Abstract
Thrombolysis is widely used to intervene in acute ischemic stroke, but reestablishment of circulation may paradoxically initiate a reperfusion injury. Here we describe studies with mice lacking protein kinase Cδ (PKCδ) showing that absence of this enzyme markedly reduces reperfusion injury following transient ischemia. This was associated with reduced infiltration of peripheral blood neutrophils into infarcted tissue and with impaired neutrophil adhesion, migration, respiratory burst, and degranulation in vitro. Total body irradiation followed by transplantation with bone marrow from PKCδ-null mice donors reduced infarct size and improved neurological outcome in WT mice, whereas marrow transplantation from WT donors increased infarction and worsened neurological scores in PKCδ-null mice. These results indicate an important role for neutrophil PKCδ in reperfusion injury and strongly suggest that PKCδ inhibitors could prove useful in the treatment of stroke.
Introduction
Ischemic stroke is the most common fatal neurological disease and the leading cause of long-term disability in the United States (1). Fibrinolytic agents such as tissue plasminogen activator (t-PA) are currently the only drugs approved for pharmacological intervention to reverse acute ischemic stroke. Early treatment can be effective in reducing ischemic damage, but a limiting complication is the occurrence of tissue injury caused by reperfusion following recanalization of the occluded vessel (2, 3). Therefore, there is great interest in developing treatments that can limit reperfusion injury.
During reperfusion, reintroduction of oxygenated blood to ischemic tissues initiates processes leading to the generation of free radicals and, importantly, infiltration of polymorphonuclear leukocytes, also referred to as neutrophils (4). Under quiescent conditions, neutrophils circulate freely and do not interact significantly with the endothelium. In response to tissue ischemia, neutrophils adhere and migrate through the endothelium of the cerebral microvasculature. At extravascular sites neutrophils produce free radicals (O2–; OH–), release proteolytic enzymes, and stimulate cytokine release from neighboring cells, thereby promoting further recruitment of neutrophils and other leukocytes. Depletion of neutrophils (5), inhibition of neutrophil adhesion (5), or inhibition of proteolytic enzymes such as elastase that are released from neutrophils (6) reduces injury size and improves neurological deficits in experimental stroke models.
It is clear that multiple steps of neutrophil recruitment provide potential targets for therapeutic intervention. Studies using phorbol esters that activate protein kinase C (PKC) suggest that several neutrophil functions, including superoxide anion generation, cell adhesion, and degranulation, are stimulated by PKC (7). The PKC family of serine/threonine kinases is composed of at least ten isozymes with distinctive means of regulation and tissue distribution (8). Five isozymes (PKCα, βI, βII, δ, and ζ) are known to be present in human neutrophils (7). The exact functional role of these different PKC isozymes in neutrophils remains to be specified.
PKCδ is a member of the novel PKC subfamily that is activated by diacylglycerol (DAG) but not by calcium. PKCδ promotes apoptosis in various types of cultured cells (9), including neuronal cells (10). In mice that lack PKCδ, reduced cell death is associated with smooth muscle cell accumulation, which leads to accelerated arteriosclerosis of vein bypass grafts (11) and increased B cell proliferation associated with impaired antigen-induced B cell self-tolerance and autoimmune disease in later life (12, 13). Studies using PKCδ peptide inhibitors and activators indicate an important role of PKCδ in cardiac ischemia and reperfusion (14). The mechanism by which PKCδ promotes reperfusion damage in the heart remains to be demonstrated.
Despite evidence linking PKCδ with smooth muscle and B cell death and reperfusion injury in cardiomyocytes, very little is known about its role in CNS disease. To investigate the potential role of PKCδ in nervous system ischemic injury, we generated mice that lack PKCδ and studied their response to cerebral ischemia. Using a model of transient middle cerebral artery (MCA) occlusion (MCAO), we found that PKCδ-null mice show a 70% reduction in stroke size compared with WT mice. This was associated with impaired neutrophil function and reduced neutrophil migration into ischemic tissue in PKCδ-null mice. Treatment with total body irradiation followed by transplantation with bone marrow from the opposite genotype reversed the stroke phenotypes in WT and PKCδ-null mice, confirming an important role for neutrophil PKCδ in reperfusion injury.
Results
Infarct size is reduced in PKCδ-null mice after transient, but not permanent, MCAO.
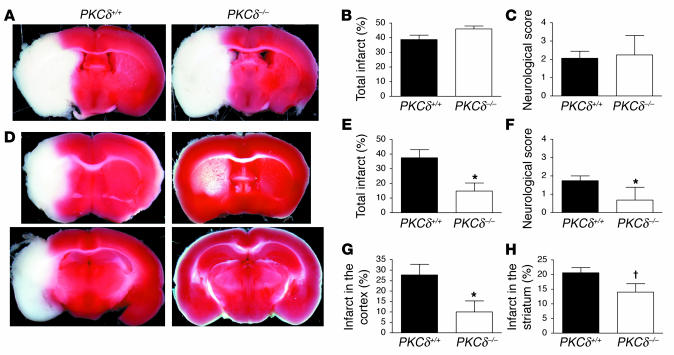
We generated mice that lack PKCδ using homologous recombination in ES cells (Figure 1). Neither full-length nor truncated PKCδ-like immunoreactivity was detected in PKCδ-null mice cells by Western blot analysis (Figure 1D and data not shown). These mice showed no gross or microscopic neuroanatomical abnormalities and no motor deficits on open field testing or on an accelerating rotarod (data not shown). To study the response of these animals to stroke, we first used a model of permanent cerebral ischemia in which an infarction in the MCA distribution is produced by occluding the vessel lumen with a suture. Placement of the suture for 20 hours resulted in cerebral infarcts of similar size in PKCδ-null and WT littermates (Figure 2, A and B). The neurological examination also showed a similar degree of impairment in both genotypes 20 hours after initiation of ischemia (Figure 2C). Since permanent occlusion, when severe, induces cell death that is mainly necrotic (15), these findings suggested that PKCδ does not play an essential role in ischemia-induced necrosis. Given the evidence for PKCδ as a mediator of apoptosis, we next subjected mice to a transient ischemia-reperfusion paradigm, in which apoptosis plays an important role in cell death (16). Interestingly, infarct size was decreased by about 65% in PKCδ-null mice compared with WT littermates (Figure 2, D and E) after 1 hour of ischemia followed by 24 hours of reperfusion. This correlated with a better neurological score in PKCδ-null mice compared with WT mice 24 hours following the ischemic period (Figure 2F). The infarct size was decreased more in the cortex than in the striatum of PKCδ-null mice (Figure 2, G and H). The attenuated infarct size in PKCδ-null mice was not due to a delay in the progression of infarction, since infarct size in PKCδ-null mice after 1 hour of ischemia and 7 days of reperfusion was similar to infarct size measured after 24 hours of reperfusion (data not shown).
