Abstract
BACKGROUND:
Electronic health record (EHR)-linked patient portals are a promising approach to facilitate shared decision-making between families of children with chronic conditions and pediatricians. This study evaluated the feasibility, acceptability, and impact of MyAsthma, an EHR-linked patient portal supporting shared decision-making for pediatric asthma.
METHODS:
We conducted a 6-month randomized controlled trial of MyAsthma at 3 primary care practices. Families were randomized to MyAsthma, which tracks families’ asthma treatment concerns and goals, children’s asthma symptoms, medication side effects and adherence, and provides decision support, or to standard care. Outcomes included the feasibility and acceptability of MyAsthma for families, child health care utilization and asthma control, and the number of days of missed school (child) and work (parent). Descriptive statistics and longitudinal regression models assessed differences in outcomes between study arms.
RESULTS:
We enrolled 60 families, 30 in each study arm (mean age 8.3 years); 57% of parents in the intervention group used MyAsthma during at least 5 of the 6 study months. Parents of children with moderate to severe persistent asthma used the portal more than others; 92% were satisfied with MyAsthma. Parents reported that use improved their communication with the office, ability to manage asthma, and awareness of the importance of ongoing attention to treatment. Parents in the intervention group reported that children had a lower frequency of asthma flares and intervention parents missed fewer days of work due to asthma.
CONCLUSIONS:
Use of an EHR-linked asthma portal was feasible and acceptable to families and improved clinically meaningful outcomes.
Keywords: asthma, patient portal, shared decision-making
What’s Known on This Subject:
Strategies are needed to engage families of chronically ill children at home in an ongoing process of shared decision-making regarding treatment that is responsive to families’ concerns and goals and children’s evolving symptoms.
What This Study Adds:
This study evaluated a novel patient portal that facilitates shared decision-making in asthma. The portal was feasible and acceptable to families, improved outcomes, and provides a model for improving care through an electronic health record portal.
Pediatric primary care practices care for a growing number of chronically ill children1; however, attendance at follow-up office visits is variable,2,3 clinical status often changes between these visits, and time-constrained office visits limit time to address families’ concerns and desired outcomes (goals) from treatment.4 As a result, strategies are needed to engage families of children at home in ongoing shared decision-making (SDM) regarding treatment that is responsive to families’ concerns and goals and the child’s symptoms. SDM involves active participation of both clinicians and families in treatment decisions, exchange of information, discussion of preferences, and joint determination of treatment plans.5 This process is associated with improved knowledge, better perceptions of health risk, and better concordance of decisions with personal values.6 However, SDM has been difficult to implement in real-world practice settings.7
One promising approach to overcome barriers to SDM involves facilitating the process through health information technology, in particular patient portals linked to electronic health records (EHRs).8,9 Despite this promise, the design of patient portals historically has focused on simple tasks, such as messaging, viewing results, and appointment scheduling, but not supporting the ongoing management of chronic conditions through a process of SDM.10–12 As a result, data on the feasibility, acceptability, and outcomes of such tools are lacking.
Pediatric asthma provides an ideal condition to examine the role of patient portals designed to foster SDM. Asthma remains the most common chronic pediatric illness, affecting >7 million children in the United States.13 The burden of asthma includes lost work for parents and school for children,14–16 lower quality of life,16,17 increased emergency department (ED) visits and hospitalizations,18 and death.19 In addition, our previous research suggests that families of US children with asthma who are able to actively communicate with clinicians from home are far more likely to report high levels of SDM,20 and that this process also may be associated with improved health and lower health care costs.21 In this study, our objective was to test the feasibility, acceptability, and impact of an innovative, EHR-linked patient portal with decision support directed at both families and clinicians on asthma outcomes. We hypothesized that parents and clinicians would find portal use both feasible and acceptable, and that clinical outcomes would improve.
Methods
Setting and Patient Population
This study was conducted within The Children’s Hospital of Philadelphia (CHOP) Pediatric Research Consortium, a primary care practice-based research network including 31 practices in 2 states.22 One urban and 2 suburban practices participated. These practices were chosen because of their previous interest in improving the coordination and outcomes of asthma care. All practices received in-person training on the portal from a physician leader (AGF) of the research team and used EpicCare (Verona, WI).
Eligible participants were children aged 6 to 12 years with persistent asthma who received care at a study site, and their parent or legal guardian. We enrolled English-speaking parents/guardians who were the primary member of their household involved in communicating with the doctor’s office and had consistent computer and Internet access.
