2 embryos at 5 DAP. Asterisk indicates a normal chloroplast in the wild type; star indicates an impaired chloroplast in the rnj-2 mutant. N, nucleolus; Nu, nucleus; CW, cell wall. Scale bars = 1 μm.
The apical-basal patterning of the rnj embryo is perturbed
The phenotypes observed in rnj embryos showed a morphologically defective transition from globular to bilateral symmetry along the apical-basal axis; there were obvious problems with the specification of shoot apical meristem (SAM), cotyledon, and hypocotyl development. To better understand the patterning defects in rnj embryos, we investigated the expression patterns of several genes known to delineate fate decisions in the embryo by using fluorescent markers.
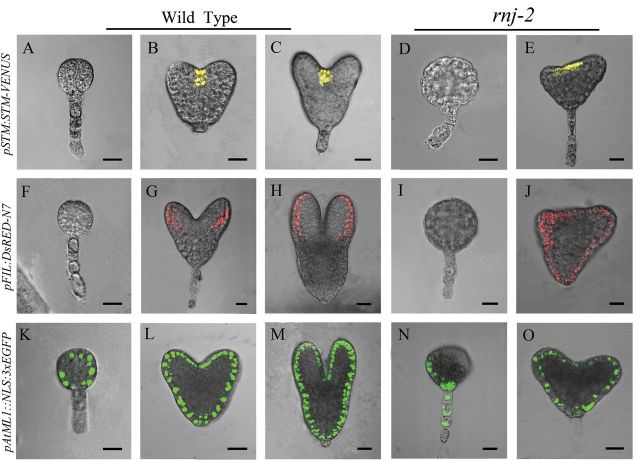
SHOOT MERISTEMLESS (STM) is a well studied gene expressed in shoot meristems that is required for meristem function (Long and Barton, 1998). Translational fusion of STM (pSTM::STM-VENUS) to the YFP variant VENUS (Heisler et al., 2005) was used to examine its expression in rnj embryos. In wild-type and rnj globular embryos, no pSTM::STM-VENUS signal could be detected (Fig. 5A, D). After the transition stage, the signal was observed in the central apical region of wild-type embryos (Fig. 5B, C). However, STM expression appeared in only one or two layers of cells in the expanded shoot meristems of rnj embryos with abnormal cotyledons (Fig. 5E). These results suggested that mutation of the RNJ gene perturbs the expression domain of the SAM-organizing gene STM.
Fig. 5.
Expression pattern of STM, FIL, and ML1 genes in wild-type and rnj embryos. (A–C) STM expression in wide-type embryos of globular stage (A), heart stage (B), and torpedo stage (C). (D, E) STM expression in rnj-2 irregular globular (D) and abnormal cotyledon (E) embryos. (F–H) FIL expression in wild-type embryos of globular stage (F), heart stage (G), and torpedo stage (H). (I, J) FIL expression in rnj-2 irregular globular (I) and abnormal cotyledon (J) embryos. (K–M) ML1 expression in wild-type embryos of globular stage (K), heart stage (L), and torpedo stage (M). (N, O) ML1 expression in rnj-2 irregular globular (N) and abnormal cotyledon (O) embryos. Scale bars = 20 μm.
Since the rnj mutant embryos appeared to have disordered or even no cotyledons, we examined the expression of the YABBY gene FILAMENTOUS FLOWER (FIL), which is known to be specifically expressed on the abaxial side of embryo cotyledon primordia (Siegfried et al., 1999), to determine whether this phenotype was due to defects in cotyledon organogenesis. In wild-type embryos, expression of FIL (pFIL::dsRED-N7) was restricted to two peripheral domains of developing cotyledons in the apical region after the transition stage (Fig. 5G, H). There was no red fluorescence in wild-type globular embryos (Fig. 5F) or in the irregular globular embryos of the rnj mutants (Fig. 5I). Rather, red fluorescence was ectopically distributed in outer cell layers of the embryos with abnormal cotyledons (Fig. 5J). This result suggested that disordered development of cotyledons may be associated with changes in FIL expression patterns.
