Abstract
Myopia is a common ocular condition, characterized by excessive elongation of the ocular globe. The prevalence of myopia continues to increase, particularly among highly educated groups, now exceeding 80% in some groups. In parallel with the increased prevalence of myopia, are increases in associated blinding ocular conditions including glaucoma, retinal detachment and macular degeneration, making myopia a significant global health concern. The elongation of the eye is closely related to the biomechanical properties of the sclera, which in turn are largely dependent on the composition of the scleral extracellular matrix. Therefore an understanding of the cellular and extracellular events involved in the regulation of scleral growth and remodeling during childhood and young adulthood will provide future avenues for the treatment of myopia and its associated ocular complications.
Keywords: sclera, extracellular matrix, myopia, emmetropization, ocular development
1. Introduction
Myopia is the most common of all ocular problems, affecting 33% of the US adult population (Vitale et al., 2008). The prevalence of myopia increased dramatically over the past forty years and, in some highly educated groups, now exceeds 80% (Lin et al., 1996). In parallel with the increase in overall myopia, there has been a rise in the prevalence of high myopia (≤−6 diopters [D]), which is associated with serious ocular complications such as posterior staphyloma, retinal degeneration, and retinal detachment (Saw et al., 2005), and which represents a leading cause of blindness worldwide (Buch et al., 2001). Furthermore, myopia is appearing with greater prevalence in young children (Vitale et al., 2008), which places these children at greater risk of developing high myopia, along with its associated complications.
The most common structural abnormality associated with myopia is excessive lengthening of the posterior segment of the ocular globe which leads to negative refractive error (myopia) due to a mismatch between the axial length and the focal length of the eye. Experimental and clinical evidence indicates that excessive ocular elongation associated with myopia is the result of altered extracellular matrix (ECM) remodeling of the scleral shell. The sclera is a dense, fibrous connective tissue that defines the size and shape of the eye. It provides a strong framework that supports the retina, withstands the expansive force generated by intraocular pressure, provides a pathway for aqueous outflow, and protects the contents of the eye from external trauma. The sclera is now known to be a dynamic tissue that undergoes constant remodeling throughout life. Results from research over the last 25 years have established that scleral remodeling is regulated by genetic and environmental influences which can have profound effects on ocular size and refraction. The purpose of this review is to provide an overview of the macromolecular composition of the sclera that is integral to its normal functions, to discuss how these elements are dis-regulated during myopia development, and to suggest potential strategies that might be considered for future therapeutic purposes.
2. Scleral structure
The sclera has regional specializations for the positioning of the cornea, the entry and exit of important nerves and blood vessels, as well as for the attachment of extraocular muscles. Despite these regional variations in structure, the sclera must be able to control eye shape during significant events that promote deformation of the globe, such as eye movements, accommodation, and intraocular pressure fluctuations. In doing so, the sclera is able to ensure stable refraction and prevent rupture of the ocular globe. The sclera meets these requirements through a specialized dense irregular connective tissue stroma composed of collagen fibrils embedded in a matrix of proteoglycans and non-collagenous glycoproteins.
2.1. Collagen
In mammals, scleral tissue contains approximately 50% collagen by weight, consisting predominantly of type I collagen (Keeley FW, 1984), which accounts for approximately 95% of the collagen present. Collagen fibrils within the sclera are organized into irregularly arranged and somewhat interwoven lamellae (Fig. 1). This lamellar organization is similar to the collagen arrangement of the cornea; however, scleral collagen fibrils are highly variable in their diameter, lamellae vary in thickness, and the orientation of each lamella is irregular with respect to neighboring lamella. Based on studies in the cornea (Birk, 1984), it is likely that the outer edges of each lamella, which are adjacent to the scleral fibroblasts, contain the most immature collagen fibrils relative to those in the centers of each collagenous lamellae.
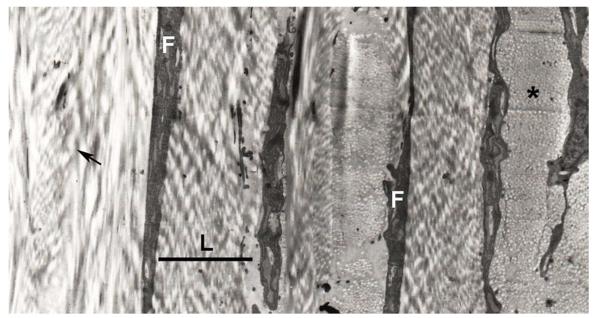
Figure 1.
Lamellar organization of the human sclera. Scleral fibroblasts (F) can be seen between irregularly arranged collagenous lamella (L). Within each lamella, collagen fibrils are oriented in the same general direction, with some running longitudinally in the plane of section (arrow), and some running perpendicular to the plane of section and seen in cross section (asterisk). The black bar indicates the width of a lamella.
In addition to type I collagen, the human sclera has been shown to contain collagen types III, IV, V, VI, VIII, XII and XIII (Rada, 2004; Sandberg-Lall, 2000; Wessel, 1997). Interestingly, high congenital myopia is pathognomonic for Stickler's Syndrome, a genetic disorder most commonly involving mutations in the collagen type II (COL2A1) gene, suggesting a major role for collagen type II in scleral development and structure (Liberfarb et al., 2003). However, although collagen type II has been identified in the sclera of embryonic mice (Savontaus, 1997), and is a major fibrillar collagen of the largely cartilaginous avian sclera, collagen type II expression has not been detected in human sclera (Young, 2003; Young et al., 2004).
2.2. Proteoglycans
Collagen fibrillogenesis, fibril orientation, size, and arrangement are influenced by a number of non-collagenous ECM components (Birk, 1981; Rada, 1993; Vogel, 1984). Specifically, proteoglycans are known modulators of collagen fibril assembly and arrangement and are found in abundance throughout the ECM of the sclera. Proteoglycans consist of a core protein with at least one attached glycosaminoglycan (GAG) side chain made up of repeating disaccharide units containing chondroitin sulfate, dermatan sulfate, heparan sulfate or keratan sulfate. The presence of sulfate residues on the GAG chains supplies considerable negative charge to proteoglycans which is important in mediating interactions with water and other extracellular proteins (e.g. collagen).
The major sulfated proteoglycans of the sclera include aggrecan, decorin, and biglycan (Rada et al., 1997). These proteoglycans show regional and age-related variations in concentration and glycosylation which are considered to be responsible for regional changes in scleral hydration and rigidity (see below) (Rada, 2000). The presence of aggrecan, a proteoglycan typically found in cartilage, in the human sclera may represent an evolutionary vestige of the cartilaginous sclera present in most vertebrates (see section 4.2 below). Additionally, the presence of aggrecan may explain the scleral involvement in systemic autoimmune disorders affecting articular cartilage such as rheumatoid arthritis and polychondritis (Sainz de la Maza et al., 1994). The human sclera also contains proteoglycan core proteins of several members of a family of related proteins termed the “small leucine-rich proteoglycans” (SLRPs) (Rada et al., 1997). All SLRPs contain a common central domain, consisting of ≈ 10 leucine-rich repeats, which have been shown to be involved in strong protein/protein interactions (Iozzo, 1997). Several members of the SLRP family, including decorin, fibromodulin, lumican, proline arginine-rich end leucine-rich repeat protein (PRELP), and biglycan have been shown to bind a variety of ECM components via their core proteins, including type I collagen where they are thought to guide matrix assembly and organization (Hedbom, 1993; Rada, 1993; Schonherr, 1995; Vogel, 1984). Direct evidence supporting the importance of proteoglycans in maintaining scleral structure and eye shape has come from studies in mice made deficient in lumican (Austin et al., 2002) or double knock-out mice deficient in both lumican and fibrmodulin (Chakravarti et al., 2003). These mice exhibited abnormalities in scleral collagen fibril diameter and organization, along with scleral thinning and an increase in axial elongation, suggesting that these extracellular matrix components are important in maintaining the biomechanical properties of the sclera (Fig. 2).
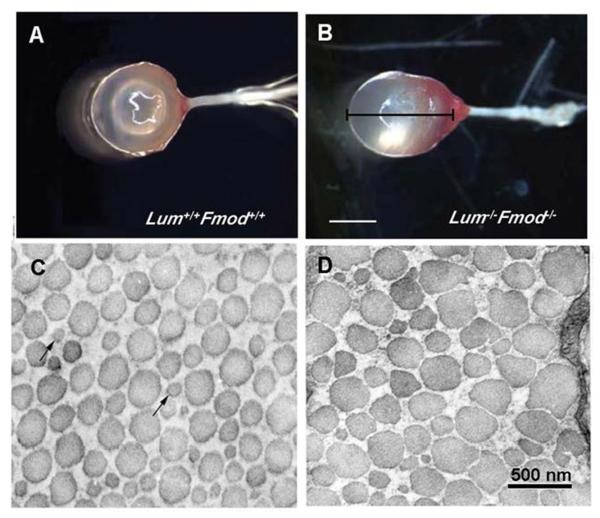
Figure 2.
