Abstract
Variation in vectorial capacity for human malaria among Anopheles mosquito species is determined by many factors, including behavior, immunity, and life history. To investigate the genomic basis of vectorial capacity and explore new avenues for vector control, we sequenced the genomes of 16 anopheline mosquito species from diverse locations spanning ~100 million years of evolution. Comparative analyses show faster rates of gene gain and loss, elevated gene shuffling on the X chromosome, and more intron losses, relative to Drosophila. Some determinants of vectorial capacity, such as chemosensory genes, do not show elevated turnover, but instead diversify through protein-sequence changes. This dynamism of anopheline genes and genomes may contribute to their flexible capacity to take advantage of new ecological niches, including adapting to humans as primary hosts.
Introduction
Malaria is a complex disease, mediated by obligate eukaryotic parasites with a life cycle requiring adaption to both vertebrate hosts and mosquito vectors. These relationships create a rich co-evolutionary triangle. Just as Plasmodium parasites have adapted to their diverse hosts and vectors, infection by Plasmodium parasites has reciprocally induced adaptive evolutionary responses in humans and other vertebrates (1), and has also influenced mosquito evolution (2). Human malaria is transmitted only by mosquitoes in the genus Anopheles, but not all species within the genus, or even all members of each vector species, are efficient malaria vectors. This suggests an underlying genetic/genomic plasticity that results in variation of key traits determining vectorial capacity within the genus.
In all, five species of Plasmodium have adapted to infect humans, and are transmitted by approximately 60 of the 450 known species of anopheline mosquitoes (3). Sequencing the genome of Anopheles gambiae, the most important malaria vector in sub-Saharan Africa, has offered numerous insights into how that species became highly specialized to live among and feed upon humans, and how susceptibility to mosquito control strategies is determined (4). Until very recently (5–7), similar genomic resources have not existed for other anophelines, limiting comparisons to individual genes or sets of genomic markers with no genome-wide data to investigate attributes associated with vectorial capacity across the genus.
Thus, we sequenced and assembled the genomes and transcriptomes of 16 anophelines from Africa, Asia, Europe, and Latin America. We chose these 16 species to represent a range of evolutionary distances from An. gambiae, a variety of geographic locations and ecological conditions, and varying degrees of vectorial capacity (8) (Fig. 1A, B). For example, Anopheles quadriannulatus, while extremely closely related to An. gambiae, feeds preferentially on bovines rather than humans, limiting its potential to transmit human malaria. Anopheles merus, Anopheles melas, Anopheles farauti, and Anopheles albimanus females can lay eggs in salty or brackish water, instead of the freshwater sites required by other species. With a focus on species most closely related to An. gambiae (9), the sampled anophelines span the three main subgenera that shared a common ancestor approximately 100 million years (MYr) ago (10).
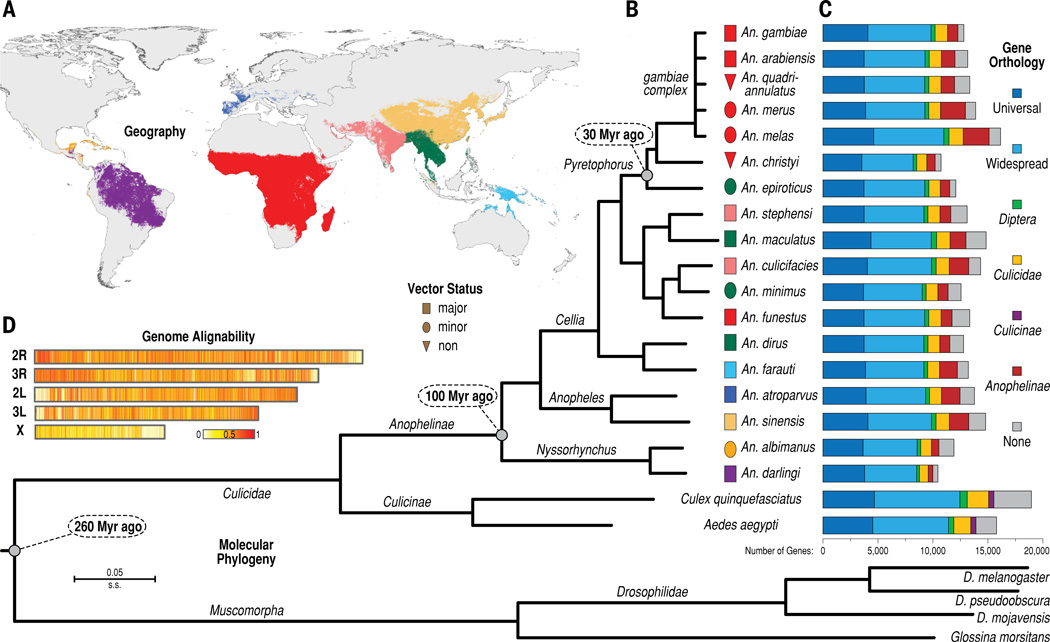
Figure 1. Geography, vector status, molecular phylogeny, gene orthology, and genome alignability of the 16 newly sequenced anopheline mosquitoes and selected other dipterans.
(A) Global geographic distributions of the 16 sampled anophelines and the previously sequenced An. gambiae and An. darlingi. Ranges are colored for each species or group of species as shown in panel B, e.g. light blue for An. farauti. (B) The maximum likelihood molecular phylogeny of all sequenced anophelines and selected dipteran outgroups. Shapes between branch termini and species names indicate vector status (rectangles, major vectors; ellipses, minor vectors, triangles, non-vectors) and are colored according to geographic ranges shown in panel A. (C) Barplots show total gene counts for each species partitioned according to their orthology profiles; from ancient genes found across insects to lineage-restricted and species-specific genes. (D) Heat map illustrating the density (in 2 kb sliding windows) of whole genome alignments along the lengths of An. gambiae chromosomal arms: from white where An. gambiae aligns to no other species, to red where An. gambiae aligns to all the other anophelines.