Study Protocol
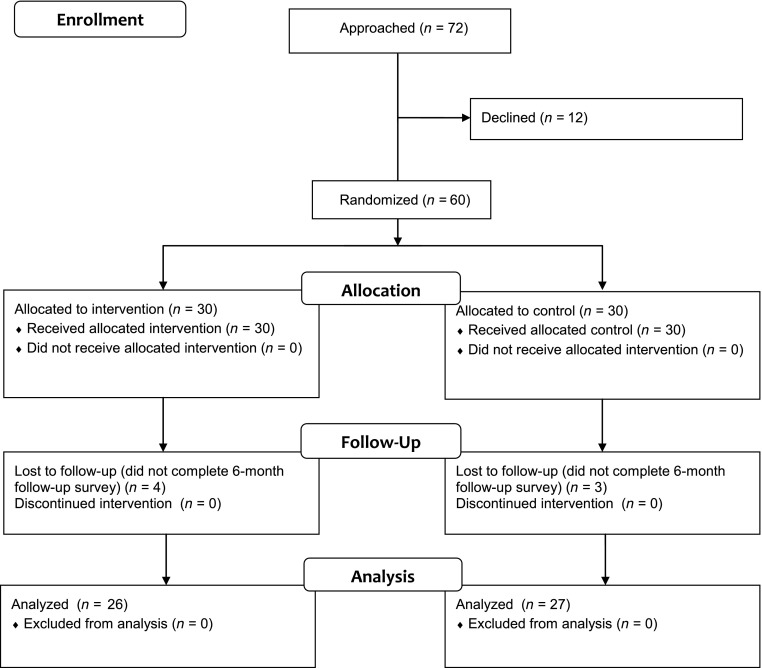
This prospective, randomized controlled trial was conducted from January to December 2013. Research staff enrolled a convenience sample of 60 families from the 3 study practices (Fig 1) either at scheduled primary care visits or separate study appointments. Eligible patients were identified by using rosters generated from the EHR and referral from clinicians and asthma coordinators at study sites. At the clinicians’ discretion, parents of children whose asthma was not a primary or current health concern were excluded, as were those not currently taking a controller medication. After informed consent, individual participants were randomized to either the EHR-linked SDM portal (MyAsthma) or usual care. A randomization sequence was generated by the study coordinator (SLM). Randomization was stratified by practice and by whether the child had mild or moderate versus severe persistent asthma. Sealed envelopes were used to ensure blinding of study staff to treatment condition before enrollment and randomization. Families randomized to the intervention were provided with brief training by study staff on MyAsthma use on enrollment and were subsequently prompted via E-mail reminders to complete a total of 6 monthly portal surveys.
FIGURE 1.
Consort diagram. The study population was a convenience sample based largely on clinician recommendation and was not designed to be representative of all children with asthma in the care network.
Intervention
MyAsthma was developed with input from families and clinicians with the goal of fostering ongoing SDM.23 MyAsthma provided decision support to both clinicians and parents. The clinician interface appeared seamlessly in the EHR, and the parent interface appeared seamlessly within MyChart, the EHR vendor’s patient portal. The features of MyAsthma (Supplemental Appendix 1) include identification of parents’ concerns and goals for asthma treatment; monthly tracking of symptoms, medication side effects, and progress toward goals; asthma educational content including videos; and access to the child’s asthma care plan. Parents were encouraged with E-mail reminders to complete monthly portal surveys with input from their affected child (Supplemental Appendix 2). In response to these surveys, families and clinicians received guideline-based decision support24 that directed them to speak to one another if asthma was not well-controlled or if there were side effects, or to continue current therapy. Survey results were tracked over time in a timeline available to families through the portal and to clinicians through the EHR. Families in the control group did not have access to the portal; however, clinicians caring for control group children had access to a clinician-focused decision support system proven effective in fostering guideline-based care.25
Outcomes
To measure acceptability and clinical outcomes, families in both groups completed outcome surveys at enrollment (after randomization) and at 3 and 6 months. Baseline outcome surveys were completed on paper and follow-ups were via phone call or secure E-mail survey (Research Electronic Data Capture [REDCap]).26 Feasibility of portal use was assessed by the proportion of participants in the intervention group who completed the portal survey each month, as a measure of whether families were able to complete the portal survey consistently. Acceptability of asthma care was assessed by the 6-month outcomes survey by using 11 Likert-scaled questions developed by the study team with face validity. Parents in the intervention group were also asked 2 questions about satisfaction with the portal and open-ended questions about their experience using the portal.