AtML1 encodes an HD-ZIP-type homeodomain protein. AtML1 transcript expression is restricted to the outermost or epidermal cell layer embryos at different developmental stages. It is known to regulate epidermal cell fate determination of embryos in Arabidopsis (Takada and Jürgens, 2007). In wild-type plants in our experiments, AtML1 expression (pAtML1::NLS-3xEGFP) was detected specifically in the outermost cell layer of embryos from the early globular stages onwards (Fig. 5K–M). AtML1 expression was confined to the epidermal cell layer in the rnj embryos with abnormal cotyledons (Fig. 5O). However, the fluorescent signal only appeared in part of the outermost cells in most of the irregular globular rnj embryos (Fig. 5N), suggesting that cell differentiation in the outermost layer may be defective.
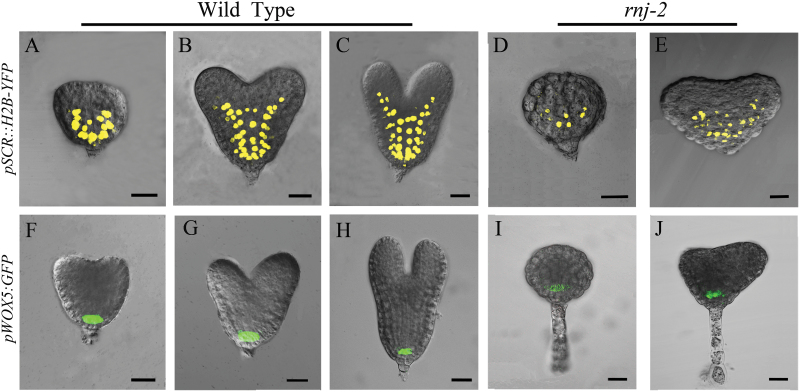
Since the apical patterning in rnj embryos was perturbed, we checked the expression of SCARECROW (SCR) and WUSCHEL-LIKE HOMEOBOX5 (WOX5) to determine whether the pattern formation in central and basal regions was altered. The SCR gene encodes a GRAS transcription factor that is only expressed in the endodermal cell layer (Wysocka-Diller et al., 2000). A transcriptional fusion of SCR to YFP (pSCR::H2B-YFP) (Heidstra et al., 2004) was introduced into rnj mutants to examine the SCR expression pattern. The results showed that SCR signals could be detected in the endodermal cells located at the central and basal domains of wild-type and rnj embryos (Fig. 6A-E). However, the fluorescent signal in rnj embryos showed abnormal cell division (Fig. 6D, E), which led to a decreased number of endodermal cells as well as a shortened embryo. These results suggested that pattern formation in the central region of the rnj embryos was disrupted.
Fig. 6.
Expression pattern of SCR and WOX5 in wild-type and rnj embryos. (A–C) SCR expression in wild-type embryos of transition stage (A), heart stage (B), and torpedo stage (C). (D, E) SCR expression in rnj-2 irregular globular (D) and abnormal cotyledon (E) embryos. (F–H) WOX5 expression in wild-type embryos of transition stage (F), heart stage (G), and torpedo stage (H). (I, J) WOX5 expression in rnj-2 irregular globular (I) and abnormal cotyledon (J) embryos. Scale bars = 20 μm.
We also examined the expression pattern of the WOX5 gene, a homeodomain transcription factor, in embryos of the wild type and rnj mutant using a transcriptional fusion of the WOX5 promoter in front of GFP (pWOX5::GFP) (Blilou et al., 2005). WOX5 is expressed specifically in the quiescent centre (QC) and is known to be involved in the maintenance of root stem cells (Haecker et al., 2004; Sarkar et al., 2007). Our results showed that the expression domain of pWOX5::GFP was not altered and was confined to the QC in both the rnj and wild-type embryos (Fig. 6F–J). Our study of SCR and WOX5 expression in rnj mutants indicated that formation of the endodermis in the central region of rnj embryos was altered, but that there was no alteration of the QC in the basal region in mutant embryos, suggesting that RNJ gene expression is required for hypocotyl formation in the central domain but is not necessary for establishment of the embryonic root.