Materials and methods summary
Genomic DNA and whole-body RNA were obtained from laboratory colonies and wild-caught specimens (tables S1-S2), with samples for nine species procured from newly established isofemale colonies to reduce heterozygosity. Illumina sequencing libraries spanning a range of insert sizes were constructed, with ~100-fold paired-end 101 base pair (bp) coverage generated for small (180 bp) and medium (1.5 kb) insert libraries and lower coverage for large (38 kb) insert libraries (table S3). DNA template for the small and medium input libraries was sourced from single female mosquitoes from each species to further reduce heterozygosity. High molecular weight DNA template for each large insert library was derived from pooled DNA obtained from several hundred mosquitoes. ALLPATHS-LG (11) genome assemblies were produced using the ‘haploidify’ option to reduce haplotype assemblies caused by high heterozygosity. Assembly quality reflected DNA template quality and homozygosity, with a mean scaffold N50 of 3.6 Mb, ranging to 18.1 Mb for An. albimanus (table S4). Despite variation in contiguity, the assemblies were remarkably complete and searches for arthropod-wide single-copy orthologs generally revealed few missing genes (fig. S1) (12).
Genome annotation with MAKER (13) supported with RNAseq transcriptomes (produced from pooled male and female larvae, pupae, and adults; table S5), and comprehensive non-coding RNA gene prediction (fig. S2), yielded relatively complete gene sets (fig. S3), with between 10,738 and 16,149 protein-coding genes identified for each species. Gene count was generally commensurate with assembly contiguity (table S6). Some of this variation in total gene counts may be attributed to the challenges of gene annotations with variable levels of assembly contiguity and supporting RNAseq data. To estimate the prevalence of erroneous gene model fusions and/or fragmentations, we compared the new gene annotations to An. gambiae gene models and found an average of 3.3% and 9.7% potentially fused and fragmented gene models, respectively. For analyses described below that may be sensitive to variation in gene model accuracy or gene set completeness, we have conducted sensitivity analyses to rule out confounding results from these factors (12).
Rapidly evolving genes and genomes
Orthology delineation identified lineage-restricted and species-specific genes, as well as ancient genes found across insect taxa, of which universal single-copy orthologs were employed to estimate the molecular species phylogeny (Fig. 1B, 1C, fig. S4). Analysis of codon frequencies in these orthologs revealed that anophelines, unlike drosophilids, exhibit relatively uniform codon usage preferences (fig. S5).
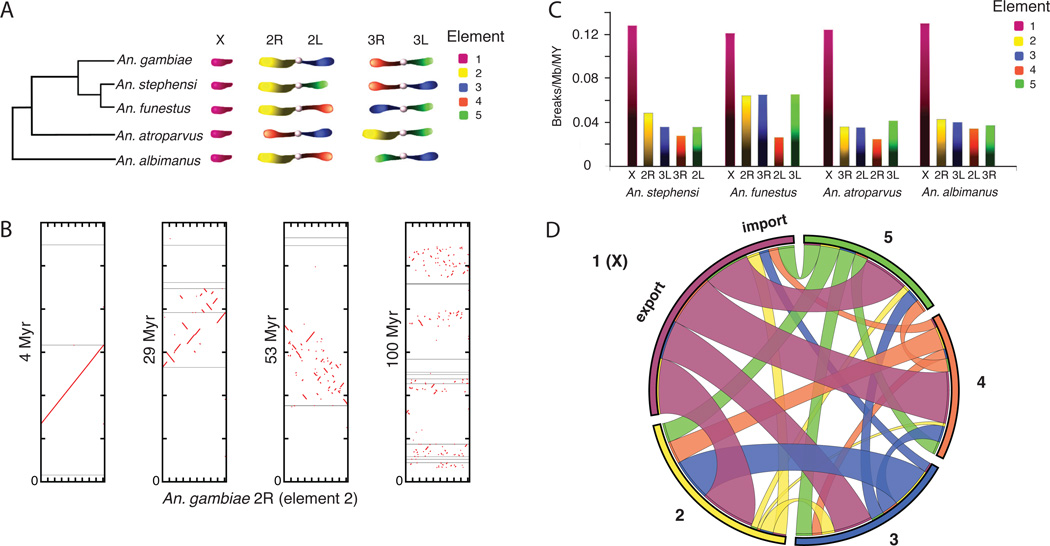
Polytene chromosomes have provided a glimpse into anopheline chromosome evolution (14). Our genome-sequence-based view confirmed the cytological observations, and offers many new insights. At the base pair level, ~90% of the non-gapped and non-masked An. gambiae genome (i.e., excluding transposable elements, as detailed in table S7) is alignable to the most closely related species, while only ~13% aligns to the most distant (Fig. 1D, fig. S6, table S8), with reduced alignability in centromeres and on the X chromosome (Fig. 1D). At chromosomal levels, mapping data anchored 35–76% of the Anopheles stephensi, Anopheles funestus, Anopheles atroparvus, and An. albimanus genome assemblies to chromosomal arms (tables S9-S12). Analysis of genes in anchored regions showed that synteny at the whole-arm level is highly conserved, despite several whole-arm translocations (Fig. 2A, table S13). In contrast, small-scale rearrangements disrupt gene colinearity within arms over time, leading to extensive shuffling of gene order over a timescale of 29 MYA or more (10, 15) (Fig. 2B, fig. S7). As in Drosophila, rearrangement rates are higher on the X chromosome than on autosomes (Fig. 2C, tables S14-S16). However, the difference is significantly more pronounced in Anopheles, where X chromosome rearrangements are 2.7-fold more frequent than autosomal rearrangements; in Drosophila, the corresponding ratio is only 1.2 (t-test, t10 = 7.3, P < 1×10−5) (fig. S8). The X chromosome is also notable for a significant degree of observed gene movement to other chromosomes relative to Drosophila (one sample proportion test, P < 2.2×10−16; Fig. 2D, tables S17-S18), as was previously noted for Anopheles relative to Aedes (16), further underscoring its distinctive evolutionary profile in Anopheles compared to other dipteran genera.