Clinical outcomes included the number of asthma ED visits, hospitalizations, and specialist and primary care visits over the 6 months of the study assessed by parent responses to the 6-month outcomes survey. We validated parental report by manual chart review for 35 children cared for at an inner-city practice. We were unable to validate results for children cared for at suburban practices because children at those sites often receive care at outside EDs and hospitals, which were not captured in the EHR. Through the EHR, we then assessed the number of prescriptions for asthma medications (albuterol, inhaled steroid, montelukast, and combination inhaled steroid/long-acting β agonists, oral steroid) received for all study participants during the 6-month study period. At baseline and 6 months, parents reported the number of school days children had missed and the number of work days lost by parents owing to the child’s asthma in the past month. Parents also completed the Parent Patient Activation Measure to measure parent activation (knowledge, skills, and confidence needed in managing child’s health care),27 Integrated Therapeutics Group Child Asthma Short Form to measure asthma-related quality of life,28 and the Asthma Control Tool (ACT), a validated measure assessing the frequency of flares and symptoms while the child is at his or her best.29
Statistical Analyses
We described the study population. To identify differences between more active portal users and other families, we compared characteristics between intervention families who used the portal at least 5 months during the study (frequent users) and those who used the portal less frequently.
To determine the portal’s impact on the parent-reported acceptability of asthma care and whether intervention families were satisfied with the portal, we calculated the proportion of parents with responses of “very much” or “completely” for each acceptability question, and compared the intervention and control groups by using χ2 tests. Open-ended questions were reviewed by multiple members of the research team and grouped into themes by consensus. Representative quotations were selected for presentation.
We then compared differences between the 2 study arms in health care use and medication use. Because visits and hospitalizations were rare and our sample size modest, we described the patterns observed without testing significance. Next, differences in the number of days of school and work missed between the study start and 6-month follow-up were compared between study arms using longitudinal negative binomial models (xtnbreg in Stata [Stata Corp, College Station, TX] ), with random intercepts for each child, with the outcome of number of days missed and with a study arm by time interaction term as the primary exposure. Initial Poisson models were overdispersed, so a negative binomial model was chosen instead. Differences in the proportion of children with days of school missed, and parents with days of work missed, were compared by using longitudinal logistic regression (xtlogit) models, also with random intercepts for each child.
To determine whether change in the Parent Patient Activation Measure, Integrated Therapeutics Group Child Asthma Short Form, and ACT over time was associated with study arm, we implemented longitudinal linear regression models (xtreg), with random intercepts for each child, score as the outcome, and the study arm by time interaction as the primary exposure.
Analyses were conducted by using Stata version 13 (Stata Corp). The CHOP Institutional Review Board approved this study. Parents provided written informed consent. Assent was obtained from children ≥7 years.
Results
Of 72 families approached, 60 enrolled in this study (30 per arm, Fig 1). Approximately half of enrolled children had moderate or severe asthma (Table 1). Twenty-six families (87%) in the intervention group and 27 families (90%) in the control completed the 6-month outcome measures. The remaining 7 families were unable to be reached by phone or E-mail.
TABLE 1.
Characteristics of Children and Parents in the Study
| Overall | Intervention | Control | |
|---|---|---|---|
| n | 60 | 30 | 30 |
| Child characteristics | |||
| Child age, y, mean (SD) | 8.3 (1.9) | 8.3 (1.9) | 8.2 (1.9) |
| Age range | 6–12 | 6–12 | 6–12 |
| Asthma severity, n (%) | |||
| Mild | 32 (53) | 18 (60) | 14 (47) |
| Moderate | 25 (42) | 11 (37) | 14 (47) |
| Severe | 3 (5) | 1 (3) | 2 (6) |
| Parent characteristics | |||
| Race, n (%) | |||
| Black/African American | 28 (47) | 13 (43) | 15 (50) |
| White | 25 (42) | 13 (43) | 12 (40) |
| Asian | 3 (5) | 3 (10) | 0 (0) |
| Other | 4 (6) | 1 (3) | 3 (10) |
| Hispanic | 4 (6) | 1 (3) | 3 (10) |
| Relationship to child, n (%) | |||
| Mother | 56 (93) | 26 (87) | 30 (100) |
| Father | 3 (5) | 3 (10) | 0 (0) |
| Other legal guardian | 1 (2) | 1 (3) | 0 (0) |
| Education, n (%) | |||
| High school graduate or less | 17 (29) | 8 (26) | 9 (31) |
| Some college/Associate’s degree | 18 (30) | 11 (37) | 7 (24) |
| Bachelor’s degree or higher | 24 (41) | 11 (37) | 13 (45) |
| Employment, n (%) | |||
| Working for wages | 45 (75) | 24 (80) | 21 (70) |
| Working without pay | 8 (13) | 3 (10) | 5 (17) |
| Unemployed | 7 (12) | 3 (10) | 4 (13) |
| Previous MyChart usera | 8 (13) | 3 (10) | 5 (17) |
No significant differences between intervention and control groups.