The rnj mutation disrupts auxin transport and responses during embryo development
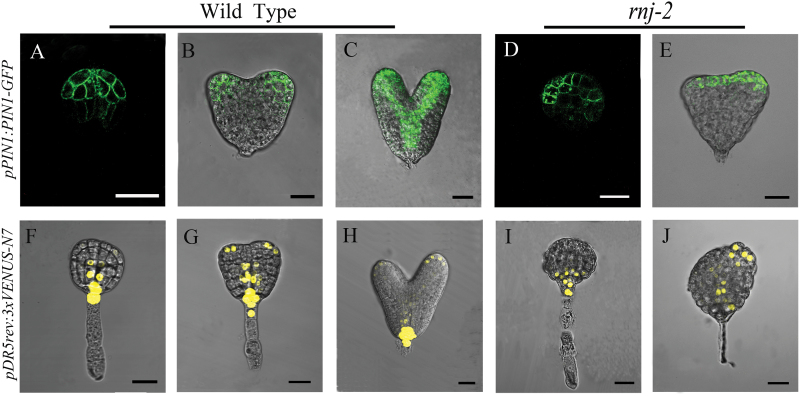
Auxin is a key regulator in the control of bilateral symmetry and the establishment of embryo patterning during embryogenesis (Liu et al., 1993). To test whether the developmental defects observed in rnj embryos were associated with changes in auxin transport and responses, we analysed pPIN1::PIN1-GFP localization and pDR5rev::3XVENUS-N7 expression in rnj embryos. PIN1 is an important auxin efflux transporter that mediates the establishment of the auxin maxima by polar localization (Paponov et al., 2005). In wild-type globular embryos, pPIN1::PIN1-GFP expression was restricted to apical cells and polarized in the plasma membrane facing the basal embryo pole (Fig. 7A). When wild-type embryos developed to the transition and heart stages, PIN1-GFP was expressed in the developing vasculature and cotyledon primordia (Fig. 7B, C). In rnj irregular globular embryos, PIN1-GFP was expressed asymmetrically in the outermost cell layer of the apical region (Fig. 7D). In rnj embryos with abnormal cotyledons, PIN1-GFP was distributed asymmetrically and was much stronger in one cotyledon primordium than the other (Fig. 7E).
Fig. 7.
Auxin transportation and distribution is disrupted during embryogenesis in rnj mutants. (A–C) PIN1 expression in wild-type embryos of globular stage (A), transition stage (B) and heart stage (C). (D–E) PIN1 expression in rnj-2 irregular globular (D) and abnormal cotyledon (E) embryos. (F–H) DR5 expression in wild-type embryos of globular stage (F), transition stage (G), and heart stage (H). (I–J) DR5 expression in rnj-2 irregular globular (I) and abnormal cotyledon (J) embryos. Scale bars = 20 μm.
DR5 is a synthetic auxin-responsive promoter, and a GFP-fused marker has been used to visualize the spatial pattern of auxin responses during embryogenesis (Friml et al., 2003). In wild-type plants, pDR5rev::3XVENUS-N7 expression was concentrated in the hypophysis of the globular embryos (Fig. 7F), and was also distributed in the cotyledon primordial tips and prevascular cells of embryos in the transition and heart stages (Fig. 7G, H). As well as the disruption of PIN1 expression pattern, pDR5::VENUS was confusedly distributed in rnj embryos. We found that pDR5rev::3XVENUS-N7 expression was restricted to the basal region of rnj irregular globular embryos (Fig. 7I). The pDR5::VENUS signal was barely detectable in the basal region of rnj embryos with abnormal cotyledons and was expressed asymmetrically at the tips of the initiating and developing cotyledon only (Fig. 7J, L). Therefore, auxin transport and responses associated with morphogenesis of cotyledons is disturbed in the rnj embryos.
In summary, we analysed the biological function of the AtRNJ gene in embryogenesis. AtRNJ is widely expressed in green tissues and reproductive organs, and its expression level is profoundly influenced by light. Homozygous rnj mutation leads to embryo death, which may be caused by impaired chloroplast development. In aborted embryos, cell division planes displayed abnormal orientation, and the apical-basal pattern was disturbed. Auxin transport and response was also altered in the mutant. Therefore, we surmise that AtRNJ may regulate embryo morphogenesis and pattern formation through controlling chloroplast development.