Figure 2. Patterns of anopheline chromosomal evolution.
(A) Anopheline genomes have conserved gene membership on chromosome arms (‘elements’; colored and labeled 1–5). Unlike Drosophila, chromosome elements reshuffle between chromosomes via translocations as intact elements, and do not show fissions or fusions. The tree depicts the supported molecular topology for the species studied. (B) Conserved synteny blocks decay rapidly within chromosomal arms as the phylogenetic distance increases between species. Moving left to right, the dotplot panels show gene-level synteny between chromosome 2R of An. gambiae (x axis) and inferred ancestral sequences (y axes; inferred using PATHGROUPS) at increasing evolutionary timescales (MYA = million years ago) estimated via an ultrametric phylogeny. Gray horizontal lines represent scaffold breaks. Discontinuity of the red lines/dots indicates rearrangement. (C) Anopheline X chromosomes exhibit higher rates of rearrangement (P < 1×10−5), measured as breaks per megabase (Mb) per million years (MY), compared with autosomes, despite a paucity of polymorphic inversions on the X. (D) The anopheline X chromosome also displays a higher rate of gene movement to other chromosomal arms than any of the autosomes. Chromosomal elements are labeled around the perimeter; internal bands are colored according to the chromosomal element source and match element colors in panels A and C. Bands are sized to indicate the relative ratio of genes imported versus exported for each chromosomal element, and the relative allocation of exported genes to other elements.
Such dynamic gene shuffling and movement may be facilitated by the multiple families of DNA transposons and LTR and non-LTR retroelements found in all genomes (table S7), as well as a weaker dosage compensation phenotype in Anopheles compared to Drosophila (17). Despite such shuffling, comparing genomic locations of orthologs can be successfully employed to reconstruct ancestral chromosomal arrangements (fig. S9) and to confidently improve assembly contiguity (tables S19-S21).
Copy number variation in homologous gene families also reveals striking evolutionary dynamism. Analysis of 11,636 gene families with CAFE 3 (18) indicates a rate of gene gain/loss at least five-fold higher than that observed for 12 Drosophila genomes (19). Overall, these Anopheles genomes exhibit a rate of gain or loss/gene/million years of 3.12×10−3 compared to 5.90×10−4 for Drosophila, suggesting substantially higher gene turnover within anophelines relative to fruit flies. This five-fold greater gain/loss rate in anophelines holds true under models that account for uncertainly in gene family sizes at the tips of the species tree due to annotation/assembly errors, and is not sensitive to inclusion or exclusion of taxa affecting the root age of the tree, nor to the exclusion of taxa with the poorest assemblies and gene sets (fig. S10, tables S22-S23). Examples include expansions of cuticular proteins in Anopheles arabiensis and neurotransmitter-gated ion channels in An. albimanus (table S24).
The evolutionary dynamism of Anopheles genes extends to their architecture. Comparisons of single-copy orthologs at deeper phylogenetic depths showed losses of introns at the root of the true fly order Diptera, and revealed continued losses as the group diversified into the lineages leading to fruit flies and mosquitoes. However, anopheline orthologs have sustained greater intron loss than drosophilids, leading to a relative paucity of introns in the genes of extant anophelines (fig. S11, table S25). Comparative analysis also revealed that gene fusion and fission played a substantial role in the evolution of mosquito genes, with apparent rearrangements affecting an average of 10.1% of all genes in the genomes of the 10 species with the most contiguous assemblies (fig. S12). Furthermore, gene boundaries can be flexible; whole genome alignments identified 325 candidates for stop-codon readthrough (fig. S13, table S26).
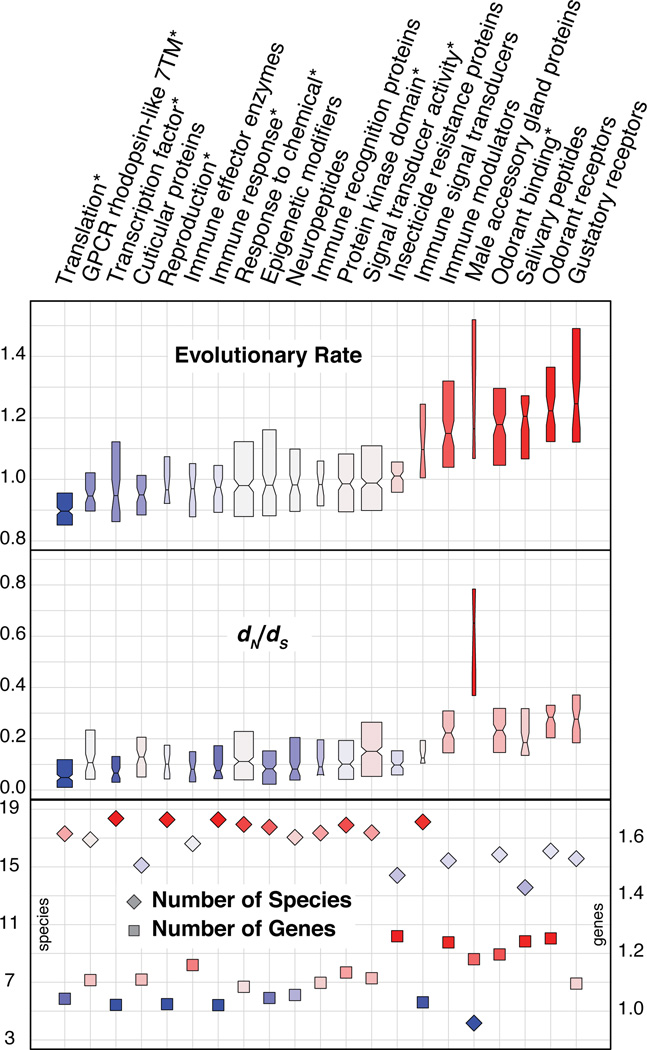
As molecular evolution of protein-coding sequences is a well-known source of phenotypic change, we compared evolutionary rates among different functional categories of anopheline orthologs. We quantified evolutionary divergence in terms of protein sequence identity of aligned orthologs and the dN/dS statistic computed using PAML (12, 20). Among curated sets of genes linked to vectorial capacity or species-specific traits against a background of functional categories defined by Gene Ontology or InterPro annotations, odorant and gustatory receptors show high evolutionary rates and male accessory gland proteins exhibit exceptionally high dN/dS ratios (Fig. 3, figs. S14-S15, tables S27-S29). Rapid divergence in functional categories related to malaria transmission and/or mosquito control strategies led us to examine the genomic basis of several facets of anopheline biology in closer detail.
