Abstract
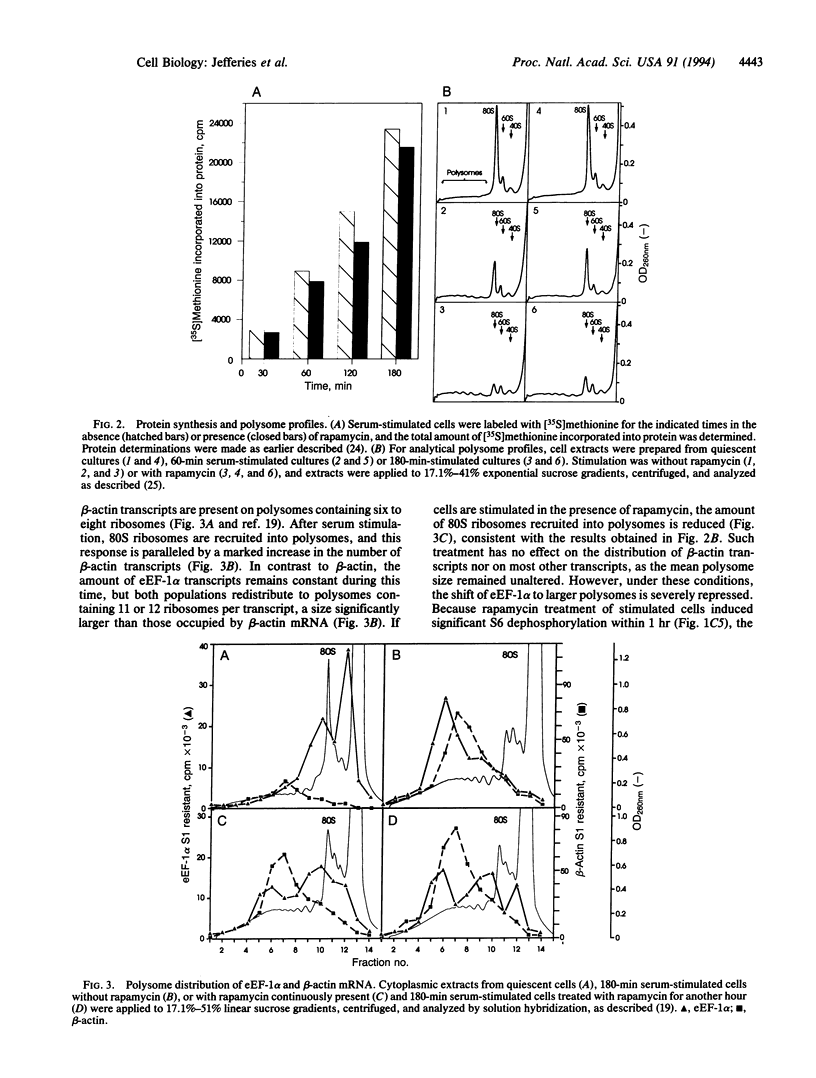
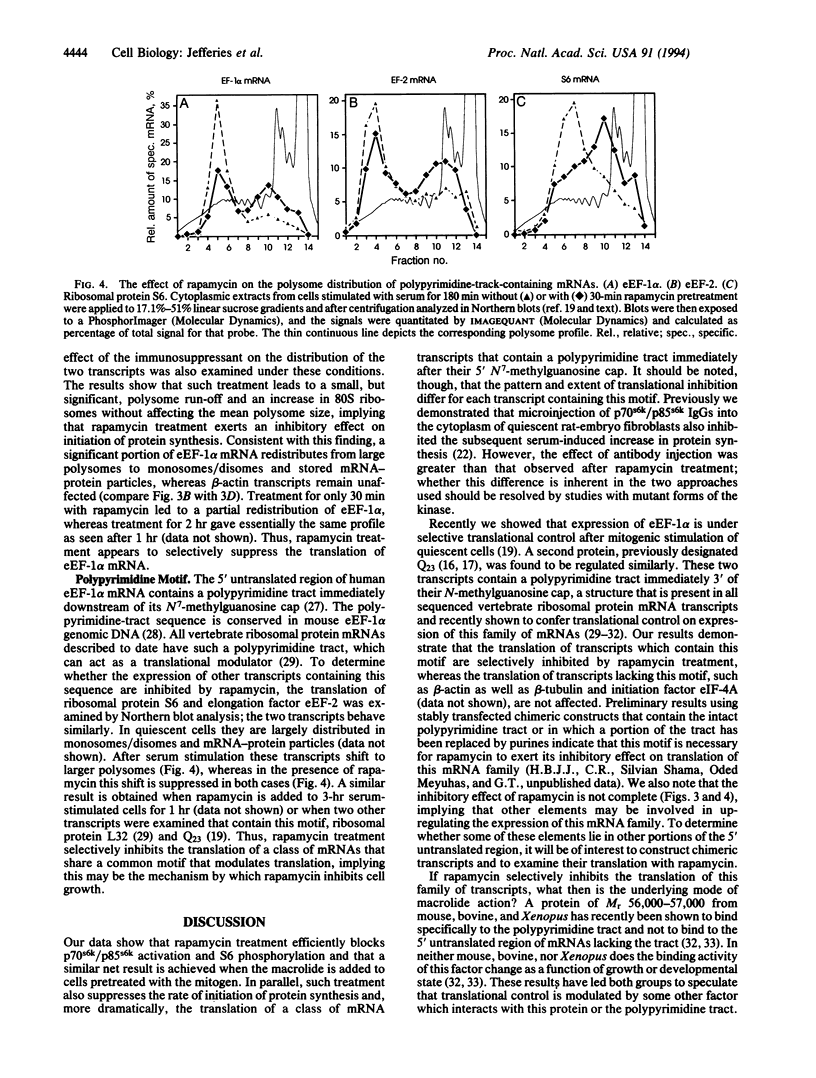
The immunosuppressant rapamycin blocks p70s6k/p85s6k activation and phosphorylation of 40S ribosomal protein S6 in Swiss 3T3 cells. The same net result is obtained when the macrolide is added 3 hr after serum stimulation. In stimulated cells p70s6k/p85s6k inactivation is achieved within minutes, whereas S6 dephosphorylation requires 1-2 hr, supporting the concept that S6 dephosphorylation results from kinase inactivation. In parallel, rapamycin treatment causes a small, but significant, reduction in the initiation rate of protein synthesis, as measured both by [35S]methionine incorporation into protein and by recruitment of 80S ribosomes into polysomes. More striking, analysis of individual mRNA transcripts revealed that rapamycin selectively suppresses the translation of a family of mRNAs that is characterized by a polypyrimidine tract immediately after their N7-methylguanosine cap, a motif that can act as a translational modulator. This family includes transcripts for ribosomal proteins, elongation factors of protein synthesis, and proteins of as-yet-unknown function. The results imply that (i) 40S ribosomes containing phosphorylated S6 may selectively recognize this motif or proteins which bind to it and (ii) rapamycin may inhibit cell growth by blocking S6 phosphorylation and, thus, translation of these mRNAs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers M. W., Williams R. T., Brown E. J., Tanaka A., Hall F. L., Schreiber S. L. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993 Oct 25;268(30):22825–22829. [PubMed] [Google Scholar]
- Ballou L. M., Siegmann M., Thomas G. S6 kinase in quiescent Swiss mouse 3T3 cells is activated by phosphorylation in response to serum treatment. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7154–7158. doi: 10.1073/pnas.85.19.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo V., Crews C. M., Vik T. A., Bierer B. E. Interleukin 2 stimulation of p70 S6 kinase activity is inhibited by the immunosuppressant rapamycin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7571–7575. doi: 10.1073/pnas.89.16.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali B., Di Cristina M., Pierandrei-Amaldi P. Interaction of proteins with the mRNA for ribosomal protein L1 in Xenopus: structural characterization of in vivo complexes and identification of proteins that bind in vitro to its 5'UTR. Nucleic Acids Res. 1993 May 25;21(10):2301–2308. doi: 10.1093/nar/21.10.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Bandi H. R., Hofsteenge J., Bussian B. M., Thomas G. Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem. 1991 Nov 25;266(33):22770–22775. [PubMed] [Google Scholar]
