Abstract
The trabecular meshwork (TM) is located in the anterior segment of the eye and is responsible for regulating the outflow of aqueous humor. Increased resistance to aqueous outflow causes intraocular pressure to increase, which is the primary risk factor for glaucoma. TM cells reside on a series of fenestrated beams and sheets through which the aqueous humor flows to exit the anterior chamber via Schlemm’s canal. The outer trabecular cells are phagocytic and are thought to function as a pre-filter. However, most of the outflow resistance is thought to be from the extracellular matrix (ECM) of the juxtacanalicular region, the deepest portion of the TM, and from the inner wall basement membrane of Schlemm’s canal. It is becoming increasingly evident that the extracellular milieu is important in maintaining the integrity of the TM. Not only have ultrastructural changes been observed in the ECM of the TM in glaucoma, and a significant number of mutations in ECM genes are known to be associated with glaucoma, but the stiffness of glaucomatous TM appears to be greater than that of normal tissue. Additionally, TGFβ2 has been found to be elevated in the aqueous humor of glaucoma patients and is assumed to be involved in ECM changes deep with the juxtacanalicular region of the TM. This review summarizes the current literature on trabecular ECM as well as the development and function of the TM. Animal models and organ culture models targeting specific ECM molecules to investigate the mechanisms of glaucoma are described. Finally, the growing number of mutations that have been identified in ECM genes and genes that modulate ECM in humans with glaucoma are documented.
Keywords: Extracellular matrix, glaucoma, trabecular meshwork, intraocular pressure, gene mutations, animal models, perfusion culture
Trabecular Meshwork Cells and Extracellular Matrix
The trabecular meshwork (TM) is a series of fenestrated beams and sheets of extracellular matrix (ECM) covered with endothelial-like TM cells, which is immediately adjacent to Schlemm’s canal (SC) (Hogan et al., 1971). Aqueous humor continuously drains through the TM at approximately 2.75 µl/min to exit the anterior chamber of the eye (Brubaker, 1991). Aqueous humor inflow is relatively constant and pressure-insensitive up to high pressure levels, thus intraocular pressure (IOP) is regulated by the resistance to aqueous humor outflow (Acott and Kelley, 2008; Brubaker, 1970, 1975; Johnson, 2006; Johnson and Erickson, 2000). Most of the resistance to aqueous humor outflow is thought to be due to ECM within the deepest portion of the TM and the basement lamina of Schlemm’s canal (SC) inner wall endothelium (Acott and Kelley, 2008; Johnson, 2006).
TM cells have different functions and ECMs depending on location within the tissue. The ECM beams in the outer uveal and corneoscleral regions are normally covered with confluent layers of TM cells and contain relatively large intratrabecular spaces, which form a convoluted fluid flow pathway to the deeper layers (Gong et al., 1996; Hogan et al., 1971; Lutjen-Drecoll and Rohen, 1996). These TM cells maintain the collagen-elastic fibers embedded in a ground substance of proteoglycans, glycosaminoglycans (GAGs), non-fibrillar collagens and several families of extracellular glycoproteins, all of which comprise the trabecular beams (Acott and Kelley, 2008; Borras, 2003; Keller et al., 2009a; Lutjen-Drecoll, 1999; Lutjen-Drecoll et al., 1989; Lutjen-Drecoll and Rohen, 1996). The cells on these beams have a robust basement membrane, and long cellular projections often traverse adjacent beams (Gong et al., 2002; Gong et al., 1996; Grierson and Lee, 1974; Grierson et al., 1978; Johnstone, 1979; Johnstone and Grant, 1973). These outer beam cells are aggressively phagocytic and are thought to function as a pre-filter, removing debris from the aqueous humor (Buller et al., 1990; Sherwood and Richardson, 1988; Tamm, 2009). Recent studies have shown that phagocytic challenge of TM cells increases the expression and activity of ECM remodeling genes (Porter et al., 2013; Porter et al., 2012).
The deepest portion of the TM, called the juxtacanalicular (JCT) or cribriform region, is approximately 7–20 µm thick and abuts the inner wall endothelium of Schlemm’s canal (SC), hence the name juxtacanalicular (Gong et al., 1996; Keller and Acott, 2013). The JCT cells reside on basement membrane and are partially embedded in an amorphous and porous 3-D ECM with some areas exposed to the open spaces of the fluid egress pathways (Acott and Kelley, 2008; Fuchshofer et al., 2006; Keller and Acott, 2013). This mixed organization has produced some controversy about the endothelial-like vs. fibroblast-like nature of JCT cells. The cells touch each other via relatively long cellular processes and frequently contact the SC inner wall cells as well (Grierson and Lee, 1974; Grierson et al., 1978; Lutjen-Drecoll, 1999). The inner wall SC cells are endothelial, forming a monolayer with very tight junctions. Interestingly, the basement membrane of SC cells is often not continuous and the degree of coverage varies somewhat between species (Lutjen-Drecoll, 1999).
Gene expression differences between TM beam cells, JCT cells and SC inner wall cells have been reported (Fuchshofer et al., 2006; O'Brien et al., 2014; Perkumas and Stamer, 2012). Many ECM proteins are present throughout the TM including in the JCT and basal lamina of the SC inner wall. Collagens, elastic fiber components, proteoglycans, GAGs, fibronectin, matricellular proteins, and many others are found at different levels in all layers. Matrix and contractile genes were highly expressed in JCT cells compared to SC cells, while ICAM-1 and fibulin-1 were relatively enriched in SC cells (O'Brien et al., 2014). SC cells, but not TM, express PECAM-1 and VE-cadherin as well as collagens I, IV, laminins, and integrin α6 (Heimark et al., 2002; VanderWyst et al., 2011).
A number of reviews focused on various aspects of the ECM of normal TM/SC have appeared in recent years and these address numerous specific details of the outflow pathway, hence the reader is referred to these sources for additional understanding of the normal ECM (Acott and Kelley, 2008; Ethier, 2002; Filla et al., 2004; Hernandez and Gong, 1996; Johnson, 2006; Keller and Acott, 2013; Keller et al., 2009a; Lutjen-Drecoll, 1999; Tamm, 2009; Yue, 1996).
Development of the TM
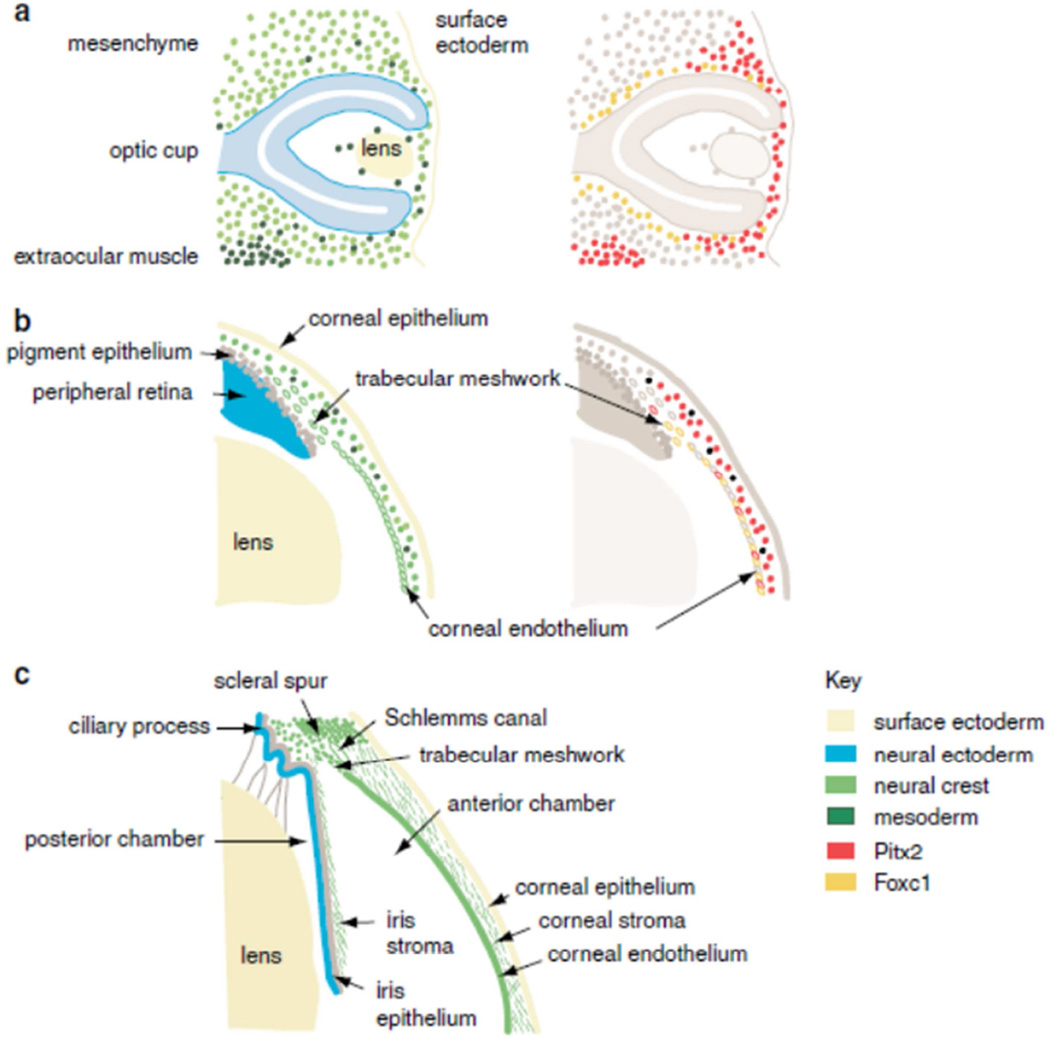
There are several studies that have investigated TM during embryonic development. When the anterior segment of the eye is developing, migrating cells from the neuroepithelium or the surface epithelium must interact with mesenchymal stem cells from the neural crest (Figure 1). Important to this process is the temporal and spatial coordination of transcription factor expression in conjunction with signaling pathways, various cytokines, and the synthesis and remodeling of unique ECM. When the required interaction does not occur, or does not occur at the appropriate time, complex developmental disorders may occur (Cvekl and Tamm, 2004). It is now thought that the migration of periocular mesenchymal cells, which partially or wholly develop into many structures of the anterior segment, occurs in three waves. The third and last migratory wave of periocular mesenchymal cells, which is not well characterized, is involved with the formation of eyelid stroma and TM (Kao et al., 2013). For a detailed review of both the embryological development of the TM and of anterior segment dysgenesis disorders, see (Cvekl and Tamm, 2004) and (Gage et al., 2005). Both neural crest and mesoderm are represented in the ocular mesenchyme (Figure 1). Early studies showed that TM cells were likely derived from the neural crest based on immunostaining with neuron-specific enolase antibodies (Tripathi and Tripathi, 1989). Fate mapping developmental studies later confirmed this by studying rats with a mutation in the transcription factor PAX6 (Matsuo et al., 1993). The anterior midbrain crest cells in these rats that did not migrate beyond the eye rudiments suggesting that the Pax-6 gene is involved in conducting migration of neural crest cells. In heterozygous PAX6 knockout mice, the anterior angle tissues do not differentiate and the mice do not develop an outflow pathway (Baulmann et al., 2002).
Figure 1. Development of the anterior segment of the eye.
(a) Optic cup stage, embryonic day 10.5 in the mouse equivalent to week 5 in human development. (b) Formation of anterior chamber, embryonic day 15.5 in the mouse equivalent to the 5th month of human gestation. (c) Mature anterior segment depicting the lens, iris, iridocorneal angle, the TM and the cornea. Key shows the color coding used to represent the embryonic origin of the anterior segment tissues in the right-hand plates, and the pattern of expression of the FOXC1 and PITX2 genes in the left-hand plates, based on published expression data. Reprinted with permission from (Sowden, 2007).
Other human developmental disorders that affect structures in the anterior segment of the eye are known as anterior segment dysgenesis disorders and include aniridia, Peters’ anomaly or Axenfeld-Rieger’s syndrome (Cvekl and Tamm, 2004; Ito and Walter, 2014; Sowden, 2007). Other groups have examined anterior segment dysgenesis in murine models (Gould and John, 2002). Understanding why these disorders occur during development has aided in elucidating the function and migration patterns of cells in the normal eye. One possibility is that transcription factors involved in the control of anterior eye morphogenesis modulate expression of signaling molecules. Mutant mouse model studies suggest that bone morphogenetic protein 4 (BMP4) and/or transforming growth factor-β (TGF-β) are directly involved in control of mesenchymal morphogenesis in the anterior segment of the eye (Chang et al., 2001; Ittner et al., 2005). The ciliary body, retinal pigment epithelium, and the iris of both embryonic and adult mouse eyes express BMP4. Haploinsufficient Bmp4 mice demonstrate various abnormalities of the ocular segment such as opacity of the cornea at the periphery, diffuse corneal haze, irregularly shaped pupils (iris), small or absent Schlemm’s canal, and hypoplastic or absent TM (Chang et al., 2001). BMP4 and OTX2 have also been implicated in TM development in humans since their absence by gene deletion causes microcornea (Takenouchi et al., 2013). Other transcription factors such as pituitary homeobox 2 (PITX2) and the Forkhead box C1 (FOXC1) are also critical to anterior segment development (Acharya et al., 2011). Mutations in these genes in humans lead to anterior segment dysgenesis, and approximately 50% of patients with these mutations will develop glaucoma. In humans, PITX2 or FOXC1 mutations during anterior eye development cause a wide variety of abnormalities with different specific clinical phenotypes (Ito and Walter, 2014). Collectively, these studies show that TM development is governed by a complex regulatory network of transcription factors and growth factors.