The sclera of lumican/fibromodulin deficient mice. Photographs of whole eyes of wildtype mice (Lum+/+ Fmod +/+) (A) and lumican/fibromodulin deficient mice (Lum−/− Fmod−/−) (B), demonstrating significantly higher axial length of deficient mice as compared with wildytpe mice. Collagen fibril morphology in the posterior sclera of wildtype mice (C) and Lum−/− Fmod −/− deficient mice (D). Collagen fibrils from deficient mice displayed abnormal small-to very large-diameter fibrils with irregular contours. Bar in B = 2 mm; Bar in D = 500 nm. From: Chakravarti S., et al. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest Ophthalmol Vis Sci 44: 2422–2432. 2003 Reproduced with permission © Association for Research in Vision and Ophthalmology.
2.3. Other glycoproteins
Elastic fibers synthesized by scleral fibroblasts are an important component of the scleral extracellular matrix, and are especially abundant in the lamina fusca and trabecular meshwork (Marshall, 1995). Elastic fibers in the mature state consist of an amorphous elastin core surrounded by longitudinally aligned microfibrils, composed of a number of glycoproteins including fibrillin, which forms a scaffold for elastin. The importance of elastic fibers in the sclera is evidenced by the scleral pathology and high myopia associated with Marfan syndrome, an autosomal dominant disorder due to mutations in the fibrillin gene (Maumenee, 1981; Robinson and Booms, 2001). Additionally, fibronectin, vitronectin, and laminin have been identified in the fetal human sclera with greatest abundance between 13 – 16 weeks of development where they generally function to facilitate cell attachment to the ECM during developmental events. Immunolabeling for these glycoproteins is subtle in the adult sclera, except for strong labeling of laminin associated with blood vessel basement membranes (Sainz de la Maza, et al., 2012).
2.4. Matrix metalloproteinases
Scleral remodeling, as with any tissue, is a dynamic process that involves continual synthesis and degradation of extracellular matrix. Matrix metalloproteinases (MMPs) are a family of neutral proteinases that can initiate the degradation of collagens and other ECM components (Woessner, 1994). Human scleral fibroblasts have been shown to express MMP-1 (interstitial collagenase), MMP-2 (gelatinase A), MMP-3 (stromeyslin) (Di Girolamo et al., 1997; Gaton et al., 2001; Lauhio et al., 1994), and MMP-9 (gelatinase B) (Di Girolamo et al., 1997) as well as the MMP inhibitor, TIMP-1 (Di Girolamo et al., 1997; Yamaoka et al., 2001).
2.5. Scleral fibroblasts
Although the composition of the scleral ECM plays a major role in determining the biomechanical properties of the sclera, several studies provide evidence that scleral cells and their interactions with the scleral matrix have an important role in modulating scleral distensibility during eye growth. Phillips and McBrien demonstrated that the tree shrew sclera has an initial increase in axial length as a response to increases in IOP, but then axial length progressively decreased over the next hour, resulting in no significant difference in axial length following 60 minutes of elevated pressure as compared with the pre-pressure length measurement (Phillips and McBrien, 2004). This reduction in axial length was attributed to the presence of α-smooth muscle acting (SMA) - containing myofibroblasts in the scleral stroma. In addition, scleral fibroblasts have also been shown to synthesize and secrete relatively large amounts of transforming growth factor beta-induced, 68kD (TGFB1p) that has the potential to modulate interactions of the scleral fibroblast with the matrix (Shelton and Rada, 2009).
3. Development of the sclera
3.1 Prenatal development
The human sclera differentiates from neural crest and mesoderm in the sixth week of human embryonic development. The majority of the sclera differentiates from neural crest that surrounds the optic cup of neuroectoderm; however, a small temporal portion of the sclera differentiates from mesoderm which also contributes to the striated extraocular muscles and vascular endothelia (Ozanics, 1982). The human sclera differentiates from anterior to posterior and from inside to outside (Duke-Elder, 1966.; Sellheyer and Spitznas, 1988; Weale, 1982). Immature collagen can be detected in the sixth week as patches of small fibrils, and elastin deposits appear in the ninth week of development and increase in amount through week 24 (Foster, 1994).
Studies have demonstrated that intraocular pressure (IOP) is necessary for proper scleral development and eye size (Coulombre, 1956; Weiss and Amprino, 1940). Decreasing IOP through the insertion of a glass cannula into the vitreous chamber results in arrested ocular growth even though development of ocular tissues follows a normal time course. In addition, it has been shown that eye size is limited by structural components of the sclera since removal of small areas of ectoderm and prescleral mesenchyme during development results in a bulging outward of the eye wall in the denuded area (Coulombre, 1956).
3.2 Postnatal development
Although the eye nearly reaches adult size by age 10 (Larsen, 1971a; Larsen, 1971b; Larsen, 1971c; Larsen, 1971d; Zadnik et al., 1994) scleral ECM components are continually being synthesized and accumulated throughout adolescence and young adulthood. The concentration of the small sulfated scleral proteoglycans, biglycan and decorin increases steadily in the human sclera from childhood through young adulthood and then appears to decline after the fourth decade (Rada, 2000). The rapid accumulation of decorin and biglycan observed in the sclera in the first four decades of life and their subsequent decrease in older tissue suggest that the matrix functions of these proteoglycans are more important in the juvenile and adolescent growth periods of the human sclera. Interestingly, the pattern of accumulation of small proteoglycans in the aging sclera is remarkably similar to the age-related proteoglycan changes observed for human articular cartilage using a competitive radioimmunoassay for decorin (DSPG-2) (Sampaio Lde et al., 1988), suggesting that postnatal scleral development mirrors the general growth and maturation of the human skeleton (Fig. 3).
Figure 3.
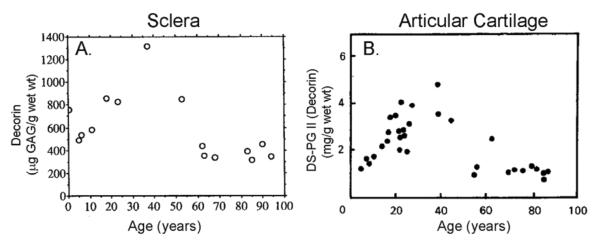
Age-related changes in decorin/DS-PG-II in human sclera and articular cartilage. A) Decorin was extracted from human sclera from ages 2 months to 94 years (n = 15), separated from other sulfated proteoglycans and quantified as micrograms of glycosaminoglycan per gram wet weight. B) Proteoglycans were extracted from normal human articular cartilage (ages 5 – 86 years, n = 32) and DS-PG-II (= decorin) was determined using a competitive radioimmunoassay. Note the striking similarity in the relative concentrations of decorin/DSPGII in the sclera and articular cartilage with increasing age. From: Rada J A, et al. Proteoglycan composition in the human sclera during growth and aging. Invest Ophthalmol Vis Sci 41: 1639–1648. 2000 Reproduced with permission © Association for Research in Vision and Ophthalmology and Sampaio LO et al., Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem J 254: 757–764. 1988. Reproduced with permission © the Biochemical Society.
Beyond the fourth decade, the scleral population of small proteoglycans (biglycan and decorin) decreases in concentration in all scleral regions. The loss of proteoglycans from the anterior sclera is associated with a coincident decrease in tissue hydration in the aging human sclera (Brown et al., 1994; Rada, 2000). In contrast to the age-related loss of small proteoglycans that occurs with increasing age, the large proteoglycan, aggrecan, remains constant over all ages and is concentrated in the posterior sclera. The retention of aggrecan in the posterior sclera of aging eyes may be related to the observation that the posterior sclera is less rigid than the anterior sclera. Due to its numerous chondroitin sulfate and keratan sulfate glycosaminoglycan side chains, aggrecan binds large amounts of water, and contributes to tissue resiliency of cartilage and its ability to withstand compressive forces. If aggrecan has a similar function in the sclera, its presence may allow the posterior sclera to remain pliable, thereby sparing the circulation to the choroid and retina through the posterior ciliary blood vessels. Decreases in aggrecan concentration would significantly reduce the glycosaminoglycan concentration in the posterior sclera and may lead to increased scleral rigidity, which has been associated with hyperopia and high myopia (Friedman, 1994). Additionally, with increasing age, the sclera increases in stiffness, due to the accumulation of nonenzymatic glycation-type cross-links of collagen fibrils with age (Coudrillier et al., 2012; Schultz et al., 2008). This age-related increase in stiffness is greatest in the anterior sclera, followed by the equatorial and then posterior sclera (Geraghty et al., 2012).
4. The sclera and myopia
Myopia is the leading cause of visual impairment in the world (Wojciechowski, 2011). Most myopia develops in children between the ages of 8 and 14 (Zadnik et al., 1994) and is produced by excessive lengthening of the vitreous chamber of the ocular globe so that the retina comes to lie behind the focal plane of the eye (Curtin, 1985; Tong et al., 2002). In highly myopic human eyes, the sclera is significantly thinner and gradually expands under the force of intraocular pressure. In high myopia, the thinner sclera and elongated globe puts individuals at increased risk of debilitating ocular disorders, such as retinal detachment, glaucoma and macular degeneration (Pan et al., 2013; Qiu et al., 2013).