Figure 3. Contrasting evolutionary properties of selected gene functional categories.
Examined evolutionary properties of orthologous groups of genes include: a measure of amino acid conservation/divergence (evolutionary rate), a measure of selective pressure (dN/dS), a measure of gene duplication in terms of mean gene copy-number per species (number of genes), and a measure of ortholog universality in terms of number of species with orthologs (number of species). Notched boxplots show medians, extend to the first and third quartiles, and their widths are proportional to the number of orthologous groups in each functional category. Functional categories derive from curated lists associated with various functions/processes as well as annotated Gene Ontology or InterPro categories (denoted by asterisks).
Insights into mosquito biology and vectorial capacity
Mosquito reproductive biology evolves rapidly and presents a compelling target for vector control. This is exemplified by the An. gambiae male accessory gland protein (Acp cluster on chromosome 3R (21, 22), where conservation is mostly lost outside the An. gambiae species complex (fig. S16). In Drosophila, male-biased genes such as Acps tend to evolve faster than loci without male-biased expression (23–25). We looked for a similar pattern in anophelines after assessing each gene for sex-biased expression using microarray and RNAseq datasets for An. gambiae (12). In contrast to Drosophila, female-biased genes show dramatically faster rates of evolution across the genus than male-biased genes (Wilcoxon rank sum test, P = 5×10−4) (fig. S17).
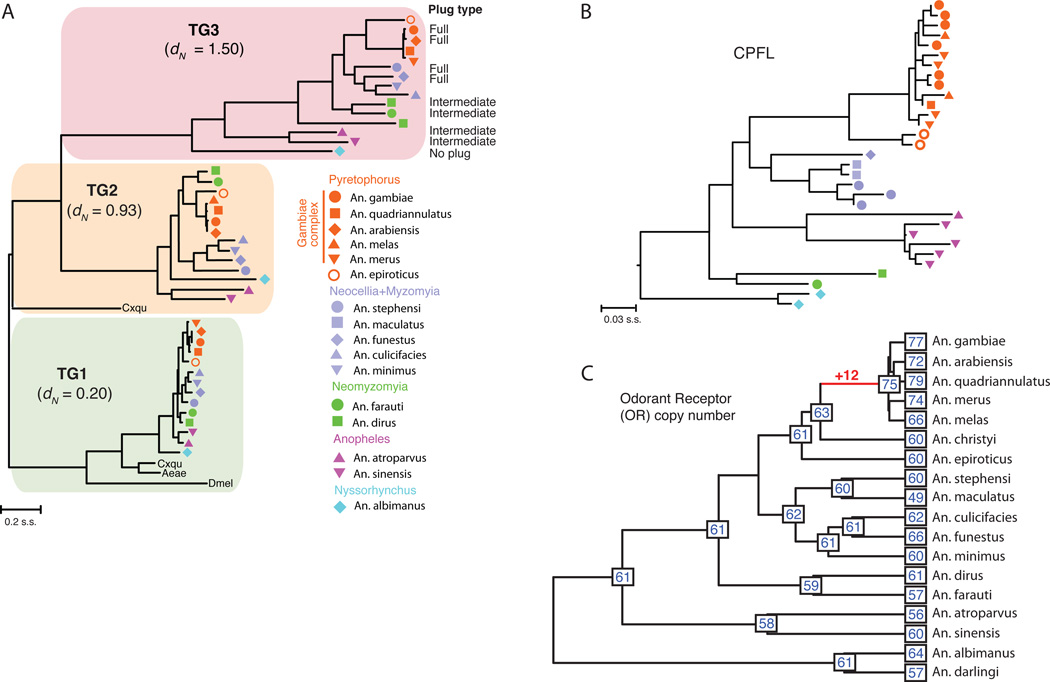
Differences in reproductive genes among anophelines may provide insight into the origin and function of sex-related traits. During copulation, An. gambiae males transfer a gelatinous mating plug, a complex of seminal proteins, lipids, and hormones that are essential for successful sperm storage by females and for reproductive success (26–28). Coagulation of the plug is mediated by a seminal transglutaminase (TG3), which is found in anophelines but is absent in other mosquito genera that do not form a mating plug (26). We examined TG3 and its two paralogs (TG1 and TG2) in the sequenced anophelines, and investigated the rate of evolution of each gene (Fig. 4A). Silent sites were saturated at the whole-genus level, making dS difficult to estimate reliably, but TG1 (the gene presumed to be ancestral due to broadest taxonomic representation) exhibited the lowest rate of amino acid change (dN = 0.20), TG2 exhibited an intermediate rate (dN = 0.93), and the anopheline-specific TG3 has evolved even more rapidly (dN = 1.50), perhaps due to male/male or male/female evolutionary conflict. Interestingly, plug formation appears to be a derived trait within anophelines, as it is not exhibited by An. albimanus and intermediate, poorly coagulated plugs were observed in taxa descending from early-branching lineages within the genus (table S30). Functional studies of mating plugs will be necessary to understand what drove the origin and rapid evolution of TG3.
