Abstract
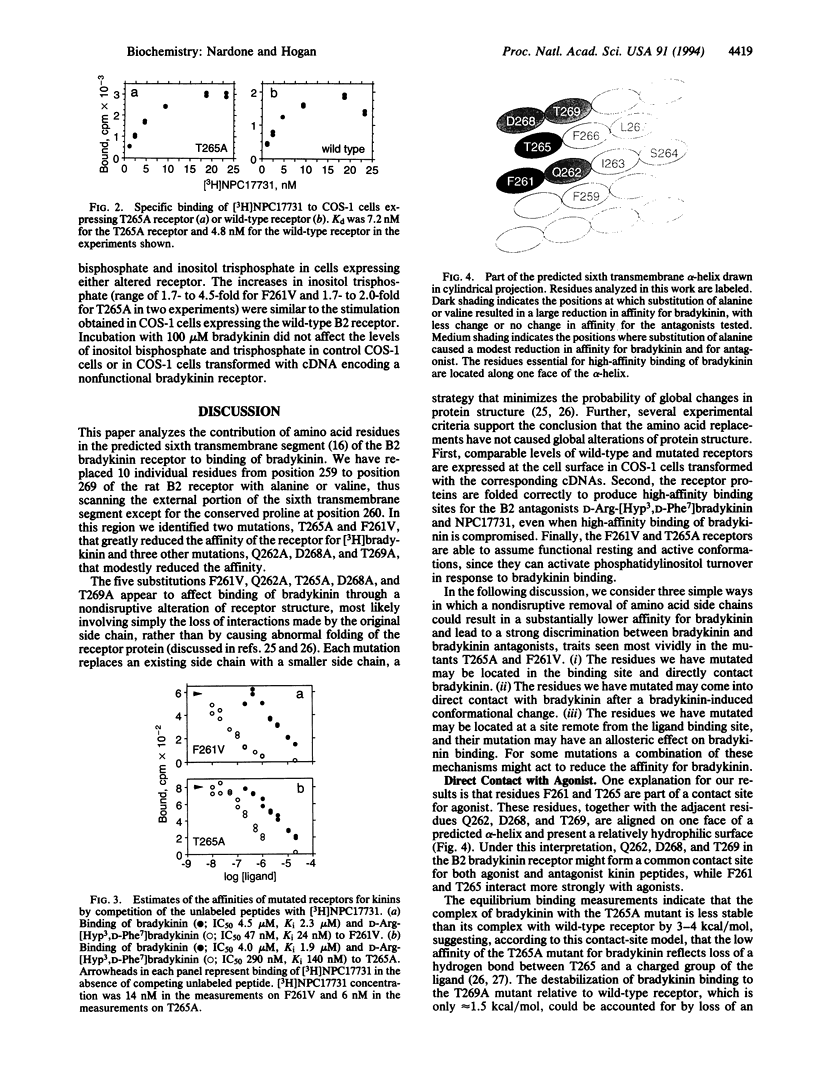
We have made mutations in the predicted sixth transmembrane segment of a rat B2 bradykinin receptor and analyzed the variant proteins by expressing them in COS-1 cells. Two amino acid substitutions reduced the affinity of the receptor for bradykinin (Phe261-->Val by 1600-fold; Thr265-->Ala by 700-fold) with comparatively little effect on the affinity for the bradykinin antagonists NPC17731 and D-Arg-[Hyp3,D-Phe7]bradykinin (where Hyp is hydroxyproline). Three other substitutions (Gln262-->Ala, Asp268-->Ala, and Thr269-->Ala) modestly reduced the affinity for bradykinin and for the antagonist D-Arg-[Hyp3,D-Phe7]bradykinin. Even the most dramatically affected mutated receptors were still able to couple, after bradykinin binding, to phosphatidylinositol turnover. The data suggest that bradykinin directly contacts the face of the sixth transmembrane helix formed by the residues Phe261, Gln262, Thr265, Asp268, and Thr269 or that this face of the helix is the site of intraprotein contacts that serve to stabilize the agonist-binding conformation of the receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993 Apr;12(4):1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathon J. M., Proud D. Bradykinin antagonists. Annu Rev Pharmacol Toxicol. 1991;31:129–162. doi: 10.1146/annurev.pa.31.040191.001021. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Farmer S. G., Steranka L. R. Bradykinin receptor antagonists. Med Res Rev. 1990 Apr-Jun;10(2):237–269. doi: 10.1002/med.2610100204. [DOI] [PubMed] [Google Scholar]
- Chan T., Lee M., Sakmar T. P. Introduction of hydroxyl-bearing amino acids causes bathochromic spectral shifts in rhodopsin. Amino acid substitutions responsible for red-green color pigment spectral tuning. J Biol Chem. 1992 May 15;267(14):9478–9480. [PubMed] [Google Scholar]
- Chung F. Z., Wang C. D., Potter P. C., Venter J. C., Fraser C. M. Site-directed mutagenesis and continuous expression of human beta-adrenergic receptors. Identification of a conserved aspartate residue involved in agonist binding and receptor activation. J Biol Chem. 1988 Mar 25;263(9):4052–4055. [PubMed] [Google Scholar]
- Eggerickx D., Raspe E., Bertrand D., Vassart G., Parmentier M. Molecular cloning, functional expression and pharmacological characterization of a human bradykinin B2 receptor gene. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1306–1313. doi: 10.1016/0006-291x(92)90445-q. [DOI] [PubMed] [Google Scholar]
- Farmer S. G. Role of kinins in airway diseases. Immunopharmacology. 1991 Jul-Aug;22(1):1–20. doi: 10.1016/0162-3109(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Leatherbarrow R. J., Wells T. N. Structure-activity relationships in engineered proteins: analysis of use of binding energy by linear free energy relationships. Biochemistry. 1987 Sep 22;26(19):6030–6038. doi: 10.1021/bi00393a013. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Cascieri M. A., Yu H., Bansal A., Swain C., Strader C. D. Amino-aromatic interaction between histidine 197 of the neurokinin-1 receptor and CP 96345. Nature. 1993 Mar 25;362(6418):350–353. doi: 10.1038/362350a0. [DOI] [PubMed] [Google Scholar]