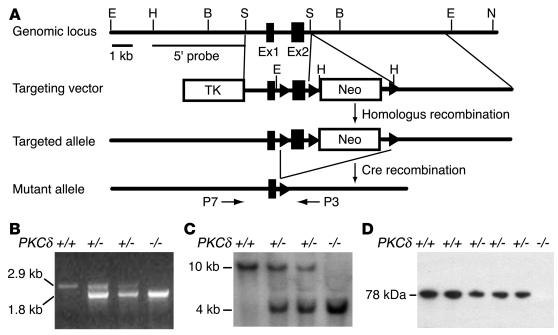
Figure 1.
Generation of PKCδ-null mice. (A) Organization of the mouse PKCδ gene, the targeting construct, and the allele resulting from homologous recombination. Boxes represent exon 1 (123 bp) and exon 2 (200 bp). Arrowheads represent loxP sequences. Only relevant restriction enzyme sites are indicated. E, EcoRI; H, HindIII; B, BamHI; S, SacI; N, NotI. (B) Verification of genotypes by PCR using the primers P7 and P3. The WT allele (+/+) generates a 2.9-kb and the mutant allele (−/−) a 1.8-kb band, as indicated. (C) Verification of genotypes by Southern blot analysis of genomic DNA digested with EcoRI from WT (+/+), heterozygous (+/ –), and homozygous mutants ( –/ –) using a 3.2-kb 5′-probe (HindIII-SacI fragment). (D) Verification of genotypes by Western blot analysis of whole brain lysates using a mAb against PKCδ. The migration of PKCδ immunoreactivity (78 kDa) is indicated.
Figure 2.
Infarct size is reduced in PKCδ-null mice after transient, but not permanent, MCAO. (A) Shown are representative images of TTC-stained brain slices after 20 hours of permanent MCAO from PKCδ+/+ and PKCδ –/ – mice. Viable tissue is stained red, whereas the ischemic area remains unstained (white). (B) Infarct size measured as a percentage of the area of the nonischemic hemisphere after 20 hours of permanent MCAO from PKCδ+/+ (n = 8) and PKCδ –/ – mice (n = 8). (C) Neurological deficit scores after 20 hours of permanent MCAO from PKCδ+/+ (n = 8) and PKCδ –/ – mice (n = 8). (D) Representative images of TTC-stained brain slices after 1 hour of MCAO and 24 hours of reperfusion from PKCδ+/+ and PKCδ –/ – mice. (E) Total infarct size after 1 hour of MCAO and 24 hours of reperfusion from PKCδ+/+ (n = 10) and PKCδ –/ – (n = 9) mice. (F) Neurological deficit scores after 1 hour of MCAO and 24 hours of reperfusion from PKCδ+/+ (n = 8) and PKCδ –/ – mice (n = 8). (G and H) Infarct size in the cerebral cortex (G) and striatum (H) after 1 hour of MCAO and 24 hours of reperfusion from PKCδ+/+ (n = 10) and PKCδ –/ – (n = 9) mice. *P < 0.05 compared with WT littermates (two-tailed, unpaired t test). P < 0.05 compared with WT littermates (one-tailed, unpaired t test).
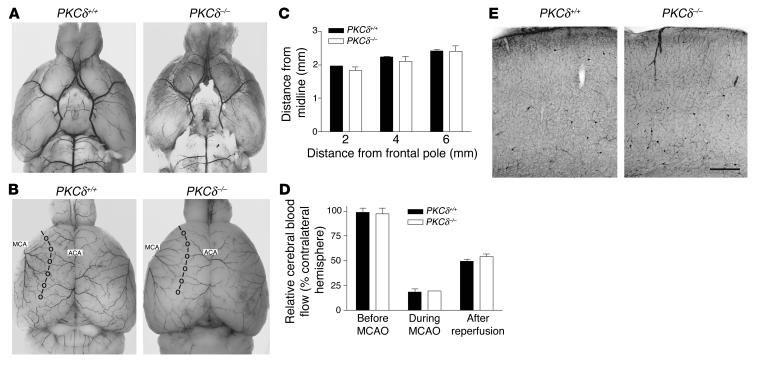
To investigate whether the attenuated infarct size in PKCδ-null mice was due to an alteration in cerebrovascular anatomy, we examined the distribution of cerebral vessels by India ink injection. No differences were observed in the origins of the MCA or other major blood vessels of the circle of Willis (Figure 3A). The distribution of the MCA territory was also similar in WT and PKCδ-null mice (Figure 3, B and C). Additionally, cerebral blood flow was similar in both genotypes before and during ischemia and after reperfusion (Figure 3D). The appearance of the brain microvasculature was also similar in both genotypes (Figure 3E).
Figure 3.