Figure 4. Phylogeny-based insights into anopheline biology.
(A) Maximum-likelihood amino acid based phylogenetic tree of three transglutaminase enzymes (TG1 (green), TG2 (yellow) and TG3 (red)) in 14 anopheline species with Culex quinquefasciatus (Cxqu), Ae. aegypti (Aeae) and D. melanogaster (Dmel) serving as outgroups. TG3 is the enzyme responsible for the formation of the male mating plug in An. gambiae, acting upon the substrate Plugin, the most abundant mating plug protein. Higher rates of evolution for plug-forming TG3 are supported by elevated levels of dN. Mating plug phenotypes are noted where known within the TG3 clade. (B) Concerted evolution in CPFL cuticular proteins. Species symbols used are the same as in panel a. In contrast to the TG1/TG2/TG3 phylogeny, CPFL paralogs cluster by sub-generic clades rather than individually recapitulating the species phylogeny. Gene family size variation among species may reflect both gene gain/loss and variation in gene set completeness. (C) Odorant receptor (OR) observed gene counts and inferred ancestral gene counts on an ultrametric phylogeny. At least 10 OR genes were gained on the branch leading to the common ancestor of the An. gambiae species complex, though the overall number of OR genes does not vary dramatically across the genus.
Proteins that constitute the mosquito cuticular exoskeleton play important roles in diverse aspects of anopheline biology, including development, ecology, and insecticide resistance, and constitute approximately 2% of all protein-coding genes (29). Comparisons among dipterans have revealed numerous amplifications of cuticular protein (CP) genes undergoing concerted evolution at physically clustered loci (30–33). We investigated the extent and timescale of gene cluster homogenization within anophelines by generating phylogenies of orthologous gene clusters (fig. S18, table S31). Throughout the genus, these gene clusters often group phylogenetically by species rather than by position within tandem arrays, particularly in a subset of clusters. These include the 3RB and 3RC clusters of CP genes (30), the CPLCG group A and CPLCW clusters found elsewhere on 3R (32), and six tandemly arrayed genes on 3L designated CPFL2 through CPFL7 (34). CPLCW genes occur in a head-to-head arrangement with CPLCG group A genes, and exhibit highly conserved intergenic sequences (fig. S19). Furthermore, transcript localization studies using in situ hybridization revealed identical spatial expression patterns for CLPCW and CPLCG group A gene pairs suggestive of co-regulation (fig. S19). For these five gene clusters, complete grouping by organismal lineage was observed for most deep nodes as well as for many individual species outside the shallow An. gambiae species complex (Fig. 4B), consistent with a relatively rapid (less than 20 million years) homogenization of sequences via concerted evolution. The emerging pattern of anopheline CP evolution is thus one of relative stasis for a majority of single-copy orthologs, juxtaposed with consistent concerted evolution of a subset of genes.
Anophelines identify hosts, oviposition sites, and other environmental cues through specialized chemosensory membrane-bound receptors. We examined three of the major gene families that encode these molecules: the odorant receptors (ORs), gustatory receptors (GRs), and variant ionotropic glutamate receptors (IRs). Given rapid chemosensory gene turnover observed in many other insects, we explored whether varying host preferences of anopheline mosquitoes could be attributed to chemosensory gene gains and losses. Unexpectedly in light of the elevated genome-wide rate of gene turnover, we found that the overall size and content of the chemosensory gene repertoire is relatively conserved across the genus. CAFE 3 (18) analyses estimated that the most recent common ancestor of the anophelines had approximately 60 genes in each of the OR and GR families, similar to most extant anophelines (Fig. 4C, fig. S20). Estimated gain/loss rates of OR and GR genes per million years (error-corrected λ = 1.3×10−3 for ORs and 2.0×10−4 for GRs) were much lower than the overall level of anopheline gene families. Similarly, we found almost the same number of antennae-expressed IRs (~20) in all anopheline genomes. Despite overall conservation in chemosensory gene numbers, we observed several examples of gene gain and loss in specific lineages. Notably, there was a net gain of at least 12 ORs in the common ancestor of the An. gambiae complex (Fig. 4C).
OR and GR gene repertoire stability may derive from their roles in several critical behaviors. Host preference differences are likely to be governed by a combination of functional divergence and transcriptional modulation of orthologs. This model is supported by studies of antennal transcriptomes in the major malaria vector An. gambiae (35), and comparisons between this vector and its morphologically identical sibling An. quadriannulatus (36), a very closely related species that plays no role in malaria transmission (despite vectorial competence) because it does not specialize on human hosts. Furthermore, we found that many subfamilies of ORs and GRs showed evidence of positive selection (19 of 53 ORs; 17 of 59 GRs) across the genus, suggesting potential functional divergence.