- Fraser C. M., Wang C. D., Robinson D. A., Gocayne J. D., Venter J. C. Site-directed mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Mol Pharmacol. 1989 Dec;36(6):840–847. [PubMed] [Google Scholar]
- Garcia K. C., Ronco P. M., Verroust P. J., Brünger A. T., Amzel L. M. Three-dimensional structure of an angiotensin II-Fab complex at 3 A: hormone recognition by an anti-idiotypic antibody. Science. 1992 Jul 24;257(5069):502–507. doi: 10.1126/science.1636085. [DOI] [PubMed] [Google Scholar]
- HAGINS W. A. Flash photolysis of rhodopsin in the retina. Nature. 1956 May 26;177(4517):989–990. doi: 10.1038/177989b0. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Hibert M. F., Trumpp-Kallmeyer S., Bruinvels A., Hoflack J. Three-dimensional models of neurotransmitter G-binding protein-coupled receptors. Mol Pharmacol. 1991 Jul;40(1):8–15. [PubMed] [Google Scholar]
- Horstman D. A., Brandon S., Wilson A. L., Guyer C. A., Cragoe E. J., Jr, Limbird L. E. An aspartate conserved among G-protein receptors confers allosteric regulation of alpha 2-adrenergic receptors by sodium. J Biol Chem. 1990 Dec 15;265(35):21590–21595. [PubMed] [Google Scholar]
- Kellis J. T., Jr, Nyberg K., Sali D., Fersht A. R. Contribution of hydrophobic interactions to protein stability. Nature. 1988 Jun 23;333(6175):784–786. doi: 10.1038/333784a0. [DOI] [PubMed] [Google Scholar]
- Kjelsberg M. A., Cotecchia S., Ostrowski J., Caron M. G., Lefkowitz R. J. Constitutive activation of the alpha 1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J Biol Chem. 1992 Jan 25;267(3):1430–1433. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Martorana P. A., Kettenbach B., Breipohl G., Linz W., Schölkens B. A. Reduction of infarct size by local angiotensin-converting enzyme inhibition is abolished by a bradykinin antagonist. Eur J Pharmacol. 1990 Jul 3;182(2):395–396. doi: 10.1016/0014-2999(90)90301-l. [DOI] [PubMed] [Google Scholar]
- McEachern A. E., Shelton E. R., Bhakta S., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R. W., Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merbs S. L., Nathans J. Absorption spectra of the hybrid pigments responsible for anomalous color vision. Science. 1992 Oct 16;258(5081):464–466. doi: 10.1126/science.1411542. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J., Insel P. A. Influence of sodium on the alpha 2-adrenergic receptor system of human platelets. Role for intraplatelet sodium in receptor binding. J Biol Chem. 1983 Mar 25;258(6):3913–3919. [PubMed] [Google Scholar]
- Nakayama T. A., Khorana H. G. Mapping of the amino acids in membrane-embedded helices that interact with the retinal chromophore in bovine rhodopsin. J Biol Chem. 1991 Mar 5;266(7):4269–4275. [PubMed] [Google Scholar]
- Nakayama T. A., Khorana H. G. Orientation of retinal in bovine rhodopsin determined by cross-linking using a photoactivatable analog of 11-cis-retinal. J Biol Chem. 1990 Sep 15;265(26):15762–15769. [PubMed] [Google Scholar]