Cerebrovascular anatomy and cerebral blood flow. (A and B) Shown are representative images of the ventral (A) and dorsal (B) surfaces of brains from PKCδ+/+ and PKCδ –/ – mice perfused with black ink. The points of anastomoses between the MCA and the ACA are circled and connected by the line of anastomoses to define the respective vascular territories. (C) Distances from the line of anastomoses to the midline in PKCδ+/+ (n = 3) and PKCδ –/ – (n = 3) mice were measured at coronal planes 2, 4, and 6 mm from the frontal pole. (D) Regional cerebral blood flow before and during 1 hour of MCAO and during the first hour of reperfusion in PKCδ+/+ (n = 3) and PKCδ –/ – (n = 3) mice. Relative cerebral blood flow was expressed as the percentage of the Doppler signal intensity of the ischemic compared with the contralateral hemisphere. (E) The microvasculature in the cortex of PKCδ+/+ and PKCδ –/ – mice revealed by NADPH diaphorase histochemistry. The scale bar corresponds to 250 & μ;m.
PKCδ has been postulated to be involved in ischemic brain injury since PKCδ mRNA and protein are induced in rat brain tissue surrounding an ischemic lesion several hours to days after transient cerebral ischemia (17). If neuronal PKCδ is responsible for the difference in stroke size between PKCδ WT and null mice, then PKCδ should be abundant in brain regions within the MCA territory of WT mice that were spared in PKCδ-null mice. In rodent brain, PKCδ is expressed predominantly in neurons of the thalamus and their axonal projections throughout the forebrain, especially in the neocortex, although much lower expression levels are also present in other areas (18). Several brain regions that were spared in PKCδ-null mice (Figure 2D), however, contain neurons with little or no PKCδ immunoreactivity in WT mice (Figure 4, A and B). The mismatch between patterns of ischemic infarction and PKCδ expression in WT mice led us away from studies of PKCδ and apoptosis in neural tissues. Instead, we considered whether other tissues outside of the brain explain differences in stroke size between WT and PKCδ-null mice.
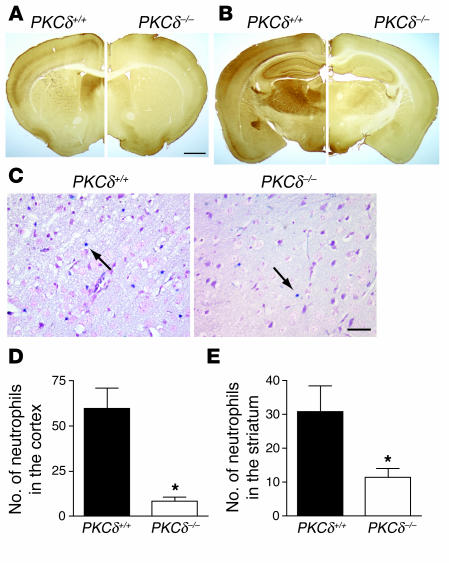
Figure 4.
Expression pattern of PKCδ in the brain and extravascular neutrophils after transient MCA occlusion. (A and B) Immunocytochemical localizations of PKCδ in the mouse brain are shown in coronal sections at bregma 0.38 mm (A) and a more posterior section at bregma –1.58 mm (B) for PKCδ+/+ (left) and PKCδ –/ – (right) mice. (C) Representative sections from PKCδ+/+ and PKCδ –/ – mice showing reduced neutrophil accumulation within infarcted tissue in the cortex of PKCδ –/ – mice after 1 hour of MCAO and 24 hours of reperfusion. Blue infiltrated neutrophils were identified in the ischemic cortex by staining for esterase activity with dichloroacetate (arrows). (D and E) Number of extravascular neutrophils in the ischemic cortex and striatum of PKCδ+/+ (n = 7) and PKCδ –/ – mice (n = 6) after transient MCAO. No esterase staining was seen in sections from nonischemic animals (data not shown). The scale bars in A and C correspond to 1 mm and 25 & μ;m, respectively. *P < 0.05 compared with WT littermates.
Attenuated neutrophil function in PKCδ-null mice.
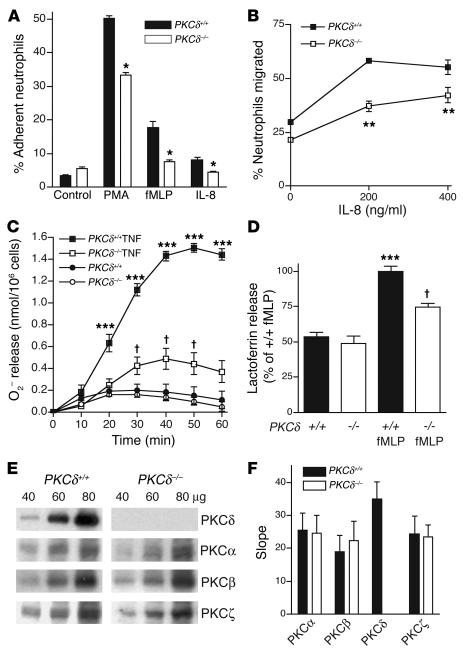
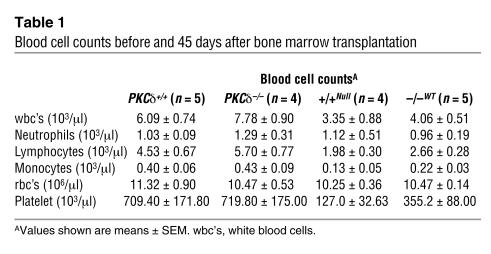
Neutrophils from the peripheral blood infiltrate into ischemic brain tissue during reperfusion and may contribute to reperfusion injury, since inhibition of neutrophil adhesion or depletion of neutrophils with anti-neutrophil adhesion Ab’s reduces apoptosis following transient ischemia (4, 19). To examine whether neutrophil infiltration was altered in PKCδ-null mice, we identified neutrophils in brain sections by chloroacetate esterase staining 24 hours after 1 hour of transient ischemia. We found approximately 80% fewer neutrophils in the cerebral cortex and 65% fewer neutrophils in the striatum of PKCδ-null mice compared with WT littermates (Figure 4, C–E). These findings correlated with in vitro studies showing reduced adhesion (Figure 5A) and migration (Figure 5B) of neutrophils from PKCδ-null mice compared with WT littermates. In addition, superoxide anion (O2–) release in response to TNF-α, formyl-Met-Leu-Phe (fMLP) peptide, or phorbol ester (Figure 5C and data not shown), and degranulation measured as lactoferrin release in response to fMLP (Figure 5D) or phorbol ester (data not shown), were reduced in neutrophils from PKCδ-null mice compared with WT mice. Despite these differences in function, there was no difference in the number of neutrophils or other cells in the peripheral blood of PKCδ-null mice when compared with WT littermates (Table 1). The abundance of other PKC isozymes expressed in neutrophils was also not altered in PKCδ-null mice compared with WT mice (Figure 5, E and F). These findings indicate an important role for PKCδ in several aspects of neutrophil function.