- Ferrari S., Bannwarth W., Morley S. J., Totty N. F., Thomas G. Activation of p70s6k is associated with phosphorylation of four clustered sites displaying Ser/Thr-Pro motifs. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7282–7286. doi: 10.1073/pnas.89.15.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Pearson R. B., Siegmann M., Kozma S. C., Thomas G. The immunosuppressant rapamycin induces inactivation of p70s6k through dephosphorylation of a novel set of sites. J Biol Chem. 1993 Aug 5;268(22):16091–16094. [PubMed] [Google Scholar]
- Flotow H., Thomas G. Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J Biol Chem. 1992 Feb 15;267(5):3074–3078. [PubMed] [Google Scholar]
- Franco R., Rosenfeld M. G. Hormonally inducible phosphorylation of a nuclear pool of ribosomal protein S6. J Biol Chem. 1990 Mar 15;265(8):4321–4325. [PubMed] [Google Scholar]
- Hammond M. L., Merrick W., Bowman L. H. Sequences mediating the translation of mouse S16 ribosomal protein mRNA during myoblast differentiation and in vitro and possible control points for the in vitro translation. Genes Dev. 1991 Sep;5(9):1723–1736. doi: 10.1101/gad.5.9.1723. [DOI] [PubMed] [Google Scholar]
- Jefferies H. B., Thomas G., Thomas G. Elongation factor-1 alpha mRNA is selectively translated following mitogenic stimulation. J Biol Chem. 1994 Feb 11;269(6):4367–4372. [PubMed] [Google Scholar]
- Kaspar R. L., Kakegawa T., Cranston H., Morris D. R., White M. W. A regulatory cis element and a specific binding factor involved in the mitogenic control of murine ribosomal protein L32 translation. J Biol Chem. 1992 Jan 5;267(1):508–514. [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz J., Hall M. N. Cyclosporin A, FK506 and rapamycin: more than just immunosuppression. Trends Biochem Sci. 1993 Sep;18(9):334–338. doi: 10.1016/0968-0004(93)90069-y. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Chung J., Fiorentino D. F., Flanagan W. M., Blenis J., Crabtree G. R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992 Jul 2;358(6381):70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- Lalanne J. L., Lucero M., le Moullec J. M. Complete sequence of mouse S6 ribosomal protein. Nucleic Acids Res. 1987 Jun 25;15(12):4990–4990. doi: 10.1093/nar/15.12.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. A., Fernandez A., Lamb N. J., Thomas G. p70s6k function is essential for G1 progression. Nature. 1993 May 13;363(6425):170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Levy S., Avni D., Hariharan N., Perry R. P., Meyuhas O. Oligopyrimidine tract at the 5' end of mammalian ribosomal protein mRNAs is required for their translational control. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3319–3323. doi: 10.1073/pnas.88.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariottini P., Amaldi F. The 5' untranslated region of mRNA for ribosomal protein S19 is involved in its translational regulation during Xenopus development. Mol Cell Biol. 1990 Feb;10(2):816–822. doi: 10.1128/mcb.10.2.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice W. G., Brunn G. J., Wiederrecht G., Siekierka J. J., Abraham R. T. Rapamycin-induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J Biol Chem. 1993 Feb 15;268(5):3734–3738. [PubMed] [Google Scholar]