Several blood feeding-related behaviors in mosquitoes are also regulated by peptide hormones (37). These peptides are synthesized, processed and released from nervous and endocrine systems and elicit their effects through binding appropriate receptors in target tissues (38). In total, 39 peptide hormones were identified from each of the sequenced anophelines (fig. S21). Interestingly, no ortholog of the well-characterized head peptide (HP) hormone of the culicine mosquito Aedes aegypti was identified in any of the assemblies. In Ae. aegypti, HP is responsible for inhibiting host seeking behavior following a blood meal (39). As anophelines broadly exhibit similar behavior (40), the absence of HP from the entire clade suggests they may have evolved a novel mechanism to inhibit excess blood feeding. Similarly, no ortholog of insulin growth factor 1 (IGF1) was identified in any anophelines even though IGF1 orthologs have been identified in other dipterans, including D. melanogaster (41) and Ae. aegypti (42). IGF1 is a key component of the insulin/insulin growth factor 1 signaling (IIS) cascade, which regulates processes including innate immunity, reproduction, metabolism and lifespan (43). Nevertheless, other members of the IIS cascade are present, and four insulin-like peptides are found in a compact cluster with gene arrangements conserved across anophelines (fig. S22). This raises questions regarding the modification of IIS signaling in the absence of IGF1 and the functional importance of this conserved genomic arrangement.
Epigenetic mechanisms impact many biological processes via modulation of chromatin structure, telomere remodeling and transcriptional control. Of the 215 epigenetic regulatory genes in D. melanogaster (44), we identified 169 putative An. gambiae orthologs (table S32), suggesting the presence of mechanisms of epigenetic control in Anopheles and Drosophila. We find, however, that retrotransposition may have contributed to the functional divergence of at least one gene associated with epigenetic regulation. The ubiquitin-conjugating enzyme E2D (orthologous to effete (45) in D. melanogaster) duplicated via retrotransposition in an early anopheline ancestor, and the retrotransposed copy is maintained in a subset of anophelines. Although the entire amino acid sequence of E2D is perfectly conserved between An. gambiae and D. melanogaster, the retrogenes are highly divergent (Fig. 5A), and may contribute to functional diversification within the genus.
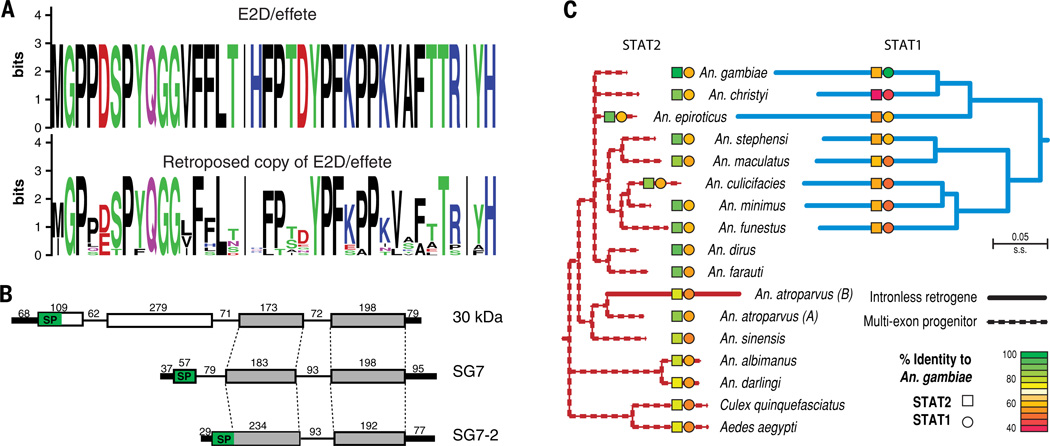
Figure 5. Genesis of novel anopheline genes.
(A) Retrotransposition of the E2D/effete gene generated a ubiquitin-conjugating enzyme at the base of the genus, which exhibits much higher sequence divergence than the original multi-exon gene. WebLogo plots contrast the amino acid conservation of the original effete gene with the diversification of the retrotransposed copy (residues 38–75; species represented are An. minimus, An. dirus, An. funestus, An. farauti, An. atroparvus, An. sinensis, An. darlingi, and An. albimanus). (B) The SG7 salivary protein-encoding gene was generated from the C-terminal half of the 30 KDa gene. SG7 then underwent tandem duplication and intron loss to generate another salivary protein, SG7-2. Numerals indicate length of segments in base pairs. (C) The origin of STAT1, a signal transducer and activator of transcription gene involved in immunity, occurred through a retrotransposition event in the Cellia ancestor after divergence from An. dirus and An. farauti. The intronless STAT1 is much more divergent than its multi-exon progenitor, STAT2, and has been maintained in all descendent species. An independent retrotransposition event created a retrogene copy in An. atroparvus, which is also more divergent than its progenitor.
Saliva is integral to blood feeding – it impairs host hemostasis and also affects inflammation and immunity. In An. gambiae the salivary proteome is estimated to contain the products of at least 75 genes, most being expressed solely in the adult female salivary glands. Comparative analyses indicate that anopheline salivary proteins are subject to strong evolutionary pressures, and these genes exhibit an accelerated pace of evolution, as well as a very high rate of gain/loss (Fig. 3, fig. S23). Polymorphisms within An. gambiae populations from limited sets of salivary genes were previously found to carry signatures of positive selection (46). Sequence analysis across the anophelines shows that salivary genes have the highest incidence of positively selected codons among the seven gene classes (fig. S24), indicating that co-evolution with vertebrate hosts is a powerful driver of natural selection in salivary proteomes. Moreover, salivary proteins also exhibit functional diversification through new gene creation. Sequence similarity, intron/exon boundaries, and secondary structure prediction point to the birth of the SG7/SG7-2 inflammation-inhibiting (47) gene family from the genomic region encoding the C-terminus of the 30 kDa protein (Fig. 5B), a collagen-binding platelet inhibitor already present in the blood-feeding ancestor of mosquitoes and black flies (48). Based on phylogenetic representation, these events must have occurred before the radiation of anophelines but after separation from the culicines.