Figure 5.
Decreased neutrophil function in PKCδ-null mice. (A) Neutrophil adhesion stimulated by PMA (100 nM), fMLP peptide (1 & μ;M), or IL-8 (200 ng/ml). *P < 0.05 compared with WT littermates (two-tailed, unpaired t tests). (B) IL-8–stimulated neutrophil migration differed by genotype [F(1, 6) = 54.8; P = 0.0003] and treatment [F(2, 6) = 63.08; P < 0.0001] without significant interaction between these factors [F(2, 6) = 3.9; NS]. **P < 0.05 compared with PKCδ+/+ at same dose (Bonferroni test). (C) TNF-α–stimulated (20 ng/ml) superoxide anion production showed main effects of treatment [F(1, 224) = 236; P < 0.0001], genotype [F(1, 224) = 506; P < 0.0001], and time [F(6, 224) = 64.6; P < 0.0001] with an interaction between these factors [F(6, 224) = 14.7; P < 0.0001]. P < 0.05 compared with unstimulated PKCδ –/ – neutrophils, and ***P < 0.05 compared with unstimulated PKCδ+/+ and stimulated PKCδ –/ – neutrophils at the same time (Newman Keuls test). (D) fMLP-stimulated (10 & μ;M) lactoferrin release differed by genotype [F(1, 12) = 15.2; P = 0.0021] and treatment [F(1, 12) = 89.01; P < 0.0001] with an interaction between these factors [F(1, 12) = 7,24; P = 0.0197]. P < 0.05 compared with unstimulated PKCδ –/ – neutrophils, and ***P < 0.05 compared with unstimulated PKCδ+/+ and stimulated PKCδ –/ – neutrophils (Bonferroni tests). (E) PKC isozyme immunoreactivity in neutrophils. (F) PKC isozyme immunoreactivity determined by regression analysis of protein concentrations and corresponding OD values on Western blots (n = 3). Assays of neutrophil function (A–D) were performed in triplicate or quadruplicate from at least three animals of each genotype.
Table 1.
Blood cell counts before and 45 days after bone marrow transplantation
Exchange of bone marrow between PKCδ WT and null mice reverses their stroke phenotypes.
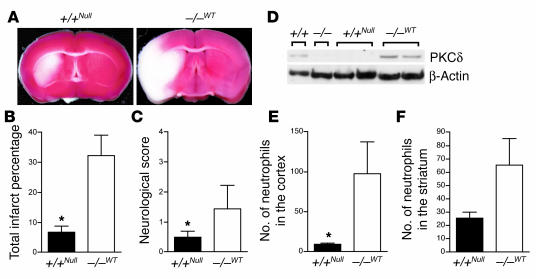
To demonstrate in vivo whether neutrophils contributed to the differences in reperfusion injury observed between PKCδ-null and WT mice, we treated mice of both genotypes with total body irradiation followed by transplantation with bone marrow from the opposite genotype. Mice were then subjected to 1 hour of cerebral ischemia followed by 24 hours of reperfusion. WT mice transplanted with PKCδ-null bone marrow showed reduced infarct size and improved neurological scores compared with PKCδ-null mice transplanted with bone marrow from WT donors (Figure 6, A–C). In addition, the number of neutrophils present in the cerebral cortex and striatum was less in WT mice transplanted with PKCδ-null bone marrow compared with PKCδ-null mice transplanted with bone marrow from WT mice (Figure 6, E and F). Survival of the transplanted tissue was verified by blood cell counts 45 days after transplantation (Table 1) and by immunoblotting with anti-PKCδ Ab’s (Figure 6D).
Figure 6.
Bone marrow transplantation reverses phenotypes in PKCδ WT and null mice. (A–C) Representative images of TTC-stained brain slices (A), infarct sizes (B), and neurological scores (C) after transient MCAO of irradiated WT mice transplanted with PKCδ-null bone marrow [(+/+NULL), n = 7] and irradiated null mice transplanted with WT marrow [( –/ –WT), n = 8]. (D) PKCδ immunoreactivity in bone marrow cells from transplanted mice to confirm the success of bone marrow transplantation. PKCδ immunoreactivity was detected in the marrow of WT (+/+) mice and irradiated null mice transplanted with WT marrow ( –/ –WT), but not in PKCδ-null mice ( –/ –) nor in irradiated WT mice transplanted with PKCδ-null bone marrow (+/+NULL). Shown are samples from one WT, one PKCδ-null, two +/+NULL, and two –/ –WT mice. Samples were normalized to β-actin immunoreactivity. (E and F) Number of extravascular neutrophils in the ischemic cortex (E) and striatum (F) of irradiated WT mice transplanted with PKCδ-null bone marrow [(+/+NULL), n = 6] and irradiated null mice transplanted with WT marrow [( –/ –WT), n = 6] after 1 hour of MCAO and 24 hours of reperfusion. *P < 0.05 compared with WT littermates.
Discussion
We believe that this study is the first to demonstrate that PKCδ is a major mediator of brain damage following transient ischemia and reperfusion in mice. Our findings also provide clear evidence that PKCδ mediates neutrophil adhesion, migration, degranulation, and superoxide generation. Impaired neutrophil function can reduce reperfusion brain injury as demonstrated, for example, in mice made deficient in Mac-1 (CD11b/CD18) (20, 21). The protective effect of PKCδ deletion was more pronounced in the cortex than in the striatum (Figure 2, G and H), which also agrees with previous studies indicating a greater role for neutrophils in damage to the cerebral cortex (22). Neutrophils are μosed to damage ischemic brain tissue by adhesion to endothelial cells and transmigration into brain parenchyma where they release oxygen-derived free radicals, phospholipases, proteases, and proinflammatory cytokines such as IL-1β and TNF-α (23). Given that PKCδ-null mice showed reduced brain injury in the setting of impaired neutrophil function, our results support further testing of inhibitors of neutrophil function as therapeutic agents to reduce infarct size and improve neurological outcome after ischemia-reperfusion injury (24, 25).