Discussion
RNJ is a plant-specific ribonuclease required for chloroplast development
In bacteria, RNJ proteins have 5′-3′ exoribonuclease activity and play important roles in rRNA maturation and 5′ stability of mRNA. Depletion of RNase J1 in Bacillus subtilis results in abolishment of 16S rRNA processing and cell viability (Mathy et al., 2007). Three other kinds of RNJ-like metallo-beta-lactamase proteins, TRZ, CPSF73-I, and Int11, have been studied in the context of reproductive development in Arabidopsis. TRZ is an endonuclease that cleaves precursor molecules at the 3′-end to generate mature functional tRNA (Schiffer et al., 2002). In Arabidopsis, four genes were found to encode tRNase Z enzymes (Supplementary Figure 1A), and AtTRZ2 was shown to be localized to chloroplasts and to be essential for embryogenesis (Canino et al., 2009). CPSF73-I has endonuclease activity and catalyses the cleavage of mRNA precursors at the 3′-end in the formation of polyadenylated mRNAs (Mandel et al., 2006). A single CPSF73-I protein is encoded in the genomes of Arabidopsis, rice, human, and yeast (Supplementary Figure 1A). Both knockdown and overexpression of CPSF73-I in Arabidopsis caused death (Xu et al., 2006). Int11 is an endonuclease that is the key catalytic component that cleaves snRNA precursors (Baillat et al., 2005). This protein was first studied in Arabidopsis, where it was designated AtCPSF73-II and was shown to be essential for early embryo development; heterozygous mutants displayed empty seed spaces as well as aborted seeds with embryos arrested at the globular stage (Xu et al., 2004). Int11 homologues can also be found in human and rice, but not in yeast (Supplementary Figure 1A). Unlike these RNJ-like metallo-beta-lactamase proteins, RNJ occurs only in the nuclear genomes of plants. Further, RNJ is highly conserved in plants (Supplementary Figure 1A). In Arabidopsis, it had been shown that RNJ has 5′-3′ exoribonuclease and endonucleolytic activities and that it is localized in chloroplasts, plastid organelles derived from a relative of an ancestral cyanobacterium through endosymbiosis. This analysis shows that the plant RNJ gene may have originated from the genome of an endosymbiotic bacterium and then been transferred to the host plant genome during evolution.
A previous study concluded that a major role of RNase J is RNA surveillance to prevent the overaccumulation of antisense RNA in chloroplasts and to regulate chloroplast gene expression during post-transcriptional processes. Repression of RNJ expression in Arabidopsis and Nicotiana benthamiana led to chlorosis in mature leaves (Sharwood et al., 2011). In our study, we found that RNJ is expressed broadly in green tissues and reproductive organs in Arabidopsis (Fig. 3) and that light induction is an important aspect of the regulation of the expression (Supplementary Figure 2). Knockout of RNJ caused white ovules (Fig. 1) and embryo lethality (Fig. 2). Observation of ultrastructures showed that aborted embryo cells displayed impaired chloroplast development (Fig. 4). All of the plastids in Arabidopsis embryos, including chloroplasts, are derived from small, non-green proplastids in meristematic cells, and the development of chloroplasts from proplastids requires the normal transcription and translation the plastid genome (Lopez-Juez and Pyke, 2005); organelle differentiation is known to be largely co-regulated by both nuclear and plastid genes (Leon et al., 1998; Woodson and Chory, 2008). In Chlamydomonas, analysis of mutants defective in chloroplast functions led to the characterization of many nuclear-encoded proteins that regulate chloroplast gene expression (Lown et al., 2001; Wostrikoff et al., 2004; Raynaud et al., 2007). In Arabidopsis, certain nuclear-encoded proteins were found to primarily control chloroplast gene expression through post-transcriptional mechanisms including transcript maturation (splicing, processing, and editing) and translation (Kleffmann et al., 2004; Saha et al., 2007; Sakamoto et al., 2008). Therefore, AtRNJ may regulate plastid gene expression at the post-transcriptional stage, which would mean that normal RNJ expression is required for chloroplast development.