Although there are a number of known genes that affect TM development as described above, the exact effect of many of these mutations on ECM is not yet clear. Much of ECM development including timing, differentiation of the tissue, and molecular interactions in the ECM has also not been fully studied. Of interest, however, is an extracellular matrix-associated protein, peroxidasin (PXDN), with peroxidase catalytic activity, which has been localized to the cornea and lens epithelial layers (Khan et al., 2011). PXDN is critical in the normal development of the lens and cornea and evidence suggests that peroxidasin may have a functional role as an anti-oxidant in protecting lens, cornea, and TM from oxidative damage (Khan et al., 2011). In other systems, mammalian peroxidasin has been localized to the endoplasmic reticulum and is secreted into the extracellular space after TGF-β-induced differentiation of fibroblasts into myofibroblasts (Nelson et al., 1994). Here it forms part of a fibril-like network with fibronectin and other ECM proteins and has been suggested to be involved in consolidation of ECM, phagocytosis, and defense.
Function of the TM
The primary function of the TM is to regulate bulk aqueous humor outflow from the anterior chamber. To do this, resistance to outflow is generated in the TM. In response to sustained elevated pressure, the TM is modified to allow greater outflow in order to reduce the pressure. IOP homeostasis is therefore defined as “corrective adjustments of the aqueous humor outflow resistance that occur in direct response to sustained pressure changes to maintain IOP within acceptable physiological ranges” (Acott et al., 2014). This definition is primarily based on evidence from perfused human anterior segment organ culture and perfused ex vivo eyes. We and others have conducted studies showing that anterior segments in perfusion organ culture can sense pressure elevation and respond by adjusting the outflow resistance (Acott and Kelley, 2008; Borras et al., 2002; Bradley et al., 2001; Keller et al., 2009a; Keller et al., 2013). In existing ex vivo model systems, doubling the pressure has not been shown to have a significant immediate effect on outflow facility (Lei et al., 2011; Millar et al., 2011; Schneemann et al., 2003; Stamer et al., 2011). However, greater pressure increases have been shown to produce facility decreases that are large and fairly immediate (Battista et al., 2008; Grierson and Lee, 1975; Hann and Fautsch, 2009; Johnstone and Grant, 1973; Vittitow and Borras, 2004). This appears to be primarily due to changes in the physical conformation of the outflow pathway where the JCT/SC inner wall distends far out into and obstructs the SC. At higher pressures, particularly when sustained, the JCT/SC forms herniations into the collector channels (Battista et al., 2008). Although there are genetic and possibly environmental contributing factors in glaucoma, the loss of IOP homeostatic capability is clearly central to progression of the disease. The inability to maintain the outflow resistance within acceptable ranges leads to sustained IOP elevation and triggers glaucomatous optic nerve damage, which is why IOP elevation remains a leading risk factor for glaucoma.
The probable site of the outflow resistance is located within the deepest portion of the JCT and the SC inner wall basement membrane (Acott and Kelley, 2008; Ethier, 2002; Johnson, 2006; Stamer and Acott, 2012). Outflow resistance is thought to be comprised primarily of ECM (Acott and Kelley, 2008; Ethier, 2002; Fuchshofer and Tamm, 2009; Johnson, 2006; Overby et al., 2009; Tamm, 2009). Soluble extracellular proteoglycans with attached GAG chains contribute to the resistance to aqueous humor outflow (Tanihara et al., 2002). Recent studies suggest that the resistance may be primarily provided by the chondroitin sulfate GAG side chains of the large proteoglycan, versican (Keller et al., 2011). Putatively, this versican-generated outflow resistance resides within 5–10 µm of the basal surface of SC inner wall cells and is supported and positioned by hyaluronan and several other versican binding proteins (Acott and Kelley, 2008; Keller et al., 2011). Since the outflow pathway, including this deepest region, contains other proteoglycans and ECM molecules, numerous candidates for contribution to the outflow resistance remain viable options. If most of the IOP drop, i.e. 6–9 mmHg, occurs across the ECM of the JCT, then SC cells might contribute and control the final 0.5–2 mmHg of pressure drop by forcing funneling through SC cellular pores (Ethier, 2002; Johnson, 2006; Johnson et al., 1992; Overby et al., 2009).
It has been long known that aqueous outflow is not uniform around the circumference of the eye. Studies utilizing tracer particles including zymosan, latex microspheres, cationic ferritin, and fluorescent Qdots indicated that segmental variations in outflow exist.(Battista et al., 2008; Buller et al., 1990; de Kater et al., 1989; Ethier and Chan, 2001; Hann et al., 2005; Hann and Fautsch, 2009; Johnson et al., 1990; Keller et al., 2011; Lu et al., 2008; Lu et al., 2011) The TM can therefore be separated into regions of high and low outflow, which appears to be consistent across species (human, monkeys, bovine, mice). Logically, there must be molecular differences between the components of each region that either affect or reflect the geographical outflow pathways. It was previously observed that there was variability in the immunostaining patterns of fibronectin and HAbp-labeling of HA chains (Floyd et al., 1985; Keller et al., 2008), but at the time the studies were performed, these distributions were not correlated with tracer particle distribution. More recently, we investigated the distribution of versican, a chondroitin sulfate (CS)-bearing proteoglycan, in the TM of human and porcine eyes (Keller et al., 2011). An inverse relationship was found between versican levels and outflow that is high versican expression was observed in low outflow regions and low versican expression was found in areas of high outflow. The matricellular protein, SPARC (secreted protein acidic and rich in cysteine), appears to display segmental variations in expression (Vranka ARVO E-abstract #3566, 2013). SPARC-null mice showed a more uniform pattern of outflow than in wild-type mice as detected by the distribution of fluorescent microbeads (Swaminathan et al., 2013).
In addition to molecular differences, morphological differences may also exist to indicate regions of outflow. The IW cells of Schlemm’s canal have intracellular pores (I-pores), and intercellular “border” pores (B-pores) that allow fluid flow between IW cells (Bill and Svedbergh, 1972; Ethier, 2002; Ethier et al., 1998; Tamm, 2009). These pores are 0.1–2 µm in diameter and allow passage of 200–500 nm particles (Johnson et al., 1990). I-pores are more common than B-pores. Recent studies indicate that B pores correlate with regions of high outflow, while I-pores don’t, suggesting that B-pores might represent the normal passage of outflow in physiological pressure conditions (Ethier ARVO E-abstract #3538, 2013). Together, these studies suggest that morphological and molecular differences contribute to the outflow pathways of the TM. Further studies will be required to elucidate the exact identities of molecules in the JCT that reflect these outflow regions.
ECM Remodeling
Forces created by increased IOP likely produce mechanical stretching or distortion of the ECM and attached cells of the JCT and SC inner wall endothelium, which then initiate a cascade of events involving matrix metalloproteinase (MMP) activity (Acott and Kelley, 2008; Bradley et al., 2001; Bradley et al., 1998). We have shown that ECM turnover, initiated by MMPs in the TM, is necessary to maintain outflow facility (Bradley et al., 2001; Bradley et al., 1998; Keller et al., 2009b). Most importantly, specific inhibition of endogenous MMPs decreases outflow, an effect that was reversible. We recently proposed the concept of “maintenance remodeling”. In the TM, certain MMPs are highly expressed during resting conditions and these may function to maintain open outflow pathways (Keller and Acott, 2013). Mechanical stretching of TM cells, or increased perfusion pressure in anterior segment organ culture, was shown to increase MMP activity and trigger numerous changes in ECM protein expression levels (Acott and Kelley, 2008; Bradley et al., 2001; Bradley et al., 1998; Keller et al., 2009b; Keller et al., 2007; Vittitow and Borras, 2004). This process took hours to days to achieve, which is compatible with ECM remodeling and IOP homeostasis following pressure elevation (Keller et al., 2009a; Vittal et al., 2005; Vittitow and Borras, 2004; WuDunn, 2001). In addition to changes in gene expression, a number of ECM genes also show differences in mRNA splice forms with pressure or stretch (Acott and Kelley, 2008; Keller et al., 2009b; Keller et al., 2007; Vittitow and Borras, 2004). It is presumed that this replacement ECM would be slightly different in composition, organization, and/or amount, in order to maintain the modified outflow resistance.
The inner wall cells of Schlemm’s canal reside on a discontinuous basement membrane, which has been shown to consist of types I and IV collagens, laminin-511 and the α6 integrin subunit. This particular integrin-laminin interaction may provide adhesive support to secure SC cells to their basement membrane (VanderWyst et al., 2011). Strong cell-matrix adhesions within the inner wall are thought to be important to maintain a continuous barrier to fluid flow and likely include additional integrin pairs such as α5β1 and α6β1 (Tervo et al., 1995; Zhou et al., 1999) and/or syndecans-3 and −4 (Filla et al., 2004). These cell-ECM adhesions are likely involved in tethering the inner wall endothelium to the basement membrane and underlying ECM, and are presumably subjected to turnover as is required to maintain the health of the tissue in relation to flow (Overby et al., 2009). Cell-cell junctions at the inner wall involving Schlemm’s canal and/or JCT cells may also be involved in maintaining the outflow resistance. MMP activation may also be linked to SC cell-cell adhesions. However, it is unknown how cell adhesion strength is affected by cell overlap and dynamic junctional remodeling (Alexander and Elrod, 2002; Overby et al., 2009).
The ECM of the TM appears to be a source of latent growth factors and other small regulatory molecules, activity of which need to be tightly regulated. One function of the JCT ECM may be to sequester these active molecules in order to functionally isolate the outflow pathway channels from aberrant modification (Keller and Acott, 2013). Maintenance remodeling by MMPs may aid removal of bound molecules to prevent saturation of the ECM. If the ECM becomes saturated, such as in response to elevated pressure, the regulatory molecules would then gain access to the outflow channels and modify the resistance accordingly.
Although our focus has been mainly on the role of ECM turnover in IOP homeostasis, other processes may be involved. A direct cellular contribution appears to affect the outflow resistance in some manner that is currently only partially understood. Numerous cell-related studies have demonstrated an association of cytoskeletal tension and relaxation with direct or indirect cellular effects on outflow, including RhoA GTPase, nitric oxide, and eNOS (endothelial nitric oxide synthase) (Overby et al., 2009; Pattabiraman et al., 2007; Pattabiraman and Rao, 2010; Sanka et al., 2007; Tian et al., 2000; Zhang et al., 2008; Zhang and Rao, 2005). Cadherins, a proteinaceous component of adherens junctions, are linked to the cytoskeleton via β-catenin and focal adhesion kinase (FAK) (Quadri, 2012). FAK may mediate cross-talk between integrin-based focal adhesions and intercellular adherens junctions to regulate endothelial barrier function of SC inner wall cells. An additional complexity recently shown to be important in this system is the varied TM cellular behavior in response to the type and rigidity of the substratum (Last et al., 2011; Russell et al., 2008; Schlunck et al., 2008; Thomasy et al., 2012).
The ECM of TM compared to ECMs of other tissues
The ECM of TM provides a microenvironment with distinctive biomechanical properties that are essential for its biological function. TM cells express certain molecules at high levels such as MMP2, tenascin C, and α-smooth muscle actin (αSMA). These proteins are typically expressed at very low levels in normal adult tissues, but are highly expressed in tissues undergoing remodeling for example during development or disease. This suggests that TM cells may have evolved transient remodeling processes as their normal function in order to maintain open flow channels in the JCT (Keller and Acott, 2013). Expression of αSMA has led some researchers to draw analogies to fibrotic tissues (Pattabiraman et al., 2014). Fibrosis is defined by the excessive accumulation of collagens and other ECM components, which leads to the over-growth, scarring or hardening of tissues (Wight and Potter-Perigo, 2011). The ECM of glaucomatous TM shows some similarities with adult wound healing such as the accumulation of sheath-derived plaques (see below). Insights into the similarities between ECMs of normal and glaucomatous TM, scarless wound healing responses, which are characteristic of fetal wounds and adult oral mucosa, scarring responses and foreign body reactions have been recently reviewed (Keller and Acott, 2013).