Resistance to insecticides and other xenobiotics has arisen independently in many anopheline species, fostered directly and indirectly by anthropogenic environmental modification. Metabolic resistance to insecticides is mediated by multiple gene families, including cytochrome P450s and glutathione-S-transferases (GSTs), which serve to generally protect against all environment stresses, both natural and anthropogenic. We manually characterized these gene families in seven anophelines spanning the genus. Despite their large size, gene numbers (87–104 P450 genes, 27–30 GST genes) within both gene families are highly conserved across all species, though lineage-specific gene duplications and losses are often seen (tables S33-S34). As with the OR and GR olfaction-related gene families, P450 and GST repertoires may be relatively constant due to the large number of roles they play in anopheline biology. Orthologs of genes associated with insecticide resistance either via up-regulation or coding variation (e.g., Cyp6m2, Cyp6p3 [Cyp6p9 in An. funestus], Gste2, Gste4) were found in all species, suggesting that virtually all anophelines likely have genes capable of conferring insecticide resistance through similar mechanisms. Unexpectedly, one member of the P450 family (Cyp18a1) with a conserved role in ecdysteroid catabolism (and consequently development and metamorphosis (49) appears to have been lost from the ancestor of the An. gambiae species complex, but is found in the genome and transcriptome assemblies of other species, indicating that the An. gambiae complex may have recently evolved an alternate mechanism for catabolizing ecdysone.
Susceptibility to malaria parasites is a key determinant of vectorial capacity. Dissecting the immune repertoire (50, 51) (table S35) into its constituent phases reveals that classical recognition genes and genes encoding effector enzymes exhibit relatively low levels of sequence divergence. Signal transducers are more divergent in sequence but are conserved in representation across species and rarely duplicated. Cascade modulators, while also divergent, are more lineage-specific and generally have more gene duplications (Fig. 3, fig. S25). A rare duplication of an immune signal transduction gene occurred through the retrotransposition of the signal transducer and activator of transcription, STAT2, to form the intronless STAT1 after the divergence of An. dirus and An. farauti from the rest of the subgenus Cellia (Fig. 5C, table S36). Interestingly, an independent retroposition event appears to have independently created another intronless STAT gene in the An. atroparvus lineage. In An. gambiae, STAT1 controls the expression of STAT2 and is activated in response to bacterial challenge (52, 53), and the STAT pathway has been demonstrated to mediate immunity to Plasmodium (53, 54), so the presence of these relatively new immune signal transducers may have allowed for rewiring of regulatory networks governing immune responses in this subset of anophelines.
Conclusion
Since the discovery over a century ago by Ronald Ross and Giovanni Battista Grassi that human malaria is transmitted by a narrow range of blood-feeding female mosquitoes, the biological basis of malarial vectorial capacity has been a matter of intense interest. Inasmuch as previous successes in the local elimination of malaria have always been accomplished wholly or in part through effective vector control, an increased understanding of vector biology is crucial for continued progress against malarial disease. These 16 new reference genome assemblies provide a foundation for additional hypothesis generation and testing to further our understanding of the diverse biological traits that determine vectorial capacity.
Supplementary Material
Acknowledgements
All sequencing reads and genome assemblies have been submitted to NCBI (umbrella BioProject ID = PRJNA67511). Genome and transcriptome assemblies are also available from VectorBase (http://vectorbase.org) and the Broad Institute (http://olive.broadinstitute.org/collections/anopheles.4).
The authors wish to acknowledge the NIH Eukaryotic Pathogen and Disease Vector Sequencing Project Working Group for guidance and development of this project. Sequence data generation was supported at the Broad Institute by the National Human Genome Research Institute (U54 HG003067). We would like to thank the many members of the Broad Institute Genomics Platform and Genome Sequencing and Analysis Program who contributed to sequencing data generation and analysis.
Footnotes
Supplementary Materials
Materials, Methods, and Information
Figs. S1 to S25
Tables S1 to S36
References and Notes
- 1.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: what makes a malaria vector? Trends Parasitol. 2010;26:130–136. doi: 10.1016/j.pt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Manguin S, C P, Mouchet J, E JL. Biodiversity of Malaria in the World. John Libbey Eurotext; 2008. [Google Scholar]
- 4.Holt RA. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 5.Marinotti O. The genome of Anopheles darlingi, the main neotropical malaria vector. Nucleic Acids Res. 2013;41:7387–7400. doi: 10.1093/nar/gkt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou D. Genome sequence of Anopheles sinensis provides insight into genetics basis of mosquito competence for malaria parasites. BMC Genomics. 2014;15:42. doi: 10.1186/1471-2164-15-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X. Genome analysis of a major urban malaria vector mosquito, Anopheles stephensi. Genome Biol. 2014;15:459. doi: 10.1186/s13059-014-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neafsey DE. The evolution of the Anopheles 16 genomes project. G3 Bethesda Md. 2013;3:1191–1194. doi: 10.1534/g3.113.006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontaine MC. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science. doi: 10.1126/science.1258524. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno M. Complete mtDNA genomes of Anopheles darlingi and an approach to anopheline divergence time. Malar. J. 2010;9:127. doi: 10.1186/1475-2875-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnerre S. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc. Natl. Acad. Sci. 2011;108:1513–1518. doi: 10.1073/pnas.1017351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supplementary material on Science Online.