Impaired chloroplasts lead to aborted embryos in rnj mutants
Our results show that embryo development in the rnj mutant is disturbed, leading to the production of irregular globular and abnormal cotyledon embryos (Fig. 2). We found that the chloroplasts in the aborted rnj embryos were impaired, having no stacked or well organized thylakoids (Fig. 4). It is known that plastids play versatile roles in plant growth and development, including during embryogenesis (Inaba and Ito-Inaba, 2010). During Arabidopsis embryogenesis, proplastids start to differentiate into chloroplasts when the embryo develops to the late globular stage (Mansfield and Briarty, 1991). Chloroplasts are an important cellular organelle that not only perform photosynthesis but are also responsible for the storage of starch and oil compounds and the synthesis of amino acids, lipids, and phytohormones (Sakamoto et al., 2008). These products are involved in many metabolic and signal transduction pathways. Therefore, it is reasonable to speculate that some of these compounds are important signalling molecules that regulate embryogenesis. Interruption of proper biogenesis and development of chloroplasts in embryos has significant deleterious effects on many fundamental biological processes. Mutations of some chloroplast-localized proteins that affect important processes in chloroplasts often lead to aborted embryogenesis at early stages (Bryant et al., 2011). One explanation for these observations is that the photosynthesis that occurs in chloroplasts supplies the energy for subsequent embryogenesis. On the other hand, mutations that disable the function of photosynthetic machinery usually lead to reduced pigmentation and result in white embryos with normal morphogenesis and patterning (Myouga et al., 2010; Bryant et al., 2011). Therefore, other metabolites such as fatty acids, amino acids, lipids, or phytohormones may participate in the signal pathways of embryogenesis. Here, we found that the rnj mutation impaired chloroplast development in Arabidopsis embryo cells, which in turn led to loss of functions important in various metabolic processes in chloroplasts. These metabolic changes may have disrupted programmed cell division and differentiation during Arabidopsis embryogenesis and resulted in aberrant embryo morphological development.
RNJ is required for embryo pattern formation through regulating chloroplast function
Our results showed that Arabidopsis rnj embryos were of two types: irregular globular embryos and abnormal cotyledon embryos (Fig. 2). We found that defective embryo development in the mutants correlated with ectopic expression of tissue-specific genes such as STM, FIL, ML1, and SCR that are restricted, respectively, to shoot meristems, the abaxial side of cotyledon primordia, the epidermal layer, and endodermal cells in wild-type embryos (Figs 5 and 6). The irregular globular embryos did not display any such tissue specialization and then developed into an amorphous agglomeration of cells. In the irregular globular embryo, no STM or FIL signals were detected, while ML1 was found to be expressed only in part of the outermost cells and SCR expression was restricted to a decreased region of endodermal cells. The other kind of rnj embryo had a pair of asymmetric cotyledons with a much larger angle between them than those of the wild type. The expression domains of STM and FIL were expanded in these asymmetric cotyledon embryos, yet ML1 and SCR were distributed in their specific cells with abnormal division patterns. These results indicated that the rnj mutation led to a perturbation of apical-basal patterning in embryos. However, the expression pattern of the QC-specific gene WOX5 was the same in both wild-type and rnj embryos (Fig. 6). Taken together, we surmise that AtRNJ plays an important role in the apical-basal patterning of embryos, but not for the formation of the QC in the embryonic root. Generating the apical-basal patterning is well known to be taken over by programmes that set a predictable sequence of cell divisions. Although the overall mechanism of embryo patterning remains relatively poorly understood, some functional components involved have been shown to participate in the regulation of this process (Capron et al., 2009; Xiang et al., 2011; Bjerkan et al., 2012; Ballesteros et al., 2013). Here, we suggest that AtRNJ may affect the expression patterns of certain tissue-specific genes by controlling chloroplast development.
It has been postulated that plastid development and gene expression are largely under nuclear control, a concept known as anterograde control. However, signals generated in plastids can be transduced to the nucleus and modulate nuclear gene expression. This retrograde regulation coordinates gene expression between the plastid and nuclear genomes, which is essential for maintaining plastid biogenesis and plastid function at optimal levels (Woodson and Chory, 2008). Although we know almost nothing about the nature of the retrograde signals and how they are transduced from plastids to the nucleus, potential candidate signal molecules and pathways have been proposed. These include tetrapyrrole intermediate biosynthesis, plastid gene expression (PGE), plastid redox state, and reactive oxygen species (ROS) (Pogson et al., 2008; Chi et al., 2013). AtRNJ is localized in the chloroplast and compensates for inefficient transcriptional termination by removal of antisense RNA, thus regulating chloroplast gene expression during post-transcriptional processes (Sharwood et al., 2011). Here, we found that the expression patterns of certain nuclear genes were altered in the absence of AtRNJ. Therefore AtRNJ may participate in retrograde control and modulate nuclear gene expression by the plastid gene expression signalling pathway, and thus plays an important role in apical-basal patterning during embryogenesis.