- Nardone J., Gerald C., Rimawi L., Song L., Hogan P. G. Identification of a B2 bradykinin receptor expressed by PC12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4412–4416. doi: 10.1073/pnas.91.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitz M., Neitz J., Jacobs G. H. Spectral tuning of pigments underlying red-green color vision. Science. 1991 May 17;252(5008):971–974. doi: 10.1126/science.1903559. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Cox B. A., Henningsen R. A., Spanoyannis A., Neve R. L. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol Pharmacol. 1991 Jun;39(6):733–739. [PubMed] [Google Scholar]
- Perlman J. H., Nussenzveig D. R., Osman R., Gershengorn M. C. Thyrotropin-releasing hormone binding to the mouse pituitary receptor does not involve ionic interactions. A model for neutral peptide binding to G protein-coupled receptors. J Biol Chem. 1992 Dec 5;267(34):24413–24417. [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steranka L. R., Farmer S. G., Burch R. M. Antagonists of B2 bradykinin receptors. FASEB J. 1989 Jul;3(9):2019–2025. doi: 10.1096/fasebj.3.9.2545496. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Candelore M. R., Hill W. S., Sigal I. S., Dixon R. A. Identification of two serine residues involved in agonist activation of the beta-adrenergic receptor. J Biol Chem. 1989 Aug 15;264(23):13572–13578. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Candelore M. R., Rands E., Hill W. S., Dixon R. A. Conserved aspartic acid residues 79 and 113 of the beta-adrenergic receptor have different roles in receptor function. J Biol Chem. 1988 Jul 25;263(21):10267–10271. [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Register R. B., Candelore M. R., Rands E., Dixon R. A. Identification of residues required for ligand binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4384–4388. doi: 10.1073/pnas.84.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Horstman D. A., Akbarali H., Limbird L. E. A point mutation of the alpha 2-adrenoceptor that blocks coupling to potassium but not calcium currents. Science. 1992 Aug 14;257(5072):977–980. doi: 10.1126/science.1354394. [DOI] [PubMed] [Google Scholar]
- Vegh A., Szekeres L., Parratt J. R. Local intracoronary infusions of bradykinin profoundly reduce the severity of ischaemia-induced arrhythmias in anaesthetized dogs. Br J Pharmacol. 1991 Oct;104(2):294–295. doi: 10.1111/j.1476-5381.1991.tb12424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Ward W. H., Timms D., Fersht A. R. Protein engineering and the study of structure--function relationships in receptors. Trends Pharmacol Sci. 1990 Jul;11(7):280–284. doi: 10.1016/0165-6147(90)90009-w. [DOI] [PubMed] [Google Scholar]
- Wess J., Gdula D., Brann M. R. Site-directed mutagenesis of the m3 muscarinic receptor: identification of a series of threonine and tyrosine residues involved in agonist but not antagonist binding. EMBO J. 1991 Dec;10(12):3729–3734. doi: 10.1002/j.1460-2075.1991.tb04941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J., Maggio R., Palmer J. R., Vogel Z. Role of conserved threonine and tyrosine residues in acetylcholine binding and muscarinic receptor activation. A study with m3 muscarinic receptor point mutants. J Biol Chem. 1992 Sep 25;267(27):19313–19319. [PubMed] [Google Scholar]