ECM Changes with Glaucoma and Glaucoma Therapies
There have been numerous studies describing the overall structure of the JCT in both normal and glaucomatous eyes. TM cells in the JCT are surrounded by fibrillar elements of the ECM to form a loose, irregular network of connective tissue (Tamm, 2009; Ueda et al., 2002). Morphological and ultrastructural ECM changes of glaucomatous TM include changes in the elastic fiber network with the presence of thickened sheath-derived plaques (Lutjen-Drecoll, 1999, 2005; Lütjen-Drecoll et al., 1981; Tektas and Lutjen-Drecoll, 2009). Sheath-derived plaques were found to be more abundant in the TM of glaucomatous eyes compared with normal (Rohen et al., 1981).The plaques were later determined to consist of a core of elastic fibers surrounded by a sheath containing fibrillin-1 and microfibrillar associated protein-1 (MAGP-1) (Gong et al., 1989; Lutjen-Drecoll et al., 1986; Ueda et al., 2002). Extending from the sheath are connecting fibrils that link to SC endothelial cells and JCT cells (Fuchshofer et al., 2006; Hann and Fautsch, 2011). A recent report confirms the presence of elastic fiber components within the JCT and within the collector channel walls suggesting a role in the support and distensibility of that region of the TM (Hann and Fautsch, 2011). The presence of such elastic-fiber or microfibril-associated molecules within the TM and outflow pathway amidst a variety of other ECM components suggests a complex role of the elastic fiber system in outflow resistance and IOP regulation pointing to a potential convergence point between different classes of ECM molecules.
ECM changes are also observed in other glaucoma subtypes. SD plaques were highly increased and there was a large accumulation of basement membrane-like material in the JCT and subendothelial layer of SC in patients with juvenile glaucoma (Furuyoshi et al., 1997). In steroid-induced glaucoma, there is an apparent accumulation of type IV collagen and fibronectin in the outer TM beams, but not in the underneath the inner wall of SC (Tawara et al., 2008). For a more in-depth description and discussion of these and other ECM changes, the readers are referred to (Tektas and Lutjen-Drecoll, 2009).
Glaucoma therapies also change ECM composition. Laser trabeculoplasty (LTP) is a non-invasive surgical treatment that alleviates elevated IOP in glaucoma patients. Several changes in the ECM have been detected following LTP. For instance, LTP was shown to increase MMP3 expression in the human TM, particularly in the JCT and insert regions of the tissue (Parshley et al., 1996). Proteomic analysis of feline TM subjected to selective LTP (SLT) resulted in elevated glycosylation levels of proteins and increased amounts of the proteoglycans biglycan, keratocan and prolargin (PRELP) compared to non-lasered tissue (Amelinckx et al., 2009). Microarray analysis of human TM cells in culture subjected to SLT identified changes in expression levels of numerous ECM genes (Izzotti et al., 2011). In addition to direct changes on ECM molecules, several cytokines are elevated by LTP treatment including tumor necrosis factor-α (TNFα), interleukin-1α (IL-1α), IL-1β and IL-8 (Alvarado et al., 2005; Bradley et al., 2000). These cytokines induce MMP expression and/or activity to increase outflow rates in perfusion culture. Thus, LTP modulates the ECM of the TM both directly and indirectly.
Involvement of TGFβ2 in Glaucoma
Over the last 20 years, there has been considerable investigation of the possible relationship between TGFβ2 and glaucoma. Although the relationship is assumed to involve ECM changes that modify the aqueous humor outflow resistance deep within the JCT region of the TM, the pertinent details remain unclear. The finding that TGFβ2 is elevated in the aqueous humor of primary open-angle glaucoma (POAG) patients triggered much of this interest (Tripathi et al., 1994). Others verified this observation and subsequently it was found that TGFβ2 decreased outflow facility after several days’ treatment in perfused ex vivo anterior segment organ cultures (Fleenor et al., 2006; Gottanka et al., 2004). Many of the effects of TGFβ2 in other tissues are on ECM turnover and deposition so it also seems likely to be important in the glaucoma-TGFβ2 relationship. Since the ECM of the JCT region is thought to be a critical component of the outflow resistance in normal and glaucomatous eyes, an ECM effect of TGFβ2 seems plausible.
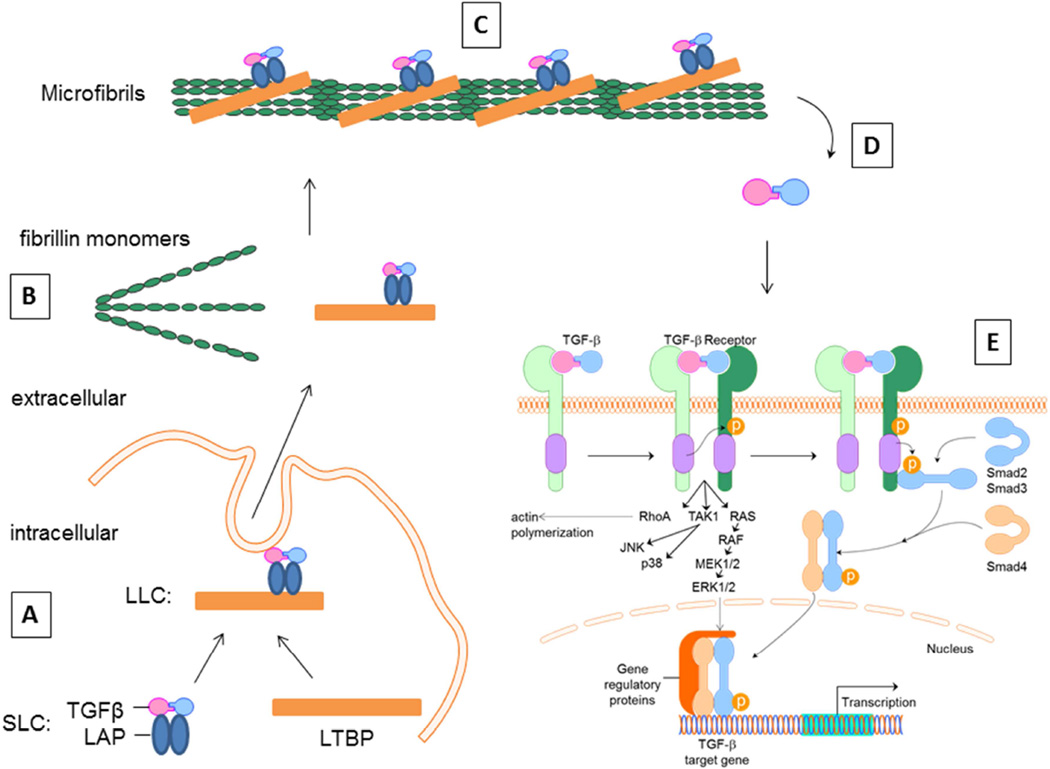
Elastic microfibrils of the ECM have been shown to be critically involved in the targeting, sequestration, and subsequent activation of latent TGFβ and bone morphogenetic protein (BMP) complexes (Figure 2) (Gregory et al., 2005; Horiguchi et al., 2012; Kuchtey and Kuchtey, 2014; Ramirez and Rifkin, 2009; Sengle et al., 2008; Sengle et al., 2011). Fibrillins are integral components of microfibrils that mediate ECM interactions with latent growth factor complexes and impart contextual specificity to TGFβ and BMP signaling (Ramirez and Rifkin, 2009; Ramirez and Sakai, 2010; Sengle et al., 2008). ECM elasticity modulates TGF-β-induced signaling and protein expression in human TM cells, reminiscent of patterns reported in glaucoma (Han et al., 2011). TGFβ superfamily prodomains have been shown to use a variety of mechanisms to target growth factors to the ECM and to control growth factor activation and signaling. BMPs are synthesized with a prodomain that confers latency to the active growth factor (Gregory et al., 2005). The mechanisms that modulate extracellular control of BMP bioavailability are less well understood, but may still require release from fibrillins to signal.
Figure 2. The role of the ECM in regulating TGFβ activation and signaling.
(A) TGFβ is produced as an inactive molecule that forms a noncovalent homodimeric complex with its own prodomain called the latency-associated peptide (LAP). LAP-TGFβ is referred to as the small latent complex (SLC). Latent TGFβ binding protein (LTBP) forms a covalent association with SLC prior to secretion. (B) The resulting large latent complex (LLC) is secreted and incorporated into the ECM during assembly, thereby preventing activation of the TGFβ molecule. (C) Transient interactions of the LLCs with fibronectin, and in the presence of heparan sulfate proteoglycans and integrins, facilitate the association of the LLC with fibrillin during microfibril assembly. The LLC is then anchored to the ECM thereby controlling TGFβ bioavailability. (D) Activation of TGFβ is thought to occur through proteolytic activity, thrombospondin or integrin binding to release the active TGFβ molecule from its prodomain. (E) Subsequent signaling occurs via binding of TGFβ receptors at the cell surface, followed by either intracellular SMAD-dependent or –independent pathways to affect TGFβ target genes. TGFβ family members, including the BMPs, follow similar synthesis, secretion and activation mechanisms. However, each individual member displays differences in their ECM binding partners, binding to cell surface receptors and activation of cell signaling pathways to induce their specific effects. Figure modified from (Horiguchi et al., 2012) and (Massam-Wu et al., 2010).
Studies of TGFβ2 signaling via both canonical and non-canonical pathways, bone morphogenetic protein (BMP)-family counter regulation, BMP antagonists like gremlin, and associated complex interactions have been described and reviewed in detail elsewhere (Fuchshofer and Tamm, 2012; Fuchshofer et al., 2007; Sethi et al., 2011a; Wordinger et al., 2007; Wordinger et al., 2014). One effect of TGFβ2 treatment is an increase in the matricellular protein, connective tissue growth factor (CTGF). There is a significant TM fibrotic phenotype in glaucoma, possibly driven by CTGF. CTGF induces matrix production and anti-CTGF treatment reduces ECM production in TM cells (Wallace et al., 2013). Interestingly, CTGF levels are increased in pseudoexfoliation glaucoma (Browne et al., 2011). Additional recent studies have highlighted the role of Rho GTPase signaling in regulating plasticity and fibrogenic activity in the TM as associated with consequent ECM effects of TGFβ and potential relationships to glaucoma (Pattabiraman et al., 2014). CTGF has also been shown to increase 24 hours after mechanical stretch (Vittal et al., 2005) or pressure elevation and other outflow enhancing agents suggestion a complex relationship between levels and outflow.
Potential mechanisms leading to ECM changes in glaucomatous TM
A prior study showed that treatment of TM cells with TGFβ2 induced all five lysyl oxidase (LOX) genes (Sethi et al., 2011b). Induction of these elastin and collagen cross-linking enzymes by TGFβ2 is likely associated with the pathological ECM changes observed in glaucomatous TM. Increases in cross-linking are consistent with the observation that glaucoma TM appears to be stiffer than age-matched normal TM (Last et al., 2011). Alternative explanations also exist. For instance, concentration of the GAG, hyaluronan, in the JCT region decreases with age and to a greater extent in diseased eyes (Knepper et al., 1996). We have shown that reduction of hyaluronan concentration not only decreases outflow rates in perfused human anterior segments, but it also reduces the levels of other ECM proteins, namely fibronectin and versican (Keller et al., 2012a; Keller et al., 2012b). Other GAGs may also affect the assembly and composition of the ECM. Chemical manipulation of sulfated GAG chains, either by inhibiting attachment of the GAGs to a protein backbone or by inhibiting sulfation, caused a greater colocalization of fibronectin with its binding partner tenascin C (Keller et al., 2008). Thus, altered GAG chain concentration or sulfation levels leads to anomalous interactions and levels of ECM molecules.
Another contributing factor to the accumulation of ECM may be altered endocytic recycling of ECM components. Following focal proteolytic degradation of ECM, partially-degraded fragments are endocytosed by cells and the fragments are either degraded intracellularly, or are recycled back into the ECM. Caveolins-1 and −2, principal components of caveolae lipid rafts, have been implicated in the endocytic recycling of fibronectin. Knockdown of CAV1 resulted in stabilization of fibronectin matrix fibrils, with a large decrease in fibronectin internalization and degradation (Hocking and Kowalski, 2002; Sottile and Chandler, 2005). Interestingly, a SNP identified between the CAV1 and CAV2 genes on chromosome 7q31 is associated with glaucoma (Loomis et al., 2014; Thorleifsson et al., 2010). We have shown that knockdown of CAV2 by application of shRNA lentivirus to human anterior segment perfusion cultures decreased outflow rates, whereas knockdown of CAV1 increased outflow rates (Aga et al., 2013 ARVO E-abstract 54:3560). Despite these opposite effects on outflow, we found that knockdown of either CAV resulted in increased MMP and ADAMTS4 levels and enzyme activities, fibronectin protein levels, actin stress fibers and α-smooth muscle actin (αSMA). Thus, caveolin-mediated endocytosis is one mechanism by which TM cells can alter the catabolism of ECM components and may contribute to the pathological changes that are observed in glaucomatous TM. A recent study showed that the archetypal glaucoma gene, myocilin, may also be involved in receptor-mediated endocytosis (McKay et al., 2013).