These parents had previously used MyChart, a patient portal with basic features including messaging and appointment scheduling, but had not previously had access to the features of MyAsthma.
Feasibility
Of the 30 families randomized to the intervention group, 14 (47%) completed the portal survey for each of the 6 months of the study, and 17 (57%) completed it 5 or more times, which we defined as frequent use; 77% completed the survey more than once. We did not observe any significant difference between frequent users and other intervention families in demographic characteristics; however, parents of children with moderate or severe asthma used the portal more frequently (75% were frequent users compared with 47% of parents whose child had mild persistent asthma). Among the 3 parents who were MyChart users before enrollment, 2 completed the portal all 6 months and 1 never completed the portal survey.
The portal identified 17 instances among 13 children of poor or uncontrolled asthma and 1 instance of medication side effects based on the results of the check-in survey. Primary care practices consistently responded to these results through 5 phone calls and 18 MyChart messages.
Acceptability
There were no significant differences between study arms in satisfaction with asthma care (data not shown). In the intervention group, 22 of the 24 parents who completed these items reported that MyAsthma made it easier to care for their child’s asthma, and that they were satisfied with the portal. Several clinically relevant themes emerged from parents’ open-ended comments. Ten of 24 parents reported that the portal made it easier to communicate with their child’s health care providers. “Communication with our providers has been much more convenient this way. I think it may have even cut out some unnecessary visits,” one said. Nine parents reported that the portal made asthma management easier, through centralizing information in a convenient location and providing tracking of symptoms over time. One mother remarked, “I loved that his records and appointments were all in one place. It made it very easy for me to keep track of everything.” Six parents reported that the portal increased their awareness of the importance of asthma management; one said, “It made me more aware of how serious his asthma could get if he did not maintain his medication administration.” In addition, 6 parents felt the portal enabled them to learn more about asthma. When asked what they liked least about MyAsthma, 3 parents reported a preference for a shorter or less frequent survey.
Asthma-Related Health Care Use
Families in the intervention group reported fewer ED visits and hospitalizations for asthma over 6 months than families in the control group (3 versus 9 and 0 versus 2, respectively) (Table 2). Only 2 intervention families reported at least 1 ED visit, compared with 6 control families, and no intervention families reported any hospitalizations. In addition, children in the intervention group had fewer visits with asthma specialists or asthma-related primary care visits (Table 2). When we repeated these analyses stratified by asthma severity to ensure that findings were not biased by severity, results were similar.
TABLE 2.
Asthma-Related Health Care Use Among Children in the Intervention and Control Groups Reported at 6-mo Follow-up
| Type of Visit | Number and Proportion of Children With ≥1 Event, n (%) | Total Count of Events | ||
| Intervention, n = 26 | Control, n = 27 | Intervention | Control | |
| Hospitalizations | 0 (0) | 1 (4) | 0 | 2 |
| ED visits | 2 (8) | 6 (22) | 3 | 9 |
| Asthma specialist visits | 8 (31) | 12 (44) | 11 | 21 |
| Primary care asthma visits | 16 (62) | 18 (67) | 29 | 41 |
Based on parent report. Twenty-six of 30 parents in the intervention group and 27 of 30 parents in the control group completed the 6-mo follow-up survey.
Medication Receipt
Children in the intervention group received more controller medications than children in the control group (1.1 prescriptions for inhaled steroids per child compared with 0.7). In contrast, children in the control group received more prescriptions for oral steroids (1.0 compared with 0.4). Use of albuterol, montelukast, and combination inhaled steroid/long-acting β agonists was similar between study arms (data not shown).
Days of School and Work Missed Due to Asthma
For all measures, intervention group children had better outcomes than control group children. Over the 6-month study period, children in the intervention group had a nonsignificant relative decrease in the number of school days missed compared with children in the control group (a relative reduction of 0.7 days per child, P = .2) (Table 3). Similarly, parents in the intervention group had a significant decrease in the number of days of work missed relative to controls (a relative reduction of 1.8 days per parent, P = .001). The intervention group also had a marginally significant relative reduction in the proportion of parents missing at least 1 day of work (a relative reduction of 13 parents, or 47%, P = .07).
TABLE 3.