In conclusion, the plant-specific nuclear gene AtRNJ encodes a chloroplast-localized ribonuclease that regulates plastid gene expression at the post-transcriptional stage and plays an essential role in chloroplast development. The rnj mutation leads to impaired chloroplast development in aborted embryos, which results in disrupted expression of certain nuclear genes. All of our results support the supposition that the RNJ gene affects embryo morphogenesis and pattern formation through controlling chloroplast development in Arabidopsis.
Supplemental material
Supplementary data can be found at JXB online.
Supplementary Table S1. Primers used for PCR.
Supplementary Figure S1. RNase J is a metallo-beta-lactamase and conserved in plants.
Supplementary Figure S2. The functional domains of RNJ with respect to the T-DNA insertions.
Supplementary Figure S3. Embryo characteristics in wild-type, rnj-1/+, and rnj-3/+ plants.
Supplementary Figure S4. The downregulation of RNJ expression levels after 24h dark treatment.
Funding
This research was supported by the National Basic Research Programme of China (2012CB944801, 2013CB126903), and the Key Grant Project of the Chinese Ministry of Education (NO. 311026).
Supplementary Material
Acknowledgements
We are very grateful to Elliot Meyerowitz (California Institute of Technology, USA) for kindly providing pDR5rev::3XVENUS-N7, pPIN1::PIN1-GFP, pSTM::STM-VENUS, and pFIL::dsRED-N7 marker lines, to Ben Scheres (University of Utrecht, The Netherlands) for pSCR::H2B-YFP and pWOX5::GFP, and to Gerd Jürgens (University of Tübingen, Germany) for pATML1::NLS-3xEGFP marker lines.
References
- Baillat D, Hakimi MA, Näär AM, Shilatifard A, Cooch N, Shiekhattar R. 2005. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123, 265–276. [DOI] [PubMed] [Google Scholar]
- Ballesteros I, Domínguez T, Sauer M, Paredes P, Duprat A, Rojo E, Sanmartín M, Sánchez-Serrano JJ. 2013. Specialized functions of the PP2A subfamily II catalytic subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in Arabidopsis. The Plant Journal 73, 862–872. [DOI] [PubMed] [Google Scholar]
- Bebrone C. 2007. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochemical Pharmacology 74, 1686–1701. [DOI] [PubMed] [Google Scholar]
- Benstein RM, Ludewig K, Wulfert S, Wittek S, Gigolashvili T, Frerigmann H, Gierth M, Flügge UI, Krueger S. 2013. Arabidopsis phosphoglycerate dehydrogenase1 of the phosphoserine pathway is essential for development and required for ammonium assimilation and tryptophan biosynthesis. The Plant Cell 25, 5011–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T, Jürgens G. 1993. The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development 118, 575–587. [Google Scholar]
- Bjerkan KN, Jung-Roméo S, Jürgens G, Genschik P, Grini PE. 2012. Arabidopsis WD repeat domain55 Interacts with DNA damaged binding protein1 and is required for apical patterning in the embryo. The Plant Cell 24, 1013–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44. [DOI] [PubMed] [Google Scholar]
- Bryant N, Lloyd J, Sweeney C, Myouga F, Meinke D. 2011. Identification of nuclear genes encoding chloroplast-localized proteins required for embryo development in Arabidopsis . Plant Physiology 155, 1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G, Bocian E, Barbezier N, Echeverría M, Forner J, Binder S, Marchfelder A. 2009. Arabidopsis encodes four tRNase Z enzymes. Plant Physiology 150, 1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A, Chatfield S, Provart N, Berleth T. 2009. Embryogenesis: pattern formation from a single cell. Arabidopsis Book 7, e0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Sun X, Zhang L. 2013. Intracellular signaling from plastid to nucleus. Annual Review of Plant Biology 64, 559–582. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Costa LM, Marshall E, Tesfaye M, et al. 2014. Central cell-derived peptides regulate early embryo patterning in flowering plants. Science 344, 168–172. [DOI] [PubMed] [Google Scholar]
- Dominski Z. 2007. Nucleases of the metallo-beta-lactamase family and their role in DNA and RNA metabolism. Critical Reviews in Biochemistry and Molecular Biology 42, 67–93. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G. 2003. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis . Nature 426, 147–153. [DOI] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. 2004. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana . Development 131, 657–668. [DOI] [PubMed] [Google Scholar]