- 13.Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics. 2011;12:491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298:1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- 15.Kamali M. Multigene phylogenetics reveals temporal diversification of major african malaria vectors. PloS One. 2014;9:e93580. doi: 10.1371/journal.pone.0093580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toups MA, Hahn MW. Retrogenes reveal the direction of sex-chromosome evolution in mosquitoes. Genetics. 2010;186:763–766. doi: 10.1534/genetics.110.118794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DA, Russell S. Role of Testis-Specific Gene Expression in Sex-Chromosome Evolution of Anopheles gambiae. Genetics. 2011;189:1117–1120. doi: 10.1534/genetics.111.133157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han MV, Thomas GWC, Lugo-Martinez J, Hahn MW. Estimating gene gain and loss rates in the presence of error in genome assembly and annotation using CAFE 3. Mol. Biol. Evol. 2013;30:1987–1997. doi: 10.1093/molbev/mst100. [DOI] [PubMed] [Google Scholar]
- 19.Hahn MW, Han MV. S.-G. Han, Gene Family Evolution across 12 Drosophila Genomes. PLoS Genet. 2007;3:e197. doi: 10.1371/journal.pgen.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 21.Dottorini T. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldini F, Gabrieli P, Rogers DW, Catteruccia F. Function and composition of male accessory gland secretions in Anopheles gambiae: a comparison with other insect vectors of infectious diseases. Pathog. Glob. Health. 2012;106:82–93. doi: 10.1179/2047773212Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assis R, Zhou Q, Bachtrog D. Sex-Biased Transcriptome Evolution in Drosophila. Genome Biol. Evol. 2012;4:1189–1200. doi: 10.1093/gbe/evs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grath S, Parsch J. Rate of amino acid substitution is influenced by the degree and conservation of male-biased transcription over 50 myr of Drosophila evolution. Genome Biol. Evol. 2012;4:346–359. doi: 10.1093/gbe/evs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry JC, Harrison PW, Mank JE. The Ontogeny and Evolution of Sex-Biased Gene Expression in Drosophila melanogaster. Mol. Biol. Evol. 2014;31:1206–1219. doi: 10.1093/molbev/msu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers DW. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldini F. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 2013;11:e1001695. doi: 10.1371/journal.pbio.1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw WR. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5854–5859. doi: 10.1073/pnas.1401715111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willis JH. Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010;40:189–204. doi: 10.1016/j.ibmb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornman RS. Annotation and analysis of a large cuticular protein family with the R&R Consensus in Anopheles gambiae. BMC Genomics. 2008;9:22. doi: 10.1186/1471-2164-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornman RS, Willis JH. Extensive gene amplification and concerted evolution within the CPR family of cuticular proteins in mosquitoes. Insect Biochem. Mol. Biol. 2008;38:661–676. doi: 10.1016/j.ibmb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornman RS, Willis JH. Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol. Biol. 2009;18:607–622. doi: 10.1111/j.1365-2583.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornman RS. Molecular evolution of Drosophila cuticular protein genes. PloS One. 2009;4:e8345. doi: 10.1371/journal.pone.0008345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togawa T, Dunn WA, Emmons AC, Nagao J, Willis JH. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem. Mol. Biol. 2008;38:508–519. doi: 10.1016/j.ibmb.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinker DC. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8260–8265. doi: 10.1073/pnas.1302562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinker DC. Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genomics. 2013;14:749. doi: 10.1186/1471-2164-14-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altstein M, Nässel DR. Neuropeptide signaling in insects. Adv. Exp. Med. Biol. 2010;692:155–165. doi: 10.1007/978-1-4419-6902-6_8. [DOI] [PubMed] [Google Scholar]
- 38.Goetze JP, Hunter I, Lippert SK, Bardram L, Rehfeld JF. Processing-independent analysis of peptide hormones and prohormones in plasma. Front. Biosci. Landmark Ed. 2012;17:1804–1815. doi: 10.2741/4020. [DOI] [PubMed] [Google Scholar]
- 39.Stracker TH, Thompson S, Grossman GL, Riehle MA, Brown MR. Characterization of the AeaHP gene and its expression in the mosquito Aedes aegypti (Diptera: Culicidae) J. Med. Entomol. 2002;39:331–342. doi: 10.1603/0022-2585-39.2.331. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira CD, Tadei WP, Abdalla FC, Paolucci Pimenta PF, Marinotti O. Multiple blood meals in Anopheles darlingi (Diptera: Culicidae) J. Vector Ecol. 2012;37:351–358. doi: 10.1111/j.1948-7134.2012.00238.x. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto N. A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev. Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides. 2006;27:2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Antonova Y, Arik AJ, Moore W, Riehle MM, Brown MR. In: Insect Endocrinology. Gilbert LI, editor. Academic Press; 2012. pp. 63–92. [Google Scholar]
- 44.Swaminathan A, Gajan A, Pile LA. Epigenetic regulation of transcription in Drosophila. Front. Biosci. Landmark Ed. 2012;17:909–937. doi: 10.2741/3964. [DOI] [PubMed] [Google Scholar]
- 45.Cipressa F. Effete, a Drosophila chromatin-associated ubiquitin-conjugating enzyme that affects telomeric and heterochromatic position effect variegation. Genetics. 2013;195:147–158. doi: 10.1534/genetics.113.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arcà B. Positive selection drives accelerated evolution of mosquito salivary genes associated with blood-feeding. Insect Mol. Biol. 2014;23:122–131. doi: 10.1111/imb.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isawa H, Yuda M, Orito Y, Chinzei Y. A mosquito salivary protein inhibits activation of the plasma contact system by binding to factor XII and high molecular weight kininogen. J. Biol. Chem. 2002;277:27651–27658. doi: 10.1074/jbc.M203505200. [DOI] [PubMed] [Google Scholar]
- 48.Calvo E. Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor. J. Biol. Chem. 2007;282:26928–26938. doi: 10.1074/jbc.M705669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guittard E. CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 2011;349:35–45. doi: 10.1016/j.ydbio.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Waterhouse RM. Evolutionary Dynamics of Immune-Related Genes and Pathways in Disease-Vector Mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartholomay LC. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barillas-Mury C, Han YS, Seeley D, Kafatos FC. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 1999;18:959–967. doi: 10.1093/emboj/18.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta L. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bahia AC. The JAK-STAT pathway controls Plasmodium vivax load in early stages of Anopheles aquasalis infection. PLoS Negl. Trop. Dis. 2011;5:e1317. doi: 10.1371/journal.pntd.0001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.