Change in Days of School and Work Missed Because of Asthma
| Number and Proportion of Children/Parents Missing ≥1 Day, n (%) | Mean Number of Days Missed per Child/Parent in Each Study Arm | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | DID | Pa | Intervention | Control | DID | Pb | |
| Days of school missed in the past month (child) | ||||||||
| Baseline | 14 (52) | 16 (57) | 1.7 | 2.3 | ||||
| At 6 mo | 7 (27) | 11 (41) | 0.6 | 1.9 | ||||
| Change | −7 (−25) | −5 (−16) | −2 (−9) | .7 | −1.1 | −0.4 | −0.7 | .2* |
| Days of work missed in the past month (parent) | ||||||||
| Baseline | 12 (46) | 8 (32) | 1.1 | 0.8 | ||||
| At 6 mo | 1 (4) | 10 (37) | <0.1 | 1.5 | ||||
| Change | −11 (−42) | +2 (+5) | −13 (−47) | .07 | −1.1 | +0.7 | −1.8 | .001* |
At baseline, n = 30 for each group. At follow-up, n = 26 for intervention, n = 27 for control. DID, difference in difference.
From longitudinal random intercept logit models (xtlogit in Stata) with a time*study arm interaction, clustering by patient; P value is for the interaction term.
From longitudinal random intercept negative binomial models (xtnbreg in Stata) with a time*study arm interaction, clustering by patient; P value is for the interaction term.
Asthma Control, Quality of Life, and Parent Activation
There were no significant differences in baseline control, quality of life, or parent activation between the 2 study arms (P > .2 for all comparisons.) Through the ACT, we found that frequency of asthma flares improved in the intervention group relative to the control group over time by 2.0 points on a 25-point scale (P = .02) (Table 4). In addition, families in the intervention group had a marginally significant improvement in symptoms during periods without flares. Although a trend toward improved quality of life in terms of daytime symptoms and functional limitations was observed for children receiving the intervention, results were not statistically significant nor were there significant changes in parent activation (Table 4). Results were similar in sensitivity analyses that excluded the 3 children with severe persistent asthma and included level of asthma severity as a covariate in regression models.
TABLE 4.
Asthma Control, Asthma-Related Quality of Life, and Parent Activation at Study Start and Follow-up Among Intervention and Control Children
| Intervention Mean (SD) | Control Mean (SD) | Difference in Difference | Pa | |
|---|---|---|---|---|
| Frequency of astdma flaresb | ||||
| Baseline | 4.7 (3.4) | 5.1 (4.4) | ||
| At 6 mo | 1.4 (2.0) | 3.8 (3.8) | ||
| Change | −3.3 | −1.3 | −2.0 | .02* |
| Asthma symptoms while at besta | ||||
| Baseline | 4.9 (4.5) | 5.0 (5.3) | ||
| At 6 mo | 2.1 (2.1) | 4.4 (5.3) | ||
| Change | −2.8 | −0.6 | −2.2 | .1 |
| Quality of lifec | ||||
| Nighttime symptoms | ||||
| Baseline | 69.3 (22.3) | 63.7 (21.4) | ||
| 6 mo | 85.0 (19.6) | 80.0 (21.3) | ||
| Change | 15.7 | 16.3 | −0.6 | .9 |
| Daytime symptoms | ||||
| Baseline | 70.3 (19.9) | 68.7 (23.3) | ||
| 6 mo | 82.3 (17.5) | 76.7 (17.5) | ||
| Change | 12.0 | 8.0 | 4.0 | .3 |
| Functional limitations | ||||
| Baseline | 77.2 (20.4) | 75.2 (21.9) | ||
| 6 mo | 86.5 (20.0) | 80.2 (24.1) | ||
| Change | 9.3 | 5.0 | 4.3 | .4 |
| Parent activationd | ||||
| Baseline | 75.8 (15.6) | 80.7 (19.8) | ||
| 3 mo | 79.0 (9.8) | 79.7 (15.5) | ||
| 6 mo | 78.1 (72.6) | 83.1 (15.9) | ||
| Change | 2.3 | 2.4 | 0.1 | .9 |
At baseline, n = 30 for each group. At follow-up, n = 26 for intervention, n = 27 for control.
From longitudinal random intercept linear regression models (xtreg in Stata) with a time*study arm interaction; P value is for the interaction term.
Both domains included 5 questions, scored from 0 to 5. The domain scores could range from 0 to 25, with higher scores indicating poorer control. For the frequency of flares domain, parents reported the number of times in the past 3 months their child had an asthma flare, a flare lasting longer than a week, started an oral steroid, stayed overnight in a hospital, or visited the ED. For the symptoms at best domain, parents reported symptoms with light activity, running or sports, at night, in the morning, and how often their child needed to take albuterol or other quick-relief inhaler.
Quality-of-life scales. Nighttime symptoms scale included 2 questions, daytime symptoms included 2 questions, functional impairment included 4 questions, all scored from 1 to 5. Domain scores were linearly transformed and could range from 0 to 100, with higher scores indicating better functioning.