Absence of PKCδ disturbed several aspects of neutrophil function, suggesting that PKCδ regulates neutrophils through several mechanisms. Particularly striking in our results was the nearly complete absence of TNF-α–induced O2– production in neutrophils from PKCδ-null mice (Figure 5C). This is especially interesting since Brown and colleagues (26) recently found that neutrophils immunodepleted of PKCδ show deficient O2– production by NADPH oxidase. PKCδ is likely to stimulate NADPH oxidase by phosphorylating the p47phox subunit, leading to assembly and activation of the enzyme complex (27). Since mice deficient in NADPH oxidase also show reduced reperfusion injury following transient ischemia (28), PKCδ regulation of NADPH oxidase is likely to be particularly important in reperfusion injury. In addition, recent evidence demonstrates that integrin-dependent T cell activation by leukocyte functional antigen 1 (LFA-1, also known as CD11a/CD18 or αLβ2) results in PKCδ-mediated phosphorylation of the LFA-1 β2 integrin cytoplasmic domain and of cytohesion, thereby promoting c-Jun–mediated transcription and activation of ERK-1 and ERK-2 MAPKs (29). These events promote differentiation of naive T cells into IFN-γ–producing Th1 cells. Since cytokine-induced neutrophil adhesion and respiratory burst can be mediated by LFA-1 (30, 31), PKCδ may also act to transduce integrin-dependent signals in neutrophils.
Although PKCδ has been implicated in radiation-induced injury (32), such a mechanism could not account for the differences we observed since the stroke phenotype was determined by the genotype of the nonirradiated donor bone marrow and not by the genotype of the irradiated recipient. Moreover, stroke volume is not altered by lethal irradiation in WT mice subjected to transient MCAO after bone marrow transplantation (28). We also considered if other bone marrow–derived cells besides neutrophils mediate reperfusion injury in transplanted WT and PKCδ-null mice. Although macrophages can be observed in and surrounding ischemic lesions in the rodent brain, they appear to be derived from resident microglia rather than from peripheral blood monocytes during the first 24 hours of reperfusion (33). Additionally, current evidence indicates that adult microglia arise from fetal macrophages rather than from adult peripheral monocytes (34). Given that anti-platelet agents such as aspirin and clopidogrel can reduce the incidence of stroke in humans (35) and reduce injury size in mouse stroke models (36, 37), we also considered whether platelet number or function was decreased in PKCδ-null mice. Although platelet counts, bleeding times, and cerebral blood flow during reperfusion were all similar in WT and PKCδ-null mice, these findings do not exclude a subtle deficit in platelet function. Considering that all clinically effective anti-platelet agents used to treat stroke prolong the bleeding time, however, our findings make it unlikely that a deficit in platelet function or number contributed to reduced reperfusion injury in PKCδ-null mice. On the other hand, in both genotypes there was a moderate decrease in platelet number following total body irradiation and bone marrow transplantation. This was more apparent in WT mice transplanted with marrow from PKCδ-null donors, which may indicate a role for PKCδ in megakaryocyte differentiation and platelet production. This difference in platelet number may have contributed to the more striking differences in stroke volume observed between the genotypes after transplantation (compare Figures 2 and 6).
In the heart PKCε contributes to protection from ischemic damage and absence of PKCε is associated with loss of ischemic preconditioning and with increased expression of PKCδ (38). Cerebral reperfusion injury in PKCδ-null mice, however, is not due to increases in PKCε since PKCε is not expressed in mouse neutrophils, and its expression in the brains of PKCδ-null and WT mice is similar (data not shown). Inhibition of PKCδ in intact rat hearts ex vivo also reduces injury due to ischemia and reperfusion; this does not involve neutrophil PKCδ, since the hearts are perfused with oxygenated buffer lacking blood cells (39). Furthermore, myocardial ischemia/reperfusion injury is similar in WT mice and in mice deficient in NADPH oxidase, whereas significant differences are observed in the cerebral ischemia/reperfusion injury between these genotypes (28). Taken together with our results, these findings indicate a unique role for neutrophil PKCδ in cerebral ischemia-reperfusion injury. This raises the intriguing possibility that PKCδ inhibitors could be useful as adjuncts to fibrinolytic therapy of acute stroke to reduce the risk of reperfusion injury and possibly lengthen the window of time during which drugs such as t-PA can be administered safely.
Methods
Generation of PKCδ-null mice.
PKCδ mutant mice were derived by homologous recombination using a targeting vector encoding loxP sequences flanking exon 3 of the mouse PKCδ gene (ENSMUSG00000021948; NCBIm32), which was introduced into 129SvJ ES cells (Figure 1A). Exon 2 includes the translation start codon. Recombinant cells were then transfected with the plasmid pPac-CRE (40), and ES cell clones lacking exon 2 were identified by Southern blot analysis and PCR. F1 generation hybrid C57Bl/6J × 129/SvJ heterozygous progeny of chimeric mice were intercrossed to generate F2 generation hybrid WT and PKCδ mutant littermates for studies. Unless otherwise stated, we used mice of both genders. The experimental mice were fed standard lab chow ad libitum. Animal care and handling procedures were approved by the Institutional Animal Care and Use Committees of the University of California San Francisco and Gallo Center in accordance with NIH guidelines.
Focal cerebral ischemia.