Focal proteolysis may unmask cryptic sites in ECM proteins. These sites are usually buried within the structure of the protein, but can become available for recognition following structural modifications in response to mechanical forces, proteolytic processing, or mutations (Davis et al., 2000). One example of cryptic site exposure in relation to glaucoma involves myocilin. Specific myocilin mutations were found to expose a cryptic peroxisomal targeting site, and the severity of these glaucoma-inducing mutations was correlated with the strength of interaction between myocilin and the peroxisomal targeting signal-1 receptor (Shepard et al., 2007).
Integrins are important receptors for cell adhesion to ECM proteins and provide critical connections between actin-mediated cell processes and the ECM (Gagen et al., 2014). The formation of cross-linked actin networks (CLANs) have been found in normal TM, but are more abundant in glaucomatous TM, and are thought to be particularly associated with steroid-induced glaucoma (Clark et al., 1994; Wordinger and Clark, 1999). Fibronectin-binding integrins found on the TM cell surface have been shown to be involved in CLAN formation, especially in response to glucocorticoids (Clark et al., 2013; Filla et al., 2011; Filla et al., 2009; Filla et al., 2006).
It was recently found that the ECM of glaucomatous TM was significantly stiffer than age-matched normal TMs (Last et al., 2011). This study used atomic force microscopy (AFM) to measure the Young’s modulus of dissected TM. Since these studies were on cadaver tissue that had been dissected for at least 2 weeks, this argues that the measurements were due to ECM compliance, rather than measuring cellular stiffness. Another recent study also measured TM stiffness, but used a different experimental approach: dissected whole TM’s were pulled in a lateral direction (Camras et al., 2014). The results showed an opposite effect where glaucomatous eyes were found to be 5-fold more compliant than normal eyes. One explanation for these apparently anomalous results may be that AFM measured stiffness of the JCT area, while the other study measured stretching of the whole TM including the beams of the corneoscleral meshwork. However, further studies are required to resolve these differences.
In cell culture, alterations in ECM rigidity influence TM cell behavior. Several groups have shown that TM cells cultured in hydrogels of varying stiffness ranging from normal in vivo stiffness (approx. 5–10 kPa) or mimicking glaucoma (approx. 70–80 kPa), result in differences in expression levels of various molecules including the appearance of actin stress fibers and αSMA, myocilin, alpha-B-crystallin, SPARC, ANGPTL-7, and transglutaminase-2 (TGM-2) (Han et al., 2011; Schlunck et al., 2008; Thomasy et al., 2012). Alterations in compliance as a tissue stiffens may be mediated by YAP and TAZ: translocation of these proteins from the cytoplasm to the nucleus modulates activity of transcription factors (Raghunathan et al., 2013). Expression levels of YAP and TAZ were inversely regulated by stiffness: YAP was down-regulated on stiffer substrates, while TAZ was up-regulated. These proteins differentially affect transcription of CTGF, TGFβ and TGM-2, which provides a direct link between alterations in tissue compliance with genes that are up-regulated in glaucoma (Morgan et al., 2013).
Glaucoma associated with mutations in ECM genes
Many human diseases are caused by mutations in ECM genes and glaucoma often manifests as part of the systemic disorder. Glaucoma is a heterogeneous group of diseases and most subtypes can be divided into 3 broad categories: open-angle (OAG), angle closure or congenital glaucoma. Here we review a select number of these genes with an emphasis on the glaucoma findings (Table 1).
Table 1.
Mutations in selected ECM and ECM modulating genes associated with glaucoma in humans.
| Gene Name | Glaucoma sub-type (if known) |
Other ocular defects | Associated Systemic Disease |
References |
|---|---|---|---|---|
| FBN1 | Primary open-angle glaucoma; Pigmentary glaucoma |
Ectopia lentis, myopia | Marfan syndrome, Weill-Marchesani syndrome |
Kuchtey, 2014; Kuchtey, 2013; Michael, 2012; Zhao, 2012; Faivre, 2003a, 2003b. |
| LTBP2 | Primary congenital glaucoma; Primary open-angle glaucoma; secondary glaucoma; pseudoexfoliation glaucoma |
Ali, 2009; Narooia-Nejad, 2009; Desir, 2010; Jelodari-Mamaghani, 2013; Krumbiegel, 2009. |
||
| LOXL1 | Pseudoexfoliation syndrome/glaucoma |
Thorleifsson, 2007; Schlotzer-Schrehardt, 2008, 2012. |
||
| ADAMTS10 | glaucoma | Ectopia lentis, myopia, spherophakia |
Weill-Marchesani syndrome |
Morales, 2009 |
| ADAMTS17 | glaucoma | Ectopia lentis, myopia, spherophakia |
Weill-Marchesani- like syndrome |
Morales, 2009 |
| COL2A1 | Open-angle glaucoma | Retinal detachment, severe myopia, anterior segment dysgenesis |
Stickler syndrome type 1 |
Tran-Viet, 2013; Snead, 2011 |
| COL8A1 | Primary open-angle glaucoma |
Thin central corneal thickness |
Desronvil, 2010 | |
| COL8A2 | Primary open-angle glaucoma |
Thin central corneal thickness |
Fuchs corneal dystrophy |
Desronvil, 2010 |
| COL11A1 | Primary angle closure glaucoma |
Stickler syndrome, Marshall syndrome |
Vithana, 2012 | |
| COL15A1 | Primary open-angle glaucoma |
Wiggs, 2013 | ||
| COL18A1 | Primary open-angle glaucoma |
Knobloch syndrome | Wiggs, 2013 | |
| Versican | Glaucoma neo- vascular glaucoma |
Vitreoretinopathy, empty vitreous cavity, retinal detachment, chorioretinal atrophy |
Wagner syndrome |
Kloeckner-Gruissem, 2006; Miyamoto, 2005; Mukhopadhyay, 2006. |
| BMP4 | Congenital glaucoma | Reigler, microcornea, nystagmus, anophthalmia; |
SHORT syndrome, microophthalmia |
Reis, 2011. |
| FGFR2 | Glaucoma | microcornea, limbal scleralization, corecoptia |
Pfeiffer syndrome | Barry, 2010. |
Mutations in the myocilin (MYOC) gene remain the most common genetic cause of glaucoma particularly juvenile open-angle glaucoma (Resch and Fautsch, 2009). Myocilin is secreted from the cell or can be released into the ECM in exosomes, small endosome-derived vesicles (Hardy et al., 2005). Several in vitro interaction experiments have evidenced the interaction of myocilin with various ECM molecules including fibronectin, laminin, decorin, and collagen types I, III, V and VI (Resch and Fautsch, 2009). Despite many years of research, the biological function of myocilin remains elusive, but it appears to affect cell adhesion and/or mitochondrial function (Borras, 2014; Sakai et al., 2007). Moreover, mutant myocilin tends to accumulate intracellularly.
There are mutations in multiple genes encoding elastic microfiber components that are associated with glaucoma (Kuchtey and Kuchtey, 2014). Fibrillin-1 mutations cause Marfan syndrome and ocular abnormalities include pigmentary glaucoma, ectopia lentis, myopia and glaucoma (Kuchtey et al., 2013; Micheal et al., 2012; Zhao et al., 2012). Fibrillin-1 mutations also cause Weill-Marchesani syndrome, which is another connective tissue disorder that includes severe myopia and glaucoma (Faivre et al., 2003a; Faivre et al., 2003b). ADAMTS10 is a member of the MMP family of proteolytic enzymes that interacts with fibrillin-1 (Kutz et al., 2011). Mutations in ADAMTS10 are associated with a dog model of POAG (Kuchtey et al., 2011), while mutations in ADAMTS10 and ADAMTS17 in humans cause myopia, ectopia lentis and glaucoma (Morales et al., 2009). Recent studies have shown that null mutations in latent transforming growth factor beta-binding protein 2 (LTBP2), which binds fibrillin microfibrils, cause primary congenital glaucoma, secondary glaucoma, POAG, or pseudoexfoliation glaucoma (Ali et al., 2009; Desir et al., 2010; Jelodari-Mamaghani et al., 2013; Krumbiegel et al., 2009; Narooie-Nejad et al., 2009). Lysyl oxidase-like 1 (LOXL1) mutations have been genetically linked to pseudoexfoliation syndrome/glaucoma (Pasutto et al., 2008; Schlotzer-Schrehardt et al., 2012; Schlotzer-Schrehardt et al., 2008; Thorleifsson et al., 2007).
Mutations in certain collagen genes have also been associated with glaucoma. Many of these mutations cause alterations in the characteristic Gly-X-Y triplet, which hinders assembly of individual collagen chains into stable trimeric fibrils. Stickler syndrome (STL) is a group of diseases caused by mutations in collagen types II, IX or XI (Snead et al., 2011). There is some phenotypic overlap with Wagner syndrome and Marshall syndrome. STL type 1 is primarily caused by mutations in the fibril-forming collagen type II gene (Tran-Viet et al., 2013). Mutations causing premature stop codons in exon 2 of COL2A1 lead to ocular-only phenotypes including retinal detachment and high myopia, but with few or no other systemic manifestations (Tran-Viet et al., 2013). The OAG in Stickler syndrome may be due to anterior segment dysgenesis or from secondary angle occlusion caused by retinal detachment and/or proliferative vitreoretinopathy (Snead et al., 2011). Collagen type XI mutations have also been associated with glaucoma. A recent GWAS study found significant association of a single nucleotide polymorphism in COL11A1 with primary angle-closure glaucoma (Vithana et al., 2012). Collagen VIII is a short-chain collagen expressed by endothelial cells and is composed of two alpha 1 chains and one alpha 2 chain. Collagen type VIII, alpha 2 (COL8A2) and collagen type VIII, alpha1 (COL8A1) gene variants are associated with POAG patients, who also exhibit thin central corneal thickness (Desronvil et al., 2010). Collagen XV is a fibril-associated collagen, and together with collagen XVIII, they are components of the basement membrane. The C-terminal non-collagenous regions of both of these molecules can be proteolytically released to generate the anti-angiogenic bioactive fragments restin (COLXV) and endostatin (COLXVIII) (Ricard-Blum, 2011). Genetic variations in collagen XV, alpha 1 (COL15A1) and collagen XVIII, alpha 1 (COL18A1) can modify the age of onset of both early and late onset POAG (Wiggs et al., 2013).
Versican is a large chondroitin sulfate-substituted proteoglycan composed of multiple domains (Wight, 2002). The globular N-terminal G1 domain interacts with hyaluronan and the C-terminal G3 domain has binding sites for multiple other ECM proteins. The G1 and G3 domains flank two large central domains, known as αGAG and βGAG, to which the chondroitin sulfate GAG chains attach. Alternative mRNA splicing of the exons encoding αGAG and βGAG leads to four mRNA transcripts. Each resulting protein product therefore contains a varying number of CS GAG chains, from 17–23 in the longest variant, to 0 for the shortest variant. Splice site mutations in versican have been shown to cause Wagner syndrome (Kloeckener-Gruissem et al., 2006; Miyamoto et al., 2005; Mukhopadhyay et al., 2006). Approximately 18% of Wagner syndrome patients also have glaucoma indicating a possible role for versican in glaucoma.
Mutations in genes that influence the ECM have also been identified. Mutations in the BMP4 gene were found to cause congenital glaucoma in a patient with SHORT syndrome (Reis et al., 2011). Also, a Pfeiffer syndrome patient with a Trp290Cys mutation in fibroblast growth factor receptor 2 (FGFR2) displayed several ocular abnormalities including microcornea, limbal scleralization, corecoptia and glaucoma (Barry et al., 2010). In other tissues, FGF signaling via FGFR2 profoundly affects ECM gene expression.
ECM manipulations that alter IOP in animal models
In recent years, focus has shifted onto animal models of glaucoma. A comprehensive summary of animal models, their mutations and glaucoma has been reviewed elsewhere (Bouhenni et al., 2012). In general, knockout mice fall into two broad categories: those which cause developmental abnormalities that are phenotypically distinct,(Gould and John, 2002) and those that show no overt outward signs of anatomical defects, but which develop glaucoma-like phenotypes postnatally. In the latter case, studies have required careful measurements of IOP over the lifetime of the mice in order to establish effects. In a few of these mice, axon loss in the optic nerve over time has also been demonstrated, which bolsters the argument that these animals are genuinely developing glaucoma. In this review, we will focus on select animal models that have targeted ECM genes.
Several studies have implicated ECM modulatory genes in causing developmental glaucoma, similar to the human disease Peter’s anomaly. For instance, heterozygous BMP4 mutant mice were found to have anterior dysgenesis and elevated IOP, leading to development of congenital glaucoma (Chang et al., 2001). Genetic ablation of the gene encoding Ext1, which is involved in the biosynthesis of the heparan sulfate GAG, led to altered TGFβ2 signaling and down-regulation of two transcription factors, FOXC1 and PITX2 (Iwao et al., 2009). In Ext1−/− mice, IOP was elevated due to iridocorneal angle dysgenesis.