4.1. The myopic human sclera
In highly myopic human eyes, the tensile strength of the sclera is reduced and the elasticity of the sclera is increased, especially at the posterior pole (Avetisov et al., 1983). Ultrastructural evaluation of the scleral stroma of highly myopic human eyes shows a more layered, lamellar structure, similar to that of the cornea (Curtin and Teng, 1958; Funata and Tokoro, 1990; McBrien et al., 2001). Because the axial length of the eye is a major determinant of the refractive status of the eye, alterations in any scleral ECM component, through genetic or environmental influences, are likely to lead to changes in scleral shape, which in turn could dramatically affect vision. However, few studies have characterized the ECM in the myopic human sclera. The sclera of the highly myopic human eye is characterized by thinning of collagen fiber bundles, as well as with a reduction in the size of the individual collagen fibrils with a preponderance of unusually small diameter fibrils averaging below 60 – 70 nm (Curtin et al., 1979; Curtin and Teng, 1958). Additionally, abnormal fibrils associated with an amorphous cementing substance and the presence of fissured or star-shaped fibrils have also been observed (Curtin, 1985; Curtin et al., 1979; Curtin and Teng, 1958) (Fig. 4). These ultramicroscopic alterations seen in the human myopic sclera suggest a derangement of the growth and organization of the collagen fibrils, either due to abnormal fibril formation or due to the presence of accentuated breakdown or catabolism of the sclera. The finding that sulfated proteoglycans continue to be synthesized and accumulate in the sclera throughout young adulthood (Rada, 2000), together with their known functions in collagen fibril assembly and organization, suggests that abnormal scleral proteoglycan biosynthesis in childhood and adolescent years may lead to disruption of the normal scleral ECM and abnormalities in ocular globe size and refraction.
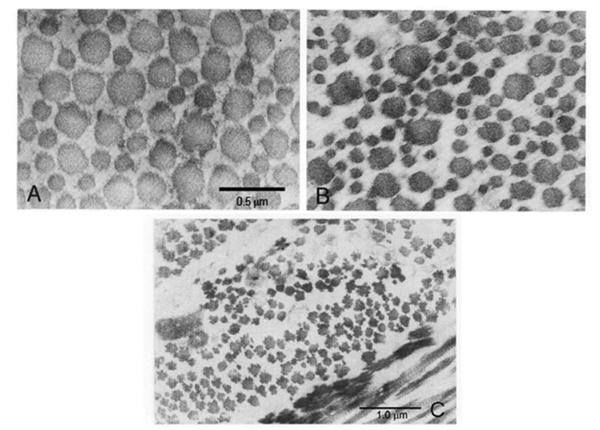
Figure 4.
Collagen fibrils in the sclera of normal and highly myopic human eyes. In contrast to normal human sclera, the highly myopic human sclera shows greater variability in collagen fibril diameters and contains an increased number of smaller diameter collagen fibrils (A, B). Additionally, an increase in unusual star-shaped fibrils and fibrils associated with amorphous cementing substance were observed on cross section (C)., B) Bar = 0.5 μ; C) Bar = 1 μm. Adapted From: Curtin BJ. The Myopias, Basic Science and Clinical Management. Philadelphia, PA, Harper & Row. Pages 256–258.
Genes responsible for myopia in association with other genetic syndromes have been identified: COL2A1 and COL11A1 for Stickler syndromes type 1 and 2 respectively (Annunen et al., 1999; Heikkinen et al., 1997), lysyl-protocollagen hydroxylase for type VI Ehlers-Danlos syndrome (Mahajan et al., 2010), COL18/A1 for Knobloch syndrome (Kainulainen et al., 1994) and fibrillin for Marfan syndrome (Paluru et al., 2003). Each of these genes is expressed in the sclera and serves as a model for possible candidate genes for non-syndromic high myopia. Recently, micro-RNA expression was compared in normal human adult eyes (55 to 80 years of age) and normal fetal eyes (24 week gestation). Interestingly, some micro-RNAs were identified that were higher in the sclera of rapidly growing fetal eyes as compared with adult eyes (Metlapally et al., 2013). It is speculated that some of these micro-RNAs may regulate scleral remodeling through their interactions with collagen and/or retinoid metabolism.
4.2. Animal models of myopia
Animal models of myopia have provided considerable insight into the mechanisms regulating eye size, refraction, and the development of myopia. In the 1970s, it was accidentally discovered that myopia could be induced in monkeys, chicks, and tree shrews by depriving the retina of form vision (Sherman et al., 1977; Wallman et al., 1978; Wiesel and Raviola, 1977). More recently the same treatment has been shown to produce myopia in mice (Barathi et al., 2008; Tkatchenko et al., 2010) and guinea pigs (Howlett and McFadden, 2006). Form vision deprivation is an “open-loop” system, in which the eye will continue to elongate at an accelerated rate for the duration of the deprivation, potentially producing eyes so enormous they bulge out of the eye socket. In contrast, Schaeffel et al. (Schaeffel et al., 1988) demonstrated through the use of positive and negative lenses of specific refractive powers that chicks could accurately compensate for imposed myopic or hyperopic defocus by modulating the axial length of the eye. Lens compensation was subsequently demonstrated in tree shrews (Norton et al., 2010), monkeys (Smith et al., 2010), guinea pigs (Howlett and McFadden, 2009), and mice (Tkatchenko et al., 2010). Remarkably, studies using animal models have demonstrated that young eyes can recover from induced myopia following removal of the diffuser or negative lens (Siegwart and Norton, 1998; Wallman and Adams, 1987). The key to this recovery is that the young eyes are still elongating as part of their postnatal (or post-hatching) development. When the diffuser or negative lens is removed, the elongated, myopic eye stops elongating, through a reversal of the scleral remodeling processes involved during myopia development. The optics of the eye continue to mature (through continued remodeling of the cornea and lens) so the focal plane gradually moves posteriorly, reducing the myopia.
Significant changes in scleral ECM synthesis, accumulation, and turnover are associated with the development of induced myopia and recovery in animal models (Norton and Rada, 1995; Rada et al., 2000; Rada et al., 1991). There are species differences in scleral structure between eutherian mammals (including humans and other primates such as the marmoset, macaque monkey, and tree shrew) and most other vertebrates, including chicks (Walls, 1942). The primary difference is that in most vertebrates, the sclera is comprised of an inner layer of cartilage and an outer fibrous layer (Fig. 5a, b). However, in eutherian mammals, the inner layer of cartilage is absent, so the entire sclera consists of the fibrous, type I collagen-dominated extracellular matrix (Fig. 5c, d). In his classic book, Walls (Walls, 1942) speculated that scleral cartilage was “allowed to disappear” in all vertebrate groups that possess a spherical eyeball (salamanders, snakes and placental mammals). Despite this difference in scleral anatomy, the fibrous sclera of mammals and the fibrous layer of the avian sclera appear to grow similarly. This could be due to ECM molecules previously believed unique to cartilage, such as aggrecan, PRELP, and cartilage olimeric matrix protein (COMP) being present in the human sclera (Young et al., 2004), suggesting that cartilaginous components have been retained in the sclera through evolution and serve important biochemical and biomechanical functions (Coster et al., 1987; Johnson et al., 2006; Rada et al., 1997). When ocular elongation accelerates, the fibrous sclera thins and loses material both in mammals (Norton and Rada, 1995; Rada et al., 2000) and birds (Gottlieb et al., 1990; Marzani and Wallman, 1997). The cartilaginous layer of the sclera of birds, however, demonstrates increased growth as the eye elongates, and this is accompanied by an increase in synthesis and accumulation of proteoglycans and an increased dry weight (Christensen and Wallman, 1991; Rada et al., 1991). At some level, all vertebrates probably use similar signaling mechanisms to control the sclera, but do so by controlling growth in the cartilage, where it is present, and by controlling remodeling in the fibrous sclera.
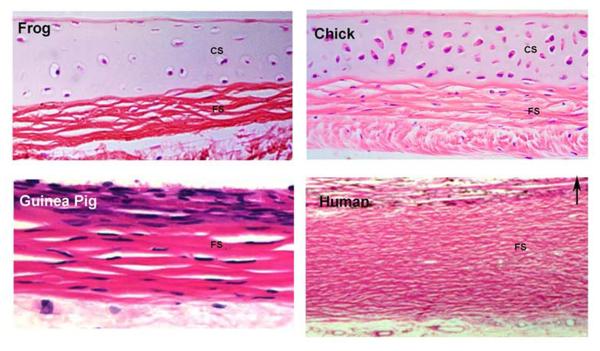
Figure 5. Vertebrate Scleras.
Light microscopic histological appearance of frog, chick, guinea pig and human scleras. Note the presence of both a cartilaginous sclera (CS) and a fibrous sclera (FS) in the frog and chick and lack of cartilage in the guinea pig and human sclera. In all micrographs, the choroidal surface of the sclera is on the top (indicated by arrow in D). Note these micrographs are not reproduced at original relative size; they are re-scaled to allow histologcial comparisons of the entire scleral thickness of each species. Frog sclera was generously donated by Dr. Allan Wiechmann (University of Oklahoma Health Science Center). Guinea pig sclera from: Simpanya et al., Expressed sequence tag analysis of guinea pig (Cavia porcellus) eye tissues for NEI Bank Mol Vis. 14:2413–2427, 2008.