The surgical procedure to induce focal cerebral ischemia is a modification based on a published protocol (41). Mice weighing 25–35 g were anesthetized with 3% isoflurane in 100% O2. Following the induction of anesthesia, isoflurane was reduced to and maintained at 1.5%. Body temperature was maintained at 35.5–37°C with a heating pad throughout the surgery. A 5-0-monofilament nylon suture rounded at the tip was inserted into external carotid artery and advanced 9.0–10 mm in the internal carotid artery (ICA) past the bifurcation of the common carotid artery (CCA), to the proximal MCA. Following 60 minutes of occlusion, the suture was removed from the CCA to induce reperfusion. After 24 hours of reperfusion, the mice were sacrificed for study. Some mice were subjected to permanent MCAO by leaving the suture in the ICA for 20 hours.
Regional cerebral blood flow.
Regional cerebral blood flow was measured by laser-Doppler flowmetry with a probe (Vasamedics Inc., St. Paul, Minnesota, USA) placed on the skull 1.5 mm lateral to the midline and 2 mm posterior to the bregma. Relative cerebral blood flow measurements were made during three different periods: after anesthesia, during a 1-hour period of MCAO, and during the first hour of reperfusion. Data are expressed as the ratio of the Doppler signal intensity of the ischemic hemisphere compared with the contralateral hemisphere.
Neurological deficits.
Neurological examinations were performed 20 hours after the induction of focal cerebral ischemia by use of a four-tiered grading system: 0, no observed neurological deficit (normal); 1, inability to walk straight (mild); 2, circling toward the paretic side (moderate); 3, falling on the paretic side (moderate-severe); 4, loss of the righting reflex (severe) (20).
Determination of infarct size.
After focal cerebral ischemia, the mice were sacrificed and the brains were rapidly removed and sliced coronally at 1-mm intervals. Brain slices were incubated for 20 minutes in 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, Missouri, USA) in PBS. Stained slices were fixed in 10% neutral buffered formalin (Sigma-Aldrich) until imaging. Brain slices were photographed using a Leica M420 microscope equipped with a Sony 3CCD color video camera. A blinded observer measured the areas of ischemic infarction and of the normal contralateral half of the brain by tracing outlines of these regions on a computer screen using NIH Image 1.6.1 software. The infarcted area was expressed as a percentage of the contralateral half of the brain to eliminate the contribution of edema to the calculation (42, 43). Total infarct area was calculated by dividing the sum of infarct areas by the sum of contralateral hemi-brain areas.
Visualization of cerebrovascular anatomy.
The mapping of cerebrovascular anatomy was performed as described (44). Higgins Black Magic waterproof ink (200–250 μl; Sanford Corp., Bellwood, Illinois, USA) was injected into the left ventricle using a 26-gauge needle, and the right atrium was cut open to release the effluent. The mice were decapitated, and the heads were soaked in 10% neutral buffered formalin (Sigma-Aldrich) for 3–4 days. The brains were carefully removed from the skulls and imaged. Points of anastomoses between anterior cerebral artery (ACA) and MCA were located by tracing the peripheral branches of these vessels. Adjacent anastomotic points were connected to identify a “line of anastomoses,” which represents the border of the territories of the ACA and the MCA. The distances from the midline to the line of anastomoses were measured at coronal planes 2, 4, and 6 mm from the frontal pole. Brain capillaries were stained using NADPH diaphorase histochemistry, which reveals both endothelial and neuronal NOS in the rodent brain. This was done by post-fixing formaldehyde-fixed tissue sections overnight in 2% glutaraldehyde in PBS at 4°C, rinsing them in PBS, and incubating them in NADPH (0.5 mg/ml; Sigma-Aldrich), nitroblue tetrazolium (0.2 mg/ml; Sigma-Aldrich), and 0.3% Triton X-100 in PBS for 4 hours at 37°C.
Immunohistochemistry.
The brains were processed as described (45). Coronal sections were incubated overnight with a primary Ab (a goat polyclonal Ab recognizing the C terminus of rat PKCδ (1:1,000–1,500; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), or a mAb (1:1,500; BD Biosciences, San Diego, California, USA) generated using a peptide comprised of amino acids 114–289 of human PKCδ, which is encoded by exons 5–10 of the human PKCδ gene (ENST00000330452); this corresponds to exons 4–9 of the mouse gene (ENSMUSG00000021948; NCBIm32). Secondary Ab’s were biotinylated donkey anti-goat or anti-mouse Ab’s (1:300; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA). Incubation for 2 hours with a secondary Ab was followed by incubation in ExtrAvidin-peroxidase complex (Sigma-Aldrich), 1:3,000 in PBS, for 2 hours. Peroxidase activity was histochemically visualized with diaminobenzidine. Slides were examined using a Nikon Eclipse E600 microscope equipped with a Spot II digital color camera. Both primary Ab’s gave similar patterns of staining in the brains of WT mice. The specificity of the PKCδ Ab’s for mouse PKCδ was verified using tissue obtained from PKCδ-null mice in which immunostaining was absent. In control experiments, omitting primary Ab’s resulted in the lack of immunostaining.
Western blot analysis.
Protein lysates were subjected to SDS-PAGE. After blotting, nitrocellulose membranes were probed with mAb’s against PKCδ, PKCα, and PKCβ (BD Transduction Laboratories, San Diego, California, USA), which were used at dilutions of 1:500, 1:1,000, and 1:250, respectively. Rabbit polyclonal Ab against PKCζ (Santa Cruz Biotechnology Inc.) was used at 0.5 & μ;g/ml. Rabbit polyclonal Ab against PKCε (SN134) was used at 1:2,000 (46). Immunoreactive bands were quantified by densitometric scanning. Linear regression analysis of protein concentrations and corresponding density values was performed, and the abundance of each isozyme was expressed as the slope of the corresponding regression line (47).
Neutrophil adhesion, migration, O2– release, and degranulation.