MYOC gene ablation in a mouse model showed no apparent ocular phenotype suggesting that MYOC mutations appear to result in gain of function activity rather than loss of function (Kim et al., 2001). Overexpression of full-length myocilin in mice also did not cause any detrimental effects (Gould et al., 2004). Conversely, a mouse model harboring the human Tyr437His glaucoma-causing mutation displayed phenotypes consistent with glaucoma (Zhou et al., 2008). These animals developed moderate elevation of IOP, some loss of retinal ganglion cells (RGCs) and axonal degeneration in the optic nerve head.
Mutations in collagen genes in mice display phenotypes consistent with glaucoma. A targeted mutation in collagen type I, which renders the molecule resistant to proteolytic cleavage by MMPs-1 and −2, was found to increase IOP in mice suggesting an association between IOP regulation and collagen turnover (Aihara et al., 2003). These mice were subsequently found to have progressive axon loss (Mabuchi et al., 2004). Aca23 mutant mice were found to contain a G770A point mutation in the COL8A2 gene (Puk et al., 2009). The mutation was mapped to a non-synonymous change, G257D, located at the critical glycine residue of the triplet, Gly-X-Y. Despite other ocular anomalies including enlarged eyes and reduced size of the corneal epithelium, stroma and Descemet’s membrane, these mice did not develop elevated IOP (Steinhart et al., 2012). Mutant mice containing a deletion in the COL4A1 gene have also been studied in relation to glaucoma (Kuo et al., 2012). These mice have a mutation in the splice acceptor site of exon 40, which leads to loss of 17 amino acids in the triple-helical domain and impaired secretion of COL4A1/COL4A2 heterotrimeric chains. IOPs in these mice were significantly different than wild-type littermates, but were variable in that some individuals had elevated pressures, while others displayed lowered IOPs (Gould et al., 2007). A mouse model of Stickler syndrome was generated by targeted heterozygous inactivation of COL2A1 and the mice had abnormalities in the ciliary body, lens and vitreous, but differed from the human disease in that there was no difference in the rate of retinal detachment (Kaarniranta et al., 2006). Thus, many of these mutant mice harboring collagen mutations have phenotypes consistent with human ocular abnormalities.
Several mouse models targeting elastic fiber components have also been studied. A mouse lacking LOXL was developed (Wiggs et al., 2014). Although the mice displayed some phentotypes similar to exfoliation syndrome, IOP was similar between wild-type and knockout mice and they did not develop glaucoma. Recent analyses of two mouse strains harboring fibrillin-1 mutations, a targeted C1039G mutation and the tight skin mutation, revealed decreased outflow facility, but no change in IOP (Kuchtey et al., 2014 ARVO abstract #3804). Both of these mouse strains had retinal damage consistent with glaucoma suggesting that fibrillin-1 deficiencies may lead to low tension glaucoma.
Mouse models targeting matricellular genes, including SPARC, thrombospondins-1 and −2, tenascins-C and –X, hevin and osteopontin, have been investigated (Chatterjee et al., 2014; Chowdhury et al., 2011; Haddadin et al., 2009; Haddadin et al., 2012; Kang et al., 2011; Keller et al., 2013). Of these, gene knockout of SPARC and both thrombospondins significantly lowered IOP in mice, while ablation of the other genes had minimal effects on IOP. Segmental outflow was more uniform in SPARC null mice suggesting a modulatory role for SPARC in IOP regulation (Swaminathan et al., 2013). There are many more matricellular proteins in the ECM of the TM, and knockout of their proteins and the effects on IOP have yet to be studied.
MMP9-deficient mice were found to have increased IOP compared to wild-type littermates (Robertson et al., 2013). Imbalances in ECM turnover profoundly affect the outflow pathways and can result in elevated IOP.
Application of virus to manipulate ECM in animal models
The use of adenovirus and lentivirus to transduce TM cells by an intracameral injection of virus into the eye are becoming increasingly popular in the quest to develop new therapeutic gene therapy solutions to elevated IOP. These studies were summarized in a recent review article (Borras, 2012). An adenovirus containing the MMP1 gene under the regulation of glucocorticoid response elements (Ad.GRE.MMP1) was found to reduce IOP in a steroid-induced elevated IOP sheep model.(Gerometta et al., 2010) Other adenoviruses that have been applied to animal models include secreted frizzled-related protein-1 (SFRP-1) (Wang et al., 2008), BMP2 (Buie et al., 2013), bioactivated human TGFβ2 (Shepard et al., 2010), active TGFβ1 (Robertson et al., 2010) and full-length or the soluble ectodomain of CD44 (Giovingo et al., 2013). All of these induced elevated IOP in mouse and/or rat eyes. Over-expression of the serine protease tissue plasminogen activator by adenovirus was found to reduce and reverse elevated IOP in steroid-induced glaucoma in mice and sheep (Gerometta et al., 2013; Kumar et al., 2013). Cochlin was identified as a protein that was up-regulated in glaucoma trabecular meshwork using proteomics (Bhattacharya et al., 2005). Recently, over-expression of cochlin using a lentiviral vector in DBA/2J mice, which exhibit age-related progressive glaucoma, increased IOP, whereas silencing cochlin via shRNA lentivirus decreased IOP (Goel et al., 2012).
ECM manipulations that alter outflow resistance in anterior segment perfusion culture
Anterior segment perfusion culture is the most widely adopted in vitro model of measuring outflow facility (Erickson-Lamy et al., 1991; Johnson and Tschumper, 1987). Many laboratories have used this system to investigate the effects of various agents on outflow. Two different model systems exist: one uses constant pressure and measures the effect on outflow rates, while the other uses a pump to produce constant flow and measures changes in pressure. Both systems are compatible with various species including human, monkey, bovine, and porcine eyes. To set up, eyes are bisected and the lens, iris, and ciliary body are removed to leave the cornea, trabecular meshwork and a thin rim of sclera to aid in clamping into the perfusion chamber. Serum-free medium is perfused. After a period of stabilization, usually overnight, agents are added to the perfusate and changes in outflow rates or pressures are measured for a period of hours to days.
Perfusion experiments have investigated the effects of the addition of exogenous proteolytic enzymes (e.g. MMPs, ADAMTS4) or their inhibitors, which results in an increase or decrease on outflow, respectively (Bradley et al., 1998; Keller et al., 2009b). Over-expression of MMP in a steroid-inducible adenovirus also increased outflow in perfused anterior segments (Spiga and Borras, 2010). Other groups have studied the effects of adding exogenous ECM proteins to perfusion culture. Application of purified recombinant HepII domain of fibronectin, a region that encompasses fibronectin type III repeats 12–14 of the full-length fibronectin molecule, increased outflow facility in human eyes (Santas et al., 2003). The HepII domain binds heparin with high affinity as well as containing a α4β1 integrin binding site (Faralli et al., 2009). While fibronectin is unlikely to comprise a component of the outflow resistance itself, it is likely to organize the resistance components in the outflow channels to maintain aqueous flow and pressures within physiological ranges. Consistent with the increased IOP in SPARC knockout mice, over-expression of SPARC in an adenovirus in human perfused anterior segments reduced outflow facility (Oh et al., 2013). Perfusion of recombinant myocilin in human anterior segments increased outflow resistance (Fautsch et al., 2006), while adenoviral overexpression of myocilin lacking the N-terminus and a portion of the olfactomedin domain had the opposite effect (Caballero et al., 2000). Relationships between Rho GTPases and TM ECM synthesis have also been shown (Pattabiraman and Rao, 2010).
Targeting glycosaminoglycan (GAG) sugar chains has been achieved using a variety of methods: (1) enzymes to specifically degrade each class of GAG (Barany and Scotchbrook, 1954; Gum et al., 1992; Hubbard et al., 1997; Knepper et al., 1984; Sawaguchi et al., 1993; Sawaguchi et al., 1992); (2) chemical agents that inhibit attachment to the protein backbone (β-xyloside), sulfate inhibition (sodium chlorate) or decrease mRNA of the biosynthetic genes and deplete cellular concentrations of the sugar monosaccharides (4MU) (Keller et al., 2008; Keller et al., 2012b); and (3) lentivirus to silence genes in the GAG biosynthetic pathway (Keller et al., 2011; Keller et al., 2012b). All of these treatments affected outflow either in human and/or pig anterior segments, but interestingly, some treatments caused opposite effects between species. This suggests anatomical differences in the ECM composition or organization that governs outflow resistance. Other studies have investigated the application of exogenous GAGs on IOP. Both single and chronic intracameral injections of chondroitin sulfate (CS) into the anterior chamber of rats led to a significant increase in IOP (Belforte et al., 2010).
Conclusions and Areas of Future Investigation
The ECM has profound effects on TM and SC cellular behavior and TM and SC cells make and organize this ECM in highly regulated patterns. Thus, cells and their ECM are inextricably linked and alterations in one compartment affect the other. Since much of the outflow resistance appears to be due to the ECM of the deepest portion of the JCT and SC inner wall basement membranes, understanding the components of the ECM, their structural organization and their regulation is of high significance. However, many questions remain unanswered. The molecular differences between high and low outflow regions as impacted by agents that change outflow facility seems to be of particular future importance. Verification of the role of versican and other related molecules to providing the actual outflow resistance is also critical since it is difficult to understand an entity like outflow resistance without knowing the molecular components. Furthermore, teasing out the interactions of cells and various specific ECM molecules and cell-cell communication is of high relevance for understanding the outflow resistance and how it is normally regulated. This is essential in order to design future glaucoma therapies. The ideal future glaucoma treatment would be one that restored the normal IOP homeostatic regulation of the outflow resistance.
Research Highlights.
-
-
Development and function of the trabecular meshwork are described in detail
-
-
Involvement of TGFβ and the extracellular matrix in glaucoma are discussed
-
-
Extracellular matrix changes with glaucoma, including compliance of the trabecular meshwork
-
-
Mutations in extracellular matrix genes associated with glaucoma in humans are detailed here
-
-
Organ culture and animal models used to study intraocular pressure and extracellular matrix are discussed
Acknowledgments
Supported by a Bright Focus National Glaucoma Research Award G2014058 (JAV), National Institute of Health grants EY021800 (MJK), EY003279, EY008247, EY010572 (TSA), EY019643 (KEK), a Sybil B. Harrington Special Scholar Award from Research to Prevent Blindness (KEK), and by an unrestricted grant to the Casey Eye Institute from Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: J. Vranka, none; M. Kelley, none; T. Acott, none; K. Keller, none.