4.3. Scleral changes in experimental myopia
Similar to the posterior sclera of the highly myopic human eye, myopia development in primates and tree shrews is associated with scleral thinning and changes in collagen fiber diameter and organization (Phillips et al., 2000; Rada et al., 2000). Biomechanical measurements of the sclera during the development of myopia and during recovery have been reported in the tree shrew and in chick. In both, there is no change in the modulus of elasticity during myopia development or recovery (Phillips et al., 2000; Phillips and McBrien, 1995; Siegwart and Norton, 1999). In tree shrews, the visco-elasticity in the sclera, as measured as the “creep rate” (continued elongation under a constant tension similar to that produced by intraocular pressure), was shown to increase significantly in form deprived eyes relative to the contralateral control eye. Within two days after a negative lens or a diffuser is put in place and begins to induce myopia, scleral creep rate increased. The increase in visco-elasticity renders the sclera more extensible, so that normal intraocular pressure may produce an enlargement of the vitreous chamber. Remarkably, when unrestricted vision was restored (recovery), the scleral creep rate decreased significantly below control levels within 2 days of removal of the diffuser, contributing to a recovery from myopia. The macromolecular changes in the sclera, directly responsible for mediating the increase in scleral creep rate during myopia development have not been identified. Changes in scleral crosslinking have been suggested to play a role controlling scleral viscoelasticity and ocular elongation. In 1994, McBrien and Norton (McBrien and Norton, 1994) demonstrated that prevention of collagen crosslinking, through the systemic administration of β-aminoproprionitrile (β-APN), resulted in markedly exaggerated elongation in form deprived eyes as compared to form deprived eyes treated with vehicle only. Interestingly, β-APN had no effect on the contralateral control eye, suggesting that additional scleral constituents are involved in restraining ocular elongation under normal visual conditions, even when scleral crosslinking is reduced. These results exemplify the dynamic nature of the sclera and compel investigation into the molecular basis of the visually driven changes in scleral biomechanics.
During myopia development, DNA synthesis has been found to be reduced (Gentle and McBrien, 1999) but DNA content in the sclera remains unchanged (Norton and Rada, 1995). There is a net loss of matrix, measured as a reduction in dry weight (McBrien et al., 2001) and a reduction in the amount of type I collagen (Gentle et al., 2003; Norton and Rada, 1995). The level of sulfated and unsulfated glycosaminoglycans (GAGs) decreases (Norton and Rada, 1995), with hyaluronan levels declining within 24 hours after a negative lens is applied in tree shrews (Moring et al., 2007). Similarly, in the common marmoset, the rate of proteoglycan synthesis in the posterior sclera is negatively correlated with the rate of vitreous chamber elongation in eyes developing myopia (accelerated elongation), hyperopia (decelerated elongation), or growing normally (Rada et al., 2000). This suggests that these ECM components are important in maintaining the biomechanical properties of the sclera. Interestingly, not all matrix proteins are reduced; type III and type V collagen is relatively unaffected (Gentle et al., 2003). Thus, it appears that there is not a modulation of growth in the fibrous sclera, but rather a remodeling that results in a loss of extracellular matrix.
In the chick model, the outer fibrous layer of the sclera also undergoes remodeling during the development of myopia, as evidenced by an increased expression of MMP-2, decreased expression of tissue inhibitor of metalloproteinase (TIMP)-2, an endogenous inhibitor of MMP-2 (Rada and Brenza, 1995; Rada et al., 1999), decreased rate of proteoglycan synthesis (Marzani and Wallman, 1997; Rada and Matthews, 1994), and overall thinning (Gottlieb et al., 1990). In contrast to the fibrous layer of chick sclera, the cartilaginous layer demonstrates increased synthesis and accumulation of DNA and of proteoglycans (particularly of aggrecan) and overall thickening during the development of myopia (Christensen and Wallman, 1991; Rada and Matthews, 1994; Rada et al., 1991). In chicks, significant increases in proteoglycan synthesis occur within one day of the start of form deprivation, presumably associated with cartilage growth, and these changes occur prior to changes in vitreous chamber elongation (Rada et al., 1992). Systemic inhibition of proteoglycan synthesis in chicks with β-xyloside significantly reduces the rate of ocular elongation in both form-deprived and contralateral control eyes (Rada et al., 2002), suggesting that increases in scleral proteoglycan synthesis and accumulation are responsible for ocular elongation during conditions of induced myopia as well as in normal post-hatch ocular growth.
In all species examined, the changes in scleral ECM synthesis and degradation are greatest at the posterior pole of the globe (Norton and Rada, 1995; Rada and Matthews, 1994; Rada et al., 2000), suggesting that these animal models of myopia accurately model the scleral changes associated with high myopia in humans. The localized response in the posterior sclera may be related to regional differences in the growth states of the scleral fibroblasts in this region or may be a reflection of a concentration of deprivation-induced changes in the retina, choroid, and sclera along the visual axis. However, this may also reflect the organization of the sclera itself because in some of these species (tree shrew, chick) the retinal region with the highest acuity (the analog of the human fovea) is not located at the posterior pole, but is found temporally.
In chicks, tree shrews, and marmosets, scleral changes during recovery from induced myopia are essentially a reversal of the scleral remodeling events associated with form-deprivation or negative-lens induced myopia. The slowed vitreous chamber elongation in the recovering eyes is associated with decreases in MMP-2 activity, increases in TIMP-2 activity, and increased proteoglycan synthesis in the fibrous sclera of marmosets and tree shrews (McBrien and Gentle, 2003; Rada et al., 2000). In addition, GAG levels, which are reduced during myopia development by negative lenses, return to normal (Moring et al., 2007). In the posterior cartilaginous sclera of chicks, there is a rapid decrease in proteoglycan synthesis within hours following restoration of unrestricted vision (Summers Rada and Hollaway, 2011). In both chicks and tree shrews, changes in scleral GAG synthesis and levels during recovery occur prior to, or at least as early as, the most rapid deceleration in vitreous chamber elongation (Moring et al., 2007; Summers Rada and Hollaway, 2011), suggesting that changes in scleral ECM remodeling are responsible for changes in ocular elongation.
5. Emmetropization and scleral remodeling
Results from clinical studies and animal studies (described above) clearly demonstrate the presence of a vision-dependent “emmetropization” mechanism that acts to minimize refractive error by controlling the axial length of eyes so that the retina comes to lie at the focal plane and images are focused on the photoreceptors. Results over the last 20 years and summarized in this review have clearly demonstrated that emmetropization controls eye growth through precise regulation of scleral extracellular matrix growth and remodeling. Interruption of the emmetropization mechanism by obscuring the visual image in children, as occurs with congenital cataract, ptosis, or vitreous hemorrhage, or with visual form deprivation in animals leads to rapid and significant myopia.
Recovery is also considered a manifestation of the emmetropization process, by which eyes actively minimize the imposed myopic defocus brought about through excessive axial elongation. However, in addition to a visual basis for modulating eye growth in recovery, there also exists a homeostatic mechanism to restore the enlarged eye to its natural shape that is due to normal elongation as part of postnatal development (described above) (Wallman and Winawer, 2004).
5.1. Local control
Many aspects of scleral ECM remodeling are speculated to be under the control of specific growth factors. The finding that age-related changes in scleral proteoglycan synthesis rates in humans are nearly identical to that observed in articular cartilage, peaking in the fourth decade of life (Rada, 2000), suggests that postnatal scleral growth, like that of other connective tissues, is under the control of systemic growth hormone or its downstream effectors, the insulin-like growth factors (IGF-I and IGF-II) (Van Wyk and Smith, 1999). However, one of the most intriguing discoveries in the past 25 years is that the scleral changes associated with visually guided postnatal ocular growth are controlled by a cascade of locally generated chemical events that are initiated in the retina and ultimately cause changes in scleral ECM remodeling (Wallman et al., 1987). If the optic nerve is severed or action potentials are blocked with tetrodotoxin (TTX), myopia can still be induced by visual deprivation or minus lenses (Norton et al., 1994; Troilo and Wallman, 1991). Furthermore, if occluders or negative lenses are fashioned to affect only a portion of the visual field, only the part of the ocular globe corresponding to the deprived or defocused portion of the retina will enlarge and become myopic (Wallman et al., 1987). Recovery from deprivation induced myopia also occurs in optic nerve sectioned eyes, although eyes tend to overshoot and become hyperopic in the absence of an intact optic nerve (Troilo and Wallman, 1991). The notion that postnatal ocular growth is regulated by an intraocular mechanism has stimulated much research on the identification of scleral growth regulators synthesized by neighboring ocular tissues.