Patient Activation included 13 questions, scored 1 to 4. Scores were transformed and could range from 0 to 100, with higher scores indicating higher activation.
Discussion
This study demonstrated the feasibility and acceptability of using an EHR-linked patient portal to foster ongoing SDM and improve outcomes for children with asthma. We found that more than half of intervention families completed portal surveys for at least 5 of the 6 study months and 77% completed the survey more than once. Ninety-two percent reported that MyAsthma made it easier to care for their child’s asthma and parents reported that the portal improved care by facilitating communication, centralizing asthma information, and increasing awareness of the importance of asthma management. Of particular importance, families randomized to receive the intervention experienced improved outcomes across many study measures.
Our results extend findings from previous clinical trials using digital systems to promote pediatric asthma self-management and improve outcomes. Multiple studies have provided digital health tools to families to assist with asthma management.30–34 These interventions variably improved outcomes, such as activity limitation,30 asthma symptoms/control,31,32,34 and missed school.34 MyAsthma extends these studies by prompting parents to share their concerns about and goals for asthma treatment with their child’s doctor, a patient-centered approach that was more tightly integrated with existing office systems and may have provided additional benefit.
Our findings also suggest several mechanisms by which asthma outcomes may have been improved through the study. By receiving regular updates on asthma control and adherence, clinicians could address barriers to treatment receipt and modify treatment plans when needed. Parents’ open-ended responses suggest that the portal facilitated communication with their primary care providers, focused attention on asthma management, and centralized information to help parents effectively manage their children’s chronic condition. Perhaps related to these tools to support parents, children in the intervention group received more prescriptions for inhaled steroids and fewer for oral steroids. Although further study is needed to detail the portal’s mechanism of action, the increased use of controller medications likely was an important approach in limiting the consequences of asthma flares, including poor control and increased use of the ED and hospital.
Much has been written regarding the concern that health information technology may increase disparities in care as more affluent families access additional tools while lower-income and minority groups fall farther behind.35–37 Instead, we found that MyAsthma was widely used by both suburban white families and urban African American families regardless of socioeconomic status. Demographic surveys increasingly show that minorities and those with less education are more likely to have smartphones38 and use them for health-related purposes.39 In the context of this trend, the results of our study demonstrate, at least in this population, the ability of digital health tools, including portals, to improve care and outcomes for diverse users.
Finally, our results underscore the value of providing decision support to families at home in addition to clinicians in the office. In this study, both control and intervention families received care at practices where providers received decision support through the EHR through a system previously shown to improve the quality of asthma care.25 Consistent with previous studies,4,40 our results demonstrate the additional value of decision support systems that engage families as well as the clinical team and justify the continued development and evaluation of decision support systems to foster shared, as opposed to simply clinician-focused, decision-making.
This study had several limitations. First, study participants were a convenience sample; some were recommended by their primary care providers and others were enrolled based on EHR rosters. As a result and because this study was confined to practices within 1 health system with an interest in improving asthma care, this sample may not be representative of the larger population of children with asthma. Nonetheless, we found that portal use was feasible and acceptable for the diverse group of families we enrolled, and this intervention may be especially relevant for the growing number of practices focused on improving asthma outcomes. Second, because of the small number of subjects in this study, randomization did not fully balance asthma severity between intervention and control subjects. However, in multiple sensitivity analyses, we found similar results. In addition, results from analyses comparing the change in control within each child over the study period consistently showed better outcomes for the intervention group. Third, this study involved a portal that engaged both parents and the clinical team. Results may not generalize to self-management tools for asthma that do not engage clinicians. Fourth, the acceptability questions had face validity, but were not pretested or otherwise validated. Fifth, the EHR was used to measure the number of prescriptions received by children in the study. This method captured all prescriptions written by providers within the CHOP network, including the ED or hospital; however, prescriptions written by outside asthma specialists might not have been present. Finally, clinical outcomes were primarily based on parent report.
Conclusions
We found that MyAsthma, an EHR-linked SDM portal for pediatric asthma, was feasible and acceptable to families, and improved outcomes. These promising early results support the use of asthma portals tightly integrated with office systems and responsive to parents’ concerns and goals to improve asthma care and outcomes.
Supplementary Material
Acknowledgments
The authors thank LeMar Davidson, Mark Ramos, Jim Massey, Trude Haecker, Tyra Bryant-Stephens, Joe Zorc, Alison Marx, Kathy Shaw, and the Chair’s Initiative from CHOP, as well as Peter White from Cincinnati Children’s Hospital Medical Center, for their support with the conduct of this research. Study data were collected and managed using REDCap electronic data capture tools hosted at CHOP. REDCap is a secure, Web-based application designed to support data capture for research studies, providing an intuitive interface for validated data entry, audit trails for tracking data manipulation and export procedures, automated export procedures for seamless data downloads to common statistical packages, and procedures for importing data from external sources.