References
- Acharya M, Huang L, Fleisch VC, Allison WT, Walter MA. A complex regulatory network of transcription factors critical for ocular development and disease. Hum Mol Genet. 2011;20:1610–1624. doi: 10.1093/hmg/ddr038. [DOI] [PubMed] [Google Scholar]
- Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM. Intraocular Pressure Homeostasis: Maintaining Balance in a High-Pressure Environment. J Ocul Pharmacol Ther. 2014 doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, Weinreb RN. Ocular hypertension in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2003;44:1581–1585. doi: 10.1167/iovs.02-0759. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat. 2002;200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009;84:664–671. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JA, Yeh RF, Franse-Carman L, Marcellino G, Brownstein MJ. Interactions between endothelia of the trabecular meshwork and of Schlemm’s canal: a new insight into the regulation of aqueous outflow in the eye. Trans Am Ophthalmol Soc. 2005;103:148–162. [PMC free article] [PubMed] [Google Scholar]
- Amelinckx A, Castello M, Arrieta-Quintero E, Lee T, Salas N, Hernandez E, Lee RK, Bhattacharya SK, Parel JM. Laser trabeculoplasty induces changes in the trabecular meshwork glycoproteome: a pilot study. J Proteome Res. 2009;8:3727–3736. doi: 10.1021/pr900294g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany EH, Scotchbrook S. Influence of testicular hyaluronidase on the resistance to flow through the angle of the anterior chamber. Acta Physiol Scand. 1954;30:240–248. doi: 10.1111/j.1748-1716.1954.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Barry GP, Ny BM, Zackai EH, Grunwald L, Forbes BJ. A case report of a patient with Pfeiffer syndrome, an FGRF 2 mutation (Trp290Cys) and unique ocular anterior segment findings. Ophthalmic Genet. 2010;31:193–195. doi: 10.3109/13816810.2010.505225. [DOI] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte N, Sande P, de Zavalia N, Knepper PA, Rosenstein RE. Effect of chondroitin sulfate on intraocular pressure in rats. Invest Ophthalmol Vis Sci. 2010;51:5768–5775. doi: 10.1167/iovs.10-5660. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Rockwood EJ, Smith SD, Bonilha VL, Crabb JS, Kuchtey RW, Robertson NG, Peachey NS, Morton CC, Crabb JW. Proteomics reveal Cochlin deposits associated with glaucomatous trabecular meshwork. J Biol Chem. 2005;280:6080–6084. doi: 10.1074/jbc.M411233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm--an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol (Copenh) 1972;50:295–320. doi: 10.1111/j.1755-3768.1972.tb05954.x. [DOI] [PubMed] [Google Scholar]
- Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003;22:435–463. doi: 10.1016/s1350-9462(03)00018-1. [DOI] [PubMed] [Google Scholar]
- Borras T. Advances in glaucoma treatment and management: gene therapy. Invest Ophthalmol Vis Sci. 2012;53:2506–2510. doi: 10.1167/iovs.12-9483o. [DOI] [PubMed] [Google Scholar]
- Borras T. The effects of myocilin expression on functionally relevant trabecular meshwork genes: a mini-review. J Ocul Pharmacol Ther. 2014;30:202–212. doi: 10.1089/jop.2013.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras T, Rowlette LL, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002;43:33–40. [PubMed] [Google Scholar]
- Bouhenni RA, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. J Biomed Biotechnol. 2012;2012:692609. doi: 10.1155/2012/692609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JM, Anderssohn AM, Colvis CM, Parshley DE, Zhu XH, Ruddat MS, Samples JR, Acott TS. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNFalpha. Invest Ophthalmol Vis Sci. 2000;41:422–430. [PubMed] [Google Scholar]
- Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- Browne JG, Ho SL, Kane R, Oliver N, Clark AF, O’Brien CJ, Crean JK. Connective Tissue Growth Factor is increased in Pseudoexfoliation Glaucoma. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-5209. [DOI] [PubMed] [Google Scholar]
- Brubaker RF. The measurement of pseudofacility and true facility by constant pressure perfusion in the normal rhesus monkey eye. Invest Ophthalmol. 1970;9:42–52. [PubMed] [Google Scholar]
- Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol. 1975;14:286–292. [PubMed] [Google Scholar]
- Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Invest Ophthalmol Vis Sci. 1991;32:3145–3166. [PubMed] [Google Scholar]
- Buie LK, Karim MZ, Smith MH, Borras T. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest Ophthalmol Vis Sci. 2013;54:5441–5455. doi: 10.1167/iovs.13-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller C, Johnson DH, Tschumper RC. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci. 1990;31:2156–2163. [PubMed] [Google Scholar]
- Caballero M, Rowlette LL, Borras T. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim Biophys Acta. 2000;1502:447–460. doi: 10.1016/s0925-4439(00)00068-5. [DOI] [PubMed] [Google Scholar]
- Camras LJ, Stamer WD, Epstein D, Gonzalez P, Yuan F. Circumferential tensile stiffness of glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55:814–823. doi: 10.1167/iovs.13-13091. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Peters M, Savinova OV, Hawes NL, Zabaleta A, Nusinowitz S, Martin JE, Davisson ML, Cepko CL, Hogan BL, John SW. Haploinsufficient Bmp4 ocular phenotypes include anterior segment dysgenesis with elevated intraocular pressure. BMC Genet. 2001;2:18. doi: 10.1186/1471-2156-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Villarreal G, Jr, Rhee DJ. Matricellular Proteins in the Trabecular Meshwork: Review and Update. J Ocul Pharmacol Ther. 2014;30:447–463. doi: 10.1089/jop.2014.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury UR, Jea SY, Oh DJ, Rhee DJ, Fautsch MP. Expression profile of the matricellular protein osteopontin in primary open-angle glaucoma and the normal human eye. Invest Ophthalmol Vis Sci. 2011;52:6443–6451. doi: 10.1167/iovs.11-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- Clark R, Nosie A, Walker T, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013;12:194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kater AW, Melamed S, Epstein DL. Patterns of aqueous humor outflow in glaucomatous and nonglaucomatous human eyes. A tracer study using cationized ferritin. Arch Ophthalmol. 1989;107:572–576. doi: 10.1001/archopht.1989.01070010586035. [DOI] [PubMed] [Google Scholar]
- Desir J, Sznajer Y, Depasse F, Roulez F, Schrooyen M, Meire F, Abramowicz M. LTBP2 null mutations in an autosomal recessive ocular syndrome with megalocornea, spherophakia, and secondary glaucoma. Eur J Hum Genet. 2010;18:761–767. doi: 10.1038/ejhg.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desronvil T, Logan-Wyatt D, Abdrabou W, Triana M, Jones R, Taheri S, Del Bono E, Pasquale LR, Olivier M, Haines JL, Fan BJ, Wiggs JL. Distribution of COL8A2 and COL8A1 gene variants in Caucasian primary open angle glaucoma patients with thin central corneal thickness. Mol Vis. 2010;16:2185–2191. [PMC free article] [PubMed] [Google Scholar]
- Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused human ocular anterior segment. Exp Eye Res. 1991;52:723–731. doi: 10.1016/0014-4835(91)90024-9. [DOI] [PubMed] [Google Scholar]
- Ethier CR. The inner wall of Schlemm’s canal. Exp Eye Res. 2002;74:161–172. doi: 10.1006/exer.2002.1144. [DOI] [PubMed] [Google Scholar]
- Ethier CR, Chan DW. Cationic ferritin changes outflow facility in human eyes whereas anionic ferritin does not. Invest Ophthalmol Vis Sci. 2001;42:1795–1802. [PubMed] [Google Scholar]
- Ethier CR, Coloma FM, Sit AJ, Johnson M. Two pore types in the inner-wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:2041–2048. [PubMed] [Google Scholar]
- Faivre L, Dollfus H, Lyonnet S, Alembik Y, Megarbane A, Samples J, Gorlin RJ, Alswaid A, Feingold J, Le Merrer M, Munnich A, Cormier-Daire V. Clinical homogeneity and genetic heterogeneity in Weill-Marchesani syndrome. Am J Med Genet A. 2003a;123A:204–207. doi: 10.1002/ajmg.a.20289. [DOI] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003b;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli JA, Schwinn MK, Gonzalez JM, Jr, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Exp Eye Res. 2009;88:689–693. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Vrabel AM, Howell KG, Loewen N, Teo WL, Poeschla EM, Johnson DH. Perfusion of his-tagged eukaryotic myocilin increases outflow resistance in human anterior segments in the presence of aqueous humor. Invest Ophthalmol Vis Sci. 2006;47:213–221. doi: 10.1167/iovs.05-0334. [DOI] [PubMed] [Google Scholar]
- Filla MS, David G, Weinreb RN, Kaufman PL, Peters DM. Distribution of syndecans 1–4 within the anterior segment of the human eye: expression of a variant syndecan-3 and matrix-associated syndecan-2. Exp Eye Res. 2004;79:61–74. doi: 10.1016/j.exer.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Filla MS, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves beta3 integrin signaling. Invest Ophthalmol Vis Sci. 2011;52:2952–2959. doi: 10.1167/iovs.10-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Schwinn MK, Sheibani N, Kaufman PL, Peters DM. Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct beta1 and beta3 integrin pathways. Invest Ophthalmol Vis Sci. 2009;50:5723–5731. doi: 10.1167/iovs.08-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filla MS, Woods A, Kaufman PL, Peters DM. Beta1 and beta3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:1956–1967. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- Floyd BB, Cleveland PH, Worthen DM. Fibronectin in human trabecular drainage channels. Invest Ophthalmol Vis Sci. 1985;26:797–804. [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009;88:683–688. doi: 10.1016/j.exer.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E, Birke M. Biochemical and morphological analysis of basement membrane component expression in corneoscleral and cribriform human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:794–801. doi: 10.1167/iovs.05-0292. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Furuyoshi N, Furuyoshi M, Futa R, Gottanka J, Lutjen-Drecoll E. Ultrastructural changes in the trabecular meshwork of juvenile glaucoma. Ophthalmologica. 1997;211:140–146. doi: 10.1159/000310781. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gagen D, Faralli JA, Filla MS, Peters DM. The role of integrins in the trabecular meshwork. J Ocul Pharmacol Ther. 2014;30:110–120. doi: 10.1089/jop.2013.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerometta R, Kumar S, Shah S, Alvarez L, Candia O, Danias J. Reduction of steroid-induced intraocular pressure elevation in sheep by tissue plasminogen activator. Invest Ophthalmol Vis Sci. 2013;54:7903–7909. doi: 10.1167/iovs.13-12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerometta R, Spiga MG, Borras T, Candia OA. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Invest Ophthalmol Vis Sci. 2010;51:3042–3048. doi: 10.1167/iovs.09-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovingo M, Nolan M, McCarty R, Pang IH, Clark AF, Beverley RM, Schwartz S, Stamer WD, Walker L, Grybauskas A, Skuran K, Kuprys PV, Yue BY, Knepper PA. sCD44 overexpression increases intraocular pressure and aqueous outflow resistance. Mol Vis. 2013;19:2151–2164. [PMC free article] [PubMed] [Google Scholar]
- Goel M, Sienkiewicz AE, Picciani R, Wang J, Lee RK, Bhattacharya SK. Cochlin, intraocular pressure regulation and mechanosensing. PLoS One. 2012;7:e34309. doi: 10.1371/journal.pone.0034309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Ruberti J, Overby D, Johnson M, Freddo TF. A new view of the human trabecular meshwork using quick-freeze, deep-etch electron microscopy. Exp Eye Res. 2002;75:347–358. [PubMed] [Google Scholar]
- Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech. 1996;33:336–367. doi: 10.1002/(SICI)1097-0029(19960301)33:4<336::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gong HY, Trinkaus-Randall V, Freddo TF. Ultrastructural immunocytochemical localization of elastin in normal human trabecular meshwork. Curr Eye Res. 1989;8:1071–1082. doi: 10.3109/02713688908997400. [DOI] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Gould DB, John SW. Anterior segment dysgenesis and the developmental glaucomas are complex traits. Hum Mol Genet. 2002;11:1185–1193. doi: 10.1093/hmg/11.10.1185. [DOI] [PubMed] [Google Scholar]
- Gould DB, Marchant JK, Savinova OV, Smith RS, John SW. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum Mol Genet. 2007;16:798–807. doi: 10.1093/hmg/ddm024. [DOI] [PubMed] [Google Scholar]
- Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bachinger HP, Sakai LY. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem. 2005;280:27970–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. Junctions between the cells of the trabecular meshwork. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192:89–104. doi: 10.1007/BF00410696. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (2) Pressures outside the physiological range (0 and 50 mmHg) Exp Eye Res. 1975;20:523–530. doi: 10.1016/0014-4835(75)90219-5. [DOI] [PubMed] [Google Scholar]
- Grierson I, Lee WR, Abraham S, Howes RC. Associations between the cells of the walls of Schlemm’s canal. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;208:33–47. doi: 10.1007/BF00406980. [DOI] [PubMed] [Google Scholar]
- Gum GG, Samuelson DA, Gelatt KN. Effect of hyaluronidase on aqueous outflow resistance in normotensive and glaucomatous eyes of dogs. Am J Vet Res. 1992;53:767–770. [PubMed] [Google Scholar]
- Haddadin RI, Oh DJ, Kang MH, Filippopoulos T, Gupta M, Hart L, Sage EH, Rhee DJ. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009;50:3771–3777. doi: 10.1167/iovs.08-2489. [DOI] [PubMed] [Google Scholar]