Neutrophils were isolated by Percoll density gradient centrifugation from mouse bone marrow as described (48). Adhesion assays were performed in 96-well Falcon plates (BD Discovery Labware, Boston, Massachusetts, USA) coated with 20% FCS (49). Purified neutrophils were fluorescently labeled with calcein AM (Molecular Probes Inc., Eugene, Oregon, USA) and incubated in the wells for 30 minutes at 37°C with the chemoattractants phorbol 12-myristate, 13-acetate (100 nM; Sigma-Aldrich), fMLP (1 μM; Sigma-Aldrich), and IL-8 (200 ng/ml; Sigma-Aldrich). The total number of cells was measured by fluorescence detection using a Cytofluor II fluorescence plate reader (PerSeptive Biosystems, Framingham, Massachusetts, USA) at 485-nm excitation and 530-nm emission wavelengths. The plates were then washed several times to remove nonadherent cells, and the fluorescence remaining on the plates was measured again. The degree of cell adhesion was expressed as a percentage calculated by dividing the amount of fluorescence after washing by the amount of fluorescence before washing.
The neutrophil chemotaxis assay (in vitro migration assay) was performed as described (50). Transwell inserts (5-& μ;m pore size polycarbonate membranes; Corning Inc., Corning, New York, USA) were filled with neutrophils in suspension and were placed into media containing indicated concentrations of IL-8 in 24-well tissue culture plates. After 1 hour of incubation in a humidified CO2 incubator at 37°C, the inserts were removed, and the number of neutrophils that migrated into the bottom of the wells was determined by FACS analysis. Parallel samples of wells loaded with suspension without inserts were included to determine the maximal signal intensity from the number of cells loaded into the Transwell inserts.
Superoxide anion (O2–) release was measured by a cytochrome c reduction assay using 20 ng/ml TNF-α (BD Biosciences) and 10 & μ;M fMLP or 100 nM PMA as a stimulus (51). Degranulation stimulated by fMLP (10 & μ;M) or PMA (100 nM) was determined by the release of the specific granule marker lactoferrin using anti-human lactoferrin Ab (Sigma-Aldrich) by ELISA (52).
Blood cell counts.
Blood was collected by decapitation, and the profiles of blood samples were analyzed using a HEMAVET 850 cell counter (CDC Technologies Inc., Oxford, Connecticut, USA).
Histological detection of neutrophils in brain.
Brain neutrophils were detected as described (21). Coronal brain slices containing the striatum were fixed in 10% neutral buffered formalin (Sigma-Aldrich) and embedded in paraffin. Tissue was cut into serial 7-& μ;m-thick sections, mounted on standard microscope slides, deparaffinized, and incubated in solution containing Naphesol AS-D and fast blue salt (Sigma-Aldrich) to reveal the presence of chloroacetate esterase activity. Sections were counterstained with nuclear fast red (Vector Laboratories, Burlingame, California, USA). Neutrophils in the cortex and striatum were counted by a blinded observer using unbiased microscopic sampling (optical dissector) in four serial, but nonadjacent, sections representing one, approximately 0.5-mm-thick coronal slice from a forebrain region (bregma coordinates were approximately 0–1) for each animal. Cells were sampled at Nikon Eclipse E600 microscope using ×40 objective. Neutrophils within blood vessels were not counted.
Bone marrow transplantation.
Bone marrow transplantation was accomplished by intravenous injection of the donor’s bone marrow into the left retro-orbital sinus of the recipients (52). Bone marrow suspensions were prepared from the cells flushed from the femurs of donors (2–5 months old). The recipients (5–8 weeks old) were given lethal doses of total body radiation with two exposures given 3 hours apart of 6 Gy from a 137Cs source. The irradiated recipients were rescued by injecting bone marrow suspensions (5 × 106 cell in 0.3 ml) from the donors within 1 hour after the irradiation. Polymyxin B (120 U/ml; Sigma-Aldrich) and neomycin (0.6 mg/ml; Sigma-Aldrich) were added into the drinking water for the transplanted mice for 20 days after transplantation to suppress pathogens.
Statistical analysis.
Quantitative data were expressed as mean plus or minus SEM. One-, two-, or three-way ANOVA with Bonferroni or Newman Keuls post-hoc tests, or two-tailed, unpaired Student’s t tests were used to assess statistical significance. In all tests, P values less than 0.05 were considered to be statistically significant.
Acknowledgments
This work was supported by NIH grants DK-58066 (C.A. Lowell), NS-35902 (D.M. Ferriero), AA-13588 (R.O. Messing), as well as by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California San Francisco (UCSF; R.O. Messing). We thank D. Wang, J. Dadgar, S. Taylor, and J. Connolly for technical support, and T.N. Mayadas (Harvard Medical School), N. Derugin, and A. Sheldon (UCSF) for technical advice.