A number of chemical changes have been identified in each of the ocular tissues during periods of accelerated eye growth (during the development of myopia) and during periods of decelerated eye growth (during recovery from induced myopia, or myopic defocus) (Fig. 6). Some of these chemical signals may interact with adjacent tissues to transmit the visual stimulus (originating in the retinal photoreceptors) downstream to the RPE, choroid and finally the sclera. For example, in chicks, insulin can inhibit recovery by inducing the RPE to synthesize diffusible molecules that prevents choroidal thickening and increases scleral GAG synthesis (Sheng et al., 2013). Insulin and muscarinic acetylcholine receptors have been identified in the choroid and sclera, suggesting that these agents have roles in mediating tissue responses outside of the retina (Penha et al., 2011; Lind et al., 1998; Liu et al., 2007). Additionally, dopamine, insulin, glucagon and GABA agonists and antagonists have been shown to have effects on the choroid and sclera leading to changes in eye size (Christian et al., 2014; Feldkaemper and Schaeffel, 2013; Feldkaemper et al., 2004; Penha et al., 2011; Zhu and Wallman, 2009).
Figure 6.
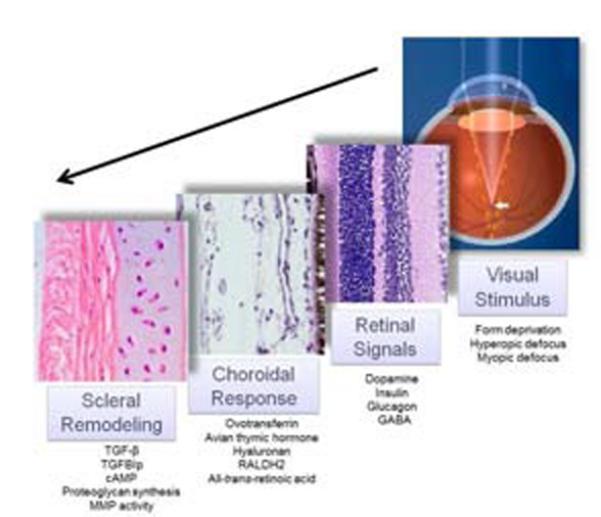
The retina-to-sclera signaling cascade. In response to visual stimuli such as form deprivation (blur), hyperopic defocus (minus lens treatment), or myopic defocus (plus lens treatment), changes in a variety of chemical moieties have been documented in the retina/RPE, choroid and sclera (listed under each tissue). Although the relationships, if any, between these tissue specific moieties have not been determined, it is likely that visual stimuli are transduced through the retina and choroid to ultimately affect scleral matrix remodeling and ocular axial length.
5.2. Choroidal regulation
As a highly vascular tissue, the choroid is responsible for the synthesis of a number of growth factors that are necessary for the development, growth, and maintenance of its elaborate vasculature. For example, choroidal endothelial and stromal cells have been shown to synthesize vascular endothelial growth factor (VEGF) (Saint-Geniez et al., 2006), basic fibroblast growth factor (bFGF or FGF-2) (Frank et al., 1996; Ogata et al., 1996) and hepatocyte growth factor (HGF) (Grierson et al., 2000). Additionally, the choroid has been shown to synthesize the matrix metalloproteinases MMP1, MMP2, and MMP3 (Steen et al., 1998). The aforementioned growth factors, MMPs, and TIMPs are secreted proteins and exert their effects through receptor-mediated interactions with neighboring cells. Therefore, in addition to their role in maintaining the choroidal vasculature, it is conceivable that these proteins could have effects on cells and tissues outside of the choroid.
Marzani and Wallman were first to demonstrate that the secreted molecules from the choroid can inhibit scleral proteoglycan synthesis and thereby have the potential to regulate the rate of ocular elongation (Marzani and Wallman, 1997). This study demonstrated that co-culture of sclera with choroids from untreated eyes inhibited proteoglycan synthesis in the cartilaginous layer of the chick sclera. Moreover, scleral proteoglycan synthesis was inhibited more by choroids isolated from recovering eyes. Conversely, sclera co-cultured with choroids isolated from myopic (form deprived) eyes demonstrated an increased rate of proteoglycan synthesis relative to that of sclera co-cultured with untreated choroids. Additionally, suprachoroidal fluid removed from recovering choroids inhibits scleral proteoglycan synthesis in vitro as compared with that of fluid isolated from control choroids (Rada et al., 2001; Rada and Palmer, 2007). Since the changes in scleral proteoglycan synthesis induced by co-culture with choroids or suprachoroidal fluid under different growth conditions mimicked those changes observed in sclera under the same visual conditions in vivo (Rada et al., 1992), these studies provided the first evidence that the choroid could be the source of scleral growth regulators involved in visually guided ocular elongation. Changes in a number of chemicals, including ovotransferrin, avian thymic hormone, hyaluronan and RALDH2, have been identified in the choroid during periods of myopia development or recovery, some of which have been speculated to participate in the retina-to sclera-chemical cascade (Fig. 6).
5.3. Retinoic acid
Retinoic acid has been implicated in the signaling cascade that modulates eye growth between the retina and the sclera (Mertz and Wallman, 2000). The chick choroid synthesizes relatively high levels of all-trans-retinoic (atRA) acid as compared with the retina or liver, and the rate of atRA synthesis is dramatically affected by the refractive state of the eye. Choroidal synthesis of atRA was shown to be increased in chick eyes during recovery from induced myopia and during compensation for imposed myopic defocus (using plus lenses), and atRA was shown to be decreased in eyes undergoing form deprivation myopia and compensation for hyperopic defocus (using minus lenses). Interestingly, the time course of the increase in choroidal atRA synthesis (Mertz and Wallman, 2000) was remarkably similar to that of the decrease in rate of sclera proteoglycan synthesis observed in the early phase of recovery from induced myopia (Summers Rada and Hollaway, 2011) (Fig. 7) suggesting a causal relationship between choroidal atRA synthesis and scleral proteoglycan synthesis.
Figure 7.
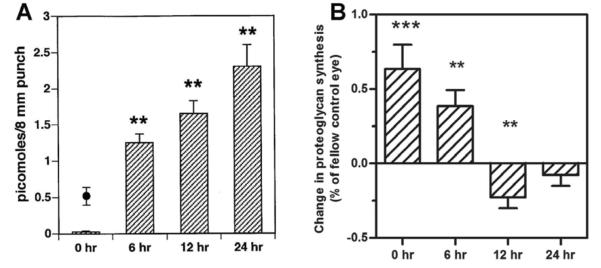
Comparison of changes in choroidal retinoic acid synthesis and scleral proteoglycan synthesis during recovery from induced myopia. A) Time course of increase in choroidal alltrans-retinoic acid (atRA) synthesis in eyes recovering from form deprivation myopia. B) Time course of decreased scleral proteoglycan synthesis in eyes recovering from form deprivation myopia. Fig. 7a from: Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res, 70(4): p. 519–527 2000. Reproduced with permission © Elsevier and Fig. 7b adapted from: Summers JA, Hollaway LR. Regulation of the biphasic decline in scleral proteoglycan synthesis during the recovery from induced myopia. Exp. Eye Res. 92(5):394–400 2011. Reproduced with permission © Elsevier.
Recently, using an ultrasensitive method of quantification [LC (liquid chromatography)/MS/MS], endogenous and newly synthesized atRA was measured in chick choroids in organ culture (Rada, 2012). In agreement with Mertz and Wallman, atRA concentration was significantly higher in cultures of choroids from eyes recovering for 24 hrs – 15 days than in cultures of paired controls. Moreover, the concentrations of atRA generated by choroids in vitro were within the range to produce significant inhibition of scleral proteoglycan synthesis (Rada, 2012) (Fig. 8). Taken together, these studies suggested that choroidal synthesis of atRA in response to visual stimuli may modulate scleral proteoglycan synthesis.
Figure 8.
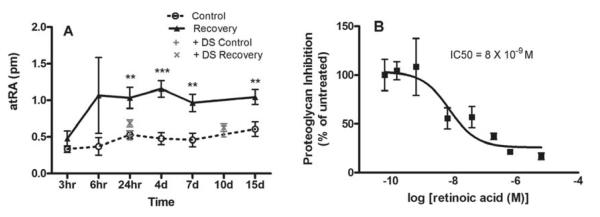
Choroidal retinoic acid as a potential scleral growth regulator. Retinoic acid was measured in organ cultures of choroids isolated from eyes recovering from induced myopia (3 hr – 15 day of unrestricted vision) using LC/MS/MS. Concentrations of retinoic acid were increased in cultures of recovering choroids following 1 – 15 days of recovery. Based on the volume of the organ cultures, the concentration of retinoic acid was determined to be ~1.45 × 10−8 M and ~5.0 × 10−9 M in recovering and control cultures, respectively (A). Comparison of a dose response curve for retinoic acid on scleral proteoglycan synthesis indicated the retinoic acid concentrations synthesized by choroids in vitro are within the range to significantly inhibit scleral proteoglycan synthesis (IC50 = 8 × 10−9 M). From: Summers JA, et al. Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest Ophthalmol Vis Sci 53: 1649–1662 2012 Reproduced with permission © Association for Research in Vision and Ophthalmology.