- Haddadin RI, Oh DJ, Kang MH, Villarreal G, Jr, Kang JH, Jin R, Gong H, Rhee DJ. Thrombospondin-1 (TSP1)-Null and TSP2-Null Mice Exhibit Lower Intraocular Pressures. Invest Ophthalmol Vis Sci. 2012;53:6708–6717. doi: 10.1167/iovs.11-9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Wecker T, Grehn F, Schlunck G. Elasticity-dependent modulation of TGF-beta responses in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:2889–2896. doi: 10.1167/iovs.10-6640. [DOI] [PubMed] [Google Scholar]
- Hann CR, Bahler CK, Johnson DH. Cationic ferritin and segmental flow through the trabecular meshwork. Invest Ophthalmol Vis Sci. 2005;46:1–7. doi: 10.1167/iovs.04-0800. [DOI] [PubMed] [Google Scholar]
- Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50:1692–1697. doi: 10.1167/iovs.08-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann CR, Fautsch MP. The elastin fiber system between and adjacent to collector channels in the human juxtacanalicular tissue. Invest Ophthalmol Vis Sci. 2011;52:45–50. doi: 10.1167/iovs.10-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KM, Hoffman EA, Gonzalez P, McKay BS, Stamer WD. Extracellular trafficking of myocilin in human trabecular meshwork cells. J Biol Chem. 2005;280:28917–28926. doi: 10.1074/jbc.M504803200. [DOI] [PubMed] [Google Scholar]
- Heimark RL, Kaochar S, Stamer WD. Human Schlemm’s canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1. Curr Eye Res. 2002;25:299–308. doi: 10.1076/ceyr.25.5.299.13495. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Gong H. Extracellular matrix of the trabecular meshwork and optic nerve head. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. 2nd ed. Mosby, St Louis: 1996. pp. 213–250. [Google Scholar]
- Hocking DC, Kowalski K. A cryptic fragment from fibronectin’s III1 module localizes to lipid rafts and stimulates cell growth and contractility. J Cell Biol. 2002;158:175–184. doi: 10.1083/jcb.200112031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Alvarado J, Weddell JE. Histology of the human eye: an atlas and textbook. Saunders: The University of Michigan; 1971. [Google Scholar]
- Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-beta function. J Biochem. 2012;152:321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard WC, Johnson M, Gong H, Gabelt BT, Peterson JA, Sawhney R, Freddo T, Kaufman PL. Intraocular pressure and outflow facility are unchanged following acute and chronic intracameral chondroitinase ABC and hyaluronidase in monkeys. Exp Eye Res. 1997;65:177–190. doi: 10.1006/exer.1997.0319. [DOI] [PubMed] [Google Scholar]
- Ito YA, Walter MA. Genomics and anterior segment dysgenesis: a review. Clin Experiment Ophthalmol. 2014;42:13–24. doi: 10.1111/ceo.12152. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, Sommer L. Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol. 2005;4:11. doi: 10.1186/jbiol29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao K, Inatani M, Matsumoto Y, Ogata-Iwao M, Takihara Y, Irie F, Yamaguchi Y, Okinami S, Tanihara H. Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF-beta2 signaling. J Clin Invest. 2009;119:1997–2008. doi: 10.1172/JCI38519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Longobardi M, Cartiglia C, Rathschuler F, Sacca SC. Trabecular meshwork gene expression after selective laser trabeculoplasty. PLoS One. 2011;6:e20110. doi: 10.1371/journal.pone.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelodari-Mamaghani S, Haji-Seyed-Javadi R, Suri F, Nilforushan N, Yazdani S, Kamyab K, Elahi E. Contribution of the latent transforming growth factor-beta binding protein 2 gene to etiology of primary open angle glaucoma and pseudoexfoliation syndrome. Mol Vis. 2013;19:333–347. [PMC free article] [PubMed] [Google Scholar]
- Johnson DH, Tschumper RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987;28:945–953. [PubMed] [Google Scholar]
- Johnson M. What controls aqueous humour outflow resistance? Exp Eye Res. 2006;82:545–557. doi: 10.1016/j.exer.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. In: Albert DM, Jakobiec F, editors. Principles and Practice of Ophthalmology. WB Saunders Co: Philadelphia; 2000. pp. 2577–2595. [Google Scholar]
- Johnson M, Johnson DH, Kamm RD, DeKater AW, Epstein DL. The filtration characteristics of the aqueous outflow system. Exp Eye Res. 1990;50:407–418. doi: 10.1016/0014-4835(90)90142-h. [DOI] [PubMed] [Google Scholar]
- Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- Johnstone MA. Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm’s canal. Invest Ophthalmol Vis Sci. 1979;18:44–51. [PubMed] [Google Scholar]
- Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- Kaarniranta K, Ihanamaki T, Sahlman J, Pulkkinen H, Uusitalo H, Arita M, Tammi R, Lammi MJ, Helminen HJ. A mouse model for Stickler’s syndrome: ocular phenotype of mice carrying a targeted heterozygous inactivation of type II (pro)collagen gene (Col2a1) Exp Eye Res. 2006;83:297–303. doi: 10.1016/j.exer.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Kang MH, Oh DJ, Rhee DJ. Effect of hevin deletion in mice and characterization in trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2187–2193. doi: 10.1167/iovs.10-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WW, Liu H, Zhang J. Wakayama symposium: challenges of future research in ocular surface cell biology. Ocul Surf. 2013;11:19–24. doi: 10.1016/j.jtos.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Acott TS. The juxtacanalicular region of ocular trabecular meshwork: A tissue with a unique extracellular matrix and specialized function. J. Ocular Biol. 2013;1:10. [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009a;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Acott TS. Differential effects of ADAMTS-1, −4, and −5 in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009b;50:5769–5777. doi: 10.1167/iovs.09-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Kelley MJ, Acott TS. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facility in perfusion culture. Invest Ophthalmol Vis Sci. 2008;49:2495–2505. doi: 10.1167/iovs.07-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Vranka JA, Acott TS. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52:5049–5057. doi: 10.1167/iovs.10-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2007;48:1164–1172. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- Keller KE, Sun YY, Vranka JA, Hayashi L, Acott TS. Inhibition of hyaluronan synthesis reduces versican and fibronectin levels in trabecular meshwork cells. PLoS One. 2012a;7:e48523. doi: 10.1371/journal.pone.0048523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Sun YY, Yang YF, Bradley JM, Acott TS. Perturbation of hyaluronan synthesis in the trabecular meshwork and the effects on outflow facility. Invest Ophthalmol Vis Sci. 2012b;53:4616–4625. doi: 10.1167/iovs.12-9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Vranka JA, Haddadin RI, Kang MH, Oh DJ, Rhee DJ, Yang YF, Sun YY, Kelley MJ, Acott TS. The effects of tenascin C knockdown on trabecular meshwork outflow resistance. Invest Ophthalmol Vis Sci. 2013;54:5613–5623. doi: 10.1167/iovs.13-11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Rudkin A, Parry DA, Burdon KP, McKibbin M, Logan CV, Abdelhamed ZI, Muecke JS, Fernandez-Fuentes N, Laurie KJ, Shires M, Fogarty R, Carr IM, Poulter JA, Morgan JE, Mohamed MD, Jafri H, Raashid Y, Meng N, Piseth H, Toomes C, Casson RJ, Taylor GR, Hammerton M, Sheridan E, Johnson CA, Inglehearn CF, Craig JE, Ali M. Homozygous mutations in PXDN cause congenital cataract, corneal opacity, and developmental glaucoma. Am J Hum Genet. 2011;89:464–473. doi: 10.1016/j.ajhg.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B, Bartholdi D, Abdou MT, Zimmermann DR, Berger W. Identification of the genetic defect in the original Wagner syndrome family. Mol Vis. 2006;12:350–355. [PubMed] [Google Scholar]
- Knepper PA, Farbman AI, Telser AG. Exogenous hyaluronidases and degradation of hyaluronic acid in the rabbit eye. Invest Ophthalmol Vis Sci. 1984;25:286–293. [PubMed] [Google Scholar]
- Knepper PA, Goossens W, Palmberg PF. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1996;37:2414–2425. [PubMed] [Google Scholar]
- Krumbiegel M, Pasutto F, Mardin CY, Weisschuh N, Paoli D, Gramer E, Zenkel M, Weber BH, Kruse FE, Schlotzer-Schrehardt U, Reis A. Exploring functional candidate genes for genetic association in german patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2009;50:2796–2801. doi: 10.1167/iovs.08-2339. [DOI] [PubMed] [Google Scholar]
- Kuchtey J, Chang TC, Panagis L, Kuchtey RW. Marfan syndrome caused by a novel FBN1 mutation with associated pigmentary glaucoma. Am J Med Genet A. 2013;161A:880–883. doi: 10.1002/ajmg.a.35838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchtey J, Kuchtey RW. The microfibril hypothesis of glaucoma: implications for treatment of elevated intraocular pressure. J Ocul Pharmacol Ther. 2014;30:170–180. doi: 10.1089/jop.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchtey J, Olson LM, Rinkoski T, Mackay EO, Iverson TM, Gelatt KN, Haines JL, Kuchtey RW. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genet. 2011;7:e1001306. doi: 10.1371/journal.pgen.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Shah S, Tang HM, Smith M, Borras T, Danias J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS One. 2013;8:e72447. doi: 10.1371/journal.pone.0072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:R97–R110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz WE, Wang LW, Bader HL, Majors AK, Iwata K, Traboulsi EI, Sakai LY, Keene DR, Apte SS. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J Biol Chem. 2011;286:17156–17167. doi: 10.1074/jbc.M111.231571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, Fautsch MP, Murphy CJ, Russell P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011;52:1865–1871. doi: 10.1167/iovs.10-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis SJ, Kang JH, Weinreb RN, Yaspan BL, Cooke Bailey JN, Gaasterland D, Gaasterland T, Lee RK, Lichter PR, Budenz DL, Liu Y, Realini T, Friedman DS, McCarty CA, Moroi SE, Olson L, Schuman JS, Singh K, Vollrath D, Wollstein G, Zack DJ, Brilliant M, Sit AJ, Christen WG, Fingert J, Kraft P, Zhang K, Allingham RR, Pericak-Vance MA, Richards JE, Hauser MA, Haines JL, Pasquale LR, Wiggs JL. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology. 2014;121:508–516. doi: 10.1016/j.ophtha.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–281. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Zhang Y, Freddo TF, Gong H. Similar hydrodynamic and morphological changes in the aqueous humor outflow pathway after washout and Y27632 treatment in monkey eyes. Exp Eye Res. 2011;93:397–404. doi: 10.1016/j.exer.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Investigative Ophthalmology & Visual Science. 1981;21:563–573. [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–147. doi: 10.1016/0014-4835(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Rohen JW. Morphology of Aqueous Outflow Pathways in Normal and Glaucomatous Eyes. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. Mosby: 1996. pp. 89–123. [Google Scholar]
- Lutjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW. Quantitative analysis of ‘plaque material’ in the inner- and outer wall of Schlemm’s canal in normal- and glaucomatous eyes. Exp Eye Res. 1986;42:443–455. doi: 10.1016/0014-4835(86)90004-7. [DOI] [PubMed] [Google Scholar]
- Mabuchi F, Lindsey JD, Aihara M, Mackey MR, Weinreb RN. Optic nerve damage in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2004;45:1841–1845. doi: 10.1167/iovs.03-1008. [DOI] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. J Cell Sci. 2010;123:3006–3018. doi: 10.1242/jcs.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Osumi-Yamashita N, Noji S, Ohuchi H, Koyama E, Myokai F, Matsuo N, Taniguchi S, Doi H, Iseki S, et al. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- McKay BS, Congrove NR, Johnson AA, Dismuke WM, Bowen TJ, Stamer WD. A role for myocilin in receptor-mediated endocytosis. PLoS One. 2013;8:e82301. doi: 10.1371/journal.pone.0082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheal S, Khan MI, Akhtar F, Weiss MM, Islam F, Ali M, Qamar R, Maugeri A, den Hollander AI. Identification of a novel FBN1 gene mutation in a large Pakistani family with Marfan syndrome. Mol Vis. 2012;18:1918–1926. [PMC free article] [PubMed] [Google Scholar]
- Millar JC, Clark AF, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011;52:685–694. doi: 10.1167/iovs.10-6069. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Inoue H, Sakamoto Y, Kudo E, Naito T, Mikawa T, Mikawa Y, Isashiki Y, Osabe D, Shinohara S, Shiota H, Itakura M. Identification of a novel splice site mutation of the CSPG2 gene in a Japanese family with Wagner syndrome. Invest Ophthalmol Vis Sci. 2005;46:2726–2735. doi: 10.1167/iovs.05-0057. [DOI] [PubMed] [Google Scholar]
- Morales J, Al-Sharif L, Khalil DS, Shinwari JM, Bavi P, Al-Mahrouqi RA, Al-Rajhi A, Alkuraya FS, Meyer BF, Al Tassan N. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am J Hum Genet. 2009;85:558–568. doi: 10.1016/j.ajhg.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFbeta have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Exp Eye Res. 2013;115:1–12. doi: 10.1016/j.exer.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Nikopoulos K, Maugeri A, de Brouwer AP, van Nouhuys CE, Boon CJ, Perveen R, Zegers HA, Wittebol-Post D, van den Biesen PR, van der Velde-Visser SD, Brunner HG, Black GC, Hoyng CB, Cremers FP. Erosive vitreoretinopathy and wagner disease are caused by intronic mutations in CSPG2/Versican that result in an imbalance of splice variants. Invest Ophthalmol Vis Sci. 2006;47:3565–3572. doi: 10.1167/iovs.06-0141. [DOI] [PubMed] [Google Scholar]