Footnotes
Dr Fiks contributed to the conception and design of the study, acquisition of data, analysis and interpretation of the data, drafted the manuscript, and approved the final manuscript as submitted; Ms Mayne contributed to the acquisition of data, analysis and interpretation of data, drafting the manuscript, and approved the final manuscript as submitted; Mr Karavite, Mr Suh, and Mr O’Hara contributed to the acquisition of data, critically reviewed the manuscript, and approved the manuscript as submitted; Drs Localio, Ross, and Grundmeier contributed to the conception and design of the study, analysis and interpretation of data, critically reviewed the manuscript, and approved the final manuscript as submitted.
This trial has been registered at clinicaltrials.gov (identifier NCT01715389).
FINANCIAL DISCLOSURE: Dr Fiks is a co-inventor of the “Care Assistant” software that was used to implement the portal in the electronic medical record in this study. He holds no patent on the software and has earned no money from this invention. No licensing agreement exists. Results have been independently verified by Russell Localio. Dr Grundmeier is a co-inventor of the “Care Assistant” software that was used to implement the portal in the electronic medical record in this study. He holds no patent on the software and has earned no money from this invention. No licensing agreement exists. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This project was supported by the Chair’s Initiative Grant and the William Wikoff Smith Endowed Chair in Pediatric Genomics from Children’s Hospital of Philadelphia, and by award number K23HD059919 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. None of the sponsors participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of Children’s Hospital of Philadelphia or National Institute of Child Health and Human Development. This study also was supported by the Chronic Care Initiative, a Pennsylvania state learning collaborative begun by the Pennsylvania Governor’s Office of Healthcare Reform in 2008 and now supported by the Centers for Medicare and Medicaid Services. The Pediatric Research Consortium was established with funding from the Agency for Health Care Research and Quality, and is part of the Center for Pediatric Practice Research and Learning, funded by the Agency for Health Care Research and Quality (1P30HS021645). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Drs Fiks and Grundmeier are co-inventors of the “Care Assistant” software that was used to implement the portal in the electronic medical record in this study. They hold no patent on the software and have earned no money from this invention. No licensing agreement exists. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623–630 [DOI] [PubMed] [Google Scholar]
- 2.Andrews R, Morgan JD, Addy DP, McNeish AS. Understanding non-attendance in outpatient paediatric clinics. Arch Dis Child. 1990;65(2):192–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabana MD, Bruckman D, Bratton SL, Kemper AR, Clark NM. Association between outpatient follow-up and pediatric emergency department asthma visits. J Asthma. 2003;40(7):741–749 [DOI] [PubMed] [Google Scholar]
- 4.Fiks AG, Grundmeier RW, Mayne S, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–692 [DOI] [PubMed] [Google Scholar]
- 6.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg MW, Van Busum K, Wexler R, Bowen M, Schneider EC. A demonstration of shared decision making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood). 2013;32(2):268–275 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Medicare and Medicaid Services. Medicaid electronic health record incentive payments for eligible professionals. 2010. Available at: http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/index.html?redirect=/ehrincentiveprograms/. Accessed January 29, 2015
- 9.Institute of Medicine. Committee on Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009 [Google Scholar]
- 10.Ammenwerth E, Schnell-Inderst P, Hoerbst A. The impact of electronic patient portals on patient care: a systematic review of controlled trials. J Med Internet Res. 2012;14(6):e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourgeois FC, Mandl KD, Shaw D, Flemming D, Nigrin DJ. Mychildren's: integration of a personally controlled health record with a tethered patient portal for a pediatric and adolescent population. AMIA Annu Symp Proc. 2009;2009:65–69 [PMC free article] [PubMed]
- 12.Silvestre AL, Sue VM, Allen JY. If you build it, will they come? The Kaiser Permanente model of online health care. Health Aff (Millwood). 2009;28(2):334–344 [DOI] [PubMed] [Google Scholar]
- 13.Bloom B, Cohen RA, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2010. Vital Health Stat 10. 2011;(250):1–80 [PubMed] [Google Scholar]
- 14.Dean BB, Calimlim BM, Kindermann SL, Khandker RK, Tinkelman D. The impact of uncontrolled asthma on absenteeism and health-related quality of life. J Asthma. 2009;46(9):861–866 [DOI] [PubMed] [Google Scholar]