In guinea pigs and primates, atRA synthesis is increased in the choroid/sclera (McFadden et al., 2004) and RPE/choroid (Troilo et al., 2006), respectively, during the development of myopia, a condition that is also associated with decreased scleral proteoglycan synthesis. However, in contrast to chicks, decreased proteoglycan synthesis in the mammalian sclera is associated with increased axial elongation (Norton and Rada, 1995; Rada et al., 2000). Similar to chicks, atRA has been demonstrated to inhibit proteoglycan synthesis in the primate sclera (Troilo et al., 2006). Therefore, in both chicks and primates, the visually induced changes in choroidal atRA synthesis and concentration are consistent with the known changes in scleral proteoglycan synthesis that occur during visually guided ocular growth and may represent an evolutionarily conserved mechanism for visually guided ocular growth regulation. How the same visual stimuli (such as hyperopic defocus) can cause opposite changes in choroidal atRA synthesis in chicks and primates is unknown, but suggests the presence of additional regulatory proteins in the cascade between the retina and choroid that differ between primates and chicks.
Tissue concentrations of atRA are tightly controlled by synthesizing and catabolizing enzymes, binding proteins and nuclear receptors (Napoli, 2011). Two studies identified significant increases in choroidal retinaldehyde dehydrogenase 2 (RALDH2) mRNA expression during recovery from induced myopia and during compensation for lens induced myopia (+7D lens) (Rada, 2012; Simon et al., 2004), suggesting that increased synthesis of atRA in choroids of recovering eyes is due to increased RALDH2 enzyme activity. The identification of atRA and its synthesizing enzyme, RALDH2, as possible mediators of visually induced changes in scleral remodeling and eye size has provided new potential molecular targets for the control of myopia development.
5.5. Other factors involved in scleral remodeling
The multifunctional cytokine, transforming growth factor-beta (TGF-β) has been implicated in regulating scleral ECM changes associated with the development of myopia. Gene expression of three isoforms of TGF-β (TGF-β1, TGF-β2 and TGF-β3) has been shown to rapidly decrease in the sclera of tree shrew eyes in response to form deprivation (McBrien et al., 2009). Moreover, comparisons of primary cultures of scleral fibroblasts grown in the presence of TGF-β isoforms at concentrations comparable to that observed in normal and myopic sclera in vivo indicate that the decrease in TGF-β isoforms results in decreases in collagen and proteoglycan synthesis similar to that observed in the sclera of myopic tree shrew eyes (McBrien, 2013).
Cyclic AMP (cAMP) has also been implicated in scleral ECM changes associated with myopia development through modulating scleral collagen levels. In guinea pigs, cAMP levels were increased in the sclera during form deprivation myopia and decreased during recovery (Tao et al., 2013). Intraocular treatment with an adenylate cyclase (AC) activator, forskolin, resulted in a myopic shift accompanied by a concomitant decrease in collagen I, III, and V transcript levels. In addition, application of forskolin to human scleral fibroblasts in vitro resulted in decreased collagen I, III and V transcript levels (Tao et al., 2013). This effect was attenuated by an AC inhibitor.
It has been shown that GABA receptors are located in chick ocular tissues, including the cornea, RPE, and cartilaginous and fibrous sclera, suggesting a potential role for GABA in mediating scleral remodeling (Cheng et al., 2011; Cheng et al., 2012; Cheng et al., 2013). Treatment of cultured chick scleral fibroblasts with GABA agonists resulted in scleral cell proliferation (as evidenced by increased DNA content in cell cultures), as well as a decrease in scleral GAG synthesis (Christian et al., 2014). An opposite effect was observed when scleral fibroblasts were treated with GABA antagonists. Moreover, when chick scleral fibroblasts were co-cultured with retina, RPE, and/or choroid from eyes previously induced to become myopic through lens compensation, GABA agonists mimicked the effect of myopic tissue on sclera (increased cell proliferation, decreased GAG synthesis), while antagonists blocked the effect of myopic tissue on sclera (Christian et al., 2014). This suggests that substrates for the GABA receptor are involved in scleral changes associated with myopia.
7. Conclusions and future directions
Research highlighted in this review clearly demonstrates that the sclera is a dynamic tissue, capable of responding rapidly to changes in the visual environment to affect changes in ocular size and refraction. Research over the last decade has identified several genes in the sclera of several animal species, including humans, which are associated with the development of myopia. Results from clinical and experimental studies clearly demonstrate the presence of an emmetropization mechanism that acts to minimize refractive error though alterations in scleral remodeling during postnatal ocular growth. In human populations, emmetropization is often dysregulated, presumably, due to environmental and/or genetic influences, leading to the development of myopia. An understanding of the chemical basis of emmetropization, as well as the genetic and environmental factors that impact this mechanism are necessary for the development of successful therapeutic strategies to slow or prevent myopia in children. Therapies designed to slow the loss of extracellular matrix in the human sclera, through inhibition of MMP activity, stimulation of proteoglycan and collagen synthesis, or increase in collagen crosslinking, would be logical approaches to slow the progression of myopia. The identification of RALDH2 as a visually regulated enzyme that, through its synthesis of all-trans-retinoic acid, is a potent regulator of scleral extracellular matrix remodeling provides new avenues for the development of new approaches to slow or prevent the progression of myopia in children.
Highlights.
The sclera is a dense connective tissue that defines ocular size and shape.
The biomechanical properties of the sclera are determined by its ECM.
Myopia is characterized by scleral thinning and ocular elongation.
Defects in scleral ECM remodeling lead to myopia in humans and animal models.
Scleral remodeling is regulated in part by a retina-to-sclera chemical cascade.
Acknowledgements
The majority of data presented in this review came from projects funded by the National Eye Institute at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annunen S, Korkko J, Czarny M, Warman ML, Brunner HG, Kaariainen H, Mulliken JB, Tranebjaerg L, Brooks DG, Cox GF, Cruysberg JR, Curtis MA, Davenport SL, Friedrich CA, Kaitila I, Krawczynski MR, Latos-Bielenska A, Mukai S, Olsen BR, Shinno N, Somer M, Vikkula M, Zlotogora J, Prockop DJ, Ala-Kokko L. Splicing mutations of 54-bp exons in the COL11A1 gene cause Marshall syndrome, but other mutations cause overlapping Marshall/Stickler phenotypes. Am J Hum Genet. 1999;65:974–983. doi: 10.1086/302585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin BA, Coulon C, Liu CY, Kao WW, Rada JA. Altered collagen fibril formation in the sclera of lumican-deficient mice. Invest Ophthalmol Vis Sci. 2002;43:1695–1701. [PubMed] [Google Scholar]
- Avetisov ES, Savitskaya NF, Vinetskaya MI, Iomdina EN. A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metab Pediatr Syst Ophthalmol. 1983;7:183–188. [PubMed] [Google Scholar]
- Barathi VA, Boopathi VG, Yap EP, Beuerman RW. Two models of experimental myopia in the mouse. Vision Res. 2008;48:904–916. doi: 10.1016/j.visres.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Birk DE, Lande MA. Corneal and scleral collagen fiber formation in vitro. Biochim Biophys Acta. 1981;670:362–369. doi: 10.1016/0005-2795(81)90108-2. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J. Cell Biol. 1984;99:2024–2033. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CT, Vural M, Johnson M, Trinkaus-Randall V. Age-related changes of scleral hydration and sulfated glycosaminoglycans. Mech Ageing Dev. 1994;77:97–107. doi: 10.1016/0047-6374(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Buch H, Vinding T, Nielsen NV. Prevalence and causes of visual impairment according to World Health Organization and United States criteria in an aged, urban Scandinavian population: the Copenhagen City Eye Study. Ophthalmology. 2001;108:2347–2357. doi: 10.1016/s0161-6420(01)00823-5. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, Birk DE. Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest Ophthalmol Vis Sci. 2003;44:2422–2432. doi: 10.1167/iovs.02-0783. [DOI] [PubMed] [Google Scholar]
- Cheng ZY, Chebib M, Schmid KL. rho1 GABAC receptors are expressed in fibrous and cartilaginous layers of chick sclera and located on sclera fibroblasts and chondrocytes. Journal of neurochemistry. 2011;118:281–287. doi: 10.1111/j.1471-4159.2011.07300.x. [DOI] [PubMed] [Google Scholar]
- Cheng ZY, Chebib M, Schmid KL. Identification of GABA receptors in chick cornea. Molecular vision. 2012;18:1107–1114. [PMC free article] [PubMed] [Google Scholar]
- Cheng ZY, Wang XP, Schmid KL, Liu L. Identification of GABA receptors in chick retinal pigment epithelium. Neuroscience letters. 2013;539:43–47. doi: 10.1016/j.neulet.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Christensen AM, Wallman J. Evidence that increased scleral growth underlies visual deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 1991;32:2143–2150. [PubMed] [Google Scholar]