- Heidstra R, Welch D, Scheres B. 2004. Mosaic analyses using marked activation and deletion clones dissect Arabidopsis SCARECROW action in asymmetric cell division. Genes and Development 18, 1964–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Current Biology 15, 1899–1911. [DOI] [PubMed] [Google Scholar]
- Huang JB, Liu H, Chen M, et al. 2014. ROP3 GTPase contributes to polar auxin transport and auxin responses and is important for embryogenesis and seedling growth in Arabidopsis. The Plant Cell 10.1105/tpc.114.127902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T, Ito-Inaba Y. 2010. Versatile roles of plastids in plant growth and development. Plant and Cell Physiology 51, 1847–1853. [DOI] [PubMed] [Google Scholar]
- Inoue H, Li M, Schnell DJ. 2013. An essential role for chloroplast heat shock protein 90 (Hsp90C) in protein import into chloroplasts. Proceedings of the National Academy of Sciences, USA 110, 3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Bayer M, Lukowitz W. 2011. Taking the very first steps: from polarity to axial domains in the early Arabidopsis embryo. Journal of Experimental Botany 62, 1687–1697. [DOI] [PubMed] [Google Scholar]
- Keeling PJ. 2010. The endosymbiotic origin, diversification and fate of plastids. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 729–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjölander K, Gruissem W, Baginsky S. 2004. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Current Biology 14, 354–362. [DOI] [PubMed] [Google Scholar]
- Leon P, Arroyo A, Mackenzie S. 1998. Nuclear control of plastid and mitochondrial development in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 49, 453–480. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu Z, Chua NH. 1993. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. The Plant Cell 5, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Barton MK. 1998. The development of apical embryonic pattern in Arabidopsis . Development 125, 3027–3035. [DOI] [PubMed] [Google Scholar]
- Lopez-Juez E. 2007. Plastid biogenesis, between light and shadows. Journal of Experimental Botany 58, 11–26. [DOI] [PubMed] [Google Scholar]
- Lopez-Juez E, Pyke KA. 2005. Plastids unleashed: their development and their integration in plant development. The International Journal of Developmental Biology 49, 557–577. [DOI] [PubMed] [Google Scholar]
- Lown FJ, Watson AT, Purton S. 2001. Chlamydomonas nuclear mutants that fail to assemble respiratory or photosynthetic electron transfer complexes. Biochemical Society Transactions 29, 452–455. [DOI] [PubMed] [Google Scholar]
- Mandel CR, Kaneko S, Zhang H, Gebauer D, Vethantham V, Manley JL, Tong L. 2006. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 444, 953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfield IW, Devlin PF, Jen CH, Westhead DR, Gilmartin PM. 2007. Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiology 143, 941–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG. 1991. Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Canadian Journal of Botany 69, 461–476. [Google Scholar]
- Mathy N, Bénard L, Pellegrini O, Daou R, Wen T, Condon C. 2007. 5′-to-3′ exoribonuclease activity in bacteria: role of RNase J1 in rRNA maturation and 5′ stability of mRNA. Cell 129, 681–692. [DOI] [PubMed] [Google Scholar]
- Meinke D, Muralla R, Sweeney C, Dickerman A. 2008. Identifying essential genes in Arabidopsis thaliana . Trends in Plant Science 13, 483–491. [DOI] [PubMed] [Google Scholar]
- Myouga F, Akiyama K, Motohashi R, Kuromori T, Ito T, Iizumi H, Ryusui R, Sakurai T, Shinozaki K. 2010. The Chloroplast Function Database: a large-scale collection of Arabidopsis Ds/Spm- or T-DNA-tagged homozygous lines for nuclear-encoded chloroplast proteins, and their systematic phenotype analysis. The Plant Journal 61, 529–542. [DOI] [PubMed] [Google Scholar]
- Paponov IA, Teale WD, Trebar M, Blilou I, Palme K. 2005. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends in Plant Science 10, 170–177. [DOI] [PubMed] [Google Scholar]
- Pogson BJ, Woo NS, Förster B, Small ID. 2008. Plastid signalling to the nucleus and beyond. Trends in Plant Science 13, 602–609. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhao J. 2006. Localization of arabinogalactan proteins in egg cells, zygotes, and two-celled proembryos and effects of beta-D-glucosyl Yariv reagent on egg cell fertilization and zygote division in Nicotiana tabacum L . Journal of Experimental Botany 57, 2061–2074. [DOI] [PubMed] [Google Scholar]