- Narooie-Nejad M, Paylakhi SH, Shojaee S, Fazlali Z, Rezaei Kanavi M, Nilforushan N, Yazdani S, Babrzadeh F, Suri F, Ronaghi M, Elahi E, Paisan-Ruiz C. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum Mol Genet. 2009;18:3969–3977. doi: 10.1093/hmg/ddp338. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. Embo J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien ET, Wang Y, Ying H, Yue BY. Differential expression of genes in cells cultured from juxtacanalicular trabecular meshwork and Schlemm’s canal. J Ocul Pharmacol Ther. 2014;30:291–299. doi: 10.1089/jop.2013.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DJ, Kang MH, Ooi YH, Choi KR, Sage EH, Rhee DJ. Overexpression of SPARC in human trabecular meshwork increases intraocular pressure and alters extracellular matrix. Invest Ophthalmol Vis Sci. 2013;54:3309–3319. doi: 10.1167/iovs.12-11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88:656–670. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshley DE, Bradley JM, Fisk A, Hadaegh A, Samples JR, Van Buskirk EM, Acott TS. Laser trabeculoplasty induces stromelysin expression by trabecular juxtacanalicular cells. Invest Ophthalmol Vis Sci. 1996;37:795–804. [PubMed] [Google Scholar]
- Pasutto F, Krumbiegel M, Mardin CY, Paoli D, Lammer R, Weber BH, Kruse FE, Schlotzer-Schrehardt U, Reis A. Association of LOXL1 common sequence variants in German and Italian patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1459–1463. doi: 10.1167/iovs.07-1449. [DOI] [PubMed] [Google Scholar]
- Pattabiraman PP, Maddala R, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J Cell Physiol. 2014;229:927–942. doi: 10.1002/jcp.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Pecen PE, Rao PV. MRP4-mediated regulation of intracellular cAMP and cGMP levels in trabecular meshwork cells and homeostasis of intraocular pressure. Invest Ophthalmol Vis Sci. 2007;54:1636–1649. doi: 10.1167/iovs.12-11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–C763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkumas KM, Stamer WD. Protein markers and differentiation in culture for Schlemm’s canal endothelial cells. Exp Eye Res. 2012;96:82–87. doi: 10.1016/j.exer.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Lin Y, Liton PB. Cathepsin B is up-regulated and mediates extracellular matrix degradation in trabecular meshwork cells following phagocytic challenge. PLoS One. 2013;8:e68668. doi: 10.1371/journal.pone.0068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KM, Epstein DL, Liton PB. Up-regulated expression of extracellular matrix remodeling genes in phagocytically challenged trabecular meshwork cells. PLoS One. 2012;7:e34792. doi: 10.1371/journal.pone.0034792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puk O, Dalke C, Calzada-Wack J, Ahmad N, Klaften M, Wagner S, de Angelis MH, Graw J. Reduced corneal thickness and enlarged anterior chamber in a novel ColVIIIa2G257D mutant mouse. Invest Ophthalmol Vis Sci. 2009;50:5653–5661. doi: 10.1167/iovs.09-3550. [DOI] [PubMed] [Google Scholar]
- Quadri SK. Cross talk between focal adhesion kinase and cadherins: role in regulating endothelial barrier function. Microvasc Res. 2012;83:3–11. doi: 10.1016/j.mvr.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Dreier B, Reilly CM, Thomasy SM, Wood JA, Ly I, Tuyen BC, Hughbanks M, Murphy CJ, Russell P. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Invest Ophthalmol Vis Sci. 2013;54:378–386. doi: 10.1167/iovs.12-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010;339:71–82. doi: 10.1007/s00441-009-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LM, Tyler RC, Schilter KF, Abdul-Rahman O, Innis JW, Kozel BA, Schneider AS, Bardakjian TM, Lose EJ, Martin DM, Broeckel U, Semina EV. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet. 2011;130:495–504. doi: 10.1007/s00439-011-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009;88:704–712. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JV, Golesic E, Gauldie J, West-Mays JA. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest Ophthalmol Vis Sci. 2010;51:308–318. doi: 10.1167/iovs.09-3380. [DOI] [PubMed] [Google Scholar]
- Robertson JV, Siwakoti A, West-Mays JA. Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice. Mol Vis. 2013;19:684–695. [PMC free article] [PubMed] [Google Scholar]
- Rohen JW, Futa R, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–585. [PubMed] [Google Scholar]
- Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Invest Ophthalmol Vis Sci. 2008;49:629–635. doi: 10.1167/iovs.07-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Shen X, Koga T, Park BC, Noskina Y, Tibudan M, Yue BY. Mitochondrial association of myocilin, product of a glaucoma gene, in human trabecular meshwork cells. J Cell Physiol. 2007;213:775–784. doi: 10.1002/jcp.21147. [DOI] [PubMed] [Google Scholar]
- Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:2105–2114. doi: 10.1167/iovs.06-1089. [DOI] [PubMed] [Google Scholar]
- Santas AJ, Bahler C, Peterson JA, Filla MS, Kaufman PL, Tamm ER, Johnson DH, Peters DM. Effect of heparin II domain of fibronectin on aqueous outflow in cultured anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2003;44:4796–4804. doi: 10.1167/iovs.02-1083. [DOI] [PubMed] [Google Scholar]
- Sawaguchi S, Lam TT, Yue BY, Tso MO. Effects of glycosaminoglycan-degrading enzymes on bovine trabecular meshwork in organ culture. J Glaucoma. 1993;2:80–86. [PubMed] [Google Scholar]
- Sawaguchi S, Yue BY, Yeh P, Tso MO. Effects of intracameral injection of chondroitinase ABC in vivo. Arch Ophthalmol. 1992;110:110–117. doi: 10.1001/archopht.1992.01080130112037. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Hammer CM, Krysta AW, Hofmann-Rummelt C, Pasutto F, Sasaki T, Kruse FE, Zenkel M. LOXL1 deficiency in the lamina cribrosa as candidate susceptibility factor for a pseudoexfoliation-specific risk of glaucoma. Ophthalmology. 2012;119:1832–1843. doi: 10.1016/j.ophtha.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Pasutto F, Sommer P, Hornstra I, Kruse FE, Naumann GO, Reis A, Zenkel M. Genotype-correlated expression of lysyl oxidase-like 1 in ocular tissues of patients with pseudoexfoliation syndrome/glaucoma and normal patients. Am J Pathol. 2008;173:1724–1735. doi: 10.2353/ajpath.2008.080535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunck G, Han H, Wecker T, Kampik D, Meyer-ter-Vehn T, Grehn F. Substrate rigidity modulates cell matrix interactions and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2008;49:262–269. doi: 10.1167/iovs.07-0956. [DOI] [PubMed] [Google Scholar]
- Schneemann A, Leusink-Muis A, van den Berg T, Hoyng PF, Kamphuis W. Elevation of nitric oxide production in human trabecular meshwork by increased pressure. Graefes Arch Clin Exp Ophthalmol. 2003;241:321–326. doi: 10.1007/s00417-003-0638-4. [DOI] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Jain A, Zode GS, Wordinger RJ, Clark AF. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011a;52:5251–5259. doi: 10.1167/iovs.11-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011b;52:5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- Sherwood ME, Richardson TM. Phagocytosis by trabecular meshwork cells: sequence of events in cats and monkeys. Exp Eye Res. 1988;46:881–895. doi: 10.1016/s0014-4835(88)80040-x. [DOI] [PubMed] [Google Scholar]
- Snead MP, McNinch AM, Poulson AV, Bearcroft P, Silverman B, Gomersall P, Parfect V, Richards AJ. Stickler syndrome, ocular-only variants and a key diagnostic role for the ophthalmologist. Eye (Lond) 2011;25:1389–1400. doi: 10.1038/eye.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell. 2005;16:757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 2007;21:1310–1318. doi: 10.1038/sj.eye.6702852. [DOI] [PubMed] [Google Scholar]
- Spiga MG, Borras T. Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3029–3041. doi: 10.1167/iovs.09-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart MR, Cone FE, Nguyen C, Nguyen TD, Pease ME, Puk O, Graw J, Oglesby EN, Quigley HA. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol Vis. 2012;18:1093–1106. [PMC free article] [PubMed] [Google Scholar]
- Swaminathan SS, Oh DJ, Kang MH, Ren R, Jin R, Gong H, Rhee DJ. Secreted protein acidic and rich in cysteine (SPARC)-null mice exhibit more uniform outflow. Invest Ophthalmol Vis Sci. 2013;54:2035–2047. doi: 10.1167/iovs.12-10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Nishina S, Kosaki R, Torii C, Furukawa R, Takahashi T, Kosaki K. Concurrent deletion of BMP4 and OTX2 genes, two master genes in ophthalmogenesis. Eur J Med Genet. 2013;56:50–53. doi: 10.1016/j.ejmg.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Koga T, Yano T, Kimura A. Proteoglycans in the eye. Cornea. 2002;21:S62–S69. doi: 10.1097/01.ico.0000263121.45898.d2. [DOI] [PubMed] [Google Scholar]
- Tawara A, Tou N, Kubota T, Harada Y, Yokota K. Immunohistochemical evaluation of the extracellular matrix in trabecular meshwork in steroid-induced glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1021–1028. doi: 10.1007/s00417-008-0800-0. [DOI] [PubMed] [Google Scholar]
- Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Tervo K, Paallysaho T, Virtanen I, Tervo T. Integrins in human anterior chamber angle. Graefes Arch Clin Exp Ophthalmol. 1995;233:291–295. doi: 10.1007/BF00177651. [DOI] [PubMed] [Google Scholar]
- Thomasy SM, Wood JA, Kass PH, Murphy CJ, Russell P. Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53:952–958. doi: 10.1167/iovs.11-8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DS, Tam PO, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JC, Karwatowski WS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- Tran-Viet KN, Soler V, Quiette V, Powell C, Yanovitch T, Metlapally R, Luo X, Katsanis N, Nading E, Young TL. Mutation in collagen II alpha 1 isoforms delineates Stickler and Wagner syndrome phenotypes. Mol Vis. 2013;19:759–766. [PMC free article] [PubMed] [Google Scholar]
- Tripathi BJ, Tripathi RC. Neural crest origin of human trabecular meshwork and its implications for the pathogenesis of glaucoma. Am J Ophthalmol. 1989;107:583–590. doi: 10.1016/0002-9394(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- VanderWyst SS, Perkumas KM, Read AT, Overby DR, Stamer WD. Structural basement membrane components and corresponding integrins in Schlemm’s canal endothelia. Mol Vis. 2011;17:199–209. [PMC free article] [PubMed] [Google Scholar]
- Vithana EN, Khor CC, Qiao C, Nongpiur ME, George R, Chen LJ, Do T, Abu-Amero K, Huang CK, Low S, Tajudin LS, Perera SA, Cheng CY, Xu L, Jia H, Ho CL, Sim KS, Wu RY, Tham CC, Chew PT, Su DH, Oen FT, Sarangapani S, Soumittra N, Osman EA, Wong HT, Tang G, Fan S, Meng H, Huong DT, Wang H, Feng B, Baskaran M, Shantha B, Ramprasad VL, Kumaramanickavel G, Iyengar SK, How AC, Lee KY, Sivakumaran TA, Yong VH, Ting SM, Li Y, Wang YX, Tay WT, Sim X, Lavanya R, Cornes BK, Zheng YF, Wong TT, Loon SC, Yong VK, Waseem N, Yaakub A, Chia KS, Allingham RR, Hauser MA, Lam DS, Hibberd ML, Bhattacharya SS, Zhang M, Teo YY, Tan DT, Jonas JB, Tai ES, Saw SM, Hon do N, Al-Obeidan SA, Liu J, Chau TN, Simmons CP, Bei JX, Zeng YX, Foster PJ, Vijaya L, Wong TY, Pang CP, Wang N, Aung T. Genome-wide association analyses identify three new susceptibility loci for primary angle closure glaucoma. Nat Genet. 2012;44:1142–1146. doi: 10.1038/ng.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Clark AF, Lipson KE, Andrews D, Crean JK, O’Brien CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Invest Ophthalmol Vis Sci. 2013;54:7836–7848. doi: 10.1167/iovs.13-12494. [DOI] [PubMed] [Google Scholar]
- Wang WH, McNatt LG, Pang IH, Millar JC, Hellberg PE, Hellberg MH, Steely HT, Rubin JS, Fingert JH, Sheffield VC, Stone EM, Clark AF. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Howell GR, Linkroum K, Abdrabou W, Hodges E, Braine CE, Pasquale LR, Hannon GJ, Haines JL, John SW. Variations in COL15A1 and COL18A1 influence age of onset of primary open angle glaucoma. Clin Genet. 2013;84:167–174. doi: 10.1111/cge.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Pawlyk B, Connolly E, Adamian M, Miller JW, Pasquale LR, Haddadin RI, Grosskreutz CL, Rhee DJ, Li T. Disruption of the blood-aqueous barrier and lens abnormalities in mice lacking lysyl oxidase-like 1 (LOXL1) Invest Ophthalmol Vis Sci. 2014;55:856–864. doi: 10.1167/iovs.13-13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–G955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999;18:629–667. doi: 10.1016/s1350-9462(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Sharma T, Clark AF. The role of TGF-beta2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J Ocul Pharmacol Ther. 2014;30:154–162. doi: 10.1089/jop.2013.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr Eye Res. 2001;22:394–397. doi: 10.1076/ceyr.22.5.394.5500. [DOI] [PubMed] [Google Scholar]
- Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol. 1996;40:379–390. doi: 10.1016/s0039-6257(96)80066-x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008;295:C1057–C1070. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Rao PV. Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci. 2005;46:4130–4138. doi: 10.1167/iovs.05-0164. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Jin TB, Liu QB, Chen C, Hu HT. Ophthalmic findings in a family with early-onset isolated ectopia lentis and the p.Arg62Cys mutation of the fibrillin-1 gene (FBN1) Ophthalmic Genet. 2012;34:21–26. doi: 10.3109/13816810.2012.718029. [DOI] [PubMed] [Google Scholar]
- Zhou L, Maruyama I, Li Y, Cheng EL, Yue BY. Expression of integrin receptors in the human trabecular meshwork. Curr Eye Res. 1999;19:395–402. doi: 10.1076/ceyr.19.5.395.5297. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1932–1939. doi: 10.1167/iovs.07-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]