- Morice W. G., Wiederrecht G., Brunn G. J., Siekierka J. J., Abraham R. T. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J Biol Chem. 1993 Oct 25;268(30):22737–22745. [PubMed] [Google Scholar]
- Nygård O., Nilsson L. Translational dynamics. Interactions between the translational factors, tRNA and ribosomes during eukaryotic protein synthesis. Eur J Biochem. 1990 Jul 20;191(1):1–17. doi: 10.1111/j.1432-1033.1990.tb19087.x. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Ballou L. M., Thomas G. Differential regulation of S6 phosphorylation by insulin and epidermal growth factor in Swiss mouse 3T3 cells: insulin activation of type 1 phosphatase. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4720–4724. doi: 10.1073/pnas.85.13.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palen E., Traugh J. A. Phosphorylation of ribosomal protein S6 by cAMP-dependent protein kinase and mitogen-stimulated S6 kinase differentially alters translation of globin mRNA. J Biol Chem. 1987 Mar 15;262(8):3518–3523. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Reinhard C., Thomas G., Kozma S. C. A single gene encodes two isoforms of the p70 S6 kinase: activation upon mitogenic stimulation. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4052–4056. doi: 10.1073/pnas.89.9.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W. W., Bragg P. W., Corrias M. V., Reddy N. S., Dholakia J. N., Wahba A. J. Expression of a gene for mouse eucaryotic elongation factor Tu during murine erythroleukemic cell differentiation. Mol Cell Biol. 1987 Nov;7(11):3929–3936. doi: 10.1128/mcb.7.11.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Bowman P. D., Gordon J. The isolation and analysis of polysomes and ribosomal RNA from cells growing in monolayer culture. Exp Cell Res. 1977 Sep;108(2):253–258. doi: 10.1016/s0014-4827(77)80032-3. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Gordon J. Multiple phosphorylation of ribosomal protein S6 during transition of quiescent 3T3 cells into early G1, and cellular compartmentalization of the phosphate donor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3952–3956. doi: 10.1073/pnas.76.8.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Thomas G., Luther H. Transcriptional and translational control of cytoplasmic proteins after serum stimulation of quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5712–5716. doi: 10.1073/pnas.78.9.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Thomas G. Translational control of mRNA expression during the early mitogenic response in Swiss mouse 3T3 cells: identification of specific proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2137–2144. doi: 10.1083/jcb.103.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetsuki T., Naito A., Nagata S., Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. J Biol Chem. 1989 Apr 5;264(10):5791–5798. [PubMed] [Google Scholar]