- Raynaud C, Loiselay C, Wostrikoff K, Kuras R, Girard-Bascou J, Wollman FA, Choquet Y. 2007. Evidence for regulatory function of nucleus-encoded factors on mRNA stabilization and translation in the chloroplast. Proceedings of the National Academy of Sciences, USA 104, 9093–9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Liu Y, Chen H, Li G, Zhang X, Zhao J. 2012. Type 4 metallothionein genes are involved in regulating Zn ion accumulation in late embryo and in controlling early seedling growth in Arabidopsis . Plant, Cell and Environment 35, 770–789. [DOI] [PubMed] [Google Scholar]
- Reyes-Prieto A, Weber AP, Bhattacharya D. 2007. The origin and establishment of the plastid in algae and plants. Annual Review of Genetics 41, 147–168. [DOI] [PubMed] [Google Scholar]
- Ruuska SA, Schwender J, Ohlrogge JB. 2004. The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiology 136, 2700–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D, Prasad AM, Srinivasan R. 2007. Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiology and Biochemistry 45, 521–534. [DOI] [PubMed] [Google Scholar]
- Sakamoto W, Miyagishima SY, Jarvis P. 2008. Chloroplast biogenesis: control of plastid development, protein import, division and inheritance. Arabidopsis Book 6, e0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. 2007. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814. [DOI] [PubMed] [Google Scholar]
- Schiffer S, Rösch S, Marchfelder A. 2002. Assigning a function to a conserved group of proteins: the tRNA 3′-processing enzymes. The EMBO Journal 21, 2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinski J, König N, Wellmeyer B, Hanke GT, Linke V, Neuhaus HE, Scheibe R. 2014. The plastid-localized NAD-dependent malate dehydrogenase is crucial for energy homeostasis in developing Arabidopsis thaliana seeds. Molecular Plant 7, 170–186. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, Halpert M, Luro S, Schuster G, Stern DB. 2011. Chloroplast RNase J compensates for inefficient transcription termination by removal of antisense RNA. RNA 17, 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. 1999. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis . Development 126, 4117–4128. [DOI] [PubMed] [Google Scholar]
- Takada S, Jürgens G. 2007. Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development 134, 1141–1150. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Teakle GR, Manfield IW, Graham JF, Gilmartin PM. 2002. Arabidopsis thaliana GATA factors: organisation, expression and DNA-binding characteristics. Plant Molecular Biology 50, 43–57. [DOI] [PubMed] [Google Scholar]
- Tzafrir I, Pena-Muralla R, Dickerman A, et al. 2004. Identification of genes required for embryo development in Arabidopsis . Plant Physiology 135, 1206–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendrich JR, Weijers D. 2013. The Arabidopsis embryo as a miniature morphogenesis model. New Phytologist 199, 14–25. [DOI] [PubMed] [Google Scholar]
- Woodson JD, Chory J. 2008. Coordination of gene expression between organellar and nuclear genomes. Nature Reviews Genetics 9, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostrikoff K, Girard-Bascou J, Wollman FA, Choquet Y. 2004. Biogenesis of PSI involves a cascade of translational autoregulation in the chloroplast of Chlamydomonas . The EMBO Journal 23, 2696–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN. 2000. Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127, 595–603. [DOI] [PubMed] [Google Scholar]
- Xiang D, Yang H, Venglat P, et al. 2011. POPCORN functions in the auxin pathway to regulate embryonic body plan and meristem organization in Arabidopsis. The Plant Cell 23, 4348–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Ye X, Li QQ. 2004. AtCPSF73-II gene encoding an Arabidopsis homolog of CPSF 73kDa subunit is critical for early embryo development. Gene 324, 35–45. [DOI] [PubMed] [Google Scholar]
- Xu R, Zhao H, Dinkins RD, Cheng X, Carberry G, Li QQ. 2006. The 73 kD subunit of the cleavage and polyadenylation specificity factor (CPSF) complex affects reproductive development in Arabidopsis. Plant Molecular Biology 61, 799–815. [DOI] [PubMed] [Google Scholar]
- Yuan J, Chen D, Ren Y, Zhang X, Zhao J. 2008. Characteristic and expression analysis of a metallothionein gene, OsMT2b, down-regulated by cytokinin suggest functions in root development and seed embryo germination of rice (Oryza sativa L.). Plant Physiology 146, 1637–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.