- Christian PG, Harkin DG, Schmid KL. GABAergic agents modify the response of chick scleral fibroblasts to myopic and hyperopic eye cup tissues. Curr Eye Res. 2014;39:172–187. doi: 10.3109/02713683.2013.834941. [DOI] [PubMed] [Google Scholar]
- Coster L, Rosenberg LC, van der Rest M, Poole AR. The dermatan sulfate proteoglycans of bovine sclera and their relationship to those of articular cartilage. An immunological and biochemical study. J Biol Chem. 1987;262:3809–3812. [PubMed] [Google Scholar]
- Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012;53:1714–1728. doi: 10.1167/iovs.11-8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre AJ. The role of intraocular pressure in the development of the chick eye. I. Control of eye size. Journal of Experimental Zoology. 1956;133:211–225. [Google Scholar]
- Curtin BJ. The Myopias: Basic Science and Clinical Management. Harper & Row; Philadelphia, PA: 1985. [Google Scholar]
- Curtin BJ, Iwamoto T, Renaldo DP. Normal and staphylomatous sclera of high myopia. An electron microscopic study. Arch Ophthalmol. 1979;97:912–915. doi: 10.1001/archopht.1979.01020010470017. [DOI] [PubMed] [Google Scholar]
- Curtin BJ, Teng CC. Scleral changes in pathological myopia. Trans Am Acad Ophthalmol Otolaryngol. 1958;62:777–788. discussion 788–790. [PubMed] [Google Scholar]
- Di Girolamo N, Lloyd A, McCluskey P, Filipic M, Wakefield D. Increased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultured human scleral fibroblasts. Am J Pathol. 1997;150:653–666. [PMC free article] [PubMed] [Google Scholar]
- Duke-Elder S, Cook CH. Normal and abnormal development. In: Duke-Elder S, editor. System of Ophthalmology. Vol. 3. CV Mosby; St. Louis: 1966. pp. 1–77. [Google Scholar]
- Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Burkhardt E, Schaeffel F. Localization and regulation of glucagon receptors in the chick eye and preproglucagon and glucagon receptor expression in the mouse eye. Exp Eye Res. 2004;79:321–329. doi: 10.1016/j.exer.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Foster CS, Sainz de la Maza M. The sclera. Springer-Verlag; New York: 1994. [Google Scholar]
- Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403. doi: 10.1016/s0002-9394(14)72066-5. [DOI] [PubMed] [Google Scholar]
- Friedman E. Aging changes of the the sclera. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology: Basic Sciences. WB Saunders; Philadelphia: 1994. pp. 726–728. [Google Scholar]
- Funata M, Tokoro T. Scleral change in experimentally myopic monkeys. Graefes Arch Clin Exp Ophthalmol. 1990;228:174–179. doi: 10.1007/BF00935729. [DOI] [PubMed] [Google Scholar]
- Gaton DD, Sagara T, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F(2 alpha)-isopropyl ester treatment. Arch Ophthalmol. 2001;119:1165–1170. doi: 10.1001/archopht.119.8.1165. [DOI] [PubMed] [Google Scholar]
- Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278:16587–16594. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- Gentle A, McBrien NA. Modulation of scleral DNA synthesis in development of and recovery from induced axial myopia in the tree shrew. Exp Eye Res. 1999;68:155–163. doi: 10.1006/exer.1998.0587. [DOI] [PubMed] [Google Scholar]
- Geraghty B, Jones SW, Rama P, Akhtar R, Elsheikh A. Age-related variations in the biomechanical properties of human sclera. J Mech Behav Biomed Mater. 2012;16:181–191. doi: 10.1016/j.jmbbm.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr Eye Res. 1990;9:1157–1165. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- Grierson I, Heathcote L, Hiscott P, Hogg P, Briggs M, Hagan S. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19:779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Hedbom H, Heinegard D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J. Biol. Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- Heikkinen J, Toppinen T, Yeowell H, Krieg T, Steinmann B, Kivirikko KI, Myllyla R. Duplication of seven exons in the lysyl hydroxylase gene is associated with longer forms of a repetitive sequence within the gene and is a common cause for the type VI variant of Ehlers-Danlos syndrome. Am J Hum Genet. 1997;60:48–56. [PMC free article] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46:267–283. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The familyl of the small leucine-rich proteoglycans:key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Young TL, Rada JA. Small leucine rich repeat proteoglycans (SLRPs) in the human sclera: identification of abundant levels of PRELP. Mol Vis. 2006;12:1057–1066. [PubMed] [Google Scholar]
- Kainulainen K, Karttunen L, Puhakka L, Sakai L, Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet. 1994;6:64–69. doi: 10.1038/ng0194-64. [DOI] [PubMed] [Google Scholar]
- Keeley FW, Vesely S MJ. Characterization of collagen from normal human sclera. Exp Eye Res. 1984;39:533–542. doi: 10.1016/0014-4835(84)90053-8. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. 1. Ultrasonic measurement of the depth of the anterior chamber from birth to puberty. Acta Ophthalmol (Copenh) 1971a;49:239–262. doi: 10.1111/j.1755-3768.1971.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. 3. Ultrasonic measurement of the posterior segment (axial length of the vitreous) from birth to puberty. Acta Ophthalmol (Copenh) 1971b;49:441–453. doi: 10.1111/j.1755-3768.1971.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. II. Ultrasonic measurement of the axial diameter of the lens and the anterior segment from birth to puberty. Acta Ophthalmol (Copenh) 1971c;49:427–440. doi: 10.1111/j.1755-3768.1971.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Larsen JS. The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol (Copenh) 1971d;49:873–886. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- Lauhio A, Konttinen YT, Salo T, Tschesche H, Lahdevirta J, Woessner F, Jr., Golub LM, Sorsa T. Placebo-controlled study of the effects of three-month lymecycline treatment on serum matrix metalloproteinases in reactive arthritis. Ann N Y Acad Sci. 1994;732:424–426. doi: 10.1111/j.1749-6632.1994.tb24774.x. [DOI] [PubMed] [Google Scholar]
- Liberfarb RM, Levy HP, Rose PS, Wilkin DJ, Davis J, Balog JZ, Griffith AJ, Szymko-Bennett YM, Johnston JJ, Francomano CA, Tsilou E, Rubin BI. The Stickler syndrome: genotype/phenotype correlation in 10 families with Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1. Genet Med. 2003;5:21–27. doi: 10.1097/00125817-200301000-00004. [DOI] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Lee YC, Hung PT, Hou PK. Changes in ocular refraction and its components among medical students--a 5-year longitudinal study. Optom Vis Sci. 1996;73:495–498. doi: 10.1097/00006324-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Lind GJ, Chew SJ, Marzani D, Wallman J. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1998;39:2217–2231. [PubMed] [Google Scholar]
- Liu Q, Wu J, Wang X, Zeng J. Changes in muscarinic acetylcholine receptor expression in form deprivation myopia in guinea pigs. Mol Vis. 2007;13:1234–1244. [PubMed] [Google Scholar]
- Mahajan VB, Olney AH, Garrett P, Chary A, Dragan E, Lerner G, Murray J, Bassuk AG. Collagen XVIII mutation in Knobloch syndrome with acute lymphoblastic leukemia. Am J Med Genet A. 2010;152A:2875–2879. doi: 10.1002/ajmg.a.33621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GE. Human scleral elastic system: an immunoelectron microscopic study. Br J Ophthalmol. 1995;79:57–64. doi: 10.1136/bjo.79.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- Maumenee IH. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc. 1981;79:684–733. [PMC free article] [PubMed] [Google Scholar]
- McBrien NA. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Experimental eye research. 2013;114:128–140. doi: 10.1016/j.exer.2013.01.014. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Cornell LM, Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42:2179–2187. [PubMed] [Google Scholar]
- McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22:307–338. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009;86:E23–30. doi: 10.1097/OPX.0b013e3181940669. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree shrew. Exp Eye Res. 1994;59:475–486. doi: 10.1006/exer.1994.1133. [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44:643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- Metlapally R, Gonzalez P, Hawthorne FA, Tran-Viet KN, Wildsoet CF, Young TL. Scleral micro-RNA signatures in adult and fetal eyes. PLoS One. 2013;8:e78984. doi: 10.1371/journal.pone.0078984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr. The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994;11:143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Ogata N, Matsushima M, Takada Y, Tobe T, Takahashi K, Yi X, Yamamoto C, Yamada H, Uyama M. Expression of basic fibroblast growth factor mRNA in developing choroidal neovascularization. Curr Eye Res. 1996;15:1008–1018. doi: 10.3109/02713689609017649. [DOI] [PubMed] [Google Scholar]
- Ozanics V, Jakobiec FA. Prenatal development of the eye and its anexa. In: Tasman W, Jaeger EA, editors. Duane's Foundations of Clinical Ophthalmology. Lippincott; Philadelphia: 1982. pp. 1–93. [Google Scholar]
- Paluru P, Ronan SM, Heon E, Devoto M, Wildenberg SC, Scavello G, Holleschau A, Makitie O, Cole WG, King RA, Young TL. New locus for autosomal dominant high myopia maps to the long arm of chromosome 17. Invest Ophthalmol Vis Sci. 2003;44:1830–1836. doi: 10.1167/iovs.02-0697. [DOI] [PubMed] [Google Scholar]