- 15.Laforest L, Yin D, Kocevar VS, et al. Association between asthma control in children and loss of workdays by caregivers. Ann Allergy Asthma Immunol. 2004;93(3):265–271 [DOI] [PubMed] [Google Scholar]
- 16.Schmier JK, Manjunath R, Halpern MT, Jones ML, Thompson K, Diette GB. The impact of inadequately controlled asthma in urban children on quality of life and productivity. Ann Allergy Asthma Immunol. 2007;98(3):245–251 [DOI] [PubMed] [Google Scholar]
- 17.Guilbert TW, Garris C, Jhingran P, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. 2011;48(2):126–132 [DOI] [PubMed] [Google Scholar]
- 18.Akinbami L, Centers for Disease Control and Prevention National Center for Health Statistics . The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;(381):1–24 [PubMed] [Google Scholar]
- 19.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in admissions for pediatric status asthmaticus in New Jersey over a 15-year period. Pediatrics. 2010;126(4). Available at: www.pediatrics.org/cgi/content/full/126/4/e904 [DOI] [PubMed] [Google Scholar]
- 20.Fiks AG, Localio AR, Alessandrini EA, Asch DA, Guevara JP. Shared decision-making in pediatrics: a national perspective. Pediatrics. 2010;126(2):306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiks AG, Mayne S, Localio AR, Alessandrini EA, Guevara JP. Shared decision-making and health care expenditures among children with special health care needs. Pediatrics. 2012;129(1):99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiks AG, Grundmeier RW, Margolis B, et al. Comparative effectiveness research using the electronic medical record: an emerging area of investigation in pediatric primary care. J Pediatr. 2012;160(5):719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiks AG, Mayne S, Karavite DJ, DeBartolo E, Grundmeier RW. A shared e-decision support portal for pediatric asthma. J Ambul Care Manage. 2014;37(2):120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007
- 25.Bell LM, Grundmeier R, Localio R, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/125/4/e770 [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 pt 1):1918–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukstein DA, McGrath MM, Buchner DA, Landgraf J, Goss TF. Evaluation of a short form for measuring health-related quality of life among pediatric asthma patients. J Allergy Clin Immunol. 2000;105(2 pt 1):245–251 [DOI] [PubMed] [Google Scholar]
- 29.Zorc JJ, Pawlowski NA, Allen JL, et al. Development and validation of an instrument to measure asthma symptom control in children. J Asthma. 2006;43(10):753–758 [DOI] [PubMed] [Google Scholar]
- 30.Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self-management behaviors of inner-city children: a randomized trial of the Health Buddy interactive device and an asthma diary. Arch Pediatr Adolesc Med. 2002;156(2):114–120 [DOI] [PubMed] [Google Scholar]
- 31.Gustafson D, Wise M, Bhattacharya A, et al. The effects of combining Web-based eHealth with telephone nurse case management for pediatric asthma control: a randomized controlled trial. J Med Internet Res. 2012;14(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jan RL, Wang JY, Huang MC, Tseng SM, Su HJ, Liu LF. An Internet-based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemed J E Health. 2007;13(3):257–268 [DOI] [PubMed] [Google Scholar]
- 33.Joseph CL, Ownby DR, Havstad SL, et al. Research team members . Evaluation of a web-based asthma management intervention program for urban teenagers: reaching the hard to reach. J Adolesc Health. 2013;52(4):419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph CL, Peterson E, Havstad S, et al. Asthma in Adolescents Research Team . A web-based, tailored asthma management program for urban African-American high school students. Am J Respir Crit Care Med. 2007;175(9):888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viswanath K, Kreuter MW. Health disparities, communication inequalities, and eHealth. Am J Prev Med. 2007;32(suppl 5):S131–S133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorence DP, Park H, Fox S. Racial disparities in health information access: resilience of the Digital Divide. J Med Syst. 2006;30(4):241–249 [DOI] [PubMed] [Google Scholar]
- 37.Shields AE, Shin P, Leu MG, et al. Adoption of health information technology in community health centers: results of a national survey. Health Aff (Millwood). 2007;26(5):1373–1383 [DOI] [PubMed] [Google Scholar]
- 38.Smith A. Smartphone Ownership 2012. Washington, DC: Pew Internet and American Life Project; 2012 [Google Scholar]
- 39.Fox S. Mobile Health 2012. Washington, DC: Pew Internet and American Life Project; 2012 [Google Scholar]
- 40.Adams WG, Fuhlbrigge AL, Miller CW, et al. TLC-Asthma: an integrated information system for patient-centered monitoring, case management, and point-of-care decision support. AMIA Annu Symp Proc. 2003:1–5 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.