Footnotes
Nonstandard abbreviations used: anterior cerebral artery (ACA); common carotid artery (CCA); diacylglycerol (DAG); formyl-Met-Leu-Phe peptide (fMLP); internal carotid artery (ICA); leukocyte functional antigen 1 (LFA-1); middle cerebral artery (MCA); middle cerebral artery occlusion (MCAO); protein kinase C (PKC); tissue plasminogen activator (t-PA); 2,3,5-triphenyltetrazolium chloride (TTC).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Stapf C, Mohr JP. Ischemic stroke therapy. Annu. Rev. Med. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- 2.Becker KJ. Targeting the central nervous system inflammatory response in ischemic stroke. Curr. Opin. Neurol. 2001;14:349–353. doi: 10.1097/00019052-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J. Cereb. Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- 4.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: the role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43:1382–1396. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh SP, Gough MJ, Homer-Vanniasinkam S. The role of the neutrophil in ischaemia-reperfusion injury: potential therapeutic interventions. Cardiovasc. Res. 1998;6:112–118. doi: 10.1016/s0967-2109(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 6.Ueno M, et al. Effect of a neutrophil elastase inhibitor (ONO-5046 Na) on ischemia/reperfusion injury using the left-sided heterotopic canine heart transplantation model. J. Heart Lung Transplant. 2001;20:889–896. doi: 10.1016/s1053-2498(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid. Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu. Rev. Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 9.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase C delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 10.Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J. Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leitges M, et al. Exacerbated vein graft arteriosclerosis in protein kinase Cδ-null mice. J. Clin. Invest. 2001;108:1505–1512. doi:10.1172/JCI200112902. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto A, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 13.Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860–865. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- 14.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch. Biochem. Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Murakami K, Kondo T, Chan PH. Reperfusion following focal cerebral ischemia alters distribution of neuronal cells with DNA fragmentation in mice. Brain Res. 1997;751:160–164. doi: 10.1016/s0006-8993(97)00029-2. [DOI] [PubMed] [Google Scholar]
- 16.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem. Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen S, Roivainen R, Keinänen R, Hökfelt T, Koistinaho J. Specific induction of protein kinase C delta subspecies after transient middle cerebral artery occlusion in the rat brain: inhibition by MK-801. J. Neurosci. 1996;16:6236–6245. doi: 10.1523/JNEUROSCI.16-19-06236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merchenthaler I, Liposits Z, Reid JJ, Wetsel WC. Light and electron microscopic immunocytochemical localization of PKCδ immunoreactivity in the rat central nervous system. J. Comp. Neurol. 1993;336:378–399. doi: 10.1002/cne.903360306. [DOI] [PubMed] [Google Scholar]
- 19.Chopp M, Li Y, Jiang N, Zhang RL, Prostak J. Ab’s against adhesion molecules reduce apoptosis after transient middle cerebral artery occlusion in rat brain. J. Cereb. Blood Flow Metab. 1996;16:578–584. doi: 10.1097/00004647-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Prestigiacomo CJ, et al. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke. 1999;30:1110–1117. doi: 10.1161/01.str.30.5.1110. [DOI] [PubMed] [Google Scholar]
- 21.Soriano SG, et al. Mice deficient in Mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 22.Shimakura A, et al. Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res. 2000;858:55–60. doi: 10.1016/s0006-8993(99)02431-2. [DOI] [PubMed] [Google Scholar]
- 23.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation — the two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZG, Chopp M, Tang WX, Jiang N, Zhang RL. Postischemic treatment (2–4 h) with anti-CD11b and anti-CD18 monoclonal Ab’s are neuroprotective after transient (2 h) focal cerebral ischemia in the rat. Brain Res. 1995;698:79–85. doi: 10.1016/0006-8993(95)00830-j. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, et al. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- 26.Brown GE, Stewart MQ, Liu H, Ha VL, Yaffe MB. A novel assay system implicates PtdIns(3, 4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol. Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 27.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22 2002phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 28.Walder CE, et al. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- 29.Perez OD, et al. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat. Immunol. 2003;4:1083–1092. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 30.Decleva E, Dri P, Menegazzi R, Busetto S, Cramer R. Evidence that TNF-induced respiratory burst of adherent PMN is mediated by integrin alpha(L)beta(2) J. Leukoc. Biol. 2002;72:718–726. [PubMed] [Google Scholar]
- 31.Takami M, Terry V, Petruzzelli L. Signaling pathways involved in IL-8-dependent activation of adhesion through Mac-1. J. Immunol. 2002;168:4559–4566. doi: 10.4049/jimmunol.168.9.4559. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida K, Wang HG, Miki Y, Kufe D. Protein kinase Cdelta is responsible for constitutive and DNA damage-induced phosphorylation of Rad9. EMBO J. 2003;22:1431–1441. doi: 10.1093/emboj/cdg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehrmann E, Christensen T, Zimmer J, Diemer NH, Finsen B. Microglial and macrophage reactions mark progressive changes and define the penumbra in the rat neocortex and striatum after transient middle cerebral artery occlusion. J. Comp. Neurol. 1997;386:461–476. [PubMed] [Google Scholar]
- 34.Kaur C, Hao AJ, Wu CH, Ling EA. Origin of microglia. Microsc. Res. Tech. 2001;54:2–9. doi: 10.1002/jemt.1114. [DOI] [PubMed] [Google Scholar]
- 35.Albers GW, Amarenco P. Combination therapy with clopidogrel and aspirin: can the CURE results be extrapolated to cerebrovascula patients? Stroke. 2001;32:2948–2949. doi: 10.1161/hs1201.100829. [DOI] [PubMed] [Google Scholar]
- 36.Pinsky DJ, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 2002;109:1031–1040. doi:10.1172/JCI200210649. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhri TF, et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J. Clin. Invest. 1998;102:1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray MO, et al. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J. Biol. Chem. 2004;279:3596–3604. doi: 10.1074/jbc.M311459200. [DOI] [PubMed] [Google Scholar]
- 39.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi M, et al. Efficient production of Cre-mediated site-directed recombinants through the utilization of the puromycin resistance gene, pac: a transient gene-integration marker for ES cells. Nucleic Acids Res. 1998;26:679–680. doi: 10.1093/nar/26.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derugin N, Ferriero DM, Vexler ZS. Neonatal reversible focal cerebral ischemia: a new model. Neurosci. Res. 1998;32:349–353. doi: 10.1016/s0168-0102(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 42.Swanson RA, et al. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 43.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 44.Panahian N, Maines MD. Assessment of induction of biliverdin reductase in a mouse model of middle cerebral artery occlusion. Brain Res. Brain Res. Protoc. 2000;6:53–70. doi: 10.1016/s1385-299x(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 45.Kharazia VN, Jacobs KM, Prince DA. Light microscopic study of GluR1 and calbindin expression in interneurons of neocortical microgyral malformations. Neuroscience. 2003;120:207–218. doi: 10.1016/s0306-4522(03)00282-3. [DOI] [PubMed] [Google Scholar]
- 46.Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J. Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter HJ, McMahon T, Dadgar J, Wang D, Messing RO. Ethanol regulates calcium channel subunits by protein kinase C delta-dependent and -independent mechanisms. J. Biol. Chem. 2000;275:25717–25722. doi: 10.1074/jbc.M910282199. [DOI] [PubMed] [Google Scholar]
- 48.Lowell CA, Berton G. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7580–7584. doi: 10.1073/pnas.95.13.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones SL, Wang J, Turck CW, Brown EJ. A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9331–9336. doi: 10.1073/pnas.95.16.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 51.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J. Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mocsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J. Immunol. 1999;162:1120–1126. [PubMed] [Google Scholar]