- Pan CW, Cheung CY, Aung T, Cheung CM, Zheng YF, Wu RY, Mitchell P, Lavanya R, Baskaran M, Wang JJ, Wong TY, Saw SM. Differential associations of myopia with major age-related eye diseases: the Singapore Indian Eye Study. Ophthalmology. 2013;120:284–291. doi: 10.1016/j.ophtha.2012.07.065. [DOI] [PubMed] [Google Scholar]
- Penha AM, Schaeffel F, Feldkaemper M. Insulin, insulin-like growth factor-1, insulin receptor, and insulin-like growth factor-1 receptor expression in the chick eye and their regulation with imposed myopic or hyperopic defocus. Mol Vis. 2011;17:1436–1448. [PMC free article] [PubMed] [Google Scholar]
- Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000;41:2028–2034. [PubMed] [Google Scholar]
- Phillips JR, McBrien NA. Form deprivation myopia: elastic properties of sclera. Ophthalmic Physiol Opt. 1995;15:357–362. [PubMed] [Google Scholar]
- Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci. 2004;45:758–763. doi: 10.1167/iovs.03-0732. [DOI] [PubMed] [Google Scholar]
- Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population. Investigative ophthalmology & visual science. 2013;54:830–835. doi: 10.1167/iovs.12-11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Achen VR, Perry CA, Fox PW. Proteoglycans in the human sclera. Evidence for the presence of aggrecan. Invest Ophthalmol Vis Sci. 1997;38:1740–1751. [PubMed] [Google Scholar]
- Rada JA, Achen VR, Penugonda S, et al. Proteoglycan composition in the human sclera during growth and aging. Invest Ophthalmol Vis Sci. 2000;41:1639–1648. [PubMed] [Google Scholar]
- Rada JA, Brenza HL. Increased latent gelatinase activity in the sclera of visually deprived chicks. Invest Ophthalmol Vis Sci. 1995;36:1555–1565. [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (Lumican and Decorin) core proteins. Exp. Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Rada JA, Hollaway LY, Li N, Napoli J. Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest Ophthalmol Vis Sci. 2012;53:1649–1662. doi: 10.1167/iovs.11-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Huang Y, Rada KG. Identification of choroidal ovotransferrin as a potential ocular growth regulator. Curr Eye Res. 2001;22:121–132. doi: 10.1076/ceyr.22.2.121.5525. [DOI] [PubMed] [Google Scholar]
- Rada JA, Johnson JM, Achen VR, Rada KG. Inhibition of scleral proteoglycan synthesis blocks deprivation-induced axial elongation in chicks. Exp Eye Res. 2002;74:205–215. doi: 10.1006/exer.2001.1113. [DOI] [PubMed] [Google Scholar]
- Rada JA, Johnson JM. In: Sclera. Cornea J. Krachmer, Mannis M, Holland E., editors. Mosby; St. Louis: 2004. [Google Scholar]
- Rada JA, Matthews AL. Visual deprivation upregulates extracellular matrix synthesis by chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1994;35:2436–2447. [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41:2050–2058. [PubMed] [Google Scholar]
- Rada JA, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest Ophthalmol Vis Sci. 2007;48:2957–2966. doi: 10.1167/iovs.06-1051. [DOI] [PubMed] [Google Scholar]
- Rada JA, Perry CA, Slover ML, Achen VR. Gelatinase A and TIMP-2 expression in the fibrous sclera of myopic and recovering chick eyes. Invest Ophthalmol Vis Sci. 1999;40:3091–3099. [PubMed] [Google Scholar]
- Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- Robinson PN, Booms P. The molecular pathogenesis of the Marfan syndrome. Cell Mol Life Sci. 2001;58:1698–1707. doi: 10.1007/PL00000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Maldonado AE, D'Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47:3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- Sainz de la Maza M, Tauber J, Foster CS. The sclera. 2nd ed. Springer-Verlag; New York: 2012. [Google Scholar]
- Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis associated with rheumatoid arthritis and with other systemic immune-mediated diseases. Ophthalmology. 1994;101:1281–1286. discussion 1287–1288. [PubMed] [Google Scholar]
- Sampaio Lde O, Bayliss MT, Hardingham TE, Muir H. Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem J. 1988;254:757–764. doi: 10.1042/bj2540757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg-Lall M, Hagg PO, Wahlstrom I, Pihlajaniemi T. Type XIII collagen is widely expressed in the adult and developing human eye and accentuated in the ciliary muscle, the optic nerve and the neural retina. Exp Eye Res. 2000;70:775–787. doi: 10.1006/exer.1998.0826. [DOI] [PubMed] [Google Scholar]
- Savontaus M, Ihanamaki T, Metsaranta M, Vuorio E, Sandberg-Lall M. Localization of type II collagen mRNA isoforms in the developing eyes of normal and transgenic mice with a mutation in type II collagen gene. Invest Ophthalmol Vis Sci. 1997;38:930–942. [PubMed] [Google Scholar]
- Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schonherr E, Witsch-Prehm P, Harrach B, et al. Interaction of biglycan with type I collagen. J. Biol. Chem. 1995;270:2776–2783. doi: 10.1074/jbc.270.6.2776. [DOI] [PubMed] [Google Scholar]
- Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM. Structural factors that mediate scleral stiffness. Invest Ophthalmol Vis Sci. 2008;49:4232–4236. doi: 10.1167/iovs.08-1970. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Spitznas M. Development of the human sclera. A morphological study. Graefes Arch Clin Exp Ophthalmol. 1988;226:89–100. doi: 10.1007/BF02172725. [DOI] [PubMed] [Google Scholar]
- Shelton L, Rada JA. Inhibition of human scleral fibroblast cell attachment to collagen type I by TGFBIp. Invest Ophthalmol Vis Sci. 2009;50:3542–3552. doi: 10.1167/iovs.09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng C, Zhu X, Wallman J. In vitro effects of insulin and RPE on choroidal and scleral components of eye growth in chicks. Experimental eye research. 2013;116:439–448. doi: 10.1016/j.exer.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–157. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr., Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Simon P, Feldkaemper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcriptional changes of retinal and choroidal TGFbeta-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis. 2004;10:588–597. [PubMed] [Google Scholar]
- Simpanya MF, Wistow G, Gao J, David LL, Giblin FJ, Mitton KP. Expressed sequence tag analysis of guinea pig (Cavia porcellus) eye tissues for NEIBank. Mol Vis. 2008;14:2413–2427. [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010;51:3864–3873. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen B, Sejersen S, Berglin L, Seregard S, Kvanta A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998;39:2194–2200. [PubMed] [Google Scholar]
- Summers Rada JA, Hollaway LR. Regulation of the biphasic decline in scleral proteoglycan synthesis during the recovery from induced myopia. Exp Eye Res. 2011;92:394–400. doi: 10.1016/j.exer.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Pan M, Liu S, Fang F, Lu R, et al. cAMP Level Modulates Scleral Collagen Remodeling, a Critical Step in the Development of Myopia. PLoS ONE. 2013 doi: 10.1371/journal.pone.0071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, Tkatchenko AV. Mouse experimental myopia has features of primate myopia. Invest Ophthalmol Vis Sci. 2010;51:1297–1303. doi: 10.1167/iovs.09-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Wong EH, Chan YH, Balakrishnan V. A multiple regression approach to study optical components of myopia in Singapore school children. Ophthalmic Physiol Opt. 2002;22:32–37. doi: 10.1046/j.1475-1313.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47:1768–1777. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- Van Wyk JJ, Smith EP. Insulin-like growth factors and skeletal growth: possibilities for therapeutic interventions. J Clin Endocrinol Metab. 1999;84:4349–4354. doi: 10.1210/jcem.84.12.6201. [DOI] [PubMed] [Google Scholar]
- Vitale S, Ellwein L, Cotch MF, Ferris FL, 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999—2004. Arch Ophthalmol. 2008;126:1111–1119. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Walls G. The vertebrate eye and its adaptive radiations. The Cranbrook Press, Bloomfield Hills; 1942. [Google Scholar]
- Weale RA. A Biography of the Eye. Lewis: , London: 1982. [Google Scholar]
- Weiss P, Amprino R. The Effect of Mechanical Stress on the Differentiation of Scleral Cartilage in Vitro and in the Embryo. Growth. 1940;4:245–258. [Google Scholar]
- Wessel H, Anderson S, Fitae D, Halvas E, Hempel J, SundarRaj N. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci. 1997;38:2408–2422. [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266:66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr. The family of matrix metalloproteinases. Ann N Y Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka A, Matsuo T, Shiraga F, Ohtsuki H. TIMP-1 production by human scleral fibroblast decreases in response to cyclic mechanical stretching. Ophthalmic Res. 2001;33:98–101. doi: 10.1159/000055651. [DOI] [PubMed] [Google Scholar]
- Young TL, Guo XD, King RA, Johnson JM, Rada JA. Identification of genes expressed in a human scleral cDNA library. Mol Vis. 2003;9:508–514. [PubMed] [Google Scholar]
- Young TL, Scavello GS, Paluru PC, Choi JD, Rappaport EF, Rada JA. Microarray analysis of gene expression in human donor sclera. Mol Vis. 2004;10:163–176. [PubMed] [Google Scholar]
- Zadnik K, Satariano WA, Mutti DO, Sholtz RI, Adams AJ. The effect of parental history of myopia on children's eye size. JAMA. 1994;271:1323–1327. [PubMed] [Google Scholar]
- Zhu X, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]